Abstract
Wnt signaling controls cell specification and fate during development and adult tissue homeostasis by converging on a small family of DNA binding factors, the T-cell factor/lymphoid enhancer factor (TCF/LEF) family. In response to Wnt signals, TCF/LEF members undergo a transcriptional switch from repression to activation mediated in part by nuclear β-catenin binding and recruitment of co-activator complexes. In mammals, the specificity and fine tuning of this transcriptional switch is also achieved by the cell-context-dependent expression of four members (TCF7, TCF7L1, TCF7L2, and LEF1) and numerous variants, which display differential DNA binding affinity and specificity, repression strength, activation potential, and regulators. TCF7/LEF1 variants are generated by alternative promoters, alternative exon cassettes, and alternative donor/acceptor splicing sites, allowing combinatorial insertion/exclusion of modular functional and regulatory domains. In this review we present mounting evidence for the interdependency of TCF7/LEF1 variant expression and functions with cell lineage and cell state. We also illustrate how the p53 and nuclear receptor family of transcription factors, known to control cell fate and to inhibit Wnt signaling, may participate in the fine tuning of TCF7/LEF1 repression/activation potentials.
Keywords: alternative splicing, cell differentiation, Wnt signaling, transcription factor, nuclear receptor, p53
I. INTRODUCTION
Wnts and Wnt signaling control cell specification and fate during embryonic development and adult tissue homeostasis. Inappropriate spatiotemporal regulation of Wnt signaling results in numerous developmental defects, and its deregulation in adult tissues is highly associated with cancer initiation and progression.1–4 Among the different signaling pathways elicited by Wnt proteins,5–8 the canonical Wnt/β-catenin signaling pathway has been directly implicated in the maintenance of adult stem-cell pools and tissue regeneration,9 as well as in colorectal and hepatocellular carcinoma.2–4 This pathway is initiated by β-catenin stabilization in the cytoplasm and subsequent accumulation in the nucleus, where β-catenin interacts with T-cell factor/lymphoid enhancer factor (TCF/LEF) DNA binding effectors to modulate the transcription of numerous target genes involved in cell cycle progression, extracellular matrix remodeling, cell adhesion, and cell differentiation. Yet, it has become evident that the transcriptional programs elicited are very diverse, albeit specific in a tissue, cell-type, and cell-state dependent manner, and that consequently the outcomes vary markedly from stem-cell renewal and cancer initiation2–4,10–12 to establishment of cell senescence and tissue aging.13–17
Surprisingly, despite these diverse and specific functions, the TCF/LEF family is actually a rather small subset of the high mobility group (HMG) box protein family, with only one member present in Drosophila (dTCF/Pan) and C. elegans (POP1), and four members in most vertebrates: TCF7 (TCF1), TCF7L1 (TCF3), TCF7L2 (TCF4), and LEF1 (TCF7L3). They are all able to bind with high affinity specific WWCAAAG DNA sequences, also called Wnt-responsive elements (WRE), present in numerous promoters and other transcriptional regulatory regions such as enhancers. 18,19 Like other HMG box proteins they are devoid of transcriptional activity per se but serve as DNA-anchored platforms to recruit transcriptional complexes and chromatin remodeling complexes, and allow by virtue of their DNA bending properties the formation of DNA loops favoring the concentration of distant transcriptional complexes.18–21 TCF/LEFs interact with both co-repressor and coactivator complexes, with β-catenin probably the most potent activator identified.22–24 A transcriptional switch from repression to activation is the prevailing model for β-catenin-dependent target gene activation.25,26 In the absence of Wnt signals, TCF/LEF factors are associated with ubiquitous co-repressors such as the transducin-like enhancer of slip (TLE)/Groucho family members and actively repress transcription of target genes.27–29 Upon Wnt signals, β-catenin interacts with TCF/LEF and concomitantly displaces or favors the release of the co-repressor TLE/Groucho and promotes target gene activation by recruiting activator complexes (e.g., pygopus, mediator, p300/CBP) and chromatin remodeling complexes (e.g., Brg1/SWI/SNF, p300/CBP) (see review by Archbold et al.30 and references therein). Although this model provides a mechanistic framework, it is insufficient to fully render the biological diversity and specificity of TCF/LEF activities including β-catenin-independent activities and differential responses to Wnt signaling,31–33 which may be mediated by derepression rather than activation.34,35
The differential expression of each TCF7/LEF1 member during development is partly responsible for the specific defects evidenced by loss of functions in animal models (see recent review by Grigoryan et al.1 and references therein). Nonetheless, phenotypic rescue experiments have revealed that the lack of redundancy was also inherent to differential if not opposite activities,36 with TCF7L1 behaving mainly as a repressor and LEF1 as an activator, while TCF7 and TCF7L2 display dual transcriptional effects depending on the assays and biological systems used35,37–42 as well as the isoform studied.35,43–49 Indeed, all TCF7/LEF1 factors display complex expression patterns combining various strategies to increase spatiotemporal diversity from a single gene, including tissue-specific enhancers, alternative promoters, alternative exon cassette splicing, and alternative donor/acceptor splicing site (Fig. 1). The combination of regulated transcription and RNA processing allows the versatile expression of a myriad of protein variants with diverse arrangements of both common and unique modular domains. The community is starting to recognize and decipher the biological relevance of these variants in the specificity and regulation of the TCF7/LEF1 transcriptional repression/activation switch. Indeed, all the cellular processes involved from transcription to post-translational modifications are also cell context dependent and under the control of feedback mechanisms and crosstalk with various signaling pathways such as nuclear receptors (NR) and p53 family members, which are known to regulate cell specification50–52 and fate,53–57 respectively, and to modulate Wnt signaling.58–63
FIGURE 1.
Cell context–dependent mechanisms generating TCF7/LEF1 diversity in expression and functions. The expression of TCF7/LEF1 members and variants with differential transcriptional activity and specificity is controlled (i) at the transcriptional level by local chromatin states, enhancers, and alternative promoters,85–87 and (ii) at co-transcriptional and post-transcriptional RNA processing levels by alternative splicing of alternative exon cassettes and alternative donor/acceptor splicing sites.30,31,113 Although TCF7/LEF1 RNA isoforms are likely to be differentially regulated at other post-transcriptional levels, e.g., RNA isoform stability, they are not documented so far. Similarly, differential translation mechanisms have been shown only for LEF1 RNA isoforms, which use either a cap-dependent or a cap-independent internal ribosome entry site (IRES) mechanism.239 The resulting TCF7/LEF1 protein variants display differential DNA binding specificity and transcriptional activity both by changes in intrinsic domains and interacting partners30,31,113,155,157 and are subjected to differential post-translational modifications47,140 and cellular localizations.47 All these steps are highly dependent on cell states and cell types, which are controlled by various signaling pathways and their associated family of transcription factors such as the nuclear receptor and p53 families as well as the TCF7/LEF1 members and variants themselves.30
II. TISSUE-SPECIFIC AND CELL CONTEXT–DEPENDENT EXPRESSION AND FUNCTIONS OF TCF7/LEF1 MEMBERS AND VARIANTS
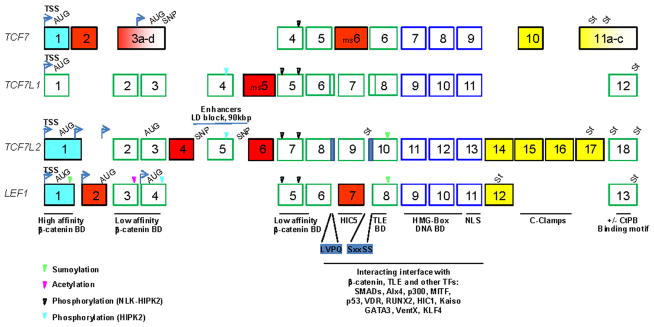
Although TCF7/LEF1 alternatively spliced exons may also affect the rate and/or exon choice for downstream RNA processing, the nuclear retention of partially mature RNA, RNA stability, cytosolic RNA localization to specific RNA-binding complexes (e.g., P-bodies) and miRNA-dependent regulation of translation (Fig. 1), these mechanisms and roles remain to be investigated. In this section, we will primarily review and discuss their roles in the creation of cell context–dependent diversity in TCF7/LEF1 functional and regulatory domains, hence in the TCF7/LEF1 repressor/activator balance. Since TCF7L2 is the most versatile member of the family and the only one able to rescue a POP1-deficient phenotype in C. elegans during the first asymmetric mesodermal/endodermal cell specification,64 we will particularly focus on the cell lineage dependent expression of TCF7L2 variants and their potential roles in the reinforcement of cell lineage commitments. The organization and exon equivalence of TCF7/LEF1 genes are shown in Fig. 2; for clarity, the latest numbering of TCF7L2 and LEF1 exons will be used throughout this review. The exon equivalence between the TCF7/LEF1 members is based upon their sequential organization in the genome starting from the upstream transcription start sites (TSS), sequence homologies, and exon/exon junction conservation (Fig. 2).
FIGURE 2.
Schematic representation of human TCF7/LEF1 transcripts organization and isoforms generated by alternative promoter and transcription start sites (TSS) (light blue), alternative in frame exon cassettes (red), alternative in-frame splicing (blue), alternative out of frame splicing and alternative exon cassettes changing the open-reading frames (yellow), and stop codon locations (St). Mouse (ms) alternative exon cassettes not conserved118 or not yet identified in humans are represented. The locations of the corresponding translation initiation AUG codon are indicated as well as the functions of the encoded domains. In particular the correspondence between exon and the delineated interaction domains of TCF7/LEF1 with other transcriptional effectors is specified. The small encoded sequences LVPQ and SxxSS present in TCF7L1, absent in TCF7 and LEF1, and generated by alternative splicing in TCF7L2 are indicated. The SNPs in the TCF7 coding region and in the TCF7L2 noncoding regions that have been associated with diseases are represented as well as the location of the 90-kbps region of TCF7L2 in linkage disequilibrium (LD) in T2D and containing tissue-specific enhancer activities.85–87
A. Tissue-Specific TCF7L2 Enhancers, Common TCF7L2 Polymorphisms, and Individual Susceptibility to Diseases
A nonsynonymous single nucleotide polymorphism (SNP), C888A at the 3′-end of the Exons 3a–d (Fig. 2), a sequence common to all isoforms, has been associated with type-1 diabetes (T1D),65–68 an autoimmune disease characterized by the degeneration of pancreatic β-islet cells.69 The resulting Pro-to-Thr conversion is present in all TCF7 variants including the major ΔN-TCF7 variants expressed in activated T lymphocytes,70 but the functional consequences are not known. In contrast to TCF7, several common noncoding polymorphisms of the TCF7L2 locus have been reproducibly associated with increased risk of developing type-2 diabetes (T2D)71–74 and its vascular complications, i.e., atherosclerosis and coronary diseases.75,76 On the other hand, the association of TCF7L2 polymorphisms with breast77–79 and colon80,81 carcinomas, which are also highly influenced by obesity and T2D,82–84 remain controversial. The major T2D-associated TCF7L2 SNP, rs7903146 C/T, is located within a region of the large intron-4 corresponding to a pancreatic β-islet-specific open-chromatin region with enhancer activities that are increased in in vitro reporter assays when the T2D-risk allele (T) is present.85,86 The existence of pancreatic-specific TCF7L2 enhancers within the 90 kbps of the T2D-susceptibility locus has been confirmed in vivo by BAC enhancer trapping scan in mouse transgenic reporter lines, though TCF7L2 expression was lost in adult mouse pancreatic β islet.87 In the same screen, additional tissue-specific enhancer activities were revealed in the brain, intestine, stomach, and limbs,87 confirming earlier studies of Tcf7l2 expression during mouse embryonic development, and in adult intestine and brain.88–90 However, no significant changes in TCF7L2 overall expression were found associated with TCF7L2 polymorphisms in pancreatic tissues and isolated β islets in most studies.91–95 In contrast, a significant decrease in the levels of a TCF7L2 alternative exon, Exon 17 (Fig. 2), which is enriched in neuro-endocrine tissues,96 was noted in pancreatic tissues and isolated pancreatic β islets by Prokunina-Olsson et al.92 but not in other studies.93,94 Osmark et al. reported an increase of the alternative Exon-4 form together with up-regulation of TCF7L2 expression in T2D patients independently of TCF7L2 polymorphic genotypes.93 These conflicting results can be explained by a complex and cell-type-specific expression of TCF7L2 variants that cannot easily be investigated in most tissues and will likely require analysis at the single-cell level. Whether TCF7L2 tissue-specific enhancers influence TCF7L2 alternative exon inclusion/exclusion during transcription-coupled alternative splicing events remains an interesting possibility warranting further investigation.
B. Cell-State-Dependent Usage of Alternative Promoters and β-Catenin Interactions
Most TCF7/LEF1 family members display alternative promoters (Fig. 2), which are responsible for the expression of RNA isoforms with or without at least the first exon.31,97–100 The relative levels of these ΔExon-1 RNA isoforms appear more prominent for TCF7100,101 and LEF197,98,102,103 than TCF7L2 in different tissues and cell types,41,49,92,104 including breast (Boyechko, submitted) and colon47 cancer cell lines. The usage of TCF7 and LEF1 alternative promoters in different cell types is linked to their cell state, with in general a switch from full-length variants in proliferating immature cells to ΔN-variants during cell differentiation and associated cell cycle arrest in the thymus, peripheral T lymphoid organs, intestine, and colon.2,47,70 In bone, LEF1 is expressed in proliferating immature osteoblasts, where it inhibits osteogenesis, but upon differentiation its expression is down-regulated105 and ΔN-LEF1 variants are up-regulated in response to BMP2 signals through RUNX2-dependent but SMAD4-independent activation of the P2 alternative promoter.102 In contrast, both LEF1 and TCF7 are re-expressed in colon carcinoma cells, where they amplify the deregulation of Wnt signaling. 47,98 This specific up-regulation of LEF1 depends on the presence of TCF7/LEF1 DNA-binding sites within Exon 1 that participate in local chromatin remodeling at the upstream P1 promoter in a β-catenin-dependent manner,99 while the downstream P2 promoter located between Exon 3 and 4 is repressed by the transcription factor Yin Yang (YY)-1 even though it contains also TCF7/LEF1 DNA-binding sites.106 Of note, one important conserved feature of all TCF7/LEF1 genes across species is the presence of functional TCF7/LEF1 DNA-binding sites in their transcriptional regulatory sequences, which mediate feedback and crosstalk between them.35,42,107–109
Exon 1 encodes the N-terminal domain involved in high-affinity binding of nuclear β-catenin (Figs. 2 and 3).110–112 Deletion of this domain is sufficient to produce ΔN-variants that prevent β-catenin-dependent activation of Wnt-target genes in many biological systems.30,31,37,98,113 However, extended interactions of β-catenin have been identified in LEF1, within the conserved N-terminal domains encoded by Exon 2 and 3 and within the conserved central region and HMG box domain when bound to DNA (Fig. 2).25 These extended areas of low-affinity β-catenin/TCF interactions overlapping with TLE/Groucho binding domain25 and the absence of detectable β-catenin/TCF/TLE ternary complexes25,114 are at the basis of the current model of a repression/activation switch by β-catenin-induced displacement of TLE/Groucho co-repressor complexes.25,30 Another low-affinity β-catenin binding site, encompassing a conserved TCF7/LEF1 domain within the context-dependent regulatory region, was recently identified in ΔN-LEF1 based upon its ability to still interact with β-catenin in vitro, and to enhance the expression of differentiation Wnt-target genes in mesenchymal and osteoblastic cell lines.103 Although the latter implicates that β-catenin-dependent activation of ΔN-LEF1 might still occur, the net effect of bone-targeted ΔN-LEF1 expression in the mouse is to promote osteogenesis.103 Similarly, the absence of β-catenin/LEF1 interaction, via mutations of LEF1 within the N-terminal β-catenin binding domain, is associated with increased sebaceous cell differentiation and development of sebaceous tumors in humans.115,116 In the mouse, the targeted expression of ΔN-LEF1 in the skin also promotes sebaceous tumor formation and alteration of the tumor-suppressor p53 cascade.115 Moreover, these in vivo data emphasize the crucial roles of Wnt signaling in cell lineage specification and their dose dependency, 51,115 which may both rely on the balance of TCF7/LEF1 repressor/activator members and variants expressed at a given time during lineage specification.
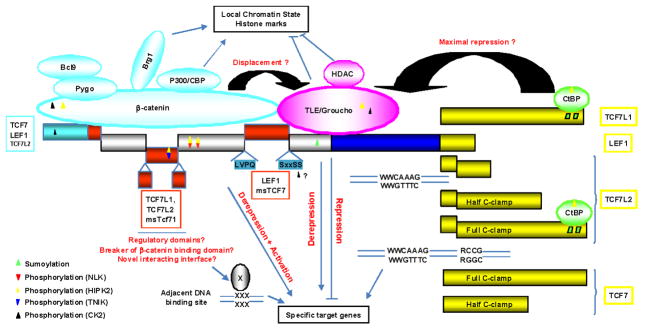
FIGURE 3.
Schematic representation of the modular structure of TCF7/LEF1 members and variants, and their association with the b-catenin activator complexes and TLE/Groucho repressor complexes. The alternative N-terminal β-catenin binding domain (light blue), the alternative domain (red) and alternative short sequences (blue) within the context-dependent regulatory region are indicated, as well as the conserved HMG box DNA binding domain and NLS (dark blue). The alternative C-termini with difference in sizes, presence of half and full c-clamps (yellow), and presence of CtBP binding motif (green) are indicated for each TCF7/LEF1 member as well as their specific DNA recognition sequence. The mechanisms of increased repression via CtBP and activation via displacement of TLE/Groucho are represented, as well as cooperation or transrepression on adjacent DNA-bound transcription factor. Some of the post-translational modifications of TCF7/LEF1 factors, β-catenin, and TLE/Groucho known to affect the transcriptional outcome are color coded as indicated.
C. Cell-State-Dependent Insertion/Exclusion of TCF7L2, TCF7L1, and LEF1 Alternative Exon Cassettes Within the Cell Context–Dependent Regulatory Region
TCF7L1 and TCF7L2 share an additional conserved exon, Exon 4 and Exon 5 respectively, which is mainly nonalternative and responsible for the bulk of their divergence in the context-dependent regulatory region from TCF7 and LEF1 (Fig. 2). Additional and highly alternative exon cassettes, Exon 4 and Exon 6 (Fig. 2), occur in human, mouse, and bovine TCF7L2 transcripts (Refs. 35, 92, 93, 104, Boyechko et al., submitted), while only the alternative Exon-4 cassette has been described in zebrafish117 and xenopus Tcf7l2 transcripts.90 In mammalian cells the levels of Exon-4 variant are highly correlated with the differentiated states of the cells independently of their cell type (Ref. 35 and Boyechko et al., submitted). TCF7L2 Exon 4 is present in >50% of the transcripts in primary macrovascular endothelial cells and epithelial committed/differentiated carcinoma cells, at intermediate levels (25–30% of the transcripts) in adipocytes, myocytes, blood cells, and microvascular endothelial cells, and <10% of the transcripts in breast cancer cell lines with stem-like properties, in some hepatocarcinoma cell lines, and in the embryonic stem-like HEK293 cells (Refs. 35, 49, 93, and Boyechko et al., submitted). Lower levels of Tcf7l2 Exon 4 were also noted in heart, brain, and mES cells.43 In contrast, TCF7L2 Exon 6 is a minor alternative exon both in embryonic and adult cells (Refs. 35, 92, Boyechko et al., submitted). A Tcf7L1 alternative exon cassette, msExon 5 (Fig. 2), was recently identified in mouse embryonic stem cells (ESC) on the basis of its decreased insertion levels during mESC differentiation. 118 Tcf7l1 Exon5 encodes a small (14 AAs) domain, which confers an increased ability to repress Oct4,118 a pluripotency core transcription factor,119 and a specific repression of AP1 family members such as Jun and ATF3 in differentiated mES cells.118 Although this alternative cassette appears not conserved in human ES cells,118 it is worth noting that the alternative Exon-6 cassette in human TCF7L2 encodes a similar though larger domain (Figs. 2 and 3) with low levels of expression in all human embryonic and adult cells tested thus far (Ref. 35, Boyechko et al., submitted). In view of their regulated expression during cell differentiation and also in response to p53 activation for TCF7L2 (see below), it is of interest to define how they may regulate the transcriptional repression-to-activation switch of TCF7L1 and TCF7L2.
Compared to LEF1, the domains in TCF7L2 encoded by the no alternative Exon 5 (37AA) and the alternative Exon 4 (23 AA) and Exon 6 (47AA) further extend the context-dependent regulatory region between the N-terminal β-catenin-binding domain and the low-affinity β-catenin-binding site and TLE/Groucho-binding domain25 (Figs. 2 and 3). The Exon-4 insertion was shown to dampen the transcriptional activity of stable β-catenin43 and to repress35 several Wnt-target gene-promoter reporters. This extension may thus be sufficient to alter the stability of β-catenin/TCF interaction and/or to promote the binding of novel cofactors by providing some organized structures (e.g., small α-helices) to the otherwise disorganized context-dependent regulatory region.25,120 Indeed, only the conserved TCF7/LEF1 HMG-Box domains fold in organized structures favorable to protein-protein interactions, when bound to DNA.21,25 Accordingly most interacting proteins for which the domain of interaction is known, interact with the HMG-box domain and surrounding sequences (Fig. 2),28,29,121–131 except β-catenin, which binds the N-terminal domain even in an extended and flexible conformation.25,111,112 Another exception is the LIM-domain protein HIC5, a focal adhesion and stress regulated nucleocytoplasmic shuttling protein, which was identified as an interacting partner of TCF7/LEF1 factors depending on the presence of the alternative Exon 7 in LEF1 (Fig. 2).132 The Exon 7–encoded domain is conserved and necessary for β-catenin-dependent activation of TCF7/LEF1-reporter and -target genes such as fibronectin.38,132 HIC5 interaction with TCF7/LEF1 members and variants containing this conserved domain inhibits β-catenin/TCF transcriptional activity. 132 In view of the overlaps between the HIC5 interaction domain and the low-affinity β-catenin binding site (Fig. 2),25 the HIC5 interaction may prevent and/or weaken β-catenin binding since the LEF1 alternative Exon 7 was shown dispensable for TLE/Groucho repression in reporter assays.29
Interestingly, like the alternative TCF7L2 Exon 4, the alternative insertion of LEF1 Exon 7 is also a regulated event during maturation of T-lymphocytes. 133 At the pre-T-cell receptor (TCR) signaling stage, the insertion of LEF1 Exon 7 is increased, which allows the expression of LEF1 variants with greater transcriptional activity on the TCR enhancer, a crucial step in T-lymphocyte maturation. 134 Moreover, the splicing factor implicated in the insertion of LEF1 Exon 7 during this specific differentiation step was identified as CELF2, a splicing factor recognizing UG-rich intronic sequences flanking Exon 7 and whose expression was also up-regulated during the pre-TCR signaling stage.133 This is the first splicing factor directly implicated in TCF7/LEF1 RNA processing but likely not the only one, in view of the biological relevance of TCF7/LEF1 alternative expression at discrete steps of cell specification and differentiation. Future investigations aiming at delineating a map of the splicing events and associated splicing factors are warranted.
D. Alternative Splicing Events Within TCF7/LEF1 Context-Dependent Regulatory Domains and Signal-Regulated Post-Translational Modifications
The disordered structures of TCF7/LEF1 context-dependent regulatory regions provide the flexibility that is needed for transient interactions and modifications by regulatory enzymes such as kinases and ubiquitin and SUMO ligases.135 It is likely that all alternative domains and sequences are necessary to either provide additional and specific regulatory sites or to modulate common regulatory sites. All known post-translational modifications of TCF7/LEF1 factors have been mapped to the N-terminus and context-dependent regulatory region (Figs. 2 and 3). CK1δ and CK2 phosphorylations of LEF1 on S40 in the N-terminal β-catenin binding domain136,137 are dispensable for β-catenin/LEF1 interaction in vitro,120 and thus their regulatory roles on LEF1 activity remain to be confirmed.
The small SxxSS sequence flanking the HIC5 interacting domain132 and juxtaposing the specific TLE/Groucho interaction motif,29 which is always (TCF7L1), alternate (TCF7L2), or never inserted (TCF7, LEF1) via usage of alternative splicing donor sites (Figs. 2 and 3), has been recognized for a long time as a main determinant in the repression/activation potential of TCF7/LEF1 factors.37,38,46,49 Although it is dispensable for TLE/Groucho interaction29 and DNA binding affinity,46 it affects in a phosphorylation-dependent manner the formation of β-catenin/TCF/DNA complex and subsequent transcriptional activation.46 Surprisingly, there are no reports of a direct phosphorylation of the SxxSS sequence in the repressor TCF7L1 and TCF7L2-SxxSS variants, and the possibility advanced by Pukrop et al.46 that this sequence may be a kinase interacting domain is a reasonable alternative.
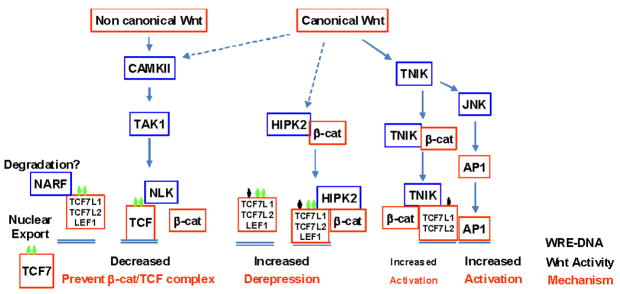
Three kinases and their phosphorylation sites have been identified in TCF7/LEF1 context-dependent regulatory regions, both in common and specific modular domains (Figs. 2 and 3): Nemo-like kinase (NLK),138,139 homeodomain interacting protein kinase (HIPK)2,34,140 and Traf2/Nck-interacting kinase (TNIK).141,142 Their respective Wnt-signaling cascade and outcomes on TCF7/LEF1 repression/activation switch are summarized in Fig. 4. They are all likely to occur simultaneously and concurrently within the same cells, in particular in response to canonical Wnts, in a gradient-dependent manner.143 These three kinases interact directly with TCF7L2 and/or TCF7L1 in vitro and in vivo, but β-catenin interactions are also required for TNIK nuclear localization and for HIPK2 interaction with TCF7L1/TCF7L2.34,140–142,144 In the most divergent part of the context-dependent regulatory region, three HIPK2 phosphorylation sites (in P2 and P3) (Fig. 5A)34 are common, with either the two identified NLK sites145 located within a conserved domain containing a low affinity β-catenin binding site103 or with the identified TNIK site142 located within the specific and nonalternative TCF7L2/TCF7L1 domain (Figs. 3, 5: P2 site). Of note, both NLK and HIPK2 are proline directed kinases belonging to the large mitogen-activated protein (MAP) kinase family, while TNIK belongs to a small subfamily of the Ste20 kinase family.
FIGURE 4.
Wnt signaling kinase cascades converging on TCF7/LEF1 phosphorylation and influencing their transcriptional repression-activation switch. Noncanonical Wnts5 as well as low canonical Wnt gradients143 activate a Ca2+ calmodulin-dependent kinase (CAMK) II/TGFβ-activated kinase (TAK) I cascade that activates Nemo-like kinase (NLK) in the nucleus, which phosphorylates all TCF7/LEF1 factors on two conserved sites in the context-dependent regulatory region and inhibits canonical Wnt activity by preventing formation of β-catenin/TCF complex138,139,145 and triggering nuclear export of TCF747 and/or proteosomal degradation via the NLK-associated ring finger (NARF) ubiquityl-ligase.153 Canonical Wnts also activate two parallel signaling cascades dependent on β-catenin stabilization and its subsequent nuclear translocation in association with two upstream kinases, the Traf2-Nck-interacting kinase (TNIK)141,142 and homeodomain interacting protein kinase (HIPK)2,34,140 which can interact and differentially phosphorylate TCF7/LEF1 factors depending on the site conservation. TCF7 is an unlikely target of both HIPK2 and TNIK, while LEF1 is an unlikely target of TNIK. Multiple HIPK2 phosphorylation sites with variable numbers are present on LEF1<TCF7L2<TCF7L1.34,140 The TNIK phosphorylation site identified overlaps with one of the HIPK2 sites, which suggests that the amplitude of the transcriptional outcome may result from a balance between both TCF7/LEF1 repressor vs. activator and HIPK2 vs. TNIK activation, since only TNIK-dependent TCF7L1/TCF7L2 phosphorylation increases β-catenin/TCF activity141,142 and also AP1 via activation of JNK.147 In contrast HIPK2-dependent phosphorylation of TCF7L1, TCF7L2, and LEF134,140 results in the release of TCFs from their cognate DNA binding site and allows derepression of target genes. This release may be mediated by targeted degradation of TCFs as well as by the degradation of the co-activator β-catenin152,240 and co-repressors TLE/Groucho149,241 and CtBP,242 which are all HIPK2 targets too.
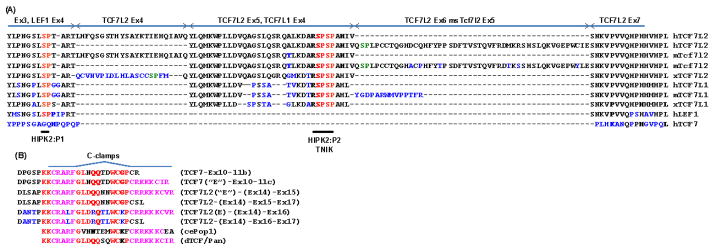
FIGURE 5.
Sequences and homologies of specific and alternative domains of TCF7/LEF1 members and variants in the context-dependent regulatory regions (A) and C-termini (B). In (A) the Exon-Exon junctions are indicated and numbered as in Fig. 1. The divergent and absent AAs are in blue and dash, respectively. The HIPK2 phosphorylation sites, P1 and P2, and the overlapping TNIK phosphorylation site, are indicated in red,34,142 and the putative phosphorylation sites within the alternative domains of TCF7L2 are in green. In (B) the AA forming (pink) and surrounding (red) the full and half c-clamps are indicated.31 The AAs distinguishing the c-clamps encoded by TCF7L2 Exon 16 are labeled in blue. The insertion or not of the Exon 14 in front of either Exon 15 or Exon 16 is neutral and does not change the following open-reading frames. The Exon 15 and Exon 16 are mainly mutually exclusive alternative exons (Boyechko et al, submitted). Thus the RNA isoforms containing Exons 15 and 16 and their encoded variants are rare and not reported in this alignment, but their sequences can be found in Weise et al.43 The co-insertion of the alternative Exon 17 with either Exon 15 or Exon 16 changes the following open-reading frames, resulting in protein variants with half c-clamps.
In agreement with the conservation of phosphorylation sites, both LEF1 and TCF7 are also phosphorylated by NLK,47,145 while TCF7 is not a target of HIPK2 within the context-dependent regulatory domain.140 Although phosphorylation of LEF1 by HIPK2 on a P2 site has also been proposed, 140 from the sequence and exon/domain localizations (Fig. 5A), it is more likely an equivalent of the P1 site in TCF7L1/TCF7L2, and thus P2 is specific for TCF7L1 and TCF7L2 repressors. An equivalent TCF7L1/2 P2 site (SPSP) is also missing in dTCF, and only armadillo/β-catenin, but not dTCF/Pan, was found phosphorylated by HIPK2.146 Nonetheless, the number of xTCF3 phosphorylation sites appears critical for the full response to canonical Wnt8 in xenopus animal cap explants, with four major sites (P2 to P4) located in the context-dependent regulatory region,34 while LEF1 contains only two sites and is accordingly less phosphorylated in mammalian cells.140 In the same region, the major TCF7L2 variants contain three sites, and the insertion of the alternative TCF7L2-Exon 6 domain may provide an additional “SP” phosphorylation site adjacent to P2, increasing the density of target sites for HIPK2 (Fig. 5A). Since HIPK2-dependent phosphorylation of TCF7L1 and TCF7L2 results in their dissociation from DNA and de-repression of target genes,140 increased or decreased susceptibility of TCF7L2 variants to undergo HIPK2-dependent phosphorylation is likely to affect their occupancy on target gene promoters/enhancers. This will not only affect the basal repressive state of the target gene, but will also create a primed state for further activation from adjacent transcription factor response elements and/or, as proposed by Hikasa et al., by a repressor (TCF7L1/2) to activator (TCF7) switch on TCF7/LEF1 binding sites.140 Both models of activation after de-repression are nonetheless highly cell-context dependent and also gene-context dependent. At this point it is worth noting that with only one activated kinase like HIPK2, the overall outcome on Wnt target gene and activity will directly depend not only on the balance of TCF7/LEF1 repressor vs. activator members but also their variants.
A Tcf7l1/Tcf7 switch has recently been implicated in the control of Wnt3a-induced stem-cell renewal.36 In this setting, the release of Tcf7l1 repression from specific stem-cell Wnt-target genes, like Nanog and the orphan nuclear receptor Nr5a2, is mediated firstly in a Tcf7l1–β-catenin-dependent manner, followed secondly by a β-catenin–Tcf7-dependent activation.36 Although a role for a kinase and/or Tcf7l1 phosphorylation in Tcf7l1-target gene de-repression in embryonic stem-cells remains to be established, the first event is, however, reminiscent of Wnt3a-induced de-repression of xTCF3-target genes during axis specification in early xenopus embryos.34 Unlike TCF7L2 Exon 6, the mouse alternative Tcf7l1 Exon 5, which confers increased repressor activity in embryonic stem cells,118 encodes a domain lacking a putative S/TP site (Fig. 5A). Whether this small 14AA sequence modulates Tcf7l1 de-repression by altering β-catenin/Tcf7l1 interaction, by preventing changes in Tcf7l1 conformation, or by its involvement in the formation of a specific repressor complex, awaits further investigation.
Nonetheless, this switch from TCF7L1 repression to TCF7 activation36,140 depends also on the availability of TCF7, which may be not expressed, competing with TCF7L2 repressor variants, or even exported from the nucleus.47 Indeed, Wnt-dependent activation of the CAMKII/NLK cascade in colon cancer cells results in the nuclear export of ΔN-TCF7E variants, which alleviates their growth inhibitory effects.47 Similarly, the availability of AP1 after TNIK/JNK activation147 and the proximity of AP1 and TCF7L2 binding sites in 15% of TCF7L2 target genes42 can drive strong activated states of Wnt target genes after derepression priming (Fig. 4), as seen, for example, in the co-requirement of both AP1 and β-catenin/TCF complexes for full activation of matrix-metalloproteinase-7 gene expression.148 Those availabilities and crosstalk may explain the different outcomes of TCF7L2 phosphorylation by TNIK (activation), HIPK2 (de-repression), and NLK (inhibition) (Fig. 4). However, it is still unclear whether TNIK and NLK also drive de-repression of Wnt target gene to the same extent as HIPK2, and thus there are questions about the importance of multiple HIPK2 phosphorylation sites in TCF7L1 and TCF7L2 for their dissociation from DNA, and the fates of β-catenin/TCF and/or TCF/TLE complexes.
TNIK, like HIPK2, requires β-catenin for its interaction and phosphorylation of TCF7L2 in normal intestinal crypt141 and in colon carcinoma cells,142 and phosphorylates also the P2 site (Fig. 5A),142 but no Wnt-dependent changes in TCF7L2 native promoter occupancy and in vitro DNA-binding affinity have been reported.141,142 On the other hand, NLK phosphorylates all TCF7/LEF1 members on some “S/TP” sites overlapping with HIPK2 sites (P3) (Figs. 2 and 3) and also induces a decrease of the DNA-binding activity of β-catenin/TCF complex in vitro,145 indicating similar conformational changes. Unlike HIPK2, all these NLK-driven events are β-catenin independent,138,139,145 and thus a priori TCF7/LEF1 members are phosphorylated when in repressor complex with TLE/Groucho and also with CtBP for TCF7L1 and some TCF7L2 variants. Both co-repressors are also phosphorylated by various MAP kinases, including HIPK2, whereby their co-repressor activities are attenuated, though the mechanisms may differ.149–151 HIPK2-dependent phosphorylation of TLE/Groucho can lead to its dissociation from the repressor Eyeless/Pax6 during drosophila eye morphogenesis,149 while CtBP is mainly targeted to proteosomal degradation.151 β-Catenin is also a target of HIPK2-dependent phosphorylation, but various outcomes have been observed, from a β-catenin stabilization and promotion of β-catenin/TCF activity146 to an increased β-catenin proteosomal degradation.152 Moreover, TCF7/LEF1 factors may also be targeted to proteosomal degradation after NLK-dependent phosphorylation via the NLK-associated ring finger ubiquitin ligase NARF.153 Although further investigation is clearly needed, HIPK2 activation appears to dissociate altogether TCF7/LEF1 repressor complexes, and thus the amplitude of specific target gene de-repression is likely to reflect the differential DNA binding affinities and repressor strengths of the TCF7/LEF1 members and variants. All of those are dependent upon alternative TCF7/LEF1 domains, including the SxxSS sequence38,46 and C-terminus tails.30,31,38
E. Alternative Splicing and TCF7/LEF1 DNA-Binding Affinity and Specificity and Tcf7/Lef1 Repression/Activation Potentials
Of note, although the HMG box DNA binding domain is itself highly conserved, with 99% homologies among the TCF7/LEF1 members, it is a key determinant in the stability of the ternary DNA/TCF/TLE repressor complexes. Indeed, the HMG box complete folding occurs when bound to the specific WWCAAAG DNA sequence, which dictates its interactions with the co-repressor TLE/Groucho and consequently further locks its conformation. 25 The newly identified HIPK2 phosphorylation sites are not overlapping with the TLE/Groucho interacting domain (Figs. 2 and 3),27,29 and thus the conformational changes involved in the DNA/TCF dissociation need to spread through the entire TLE/Groucho interacting domain, suggesting that the strength of TCF7/LEF1 interaction with TLE/Groucho may modulate these changes. Needless to mention, depending also on the assembled repressor complexes and corresponding chromatin remodeling, neither TLE/Groucho nor the repressor TCF7/LEF1 members may be easily accessible to kinases.154 This would also be in agreement with de-repression/activation of specific subsets of target genes in response to Wnt signaling in a cell state (e.g., stem cell vs. differentiated) but also in a cell lineage (e.g., epithelial vs. fibroblast) dependent manner. Besides TLE/Groucho, other transcriptional activators and repressors are also able to interact with the HMG box domain (see Fig. 2),31 and they are competing in most cases with the formation of TCF7/LEF1-DNA complexes in vitro.28,29,121–131 It is conceivable that when bound to adjacent sites, they may participate in the de-repression mechanism by favoring the dissociation of TCF7/LEF1 from DNA.
It is clearly established that the diverse C-terminus tails of the TCF7/LEF1 members and variants, which are generated by alternative exon cassettes and alternative splicing (Figs. 2 and 3) are directly implicated in their differential DNA-binding specificity and repression/activation potentials. 31,35,37,38,43,49,113,128,155 In particular, seminal studies have shown that deletion of the CtBP binding motifs in the xTCF3 C-terminus tail was sufficient to convert it from repressor to an LEF1-like activator,38 and that the addition of an “E” tail (c-clamp) from TCF7 or TCF7L2 to LEF1 C-terminus was sufficient to confer upon it another DNA binding specificity and affinity.128,156 The c-clamps are highly conserved sequences in the C-terminus tails of the invertebrates dTCF/Pan and POP1, and vertebrates TCF7-“E” and TCF7L2-“E” variants (Fig. 5B), which are necessary for their binding to bipartite DNA Wnt-responsive element: the classical WRE WWCAAAG plus an adjacent GC-rich sequence, the auxiliary RCCG sequences in mammals,155 and the helper sequences in drosophila. 30,157 The c-clamp itself is a bipartite cystrich domain, containing the sequences “CRARF” and “WCXXCRRKKKCIRY,” which stabilizes (clamps) the TCF/DNA complex.155 The original term “E-tail” has become somewhat improper since the “E” tail of TCF7L2 also contains CtBP binding motifs but not TCF7-E tail (Fig. 3). Moreover, in TCF7L2 there are two alternative versions of the c-clamp: the highly conserved “CRARF” c-clamp is encoded by the alternative Exon-15 cassette, while a slightly divergent “CRALF” c-clamp is encoded by the alternative Exon-16 cassette (Fig. 5).43 Based upon the crucial roles of the “CRARF” c-clamps in DNA recognition, the “CRALF” c-clamp may provide another DNA binding specificity and/or modulate DNA-binding affinity. In support of this assumption, in addition to their tissue-specific expression (see below), is the slight increases in β-catenin-dependent activation of the cdx1-promoter reporter with TCF7L2-Ex16 (CRALF) variant as compared to TCF7L2-Ex15 (CRARF).43
Only CtBP binding motifs have been identified so far in the nonalternative and large C-terminal tail of the “repressor” TCF7L1 to explain its transcriptional activity (Figs. 2 and 3).44,158 However, it is possible that specific auxiliary DNA binding domains, interacting partners, and/or regulatory sequences are required for its unique repressor functions and specific target genes in ES cells and early embryonic development.36,37,159 The “activator” LEF1 lacks both the c-clamps and CtBP binding motifs (Fig. 3), but can still repress transcription via its interaction with the co-repressor TLE/Groucho,28 and its target genes are either activated or down-regulated in vivo.30,31 There are nonetheless two alternative LEF-1 C-termini for which differential activities have not been reported yet. The skipping of the alternative TCF7–Exon 10 (TCF7-ΔEx10) and TCF7L2–Exons 14 to 17 (TCF7L2-ΔEx14-17) results in the expression of LEF1-like activator variants, i.e., with short C-termini, although other regulatory functions and/or interacting partners may also exist (Figs. 2 and 3). While TCF7ΔEx10 behaves as an LEF1-like activator,30,31 the “activator” potential of TCF7L2-ΔEx14-17 is not obvious, since in concert with stable β-catenin forms it has been shown to increase artificial β-catenin/TCF-reporters49 but not Wnt target gene-promoter reporters, irrespective of the presence/absence of the auxiliary RCCG sites in the promoter sequences.35,43 All the TCF7 and TCF7L2 variants with half c-clamps, i.e., with only the “CRARF” motif, have lost the “E” tail property (Fig. 5),43,155 further stressing the crucial roles of the DNA/TCF complex stability in the repression/activation potential of TCF7/LEF1 members and consequently in their de-repression potential as well.
Nonetheless, reporter assays are not as informative as expected for analyzing subtle differences in DNA-binding affinity and repressor activity, since the chromatin structure is only partially reconstituted on artificial promoters and some Wnt target reporters display even the opposite regulation of that observed in vivo.30 Also, preformed β-catenin/TCF complexes are the key players in reporter assays conducted with overexpression of activated β-catenin and TCF7/LEF1 members and variants, thereby bypassing important steps in the regulation of Wnt target genes, e.g., de-repression and/or displacement of the repressor complex (Fig. 3). In vitro DNA-binding assays have previously shown that β-catenin/Tcf7l2 complexes display higher DNA-binding affinity for the classical WRE and cannot grasp differences in the DNA-binding affinity of Tcf7l2 variants, even in the presence of the auxiliary RCCG sequence, while clear differences in native promoter occupancy by ChIp assays can be observed among ectopically expressed Tcf7l2 variants.43 Unlike TCF7L1, which is mostly nonalternative, the other TCF7/LEF1 members are expressed with alternative N- and C-termini in at least four different versions for LEF1 and up to at least 10 different versions for TCF7L2. Therefore, the upcoming challenges are to develop specific tools distinguishing each TCF7L2 variant, like the recently developed specific TCF7–“E” tail antibodies,47 to further define their biological roles in vivo and determine whether the complex regulation of TCF7L2 alternative splicing participates in a transcriptional repression/activation switch in response to Wnt signaling but also in response to other differentiation and/or stress cascades.
F. Cell Lineages and Insertion/Exclusion of the Alternative Exon Cassettes Encoding TCF7L2 C-Termini
TCF7L2, but not TCF7L1 and LEF1, is able to rescue POP1(zu189) phenotype in C. elegans.64 POP1 activities are crucial for the transition between the first asymmetric division and the specification of the single endodermal precursor and single mesodermal precursor in response to Wnt signals at the blastula stage. A POP1 transcriptional repression-to-activation switch governs the specific up-regulation of endodermal genes in the Wnt-sensitive endodermal precursor, while the POP1-dependent repression of endodermal genes is reinforced in the Wnt nonresponsive mesodermal precursor. The POP1(zu189) mutant is defective in the reinforcement of endodermal gene repression in the mesodermal precursor, and thus both precursors undergo an endodermal fate in response to Wnt signaling. Interestingly, the TCF7L2 variant used in these studies corresponds to TCF7L2-Ex15 (CRARF) variant, which thus can repress the expression of endodermal Wnt target genes and promote a mesodermal fate like POP1.64 From thorough analyses of TCF7L2 alternative exon expression in various cell types, a tissue-specific expression of TCF7L2 variants has recently emerged (Refs. 35, 43, 89, 92, 93, 96, 160, and Boyechko et al., submitted). TCF7L2-Ex15 (CRARF) variant is predominantly expressed in mesenchymal-derived cells and tissues such as adipocytes, myoblasts, and skeletal muscle.93,94,160 Based on the conservation of TCF7L2 expression and impact on cell differentiation across species, TCF7L2-Ex15 (CRARF) variant has likely retained its POP1 ancestral developmental property, i.e., to repress specifically endodermal/epithelial genes and features. It will be interesting to see how other TCF7L2 variants may substitute POP1 for the activation of endodermal genes and/or repression of mesodermal genes.
The alternative Exon 15 and Exon 16 are mutually exclusive exons, i.e., only rare TCF7L2 RNA isoforms have both Exon 15 and Exon 16 inserted, while the alternative in frame Exon 14 is more frequently inserted with either of them (Figs. 3 and 5). This mutually exclusive insertion is in agreement with—and also the cause of—their tissue-specific expression and cell lineage–dependent expression (Boyechko et al., submitted). The alternative TCF7L2-Ex16 (CRALF) is the major variant in epithelial lineage committed cells, such as breast cancer cell lines with myo-epithelial and luminal cell phenotypes, while the alternative Exon 15 is predominantly inserted in breast cancer cell lines with mesenchymal phenotypes (Boyechko et al, submitted). The predominance of the alternative Exon 16 was also noted in normal epithelial tissues such as isolated pancreatic β-islets,93 but not in hepatocellular carcinoma cell lines, in which the alternative Exon 15 is instead prominent. 49 However, in the latter study, higher levels of TCF7L2-Ex16 insertion were observed in cell lines with more epithelial differentiated features.49 Activation of the canonical Wnt3A signaling pathway decreases the Exon-16 insertion in embryonic stem-like HEK293 cells, while the noncanonical Wnt13A161 and stable β-catenin forms have no effect (Boyechko et al, submitted). The promotion of mesenchymal-to-epithelial transition in breast cancer stem-like cells via either down-regulation of TAp63 or increased cell density results in the specific up-regulation of TCF7L2-Ex16 insertion (Boyechko et al., submitted). Collectively, these studies corroborate the epithelial specific expression of TCF7L2-Ex16 (CRALF) variants, which in mammary epithelial committed cells is also accompanied by the inclusion of the alternative Exon 4. The combined Exon 4/Exon 16 signatures clearly distinguish epithelial breast cancer cell lines from stem-like and mesenchymal-like breast cancer cells (Boyechko et al., submitted). Of note, normal mesenchymal-derived cells display combined Exon 4/Exon 15, while cancer cell lines with a stem-like/mesenchymal phenotype have reduced Exon 4 insertion, which is consistent with the alternative Exon 4 insertion in a cell state–dependent manner but cell type–independent manner. As opposed to TCF7L2-Ex15 (CRARF) variant and its role in repressing endodermal/epithelial genes, TCF7L2-Ex16 (CRALF) variant may participate either in the repression of mesodermal/mesenchymal genes or in the activation of endodermal/epithelial gene expression. The latter possibility is supported by the increase of cdx1-promoter reporter activation by Tcf7l2-Ex16 variant,43 since cdx1 is an homeobox transcription factor implicated in endodermal tissue development such as small and large intestine, and required for the maintenance of epithelial cell differentiation.162
The alternative Exon 17 is rarely present because of its neuro-endocrine tissue–specific insertion in some regions of the brain, pancreas, and gut.43,89,92,96 On the other hand, the inclusion of the alternative Exon 14 is predominant in microvascular and plastic endothelial cells35 and in cell lines with neural/neuronal differentiation potentials, such as the embryonic stem-like HEK293 cells and the melanocytic MDA-MB-435 cancer cell line (Boyechko et al., submitted). Although Tcfl2 expression is detected in mouse ES cells,36,43 it is specifically up-regulated during the neural stages, including in zebrafish and xenopus embryos.87,117 All the “xTCF4” variants originally cloned during xenopus gastrulation correspond in fact to TCF7L2-Ex14 variants, suggesting again that the adult tissue–specific expression of TCF7L2 alternative Exon 14 may have retained the developmental regulation by neural-specific splicing factors, and perhaps the function as well. These TCF7L2-Ex14 variants lack both the c-clamps and CtBP binding motifs, but surprisingly can substitute for TCF7L1-dependent repression of mesodermal genes in xenopus embryos.37
TCF7L2-ΔEx14-17 variants, generated by the skipping of all alternative exon cassettes (Figs. 2 and 3), are up-regulated in all the cell lines and primary cells in culture as compared to normal tissue. TCF7L2-ΔEx14-17 variants are also strongly expressed in numerous stem-like cancer cell lines (Ref. 104, Boyechko et al., submitted) and are increased in the embryonic stem-like HEK293 cells in response to lithium,35 an activator of both canonical Wnt signaling163 and p53 cascade.164 Canonical Wnt3A signaling decreases only the alternative Exon 16, and p53 up-regulation decreases mainly the alternative Exon 14, and thus neither provokes by itself an overall Ex14-17 skipping like lithium (Boyechko et al., submitted). Nonetheless, while the inclusion of one specific alternative Exon 14-17 is favored during lineage commitment and further differentiation, their skipping in concert with reduced Exon 4 insertions characterizes inversely undifferentiated cancer cells with stem-like cell properties. In mouse ES cells, the Tcf7l2 RNA isoform profile defined by Weise et al.43 is also consistent with the expression of TCF7L2 variants lacking altogether the alternative Exon 4 and the alternative Exons 14 to 17. It is conceivable, since the resulting TCF7L2-ΔEx4ΔEx14-17 variant lacks significant transcriptional activity towards a variety of Wnt target genes,35,43 that it may constitute a permissive/nonrepressive form participating in the maintenance of undifferentiated states (Fig. 6).
FIGURE 6.
Schematic representation of the working model of TCF7L2 alternative exon tissue-specific expression and of corresponding TCF7L2 variant activity on differentiation programs.
A decrease of TCF7L2 Exon 4 insertion was detected by exon-junction microarray in human prostate epithelial cells undergoing an epithelial-to-mesenchymal transition caused by the knockdown of the epithelial-specific splicing factors ESRP1/2.165 TAK1, a TGFβ and Wnt-signaling upstream activator of TNIK (Fig. 4), also displays differentially expressed variants in these ESRP1/2 knockdown cells, but surprisingly, changes in TCF7L2-Exon 14–17 alternative splicing, and more particularly their skipping, were not detected in the same screen.165 This may be explained by an incomplete coverage of TCF7L2 exon junctions in the microarray. Nonetheless, changes in TCF7L2–Exon 4 levels were not detected in the reverse experiments with ESRP1/2 ectopic expression in mesenchymal-like breast cancer MDA-MB-231 cells, while several epithelial markers and splice variants were re-expressed.165 Whether increased TCF7L2 exon skipping, including Exon 4 skipping, is a also a response to stress such as a DNA damage-like cascade initiated by lithium, for example, and p53 activation and/or an anoikis-like cascade initiated by disruption of cell-cell adhesion, remain to be answered. But in view of the important crosstalk of Wnt signaling with p53 cascade via HIPK2 activation,34,140,166 and its dependency on p53 status, regulation of TCF7L2 alternative splicing will certainly be the subject of future investigations. Indeed, regulation of TCF7L2 alternative splicing is a key component in the control of the balance of TCF7L2 variants with differential repressor/activator potentials, and hence cell context–dependent response to Wnt signaling. Moreover, TCF7L2 alternative splicing is likely a direct or indirect target of deregulation in cancer, in agreement with its tumor suppressor role in colon and breast carcinoma, 33,167 but also in all chronic diseases with which TCF7L2 polymorphisms have been associated.71–76
III. Alternative RNA Splicing and Crosstalk of Wnt Signaling and Nuclear Receptors in the Control of Cell Fate
Like transcriptional control of gene expression, regulation of constitutive and alternative RNA splicing relies on cis-acting core and auxiliary elements respectively located in flanking intronic sequences and even in exons for some splicing inhibitory events. During transcription, the core elements are recognized by the spliceosome machinery, while the auxiliary elements recruit in a sequence-specific manner RNA-binding factors and associated proteins, the splicing regulators, which display either ubiquitous or tissue-specific expression.168,169 Alternative splicing is regulated by the availability of the splicing factors—e.g., CELF2 up-regulation precedes LEF1–Exon 7 inclusion during T-lymphocyte differentiation133—but also, more generally, by the balance of splicing factors and regulators, which enhance or inhibit splicing events and their intricate auto- and crossregulation by alternative splicing.169,170 Alternative promoter and rate of RNA polymerase II elongation activity also parallel the choice of alternative splicing events.171–173 As such, all transcription factors involved in the control of cell fate and regulating TCF7/LEF1 transcription are prone to affect concomitantly their alternative RNA splicing. Moreover, coordinated regulations of factors involved in the same biological processes apply also for RNA processing.168,169 For instance Wingless signaling in drosophila S2 cells is accompanied by the usage of an alternative promoter for its own receptor dFz2 and by alternative exon skipping of unfulfilled/hr51, an NR critical for neuron development in the mushroom body and axon guidance, which further emphasizes the interconnections between Wnt and NR signaling during cell differentiation. 174 In this section we explore the known and possible crosstalk of NRs and Wnt signaling at the level of alternative RNA splicing.
Various NR signaling pathways control cell specification and fate during development in a spatio-temporal-dependent manner, as well as diverse physiological functions in the adult, e.g., regulation of circadian rhythm, maturation of the mammary glands, and inflammation.62,63 The biological diversity and specificity of the NR family of Zn2+ finger transcription factors is conferred by 48 different members able to bind specific repeated DNA sequences either as homodimers, e.g., estrogen receptor (ER/NR3A1), or as heterodimers with the retinoid x receptors (RXRα-γ/NR2B1-3), e.g., vitamin D receptor (VDR/NR1I1). The NRs recognize and are activated by specific ligands, which are mostly lipophilic compounds, e.g., steroid hormones, vitamin D, and retinoids.62 Most of the NRs are nuclear, bound to their cognate DNA-response element and associated with the ubiquitous co-repressors NCoR and SMRT in the absence of ligands.175 Additional tissue-specific and developmentally regulated co-repressors, such as HR (Hairless) for VDR, also provide spatio-temporal NR repression.176 Upon ligand binding, the NRs undergo a transcriptional switch from repression to activation via ligand-induced conformational changes, leading to the release of the co-repressor complexes and subsequent binding of co-activators— e.g., SRC-1-3, which will further recruit chromatin remodeling complexes, histone acetyltransferase, e.g., p300/CBP and the basal transcription machinery through the mediator.177
Crosstalk between the NR and Wnt signaling can occur indirectly through competition for co-activators and co-repressors and through Wnt-dependent activation of signaling kinase cascades, for example JNK (Fig. 4), Akt, GSK3β, and PKA, to cite a few, which will regulate the activity of the NRs, co-activators, and co-repressors.62 On the other hand, estradiol can also initiate the Akt/GSK3β and MAP kinase cascades in an ER-dependent manner at the plasma membrane and cytoplasm, as well as a redox-dependent cascade by an intrinsic antioxidant activity.178 In fact, the NRs intersect with Wnt signaling at multiple levels and for most, not surprisingly, the outcomes are cell-context dependent. Detailed reviews of direct crosstalk by the NR-dependent regulation of Wnt signaling component expression, NR interaction with β-catenin and/or TCF7/LEF1, and their synergistic effects on NR transcriptional activity, but in general repression of β-catenin/TCF activity, can be found in Beildeck et al. and Mulholland et al. (Refs. 62, 63, and references therein).
A. Crosstalk During the Coupling of Transcription and RNA Splicing
The search for NR co-activators and/or interacting partners has led to the discovery that numerous transcription elongation factors (e.g., 7SK RNA ) and splicing factors associated with different small nuclear ribonucleoprotein particles (snRNP) of the spliceosome were present in the complexes.179 Several of these splicing factors contain either a LxxLL or FxxLF motif whereby they interact directly with NRs: the dead-box helicases DDX5 (p68) and DDX17 (p72), the core factor SF1, which recognizes the branch point sequence of the 3′ splice site, Fus/TLS protein, the p54nrb(NONO)/PSF heterodimer, and the prp4 kinase/SRPK1.180 An interaction of ERα with SF3a p120 of the U2-snRNP complex involves the N-terminal ligand-independent AF1 transactivation domain in a S118 phosphorylation-dependent manner.181 The splicing factor SKIP (Ski-interacting protein), which was also identified as a VDR coactivator (NCOA62), interacts preferentially with NR heterodimer, e.g., VDR/RXR, in a ligand-enhanced manner by an unusual binding site involving the α-helix 10 of the ligand-binding domain.182,183 This allows the formation of ternary complexes NR/SKIP with coactivators, e.g., SRC1, but also with other splicing factors, which bind the C-terminal ligand-dependent AF2 transactivation domain.182,183 NR coactivators and co-repressors themselves serve as platforms for additional complexes such as Sin3A/HDAC repressor complexes but also the coactivator CARM1/PRMT4, which is a methyltransferase implicated in the coupling of RNA transcription and splicing.184
CARM1 was shown to interact also with β-catenin, 185 and its recruitment to a subset of Wnt target genes allows histone H3-R17 dimethylation and activation of transcription.186 It is not known yet whether β-catenin is methylated upon CARM1 interaction like most of CARM1 substrates and whether RNA splicing is also activated. CARM1 auto-methylation is the coupling event with RNA splicing regulation187 and in the NR case, CARM1 auto-activation is dependent upon its interaction with SRC1 (p160 co-activator) and promotes exon skipping.188 High CARM1 expression has been found in colon carcinoma cells,186 which display altered RNA splicing, including alternative promoter and splicing of TCF7/LEF1.47,98,189 Similarly, TCF7L2 expression is up-regulated in colon carcinoma, and alteration of splicing events have been reported.44,104 TCF7L2 transcription is dependent on both β-catenin/TCF and VDR regulations,190,191 which makes CARM1 an interesting candidate to mediate the alternative splicing events occurring in colon carcinoma cells for TCF7/LEF1 members.
Although less documented, there is some evidence for an association of β-catenin and/or TCF7/LEF1 with RNA splicing factors associated with the spliceosome. Fus/TLS, DDX5, and p54nrb/NONO have been found associated with active β-catenin forms in colon carcinoma cells, and overexpression of Fus/TLS has been shown to inhibit β-catenin/TCF activity but to increase ERβ alternative splicing.192 These ERβ splicing variants result in a partial deletion of the ligand binding domain, and similar ERα splicing variants are also highly expressed in breast cancer.193 Fus and its interacting partner FusIP are also targets of the GSK3β-degradation complex, and their stability is increased by lithium.194 Abnormal stability of both Fus/TLS and TAR-DNA binding protein (TDP)-43 and their cytoplasmic aggregation is responsible for the development of a neurodegenerative disease, amyotrophic lateral sclerosis (ALS).195 Fus is also a target of the DNA-damage cascade via ATM phosphorylation and inhibits cell cycle progression.196 Its androgen-dependent down-regulation may participate in prostate cancer development.197
In intestinal epithelial cells, the expression of SF1 is dependent upon their differentiated states and participates in the shutoff of β-catenin/TCF signaling during differentiation of crypt stem cells to mature villous epithelial cells.198 SF1 expression is also increased by the ectopic expression of the dominant negative ΔN-TCF7L2-ΔEx14-17 and by butyrate, a PPARγ ligand and differentiating agent in colon carcinoma cells.198 Similarly to Fus/TLS, SF1 up-regulation is accompanied by increased ERβ alternative splicing,198 and whether SF1 and Fus/TLS are also involved in the control of endogenous TCF7L2, LEF1, and TCF7 alternative variants expressed in colon carcinoma cells is of particular interest in view of their relationship with epithelial differentiation and deregulation in cancer. Of note, several splicing factors, including Fus/TLS and DDX5, were found in an RNA complex with the IRES sequence of LEF1, indicating that they may also affect translation of full-length LEF1 variant.199
DDX5 is a nucleocytoplasmic shuttling protein, which is phosphorylated by the nonreceptor tyrosine kinase c-abl downstream of the PDGF and TNFα signaling cascades.200 When phosphorylated, DDX5 prevents β-catenin GSK3β-dependent phosphorylation and degradation, allowing β-catenin/TCF activity in a Wnt-independent manner.201 Phosphorylated DDX5 is also found associated with the promoter sequences of cyclin-D1 and c-myc, two Wnt target genes, and increases their expression.202 DDX5 couples transcription and splicing via its interaction with RNA Pol-II carboxyl-terminal domain (CTD) and its RNA helicase activity, which is necessary for unwinding RNA secondary structures and nascent RNA pairing.200 The helicase activity of DDX5 and DDX17 is dispensable for NR co-activation, while their RNA binding domain is required for the recruitment of the noncoding RNA, SRA, to the activation complex such as with ERα.203 ERα interaction with DDX5 and DDX17 is also crucial for estrogen-induced alternative promoter switch of target genes even when the global expression is not significantly affected.204 The usage of alternative promoters appears dependent upon the presence of ERα response elements with adjacent binding sites for CTCF,204 a factor implicated in gene insulation and hetero-/euchromatin boundary formation and remodeling, and the recruitment of both DDX5/DDX17 heterodimers and the RNA coactivator SRA.205 The alternative promoters of ERα coactivator NCOA7/ERAP140, RARα, GR, and also TP73, are among the novel ERα-target genes in breast cancer MCF-7 cells.204 Whether such a mechanism may apply also to TCF7/LEF1 binding sites in the proximity of CTCF binding sites, e.g., around enhancers, is an intriguing possibility especially for the regulation of LEF1 and TCF7 alternative promoters in colon carcinoma cells,47,98 in which DDX5 and DDX17 are also highly expressed.200
B. Crosstalk During Regulation of Constitutive and Alternative Splicing Events
ALY, a component of the TREX complex, which couples RNA splicing and RNA export,206,207 was found associated with LEF1 and behaving as a coactivator for LEF1 transcriptional regulation of the TCRα enhancer in a β-catenin-independent manner. 208,209 Interestingly, the RNA export activity of ALY is also enhanced by Akt1, but not Akt2, phosphorylation, which allows binding of nuclear phosphosinositides onto ALY Gly/Arg (GR) dipeptide repeats and its localization to nuclear speckles.210 Of note, nuclear speckles are thought to be the storage, assembly, and post-translational modifications of splicing factors that are released and recruited onto the nascent pre-mRNA localized in perichromatin fibrils, in which transcription elongation and RNA splicing take place.211
Pinin, a nucleocytoplasmic shuttling protein, was found associated with the co-repressor CtBP2 in nuclear speckles. Pinin attenuates the activity of various repressor/CtBP complexes, leading to derepression of the E-cadherin gene and increase of E-cadherin RNA splicing in a promoter-dependent manner.212,213 Pinin down-regulation up-regulates TCF7L2 expression in intestinal and corneal epithelial cells as well as increases active β-catenin forms.214,215 A Pinin/CtBP2/β-catenin complex has been found present on cdx2 promoter and proposed to mediate cdx2 expression in intestinal epithelial cells.214 The effects of Pinin on the expression of TCF7L2 variants and alternative RNA splicing were not tested, but future studies are warranted. Indeed, while both ALY and Pinin are transiently present in the exon-junction complexes,216 only Pinin is directly involved in pre-mRNA splicing activation.217 In particular, Pinin contains Ser/Arg (SR) dipeptide repeats like numerous splicing regulators, e.g., SRp20 and the co-activator SKIP, whereby they can form heterodimers in a phosphorylation-dependent manner.218 Pinin has been shown to interact directly with splicing factors associated with the spliceosome, e.g., DDX5,216 which regulates both constitutive and alternative RNA splicing, as well as with spliceosome regulatory complexes, e.g., the general activator RNPS1, which forms the apoptosis- and splicing-associated protein complex (ASAP) with SAP18, a component of the Sin3a/HDAC complex, and Acinus.219,220 Acinus interacts also with the RARα N-terminal AF1 transactivation domain and represses RAR target genes in a ligand-independent manner by recruiting co-repressors such as CtBP2.221,222 Its phosphorylation by SR protein kinase (SRPK)2 within the SR domain favors its release from the nuclear speckles and its transcriptional/splicing activities.223 On the other hand, Akt1-dependent phosphorylation prevents Acinus C-terminal cleavage by caspase-3,224 a crucial event for the activation of the ASAP complex during apoptosis.225 Whether Acinus participates in DNA condensation or in DNA fragmentation during cell death is still debated.225,226 Intriguingly, SAP18 homolog in drosophila is associated with the endocytic compartment, where it may play a role in autophagy,227 and thus SAP18 via its recruitment to the ASAP complex may also participate in the autophagic-dependent cell-death cascade. In the same line, Pinin is a desmosomeassociated protein when not in the nucleus,228 and thus all these RNA-processing auxiliary regulators are thought to constitute diverse stress sensors and effectors of stress-induced gene expression programs, including alternative splicing.189
Pinin is also present with SKIP in a complex with Smad nuclear interacting protein (SNIP1), TRAP150/THARP3, and the cell-death promoting-transcription repressor BCAFL1/Btf.229 This SNIP1/SKIP complex controls the stability of cyclin-D1 spliced RNA prior to its nuclear export, but not per se pre-mRNA splicing.229 SNIP1 also controls p53 expression in response to UV stress and modulates the activity of the DNA-damage ATR kinase on numerous stress-related targets including p53.230 Moreover, SKIP splicing activity is also involved in CDKNA1/p21cip exon splicing during p53-dependent DNA-damage stress response.231 In this case, the spliceosome U2AF65 factor is specifically recruited by SKIP on the p21cip gene in proximity to an elongation RNA pol-II pause site where it binds the nascent and unspliced premRNA and favors subsequent splicing.231 In the same line, VDR is rather a pro-apoptotic NR, and its induction of p21cip expression may use a similar SKIP-dependent mechanism, since alteration of SKIP activity has been shown to increase unspliced RNA in a VDR-dependent manner.232 Also, since the composition of SKIP complexes defines its transcriptional and splicing activity, the competitive availability of its partners, like pinin with β-catenin, is likely to influence the response to stress from survival to apoptosis. Interestingly, SKIP has also been identified as a novel effector of β-catenin/TCF signaling and proposed as a possible scaffold for the TCF7/LEF1 repression-to-activation switch.233 SKIP interacts with its central SNW domain with TCF7/LEF1 members and recruits HDAC1, and the ternary repressor complex is present on c-myc promoter in the absence of Wnt signals.233 SKIP also recruits nuclear β-catenin via its C-terminal domains including NLS, and is indispensable for Wnt signaling in in vitro reporter assays and in vivo for neural crest induction in xenopus embryos.233 Retinoic acid is another crucial factor for neural crest induction, and its receptor RARα interacts in a ligand-dependent manner with the SKIP central SNW domain as well.234 One can speculate that crosstalk between RA and Wnt signaling is likely to occur at the level of SKIP, and whether synergistic activation or competition for SKIP binding is the main outcome during neuronal cell differentiation remain to be seen.
C. Crosstalk and Regulation of Splicing Factor Expression
Since both Wnt signaling and NR pathways affect cell state and cell differentiation, they certainly affect the expression of numerous splicing factors and regulators as well as tissue-specific splicing factors such as epithelial ESRP1/2.165 Yet few have been reported and studied so far. This can be explained in part by the complex expression pattern of the splicing factors themselves, with multiple alternative splicing and exon cassettes, but also by the fact that these splicing events can occur without overall changes in the RNA levels. Deep mRNA sequencing is going to fill this knowledge gap of the changes in gene expression in different tissues and conditions. However, changes in splicing factors have been reported after activation of Wnt signaling. In addition to SF1, discussed above,198 the splicing regulator SRp20/Srsf3 has also been identified as a β-catenin/TCF target gene in colon cancer cells,235 in which it participates in the aberrant splicing associated with cancer cells.189 A two-fold up-regulation of SRp20 by ectopic expression of β-catenin was sufficient to alter the RNA splicing of a CD44-minigene and produce the metastatic CD44v5 RNA isoform.236 On the other hand, another metastasis-associated splicing event, Rac1 exon3b inclusion, was inhibited by the SRp20/β-catenin cascade but increased by the ASF/SF2(Srsf1)/Akt cascade.236 These somewhat opposite functions of Srp20/Srsf3 on two metastasis markers exemplify the challenges of RNA splicing regulation and outcomes.
Indeed, none of these splicing regulators function alone, but participate in small complexes in the enhancement or inhibition of RNA splicing events. The auxiliary complex composition will depend on both the RNA target sequences and splicing factor availability. The former implies that each splicing event on a pre-mRNA may be regulated by a specific complex, and the final mature spliced mRNA thus represents the combinatorial activity of different complexes proportionally to their complexity of alternative splicing and alternative exon cassettes. The latter relies also on the expression of tissuespecific splicing factors to confer tissue-specific expression of splice variants that in turn concur in the cell context–dependent responses to stress, Wnt signaling, but also to therapy such as the tissue-specific Fox2/RBM9/RTA splicing factor. The repressor for tamoxifen transactivation (RTA) was identified on the basis of its interaction with the ligand-independent AF1 transactivation domain of ERα, its ability to repress tamoxifen partial agonist transactivation, and its differential expression in sensitive vs. resistant breast cancer cells to tamoxifen, a therapeutic ER antagonist in breast cancer.237 Although the mechanisms of Fox2/RBM9/RTA-mediated NR repression are not fully understood, they likely involve co-repressor recruitment and transcription/splicing coupling in a cell context–dependent manner. Indeed, Fox2/RBM9/RTA is particularly expressed in breast epithelial cells and is also partly responsible for the expression of the epithelial FGFR2–Exon IIIb vs. mesenchymal FGFR2–Exon IIIc variants, and Fox2 expression changes are sufficient to mediate also epithelial/mesenchymal transitions238 like ESRP1/2.165 As such, Fox2 expression is considered as a valuable complement of ER status in breast cancer tissues both for prediction of tamoxifen-therapy resistance and for prognosis of metastatic progression.
IV. CONCLUSION
Despites all the challenges, the biological relevance of alternative splicing in development, but also its crucial role in the maintenance of adult tissue homeostasis in response to stress and its deregulation in numerous age-related diseases such as cancer189 and neurodegenerative diseases,195 ensures future investigations of the factors and mechanisms involved for each key gene and each key splicing event, including for TCF7/LEF1 members.
Acknowledgments
The author’s studies were funded by NIH R01-HL68698, NIH R21-AG031999 (CDM), NIH R01-CA129813, NIH R01-DK58196, and NIH P01-CA130821 (SWB).
ABBREVIATIONS
- BD
binding domain
- CtBP
C-terminal binding protein
- LEF
lymphoid enhancer factor
- NLS
nuclear localization signal
- NR
nuclear receptor
- RA
retinoic acid
- RAR
retinoic acid receptor
- SNP
single nucleotide polymorphism
- TGF
transforming growth factor
- TLE
transducin-like enhancer of split
- TSS
transcription start site
- VDR
vitamin D receptor
References
- 1.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22(17):2308–41. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17(1):45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 5.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5(1):367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 6.Skoglund P, Keller R. Integration of planar cell polarity and ECM signaling in elongation of the vertebrate body plan. Curr Opin Cell Biol. 2010;22(5):589–96. doi: 10.1016/j.ceb.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25(3):201–13. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10(7):468–77. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 9.Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21(11):1292–315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- 10.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19(4):379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 11.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19(2):150–8. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Roarty K, Rosen JM. Wnt and mammary stem cells: hormones cannot fly wingless. Curr Opin Pharmacol. 2010;10(6):643–9. doi: 10.1016/j.coph.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams PD, Enders GH. Wnt-signaling and senescence: A tug of war in early neoplasia. Cancer Biol Ther. 2008;7(11):1706–11. doi: 10.4161/cbt.7.11.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317(5839):803–6. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 16.Zhang DY, Wang HJ, Tan YZ. Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PLoS One. 2011;6(6):e21397. doi: 10.1371/journal.pone.0021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binet R, Ythier D, Robles AI, Collado M, Larrieu D, Fonti C, Brambilla E, Brambilla C, Serrano M, Harris CC, Pedeux R. WNT16B is a new marker of cellular senescence that regulates p53 activity and the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 2009;69(24):9183–91. doi: 10.1158/0008-5472.CAN-09-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes TL, Thomas MB, Dusing MR, Valerius MT, Potter SS, Wiginton DA. An enhancer LEF-1/TCF-1 site is essential for insertion site-independent transgene expression in thymus. Nucleic Acids Res. 1996;24(24):5034– 44. doi: 10.1093/nar/24.24.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giese K, Kingsley C, Kirshner JR, Grosschedl R. Assembly and function of a TCR alpha enhancer complex is dependent on LEF-1-induced DNA bending and multiple protein-protein interactions. Genes Dev. 1995;9(8):995– 1008. doi: 10.1101/gad.9.8.995. [DOI] [PubMed] [Google Scholar]
- 20.Love JJ, Li X, Case DA, Giese K, Grosschedl R, Wright PE. Structural basis for DNA bending by the architectural transcription factor LEF-1. Nature. 1995;376(6543):791–5. doi: 10.1038/376791a0. [DOI] [PubMed] [Google Scholar]
- 21.van Houte L, van Oers A, van de Wetering M, Dooijes D, Kaptein R, Clevers H. The sequence-specific high mobility group 1 box of TCF-1 adopts a predominantly alpha-helical conformation in solution. J Biol Chem. 1993;268(24):18083–7. [PubMed] [Google Scholar]
- 22.Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 1997;11(18):2359–70. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88(6):789–99. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 24.Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385(6619):829–33. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 25.Daniels DL, Weis WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12(4):364–71. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- 26.Billin AN, Thirlwell H, Ayer DE. Beta-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol Cell Biol. 2000;20(18):6882–90. doi: 10.1128/mcb.20.18.6882-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destrée O, Clevers H. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395(6702):608–12. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- 28.Brantjes H, Roose J, van De Wetering M, Clevers H. All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res. 2001;29(7):1410–9. doi: 10.1093/nar/29.7.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arce L, Pate KT, Waterman ML. Groucho binds two conserved regions of LEF-1 for HDAC-dependent repression. BMC Cancer. 2009;9(1):159. doi: 10.1186/1471-2407-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Archbold HC, Chen L, Cadigan KM. How do they do Wnt they do?: regulation of transcription by the Wnt/β-catenin pathway. Acta Physiol (Oxf) 2011 doi: 10.1111/j.1748-1716.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- 31.Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25(57):7492–504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- 32.Prieve MG, Waterman ML. Nuclear localization and formation of beta-catenin-lymphoid enhancer factor complexes are not sufficient for activation of gene expression. Mol Cell Biol. 1999;19(6):4503–15. doi: 10.1128/mcb.19.6.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang W, Dodge M, Gundapaneni D, Michnoff C, Roth M, Lum L. A genome-wide RNAi screen for Wnt/beta-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proc Natl Acad Sci U S A. 2008;105(28):9697–702. doi: 10.1073/pnas.0804709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell. 2010;19(4):521–32. doi: 10.1016/j.devcel.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Struewing I, Boyechko T, Barnett C, Beildeck M, Byers SW, Mao CD. The balance of TCF7L2 variants with differential activities in Wnt-signaling is regulated by lithium in a GSK3beta-independent manner. Biochem Biophys Res Commun. 2010;399 (2):245–50. doi: 10.1016/j.bbrc.2010.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol. 2011;13(7):762–70. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F, van den Broek O, Destrée O, Hoppler S. Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/beta-catenin signalling in mesoderm development. Development. 2005;132(24):5375–85. doi: 10.1242/dev.02152. [DOI] [PubMed] [Google Scholar]
- 38.Gradl D, König A, Wedlich D. Functional diversity of Xenopus lymphoid enhancer factor/T-cell factor transcription factors relies on combinations of activating and repressing elements. J Biol Chem. 2002;277(16):14159–71. doi: 10.1074/jbc.M107055200. [DOI] [PubMed] [Google Scholar]
- 39.Levy L, Neuveut C, Renard CA, Charneau P, Branchereau S, Gauthier F, Van Nhieu JT, Cherqui D, Petit-Bertron AF, Mathieu D, Buendia MA. Transcriptional activation of interleukin-8 by beta-catenin-Tcf4. J Biol Chem. 2002;277(44):42386–93. doi: 10.1074/jbc.M207418200. [DOI] [PubMed] [Google Scholar]
- 40.Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437(7056):281–5. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- 41.Shulewitz M, Soloviev I, Wu T, Koeppen H, Polakis P, Sakanaka C. Repressor roles for TCF-4 and Sfrp1 in Wnt signaling in breast cancer. Oncogene. 2006;25(31):4361–9. doi: 10.1038/sj.onc.1209470. [DOI] [PubMed] [Google Scholar]
- 42.Hatzis P, van der Flier LG, van Driel MA, Guryev V, Nielsen F, Denissov S, Nijman IJ, Koster J, Santo EE, Welboren W, Versteeg R, Cuppen E, van de Wetering M, Clevers H, Stunnenberg HG. Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol Cell Biol. 2008;28(8):2732–44. doi: 10.1128/MCB.02175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weise A, Bruser K, Elfert S, Wallmen B, Wittel Y, Wöhrle S, Hecht A. Alternative splicing of Tcf7l2 transcripts generates protein variants with differential promoter-binding and transcriptional activation properties at Wnt/{beta}-catenin targets. Nucleic Acids Res. 2009;38(6):1964–81. doi: 10.1093/nar/gkp1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuilliere-Dartigues P, El-Bchiri J, Krimi A, Buhard O, Fontanges P, Fléjou JF, Hamelin R, Duval A. TCF-4 isoforms absent in TCF-4 mutated MSI-H colorectal cancer cells colocalize with nuclear CtBP and repress TCF-4-mediated transcription. Oncogene. 2006;25(32):4441–8. doi: 10.1038/sj.onc.1209471. [DOI] [PubMed] [Google Scholar]
- 45.Le Bacquer O, Shu L, Marchand M, Neve B, Paroni F, Kerr Conte J, Pattou F, Froguel P, Maedler K. TCF7L2 splice variants have distinct effects on beta-cell turnover and function. Hum Mol Genet. 2011;20(10):1906–15. doi: 10.1093/hmg/ddr072. [DOI] [PubMed] [Google Scholar]
- 46.Pukrop T, Gradl D, Henningfeld KA, Knochel W, Wedlich D, Kuhl M. Identification of two regulatory elements within the high mobility group box transcription factor XTCF-4. J Biol Chem. 2001;276(12):8968–78. doi: 10.1074/jbc.M007533200. [DOI] [PubMed] [Google Scholar]
- 47.Najdi R, Syed A, Arce L, Theisen H, Ting JH, Atcha F, Nguyen AV, Martinez M, Holcombe RF, Edwards RA, Marsh JL, Waterman ML. A Wnt kinase network alters nuclear localization of TCF-1 in colon cancer. Oncogene. 2009;28(47):4133–46. doi: 10.1038/onc.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennell JA, O’Leary EE, Gummow BM, Hammer GD, MacDougald OA. T-cell factor 4N (TCF-4N), a novel isoform of mouse TCF-4, synergizes with beta-catenin to coactivate C/EBPalpha and steroidogenic factor 1 transcription factors. Mol Cell Biol. 2003;23(15):5366–75. doi: 10.1128/MCB.23.15.5366-5375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsedensodnom O, Koga H, Rosenberg SA, Nambotin SB, Carroll JJ, Wands JR, Kim M. Identification of T-cell factor-4 isoforms that contribute to the malignant phenotype of hepatocellular carcinoma cells. Exp Cell Res. 2011;317(7):920–31. doi: 10.1016/j.yexcr.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci U S A. 2007;104(22):9428–33. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pálmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS One. 2008;3(1):e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins SJ. Retinoic acid receptors, hematopoiesis and leukemogenesis. Curr Opin Hematol. 2008;15(4):346–51. doi: 10.1097/MOH.0b013e3283007edf. [DOI] [PubMed] [Google Scholar]
- 53.Fan Y, Lee TV, Xu D, Chen Z, Lamblin AF, Steller H, Bergmann A. Dual roles of Drosophila p53 in cell death and cell differentiation. Cell Death Differ. 2010;17(6):912–21. doi: 10.1038/cdd.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Junttila MR, Evan GI. p53—a Jack of all trades but master of none. Nat Rev Cancer. 2009;9(11):821–9. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- 55.Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol. 2007;17(6):286–91. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10(4):431–42. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 57.Collavin L, Lunardi A, Del Sal G. p53-family proteins and their regulators: hubs and spokes in tumor suppression. Cell Death Differ. 2010;17(1):901–11. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- 58.Prowald A, Cronauer MV, von Klot C, Eilers T, Rinnab L, Herrmann T, Spindler KD, Montenarh M, Jonas U, Burchardt M. Modulation of beta-catenin-mediated TCF-signalling in prostate cancer cell lines by wild-type and mutant p53. Prostate. 2007;67(16):1751–60. doi: 10.1002/pros.20660. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Shou J, Chen X. Dickkopf-1, an inhibitor of the Wnt signaling pathway, is induced by p53. Oncogene. 2000;19(14):1843–8. doi: 10.1038/sj.onc.1203503. [DOI] [PubMed] [Google Scholar]
- 60.Sadot E, Geiger B, Oren M, Ben-Ze’ev A. Down-regulation of beta-catenin by activated p53. Mol Cell Biol. 2001;21(20):6768–81. doi: 10.1128/MCB.21.20.6768-6781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cagatay T, Ozturk M. P53 mutation as a source of aberrant beta-catenin accumulation in cancer cells. Oncogene. 2002;21(52):7971–80. doi: 10.1038/sj.onc.1205919. [DOI] [PubMed] [Google Scholar]
- 62.Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26(7):898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- 63.Beildeck ME, Gelmann EP, Byers SW. Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway. Exp Cell Res. 2010;316(11):1763–72. doi: 10.1016/j.yexcr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robertson SM, Lo MC, Odom R, Yang XD, Medina J, Huang S, Lin R. Functional analyses of vertebrate TCF proteins in C. elegans embryos. Dev Biol. 2011;355(1):115–23. doi: 10.1016/j.ydbio.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noble JA, White AM, Lazzeroni LC, Valdes AM, Mirel DB, Reynolds R, Grupe A, Aud D, Peltz G, Erlich HA. A polymorphism in the TCF7 gene, C883A, is associated with type 1 diabetes. Diabetes. 2003;52(6):1579–82. doi: 10.2337/diabetes.52.6.1579. [DOI] [PubMed] [Google Scholar]
- 66.Cooper JD, Smyth DJ, Bailey R, Payne F, Downes K, Godfrey LM, Masters J, Zeitels LR, Vella A, Walker NM, Todd JA. The candidate genes TAF5L, TCF7, PDCD1, IL6 and ICAM1 cannot be excluded from having effects in type 1 diabetes. BMC Med Genet. 2007;8(1):71. doi: 10.1186/1471-2350-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erlich HA, Valdes AM, Julier C, Mirel D, Noble JA Consortium TIDG. Evidence for association of the TCF7 locus with type I diabetes. Genes Immun. 2009;10(Suppl 1):S54–9. doi: 10.1038/gene.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Julier C, Akolkar B, Concannon P, Morahan G, Nierras C, Pugliese A Type I Diabetes Genetics Consortium. The Type I Diabetes Genetics Consortium ‘Rapid Response’ family-based candidate gene study: strategy, genes selection, and main outcome. Genes Immun. 2009;10(Suppl 1):S121–7. doi: 10.1038/gene.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91(1):79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 70.Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells downregulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176(3):1439–46. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- 71.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38(3):320–3. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 72.Zhang C, Qi L, Hunter DJ, Meigs JB, Manson JE, van Dam RM, Hu FB. Variant of transcription factor 7-like 2 (TCF7L2) gene and the risk of type 2 diabetes in large cohorts of U.S. women and men. Diabetes. 2006;55(9):2645–8. doi: 10.2337/db06-0643. [DOI] [PubMed] [Google Scholar]
- 73.Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PI, Shuldiner AR, Knowler WC, Nathan DM, Altshuler D Diabetes Prevention Program Research Group. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355(3):241–50. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saxena R, Gianniny L, Burtt NP, Lyssenko V, Giuducci C, Sjögren M, Florez JC, Almgren P, Isomaa B, Orho-Melander M, Lindblad U, Daly MJ, Tuomi T, Hirschhorn JN, Ardlie KG, Groop LC, Altshuler D. Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes. 2006;55(10):2890–5. doi: 10.2337/db06-0381. [DOI] [PubMed] [Google Scholar]
- 75.Muendlein A, Saely CH, Geller-Rhomberg S, Sonderegger G, Rein P, Winder T, Beer S, Vonbank A, Drexel H. Single nucleotide polymorphisms of TCF7L2 are linked to diabetic coronary atherosclerosis. PLoS One. 2011;6(3):e17978. doi: 10.1371/journal.pone.0017978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sousa AG, Marquezine GF, Lemos PA, Martinez E, Lopes N, Hueb WA, Krieger JE, Pereira AC. TCF7L2 polymorphism rs7903146 is associated with coronary artery disease severity and mortality. PLoS One. 2009;4(11):e7697. doi: 10.1371/journal.pone.0007697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goode EL, Szabo C, Prokunina-Olsson L, Vierkant RA, Fredericksen ZS, Collins FS, White KL, Schmidt M, Fridley BL, Couch FJ. No association between a candidate TCF7L2 variant and risk of breast or ovarian cancer. BMC Cancer. 2009;9(1):312. doi: 10.1186/1471-2407-9-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naidu R, Yip CH, Taib NA. Genetic variations in transcription factor 7-like 2 (TCF7L2) gene: association of TCF7L2 rs12255372(G/T) or rs7903146(C/T) with breast cancer risk and clinico-pathological parameters. Med Oncol. 2011 doi: 10.1007/s12032-011-9837-8. [DOI] [PubMed] [Google Scholar]
- 79.Chen F, Wilkens LR, Monroe KR, Stram DO, Kolonel LN, Henderson BE, Le Marchand L, Haiman CA. No association of risk variants for diabetes and obesity with breast cancer: the Multiethnic Cohort and PAGE studies. Cancer Epidemiol Biomarkers Prev. 2011;20(5):1039–42. doi: 10.1158/1055-9965.EPI-11-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hazra A, Fuchs CS, Chan AT, Giovannucci EL, Hunter DJ. Association of the TCF7L2 polymorphism with colorectal cancer and adenoma risk. Cancer Causes Control. 2008;19(9):975–80. doi: 10.1007/s10552-008-9164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slattery ML, Folsom AR, Wolff R, Herrick J, Caan BJ, Potter JD. Transcription factor 7-like 2 polymorphism and colon cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(4):978–82. doi: 10.1158/1055-9965.EPI-07-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alokail MS, Al-Daghri NM, Al-Attas OS, Hussain T. Combined effects of obesity and type 2 diabetes contribute to increased breast cancer risk in premenopausal women. Cardiovasc Diabetol. 2009;8(1):33. doi: 10.1186/1475-2840-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kabat GC, Kim M, Chlebowski RT, Khandekar J, Ko MG, McTiernan A, Neuhouser ML, Parker DR, Shikany JM, Stefanick ML, Thomson CA, Rohan TE. A longitudinal study of the metabolic syndrome and risk of post-menopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2046–53. doi: 10.1158/1055-9965.EPI-09-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes. 2008;116(Suppl 1):S4–S6. doi: 10.1055/s-2008-1081488. [DOI] [PubMed] [Google Scholar]
- 85.Stitzel ML, Sethupathy P, Pearson DS, Chines PS, Song L, Erdos MR, Welch R, Parker SC, Boyle AP, Scott LJ, Margulies EH, Boehnke M, Furey TS, Crawford GE, Collins FS NISC Comparative Sequencing Program. Global epigenomic analysis of primary human pancreatic islets provides insights into type 2 diabetes susceptibility loci. Cell Metab. 2010;12(5):443–55. doi: 10.1016/j.cmet.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaulton KJ, Nammo T, Pasquali L, Simon JM, Giresi PG, Fogarty MP, Panhuis TM, Mieczkowski P, Secchi A, Bosco D, Berney T, Montanya E, Mohlke KL, Lieb JD, Ferrer J. A map of open chromatin in human pancreatic islets. Nat Genet. 2010;42(3):255–9. doi: 10.1038/ng.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Savic D, Ye H, Aneas I, Park SY, Bell GI, Nobrega MA. Alterations in TCF7L2 expression define its role as a key regulator of glucose metabolism. Genome Res. 2011;21(9):1417–25. doi: 10.1101/gr.123745.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barker N, Huls G, Korinek V, Clevers H. Restricted high level expression of Tcf-4 protein in intestinal and mammary gland epithelium. Am J Pathol. 1999;154(1):29–35. doi: 10.1016/S0002-9440(10)65247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nazwar TA, Glassmann A, Schilling K. Expression and molecular diversity of Tcf7l2 in the developing murine cerebellum and brain. J Neurosci Res. 2009;87(7):1532–46. doi: 10.1002/jnr.21989. [DOI] [PubMed] [Google Scholar]
- 90.König A, Gradl D, Kühl M, Wedlich D. The HMG-box transcription factor XTcf-4 demarcates the forebrain-midbrain boundary. Mech Dev. 2000;93(1–2):211–4. doi: 10.1016/s0925-4773(00)00266-5. [DOI] [PubMed] [Google Scholar]
- 91.Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P, Sjögren M, Ling C, Eriksson KF, Lethagen AL, Mancarella R, Berglund G, Tuomi T, Nilsson P, Del Prato S, Groop L. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest. 2007;117(8):2155–63. doi: 10.1172/JCI30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prokunina-Olsson L, Welch C, Hansson O, Adhikari N, Scott LJ, Usher N, Tong M, Sprau A, Swift A, Bonnycastle LL, Erdos MR, He Z, Saxena R, Harmon B, Kotova O, Hoffman EP, Altshuler D, Groop L, Boehnke M, Collins FS, Hall JL. Tissue-specific alternative splicing of TCF7L2. Hum Mol Genet. 2009;18(20):3795–804. doi: 10.1093/hmg/ddp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Osmark P, Hansson O, Jonsson A, Rönn T, Groop L, Renström E. Unique splicing pattern of the TCF7L2 gene in human pancreatic islets. Diabetologia. 2009;52(5):850–4. doi: 10.1007/s00125-009-1293-z. [DOI] [PubMed] [Google Scholar]
- 94.Mondal AK, Das SK, Baldini G, Chu WS, Sharma NK, Hackney OG, Zhao J, Grant SF, Elbein SC. Genotype and tissue-specific effects on alternative splicing of the transcription factor 7-like 2 gene in humans. J Clin Endocrinol Metab. 2010;95(3):1450–7. doi: 10.1210/jc.2009-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CH, Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet. 2009;18(13):2388–99. doi: 10.1093/hmg/ddp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prokunina-Olsson L, Hall JL. Evidence for neuroendocrine function of a unique splicing form of TCF7L2 in human brain, islets and gut. Diabetologia. 2010;53(4):712– 6. doi: 10.1007/s00125-009-1640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hovanes K, Li TW, Waterman ML. The human LEF-1 gene contains a promoter preferentially active in lymphocytes and encodes multiple isoforms derived from alternative splicing. Nucleic Acids Res. 2000;28(9):1994–2003. doi: 10.1093/nar/28.9.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe RF, Waterman ML. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28(1):53–7. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 99.Li TW, Ting JH, Yokoyama NN, Bernstein A, van de Wetering M, Waterman ML. Wnt activation and alternative promoter repression of LEF1 in colon cancer. Mol Cell Biol. 2006;26(14):5284–99. doi: 10.1128/MCB.00105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van de Wetering M, Castrop J, Korinek V, Clevers H. Extensive alternative splicing and dual promoter usage generate Tcf-1 protein isoforms with differential transcription control properties. Mol Cell Biol. 1996;16(3):745–52. doi: 10.1128/mcb.16.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van de Wetering M, Oosterwegel M, Holstege F, Dooyes D, Suijkerbuijk R, Geurts van Kessel A, Clevers H. The human T cell transcription factor-1 gene. Structure, localization, and promoter characterization. J Biol Chem. 1992;267(12):8530–6. [PubMed] [Google Scholar]
- 102.Hoeppner LH, Secreto F, Jensen ED, Li X, Kahler RA, Westendorf JJ. Runx2 and bone morphogenic protein 2 regulate the expression of an alternative Lef1 transcript during osteoblast maturation. J Cell Physiol. 2009;221(2):480–9. doi: 10.1002/jcp.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoeppner LH, Secreto FJ, Razidlo DF, Whitney TJ, Westendorf JJ. Lef1DeltaN binds beta-catenin and increases osteoblast activity and trabecular bone mass. J Biol Chem. 2011;286(13):10950–9. doi: 10.1074/jbc.M110.165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duval A, Rolland S, Tubacher E, Bui H, Thomas G, Hamelin R. The human T-cell transcription factor-4 gene: structure, extensive characterization of alternative splicings, and mutational analysis in colorectal cancer cell lines. Cancer Res. 2000;60(14):3872–9. [PubMed] [Google Scholar]
- 105.Kahler RA, Galindo M, Lian J, Stein GS, van Wijnen AJ, Westendorf JJ. Lymphocyte enhancer-binding factor 1 (Lef1) inhibits terminal differentiation of osteoblasts. J Cell Biochem. 2006;97(5):969–83. doi: 10.1002/jcb.20702. [DOI] [PubMed] [Google Scholar]
- 106.Yokoyama NN, Pate KT, Sprowl S, Waterman ML. A role for YY1 in repression of dominant negative LEF-1 expression in colon cancer. Nucleic Acids Res. 2010;38(19):6375–88. doi: 10.1093/nar/gkq492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koenig SF, Lattanzio R, Mansperger K, Rupp RA, Wedlich D, Gradl D. Autoregulation of XTcf-4 depends on a Lef/Tcf site on the XTcf-4 promoter. Genesis. 2008;46(2):81–6. doi: 10.1002/dvg.20363. [DOI] [PubMed] [Google Scholar]
- 108.Kunz M, Herrmann M, Wedlich D, Gradl D. Autoregulation of canonical Wnt signaling controls midbrain development. Dev Biol. 2004;273(2):390–401. doi: 10.1016/j.ydbio.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 109.Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, Goldschmeding R, Logtenberg T, Clevers H. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285(5435):1923–6. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- 110.Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu W. Crystal structure of a beta-catenin/BCL9/Tcf4 complex. Mol Cell. 2006;24(2):293–300. doi: 10.1016/j.molcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 111.Graham TA, Ferkey DM, Mao F, Kimelman D, Xu W. Tcf4 can specifically recognize beta-catenin using alternative conformations. Nat Struct Biol. 2001;8(12):1048–12. doi: 10.1038/nsb718. [DOI] [PubMed] [Google Scholar]
- 112.Graham TA, Weaver C, Mao F, Kimelman D, Xu W. Crystal structure of a beta-catenin/Tcf complex. Cell. 2000;103(6):885–96. doi: 10.1016/s0092-8674(00)00192-6. [DOI] [PubMed] [Google Scholar]
- 113.Hoppler S, Kavanagh CL. Wnt signalling: variety at the core. J Cell Sci. 2007;120(Pt3):385–93. doi: 10.1242/jcs.03363. [DOI] [PubMed] [Google Scholar]
- 114.Sierra J, Yoshida T, Joazeiro CA, Jones KA. The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 2006;20(5):586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Niemann C, Owens DM, Schettina P, Watt FM. Dual role of inactivating Lef1 mutations in epidermis: tumor promotion and specification of tumor type. Cancer Res. 2007;67(7):2916–21. doi: 10.1158/0008-5472.CAN-06-3427. [DOI] [PubMed] [Google Scholar]
- 116.Takeda H, Lyle S, Lazar AJ, Zouboulis CC, Smyth I, Watt FM. Human sebaceous tumors harbor inactivating mutations in LEF1. Nat Med. 2006;12(4):395–97. doi: 10.1038/nm1386. [DOI] [PubMed] [Google Scholar]
- 117.Young RM, Reyes AE, Allende ML. Expression and splice variant analysis of the zebrafish tcf4 transcription factor. Mech Dev. 2002;117(1–2):269–73. doi: 10.1016/s0925-4773(02)00180-6. [DOI] [PubMed] [Google Scholar]
- 118.Salomonis N, Schlieve CR, Pereira L, Wahlquist C, Colas A, Zambon AC, Vranizan K, Spindler MJ, Pico AR, Cline MS, Clark TA, Williams A, Blume JE, Samal E, Mercola M, Merrill BJ, Conklin BR. Alternative splicing regulates mouse embryonic stem cell pluripotency and differentiation. Proc Natl Acad Sci U S A. 2010;107(23):10514–9. doi: 10.1073/pnas.0912260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun J, Weis WI. Biochemical and structural characterization of β-catenin interactions with nonphosphorylated and CK2-phosphorylated Lef-1. J Mol Biol. 2011;405(2):519–30. doi: 10.1016/j.jmb.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hossain MB, Hosokawa H, Hasegawa A, Watarai H, Taniguchi M, Yamashita M, Nakayama T. Lymphoid enhancer factor interacts with GATA-3 and controls its function in T helper type 2 cells. Immunology. 2008;125(3):377–86. doi: 10.1111/j.1365-2567.2008.02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ruzov A, Hackett JA, Prokhortchouk A, Reddington JP, Madej MJ, Dunican DS, Prokhortchouk E, Pennings S, Meehan RR. The interaction of xKaiso with xTcf3: a revised model for integration of epigenetic and Wnt signalling pathways. Development. 2009;136(5):723–7. doi: 10.1242/dev.025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kahler RA, Westendorf JJ. Lymphoid enhancer factor-1 and beta-catenin inhibit Runx2-dependent transcriptional activation of the osteocalcin promoter. J Biol Chem. 2003;278(14):11937–44. doi: 10.1074/jbc.M211443200. [DOI] [PubMed] [Google Scholar]
- 124.Luderer HF, Gori F, Demay MB. Lymphoid enhancer-binding factor-1 (LEF1) interacts with the DNA-binding domain of the vitamin D receptor. J Biol Chem. 2011;286(21):18444–51. doi: 10.1074/jbc.M110.188219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yasumoto K, Takeda K, Saito H, Watanabe K, Takahashi K, Shibahara S. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. 2002;21(11):2703–14. doi: 10.1093/emboj/21.11.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Drewelus I, Göpfert C, Hippel C, Dickmanns A, Damianitsch K, Pieler T, Dobbelstein M. p63 antagonizes Wnt-induced transcription. Cell Cycle. 2010;9(3):580–87. doi: 10.4161/cc.9.3.10593. [DOI] [PubMed] [Google Scholar]
- 127.Snider L, Thirlwell H, Miller JR, Moon RT, Groudine M, Tapscott SJ. Inhibition of Tcf3 binding by I-mfa domain proteins. Mol Cell Biol. 2001;21(5):1866–73. doi: 10.1128/MCB.21.5.1866-1873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hecht A, Stemmler MP. Identification of a promoter-specific transcriptional activation domain at the C terminus of the Wnt effector protein T-cell factor 4. J Biol Chem. 2003;278(6):3776–85. doi: 10.1074/jbc.M210081200. [DOI] [PubMed] [Google Scholar]
- 129.Labbé E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci U S A. 2000;97(15):8358–63. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Valenta T, Lukas J, Doubravska L, Fafilek B, Korinek V. HIC1 attenuates Wnt signaling by recruitment of TCF-4 and beta-catenin to the nuclear bodies. EMBO J. 2006;25(11):2326–37. doi: 10.1038/sj.emboj.7601147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boras K, Hamel PA. Alx4 binding to LEF-1 regulates N-CAM promoter activity. J Biol Chem. 2002;277(2):1120–7. doi: 10.1074/jbc.M109912200. [DOI] [PubMed] [Google Scholar]
- 132.Ghogomu SM, van Venrooy S, Ritthaler M, Wedlich D, Gradl D. HIC-5 is a novel repressor of lymphoid enhanc er factor/T-cell factor-driven transcription. J Biol Chem. 2006;281(3):1755–64. doi: 10.1074/jbc.M505869200. [DOI] [PubMed] [Google Scholar]
- 133.Mallory MJ, Jackson J, Weber B, Chi A, Heyd F, Lynch KW. Signal- and development-dependent alternative splicing of LEF1 in T cells is controlled by CELF2. Mol Cell Biol. 2011;11(2):2184–95. doi: 10.1128/MCB.05170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carlsson P, Waterman ML, Jones KA. The hLEF/TCF-1 alpha HMG protein contains a context-dependent transcriptional activation domain that induces the TCR alpha enhancer in T cells. Genes Dev. 1993;7(12A):2418–30. doi: 10.1101/gad.7.12a.2418. [DOI] [PubMed] [Google Scholar]
- 135.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6(3):197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 136.Wang S, Jones KA. CK2 controls the recruitment of Wnt regulators to target genes in vivo. Curr Biol. 2006;16(22):2239–44. doi: 10.1016/j.cub.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 137.Hämmerlein A, Weiske J, Huber O. A second protein kinase CK1-mediated step negatively regulates Wnt signalling by disrupting the lymphocyte enhancer factor-1/beta-catenin complex. Cell Mol Life Sci. 2005;62(5):606–18. doi: 10.1007/s00018-005-4507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23(1):131–9. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature. 1999;399(6738):798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- 140.Hikasa H, Sokol SY. Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. J Biol Chem. 2011;286(14):12093–100. doi: 10.1074/jbc.M110.185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mahmoudi T, Li VS, Ng SS, Taouatas N, Vries RG, Mohammed S, Heck AJ, Clevers H. The kinase TNIK is an essential activator of Wnt target genes. EMBO J. 2009;28(21):3329–40. doi: 10.1038/emboj.2009.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shitashige M, Satow R, Jigami T, Aoki K, Honda K, Shibata T, Ono M, Hirohashi S, Yamada T. Traf2- and Nck-interacting kinase is essential for Wnt signaling and colorectal cancer growth. Cancer Res. 2010;70(12):5024– 33. doi: 10.1158/0008-5472.CAN-10-0306. [DOI] [PubMed] [Google Scholar]
- 143.Nalesso G, Sherwood J, Bertrand J, Pap T, Ramachandran M, De Bari C, Pitzalis C, Dell’accio F. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J Cell Biol. 2011;193(3):551–64. doi: 10.1083/jcb.201011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Satow R, Shitashige M, Jigami T, Honda K, Ono M, Hirohashi S, Yamada T. Traf2- and Nck-interacting kinase is essential for canonical Wnt signaling in Xenopus axis formation. J Biol Chem. 2010;285(34):26289–94. doi: 10.1074/jbc.M109.090597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23(4):1379–89. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee W, Swarup S, Chen J, Ishitani T, Verheyen EM. Homeodomain-interacting protein kinases (Hipks) promote Wnt/Wg signaling through stabilization of beta-catenin/Arm and stimulation of target gene expression. Development. 2009;136(2):241–51. doi: 10.1242/dev.025460. [DOI] [PubMed] [Google Scholar]
- 147.Fu CA, Shen M, Huang BC, Lasaga J, Payan DG, Luo Y. TNIK, a novel member of the germinal center kinase family that activates the c-Jun N-terminal kinase pathway and regulates the cytoskeleton. J Biol Chem. 1999;274(43):30729–37. doi: 10.1074/jbc.274.43.30729. [DOI] [PubMed] [Google Scholar]
- 148.Rivat C, Le Floch N, Sabbah M, Teyrol I, Redeuilh G, Bruyneel E, Mareel M, Matrisian LM, Crawford HC, Gespach C, Attoub S. Synergistic cooperation between the AP-1 and LEF-1 transcription factors in activation of the matrilysin promoter by the src oncogene: implications in cellular invasion. FASEB J. 2003;17(12):1721–3. doi: 10.1096/fj.03-0132fje. [DOI] [PubMed] [Google Scholar]
- 149.Choi CY, Kim YH, Kim YO, Park SJ, Kim EA, Riemenschneider W, Gajewski K, Schulz RA, Kim Y. Phosphorylation by the DHIPK2 protein kinase modulates the corepressor activity of Groucho. J Biol Chem. 2005;280(22):21427–36. doi: 10.1074/jbc.M500496200. [DOI] [PubMed] [Google Scholar]
- 150.Hasson P, Egoz N, Winkler C, Volohonsky G, Jia S, Dinur T, Volk T, Courey AJ, Paroush Z. EGFR signaling attenuates Groucho-dependent repression to antagonize Notch transcriptional output. Nat Genet. 2005;37(1):101–5. doi: 10.1038/ng1486. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Q, Yoshimatsu Y, Hildebrand J, Frisch SM, Goodman RH. Homeodomain interacting protein kinase 2 promotes apoptosis by downregulating the transcriptional corepressor CtBP. Cell. 2003;115(2):177–86. doi: 10.1016/s0092-8674(03)00802-x. [DOI] [PubMed] [Google Scholar]
- 152.Kim EA, Kim JE, Sung KS, Choi DW, Lee BJ, Choi CY. Homeodomain-interacting protein kinase 2 (HIPK2) targets beta-catenin for phosphorylation and proteasomal degradation. Biochem Biophys Res Commun. 2010;394(4):966–71. doi: 10.1016/j.bbrc.2010.03.099. [DOI] [PubMed] [Google Scholar]
- 153.Yamada M, Ohnishi J, Ohkawara B, Iemura S, Satoh K, Hyodo–Miura J, Kawachi K, Natsume T, Shibuya H. NARF, an nemo-like kinase (NLK)-associated ring finger protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF) J Biol Chem. 2006;281(30):20749–60. doi: 10.1074/jbc.M602089200. [DOI] [PubMed] [Google Scholar]
- 154.Cinnamon E, Paroush Z. Context-dependent regulation of Groucho/TLE-mediated repression. Curr Opin Genet Dev. 2008;18(5):435–40. doi: 10.1016/j.gde.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 155.Atcha FA, Syed A, Wu B, Hoverter NP, Yokoyama NN, Ting JH, Munguia JE, Mangalam HJ, Marsh JL, Waterman ML. A unique DNA binding domain converts T-cell factors into strong Wnt effectors. Mol Cell Biol. 2007;27(23):8352–63. doi: 10.1128/MCB.02132-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Atcha FA, Munguia JE, Li TW, Hovanes K, Waterman ML. A new beta-catenin-dependent activation domain in T cell factor. J Biol Chem. 2003;278(18):16169–75. doi: 10.1074/jbc.M213218200. [DOI] [PubMed] [Google Scholar]
- 157.Chang MV, Chang JL, Gangopadhyay A, Shearer A, Cadigan KM. Activation of wingless targets requires bipartite recognition of DNA by TCF. Curr Biol. 2008;18(23):1877–81. doi: 10.1016/j.cub.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Valenta T, Lukas J, Korinek V. HMG box transcription factor TCF-4’s interaction with CtBP1 controls the expression of the Wnt target Axin2/Conductin in human embryonic kidney cells. Nucleic Acids Res. 2003;31(9):2369–80. doi: 10.1093/nar/gkg346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22(6):746–55. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Prokunina-Olsson L, Kaplan LM, Schadt EE, Collins FS. Alternative splicing of TCF7L2 gene in omental and subcutaneous adipose tissue and risk of type 2 diabetes. PLoS One. 2009;4(9):e7231. doi: 10.1371/journal.pone.0007231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Struewing IT, Toborek A, Mao CD. Mitochondrial and nuclear forms of Wnt13 are generated via alternative promoters, alternative RNA splicing, and alternative translation start sites. J Biol Chem. 2006;281(11):7282. doi: 10.1074/jbc.M511182200. [DOI] [PubMed] [Google Scholar]
- 162.Soubeyran P, André F, Lissitzky JC, Mallo GV, Moucadel V, Roccabianca M, Rechreche H, Marvaldi J, Dikic I, Dagorn JC, Iovanna JL. Cdx1 promotes differentiation in a rat intestinal epithelial cell line. Gastroenterology. 1999;117(6):1326–38. doi: 10.1016/s0016-5085(99)70283-0. [DOI] [PubMed] [Google Scholar]
- 163.Hedgepeth C, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol. 1997;185(1):82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 164.Mao CD, Hoang P, DiCorleto PE. Lithium inhibits cell cycle progression and induces stabilization of p53 in bovine aortic endothelial cells. J Biol Chem. 2001;276(28):26180–8. doi: 10.1074/jbc.M101188200. [DOI] [PubMed] [Google Scholar]
- 165.Warzecha CC, Jiang P, Amirikian K, Dittmar KA, Lu H, Shen S, Guo W, Xing Y, Carstens RP. An ESRP-regulated splicing programme is abrogated during the epithelial-mesenchymal transition. EMBO J. 2010;29(19):3286– 300. doi: 10.1038/emboj.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Puca R, Nardinocchi L, Givol D, D’Orazi G. Regulation of p53 activity by HIPK2: molecular mechanisms and therapeutical implications in human cancer cells. Oncogene. 2010;29(31):4378–87. doi: 10.1038/onc.2010.183. [DOI] [PubMed] [Google Scholar]
- 167.Angus-Hill ML, Elbert KM, Hidalgo J, Capecchi MR. T-cell factor 4 functions as a tumor suppressor whose disruption modulates colon cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. 2011;108(12):4914–9. doi: 10.1073/pnas.1102300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Licatalosi DD, Darnell RB. RNA processing and its regulation: global insights into biological networks. Nat Rev Genet. 2010;11(1):75–87. doi: 10.1038/nrg2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Blencowe BJ. Alternative splicing: New insights from global analysis. Cell. 2006;126(1):37–47. doi: 10.1016/j.cell.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 170.Gabut M, Chaudhry S, Blencowe BJ. SnapShot: The splicing regulatory machinery. Cell. 2008;133(1):192.e1. doi: 10.1016/j.cell.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 171.Furger A, O’Sullivan JM, Binnie A, Lee BA, Proudfoot NJ. Promoter proximal splice sites enhance transcription. Genes Dev. 2002;16(21):2792–9. doi: 10.1101/gad.983602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kornblihtt AR. Coupling transcription and alternative splicing. Adv Exp Med Biol. 2007;623:175–89. doi: 10.1007/978-0-387-77374-2_11. [DOI] [PubMed] [Google Scholar]
- 173.Kornblihtt AR. Promoter usage and alternative splicing. Curr Opin Cell Biol. 2005;17(3):262–8. doi: 10.1016/j.ceb.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 174.Hartmann B, Castelo R, Blanchette M, Boue S, Rio DC, Valcárcel J. Global analysis of alternative splicing regulation by insulin and wingless signaling in Drosophila cells. Genome Biol. 2009;10(1):R11. doi: 10.1186/gb-2009-10-1-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89(3):373–80. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 176.Hsieh JC, Sisk JM, Jurutka PW, Haussler CA, Slater SA, Haussler MR, Slater SA, Haussler MR, Thompson CC. Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J Biol Chem. 2003;278(40):38665–74. doi: 10.1074/jbc.M304886200. [DOI] [PubMed] [Google Scholar]
- 177.York B, O’Malley BW. Steroid receptor coactivator (SRC) family: masters of systems biology. J Biol Chem. 2010;285(50):38743–50. doi: 10.1074/jbc.R110.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Manthey D, Behl C. From structural biochemistry to expression profiling: neuroprotective activities of estrogen. Neuroscience. 2006;138(3):845–50. doi: 10.1016/j.neuroscience.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 179.Auboeuf D, Batsché E, Dutertre M, Muchardt C, O’Malley BW. Coregulators: transducing signal from transcription to alternative splicing. Trends Endocrinol Metab. 2007;18(3):122–9. doi: 10.1016/j.tem.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 180.Auboeuf D, Hönig A, Berget SM, O’Malley BW. Coordinate regulation of transcription and splicing by steroid receptor coregulators. Science. 2002;298(5592):416–9. doi: 10.1126/science.1073734. [DOI] [PubMed] [Google Scholar]
- 181.Masuhiro Y, Mezaki Y, Sakari M, Takeyama K, Yoshida T, Inoue K, Yanagisawa J, Hanazawa S, O’malley BW, Kato S. Splicing potentiation by growth factor signals via estrogen receptor phosphorylation. Proc Natl Acad Sci U S A. 2005;102(23):8126–31. doi: 10.1073/pnas.0503197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang C, Baudino TA, Dowd DR, Tokumaru H, Wang W, NMP Ternary complexes and cooperative interplay between NCoA-62/Ski-interacting protein and steroid receptor coactivators in vitamin D receptor-mediated transcription. J Biol Chem. 2001;276(44):40614–20. doi: 10.1074/jbc.M106263200. [DOI] [PubMed] [Google Scholar]
- 183.Barry JB, Leong GM, Church WB, Issa LL, Eisman JA, Gardiner EM. Interactions of SKIP/NCoA-62, TFIIB, and retinoid X receptor with vitamin D receptor helix H10 residues. J Biol Chem. 2003;278(10):8224–8. doi: 10.1074/jbc.C200712200. [DOI] [PubMed] [Google Scholar]
- 184.Cheng D, Côté J, Shaaban S, Bedford MT. The arginine methyltransferase CARM1 regulates the coupling of transcription and mRNA processing. Mol Cell. 2007;25(1):71–83. doi: 10.1016/j.molcel.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 185.Koh SS, Li H, Lee YH, Widelitz RB, Chuong CM, Stallcup MR. Synergistic coactivator function by coactivator-associated arginine methyltransferase (CARM) 1 and beta-catenin with two different classes of DNA-binding transcriptional activators. J Biol Chem. 2002;277(29):26031–5. doi: 10.1074/jbc.M110865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ou CY, LaBonte MJ, Manegold PC, So AY, Ianculescu I, Gerke DS, Yamamoto KR, Ladner RD, Kahn M, Kim JH, Stallcup MR. A coactivator role of CARM1 in the dysregulation of β-catenin activity in colorectal cancer cell growth and gene expression. Mol Cancer Res. 2011;9(5):660–70. doi: 10.1158/1541-7786.MCR-10-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kuhn P, Chumanov R, Wang Y, Ge Y, Burgess RR, Xu W. Automethylation of CARM1 allows coupling of transcription and mRNA splicing. Nucleic Acids Res. 2011;39(7):2717–26. doi: 10.1093/nar/gkq1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284(5423):2174–7. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 189.David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. 2010;24(21):2343–64. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Beildeck ME, Islam M, Shah S, Welsh J, Byers SW. Control of TCF-4 expression by VDR and vitamin D in the mouse mammary gland and colorectal cancer cell lines. PLoS One. 2009;4(11):e7872. doi: 10.1371/journal.pone.0007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I. Upregulation of TCF4 expression as a transcriptional target of beta-catenin/p300 complexes during trans-differentiation of endometrial carcinoma cells. Lab Invest. 2005;85(6):768–79. doi: 10.1038/labinvest.3700273. [DOI] [PubMed] [Google Scholar]
- 192.Sato S, Idogawa M, Honda K, Fujii G, Kawashima H, Takekuma K, Hoshika A, Hirohashi S, Yamada T. beta-catenin interacts with the FUS proto-oncogene product and regulates pre-mRNA splicing. Gastroenterology. 2005;129(4):1225–36. doi: 10.1053/j.gastro.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 193.van der Vaart M, Schaaf MJ. Naturally occurring C-terminal splice variants of nuclear receptors. Nucl Recept Signal. 2009;7(1):e007. doi: 10.1621/nrs.07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim NG, Xu C, Gumbiner BM. Identification of targets of the Wnt pathway destruction complex in addition to betacatenin. Proc Natl Acad Sci U S A. 2009;106(13):5165– 70. doi: 10.1073/pnas.0810185106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19(R1):R46–64. doi: 10.1093/hmg/ddq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gardiner M, Toth R, Vandermoere F, Morrice NA, Rouse J. Identification and characterization of FUS/TLS as a new target of ATM. Biochem J. 2008;415(2):297–307. doi: 10.1042/BJ20081135. [DOI] [PubMed] [Google Scholar]
- 197.Brooke GN, Culley RL, Dart DA, Mann DJ, Gaughan L, McCracken SR, Robson CN, Spencer-Dene B, Gamble SC, Powell SM, Wait R, Waxman J, Walker MM, Bevan CL. FUS/TLS is a novel mediator of androgen-dependent cell-cycle progression and prostate cancer growth. Cancer Res. 2011;71(3):914–24. doi: 10.1158/0008-5472.CAN-10-0874. [DOI] [PubMed] [Google Scholar]
- 198.Shitashige M, Naishiro Y, Idogawa M, Honda K, Ono M, Hirohashi S, Yamada T. Involvement of splicing factor-1 in beta-catenin/T-cell factor-4-mediated gene trans-activation and pre-mRNA splicing. Gastroenterology. 2007;132(3):1039–54. doi: 10.1053/j.gastro.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 199.Tsai BP, Wang X, Huang L, Waterman ML. Quantitative profiling of in vivo-assembled RNA-protein complexes using a novel integrated proteomic approach. Mol Cell Proteomics. 2011;10(4):M110.007385. doi: 10.1074/mcp.M110.007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Janknecht R. Multi-talented DEAD-box proteins and potential tumor promoters: p68 RNA helicase (DDX5) and its paralog, p72 RNA helicase (DDX17) Am J Transl Res. 2010;2(3):223–34. [PMC free article] [PubMed] [Google Scholar]
- 201.Yang L, Lin C, Liu ZR. P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell. 2006;127(1):139– 55. doi: 10.1016/j.cell.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 202.Yang L, Lin C, Zhao S, Wang H, Liu ZR. Phosphorylation of p68 RNA helicase plays a role in platelet-derived growth factor-induced cell proliferation by up-regulating cyclin D1 and c-Myc expression. J Biol Chem. 2007;282(23):16811–9. doi: 10.1074/jbc.M610488200. [DOI] [PubMed] [Google Scholar]
- 203.Watanabe M, Yanagisawa J, Kitagawa H, Takeyama K, Ogawa S, Arao Y, Suzawa M, Kobayashi Y, Yano T, Yoshikawa H, Masuhiro Y, Kato S. A subfamily of RNA-binding DEAD-box proteins acts as an estrogen receptor alpha coactivator through the N-terminal activation domain (AF-1) with an RNA coactivator, SRA. EMBO J. 2001;20(6):1341–52. doi: 10.1093/emboj/20.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 204.Dutertre M, Gratadou L, Dardenne E, Germann S, Samaan S, Lidereau R, Driouch K, de la Grange P, Auboeuf D. Estrogen regulation and physiopathologic significance of alternative promoters in breast cancer. Cancer Res. 2010;70(9) doi: 10.1158/0008-5472.CAN-09-3988. [DOI] [PubMed] [Google Scholar]
- 205.Yao H, Brick K, Evrard Y, Xiao T, Camerini-Otero RD, Felsenfeld G. Mediation of CTCF transcriptional insulation by DEAD-box RNA-binding protein p68 and steroid receptor RNA activator SRA. Genes Dev. 2010;24(22):2543–55. doi: 10.1101/gad.1967810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Strässer K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondón AG, Aguilera A, Struhl K, Reed R, Hurt E. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 2002;417(6886):304–8. doi: 10.1038/nature746. [DOI] [PubMed] [Google Scholar]
- 207.Valencia P, Dias AP, Reed R. Splicing promotes rapid and efficient mRNA export in mammalian cells. Proc Natl Acad Sci U S A. 2008;105(9):3386–91. doi: 10.1073/pnas.0800250105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bruhn L, Munnerlyn A, Grosschedl R. ALY, a context-dependent coactivator of LEF-1 and AML-1, is required for TCRalpha enhancer function. Genes Dev. 1997;11(5):640–53. doi: 10.1101/gad.11.5.640. [DOI] [PubMed] [Google Scholar]
- 209.Hsu SC, Galceran J, Grosschedl R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 sig naling and association with beta-catenin. Mol Cell Biol. 1998;18(8):4807–18. doi: 10.1128/mcb.18.8.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Okada M, Jang SW, Ye K. Akt phosphorylation and nuclear phosphoinositide association mediate mRNA export and cell proliferation activities by ALY. Proc Natl Acad Sci U S A. 2008;105(25):8649–54. doi: 10.1073/pnas.0802533105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Spector DL, Lamond AI. Nuclear speckles. Cold Spring Harb Perspect Biol. 2011;3(2) doi: 10.1101/cshperspect.a000646. pii: a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Alpatov R, Munguba GC, Caton P, Joo JH, Shi Y, Shi Y, Hunt ME, Sugrue SP. Nuclear speckle-associated protein Pnn/DRS binds to the transcriptional corepressor CtBP and relieves CtBP-mediated repression of the E-cadherin gene. Mol Cell Biol. 2004;24(23):10223–35. doi: 10.1128/MCB.24.23.10223-10235.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Alpatov R, Shi Y, Munguba GC, Moghimi B, Joo JH, Bungert J, Sugrue SP. Corepressor CtBP and nuclear speckle protein Pnn/DRS differentially modulate transcription and splicing of the E-cadherin gene. Mol Cell Biol. 2008;28(5):1584–95. doi: 10.1128/MCB.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Joo JH, Taxter TJ, Munguba GC, Kim YH, Dhaduvai K, Dunn NW, Degan WJ, Oh SP, Sugrue SP. Pinin modulates expression of an intestinal homeobox gene, Cdx2, and plays an essential role for small intestinal morphogenesis. Dev Biol. 2010;345(2):191–203. doi: 10.1016/j.ydbio.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Joo JH, Kim YH, Dunn NW, Sugrue SP. Disruption of mouse corneal epithelial differentiation by conditional inactivation of pnn. Invest Ophthalmol Vis Sci. 2010;51(4):1927–34. doi: 10.1167/iovs.09-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sakashita E, Tatsumi S, Werner D, Endo H, Mayeda A. Human RNPS1 and its associated factors: a versatile alternative pre-mRNA splicing regulator in vivo. Mol Cell Biol. 2004;24(3):1174–87. doi: 10.1128/MCB.24.3.1174-1187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang P, Lou PJ, Leu S, Ouyang P. Modulation of alternative pre-mRNA splicing in vivo by pinin. Biochem Biophys Res Commun. 2002;294(2):448–55. doi: 10.1016/S0006-291X(02)00495-3. [DOI] [PubMed] [Google Scholar]
- 218.Lin S, Fu XD. SR proteins and related factors in alternative splicing. Adv Exp Med Biol. 2007;623(1):107–22. doi: 10.1007/978-0-387-77374-2_7. [DOI] [PubMed] [Google Scholar]
- 219.Schwerk C, Prasad J, Degenhardt K, Erdjument-Bromage H, White E, Tempst P, Kidd VJ, Manley JL, Lahti JM, Reinberg D. ASAP, a novel protein complex involved in RNA processing and apoptosis. Mol Cell Biol. 2003;23(8):2981–90. doi: 10.1128/MCB.23.8.2981-2990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tange TØ, Shibuya T, Jurica MS, Moore MJ. Biochemical analysis of the EJC reveals two new factors and a stable tetrameric protein core. RNA. 2005;11(12):1869–83. doi: 10.1261/rna.2155905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Vucetic Z, Zhang Z, Zhao J, Wang F, Soprano KJ, Soprano DR. Acinus-S’ represses retinoic acid receptor (RAR)-regulated gene expression through interaction with the B domains of RARs. Mol Cell Biol. 2008;28(8):2549–58. doi: 10.1128/MCB.01199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chan CB, Liu X, Jang SW, Hsu SI, Williams I, Kang S, Chen J, Ye K. NGF inhibits human leukemia proliferation by downregulating cyclin A1 expression through promoting acinus/CtBP2 association. Oncogene. 2009;28(43):3825–36. doi: 10.1038/onc.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jang SW, Yang SJ, Ehlén A, Dong S, Khoury H, Chen J, Persson JL, Ye K. Serine/arginine protein-specific kinase 2 promotes leukemia cell proliferation by phosphorylating acinus and regulating cyclin A1. Cancer Res. 2008;68(12):4559–70. doi: 10.1158/0008-5472.CAN-08-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hu Y, Yao J, Liu Z, Liu X, Fu H, Ye K. Akt phosphorylates acinus and inhibits its proteolytic cleavage, preventing chromatin condensation. EMBO J. 2005;24(20):3543– 54. doi: 10.1038/sj.emboj.7600823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y, Tsujimoto Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature. 1999;401(6749):168–73. doi: 10.1038/43678. [DOI] [PubMed] [Google Scholar]
- 226.Joselin AP, Schulze-Osthoff K, Schwerk C. Loss of Acinus inhibits oligonucleosomal DNA fragmentation but not chromatin condensation during apoptosis. J Biol Chem. 2006;281(18):12475–84. doi: 10.1074/jbc.M509859200. [DOI] [PubMed] [Google Scholar]
- 227.Haberman AS, Akbar MA, Ray S, Krämer H. Drosophila acinus encodes a novel regulator of endocytic and autophagic trafficking. Development. 2010;137(13):2157– 66. doi: 10.1242/dev.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Brandner JM, Reidenbach S, Franke WW. Evidence that “pinin”, reportedly a differentiation-specific desmosomal protein, is actually a widespread nuclear protein. Differentiation. 1997;62(3):119–27. doi: 10.1046/j.1432-0436.1997.6230119.x. [DOI] [PubMed] [Google Scholar]
- 229.Bracken CP, Wall SJ, Barré B, Panov KI, Ajuh PM, Perkins ND. Regulation of cyclin D1 RNA stability by SNIP1. Cancer Res. 2008;68(18):7621–8. doi: 10.1158/0008-5472.CAN-08-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Roche KC, Rocha S, Bracken CP, Perkins ND. Regulation of ATR-dependent pathways by the FHA domain containing protein SNIP1. Oncogene. 2007;26(31):4523–30. doi: 10.1038/sj.onc.1210233. [DOI] [PubMed] [Google Scholar]
- 231.Chen Y, Zhang L, Jones KA. SKIP counteracts p53-mediated apoptosis via selective regulation of p21Cip1 mRNA splicing. Genes Dev. 2011;25(7):701–16. doi: 10.1101/gad.2002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang C, Dowd DR, Staal A, Gu C, Lian JB, van Wijnen AJ, Stein GS, MacDonald PN. Nuclear coactivator-62 kDa/Ski-interacting protein is a nuclear matrix-associated coactivator that may couple vitamin D receptor-mediated transcription and RNA splicing. J Biol Chem. 2003;278(37):35325–36. doi: 10.1074/jbc.M305191200. [DOI] [PubMed] [Google Scholar]
- 233.Wang Y, Fu Y, Gao L, Zhu G, Liang J, Gao C, Huang B, Fenger U, Niehrs C, Chen YG, Wu W. Xenopus skip modulates Wnt/beta-catenin signaling and functions in neural crest induction. J Biol Chem. 2010;285(14):10890–901. doi: 10.1074/jbc.M109.058347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kang MR, Lee SW, Um E, Kang HT, Hwang ES, Kim EJ, Um SJ. Reciprocal roles of SIRT1 and SKIP in the regulation of RAR activity: implication in the retinoic acid-induced neuronal differentiation of P19 cells. Nucleic Acids Res. 2010;38(3):822–31. doi: 10.1093/nar/gkp1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gonçalves V, Matos P, Jordan P. The beta-catenin/TCF4 pathway modifies alternative splicing through modulation of SRp20 expression. RNA. 2008;14(12):2538–49. doi: 10.1261/rna.1253408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gonçalves V, Matos P, Jordan P. Antagonistic SR proteins regulate alternative splicing of tumor-related Rac1b downstream of the PI3-kinase and Wnt pathways. Hum Mol Genet. 2009;18(19):3696–707. doi: 10.1093/hmg/ddp317. [DOI] [PubMed] [Google Scholar]
- 237.Norris JD, Fan D, Sherk A, McDonnell DP. A negative coregulator for the human ER. Mol Endocrinol. 2002;16(3):459–68. doi: 10.1210/mend.16.3.0787. [DOI] [PubMed] [Google Scholar]
- 238.Baraniak AP, Chen JR, Garcia-Blanco MA. Fox-2 mediates epithelial cell-specific fibroblast growth factor receptor 2 exon choice. Mol Cell Biol. 2006;26(4):1209–22. doi: 10.1128/MCB.26.4.1209-1222.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jimenez J, Jang GM, Semler BL, Waterman ML. An internal ribosome entry site mediates translation of lymphoid enhancer factor-1. RNA. 2005;11(9):1385–99. doi: 10.1261/rna.7226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wei G, Ku S, Ma GK, Saito S, Tang AA, Zhang J, Mao JH, Appella E, Balmain A, Huang EJ. HIPK2 represses beta-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc Natl Acad Sci U S A. 2007;104(32):13040–5. doi: 10.1073/pnas.0703213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee W, Andrews BC, Faust M, Walldorf U, Verheyen EM. Hipk is an essential protein that promotes Notch signal transduction in the Drosophila eye by inhibition of the global co-repressor Groucho. Dev Biol. 2009;325(1):263–72. doi: 10.1016/j.ydbio.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 242.Zhang Q, Nottke A, Goodman RH. Homeodomain-interacting protein kinase-2 mediates CtBP phosphorylation and degradation in UV-triggered apoptosis. Proc Natl Acad Sci U S A. 2005;102(8):2802–7. doi: 10.1073/pnas.0409373102. [DOI] [PMC free article] [PubMed] [Google Scholar]