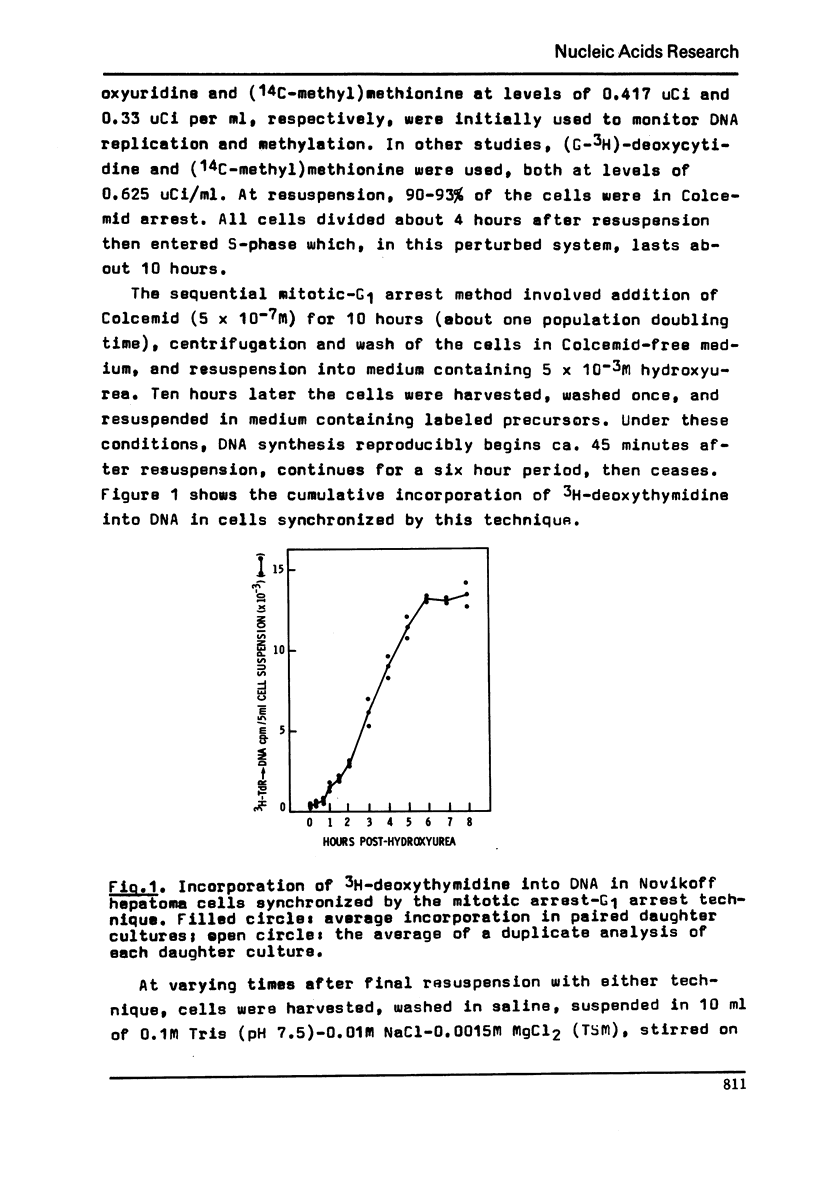
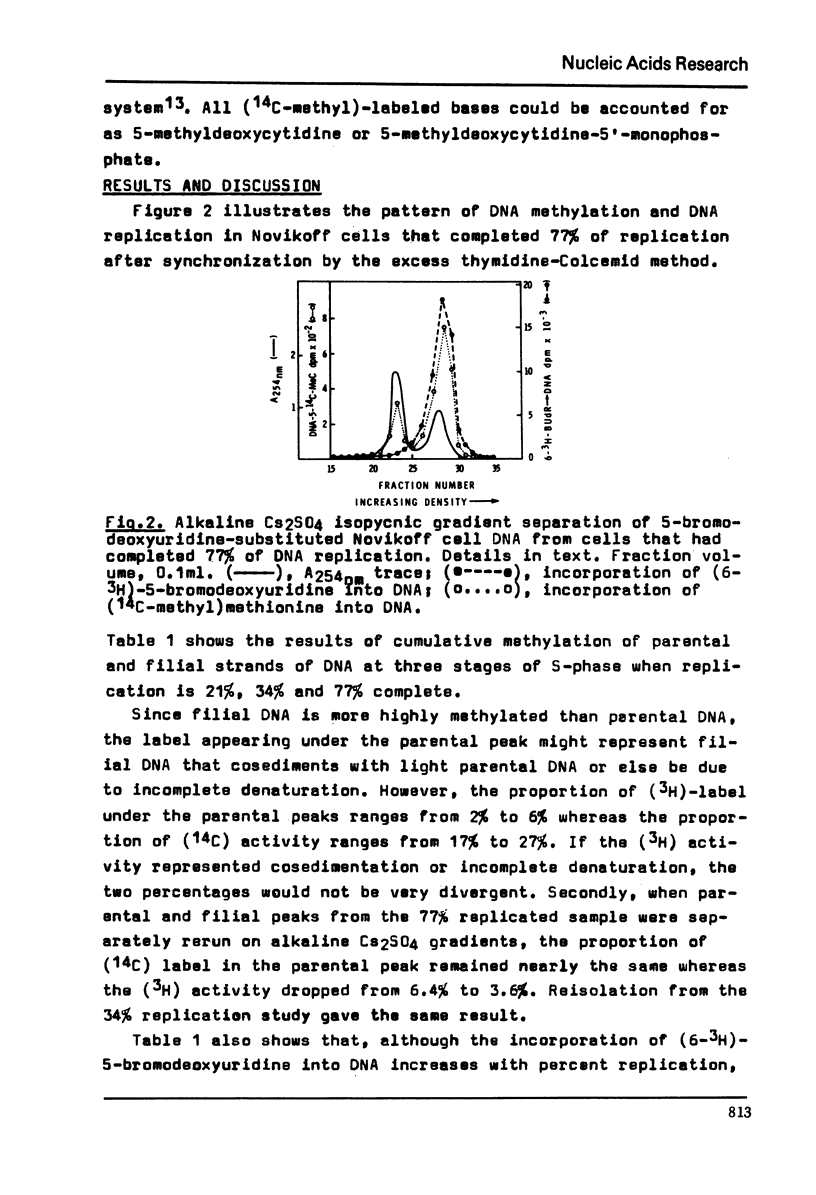
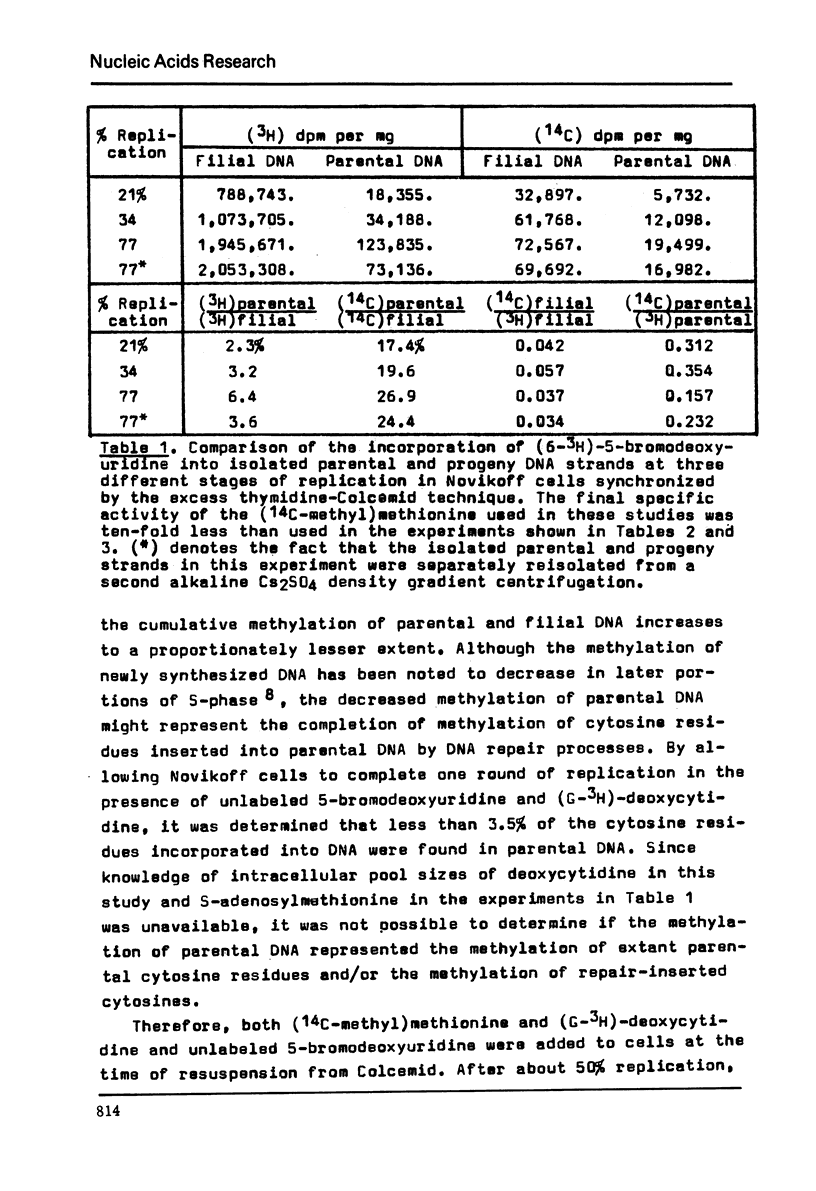
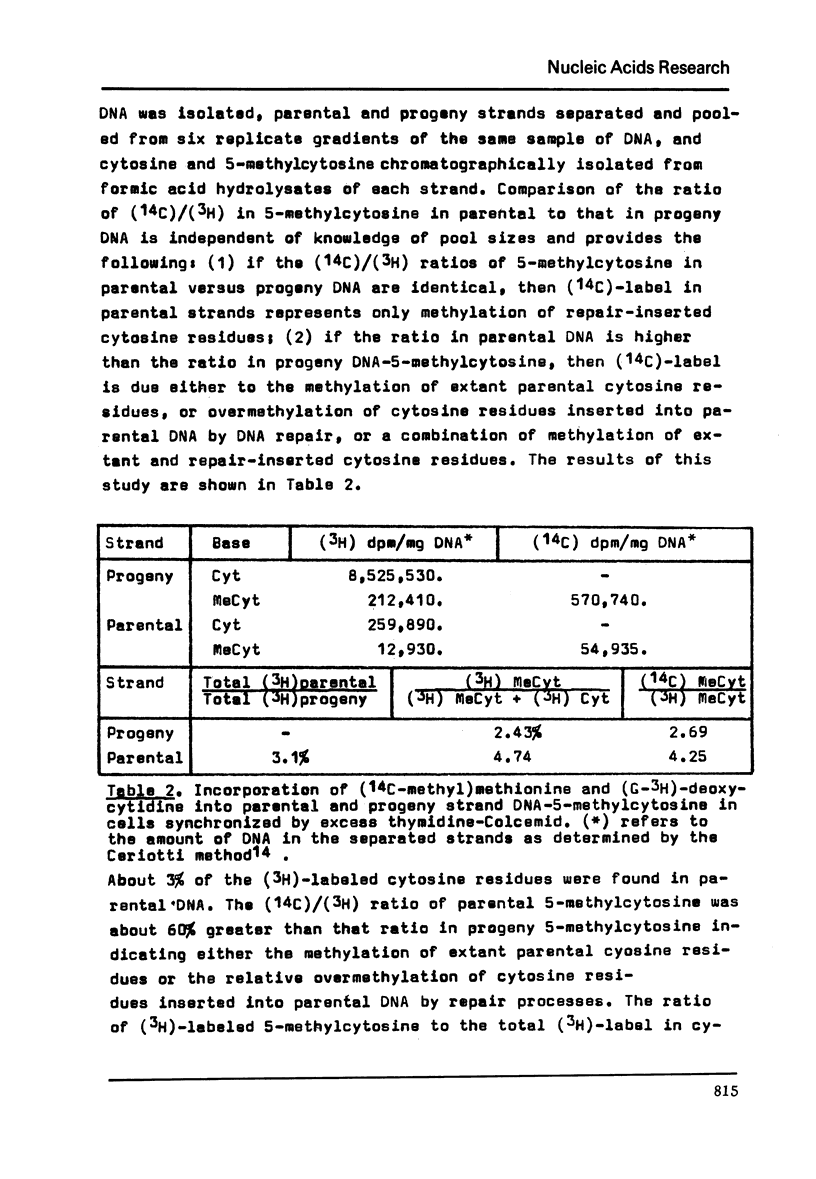
Abstract
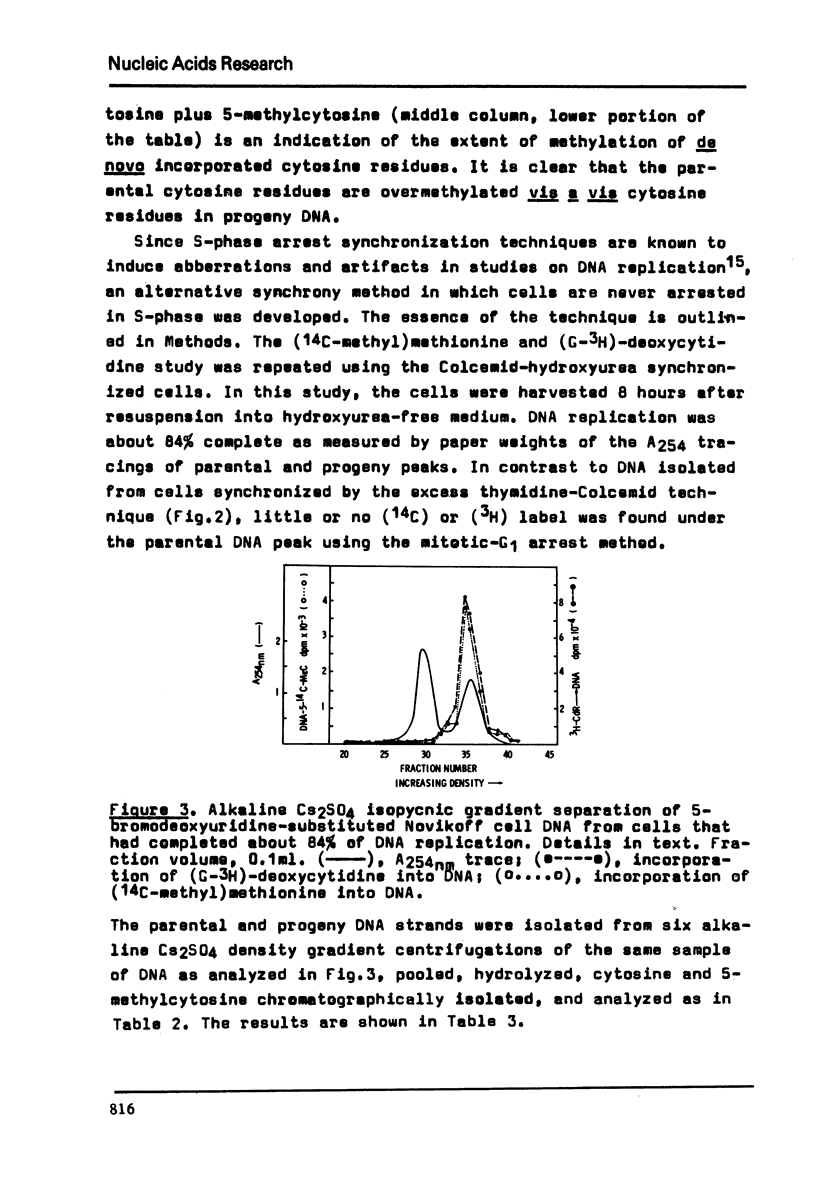
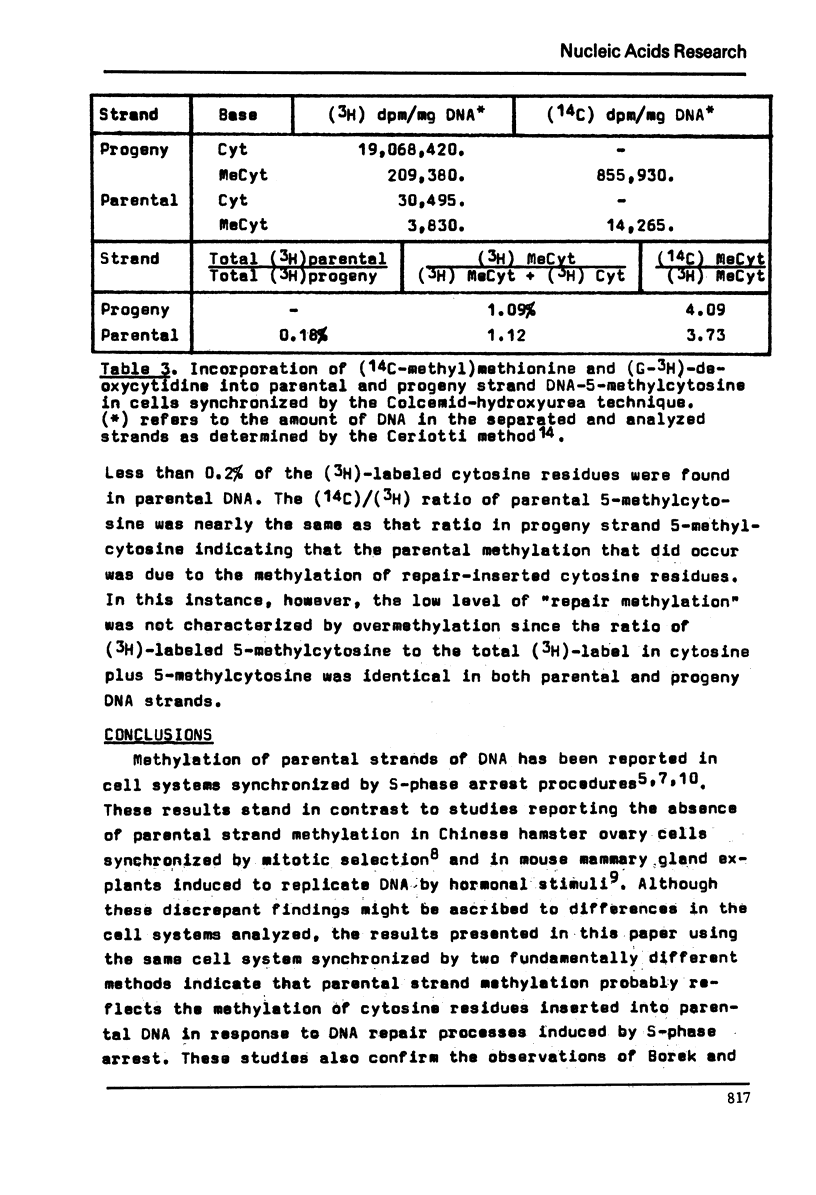
Parental and filial DNA strands were isolated from a Novikoff rat hepatoma cell line, synchronized by S-phase arrest with excess thymidine, that had completed up to one round of DNA replication in the presence of (14-C-methyl)methionine and (6-3-H) bromodeoxyuridine. Both strands were methylated, the proportion of total methyl label in parental DNA increasing slightly with time in S-phase. The studies were repeated with (14-C-methyl)methionine and (3-H)deoxycytidine to determine if parental methylation occurred on extant or repair-inserted cytosine residues. Both (14-C) and (3-H) were found in parental DNA. The (14-C)/(3-H) ration of parental DNA-5-methylcytosine was about twice that in filial DNA while the (3-H) data showed twice the concentration of 5-methylcytosine in parental compared to filial DNA. Thus parental methylation occurred on repair-inserted cytosine residues and resulted in overmethylation. That the DNA damage and repair was due to 5-phase arrest was shown by repeating the studies using a sequential mitotic-G1 arrest method. With this method little (14-C) or (3-H) was found in parental DNA. We conclude that S-phase arrest leads to DNA damage and repair with subsequent overmethylation of repair-inserted cytosines; that sequential mitotic-G1 arrest minimizes DNA damage; and, that the latter technique, suitable for synchronization of large quantities of cells, may prove useful in relatively artifact-free studies of eukaryotic DNA replication.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L. The relationship between synthesis and methylation of DNA in mouse fibroblasts. Biochim Biophys Acta. 1971 Dec 16;254(2):205–212. doi: 10.1016/0005-2787(71)90829-x. [DOI] [PubMed] [Google Scholar]
- Billen D. Methylation of the bacterial chromosome: an event at the "replication point"? J Mol Biol. 1968 Feb 14;31(3):477–486. doi: 10.1016/0022-2836(68)90422-1. [DOI] [PubMed] [Google Scholar]
- Burdon R. H., Adams R. L. The in vivo methylation of DNA in mouse fibroblasts. Biochim Biophys Acta. 1969 Jan 21;174(1):322–329. doi: 10.1016/0005-2787(69)90257-3. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- Evans H. H., Evans T. E., Littman S. Methylation of parental and progeny DNA strands in Physarum polycephalum. J Mol Biol. 1973 Mar 15;74(4):563–572. doi: 10.1016/0022-2836(73)90047-8. [DOI] [PubMed] [Google Scholar]
- Kappler J. W. The kinetics of DNA methylation in cultures of a mouse adrenal cell line. J Cell Physiol. 1970 Feb;75(1):21–31. doi: 10.1002/jcp.1040750104. [DOI] [PubMed] [Google Scholar]
- Ryan A. M., Borek E. Methylation of DNA in ultraviolet-irradiated bacteria. Biochim Biophys Acta. 1971 Jun 30;240(2):203–214. doi: 10.1016/0005-2787(71)90659-9. [DOI] [PubMed] [Google Scholar]
- Scarano E., Iaccarino M., Grippo P., Winckelmans D. On methylation of DNA during development of the sea urchin embryo. J Mol Biol. 1965 Dec;14(2):603–607. doi: 10.1016/s0022-2836(65)80211-x. [DOI] [PubMed] [Google Scholar]
- Schein A., Berdahl B. J., Low M., Borek E. Deficiency of the DNA of Micrococcus radiodurans in methyladenine and methylcytosine. Biochim Biophys Acta. 1972 Jul 20;272(3):481–485. doi: 10.1016/0005-2787(72)90400-5. [DOI] [PubMed] [Google Scholar]
- Schneiderman M. H., Billen D. Methylation rapidly reannealing DNA during the cell cycle of Chinese hamster cells. Biochim Biophys Acta. 1973 May 18;308(3):352–360. doi: 10.1016/0005-2787(73)90327-4. [DOI] [PubMed] [Google Scholar]
- Sneider T. W. Methylation of mammalian deoxyribonucleic acid. II. The distribution of 5-methylcytosine in pyrimidine deoxyribonucleotide clusters in Novikoff hepatoma cell deoxyribonucleic acid. J Biol Chem. 1971 Aug 10;246(15):4774–4783. [PubMed] [Google Scholar]
- Sneider T. W., Potter V. R. Methylation of mammalian DNA: studies on Novikoff hepatoma cells in tissue culture. J Mol Biol. 1969 Jun 14;42(2):271–284. doi: 10.1016/0022-2836(69)90043-6. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Spielvogel R. L. Methylation of deoxyribonucleic acid during hormonal stimulation of mammary cells in vitro. J Biol Chem. 1971 Jun 25;246(12):3835–3840. [PubMed] [Google Scholar]
- Walters R. A., Tobey R. A., Ratliff R. L. Cell-cycle-dependent variations of deoxyribonucleoside triphosphate pools in Chinese hamster cells. Biochim Biophys Acta. 1973 Sep 7;319(3):336–347. doi: 10.1016/0005-2787(73)90173-1. [DOI] [PubMed] [Google Scholar]


