Abstract
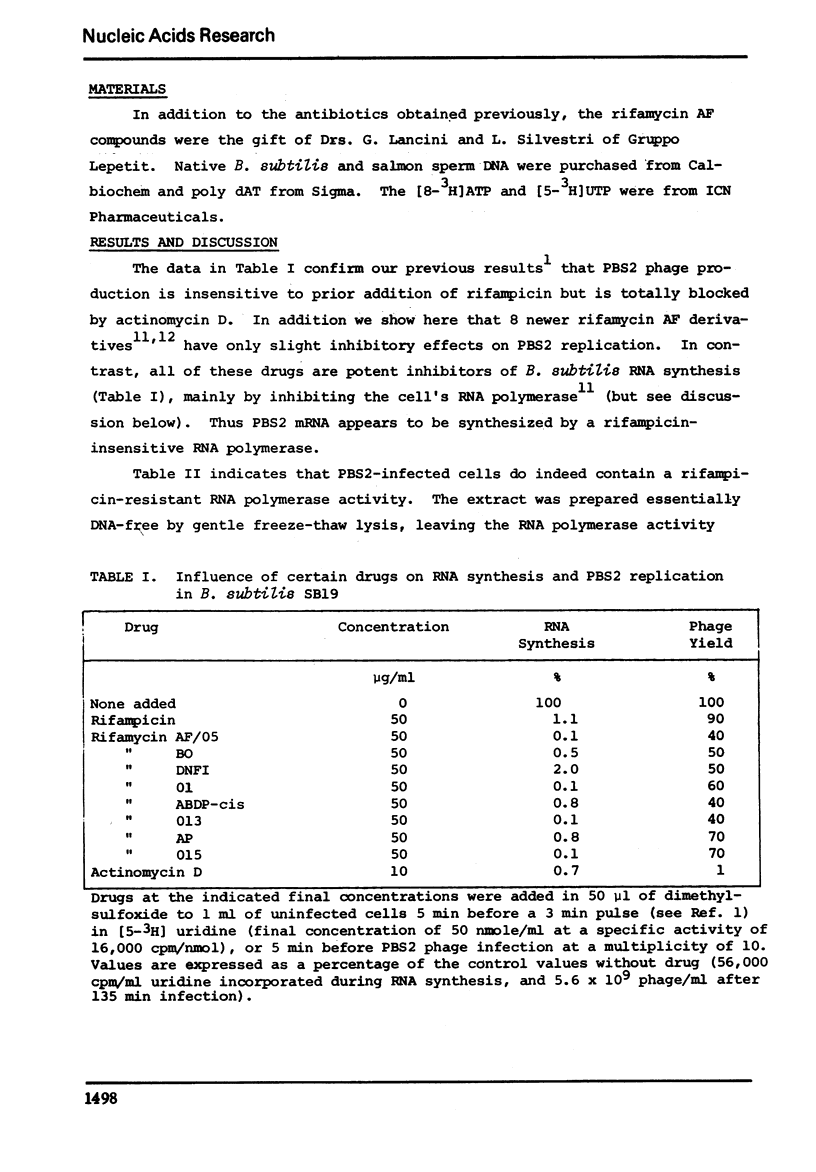
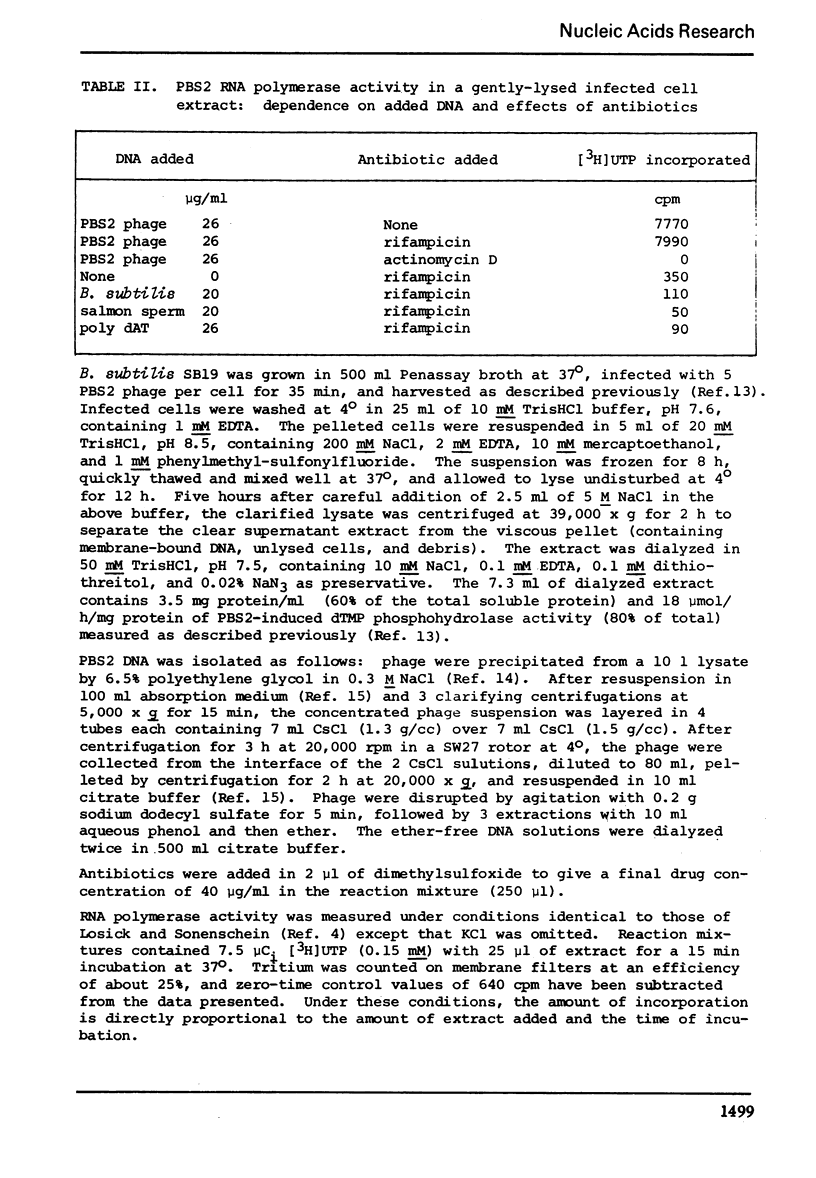
Bacteriophage PBS2 replication is unaffected by rifampicin and other rifamycin derivatives, which are potent inhibitors of Bacillus subtilis RNA synthesis. Extracts of gently-lysed infected cells contain a DNA-dependent RNA polymerase activity which is specific for uracil-containing PBS2 DNA. The PBS2-induced RNA polymerase is insensitive to rifamycin derivatives which inhibit the host's RNA polymerase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J., Hermoso J. M., Vinuela E., Salas M. Purification and properties of DNA-dependent RNA polymerase from Bacillus subtilis vegetative cells. Eur J Biochem. 1971 Aug 25;21(4):526–535. doi: 10.1111/j.1432-1033.1971.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Barrett K. J., Gibbs W., Calendar R. A transcribing activity induced by satellite phage P4. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2986–2990. doi: 10.1073/pnas.69.10.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M. J., Ring J. Characterization of T7 specific RNA polymerase. 3. Inhibition by derivatives of rifamycin SV. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1129–1136. doi: 10.1016/0006-291x(72)90330-0. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Bautz F. A., Bautz E. K. Different template specificities of phage T3 and T7 RNA polymerases. Nat New Biol. 1971 Mar 17;230(11):94–96. doi: 10.1038/newbio230094a0. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Maia J. C.C., Kerjan P., Szulmajster J. DNA-dependent RNA polymerase from vegetative cells and from spores of Bacillus subtilis. IV. Subunit composition. FEBS Lett. 1971 Mar 22;13(5):269–274. doi: 10.1016/0014-5793(71)80238-7. [DOI] [PubMed] [Google Scholar]
- Oasa S., Tsugita A. Poly A synthesizing activity in a constitutive subunit of RNA polymerase. Nat New Biol. 1972 Nov 8;240(97):35–38. doi: 10.1038/newbio240035a0. [DOI] [PubMed] [Google Scholar]
- Price A. R., Cook S. J. New deoxyribonucleic acid polymerase induced by Bacillus subtilis bacteriophage PBS2. J Virol. 1972 Apr;9(4):602–610. doi: 10.1128/jvi.9.4.602-610.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. R., Fogt S. M. Deoxythymidylate phosphohydrolase induced by bacteriophage PBS2 during infection of Bacillus subtilis. J Biol Chem. 1973 Feb 25;248(4):1372–1380. [PubMed] [Google Scholar]
- Price A. R., Frabotta M. Resistance of bacteriophage PBS2 infection to rifampicin, an inhibitor of Bacillus subtilis RNA synthesis. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1578–1585. doi: 10.1016/0006-291x(72)90894-7. [DOI] [PubMed] [Google Scholar]
- Rima B. K., Takahashi I. The synthesis of nucleic acids in Bacillus subtilis infected with phage PBS 1. Can J Biochem. 1973 Sep;51(9):1219–1224. doi: 10.1139/o73-161. [DOI] [PubMed] [Google Scholar]
- Riva S., Fietta A., Silvestri L. G. Mechanism of action of a rifamycin derivative (AF-013) which is active on the nucleic acid polymerases insensitive to rifampicin. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1263–1271. doi: 10.1016/0006-291x(72)90604-3. [DOI] [PubMed] [Google Scholar]
- Riva S., Silvestri L. G. Rifamycins: a general view. Annu Rev Microbiol. 1972;26:199–224. doi: 10.1146/annurev.mi.26.100172.001215. [DOI] [PubMed] [Google Scholar]
- Tomita F., Takahashi I. A novel enzyme, dCTP deaminase, found in Bacillus subtilis infected with phage PBS I. Biochim Biophys Acta. 1969 Mar 18;179(1):18–27. doi: 10.1016/0005-2787(69)90117-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


