Abstract
Impairment in two blood-brain barrier (BBB) efflux transporters, p-glycoprotein (Pgp) and low-density lipoprotein receptor-related protein-1 (LRP-1) are thought to contribute to the progression of Alzheimer’s disease (AD) by resulting in the brain accumulation of their substrate amyloid beta peptide (Aβ). The initial cause of impaired efflux, however, is unknown. We have shown that induction of systemic inflammation by intraperitoneal administration of lipopolysaccharide impairs the efflux of Aβ from the brain, suggesting that systemic inflammation could be one such initiator. In this study, we determined whether pre-administration of the antioxidant N-aceytlcysteine (Nac) has a protective effect against LPS-induced Aβ transporter dysfunction. Our findings were that Nac protected against LPS-induced Aβ transport dysfunction at the BBB through an LRP-1-dependent and Pgp-independent mechanism. This was associated with Nac exerting antioxidant effects in the periphery but not the brain, despite an increased rate of entry of Nac into the brain following LPS. We also found that Nac pre-administration resulted in lower blood levels of the cytokines and chemokines interferon-γ, interleukin-10, CCL2, CCL4, and CL5, but only lowered CCL4 in the cerebral cortex and hippocampus. Finally, we observed that hippocampal cytokine responses to LPS were decreased compared to cortex. These findings demonstrate a novel mechanism by which antioxidants prevent Aβ accumulation in the brain caused by inflammation, and therefore protect against AD.
Keywords: Amyloid beta, Alzheimer’s disease, neuroinflammation, oxidative stress, lipopolysaccharide, LRP-1, Pgp, N-acetylcysteine
Introduction
Accumulation of the amyloid beta peptide (Aβ) in the central nervous system (CNS) is thought to play a causative role in Alzheimer’s disease (AD) (Hardy and Selkoe, 2002). The events leading to pathological Aβ accumulation in the brain in the majority of AD cases are, however, unknown. One potential mechanism that would contribute to Aβ accumulation in the brain is defective clearance across the blood-brain barrier (BBB) (Deane et al., 2009). Two predominant efflux transporters have been implicated in this process: low-density lipoprotein receptor-related protein-1 (LRP-1) and p-glycoprotein (Pgp) (Brenn et al., 2011; Cirrito et al., 2005; Donahue et al., 2006; Hartz et al., 2010; Jaeger et al., 2009; Pflanzner et al., 2011; Shibata et al., 2000; van Assema et al., 2012). LRP-1 is predominantly expressed at the abluminal (brain-facing) surface of the brain endothelial cell, whereas Pgp is predominantly expressed at the luminal (blood-facing) membrane. Therefore, a working model has been proposed where Aβ in the brain interstitial fluid (ISF) is first internalized by LRP-1, and then is exported out of the endothelial cell by Pgp (Hartz et al., 2010). Both transporters become deficient in AD (Donahue et al., 2006; Hartz et al., 2010; Shibata et al., 2000; van Assema et al., 2012), and for this reason represent likely players in AD progression.
Inflammation and oxidative stress are associated with AD as well as many of its risk factors, including aging, obesity, traumatic brain injury, hypertension, high cholesterol, and diabetes (Akiyama et al., 2000; Alzheimer's Association, 2012; Sultana and Butterfield, 2010). Aβ itself can cause oxidative stress and inflammation in the brain (Butterfield, 2002; Salminen et al., 2009), however it is also possible that oxidative stress and inflammation could act upstream of Aβ to exert neurotoxic effects (Lee et al., 2008; Tamagno et al., 2011). Although mechanistic links between inflammation, oxidative stress, and Aβ accumulation in AD have been extensively studied in the CNS, less is understood about how inflammation and oxidative stress in the periphery contribute to AD. Clinical studies have shown that elevated inflammatory markers in the blood confer increased AD risk and can accelerate cognitive decline, suggesting that systemic inflammation plays a role in the onset and progression of AD (Holmes et al., 2009; Tan et al., 2007).
The BBB is simultaneously exposed to both CNS and peripheral compartments, and thus is affected by signals from either compartment. Both inflammation and oxidative stress have been shown to alter BBB function (Abbott, 2000; Banks and Erickson, 2010; Freeman and Keller, 2012; Haorah et al., 2007). Therefore, inflammation and oxidative stress in the brain or periphery could induce BBB changes that contribute to AD, such as Aβ transporter dysfunction. This is supported by the finding that systemic inflammation induced by intraperitoneal injection of lipopolysaccharide in young mice is sufficient to impair Aβ efflux from the brain (Jaeger et al., 2009). The Aβ transporter Pgp also becomes impaired following LPS (Jin et al., 2011; Salkeni et al., 2009). Furthermore, increased oxidative damage to LRP-1 has been observed in AD hippocampus compared to age-matched controls, suggesting that LRP-1 is functionally impaired in AD (Owen et al., 2010).
Oxidative stress is an important mediator of CNS damage during systemic inflammation (Berg et al., 2011), and antioxidants may protect the CNS during this process. This is supported by the finding that the antioxidant N-acetylcysteine protects against memory impairment in rats that survived sepsis (Barichello et al., 2007). The antioxidant Nac also has protective effects in rodent models of AD (Farr et al., 2003; Huang et al., 2010), and is associated with improved cognitive performance in a preliminary human AD trial (Adair et al., 2001). Following intraperitoneal administration, Nac rapidly accumulates in peripheral organs (McLellan et al., 1995). More sensitive methods show, however, that Nac crosses the BBB and therefore can act locally in the CNS (Farr et al., 2003). ROS produced in response to inflammatory stimuli can exacerbate inflammation by activating a number of signaling molecules, including NFk-B, AP-1 MAPK (Hsu and Wen, 2002; Lo et al., 1996; Morgan and Liu, 2011), and promoting inflammasome activation (Martinon, 2010). For this reason, antioxidants such as Nac have anti-inflammatory effects by limiting the ability of ROS to potentiate inflammatory cytokine production (Palacio et al., 2011).Therefore, Nac could protect against damage to the CNS by limiting inflammation and oxidative stress in both the CNS and peripheral compartments. Due to the anatomical location of the BBB, signals from either the peripheral tissue or central nervous system compartment could alter its function. This suggests that the neuroprotective effects of Nac may be through suppression of inflammatory and oxidative stress signals to the BBB. In this study, we determined whether administering Nac prior to inducing systemic inflammation with LPS protected against impairment in Aβ transport across the BBB, and through which transporters this effect was mediated. We also measured markers of oxidative stress and cytokine profiles in serum, as well as the cortex and hippocampus as these are brain regions susceptible to damage in both inflammation and AD. Our findings were that Nac protected against LRP-1 but not Pgp dysfunction at the BBB, and this was associated with reduced oxidative stress and inflammation predominantly in serum. We also observed significantly different cytokine responses in cortex vs. hippocampus. These results demonstrate a novel mechanism by which Nac protects against BBB dysfunction under conditions of systemic inflammation, and provide insight on the relationships between systemic inflammation and AD.
Methods
Animal use and treatment regimens
All animal protocols were performed in an AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) accredited facility and approved by the animal committee of the VA and St Louis University Medical Centers. Male CD-1 mice were purchased from Charles River and kept on a 12/12 hour light/dark cycle with food and water freely available. Mice at 6–8 weeks of age were treated with three intraperitoneal (IP) injections of 3mg/kg LPS from Salmonella typhimurium (Sigma, St. Louis, MO) dissolved in sterile normal saline over a 24-hour period as previously described (Jaeger et al., 2009). The first injection was given in the morning, and the second and third injections were given at 6 and 24 hours following the first injection, respectively. Some groups of mice were also pretreated with 100 mg/kg Nac in sterile normal saline 30 minutes prior to each saline or LPS injection. All mice were studied at 28 hours following the first injection of LPS. Mice given LPS displayed overt sickness behavior and weight loss, however no mice died as a result of treatment. A total of 281 mice were used in this study: 68 for measurement of Aβ efflux, 28 for measurement of Nac influx, 65 for alpha-2 macroglobulin (a2M) efflux, 47 for verapamil brain perfusion studies, 36 for oxidative stress measures, and 37 for measurement of cytokines.
Iodination of Aβ, albumin, and α2M
Murine Aβ1-42 was purchased from Bachem (Torrance, CA) and bovine serum albumin (BSA) and human α2M from Sigma (St. Louis, MO). Lyophilized Aβ peptide was resuspended at a concentration of 1mg/ml in 0.1M ammonium hydroxide to prevent aggregation, aliquotted, and stored frozen at −80°C for up to three months. To enable LRP-1 binding, human α2M was activated with methylamine according to methods described previously (Imber and Pizzo, 1981), and stored at −20°C. 5 µg of Aβ, activated a2M (a2m*), or albumin was labeled with 0.5 mCi 125I or 2mCi 131I (Perkin Elmer) using the chloramine-T method (Greenwood et al., 1963), and purified on a Sephadex G-10 column (Sigma). To assess the purity of iodinated proteins, an aliquot of the labeled protein fraction was precipitated in 15% trichloroacetic acid. All iodinated compounds consistently showed greater than 95% activity in the precipitate, and iodinated Aβ and α2M* were always used within 24 hours of radioactive labeling. The iodinated versions are referred to as I-Aβ, I-α2M*, and Ialbumin.
Measurement of I-Aβ and I-α2M efflux from brain
To measure I-Aβ and I-α2M* efflux, labeled protein solutions were diluted to a concentration of 20,000 counts per minute (CPM)/µl in BSA/lactated Ringer’s solution. LPS/Nac-treated mice were anesthetized with 40% urethane, and 1 µl of labeled protein was injected into the lateral ventricle of the brain (ICV) by reflecting the scalp and drilling a hole 1mm lateral and 0.5mm posterior to the bregma, followed by injection at a depth of 2.5 mm using a 26g Hamilton syringe. Brains were collected 10 minutes post-injection (t10). To account for CNS distribution, an identical treatment group was overdosed with urethane, and protein was injected ICV 10 min post-mortem (t0). Brains were collected from this group 10 min post-injection. To accurately determine the activity injected, three 1µl aliquots of sample were injected into blank tubes using a Hamilton syringe (injection checks). Radioactivity in brain and injection checks was measured using a Wizard2 automatic gamma counter (Perkin Elmer, Waltham MA). Brain efflux was calculated by first determining the percent of injected material remaining in brain in t10 and t0 groups:
%Inj/brain = 100(CPM in brain/CPM injection check)
Delta values were calculated by subtracting individual values of %Inj/brain from each t10 group from the average %Inj/brain of each t0 group:
Delta %Inj/brain = (Average %Inj/brain t0)-(%Inj/brain t10)
Measurement of Nac uptake by brain
1×106 CPM of 14C-Nac (American Radiolabeled Chemicals, St. Louis, MO) and I-albumin were prepared in BSA/lactated Ringer’s solution and co-injected in the jugular vein of mice treated with LPS or saline. Whole brains and blood from the carotid artery were collected at 1, 2, 5, 10, 15, 20, and 30 minutes. Blood was allowed to clot, spun at 5000g to separate serum from blood cells, and 50 µl serum was analyzed by gamma counting or LSC. Brains from both groups were analyzed by gamma counting for 125I-albumin, then solubilized and suspended in liquid scintillation cocktail for counting 14C-Nac activity. To exclude signal from I-albumin, counts were gated between 60 and 156 keV. Triplicate counts from an injection volume (injection check) of 14C-Nac were measured in brain or serum matrix to account for differences in quenching. Brain and serum values were then expressed as a percent of the average injection check (%Inj). The rate of I-albumin and 14C-Nac brain uptake was determined using multiple-time regression analysis (Patlak et al., 1983). This analysis method determines the influx rate of a substance by plotting the brain/serum ratio vs. a corrected time parameter called exposure time. This correction is necessary to negate the influence of peripheral clearance of a substance from blood that would otherwise overestimate the influx rate. Exposure time was calculated from the formula:
where t equals experimental clock time, Cp represents the level of radioactivity in the serum over time and Cpt is the level of radioactivity in the serum at time t. Brain/serum ratios were then calculated from the following formula:
Tissue/serum ratio = (%Inj/g brain)/(%Inj/µl serum)
To correct for alterations in vascular space and/or vascular permeability which occur with LPS administration, tissue/serum ratios for I-albumin were subtracted from those for 14C-Nac. The corrected tissue/serum ratios were plotted against exposure time calculated for 14C-Nac, and the unidirectional influx rate (Ki) determined from the slope of the linear portion of the curve. %Inj/g brain was calculated for 14C-Nac from the corrected tissue/serum ratio:
%Inj/g brain = (corrected tissue/serum ratio)(%Inj/ µl serum)
Measurement of verapamil influx
Mice were anesthetized with an intraperitoneal injection of 40% urethane solution, and influx of the Pgp ligand verapamil was measured using the cardiac perfusion method (Smith and Allen, 2003). In brief, the heart was exposed via thoracotomy, the descending aorta clamped, and the jugulars cut bilaterally to prevent infused solution from returning to the heart. The left ventricle of the heart was then infused with Zlokovic’s buffer (7.19 g/l NaCl, 0.3 g/l KCl, 0.28 g/l CaCl2, 2.1 g/l NaHCO3, 0.16 g/l KH2PO4, 0.17 g/l anhydrous MgCl2, 0.99 g/l D-glucose, and 10 g/l bovine serum albumin added the day of perfusion) at a rate of 2 ml/min for 2 min. The perfusion contained 100,000 disintigrations per minute (dpm)/ml of 3H-verapamil and 100,000 dpm/ml of 14C-sucrose as a marker of vascular space/BBB permeability. Periodic sampling of perfusion fluid from the tip of the catheter was performed to determine the exact concentration of radioactivity being perfused (injection checks). The mouse was immediately decapitated at the end of the perfusion time, and the brain harvested, weighed, and then placed in a glass vial. Brains were solubilized, and then brains and injection checks were counted for 3H and 14C isotopes using a dual counting program in a liquid scintillation counter (Packard). The brain/perfusion ratio (µl/g) was calculated using the equation:
Brain/perfusion ratio: (DPM/g brain)/(DPM/µl perfusate)
This equation was used to calculate the ratios for both 3H-verapamil and 14C-sucrose. The 14C-sucrose values were subtracted from the values for 3H-verapamil to yield the amount of 3H-verapamil which had entered the brain. As verapamil is a substrate of the efflux pump P-gp, the activity of P-gp is inversely related to the brain/perfusion ratio.
Dot blot measurements of protein carbonyl, HNE-protein adducts, and 3-NT
Protein was extracted from hemibrains by homogenizing in 5 volumes of ice-cold lysis buffer (PBS plus 1% Triton X-100, 5mM EDTA, 1mM PMSF, and protease inhibitor cocktail) followed by shaking vigorously for 30 minutes at 4° C. Extracts were then centrifuged at 20,000g for 10 minutes at 4° C, and supernatants were used for protein analysis. Protein was quantified in all extracts by BCA assay (Thermo Scientific). Measurement of protein carbonyl was conducted according to oxyblot kit instructions (Millipore, Temecula, CA). 32 ng of derivitized protein per sample was loaded on a nitrocellulose membrane in duplicate using a dot blot apparatus (Biorad, Hercules CA), then blocked and probed with primary and secondary antibodies according to kit instructions. HNE-adducts and 3-NT were measured by loading 1µg of protein on a nitrocellulose membrane, blocking with 5% milk, and probing with an anti-HNE antibody (alpha-diagnostics 2µg/ml in 5% milk) or anti 3-NT (Millipore; 2µg/ml in 5% milk) overnight at 4°C. The membranes were then washed and probed with anti-rabbit secondary (Santa Cruz, 1:5000) for 1 hour at room temperature. Immunoreactive spots on both membranes were visualized using West Pico chemiluminescent substrate (Thermo Scientific) and all images were captured using an ImageQuant LAS4000 CCD imaging system (GE Life Sciences, Piscataway, NJ). Densitometric analysis was done using IQTL software (GE Life Sciences).
Measurement of oxidized and reduced glutathione in brain
Total and oxidized glutathione was measured in hemibrains using a colorimetric glutathione assay kit from Cayman chemicals. Brains were extracted in MES buffer and deproteinated according to kit instructions, then stored at −20 c until the assays were performed. On the day of assay, the deproteinated extracts were thawed, and diluted in MES buffer prior to assay with or without derivitization 2-vinylpyridine to measure oxidized and total glutathione, respectively. Glutathione signal was measured using a kinetic method of absorbance detection, and the levels of reduced glutathione were calculated by subtracting oxidized glutathione from total levels.
Cytokine and chemokine measurements in brain and serum
Serum, cortex, and hippocampus were collected from mice treated with LPS/Nac, and stored frozen until assay/extraction. To prevent contamination of blood cytokines in brain measurements, the upper circulatory system of each mouse was perfused with ice-cold PBS prior to removing the brain. Brain tissues were extracted for soluble protein by homogenizing in a 5× volume of extraction buffer (TBS plus 0.2% Triton X-100, 2mM EDTA, 1mM PMSF, and protease inhibitor cocktail). Homogenates were then processed and total protein quantified by BCA assay. A panel of 23 cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-17, Eotaxin (CCL11), G-CSF, GM-CSF, IFN-γ, KC (CXCL1), MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), and TNF-α) were measured in brain and serum samples using a murine Bioplex assay kit from Biorad. All samples were diluted 1:3 in sample diluent provided in the kit, and processed according to assay directions. Plates were read on a Bioplex 200 (Biorad). Cytokines which were undetected for a sample were assigned a value of zero, and only cytokines which were greater than 50% detectable in any group were considered for analysis.
Statistical analysis
All statistical analyses were done using Prism 5 software (GraphPad Inc, San Diego, CA). Data on Nac influx were analyzed by linear regression, and the remaining data were analyzed by two-tailed Student’s t-tests (two groups), one-sample t-test (for cytokines which were not detected in the absence of LPS stimulation), one way ANOVA and Newman-Keuls multiple comparisons test (more than two groups, one tissue), or two-way ANOVA with Bonferroni post-test for multiple comparisons (cortex vs. hippocampus comparisons). Bar graphs are expressed as mean +/− SEM.
Results
Effects of Nac on LPS-induced deficits in Aβ efflux and efflux transporter function
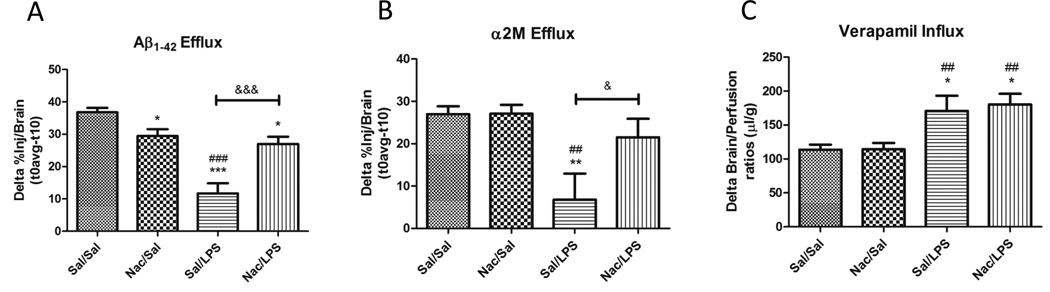
Previously, we have observed a decreased efflux of ICV-injected I-Aβ from the brain following repeated injections of IP LPS. Using the same regimen, we determined whether pre-administration of Nac prior to each LPS injection protected against the LPS-induce efflux deficit. Figure 1a shows that Nac protects against the efflux deficit in Aβ induced by LPS. We next determined whether LPS impaired the Aβ efflux transporters LRP-1 and Pgp, and whether Nac protected against LPS effects. Figure 1b shows that LPS significantly inhibited brain efflux of ICV-injected I- α2M*, an LRP-1 ligand, and that Nac protected against this inhibition. To test whether this effect was also observed for Pgp, a brain perfusion method was used which prevents interaction of the drug with serum binding proteins that limit its BBB influx. Included in the perfusate were the Pgp ligand 3H verapamil, and 14C sucrose that was used to correct for changes in vascular space and BBB permeability. Because Pgp limits the brain influx of verapamil, increased brain levels are representative of decreased Pgp function. Our findings, shown in Figure 1c demonstrate that LPS causes Pgp dysfunction that is not blocked by Nac. Although Nac was protective against LPS-induced decreases in Aβ efflux, we did observe a slight, but statistically significant decrease in Aβ efflux in the Nac control group. This treatment did not alter LRP-1 or Pgp function, and therefore indicates that inhibition of Aβ efflux by Nac alone is through a process independent of LRP-1 or Pgp. Together, these data demonstrate that the protective effects of Nac against LPS-induced decreases in Aβ transport are mediated through LRP-1 but not Pgp.
Figure 1. Effects of Nac pre-administration on LPS-induced dysfunction of Aβ transport across the BBB.
Disappearance of ICV-injected I-Aβ (A) or I-a2M* (B), an LRP-1 ligand, from brain was measured at 10 minutes and subtracted from the average activity remaining at time 0. The difference is shown, and reflects brain efflux; n= 7–11 per group. Influx of 3H verapamil, a Pgp ligand, was measured using the in situ brain perfusion method (C). Uptake into brain was expressed as the brain/perfusion ratio, and corrected for changes in vascular permeability by subtracting uptake values for 14C sucrose. Increased brain/perfusion ratios indicate decreased Pgp function; n=11–13 per group. All data were analyzed by one way ANOVA and Newman-Keuls multiple comparisons tests; *p < 0.05, **p < 0.05, ***p < 0.001 vs. Sal/Sal; ##p < 0.01, ###p < 0.001 vs. Nac/Sal; &p < 0.05, &&&p < 0.001 vs. group indicated.
Effects of Nac on LPS-induced oxidative stress in brain and serum
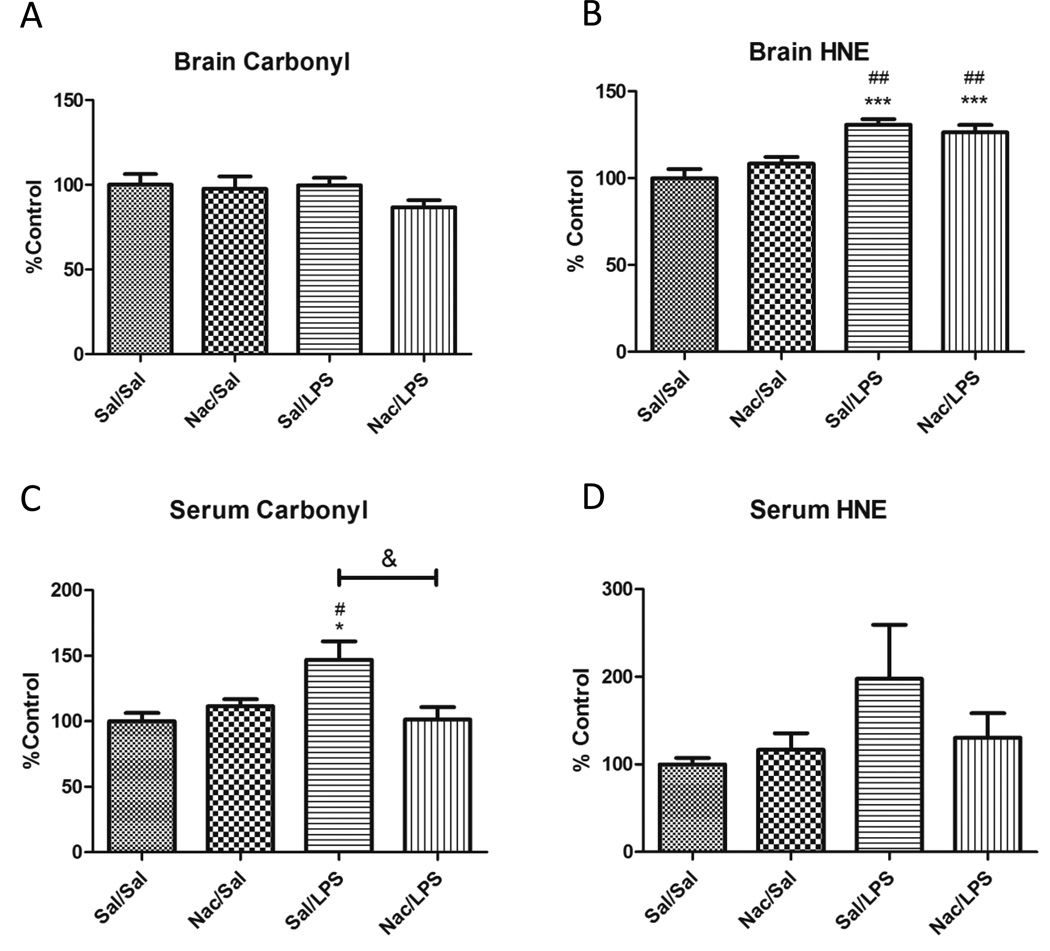
Systemic inflammation induced by LPS causes increased oxidative stress in peripheral tissues as well as in the brain. Oxidative stress in the brain or periphery could influence BBB function due to its anatomical location in both compartments. Therefore, we determined whether Nac had protective effects against LPS-induced oxidative stress in both brain and serum. We used three protein markers of oxidative/nitrative stress in our study: carbonyl, HNE, and 3-NT. We also measured the ratio of oxidized to reduced glutathione in brains only, as serum levels of oxidized glutathione are too low to be measured by this method. Figure 2 shows the results of our measurements of protein markers of oxidative stress. Only carbonyl and HNE are shown, as 3-NT was not detectable by this method even though we did detect signal from peroxynitrite-treated BSA. In brain, LPS did not increase levels of protein carbonyl (Figure 2a), but significantly increased levels of HNE-modified protein (Figure 2b). Nac did not protect against the LPS-induced increase in HNE in the brain. We saw no significant differences in the CNS ratios of oxidized to reduced glutathione in any of our treatments (data not shown). In serum, LPS caused a significant increase in protein carbonyl (Figure 2c), and Nac blocked this effect. Although trends were similar for HNE, these did not reach statistical significance (Figure 2d). Together, these results indicate that Nac is primarily acting in the periphery to limit oxidative stress in our model.
Figure 2. Effects of LPS and Nac on oxidative stress markers in brain and serum.
Protein carbonyl (A and C) and HNE adducts (B and D) in brain (A and B) and serum (C and D) were measured by slot blot, n=6–9. Nac reduced oxidative stress in serum but not brain. Data analyzed by one way ANOVA and Newman-Keuls Multiple Comparisons test; *p < 0.05, ***p < 0.001 vs. Sal/Sal, #p < 0.05, ###p < 0.001 vs. Nac/Sal, &p < 0.05 vs. group indicated.
LPS effects on Nac uptake into brain
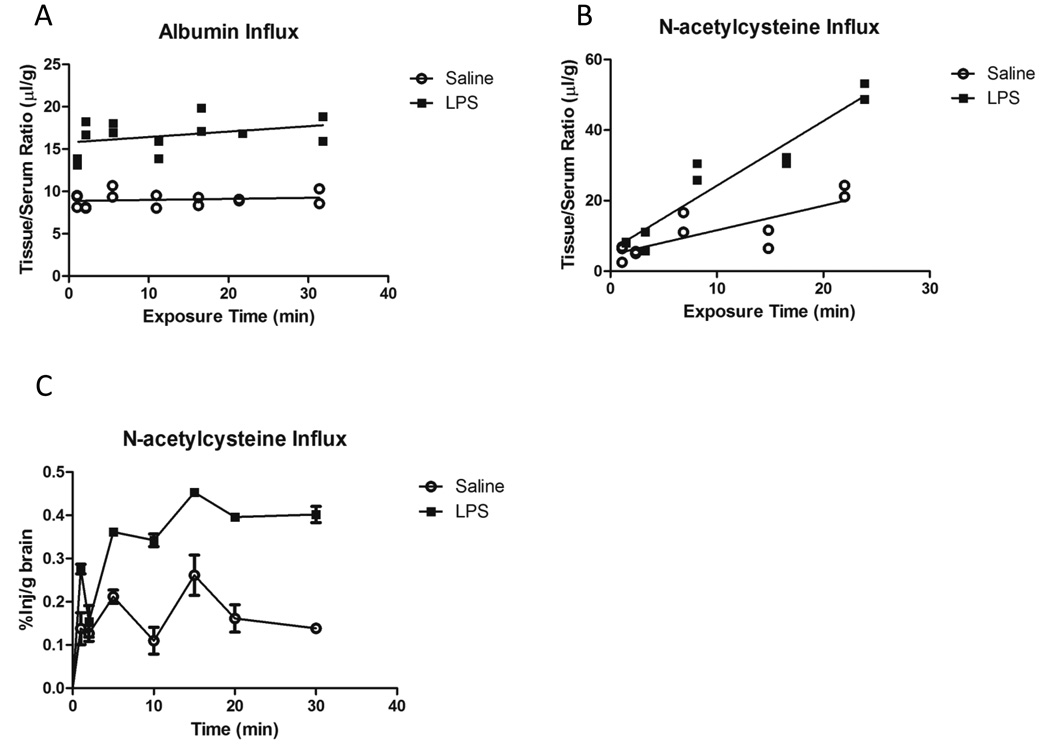
Because Nac crosses the BBB (Farr et al., 2003), it is possible that the inability of Nac to lower HNE-modified proteins in brain following LPS is due to LPS effects on BBB transport of Nac. To test this, we compared the influx of 14C-Nac in mice treated with saline or LPS. To correct for changes in vascular space and BBB permeability, we included I-albumin as a tracer in the injection. Figure 3a shows that the y-intercept of the I-albumin time curve is significantly elevated with LPS, reflecting an increase in vascular space and BBB permeability. Tissue/Serum ratios for 14C-Nac were corrected for these changes by subtracting I-albumin values, and the differences are shown in Figure 3b. The unidirectional influx constant (slope of the line) for Nac following LPS treatment (1.833 ± 0.1970 µl/g-min, r2=0.9154) was significantly increased compared to that of saline (0.6954 ± 0.1663 µl/g-min, r2=0.6601). Figure 3c shows brain uptake data represented as %Injection/g of brain vs. time. Values peaked at approximately 0.25 and 0.40 %Inj/g for saline and LPS, respectively. These data show that LPS increases the BBB permeability of Nac, and therefore does not explain the inability of Nac to protect against oxidative stress in the brain in our LPS model.
Figure 3. Brain influx of I-albumin and 14C-Nac following LPS treatment.
A) Albumin showed no significant difference in its influx rate (0.01218 ± 0.02102 µl/g-min Sal vs. 0.06413 ± 0.05179 µl/g-min LPS). A significant difference in the Y-intercept was observed (p < 0.001). B) Nac showed a significant difference in its influx rate (0.6954 ± 0.1663 µl/g-min Sal vs. 1.833 ± 0.1970 µl/g-min LPS; p < 0.001). Data analyzed by linear regression. C) Data represented as %Inj/g vs. clock time. A peak uptake of approximately 0.25 and 0.4 %Inj/g was observed for saline and LPS groups, respectively.
Effects of Nac on LPS-induced cytokine levels in cortex, hippocampus, and serum
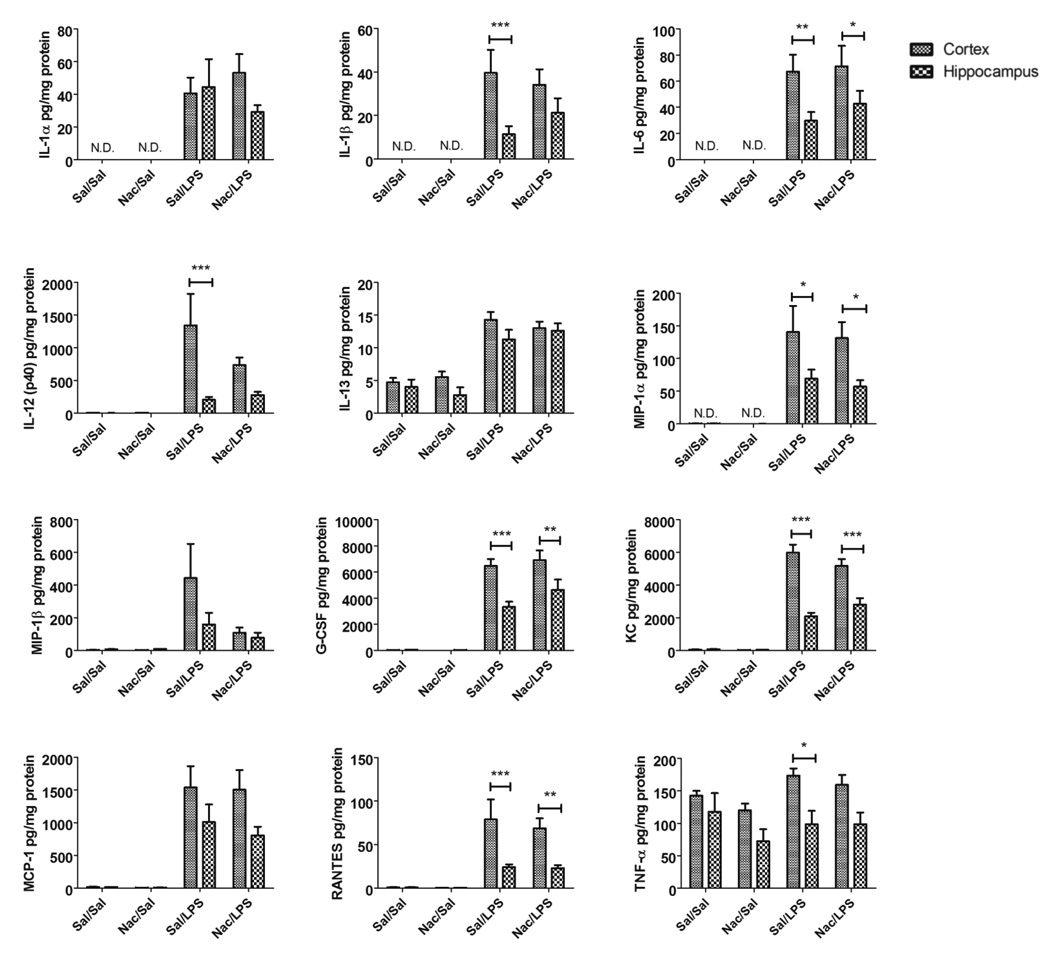
In addition to its antioxidant properties, Nac is also anti-inflammatory. This could have important effects on cytokine interactions with the BBB. Therefore, we determined whether Nac alters cytokine responses in serum or in two of the brain regions that are implicated in both AD and sepsis: the cortex and hippocampus. Of 23 cytokines measured in these tissues, LPS significantly increased the expression of 13 in serum, 12 in cortex, and 11 in hippocampus (Supplementary figures 1–3 and Table I, see table entries with one, two, or three asterisks). Nac pre-administration significantly suppressed the cytokines and chemokines IL-10, IFN-γ, MIP-1β, MCP-1, and RANTES in serum, but only the cytokine MIP-1β in cortex and hippocampus (Supplementary figures 1–3 and Table I, see table entries with two asterisks). KC levels were significantly potentiated in hippocampus when Nac was given prior to LPS (Supplementary figure 3 and Table I, see table entries with three asterisks). Detectable cytokines which were not increased with LPS at the time point studied included IL-2, IL-3, IL-5, IL-12(p70), IL-13, IL-17, GM-CSF, and TNF-α in serum, and TNF-α in cortex and hippocampus. In each treatment group, we also compared cytokine responses in cortex compared to hippocampus. We found no significant differences in cytokine levels between regions without LPS stimulation with the exception of eotaxin, which was not detected in hippocampus in any of our treatments. The hippocampus expressed significantly lower levels of the cytokines and chemokines IL-12(p40), MIP-1α, GCSF, KC, RANTES, and TNF-α compared to cortex under LPS-stimulated conditions (Figure 4). For all analytes except IL-12 (p40), and TNF-α, these differences remained significant when mice were also pre-treated with Nac. These results show that Nac exerts its anti-inflammatory properties predominantly in the serum compartment, and that cortical cytokine responses are increased compared to hippocampus in our model.
Table I. Summary of cytokine responses to LPS and Nac in cortex, hippocampus, and serum.
Cytokines that were significantly elevated following LPS treatment and unaffected by Nac are marked with a single asterisk, two asterisks indicate that elevations induced by LPS were significantly inhibited by Nac, and three asterisks indicate that LPS-induced elevations were significantly potentiated by Nac. An absence of asterisk indicates baseline levels were either undetectable (brain IL-10, INF-gamma, eotaxin) or detectable but that LPS had no effect (serum IL-13). All data were analyzed by one way ANOVA with Newman-Keuls multiple comparisons test, n=8–10 per group, p-value cutoff was 0.05.
| Cytokine/Chemokine | Serum | Cortex | Hippocampus |
|---|---|---|---|
| IL-1α | * | * | * |
| IL-1β | * | * | * |
| IL-6 | * | * | * |
| IL-10 | ** | ||
| IL-12 (p40) | * | * | * |
| IL-13 | * | * | |
| Eotaxin (CCL11) | * | * | |
| IFN-γ | ** | ||
| MIP-1α | * | * | * |
| MIP-1β | ** | ** | ** |
| G-CSF | * | * | * |
| KC (CXCL1) | * | * | *** |
| MCP-1 (CCL2) | ** | * | * |
| RANTES (CCL-5) | ** | * | * |
Figure 4. Comparison of cytokine levels in cortex vs. hippocampus.
Cytokines found to be significantly elevated at the protein level in both cortex and hippocampus by LPS were compared to determine whether there was a significant difference in cytokine expression between brain regions in any of the treatment groups. Data were analyzed by two-way ANOVA followed by Bonferroni multiple-comparisons tests, n=8–10 per group; *p < 0.05, **p < 0.05, ***p < 0.001 vs. group indicated.
Discussion
In this study, we have shown that Nac protects against the LPS-induced inhibition of Aβ brain-to-blood efflux through an LRP-1-dependent and Pgp-independent mechanism. This was associated with reductions in oxidative stress markers in serum but not brain, and altered cytokine profiles in serum, cortex, and hippocampus. We also found that the magnitude of increase for many cytokines following LPS was higher in cortex than hippocampus. These results highlight important mechanistic considerations for the regulation of Aβ transport across the BBB during inflammation, and accumulation of Aβ in the brain in AD.
Deficits in both LRP-1 and Pgp at the BBB have been proposed to cause Aβ accumulation in the brain (Cirrito et al., 2005; Hartz et al., 2010; Jaeger et al., 2009; Shibata et al., 2000). Decreased efflux has also been reported for Aβ during systemic inflammation (Jaeger et al., 2009). We have recently shown that LPS does not significantly alter the expression of LRP-1 or Pgp in isolated brain microvessels (Erickson et al., 2012). Our findings here and elsewhere show, however, that both LRP-1 and Pgp are functionally inhibited by LPS (Erickson et al., 2012; Jin et al., 2011; Salkeni et al., 2009). Because Pgp is highly expressed in brain endothelial cells, decreased function of Pgp is most likely explained by post-translational effects. LRP-1, however, was found to be downregulated and mislocalized in cultured brain endothelial cells and upregulated in cultured pericytes, indicating that LRP-1 deficits at the brain endothelial cell are masked by pericyte overexpression in isolated microvessels (Erickson et al., 2012; Kovac et al., 2011). In this study, we found that LRP-1 and Pgp at the BBB are regulated by different mechanisms during inflammation. LRP-1 dysfunction was inhibited by Nac pretreatment, whereas Nac did not protect against Pgp dysfunction. Furthermore, despite our observations that LPS inhibited Pgp function by about 50%, Nac pretreatment resulted in Aβ efflux comparable to that of the Nac control group. This shows that in our model of systemic inflammation, inhibition of Aβ transport that is blocked by Nac is mediated through LRP-1 rather than Pgp. Recently, it has been reported that the LRP-1 ligand APOE protects against BBB disruption by inhibiting the overexpression of cyclophilin A in the pericyte (Bell et al., 2012). Cyclophilin A is also upregulated by ROS, and participates in the vascular inflammatory process by activating NF-kB signaling (Bell et al., 2012; Satoh et al., 2010). Therefore, cyclophillin A may be a potentially important mediator of inflammatory processes which regulate BBB transport. Despite our finding that Nac protects LRP-1 dysfunction during inflammation, Nac treatment alone (i.e., in the absence of LPS treatment) had a slight, but significant inhibitory effect on Aβ efflux. This effect was not observed for α2M or verapamil, indicating that this effect is LRP-1 and Pgp independent. Furthermore, our delta value for Aβ efflux was approximately 36% higher than that of α2M, raising the possibility that there is an undiscovered, Nac-sensitive Aβ transporter at the BBB. Recently, additional transporters have been identified at the BBB which participate in Aβ efflux including the cellular prion protein (Pflanzner et al., 2012) and multidrug transporters ABCG2 and ABCG4 (Do et al., 2012). Whether Nac inhibits these transporters at the BBB is unknown, but would be an important question to address in future studies.
Characterization of the protective effects of Nac against LPS revealed that oxidative stress was inhibited in the serum but not the brain, despite our observations of increased BBB transport of Nac. This finding has multiple explanations. First, Nac is a hydrophilic molecule, and has a tendency to concentrate in the cytoplasm. Therefore, its inability to lower HNE formation in the brain could be explained by a lack of proximity to cell membranes where HNE is generated. A second possibility is that although Nac can cross the BBB, it may not reach sufficient levels in brain to exert protective effects under the conditions studied. We observed that the peak brain uptake of Nac ranged between 0.25% and 0.4% of the total injection per gram of brain tissue, depending on whether mice were treated with saline or LPS. For a mouse brain weighing 0.5g, the percent of Nac taken up into brain would therefore be 0.1% for saline and 0.2% for LPS of the total injection. Less sensitive autoradiography-based methods have shown that Nac concentrates in peripheral organs, with no detectable uptake in the CNS (McLellan et al., 1995). Therefore, the low level of CNS uptake of Nac observed in this study and elsewhere could explain the inability of an acute regimen of Nac to block oxidative damage in the CNS. Despite this, Nac has protective effects in the brains of rodents with AD (Farr et al., 2003; Fu et al., 2006; Huang et al., 2010), and its ability to increase CNS glutathione levels indicates that its action is local (Pocernich et al., 2000). These models, however, used chronic pretreatment regimens of Nac which occurred over the duration of weeks. This raises a third possibility: that the effectiveness of Nac to limit oxidative stress in the brain requires chronic treatment. Despite the inability of Nac to lower oxidative stress in the brain after LPS in our model, limiting oxidative stress in the periphery during inflammation was sufficient to protect LRP-1 function. This highlights a novel mechanism by which Nac could protect against AD.
In light of these findings, an interesting question is whether peripheral oxidative stress could mediate LRP-1 dysfunction in other diseases that increase AD risk. LRP-1 dysfunction at the BBB is also observed in a rodent model of streptozotozin-induced diabetes (Hong et al., 2009), which has increased serum markers of oxidative stress (Sen et al., 2011). Increased serum markers of oxidative stress have been observed in individuals with AD and mild cognitive impairment (MCI) (Padurariu et al., 2010), as well as metabolic syndrome (Furukawa et al., 2004; Hansel et al., 2004) which is a risk factor for AD (Alzheimer's Association, 2012). A recent study revealed an important mechanistic link by which oxidative stress contributes to AD pathology in a mouse model of hypertension by activating the receptor for advanced glycation endproducts at the BBB (Carnevale et al., 2012). It was not determined whether Aβ efflux is also impaired in this model, but could be a likely consequence of RAGE activation due to downstream inflammatory signaling. Importantly, AD pathology following hypertension was blocked by enteral antioxidant treatment (Carnevale et al., 2012). The peripheral actions of Nac may therefore be important parameters to consider for individuals with risk factors for AD such as systemic inflammation and metabolic syndrome (Cunningham, 2011; Tan et al., 2007; Yaffe et al., 2004), due to the association with decreased BBB efflux of Aβ.
Nac also showed protective effects through its anti-inflammatory properties in our model. These findings are consistent with reports of anti-inflammatory effects of Nac in vitro (Palacio et al., 2011), and were expected due to the participation of oxidative stress in the inflammatory response. In serum, Nac pretreatment reduced the LPS-induced expression of IL-10, IFN-γ, MIP-1β, MCP-1, and RANTES, whereas in cortex and hippocampus, only MIP-1β was reduced. Therefore, the majority of anti-inflammatory effects of Nac were also restricted to the serum compartment, supporting that Nac actions in the periphery protect against LRP-1 dysfunction. The chemokines MIP-1β, MCP-1, and RANTES and the cytokine IFN-γ have all been implicated in immune cell interactions with the brain endothelium (Banks and Kastin, 1996; Dzenko et al., 2005; Huynh and Dorovini-Zis, 1993; Louboutin et al., 2011; McCarron et al., 1993; Shukaliak and Dorovini-Zis, 2000). Furthermore, Nac can inhibit cytokine-induced adhesion of immune cells to the brain endothelium (Faruqi et al., 1997). This supports the possibility that immune cell interactions with the luminal membrane of the BBB can contribute to LRP-1 dysfunction. Although Nac also reduced serum levels of the anti-inflammatory cytokine IL-10, this may reflect effects of Nac that occurred prior to the time point studied. The proinflammatory cytokine TNF-α can induce the latent production of IL-10 (Wanidworanun and Strober, 1993). TNF-α levels in serum rise rapidly following LPS treatment, and within hours return to baseline (Kakizaki et al., 1999). Therefore, we cannot rule out the possibility that reduction of IL-10 levels by Nac pretreatment were actually in reaction to decreases in TNF-α in an earlier phase of the immune response. Future studies are necessary to determine whether the effects of Nac on IL-10 are helpful or harmful to BBB function. In both cortex and hippocampus, only elevations in MIP-1β were inhibited by Nac, whereas KC was potentiated by Nac. Although elevated levels of KC would suggest neutrophil chemoattraction, this may be countered by the ability of Nac to reduce adhesion molecule expression at the BBB. Furthermore, increased IL-8 (the human cytokine analog of KC) was shown to protect against neuronal apoptosis induced by inflammation or Aβ treatment (Ashutosh et al., 2011). Therefore, hippocampal increases in KC by Nac could be neuroprotective. MIP-1β is released by brain endothelial cells following ischemia and stimulates microglial proliferation (Wang et al., 2012; Wang et al., 2011). This could therefore be an important BBB-derived cytokine which modulates CNS inflammation. Whether MIP-1β mediates any effects of LPS on BBB transport of Aβ remains to be tested, but may provide insight on the mechanism by which Nac protects against LRP-1 dysfunction in our model.
The cortex and hippocampus are two brain regions which are highly affected in both AD and systemic inflammation (Barichello et al., 2007; Braak et al., 2006; Comim et al., 2011; Sultana and Butterfield, 2010; Thal et al., 2002). In AD, however, neuropathological hallmarks of Aβ and tau show different temporal and spatial patterns of accumulation in these regions. Tau neurofibrillary tangles begin to accumulate in the hippocampus and surrounding areas, and extend throughout the neocortex as neuropathological severity increases (Braak et al., 2006). Aβ plaque, however, begins to accumulate in the neocortex first, and hippocampal pathology occurs later as the disease advances (Thal et al., 2002). This suggests that these two regions have different responses and/or susceptibilities to pathological stimuli associated with AD. Inflammation is such a stimulus, and therefore we compared LPS-induced cytokine responses in cortex to those in hippocampus. We found that basal cytokine levels in these two regions did not significantly differ from each other with the exception of eotaxin, which was undetectable in hippocampus for all treatment regimens. The elevations in levels induced by LPS administration for the cytokines IL-1β, IL-6, IL-12(p40), MIP-1α, G-CSF, KC, RANTES, and TNF-α were all significantly lower in the hippocampus than in the cortex. The statistical differences between cortex and hippocampus were lost for IL-1β, IL-12(p40), and TNF-α in groups with Nac pretreatment. These findings show that the inflammatory response to LPS is greater in the cortex than the hippocampus, a finding which may have important implications for conditions such as septic encephalopathy and AD. Furthermore, inflammation can both increase Aβ production and decrease Aβ clearance (Jaeger et al., 2009; Lee et al., 2008), raising the possibility that early neocortical Aβ accumulation in AD could be caused by inflammation in this region. Whether Aβ or tau pathologies are also present in the brains of septic encephalopathy patients is unknown, but may reveal important clues as to what could cause these pathologies in AD.
In conclusion, we have shown that Nac protects against Aβ transport dysfunction at the BBB caused by systemic inflammation. This was associated with reduction of oxidative stress in the periphery but not the brain, and these reductions in oxidative stress corresponded with cytokine reductions primarily in serum. These results are therefore suggestive of Nac mediating its protective effects prior to entering the CNS. Many predominant risk factors for AD are associated with elevated systemic inflammation and peripheral oxidative stress. Future studies are therefore necessary to determine whether antioxidants could be neuroprotective in individuals with risk factors for AD by blocking peripheral oxidative stress, lowering serum cytokine levels, and preventing LRP-1 dysfunction at the BBB. Our findings highlight the importance of considering BBB changes in response to peripheral signals as well as those in the CNS when studying etiological mechanisms of AD.
Supplementary Material
Data were analyzed by one way ANOVA and Newman-Keuls Multiple Comparisons tests, n=8–10 per group; *p < 0.05, **p < 0.05, ***p < 0.001 vs. Sal/Sal; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. Nac/Sal; &p < 0.05, &&p < 0.01 vs. group indicated.
Data were analyzed by one sample t-tests (significantly different than zero) or one way ANOVA and Newman-Keuls Multiple Comparisons tests; @@p< 0.01 vs. zero; *p < 0.05, **p < 0.05, ***p < 0.001 vs. Sal/Sal; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. Nac/Sal; &p < 0.05 vs. group indicated.
Data were analyzed by one sample t-tests (significantly different than zero) or one way ANOVA and Newman-Keuls Multiple Comparisons tests; @p< 0.05, @@@p< 0.001 vs. zero; **p < 0.05, ***p < 0.001 vs. Sal/Sal; ##p < 0.01, ###p < 0.001 vs. Nac/Sal; &p < 0.05 vs. group indicated.
Research Highlight.
N-acetylcysteine protects against LPS-induced inhibition of amyloid beta efflux across the blood-brain barrier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle A. Erickson, Email: mericks9@slu.edu.
Kim Hansen, Email: nesnah@washington.edu.
William A. Banks, Email: wabanks1@uw.edu.
References
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cellular and molecular neurobiology. 2000;20:131–147. doi: 10.1023/a:1007074420772. [DOI] [PubMed] [Google Scholar]
- Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer's disease. Neurology. 2001;57:1515–1517. doi: 10.1212/wnl.57.8.1515. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer's disease. Neurobiology of aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer'sAssociation. 2012 Alzheimer's disease facts and figures. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ashutosh Kou W, Cotter R, Borgmann K, Wu L, Persidsky R, et al. CXCL8 protects human neurons from amyloid-beta-induced neurotoxicity: relevance to Alzheimer's disease. Biochemical and biophysical research communications. 2011;412:565–571. doi: 10.1016/j.bbrc.2011.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiology of disease. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Reversible association of the cytokines MIP-1 alpha and MIP-1 beta with the endothelia of the blood-brain barrier. Neuroscience letters. 1996;205:202–206. doi: 10.1016/0304-3940(96)12410-1. [DOI] [PubMed] [Google Scholar]
- Barichello T, Machado RA, Constantino L, Valvassori SS, Reus GZ, Martins MR, et al. Antioxidant treatment prevented late memory impairment in an animal model of sepsis. Critical care medicine. 2007;35:2186–2190. doi: 10.1097/01.ccm.0000281452.60683.96. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RM, Moller K, Bailey DM. Neuro-oxidative-nitrosative stress in sepsis. J Cereb Blood Flow Metab. 2011;31:1532–1544. doi: 10.1038/jcbfm.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta neuropathologica. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenn A, Grube M, Peters M, Fischer A, Jedlitschky G, Kroemer HK, et al. Beta-Amyloid Downregulates MDR1-P-Glycoprotein (Abcb1) Expression at the Blood-Brain Barrier in Mice. International journal of Alzheimer's disease. 2011;2011:690121. doi: 10.4061/2011/690121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free radical research. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- Carnevale D, Mascio G, D'Andrea I, Fardella V, Bell RD, Branchi I, et al. Hypertension Induces Brain beta-Amyloid Accumulation, Cognitive Impairment, and Memory Deterioration Through Activation of Receptor for Advanced Glycation End Products in Brain Vasculature. Hypertension. 2012;60:188–197. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. The Journal of clinical investigation. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comim CM, Cassol-Jr OJ, Constantino LS, Felisberto F, Petronilho F, Rezin GT, et al. Alterations in inflammatory mediators, oxidative stress parameters and energetic metabolism in the brain of sepsis survivor rats. Neurochemical research. 2011;36:304–311. doi: 10.1007/s11064-010-0320-2. [DOI] [PubMed] [Google Scholar]
- Cunningham C. Systemic inflammation and delirium: important co-factors in the progression of dementia. Biochemical Society transactions. 2011;39:945–953. doi: 10.1042/BST0390945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer's disease. CNS & neurological disorders drug targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do TM, Noel-Hudson MS, Ribes S, Besengez C, Smirnova M, Cisternino S, et al. ABCG2- and ABCG4-Mediated Efflux of Amyloid-beta Peptide 1–40 at the Mouse Blood-Brain Barrier. Journal of Alzheimer's disease : JAD. 2012;30:155–166. doi: 10.3233/JAD-2012-112189. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Flaherty SL, Johanson CE, Duncan JA, 3rd, Silverberg GD, Miller MC, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta neuropathologica. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- Dzenko KA, Song L, Ge S, Kuziel WA, Pachter JS. CCR2 expression by brain microvascular endothelial cells is critical for macrophage transendothelial migration in response to CCL2. Microvascular research. 2005;70:53–64. doi: 10.1016/j.mvr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Hartvigson PE, Morofuji Y, Owen JB, Butterfield DA, Banks WA. Lipopolysaccharide impairs amyloid beta efflux from brain: altered vascular sequestration, cerebrospinal fluid reabsorption, peripheral clearance and transporter function at the blood-brain barrier. Journal of neuroinflammation. 2012;9:150. doi: 10.1186/1742-2094-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. Journal of neurochemistry. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- Faruqi RM, Poptic EJ, Faruqi TR, De La Motte C, DiCorleto PE. Distinct mechanisms for N-acetylcysteine inhibition of cytokine-induced E-selectin and VCAM-1 expression. The American journal of physiology. 1997;273:H817–H826. doi: 10.1152/ajpheart.1997.273.2.H817. [DOI] [PubMed] [Google Scholar]
- Freeman LR, Keller JN. Oxidative stress and cerebral endothelial cells: Regulation of the blood-brain-barrier and antioxidant based interventions. Biochimica et biophysica acta. 2012;1822:822–829. doi: 10.1016/j.bbadis.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu AL, Dong ZH, Sun MJ. Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain research. 2006;1109:201–206. doi: 10.1016/j.brainres.2006.06.042. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood FC, Hunter WM, Glover JS. The Preparation of I-131-Labelled Human Growth Hormone of High Specific Radioactivity. Biochem J. 1963;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. The Journal of clinical endocrinology and metabolism. 2004;89:4963–4971. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. Journal of neurochemistry. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Miller DS, Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease. Molecular pharmacology. 2010;77:715–723. doi: 10.1124/mol.109.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Liu LP, Liao JM, Wang TS, Ye FY, Wu J, et al. Downregulation of LRP1 [correction of LPR1] at the blood-brain barrier in streptozotocin-induced diabetic mice. Neuropharmacology. 2009;56:1054–1059. doi: 10.1016/j.neuropharm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- Huang Q, Aluise CD, Joshi G, Sultana R, St Clair DK, Markesbery WR, et al. Potential in vivo amelioration by N-acetyl-L-cysteine of oxidative stress in brain in human double mutant APP/PS-1 knock-in mice: toward therapeutic modulation of mild cognitive impairment. Journal of neuroscience research. 2010;88:2618–2629. doi: 10.1002/jnr.22422. [DOI] [PubMed] [Google Scholar]
- Huynh HK, Dorovini-Zis K. Effects of interferon-gamma on primary cultures of human brain microvessel endothelial cells. The American journal of pathology. 1993;142:1265–1278. [PMC free article] [PubMed] [Google Scholar]
- Imber MJ, Pizzo SV. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981;256:8134–8139. [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Hwang MC, Farr SA, Murphy MP, Fleegal-DeMotta MA, et al. Testing the neurovascular hypothesis of Alzheimer's disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. Journal of Alzheimer's disease : JAD. 2009;17:553–570. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, et al. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer's disease. Brain, behavior, and immunity. 2009;23:507–517. doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Li J, Nation RL, Nicolazzo JA. Impact of p-glycoprotein inhibition and lipopolysaccharide administration on blood-brain barrier transport of colistin in mice. Antimicrobial agents and chemotherapy. 2011;55:502–507. doi: 10.1128/AAC.01273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizaki Y, Watanobe H, Kohsaka A, Suda T. Temporal profiles of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in the plasma and hypothalamic paraventricular nucleus after intravenous or intraperitoneal administration of lipopolysaccharide in the rat: estimation by push-pull perfusion. Endocrine journal. 1999;46:487–496. doi: 10.1507/endocrj.46.487. [DOI] [PubMed] [Google Scholar]
- Kovac A, Erickson MA, Banks WA. Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. Journal of neuroinflammation. 2011;8:139. doi: 10.1186/1742-2094-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. Journal of neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Chekmasova A, Marusich E, Agrawal L, Strayer DS. Role of CCR5 and its ligands in the control of vascular inflammation and leukocyte recruitment required for acute excitotoxic seizure induction and neural damage. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:737–753. doi: 10.1096/fj.10-161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- McCarron RM, Wang L, Racke MK, McFarlin DE, Spatz M. Cytokine-regulated adhesion between encephalitogenic T lymphocytes and cerebrovascular endothelial cells. Journal of neuroimmunology. 1993;43:23–30. doi: 10.1016/0165-5728(93)90071-6. [DOI] [PubMed] [Google Scholar]
- McLellan LI, Lewis AD, Hall DJ, Ansell JD, Wolf CR. Uptake and distribution of N-acetylcysteine in mice: tissue-specific effects on glutathione concentrations. Carcinogenesis. 1995;16:2099–2106. doi: 10.1093/carcin/16.9.2099. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JB, Sultana R, Aluise CD, Erickson MA, Price TO, Bu G, et al. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Abeta accumulation in AD brain. Free radical biology & medicine. 2010;49:1798–1803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neuroscience letters. 2010;469:6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- Palacio JR, Markert UR, Martinez P. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflammation research : official journal of the European Histamine Research Society [et al] 2011;60:695–704. doi: 10.1007/s00011-011-0323-8. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Pflanzner T, Janko MC, Andre-Dohmen B, Reuss S, Weggen S, Roebroek AJ, et al. LRP1 mediates bidirectional transcytosis of amyloid-beta across the blood-brain barrier. Neurobiology of aging. 2011;32:e1–e11. doi: 10.1016/j.neurobiolaging.2010.05.025. 2323. [DOI] [PubMed] [Google Scholar]
- Pflanzner T, Petsch B, Andre-Dohmen B, Muller-Schiffmann A, Tschickardt S, Weggen S, et al. Cellular prion protein participates in amyloid-beta transcytosis across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:628–632. doi: 10.1038/jcbfm.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocernich CB, La Fontaine M, Butterfield DA. In-vivo glutathione elevation protects against hydroxyl free radical-induced protein oxidation in rat brain. Neurochemistry international. 2000;36:185–191. doi: 10.1016/s0197-0186(99)00126-6. [DOI] [PubMed] [Google Scholar]
- Salkeni MA, Lynch JL, Otamis-Price T, Banks WA. Lipopolysaccharide impairs blood-brain barrier P-glycoprotein function in mice through prostaglandin- and nitric oxide-independent pathways. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2009;4:276–282. doi: 10.1007/s11481-008-9138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer's disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Progress in neurobiology. 2009;87:181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Satoh K, Shimokawa H, Berk BC. Cyclophilin A: promising new target in cardiovascular therapy. Circ J. 2010;74:2249–2256. doi: 10.1253/circj.cj-10-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Roy M, Chakraborti AS. Ameliorative effects of glycyrrhizin on streptozotocin-induced diabetes in rats. The Journal of pharmacy and pharmacology. 2011;63:287–296. doi: 10.1111/j.2042-7158.2010.01217.x. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer's amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the bloodbrain barrier. The Journal of clinical investigation. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukaliak JA, Dorovini-Zis K. Expression of the beta-chemokines RANTES and MIP-1 beta by human brain microvessel endothelial cells in primary culture. Journal of neuropathology and experimental neurology. 2000;59:339–352. doi: 10.1093/jnen/59.5.339. [DOI] [PubMed] [Google Scholar]
- Smith QR, Allen DD. In situ brain perfusion technique. Methods Mol Med. 2003;89:209–218. doi: 10.1385/1-59259-419-0:209. [DOI] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2010;19:341–353. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Monteleone D, Tabaton M. Amyloid-beta Production: Major Link Between Oxidative Stress and BACE1. Neurotoxicity research. 2011 doi: 10.1007/s12640-011-9283-6. [DOI] [PubMed] [Google Scholar]
- Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- van Assema DM, Lubberink M, Bauer M, van der Flier WM, Schuit RC, Windhorst AD, et al. Blood-brain barrier P-glycoprotein function in Alzheimer's disease. Brain : a journal of neurology. 2012;135:181–189. doi: 10.1093/brain/awr298. [DOI] [PubMed] [Google Scholar]
- Wang J, Li PT, Du H, Hou JC, Li WH, Pan YS, et al. Tong Luo Jiu Nao injection, a traditional Chinese medicinal preparation, inhibits MIP-1beta expression in brain microvascular endothelial cells injured by oxygen-glucose deprivation. Journal of ethnopharmacology. 2012;141:151–157. doi: 10.1016/j.jep.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Wang J, Li PT, Du H, Hou JC, Li WH, Pan YS, et al. Impact of paracrine signals from brain microvascular endothelial cells on microglial proliferation and migration. Brain research bulletin. 2011;86:53–59. doi: 10.1016/j.brainresbull.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–6861. [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA : the journal of the American Medical Association. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data were analyzed by one way ANOVA and Newman-Keuls Multiple Comparisons tests, n=8–10 per group; *p < 0.05, **p < 0.05, ***p < 0.001 vs. Sal/Sal; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. Nac/Sal; &p < 0.05, &&p < 0.01 vs. group indicated.
Data were analyzed by one sample t-tests (significantly different than zero) or one way ANOVA and Newman-Keuls Multiple Comparisons tests; @@p< 0.01 vs. zero; *p < 0.05, **p < 0.05, ***p < 0.001 vs. Sal/Sal; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. Nac/Sal; &p < 0.05 vs. group indicated.
Data were analyzed by one sample t-tests (significantly different than zero) or one way ANOVA and Newman-Keuls Multiple Comparisons tests; @p< 0.05, @@@p< 0.001 vs. zero; **p < 0.05, ***p < 0.001 vs. Sal/Sal; ##p < 0.01, ###p < 0.001 vs. Nac/Sal; &p < 0.05 vs. group indicated.