Abstract
Helicobacter pylori may cause chronic gastritis, gastric cancer, or lymphoma. Myeloid antigen-presenting cells (APCs) are most likely involved in the induction and expression of the underlying inflammatory responses. To study the interaction of human APC subsets with H. pylori, we infected monocytes, monocyte-derived dendritic cells (DCs), and monocyte-derived (classically activated; M1) macrophages with H. pylori and analyzed phenotypic alterations, cytokine secretion, phagocytosis, and immunostimulation. Since we detected CD163+ (alternatively activated; M2) macrophages in gastric biopsy specimens from H. pylori-positive patients, we also included monocyte-derived M2 macrophages in the study. Upon H. pylori infection, monocytes secreted interleukin-1β (IL-1β), IL-6, IL-10, and IL-12p40 (partially secreted as IL-23) but not IL-12p70. Infected DCs became activated, as shown by the enhanced expression of CD25, CD80, CD83, PDL-1, and CCR7, and secreted IL-1β, IL-6, IL-10, IL-12p40, IL-12p70, and IL-23. However, infection led to significantly downregulated CD209 and suppressed the constitutive secretion of macrophage migration inhibitory factor (MIF). H. pylori-infected M1 macrophages upregulated CD14 and CD32, downregulated CD11b and HLA-DR, and secreted mainly IL-1β, IL-6, IL-10, IL-12p40, and IL-23. Activation of DCs and M1 macrophages correlated with increased capacity to induce T-cell proliferation and decreased phagocytosis of dextran. M2 macrophages upregulated CD14 and CD206 and secreted IL-10 but produced less of the proinflammatory cytokines than M1 macrophages. Thus, H. pylori affects the functions of human APC subsets differently, which may influence the course and the outcome of H. pylori infection. The suppression of MIF in DCs constitutes a novel immune evasion mechanism exploited by H. pylori.
INTRODUCTION
Helicobacter pylori (H. pylori) is a spiral-shaped, Gram-negative, microaerophilic bacterium that colonizes the human gastric mucus layer in approximately half of the world's population. H. pylori infection causes gastritis and is a risk factor for the development of peptic ulcers, gastric cancer, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma (9, 82). Successful colonization is accompanied by a local inflammatory response involving both innate and adaptive immunity, and cytokines such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha (TNF-α), IL-8, IL-21, gamma interferon (IFN-γ), and transforming growth factor β (TGF-β) are found at increased levels in the gastric mucosa of patients with H. pylori-associated gastritis compared with levels in healthy controls (18, 21, 50). The cellular sources of these cytokines, however, have remained largely undefined. In addition, increased expression of macrophage migration inhibitory factor (MIF)—a constitutively expressed cytokine involved in innate and adaptive immune responses (10)—by epithelial cells and leukocytes in the H. pylori-infected gastric mucosa has been shown (86).
The bacteria are rarely eliminated, and colonization usually persists throughout life. The predominant T-cell response against H. pylori is mediated by proinflammatory Th1 and Th17 CD4+ T cells (7, 23, 76), the latter being induced by IL-23, IL-1β, and IL-6 (1, 83). In spite of evidence for CD4+ T-cell-mediated protection against H. pylori infection in mouse models, Th1 and Th17 responses in humans are also present in chronic H. pylori infection as is IL-17 (19, 49). In mice, the induction of regulatory T (Treg) cells with the simultaneous suppression of Th17 cells may contribute to bacterial persistence (43). Likewise, regulatory T cells can be found in the gastric mucosa of H. pylori-infected children and, to a lesser extent, adults (38), and H. pylori-specific regulatory T cells have been shown to suppress memory CD4+ T-cell responses to H. pylori in infected individuals (51).
Myeloid antigen-presenting cells (APCs), such as monocytes, monocyte-derived and resident dendritic cells (DCs), and macrophages, are present in the H. pylori-infected mucosa and are likely involved in both the induction and maintenance of H. pylori-specific immune responses and inflammatory reactions. Originating from a common myeloid progenitor, monocytes can differentiate into tissue macrophages or DCs when crossing the endothelial barrier (69) and have a high capacity to kill H. pylori (16). Notably, different types of macrophages have been described. Besides “classically,” i.e., IFN-γ-activated macrophages, cells also can undergo a “nonclassical,” alternative activation, which can be induced by, e.g., IL-4 and IL-13. This results in macrophages with a different phenotype and different receptor expression and cytokine secretion patterns. As classically and alternatively activated macrophages go along with Th1 and Th2 cell induction, these cells have also been classified as M1 and M2 macrophages, respectively (57). Depending on the activating stimulus, M2 macrophages can be further subdivided into at least three distinct subsets that share strong IL-10-secreting properties but are able to react differently upon activation (11, 53, 55). Whereas M1 macrophages are thought to be primarily microbicidal and proinflammatory, M2 macrophages exhibit anti-inflammatory and immune-modulating functions and have been reported to be involved in responses against different bacterial and parasitic infections and in tissue remodeling (28–31, 60).
DCs are specialized in antigen capture, processing, and presentation to naïve T cells after migration to the draining lymph nodes (8). They are widely distributed in human tissues, including the gastric mucosa, and are capable of penetrating the gut epithelial monolayers to sample, e.g., luminal bacteria (71). The interaction of DCs with H. pylori gives rise to activation (maturation) of the cells, as shown by enhanced expression of CD80, CD83, CD86, and HLA-DR (47). This is associated with the secretion of IL-6, IL-8, IL-10, and IL-12 (34, 36, 47, 48, 58). IL-12 plays a key role in Th1 polarization (78), and DCs stimulated with H. pylori promote Th1 responses in vitro (35). Following in vitro infection, DCs also express CCR7 and migrate in response to CCL19 (36). Furthermore, DCs may also interact with H. pylori through CD209 (DC-SIGN) that binds H. pylori lipopolysaccharide (LPS) and promotes IL-10 secretion (13). Moreover, H. pylori stimulates DCs to induce IL-17 expression in CD4+ lymphocytes (45).
Since myeloid APCs are found in the gastric mucosa and are involved in H. pylori-specific immune responses and are, therefore, potential targets of bacterial virulence factors promoting immune evasion, we were interested in elucidating the immuno-modulating effects of H. pylori on human APCs. Accordingly, we isolated monocytes from human peripheral blood and used these cells directly and also as progenitors for DCs and for M1 and M2 macrophages. Using this approach, we were able to study the effects of H. pylori on myeloid APCs from single donors in parallel, excluding interindividual differences in these comparative assays.
MATERIALS AND METHODS
Immunohistochemistry.
Paraffin-embedded biopsy samples obtained during routine diagnostic esophago-gastro-duodenoscopy from H. pylori-infected individuals and H. pylori-negative controls were used, and immunohistochemistry was performed as described previously (60). Primary antibodies against human-CD163 (Novocastra, Newcastle, United Kingdom), CD68 (Dako, Hamburg, Germany), and stabilin-1 (Sigma, Taufkirchen, Germany) were followed by donkey anti-mouse biotin (Dianova, Hamburg, Germany). Biotin was visualized with streptavidin-alkaline phosphatase and Fast Red (both from Dako). Cell counts were determined from three biopsy specimens per sampling date as the mean cell count of 10 high-power fields (hpf) of 0.237 mm2 each.
Bacterial growth conditions and preparations.
H. pylori P12 strain was grown on solid GC agar plates (Remel, Thermo Scientific, MA) supplemented with 10% horse serum and vancomycin (Biochrom, Berlin, Germany) for 48 h under microaerobic conditions (Thermo Forma Series II, Thermo Scientific, MA) at 37°C. After growth, bacteria were passaged every day. Bacteria were harvested from day 3 to day 5 from plates with confluent growth with a sterile cotton swab and washed twice in phosphate-buffered saline ([PBS] Gibco/Invitrogen, CA). The bacteria were finally resolved in fresh RPMI 1640 medium (Gibco) containing 2 mM l-glutamine and 25 mM HEPES (Gibco), supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS), and the bacterial number was determined by measuring the optical density at 550 nm, with a value of 0.6 corresponding to 4.6 × 108 H. pylori bacteria/ml. For infection, bacteria were adjusted to a multiplicity of infection (MOI) of 10 (47).
Generation of human APCs.
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats (German Red Cross, Berlin, Germany) via Ficoll (Ficoll Paque Premium; GE Healthcare, NJ) density gradient centrifugation. Monocytes were separated by positive selection of CD14+ cells using antibody-conjugated magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's recommendations. After isolation, cells were washed once in fresh complete RPMI 1640 medium containing 2 mM l-glutamine and 25 mM HEPES (Gibco), supplemented with 10% FCS and penicillin (100 U/ml)-streptomycin (100 μg/ml). Freshly isolated monocytes expressed high levels of CD14, and the purity of the cells as determined by flow cytometry was >95%. A fraction of the cells from a single buffy coat was used directly while the other cells were differentiated to DCs or M1 or M2 macrophages as described below.
Immature monocyte-derived DCs were generated by culturing 1 × 106 monocytes/ml in complete RPMI 1640 medium containing 2 mM l-glutamine (Merck, Darmstadt, Germany, or Gibco) and 10 mM HEPES (Gibco) supplemented with 10% FCS, and penicillin (100 U/ml)-streptomycin (100 μg/ml) (Sigma) as well as 1,000 U/ml of granulocyte-macrophage colony-stimulating factor ([GM-CSF] Bayer HealthCare Pharmaceuticals, VA) and 100 U/ml recombinant IL-4 (R&D Systems, MN) (41). A total of 3 × 106 cells per well were cultivated in six-well culture plates (TPP, Trasadingen, Switzerland, or Nunc, Roskilde, Denmark). Fresh medium containing IL-4 and GM-CSF was added every second or third day to the cell cultures. On day 6 or 7, cells were harvested and washed once in fresh complete RPMI 1640 medium. The phenotype of DCs was routinely determined by flow cytometry. Immature DCs expressed high levels of CD11b and low levels of CD80, whereas CD14 was markedly downregulated. Macrophages were differentiated from monocytes by culturing 1 × 106 cells/ml for 6 days at 3 × 106 cells per well in six-well culture plates in complete RPMI 1640 medium containing 2 mM l-glutamine and 25 mM HEPES (Gibco), supplemented with 10% FCS and penicillin (100 U/ml)-streptomycin (100 μg/ml) in the presence of either 50 U/ml human GM-CSF for M1 macrophages or 50 ng/ml recombinant human macrophage colony-stimulating factor ([rhM-CSF] R&D Systems) for M2 macrophages (80). Cytokines were replenished every second or third day with a 50% medium change. Cells were harvested by incubating the adherent cells for 20 min in a PBS solution containing 5 mM EDTA (Gibco). Thereafter, cells were washed twice in PBS and finally resuspended in complete RPMI 1640 medium. Differentiation of macrophages was verified by cytokine measurement. Compared with M1 macrophages, M2 macrophages secreted high levels of IL-10 and no IL-12p40 upon LPS (derived from Escherichia coli) (100 ng/ml; Invivogen, CA) stimulation (80).
Infection of APCs.
All cells were infected following the same procedure. Cells were washed in antibiotic-free medium, and numbers of viable APCs were determined by trypan blue (Sigma) exclusion. A total of 5 × 105 cells/well of a 12-well culture plate (TPP) were incubated in 1 ml of RPMI 1640 medium supplemented with 10% FCS in the absence of antibiotics with the H. pylori P12 strain for 1 h at 37°C. LPS (100 ng/ml) was used as a control stimulus while medium alone served as a negative control. In some experiments, recombinant cytokines consisting of 10 μM prostaglandin E2 ([PGE2] Sigma-Adlrich, Steinheim, Germany), 10 ng/ml TNF-α, 10 ng/ml IL-1β, and 10 ng/ml IL-6 (all, R&D Systems) were used as activation stimulus for DCs (2, 42). Cells and bacteria were centrifuged for 5 min at 800 × g to allow synchronization. After 1 h of infection, the medium was removed, and the infection was stopped by the addition of 1 ml of fresh complete RPMI 1640 medium containing 100 μg/ml gentamicin (Sigma). The cells were finally incubated for a further 24 h at 37°C. The culture supernatants were collected and stored at −80°C.
Flow cytometry.
Surface marker expression was analyzed using a BD FACSCalibur or FACSScan flow cytometer with CellQuestPro software (BD Pharmingen, NJ). Cells were harvested and washed once in PBS containing 5% FCS and 10 mM sodium azide (fluorescence-activated cell sorting [FACS] wash) and then stained with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled monoclonal antibodies (MAbs) to CD11b, CD14, CD16, CD32, CD64, CD80, CD86, HLA-DR (BD Pharmingen, NJ), or the appropriate isotype controls (BD Pharmingen). DCs were further stained for CD25, CD83, CD209 (DC-SIGN), PDL-1 (all, BD Pharmingen), CCR7 (R&D Systems), and CD40 (Immunotech, Marseille, France), and macrophages were stained for CD206 (BD Pharmingen) expression. Cells were washed and fixed in 10% (vol/vol) formaldehyde-PBS. Data were analyzed using FlowJo (TreeStar, Inc., Ashland, OR) and CellQuest Pro software. Median fluorescence intensities (MFIs) and the percentages of positively expressing cells were determined after subtraction of the values for the isotype controls.
Phagocytosis assays.
To determine the phagocytic function of DCs and macrophages, FITC-conjugated dextran (molecular mass, 40 kDa) (Molecular Probes, Invitrogen, CA) was used (15). Cell numbers were adjusted to 5 × 104 cells in 50 μl of complete RPMI 1640 medium, and cells were preincubated on ice for 30 min. Then, the cells were incubated with 20 μg/ml dextran-FITC for 30 min at 37°C or at 4°C to detect nonspecific binding. Cells were washed three times using 500 μl of complete RPMI 1640 medium and fixed in 10% (vol/vol) formaldehyde-PBS. Median fluorescence intensities (MFIs) and the percentages of dextran-positive cells were determined by flow cytometry.
Mixed leukocyte reaction (MLR).
Allogeneic T cells labeled with carboxyfluorescein diacetate succinimidyl ester ([CFSE] Molecular Probes, Invitrogen, CA) were used to assess the capability of DCs and macrophages to induce T-cell proliferation (66). T cells were enriched from CD14− PBMCs by HLA-DR depletion using FITC-conjugated anti-human HLA-DR MAb (BD Pharmingen) and magnetic beads conjugated with anti-FITC MAb (Miltenyi Biotec) according to the manufacturer's instructions. Cells were stained with 0.25 μM CFSE in PBS for 4 min in the dark at room temperature and washed three times with complete RPMI 1640 medium. The APCs were harvested and cocultured in graded ratios starting at 1:40 with 2 × 105 CFSE-labeled T cells/well in 96-well flat bottom tissue culture plates (Nunc). T cells alone (2 × 105/well) served as controls. After 6 days, cells were harvested and stained with phycoerythrin (PE)-labeled anti-CD3 and peridinin chlorophyll protein (PerCP)-labeled anti-CD8 MAbs. Cells were washed with FACS wash and fixed in 10% (vol/vol) formaldehyde-PBS. The percentage of proliferated T cells was determined by flow cytometry as cells expressing low levels of CFSE (CFSElow). Proliferation of CD4+ T cells was determined by gating on CD3+ CD8− cells.
Detection of cytokines.
The culture supernatants of the different conditions of all cell types were monitored for cytokine levels of IL-1β, IL-6, IL-10, IL-12p40, IL-12p70, IL-23 (all, U-Cytech Biosciences, Utrecht, The Netherlands) and MIF (R&D Systems) using a sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol. The results were read by using a SpectraMax 190 plate reader (Molecular Devices, CA).
Statistics.
Statistical analyses were performed using a nonparametric Friedman test and Dunn's multiple comparison as a posttest. For the analysis of the immunohistochemistry data, a nonparametric Mann-Whitney test was used. P values of <0.05 were considered significant.
RESULTS
Alternatively activated macrophages are present in the gastric mucosa of H. pylori-positive individuals.
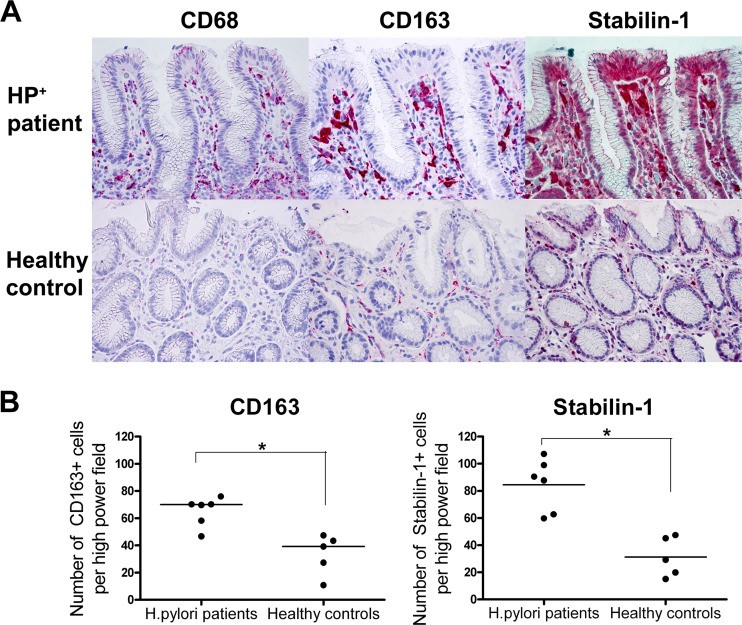
Macrophages play an important role in the maintenance of host defenses, and tissue macrophages significantly increase in numbers in the gastric mucosa during the course of an H. pylori infection (12). Here, they may play a pivotal role in the perpetuation of the inflammatory response to H. pylori, e.g., through the secretion of IL-6 (64). While M1 macrophage polarization has recently been reported for human and murine H. pylori infections (68), the roles of M2 macrophages in H. pylori infection have not been studied thus far. Therefore, we first investigated by immunohistochemistry what alternatively differentiated macrophages were present, and in what proportions, in gastric biopsy specimens from H. pylori-positive patients and compared the results to those obtained with tissue from healthy, H. pylori-negative individuals (Fig. 1A). Macrophages in general were identified by their expression of CD68 (46), and CD163 and stabilin-1 were selected as markers for M2 macrophages (27, 60, 73). Gastric mucosa from H. pylori-positive individuals contained around twice as many CD163+ and three times as many stabilin-1+ cells than tissue derived from controls (Fig. 1B). We therefore included M2 macrophages in our study of the effects of H. pylori on APCs.
Fig 1.
M2 macrophages are present in the gastric mucosal tissue of H. pylori patients. (A) Immunohistochemical analysis of the gastric mucosa of H. pylori-positive patients and healthy controls. Paraffin sections of biopsy specimens were analyzed for CD68, CD163, and stabilin-1 expression (red cells). One representative example of three experiments is shown. (B) Cell counts were determined from three biopsy specimens per sampling date as the mean cell count of 10 high-power fields of 0.237 mm2 each. *, P < 0.05 (nonparametric Mann-Whitney test).
H. pylori-infected human monocytes secrete substantial amounts of proinflammatory cytokines.
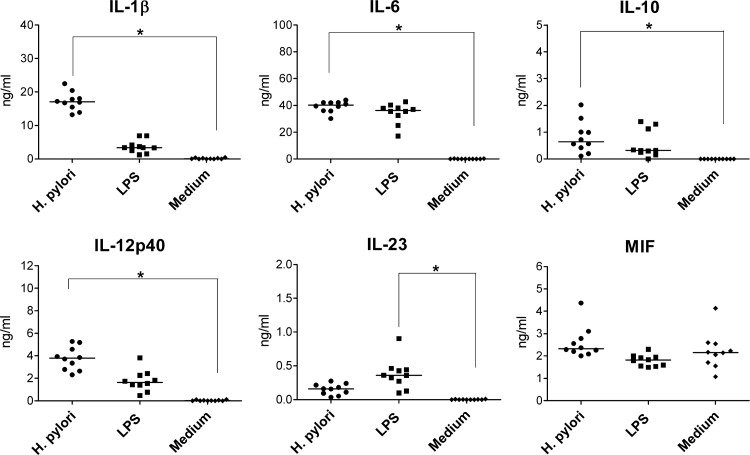
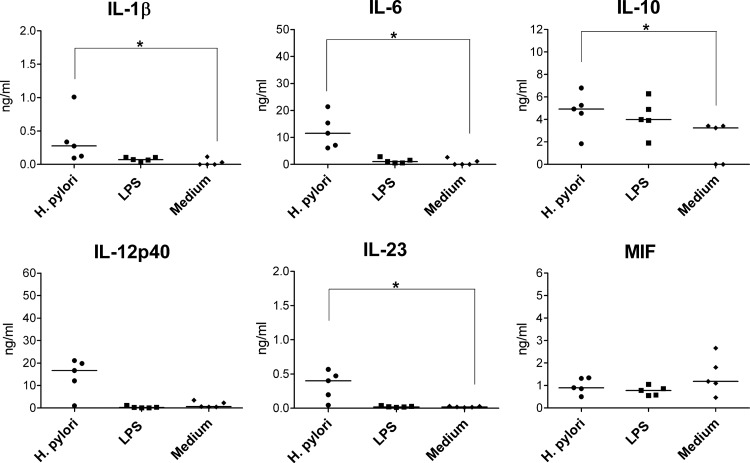
At the time of establishment of H. pylori infection, monocytes may be among the first myeloid APCs encountering the bacteria. To characterize the H. pylori-induced cytokine secretion by monocytes, we infected these cells with H. pylori for 1 h while control cells were incubated in the presence of LPS or in the absence of any stimulus. Twenty-four hours later, we collected the supernatants for cytokine analyses and monitored the phenotypes of the cells by flow cytometry. H. pylori induced the production of IL-12p40, which was partially secreted as IL-23 (Fig. 2), while IL-12p70 was not detected (data not shown). In addition, H. pylori-infected monocytes produced substantial amounts of IL-1β and IL-6 but little IL-10. Notably, infected cells produced significantly more IL-12p40, IL-23, IL-1β, IL-6, and IL-10 than uninfected control cells and also more IL-12p40 and IL-1β, but less IL-23, than cells stimulated with LPS. The production of MIF by monocytes was not altered by H. pylori or LPS. As described before for H. pylori-infected monocytes (25), we also observed considerable cell death; i.e., the vast majority of the cells died. For this reason, we were not able to characterize phenotypic or functional changes of the cells. Thus, H. pylori triggers the secretion of predominantly proinflammatory cytokines by human monocytes.
Fig 2.
Cytokines released by monocytes treated with H. pylori. A total of 5 × 105 monocytes were infected with H. pylori (MOI of 10; circles) for 1 h; the bacteria was washed out, and the cells were afterwards incubated for 24 h. Controls included monocytes treated with 100 ng/ml LPS (squares) or incubated with medium alone (diamonds). Concentrations of cytokines in the supernatants were determined by ELISA. Each symbol per condition represents the data obtained with cells from one donor. Horizontal lines show the median values of 10 experiments. *, P < 0.05 (Friedman test and Dunn's multiple comparison test).
H. pylori downregulates CD209 expression and the constitutive production of MIF by human DCs.
Some of the monocytes from each donor were differentiated in vitro to immature DCs through incubation in the presence of recombinant IL-4 and GM-CSF. DCs are prevalent in the human gastric mucosa of H. pylori-infected patients (63), and DCs isolated from biopsy specimens of infected subjects display an activated phenotype (14, 63) and are, in part, monocyte derived (45). Previous studies have shown that H. pylori is able to activate human DCs in vitro (36, 47, 48, 58). In addition to studying molecules included in prior studies, we analyzed the effects of H. pylori on the expression of CD25 (typically expressed by mature DCs) and CD209 (DC-SIGN) as well as on the secretion of MIF, which is constitutively produced by immature monocyte-derived DCs (65).
We infected immature DCs with H. pylori and observed significantly higher percentages (and higher MFIs) of cells expressing CD25, CD80, and CD83—molecules typically expressed by activated DCs—than in uninfected cells (Table 1; see also Fig. S1 in the supplemental material). Similarly, more PDL-1- and CCR7-positive cells were detected among infected DCs. Notably, H. pylori infection resulted in reduced expression of the H. pylori-LPS binding molecule, CD209, by DCs while it did not affect the expression of CD40, CD86, or HLA-DR (Table 1).
Table 1.
Increased expression of CD25, CD80, CD83, CCR7, and PDL-1 but reduced expression of CD209 by H. pylori-infected DCs
| Treatmenta | Surface molecule expression (median MFI)b |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD25 | CD40 | CD80 | CD83 | CD86 | CD209 | CCR7 | PDL-1 | HLA-DR | |
| H. pylori | 26* (18, 27) | 303 (114, 525) | 48* (15, 53) | 15* (8, 79) | 160 (44, 222) | 15* (9, 65) | 7* (3, 20) | 92* (16, 250) | 114 (71, 187) |
| LPS | 1 (0, 15) | 340 (64, 502) | 42* (16, 65) | 18* (15, 61) | 216* (45, 320) | 28 (11, 133) | 5 (1, 6) | 88 (17, 93) | 143 (106, 271) |
| Medium | 0 (0, 0) | 309 (35, 433) | 8 (3, 23) | 8 (1, 13) | 179 (16, 261) | 35 (25, 169) | 1 (0, 3) | 26 (3, 34) | 56 (55, 290) |
H. pylori, infected with H. pylori; LPS, activated by LPS; medium, incubated in medium only.
Values are based on seven independent experiments (seven donors). MFIs of isotype controls were subtracted. Values in parentheses are the 25th and 75th percentile MFIs, respectively.
, P < 0.05 (Friedman test and Dunn's multiple comparison test).
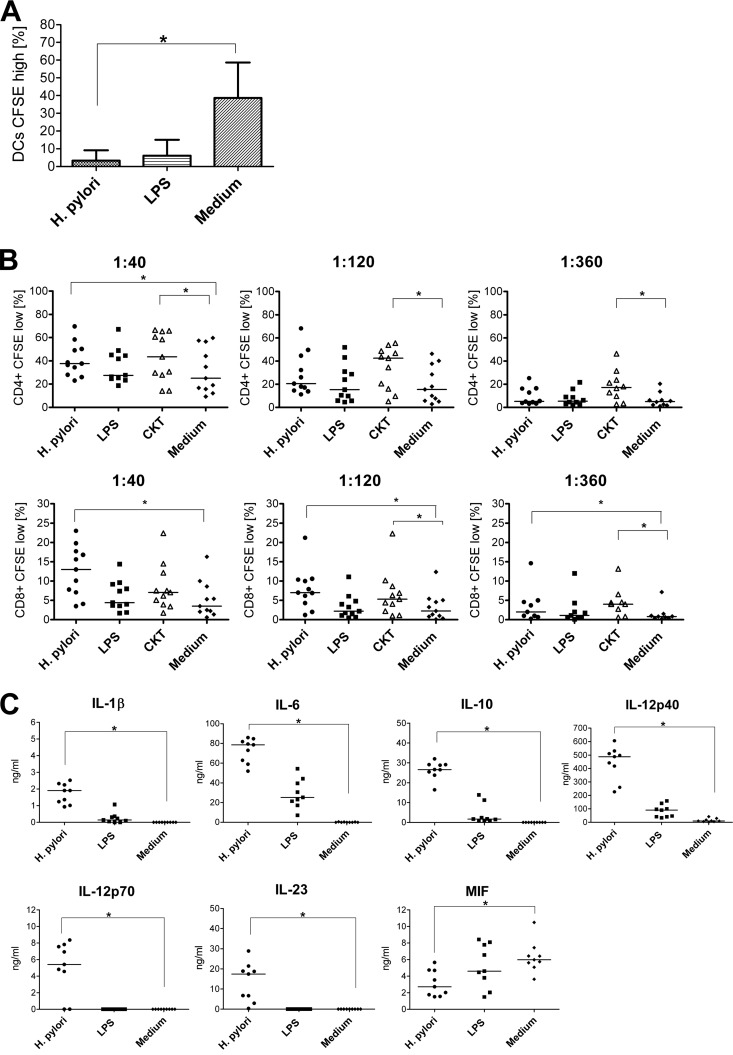
The phagocytic activity of immature DCs is considerably reduced upon activation (77). Therefore, we investigated the effects of H. pylori infection on dextran phagocytosis. Less than 5% of DCs preincubated with H. pylori or LPS were able to incorporate dextran, while it was taken up by around 40% of immature unstimulated cells (Fig. 3A).
Fig 3.
H. pylori-activated DCs are characterized by impaired phagocytosis, enhanced proliferation of allogeneic CD4+ and CD8+ T cells, and secretion of proinflammatory cytokines as well as IL-10, but reduced production of MIF. A total of 5 × 105 immature DCs were incubated with H. pylori (MOI of 10; circles), LPS (100 ng/ml; squares), recombinant cytokines ([CKT] 352 ng/ml prostaglandin E2, 10 ng/ml TNF-α, 10 ng/ml IL-1β, and 10 ng/ml IL-6; triangles), or medium alone (diamonds) for 1 h and afterwards cultured for 24 h. (A) The cells were incubated with FITC-labeled dextran for 30 min at 37°C and washed, and the uptake was determined by flow cytometry. Bars indicate the mean values ± standard deviations of data obtained for seven donors (values for unspecific binding at 4°C were subtracted). (B) DCs were incubated with allogeneic CFSE-labeled, HLA-DR-depleted T cells in graded ratios for 6 days. Percentages of proliferated T cells (CD4+ CD8+) were determined by FACS as CFSElow cells. Each symbol represents an experiment using cells from one individual donor. Horizontal lines show the median values of at least 10 experiments. (C) Concentrations of cytokines in the supernatants determined by ELISA. Each symbol per condition represents data obtained for one donor. Horizontal lines indicate the median of nine experiments.*, P < 0.05 (Friedman test and Dunn's multiple comparison test).
DC activation also coincides with an increased capability of the cells to induce T-cell proliferation. We tested this property of H. pylori-incubated DCs in allogeneic MLR assays and included as an additional positive control DCs activated by proinflammatory cytokines (2, 42). Compared with cells kept in medium alone, H. pylori-infected, but not LPS-treated, DCs gave rise to an approximately 1.5-fold increase in proliferation of CD4+ cells at a DC/T-cell ratio of 1:40 (Fig. 3B), whereas cytokine-stimulated DCs triggered the proliferation of around 20% of CD4+ T cells even at a DC/T-cell ratio of 1:360. Although proliferation of CD8+ T cells in general was lower than CD4+ T-cell proliferation, H. pylori-activated but not LPS-treated DCs induced a marked stimulatory capacity at all DC/T-cell ratios (Fig. 3B). Cytokine-activated DCs also increased CD8+ T-cell proliferation at lower DC/T-cell ratios.
DCs infected with H. pylori secreted IL-1β and IL-6 and, in comparison to monocytes, approximately 40-fold more IL-10 and 100-fold more IL-23. In contrast to monocytes, infected DCs also produced IL-12p70 but at a level approximately 3-fold lower than that of IL-23 (Fig. 3C). In contrast to the other H. pylori-infected APCs, H. pylori-infected DCs secreted approximately 50% less MIF than uninfected cells (Fig. 3C). Therefore, H. pylori activated monocyte-derived human DCs, coinciding with reduced phagocytic and increased T-cell stimulatory capacities. This process induced the secretion of proinflammatory cytokines, particularly those of the IL-12 family but also IL-10, while the production of MIF was impaired.
M1 and M2 macrophages respond differently to H. pylori infection.
To investigate the interaction of human macrophages with H. pylori, we generated two different types of human macrophages, i.e., classically activated (M1) and alternatively activated (M2) macrophages, following a standard protocol (80). M1 and M2 macrophage differentiation was verified by CD14 expression and the secretion of IL-12p40 and IL-10 upon stimulation with LPS (80).
After infection of M1 macrophages with H. pylori, we observed significantly more CD14 and CD32 expression among infected cells than in uninfected macrophages (see Fig. S2 in the supplemental material). In contrast, CD11b and HLA-DR were expressed at significantly lower levels by infected M1 macrophages than by uninfected cells. Accordingly, the MFIs for these molecules, except for HLA-DR, were similarly affected (Table 2). The expression of CD80, CD86, and CD206 (macrophage mannose receptor) as well as the expression of the Fcγ receptors (CD16 and CD64) was not altered by H. pylori infection (Table 2). We did not assess CD209 expression since this molecule is not expressed by monocyte-derived macrophages (75).
Table 2.
Increased expression of CD14 and CD32 but reduced expression of CD11b by H. pylori-infected M1 macrophages
| Treatmenta | Surface molecule expression (median MFI)b |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD11b | CD14 | CD16 | CD32 | CD64 | CD80 | CD86 | CD206 | HLA-DR | |
| H. pylori | 17* (9, 21) | 52* (26, 72) | 2 (1, 4) | 44* (38, 47) | 0 (0, 2) | 12 (1, 14) | 6 (4, 8) | 21 (0, 26) | 25 (21, 40) |
| LPS | 27 (13, 34) | 24 (18, 49) | 4 (2, 5) | 43 (37, 46) | 0 (0, 2) | 13* (3, 20) | 13 (9, 23) | 13 (0, 38) | 39 (30, 41) |
| Medium | 61 (11, 81) | 12 (8, 18) | 3 (1, 6) | 32 (28, 37) | 1 (0, 3) | 1 (0, 3) | 12 (8, 18) | 33 (0, 64) | 47 (43, 63) |
H. pylori, infected with H. pylori; LPS, stimulated with LPS; medium, treated with medium only.
Values are based on at least six independent experiments (six donors). MFIs of isotype controls were subtracted. Values in parentheses are the 25th and 75th percentile MFIs, respectively.
, P < 0.05 (Friedman test and Dunn's multiple comparison test).
Different phenotypic alterations induced by H. pylori infection were observed in M2 macrophages. Here, H. pylori induced significantly increased expression of CD14 and CD206 (see Fig. S3 in the supplemental material), and, accordingly, MFIs of these molecules were increased (Table 3). Whereas we also observed a 2-fold higher MFI for CD32 on infected cells, the expression of CD11b, CD16, CD64, CD80, CD86, or HLA-DR was not affected (Table 3).
Table 3.
Increased expression of CD14, CD32, and CD206 by H. pylori-infected M2 macrophages
| Treatmenta | Surface molecule expression (median MFI)b |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD11b | CD14 | CD16 | CD32 | CD64 | CD80 | CD86 | CD206 | HLA-DR | |
| H. pylori | 2 (2, 33) | 108* (50, 241) | 10 (5, 13) | 95* (39, 125) | 0 (0, 2) | 0 (0, 2) | 0 (0, 2) | 27* (5, 30) | 17 (13, 24) |
| LPS | 2 (1, 27) | 59 (39, 216) | 7 (4, 14) | 47 (29, 84) | 0 (0, 5) | 0 (0, 4) | 0* (0, 1) | 5 (4, 9) | 16 (11, 22) |
| Medium | 2 (2, 45) | 43 (12, 110) | 5 (2, 9) | 50 (25, 73) | 0 (0, 5) | 0 (0, 2) | 2 (1, 5) | 4 (2, 10) | 22 (14, 58) |
H. pylori, infected with H. pylori; LPS, stimulated with LPS; medium, treated with medium only.
Values are based on at least five independent experiments (five donors). MFIs of isotype controls were subtracted. Values in parentheses are the 25th and 75th percentile MFIs, respectively.
, P < 0.05 (Friedman test and Dunn's multiple comparison test).
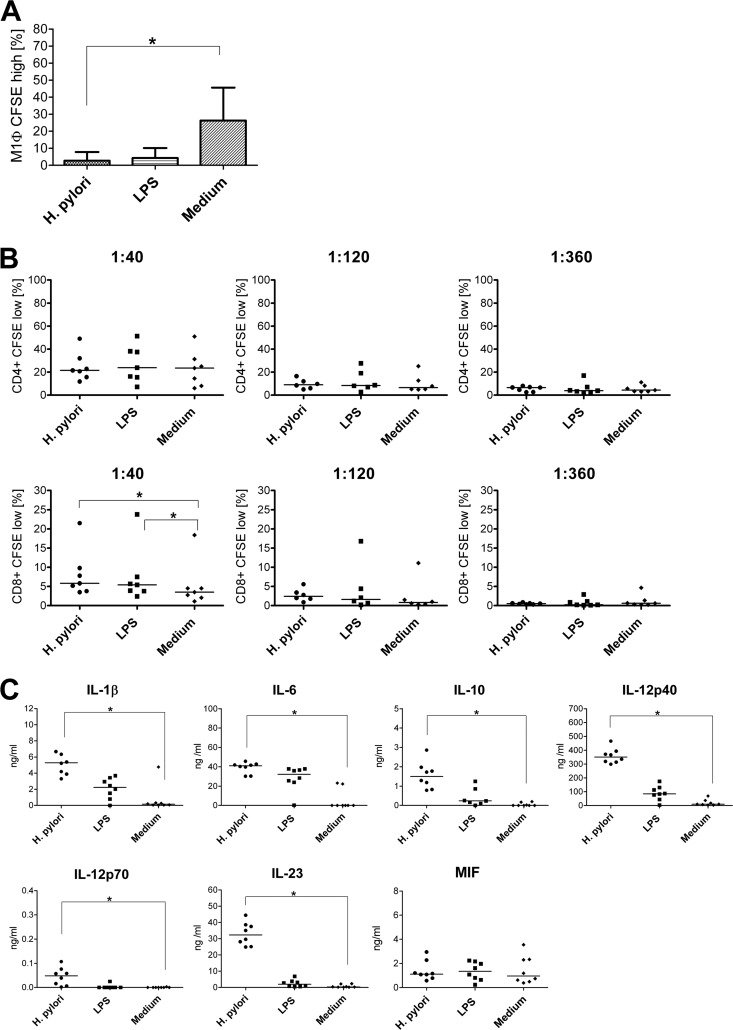
We also investigated the phagocytic properties of macrophages preincubated with H. pylori or LPS compared with those of macrophages kept in medium alone. Whereas around 25% of unstimulated M1 macrophages phagocytosed dextran, less than 3% of the cells preincubated with H. pylori or LPS were able to internalize dextran (Fig. 4A). Notably, unstimulated M2 macrophages did not take up dextran in substantial quantities, and this remained unaltered upon H. pylori infection (data not shown). In MLR assays following H. pylori or LPS exposure, M1 macrophages were not able to induce significantly more proliferation of CD4+ T cells than uninfected cells. In contrast, both H. pylori-activated and LPS-stimulated cells induced the proliferation of approximately 2-fold more CD8+ T cells at an APC/T-cell ratio of 1:40 than medium-incubated control cells (Fig. 4B). M2 macrophages generally displayed a very low capacity to stimulate T cells and induced neither CD4+ nor CD8+ T-cell proliferation (data not shown).
Fig 4.
H. pylori-activated M1 macrophages are characterized by impaired phagocytosis, enhanced proliferation of allogeneic CD8+ T cells, and secretion of mainly proinflammatory cytokines. A total of 5 × 105 M1 macrophages were incubated with H. pylori (MOI of 10; circles), LPS (100 ng/ml; squares), or medium alone (diamonds) for 1 h and afterwards cultured for 24 h. (A) The cells were incubated with FITC-labeled dextran for 30 min at 37°C and washed, and the uptake was determined by flow cytometry. Bars indicate the mean values ± standard deviations of data obtained for seven donors (values for unspecific binding at 4°C were subtracted). (B) M1 macrophages were cocultured with allogeneic CFSE-labeled, HLA-DR-depleted T cells in graded ratios for 6 days. Percentages of proliferated T cells (CD4+ CD8+) were determined by FACS as CFSElow cells. Each symbol represents an experiment using cells from one individual donor. Horizontal lines indicate the median of at least six experiments. (C) Concentrations of cytokines in the supernatants determined by ELISA. Each symbol per condition represents data obtained for one donor. Horizontal lines show the median values of eight experiments.*, P < 0.05 (Friedman test and Dunn's multiple comparison test).
M1 macrophages secreted IL-1β, IL-6, IL-10, IL-12p40, IL-12p70, IL-23, and MIF after infection (Fig. 4C). Notably, IL-12p70 was secreted at only very low concentrations (around 100-fold less than in supernatants from DCs), and a substantial proportion of IL-12p40 was produced as IL-23. In contrast, M2 macrophages produced significantly less (P < 0.05) of all proinflammatory cytokines, with the exception of MIF. While IL-12p70 was not produced by M2 macrophages at all (Fig. 5), they secreted 3-fold more IL-10 than M1 macrophages infected with H. pylori. Like DCs, both types of macrophages released lower levels of cytokines upon LPS stimulation than H. pylori-infected cells. As observed with monocytes but in contrast to DCs, H. pylori infection did not affect the secretion of MIF by M1 or M2 macrophages.
Fig 5.
H. pylori-infected M2 macrophages secrete little proinflammatory cytokines but IL-10. A total of 5 × 105 M2 macrophages were infected with H. pylori (MOI of 10; circles) for 1 h and afterwards incubated for 24 h. Controls included macrophages treated with 100 ng/ml LPS (squares) or incubated in medium alone (diamonds). Concentrations of cytokines in the supernatants were determined by ELISA. Each symbol per condition represents data obtained for one donor. Horizontal lines show the median values of five experiments. *, P < 0.05 (Friedman test and Dunn's multiple comparison test).
DISCUSSION
The exact mechanisms underlying the persistent colonization of the gastric mucosa by H. pylori and its ability to evade host immune defenses remain elusive. Myeloid APCs are involved in the induction and expression of H. pylori-specific immune responses that lead to an infiltration of the infected tissue by various types of leukocytes (87). Several studies have focused on DCs in H. pylori infection (34, 36, 47, 48, 58). However, other APCs, such as monocytes and macrophages, are also likely to be involved in H. pylori-induced inflammatory processes, and a dominance of M1 macrophages in H. pylori infections has recently been suspected based on real-time PCR analysis of gastric tissue RNA samples from infected patients (68). For our analysis of APCs in H. pylori infections, we used immunohistochemistry to investigate the presence of M2 macrophages in gastric biopsy specimens of H. pylori-positive patients and detected these cells in higher numbers than in biopsy specimens from healthy donors. We compared four different types of myeloid APCs, i.e., monocytes and three leukocyte subsets derived from these (DCs and M1 and M2 macrophages) for their interaction with H. pylori. Although the APC subsets differed regarding the H. pylori-induced alterations in phenotype and their functional profiles, all APCs were activated by this interaction.
Monocytes may serve as early immune modulators in H. pylori infection but may also differentiate into macrophages or DCs upon migration into inflamed tissues (69). In our study, infected monocytes produced IL-1β, IL-6, IL-10, IL-12p40, and IL-23 but not IL-12p70, while the constitutive production of MIF by cells was not altered by H. pylori infection. The release of IL-6 and IL-12 by monocytes upon H. pylori exposure has been described before (3). H. pylori may activate human monocytes in an LPS-dependent and also -independent manner through the production of other soluble surface proteins (52). For example, the H. pylori urease has been shown to stimulate the production of IL-1β, IL-6, IL-8, and TNF-α by monocytes (37). Several pathogen recognition receptors could be involved in H. pylori-induced activation of APCs. Toll-like receptor 2 (TLR2) is expressed by human myeloid APCs (20, 39, 74) and can be triggered by the neutrophil-activating protein (NAP) of H. pylori (22). Monocytes secrete IL-12 p40 and IL-23 upon NAP stimulation (22), and we have observed similar results. No APCs used in our study express TLR9 (39, 54, 74); thus, this receptor is not involved in the effects of H. pylori on APCs that we observed in our experiments. Notably, H. pylori could also be sensed by APCs through other pathogen recognition receptors. For example, gastric epithelial cells recognize Cag pathogenicity island (PAI)-positive bacteria via the NOD1 receptor (81), and NOD1 regulates the direct killing of the bacteria by the antimicrobial peptides, human β-defensins (33). In addition to these stimulating virulence factors, cholesterol has previously been identified as an H. pylori-specific virulence component and was proposed to mediate immune evasion, possibly by interfering with phagocytosis, as shown for mouse APCs by our group (85). For this reason, we also analyzed the effects of H. pylori infection on the different APCs used in this study with respect to the cholesterol metabolism of the bacteria by using mutant bacteria lacking cholesterol-α-glucosyl transferase activity. However, we did not detect any major differences compared with wild-type bacteria (data not shown).
We observed phenotypic changes in DCs upon interaction with H. pylori. The upregulation of CD80, CD83, CD86, CCR7, and PDL-1 by infected DCs has previously been observed by others (36, 47, 48, 58). Additionally, we observed reduced expression of CD209 (DC-SIGN), which is also expressed on gastric DCs (13, 14, 63). H. pylori LPS can become modified to contain Lewis blood group antigens (59) that play a role in the attachment to epithelial cells (4). CD209 may play an important role in H. pylori-specific immunity. It has been demonstrated to have a high affinity for, inter alia, H. pylori (5) by the recognition of glycan antigen Lewis x (79). The interaction with CD209 contributes to an anti-inflammatory environment through the release of IL-10 (32) and, thereby, to modulation of the T helper cell balance (13). In our study, DCs exposed to H. pylori secreted considerably more IL-10 than LPS-treated DCs. The downregulation of CD209 in this context may be the result of the interaction of the cells with the bacteria through this receptor as CD209-ligand complexes are rapidly internalized after binding (24).
DCs may take up and internalize exogenous particles through various mechanisms (8). However, the phagocytic capacities of DCs are usually downregulated upon activation (77), and we observed this for both H. pylori-infected and LPS-treated DCs.
We detected approximately 1.5-fold increased proliferation of allogeneic CD4+ T cells after H. pylori activation of DCs. This proliferation was lower than with cytokine-stimulated DCs and required a DC/T-cell ratio of at least 1:40. We also observed a low, but significant, proliferation of CD8+ T cells after DC activation by H. pylori in the mixed leukocyte assays, even at the lowest DC/T-cell ratio of 1:360. Information on CD8+ T cells during the course of H. pylori infection is scarce; however, these cells may be a major source of IFN-γ (67).
H. pylori-exposed DCs secreted IL-12p70. Similar to data demonstrated by Hansson et al. (36) but in contrast to another recent study (58), CD40 ligation was not required for this process. Apparent discrepancies in the published data might be due to different experimental conditions. Notably, DCs are crucial during the early induction of immune responses, when they are most likely the driving force for the induction of the Th1 immune response that is characteristic of H. pylori-infection (14). However, at these early stages of infection, specific CD40 ligand-expressing T cells that might provide this signal are rare. Thus, CD40-independent IL-12p70 production, as observed here, might well mimic the physiological situation. In contrast to the other proinflammatory cytokines analyzed, we observed significant suppression of MIF production by DCs. MIF plays an essential role in H. pylori-induced inflammation (84, 86). In mice, MIF is significantly upregulated during H. pylori infection and is associated with gastritis, and MIF knockout mice express a reduced Th1 immune response and are protected against gastritis (84). Thus, reduced MIF production by DCs upon H. pylori infection may reduce the risk of a strong inflammatory response and thereby favor the establishment of a chronic infection. Furthermore, blocking MIF production inhibits macrophage accumulation (40).
Upon infection, M1 macrophages showed increased CD14 expression, which might affect the sensing of the bacteria by the cells. Additionally, infected M1 macrophages expressed CD32 at significantly higher levels than uninfected cells. CD32 can be expressed as activating Fcγ receptor IIA (Fcγ-RIIA) or as inhibitory Fcγ-RIIB, which could be coexpressed by the same cell (70). Downregulation of Fcγ-RIIB on monocytes has been reported in patients with H. pylori-associated immune thrombocytopenia purpura (6). However, since we employed flow cytometry to analyze CD32 expression here, we were unable to distinguish between the expression of Fcγ-RIIA and Fcγ-RIIB. H. pylori infection did not augment the T-cell stimulatory capacity of M1 macrophages. Macrophages are usually less efficient in allogeneic MLRs than DCs (72), however, and the reduced expression of HLA-DR by H. pylori-infected M1 macrophages may add to this defect. Infected macrophages produced predominantly proinflammatory cytokines, i.e., >200-fold more IL-12 than IL-10 compared with DCs, although IL-12p70 was produced only at low levels.
Similar to M1 macrophages, increased expression of CD14 and higher MFIs for CD32 were detected for M2 macrophages. In addition, the expression of CD206 (macrophage mannose receptor) was significantly enhanced. CD206 is associated with pathogen binding and phagocytosis (44, 56); however, we did not observe phagocytosis by M2 macrophages, regardless of prior H. pylori treatment. This is in accordance with a previous report on decreased internalization of latex beads by alternatively activated macrophages (61). As mycobacteria are taken up efficiently by M2 macrophages generated following the same protocol as in our study (80), phagocytic behavior of M2 macrophages may differ and depend on particle properties. Mycobacterium uptake, however, does not support T-cell proliferation (80), and consistent with these findings we also observed that M2 macrophages did not stimulate T-cell proliferation.
Infected M2 macrophages secreted less of the proinflammatory cytokines and more IL-10 than M1 macrophages. In contrast to results with M2 macrophages activated by mycobacteria (80), we also observed increased production of IL-12p40 and IL-23. The simultaneous secretion of IL-12p40 and IL-10 by M2 macrophages during H. pylori infection may suggest a mixed (wound-healing and regulatory macrophage) phenotype with overlapping properties (62).
Infected DCs and M1 macrophages secreted considerably more IL-23 than monocytes and M2 macrophages and might contribute to the induction and maintenance of the Th17 responses that can be detected in the gastric mucosa of H. pylori-infected patients (17). Notably, we were unable to detect TGF-β in the supernatants of H. pylori-infected cells (data not shown), but Th17 induction in the absence of TGF-β has been demonstrated for murine and human cells (1, 26).
In conclusion, a comparative analysis of four different types of APCs in vitro revealed considerable variation in phenotype, cytokine secretion, phagocytosis and T cell stimulating capabilities in these cells in response to H. pylori infection. During the course of infection, monocytes may be the first APCs attracted to the site of infection and may first encounter H. pylori. The secretion of IL-1β and IL-6 by these cells may recruit further immune cells, including DCs, to the site of inflammation and contribute to the maturation of DCs. DCs likely pick up antigen and migrate to the draining lymph nodes, where they induce CD4+ Th1 (by IL-12p70), Th17 (by IL-23), and Treg (by IL-10) responses. In this context, H. pylori may further hamper the induction of excessive proinflammatory responses by the suppression of MIF secretion in DCs. The peripheral inflammatory environment during chronic infection might be dominated by macrophages, where M2 macrophages may limit excessive inflammation in favor of a chronic inflammatory response.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Hummel for providing paraffin-embedded biopsy material from patients and controls as well as E. Berg for expert technical assistance.
This study was supported by the DFG (SFB 633).
Footnotes
Published ahead of print 21 May 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. 2007. Interleukins 1β and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8:942–949 [DOI] [PubMed] [Google Scholar]
- 2. Ahuja V, Eisenblätter M, Ignatius R, Stahlmann R. 2009. Ammonium perfluorooctanoate substantially alters phenotype and cytokine secretion of human monocyte-derived dendritic cells in vitro. Immunopharmacol. Immunotoxicol. 31:641–646 [DOI] [PubMed] [Google Scholar]
- 3. Amedei A, et al. 2006. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J. Clin. Invest. 116:1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Appelmelk BJ, Monteiro MA, Martin SL, Moran AP, Vandenbroucke-Grauls CMJ. 2000. Why Helicobacter pylori has Lewis antigens. Trends Microbiol. 8:565–570 [DOI] [PubMed] [Google Scholar]
- 5. Appelmelk BJ, et al. 2003. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635–1639 [DOI] [PubMed] [Google Scholar]
- 6. Asahi A, et al. 2008. Helicobacter pylori eradication shifts monocyte Fcγ receptor balance toward inhibitory FcγRIIB in immune thrombocytopenic purpura patients. J. Clin. Invest. 118:2939–2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bamford KB, et al. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482–492 [DOI] [PubMed] [Google Scholar]
- 8. Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252 [DOI] [PubMed] [Google Scholar]
- 9. Bauer B, Meyer TF. 2011. The human gastric pathogen Helicobacter pylori and its association with gastric cancer and ulcer disease. Ulcers 2011:1–23 [Google Scholar]
- 10. Baugh JA, Bucala R. 2002. Macrophage migration inhibitory factor. Crit. Care Med. 30(Suppl 1):S27–S35 [PubMed] [Google Scholar]
- 11. Benoit M, Desnues B, Mege JL. 2008. Macrophage polarization in bacterial infections. J. Immunol. 181:3733–3739 [DOI] [PubMed] [Google Scholar]
- 12. Bergin PJ, et al. 2004. Increased production of matrix metalloproteinases in Helicobacter pylori-associated human gastritis. Helicobacter 9:201–210 [DOI] [PubMed] [Google Scholar]
- 13. Bergman MP, et al. 2004. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 200:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bimczok D, et al. 2010. Human primary gastric dendritic cells induce a Th1 response to H. pylori. Mucosal Immunol. 3:260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bond E, et al. 2009. Techniques for time-efficient isolation of human skin dendritic cell subsets and assessment of their antigen uptake capacity. J. Immunol. Methods 348:42–56 [DOI] [PubMed] [Google Scholar]
- 16. Borlace GN, Butler RN, Brooks DA. 2008. Monocyte and macrophage killing of Helicobacter pylori: relationship to bacterial virulence factors. Helicobacter 13:380–387 [DOI] [PubMed] [Google Scholar]
- 17. Caruso R, et al. 2008. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur. J. Immunol. 38:470–478 [DOI] [PubMed] [Google Scholar]
- 18. Caruso R, et al. 2007. IL-21 is highly produced in Helicobacter pylori-infected gastric mucosa and promotes gelatinases synthesis. J. Immunol. 178:5957–5965 [DOI] [PubMed] [Google Scholar]
- 19. Chabaud M, et al. 1999. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42:963–970 [DOI] [PubMed] [Google Scholar]
- 20. Chavez-Sanchez L, et al. 2010. The activation of CD14, TLR4, and TLR2 by mmLDL induces IL-1β, IL-6, and IL-10 secretion in human monocytes and macrophages. Lipids Health Dis. 9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. 1991. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut 32:1473–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Elios MM, Amedei A, Cappon A, Del Prete G, de Bernard M. 2007. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent. FEMS Immunol. Med. Microbiol. 50:157–164 [DOI] [PubMed] [Google Scholar]
- 23. D'Elios MM, et al. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962–967 [PubMed] [Google Scholar]
- 24. Engering A, et al. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118–2126 [DOI] [PubMed] [Google Scholar]
- 25. Galgani M, et al. 2004. Helicobacter pylori induces apoptosis of human monocytes but not monocyte-derived dendritic cells: role of the cag pathogenicity island. Infect. Immun. 72:4480–4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghoreschi K, et al. 2010. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467:967–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goerdt S, Orfanos CE. 1999. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 10:137–142 [DOI] [PubMed] [Google Scholar]
- 28. Gordon S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35 [DOI] [PubMed] [Google Scholar]
- 29. Gordon S. 2007. The macrophage: past, present and future. Eur. J. Immunol. 37(Suppl 1):S9–S17 [DOI] [PubMed] [Google Scholar]
- 30. Gordon S, Martinez FO. 2010. Alternative activation of macrophages: mechanism and functions. Immunity 32:593–604 [DOI] [PubMed] [Google Scholar]
- 31. Gordon S, Taylor PR. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953–964 [DOI] [PubMed] [Google Scholar]
- 32. Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TBH. 2009. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat. Immunol. 10:1081–1088 [DOI] [PubMed] [Google Scholar]
- 33. Grubman A, et al. 2010. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell. Microbiol. 12:626–639 [DOI] [PubMed] [Google Scholar]
- 34. Guiney DG, Hasegawa P, Cole SP. 2003. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect. Immun. 71:4163–4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hafsi N, et al. 2004. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J. Immunol. 173:1249–1257 [DOI] [PubMed] [Google Scholar]
- 36. Hansson M, et al. 2006. Dendritic cells express CCR7 and migrate in response to CCL19 (MIP-3β) after exposure to Helicobacter pylori. Microbes Infect. 8:841–850 [DOI] [PubMed] [Google Scholar]
- 37. Harris PR, Mobley HL, Perez-Perez GI, Blaser MJ, Smith PD. 1996. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology 111:419–425 [DOI] [PubMed] [Google Scholar]
- 38. Harris PR, et al. 2008. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology 134:491–499 [DOI] [PubMed] [Google Scholar]
- 39. Hornung V, et al. 2002. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531–4537 [DOI] [PubMed] [Google Scholar]
- 40. Huang XR, et al. 2001. Macrophage migration inhibitory factor is an important mediator in the pathogenesis of gastric inflammation in rats. Gastroenterology 121:619–630 [DOI] [PubMed] [Google Scholar]
- 41. Jasny E, et al. 2008. IL-12-impaired and IL-12-secreting dendritic cells produce IL-23 upon CD154 restimulation. J. Immunol. 180:6629–6639 [DOI] [PubMed] [Google Scholar]
- 42. Jonuleit H, et al. 1997. Pro-inflammatory cytokines and prostaglandins. Eur. J. Immunol. 27:3135–3142 [DOI] [PubMed] [Google Scholar]
- 43. Kao JY, et al. 2010. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology 138:1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kerrigan AM, Brown GD. 2009. C-type lectins and phagocytosis. Immunobiology 214:562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khamri W, et al. 2010. Helicobacter pylori stimulates dendritic cells to induce interleukin-17 expression from CD4+ T lymphocytes. Infect. Immun. 78:845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Köhalmi F, Strausz J, Egerváry M, Szekeres G, TíMár J. 1997. Expression of macrophage markers in childhood and adult Langerhans histiocytosis (LCH). Orv. Hetil. 138:1399–1402 (In Hungarian.) [PubMed] [Google Scholar]
- 47. Kranzer K, et al. 2004. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect. Immun. 72:4416–4423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kranzer K, et al. 2005. Impact of Helicobacter pylori virulence factors and compounds on activation and maturation of human dendritic cells. Infect. Immun. 73:4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kurasawa K, et al. 2000. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum. 43:2455–2463 [DOI] [PubMed] [Google Scholar]
- 50. Lindholm C, Quiding-Jarbrink M, Lonroth H, Hamlet A, Svennerholm AM. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964–5971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS. 2003. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 71:1755–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mai UE, et al. 1991. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J. Clin. Invest. 87:894–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mantovani A, Sica A, Locati M. 2007. New vistas on macrophage differentiation and activation. Eur. J. Immunol. 37:14–16 [DOI] [PubMed] [Google Scholar]
- 54. Maris NA, et al. 2006. Toll-like receptor mRNA levels in alveolar macrophages after inhalation of endotoxin. Eur. Respir. J. 28:622–626 [DOI] [PubMed] [Google Scholar]
- 55. Martinez FO, Sica A, Mantovani A, Locati M. 2008. Macrophage activation and polarization. Front. Biosci. 13:453–461 [DOI] [PubMed] [Google Scholar]
- 56. Martinez-Pomares L, Linehan SA, Taylor PR, Gordon S. 2001. Binding properties of the mannose receptor. Immunobiology 204:527–535 [DOI] [PubMed] [Google Scholar]
- 57. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164:6166–617310843666 [Google Scholar]
- 58. Mitchell P, et al. 2007. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect. Immun. 75:810–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Monteiro MA, et al. 1998. Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides. J. Biol. Chem. 273:11533–11543 [DOI] [PubMed] [Google Scholar]
- 60. Moos V, et al. 2010. Impaired immune functions of monocytes and macrophages in Whipple's disease. Gastroenterology 138:210–220 [DOI] [PubMed] [Google Scholar]
- 61. Moreno JL, Mikhailenko I, Tondravi MM, Keegan AD. 2007. IL-4 promotes the formation of multinucleated giant cells from macrophage precursors by a STAT6-dependent, homotypic mechanism: contribution of E-cadherin. J. Leukoc. Biol. 82:1542–1553 [DOI] [PubMed] [Google Scholar]
- 62. Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Necchi V, Manca R, Ricci V, Solcia E. 2009. Evidence for transepithelial dendritic cells in human H. pylori active gastritis. Helicobacter 14:208–222 [DOI] [PubMed] [Google Scholar]
- 64. Odenbreit S, et al. 2006. Interleukin-6 induction by Helicobacter pylori in human macrophages is dependent on phagocytosis. Helicobacter 11:196–207 [DOI] [PubMed] [Google Scholar]
- 65. Popa C, et al. 2006. MIF production by dendritic cells is differentially regulated by Toll-like receptors and increased during rheumatoid arthritis. Cytokine 36:51–56 [DOI] [PubMed] [Google Scholar]
- 66. Quah BJC, Parish CR. 2010. The use of carboxyfluorescein diacetate succinimidyl ester (CFSE) to monitor lymphocyte proliferation. J. Vis. Exp. 44:2259 doi:10.3791/2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Quiding-Järbrink M, Lundin BS, Lönroth H, Svennerholm AM. 2001. CD4+ and CD8+ T cell responses in Helicobacter pylori-infected individuals. Clin. Exp. Immunol. 123:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Quiding-Järbrink M, Raghavan S, Sundquist M. 2010. Enhanced M1 macrophage polarization in human Helicobacter pylori-associated atrophic gastritis and in vaccinated mice. PLoS One 5:e15018 doi:10.1371/journal.pone.0015018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Randolph GJ, Jakubzick C, Qu C. 2008. Antigen presentation by monocytes and monocyte-derived cells. Curr. Opin. Immunol. 20:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ravetch JV, Bolland S. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290 [DOI] [PubMed] [Google Scholar]
- 71. Rescigno M, et al. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361–367 [DOI] [PubMed] [Google Scholar]
- 72. Sallusto F, Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schebesch C, et al. 1997. Alternatively activated macrophages actively inhibit proliferation of peripheral blood lymphocytes and CD4+ T cells in vitro. Immunology 92:478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schreibelt G, et al. 2010. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol. Immunother. 59:1573–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Soilleux EJ, et al. 2002. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71:445–457 [PubMed] [Google Scholar]
- 76. Sommer F, et al. 1998. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 66:5543–5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Steinman RM, Hemmi H. 2006. Dendritic cells: translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 311:17–58 [DOI] [PubMed] [Google Scholar]
- 78. Trinchieri G. 1998. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 70:83–243 [DOI] [PubMed] [Google Scholar]
- 79. van Die I, et al. 2003. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology 13:471–478 [DOI] [PubMed] [Google Scholar]
- 80. Verreck FA, et al. 2004. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. U. S. A. 101:4560–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Viala J, et al. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166–1174 [DOI] [PubMed] [Google Scholar]
- 82. Wilson KT, Crabtree JE. 2007. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology 133:288–308 [DOI] [PubMed] [Google Scholar]
- 83. Wilson NJ, et al. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8:950–957 [DOI] [PubMed] [Google Scholar]
- 84. Wong BL, et al. 2009. Essential role for macrophage migration inhibitory factor in gastritis induced by Helicobacter pylori. Am. J. Pathol. 174:1319–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wunder C, et al. 2006. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat. Med. 12:1030–1038 [DOI] [PubMed] [Google Scholar]
- 86. Xia HH-X, et al. 2004. Helicobacter pylori infection is associated with increased expression of macrophage migratory inhibitory factor—by epithelial cells, T cells, and macrophages—in gastric mucosa. J. Infect. Dis. 190:293–302 [DOI] [PubMed] [Google Scholar]
- 87. Zarrilli R, Ricci V, Romano M. 1999. Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage. Cell. Microbiol. 1:93–99 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.