Abstract
Chaperones and foldases in the endoplasmic reticulum (ER) ensure correct protein folding. Extensive protein-protein interaction maps have defined the organization and function of many cellular complexes, but ER complexes are under-represented. Consequently, chaperone and foldase networks in the ER are largely uncharacterized. Using complementary ER-specific methods, we have mapped interactions between ER-lumenal chaperones and foldases and describe their organization in multiprotein complexes. We identify new functional chaperone modules, including interactions between protein-disulfide isomerases and peptidyl-prolyl cis-trans-isomerases. We have examined in detail a novel ERp72-cyclophilin B complex that enhances the rate of folding of immunoglobulin G. Deletion analysis and NMR reveal a conserved surface of cyclophilin B that interacts with polyacidic stretches of ERp72 and GRp94. Mutagenesis within this highly charged surface region abrogates interactions with its chaperone partners and reveals a new mechanism of ER protein-protein interaction. This ability of cyclophilin B to interact with different partners using the same molecular surface suggests that ER-chaperone/foldase partnerships may switch depending on the needs of different substrates, illustrating the flexibility of multichaperone complexes of the ER folding machinery.
In the endoplasmic reticulum (ER)1 secretory proteins become correctly folded, and membrane complexes are assembled. To facilitate these processes, the ER contains a complex network of molecular machines including chaperones that prevent protein aggregation and foldases that directly promote folding. The functions of ER chaperones and foldases overlap in a protein maturation continuum that encompasses ER-assisted folding, ER quality control, ER-associated degradation (ERAD), and export from the ER (1, 2). Protein folding begins immediately as nascent polypeptide chains enter the ER, assisted initially by two chaperone systems: the BiP-associated, glycan-independent system and the glycan-dependent calnexin/calreticulin cycle (3). Concurrently, protein-disulfide isomerases (PDIs), and peptidyl-prolyl cis-trans-isomerases (PPIs) catalyze rate-limiting steps of protein folding (4, 5). The in vivo requirement for PDIs to fold a variety of substrate proteins is well documented (5), whereas the lumenal PPI cyclophilin B has been implicated in IgG folding (6) and recently in ERAD of soluble lumenal substrates (7). How ER chaperones and foldases function together in vivo to fold proteins is not clear, but their association into multimember complexes has been proposed to focus their activities on nascent chains (8, 9).
Although the functions of multimember protein complexes have been inferred from the functions of individual components, the lack of molecular detail limits knowledge of their mechanisms and their organization in the ER. Their specific functions may depend on how they interact with one another, as we have previously shown for calnexin and ERp57, where ERp57 is defined as the PDI that acts specifically on glycoprotein substrates bound to calnexin (10, 11). Similarly, the specific interaction between ERdj5 and EDEM/BiP defines Erdj5 as the reductase that unfolds misfolded glycoproteins that are destined for ERAD (12, 13), whereas the HPD motif of ERdj3 mediates its interactions with BiP (14, 15). N-Glycosylation as an interaction motif regulates folding and degradation of client proteins passing through the ER (2, 16, 17) but has not been shown to regulate chaperone-chaperone interactions. Post-translational modifications such as phosphorylation and acetylation are absent in the ER, suggesting that the ER requires unique mechanisms to regulate its dynamic (18) protein-protein interactions.
A more complete understanding of ER protein-protein interactions will provide insight into the specific functions of ER proteins, particularly those proteins with apparently redundant activities. In the past years, genome-wide assays using two-hybrid and affinity purifications have identified many interactions now available in curated databases (19, 20). However, because of methodological limitations, interactions between ER proteins remain under-represented, most likely because of their dependence on specific conditions for interaction, such as high calcium (21–23) and the oxidative environment of the ER (24–26). Furthermore, it is difficult to detect highly dynamic protein-protein interactions that are prevalent in the ER (18) with traditional tandem affinity purification tag approaches. Consequently, the organization of chaperone networks in the ER remains largely uncharacterized. Our approach has been to develop a new ER-specific two-hybrid method and to adapt existing methods specifically to detect protein interactions in the ER (12, 27, 28).
Here we use such a combined method that we term ER-MAP to detect protein-protein interactions in the ER. The combination of different methods allows a comprehensive detection of interactions because some protein baits are amenable to the ER membrane yeast two-hybrid, but not to affinity purification and vice versa. We previously used the in vivo ER-localized yeast two-hybrid system to detect binary protein interactions of ERp57 (27) and ERdj5 (12), whereas we have used the affinity purification method to identify a cyclophilin B-calreticulin/calnexin interaction (29). Here, we expand the number of bait proteins tested with each method, focusing primarily on interactions between chaperones and foldases in the ER (30–33).
Using the ER-MAP, we identify 75 ER protein-protein interactions, including 53 novel interactions. Consistent with its roles in multiple ER processes (9), BiP plays a central role in the ER multiprotein complex, interacting with itself and 12 distinct ER partners. We reveal that previously defined multiprotein complexes in the ER exclude a significant number of additional lumenal interacting proteins, probably because of the use of less sensitive detection methods. Increased specificity and sensitivity has allowed us to define the binary interactions within the multiprotein complexes. Most notably, we uncover six PDI-PPI complexes, including an ERp72-cyclophilin B interaction that we further define in detail. In an in vitro assay, the ERp72-cyclophilin B pair can enhance the rate of immunoglobulin G CH1/CL intermolecular disulfide bonding. By mutagenesis and NMR, we define a conserved lysine-rich surface of cyclophilin B distinct from its active site that interacts with ERp72 and GRp94. Using deletions, we show that the distinctive N-terminal acidic head of ERp72 binds to cyclophilin B, whereas GRp94 binds to cyclophilin B via a similar C-terminal acidic tail. These studies highlight the interconnectedness of the ER protein folding machinery and define novel interaction mechanisms between ER proteins.
EXPERIMENTAL PROCEDURES
Plasmids, Strains, and Cell Lines
Genes from rat or human epithelial cells were cloned by SMART PCR cDNA synthesis (BD Biosciences, Mississauga, Canada). Each cDNA was amplified from cDNA pools using gene specific primers removing the STOP codon for C-terminal fusion constructs. Genes were introduced into the two-hybrid destination vectors using Gateway technology (Invitrogen). Yeast media, culture conditions, and manipulations of yeast strains were as described (64). For bacterial expression, cDNAs were cloned into pGEX-6P-1 (GE Healthcare) using BamHI and NotI restriction sites (details in supplemental materials). For mammalian cell expression, mouse EDEM1, 2, and 3 were cloned into pCDNA3 (Invitrogen) (details in supplemental materials). HEK293 cells were transfected using FuGENE HD (Roche Applied Science) according to the manufacturer's suggested protocol and grown in Dulbecco's modified Eagle's medium supplemented with hygromycin, nonessential amino acids, 10% fetal bovine serum, and G418.
ER-MYTHS Screen
For the ER membrane yeast two-hybrid system, the lumenal part of yeast Ire1p was replaced by mammalian chaperones and foldases as indicated in supplemental Table SIII. A mating-based interaction grid was used to test all binary interactions between bait and prey fusions. Interaction of bait and prey leads to oligomerization of the cytosolic part of Ire1p, which activates its ribonuclease activity. The ribonuclease splices the mRNA of the transcriptional activator HAC1, resulting in activation of the unfolded protein response element-coupled ADE2 growth reporter. Interactions were indicated by growth on media lacking adenine. Positive interactions were determined by growth compared with controls. For details, see supplemental materials.
Pulldown Method
We chose smooth ER as a starting material to minimize ribosomal contaminants. 0.5–2.0 mg of purified recombinant bait proteins were coupled to NHS-activated Sepharose 4B beads, and interacting proteins were captured from rat liver low density microsomes (42) as described previously (29). The binding experiments were done in a calcium-supplemented, neutral pH, HEPES-acetate buffer (115 mm potassium acetate, 20 mm HEPES, pH 7.0, 0.75 mm CaCl2, 0.1% Triton X-100) we optimized for detecting ER protein-protein interactions that is similar to potassium-HEPES-magnesium buffer (65). Wild-type and mutant cyclophilin B used to determine the effects of K6A, K35A, K6A/K35A, and PentaK-A (K4A/K5A/K6A/K9A/K35A) on binding to ER partners were purified as GST fusions on glutathione-Sepharose 4B beads. 10 μl of these beads were used to bait rat smooth ER as described above, except 1% Triton X-100 was used to solubilize the ER, because we found that the interaction of cyclophilin B with its lumenal partners was not significantly affected by this increase in detergent concentration. Proteins co-purified with cyclophilin B and its mutants were eluted with 20 μl of 10 mm glutathione in 0.1 m Tris, pH 8.0. 15 μl of each supernatant was analyzed by SDS-PAGE and colloidal Coomassie staining according the manufacturer's suggested protocol (Invitrogen).
Western Blotting and LC/MS/MS Analysis
10 μl of each affinity-purified eluate was analyzed by Western blotting, alongside eluates from beads treated the same way but not exposed to rat ER. Total rat smooth ER loaded onto the beads was included as a positive control. Western blotting was performed using rabbit polyclonal antibodies against ER chaperones. For mass spectrometry, 40 μl of total eluate from beads was run into a stacking gel. The bands were excised, reduced (10 mm DTT, 10 min), alkylated (55 mm iodoacetamide, 30 min), and subjected to digestion by trypsin (12 ng/μl) overnight according to standard procedures (50). The resulting peptide digests were separated by C-18 reverse phase nano LC, followed by MS/MS on either a Bruker HCT Ultra ion trap mass spectrometer (Bruker Daltonics, Billerica, MA) or an Agilent 6510 QTOF (Agilent Technologies, Santa Clara, CA). Data files (Bruker) were formatted to mgf files with Bruker Daltonics Compass data analysis software (standard settings) and searched on either the rodent Swissprot database version 12_05_2006 or newer (217,551 sequences; 79,825,379 residues), or the NCBI rodent database version 20080510 or newer (6,512,701 sequences; 2,221,612,072 residues) using Mascot v. 2.2 (Matrix Sciences). Fixed modifications were set for iodoacetamide-modified cysteine, and oxidized methionine was chosen for variable modifications. “Semitrypsin” was chosen as cleavage, and one missed tryptic cleavage was allowed. Mass accuracies for Mascot searches of ion trap data were set at 1.4 Da for precursor ions and 0.4 Da for fragment ions. Summarized Mascot data presented in supplemental Table SII were selected, based on a cutoff at a minimum of two unique and unambiguous peptides with a p value of p < 0.05 (Mowse scores of 45 or better). Spectrum Mill (version A.03.03) searches (supplemental Tables SII and SIII) were performed using the NCBInr mammalian database (downloaded 10_03_2010), setting trypsin as used cleavage enzyme (allowing one missed cleavage), iodoacetamide-modified cysteines, allowing oxidized methionine as variable modification and searching with mass accuracies of 1.4 Da for precursor ions and 0.3 Da for fragment ions. Autovalidation was performed using standard settings. Proteins were considered identified, when two or more unique and unambiguous peptides were identified with Spectrum Mill scores above 10 (conservative setting, because Spectrum Mill version A.03.03 does not operate with ion statistics, wherefore p values are undetermined). To more readily detect less abundant interacting protein partners, total eluates were separated by SDS-PAGE and Coomassie-stained, and individual proteins bands were excised and analyzed separately by mass spectrometry as described above.
Domain Deletions and in Vitro Interaction between Purified Recombinant Proteins
The coding sequences for fragments of human ERp72 corresponding to Pro177–Gly523 (abb′), Pro283–Gly523 (bb′), Glu25–Pro177 (a0), Glu57–Pro177 (a0 no N-terminal), and Glu25–Glu57 (acidic N-terminal) were cloned into pGEX-6P-1, expressed in Escherichia coli, purified, and coupled to beads (see supplemental materials). The bait proteins were used to pull down prey in HEPES-acetate buffer by incubating 10 μl of coupled beads with 500 μl of 10 μm prey protein for 30 min, followed by three washes with 500 μl of ice-cold HEPES-acetate. The N-terminal peptide of ERp72 was purified as a GST fusion with glutathione-Sepharose 4B beads (GE Healthcare) that were used in interaction experiments with cyclophilin B as described above. Bound proteins were eluted from beads and analyzed by SDS-PAGE and Coomassie Blue staining.
Isothermal Titration Calorimetry
Experiments were carried out on a MicroCal VP-ITC titration calorimeter (Microcal, Northampton, MA), with ligand and titrant in HEPES-acetate buffer, pH 7.0, at 15 °C. The data were processed using ORIGIN and the binding isotherm fit to a single-site binding model to determine the binding constant.
NMR Sample Preparation and Titrations of Cyclophilin B with Peptides
15N-Labeled cyclophilin B for NMR was prepared and assigned as described previously (29). All of the NMR experiments were performed at 30 °C on a Bruker DRX 600 MHz spectrometer. The NMR samples contained 0.2 mm protein in 90% H2O, 10% D2O, 50 mm sodium phosphate buffer, pH 6.5, 40 mm NaCl, 1 mm EDTA, and 1 mm DTT. Analysis of the binding of the 36-amino acid peptide from the N terminus of ERp72 to cyclophilin B was carried out by comparison of chemical shifts for backbone amide signals in 1H-15N heteronuclear single quantum coherence spectra. Heteronuclear single quantum coherence spectra were recorded at 1:2, 1:1, 2:1, 5:1 peptide to protein ratios. The magnitude of amide chemical shift changes was calculated as [(D1H shift)2 + (D15N shift × 0.2)2][frax;1,2] in ppm and used to calculate approximate dissociation constants for the ERp72 and GRp94 peptides using a simple binding model.
Immunoglobulin CH1/CL Oxidation Assay
The effect of ERp72 and cyclophilin B together on the rate of disulfide bridge formation between the CH1 and CL domains of IgG was tested using a CH1-CL assembly assay essentially as described previously (6). Briefly, 25 μm recombinant CH1 and CL domains with their C-terminal cysteines reduced were incubated with ERp72 or cyclophilin B or a combination of ERp72 and cyclophilin B in PBS, pH 7.4, with 0.5 mm GSSG and 2.0 mm GSH. The assembly reaction was stopped by transfer into Laemmli sample buffer (no reducing agent) and boiled for 5 min at 95 °C. 0.5 μl of each sample was subjected to Phastgel Homogenous 20 SDS-PAGE (GE Healthcare), and the proteins were visualized by staining with Coomassie Blue. The gels were scanned in a Bio-Rad Gel Doc XR system, and background-subtracted band intensity was quantified with Quantity One software (Bio-Rad). Band intensities were normalized for each lane based on intensity of cyclophilin B or ERp72 staining added in identical quantities to each folding reaction. Under these experimental conditions, the assembly of available subunits in excess was completed within 160 min for the reaction catalyzed by cyclophilin B or ERp72 alone. Half-times (τ values) were estimated by determining the time to reach half the normalized band intensity observed at 160 min.
RESULTS
In Vitro and in Vivo Detection of ER Protein-Protein Interactions Using ER-MAP
We have used different ER-specific strategies to detect interactions: in vitro affinity purifications and an in vivo ER-MYTHS (see experimental scheme in supplemental Fig. S1). Well characterized interactions (including ERp57-calnexin, ERdj5-BiP, BiP-ERdj3, UGGT-SEP15, FKBP23-BiP, and BiP-BaP) within this set allowed us to benchmark our methods (12, 22, 27, 34, 35). We tested 11 recombinant proteins by affinity purification, 3 proteins by immunopurification using FLAG constructs, and 25 proteins by ER-MYTHS (supplemental Table SI).
For in vitro affinity purification, we used purified recombinant ER chaperones and foldases as bait to purify complexes from rat liver ER. In the case of EDEM1, 2, and 3, which are refractory to bacterial expression, epitope-tagged proteins were expressed in mammalian cells to co-purify interacting proteins. To eliminate interactions of chaperones with their misfolded substrates, only soluble and pure recombinant proteins (>90% by SDS-PAGE and Coomassie staining) were used as baits for further experiments. The 11 recombinant proteins we used have all previously been purified from E. coli as soluble, folded polypeptides (10, 22, 29, 34, 36–41) (FKBP13 see 2PBC at http://www.pdb.org). The proteins were coupled covalently to Sepharose beads and used to purify interacting partners from solubilized rat liver smooth ER (42). The ER extract was minimally diluted in a calcium-supplemented buffer to simulate conditions found in the ER.
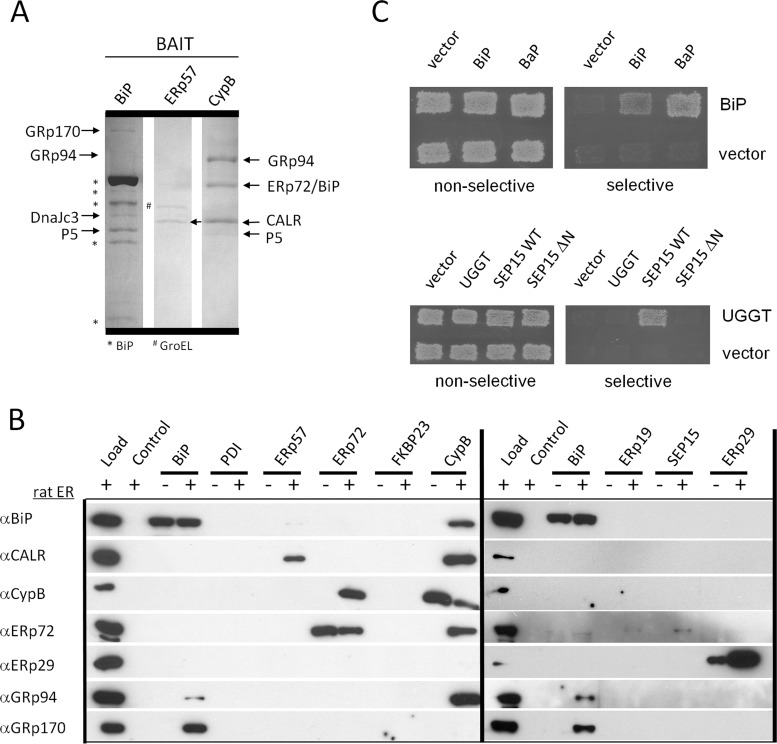
Proteins captured by the baits and identified by mass spectrometry are listed along with the specific peptides identified for each in supplemental Tables SII and SIII. We noted distinct sets of interacting proteins for each bait and confirmed the interaction of ERp57 with calreticulin (Fig. 1A and supplemental Table SII). SDS-PAGE and Coomassie staining results for BiP, ERp57, and cyclophilin B affinity purifications are shown in Fig. 1A, and EDEM1, 2, and 3 affinity purifications are shown in supplemental Fig. S4A. The BiP eluate contained enough GRp170, GRp94, DnaJc3, and P5 to observe on the gel (Fig. 1A). GRp94, ERp72/BiP, and calreticulin were identified in eluates from cyclophilin B-coupled beads (Fig. 1A). We did not detect binding partners that were above our mass spectrometry detection threshold for ERp19, SEP15, or FKBP23 baits.
Fig. 1.
ER protein interaction map by affinity and ER-MYTHS methods. A, SDS-PAGE of rat ER proteins purified by affinity to the bait proteins BiP, ERp57, and cyclophilin B. The bands were cut and identified by mass spectrometry. B, Western blots identify proteins purified from rat ER by affinity to recombinant bait proteins. Load, 20 μg of rat ER; Control, beads blocked with ethanolamine; −, no ER added; +, ER added. C, example of ER-MYTHS interactions. Upper panel, BiP interacts with itself and the nucleotide exchange factor Bap. Transformants were grown under conditions nonselective for interactions (SD-LT) and selective (SD-LTAI). Lower panel, UGGT interacts with full-length SEP15 (WT) but not with itself or N-terminally truncated SEP15ΔN (62–178aa). (See also supplemental Fig. S1 and Tables SI–SIV.)
In most cases where good quality antibodies specific to prey proteins were available, we confirmed the mass spectrometry identified interactions by Western blotting (Fig. 1B and supplemental Figs. S2 and S4B). The blots identified the same proteins as mass spectrometry except for four interactions: ERp72-SEP15 (detected by Western blot and ER-MYTHS; Fig. 1B and supplemental Table SII) and EDEM1, 2, and 3-ERdj5 (detected by Western blot, supplemental Fig. S4B). The homotypic interaction of ERp29 (43) was readily observed by Western blotting (Fig. 1B, far right lane) and was also detected by identification of rat-specific peptides of ERp29 by mass spectrometry (supplemental Table SII and Fig. S3). Altogether mass spectrometry and Western blotting of affinity purifications identified 42 heterotypic interactions and 1 homotypic interaction, including 30 interactions not previously reported in the literature (Fig. 2A and supplemental Table SIV and Fig. S5).
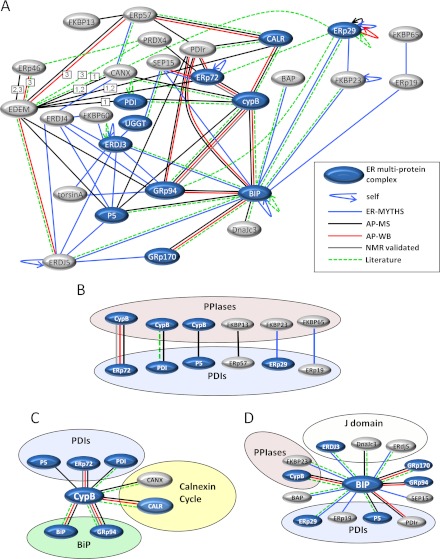
Fig. 2.
The interaction map of ER chaperones and foldases. A, combined map of all ER protein-protein interactions detected with edge color representing interactions detected by the ER-MYTHS (blue), AP-MS (affinity purification results by mass spectrometry, black), affinity purification results by Western blotting (AP-WB, red), interactions validated by NMR experiments (grey), and interactions already validated in the literature (green). Members of the previously identified ER multiprotein complex are shaded in blue. Curved arrows represent self-interactions. EDEM 1, 2, and 3 are represented by EDEM, but in cases where an interaction was not observed with all three family members, the number corresponding to the member(s) that interact is indicated in a white box on the connecting edge. B, interactions detected between PDIs and PPIs. C, all cyclophilin B interactions detected grouped by protein family with PDIs, calnexin cycle chaperones, and BiP chaperones. D, all of the BiP interactions detected, highlighting J-domain containing proteins, PDIs, and PPIs. (See also supplemental Tables SI–SIV and Figs. S2–S5.)
For the ER-localized membrane yeast two-hybrid system, the coding sequence of each protein was cloned in-frame with an Ire1p reporter construct so that the lumenal domain of yeast Ire1p was substituted with bait or prey protein (supplemental Fig. S1). The interaction of Ire1p fusions activates the unfolded protein response, resulting in the expression of a unfolded protein response-coupled growth reporter (Fig. 1C). To minimize the occurrence of unspecific interactions caused by overexpression of either bait or prey, the expression of fusion proteins was driven by the weak IRE1 promoter (44). ER-MYTHS detected 27 heterotypic and 6 homotypic interactions including 23 novel binary interactions (Fig. 2A and supplemental Fig. S5). Two of the novel interactions detected (ERp29-FKBP23 and ERp19-FKBP65) are between foldases of the PPI and PDI families (Fig. 2B).
ERMAP Dissects the ER Multiprotein Complex
In total, we detected 15 heterotypic and 4 homotypic interactions between multiprotein complex members. All of the interactions identified are depicted in Fig. 2A, where members of the multiprotein complex are shown in blue. Supplemental Table SIV shows each interaction detected by each method including interactions previously identified in the literature with PubMed Identifier reference numbers. Supplemental Fig. S5 summarizes the totals for each method. There was limited overlap between the ER-MYTHS and affinity purification techniques, mostly because of differences in amenability of specific baits to each technique (see supplemental Fig. S6). Of 28 known literature interactions, we detected 22, indicating that our method could identify the majority (79%) of known interactions.
We discovered that members of the previously identified ER multiprotein complex do not interact exclusively with one another and are much more promiscuous than previously appreciated. Still, some of the apparent boundaries between complexes involved in separate processes (such as the separation between the calnexin cycle and BiP-associated chaperones) were maintained, although less strictly than we anticipated. We found that some calnexin cycle proteins (ERp57, calnexin, and calreticulin) associate with BiP-associated proteins. However, BiP itself did not affinity purify any calnexin cycle enzymes, nor did it show any binary interactions with them by ER-MYTHS. Conversely, interactions detected with the calnexin cycle enzyme ERp57 were distinct from those observed for BiP (Fig. 2A). Cyclophilin B, on the other hand, interacted both with BiP-associated proteins (ERp72, GRp94, BiP, PDI, and P5) and with calnexin and calreticulin (Fig. 2C). Consistent with its pivotal role in ER function, BiP interacted with the most proteins of any single bait we tested, including three J-domain co-chaperones, four different PDIs, two PPIs, BaP, SEP15, GRp94, and GRp170 (Fig. 2D).
One concern when studying the ER multiprotein complex is that chaperones bind misfolded proteins as substrates, making it difficult to identify bona fide partner protein interactions (45). Although unable to specifically distinguish between substrate and partner protein interactions using ER-MAP, we identified distinct sets of interacting partners with each bait protein tested. This suggests that we did not observe general substrate-like binding of chaperones to the bait, but rather the purification of specific complexes with each bait protein (Fig. 1, A and B, and supplemental Tables SII and SIII). BiP was the only protein found in eluates from each bait tested, but it was also found to exhibit weak nonspecific binding to the Sepharose beads. For this reason, if BiP was only detected by mass spectrometry and not Western blotting in eluates from a particular bait affinity purification, it was considered to be background and excluded from the map.
ERAD-enhancing Mannosidase-like Proteins Interact with PDIs
FLAG-tagged ERAD enhancing α-1,2-mannosidase-like proteins (EDEM1, 2, and 3) co-purified several members of the PDI family, in addition to GRp94 from HEK293 total cell lysates (supplemental Table SIII). Each EDEM co-purified with P5, ERp72, and ERp57. EDEM1 also co-purified with PDI, whereas EDEM2 and EDEM3 co-purified with ERp46. In addition to the PDIs, EDEM1 and EDEM2 co-purified with calnexin and calreticulin, whereas EDEM3 did not interact with either of these lectin-like chaperones. Thus, despite differences in primary amino acid sequence outside of their mannose-6-phosphate homology domains, EDEM1, 2, and 3 interact with similar ER proteins, most of which are PDIs with a few notable exceptions.
PDIs Partner with PPIs
We identified a striking pattern of interactions between specific PDIs and PPIs. ER-MAP revealed six distinct PDI with PPI interactions (Fig. 2B). Several of the most abundant PDIs interact with PPIs, including ERp72, PDI, ERp57, and P5. We also detected interactions of FKBP23 with ERp29 (a PDI-like protein lacking oxidoreductase activity) and FKBP65 with ERp19. The specific interactions between many members of the PDI and PPI families suggest a previously unappreciated physical clustering of foldases in the ER.
ERp72 Interacts Directly with Cyclophilin B
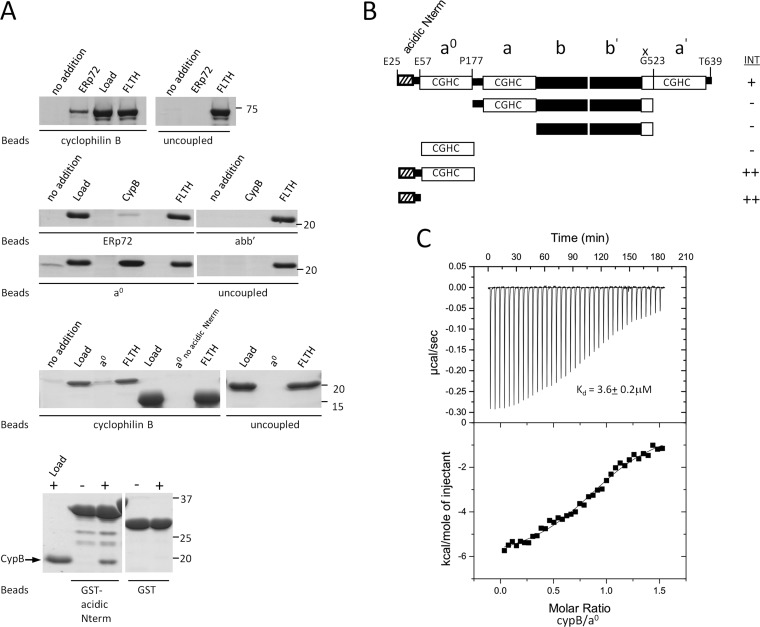
We were specifically interested in interactions between PDIs and PPIs and chose to focus on the interaction of cyclophilin B with ERp72, because both of these proteins have been recently implicated in folding at least one distinct substrate, immunoglobulin G (6, 11). In affinity purifications, ERp72 preferentially bound cyclophilin B (Fig. 1B and supplemental Table SII). In addition to ERp72, cyclophilin B also affinity-purified several other ER chaperones (Fig. 1, A and B, and supplemental Table SII). We tested for direct interaction by in vitro binding assays using purified recombinant cyclophilin B and ERp72 (Fig. 3, A and B). We performed the interaction experiment with both a cyclophilin B-bead matrix and an ERp72 bead matrix and confirmed that the interaction is direct because it occurs in the absence of additional proteins (Fig. 3A, top two panels). The ERp72-CypB interaction remained stable in the presence of 1 mm DTT (data not shown), indicating that it is not mediated by mixed disulfides between ERp72 and cyclophilin B.
Fig. 3.
ERp72 interacts directly with cyclophilin B through an N-terminal sequence that is necessary and sufficient for binding A, direct interaction experiments between ERp72 and cyclophilin B. Full-length cyclophilin B and ERp72 fragments were covalently attached to beads as an affinity matrix. Free ERp72 fragments were added to the cyclophilin B matrix, and free cyclophilin B was added to the ERp72 matrices, followed by washing, elution, SDS-PAGE, and Coomassie Blue staining. Uncoupled beads were used in each experiment as a control. The bands seen at lower molecular weights in the bottom panel were identified as degradation products of the GST-fused N-terminal region because they were not detectable immediately after purification. no addition, no prey protein added; ERp72, full-length ERp72 (Glu25–Tyr639); Load, 5% of total prey protein applied; FLTH, flow through; CypB, full-length cyclophilin B (Asp33–Glu216); abb′, Pro177–Gly523 of ERp72); a0, Glu25–Pro177 of ERp72), a0 no acidic Nterm, Glu57–Pro177 of ERp72); GST-acidic Nterm, GST-fused Glu25–Glu57 of ERp72. B, schematic representation of the domain fragments of ERp72 used to identify the part of ERp72 essential for binding to cyclophilin B. INT, interaction intensity detected (+) or not detected (−) between the ERp72 fragment and cyclophilin B. C, isothermal titration calorimetry binding isotherm for titration of ERp72 a0 E25-P177 (30 μm) with cyclophilin B (200 μm), with a resultant Kd of 3.6 ± 0.2 μm. The interaction is not disrupted by calcium below 10 mm concentration. (See also supplemental Fig. S7.)
ERp72 and Cyclophilin B Together Enhance the Rate of IgG Assembly in Vitro
We next determined whether ERp72 and cyclophilin B form a physical as well as a functional complex in the ER. Both have been shown to associate with unassembled, incompletely folded IgG heavy chains by immunoprecipitation after cross-linking (31). Recent work has demonstrated that proline isomerization of the intrinsically disordered IgG-CH1 domain precedes formation of a disulfide bond with the IgG-CL domain that is necessary for IgG secretion (6). The rate of CH1-CL folding is enhanced by cyclophilin B in vitro (6) but in its absence occurs relatively slowly (>2 h).
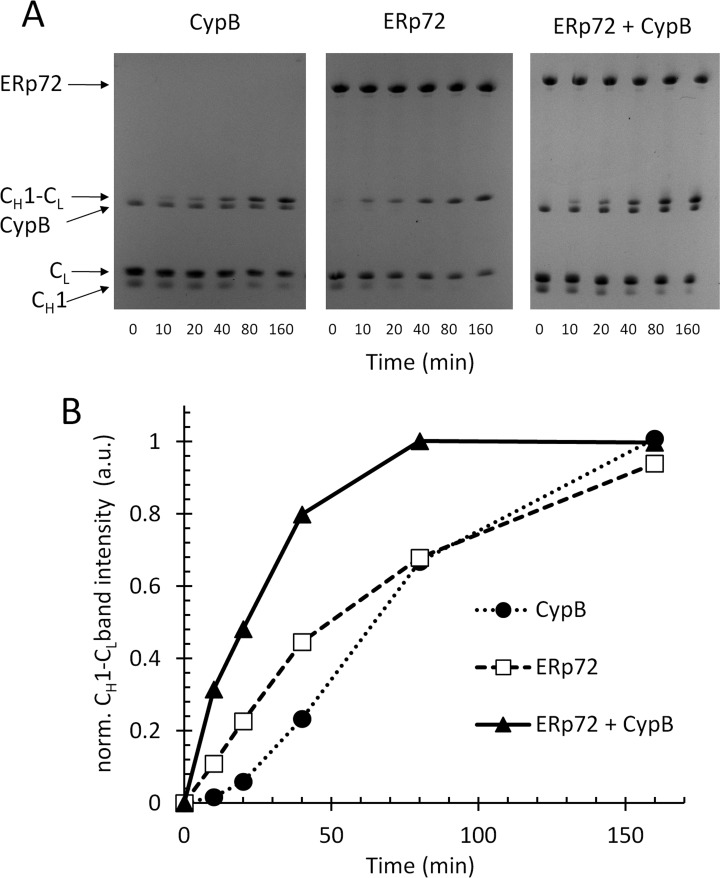
We set out to determine whether ERp72 and cyclophilin B together enhance antibody assembly in vitro. We assayed the assembly of purified recombinant CH1 (C-terminal Cys217 reduced) with purified recombinant CL (C-terminal Cys215 reduced) in the presence of ERp72, cyclophilin B, or both ERp72 and cyclophilin B by nonreducing SDS-PAGE of time points taken during the course of the folding reaction (Fig. 4A). In the denaturing gels we were able to visualize disulfide bond formation, whereas SDS dissociated noncovalent interactions between ERp72 and cyclophilin B. ERp72 and cyclophilin B together catalyzed CH1-CL assembly with a half-time (τ) of ∼21 min, whereas τ with ERp72 or cyclophilin B alone was ∼48 or 64 min, respectively (Fig. 4B). The noncatalyzed assembly of the CH1-CL heterodimer occurred in ∼2.5 h at a rate slightly slower than observed with cyclophilin B alone (not shown). The increase in assembly rate observed with ERp72 and cyclophilin B combined was not due to an increase in overall catalyst concentration, because double the concentration of ERp72 alone (supplemental Fig. S8A) or cyclophilin B alone (supplemental Fig. S8B) did not lead to any increases in assembly rate. In control experiments, we found that addition of cyclophilin B to ERp72 did not significantly enhance or inhibit ERp72 catalyzed oxidative folding of RNase A (supplemental Fig. S8C) or its reductase activity on the small molecule substrate di-Eosin-GSSG (supplemental Fig. S8D). Our data indicate that ERp72 and cyclophilin B cooperatively catalyze folding and assembly of the CH1-CL IgG heterodimer in vitro.
Fig. 4.
ERp72 and cyclophilin B cooperate in the assembly of the immunoglobulin G CH1-CL heterodimer in vitro. A, individual recombinant CH1 and CL domains of immunoglobulin G (25 μm of each), with their C-terminal cysteines reduced were incubated in a PBS redox buffer, pH 7.4, with 5 μm of ERp72 alone (middle panel), cyclophilin B alone (left panel), or a combination of ERp72 and cyclophilin B (right panel). The assembly of the CH1-CL heterodimer by disulfide bonding was assayed by nonreducing SDS-PAGE and CH1-CL band intensity was quantified for the indicated time points, plotted in B, plot of normalized CH1-CL band intensity (arbitrary units) versus time for the gels shown in A. (See also supplemental Fig. S8.)
A Novel Type of Protein-Protein Interaction in the ER
To identify the region of ERp72 responsible for its interaction with cyclophilin B, we tested purified recombinant ERp72 fragments (Fig. 3B) for their ability to interact with purified cyclophilin B. Sequential domain deletions identified the N-terminal a0 domain of ERp72 as sufficient for the interaction with cyclophilin B (Fig. 3A). We determined the affinity of the interaction between cyclophilin B and the a0 domain to be 3.6 ± 0.2 μm by isothermal titration calorimetry (Fig. 3C). Inspection of the ERp72 a0 domain sequence revealed a 36-amino acid Glu- and Asp-rich acidic sequence at its extreme N terminus. We hypothesized that this acidic N terminus may mediate an electrostatic interaction with cyclophilin B, which has an isoelectric point of 9.33. After removal of the acidic N terminus, the remaining a0 domain could no longer bind to cyclophilin B (Fig. 3A). To determine whether the acidic N terminus alone is sufficient for binding cyclophilin B in the absence of a0, we fused the motif to GST. As shown in Fig. 3A (bottom panel), the ERp72 acidic N terminus fused to GST is sufficient to bind cyclophilin B. These results demonstrate that the interaction of ERp72 with cyclophilin B is mediated by an acidic N-terminal motif of ERp72.
Because this motif of ERp72 has been suggested to be a calcium-binding site (46, 47), we tested whether calcium could inhibit the binding of ERp72 to cyclophilin B. Calcium levels are high in the ER (48), and we found that the complex was stable up to 10 mm calcium (supplemental Fig. S7), suggesting that it remains stable at calcium concentrations found in the ER lumen.
ERp72 Binds to a Positive Electrostatic Surface of Cyclophilin B
To identify the part of cyclophilin B that binds to ERp72, we titrated 15N-labeled recombinant cyclophilin B with the N-terminal motif of ERp72. The strongest chemical shift changes were for Lys4, Lys5, Lys6, Lys9, and Lys35 (Fig. 5A). These chemical shift changes map to a conserved positively charged surface of cyclophilin B (Fig. 5B) located opposite its active site (not shown in the figure). This lysine-rich binding site of cyclophilin B is the same site found to bind heparan sulfate (49), which does not affect PPI activity.
Fig. 5.
ER chaperones interact with a conserved positive electrostatic surface of cyclophilin B. A, chemical shift changes of 15N-labeled cyclophilin B upon binding to N-terminal 36-amino acid peptide of ERp72 (left panel) and C-terminal 21-amino acid peptide of GRp94 (right panel), plotted as a function of cyclophilin B sequence. B, the shifted residues of cyclophilin B Lys6, Lys9, and Lys35 (bold type) are identical from worm to human (left panel) and localize to a positively charged surface of cyclophilin B (right panel). Red indicates negative electrostatic potential, and blue indicates positive electrostatic potential. C, affinity purification of chaperones from rat ER with recombinant GST-cyclophilin B and its mutants K6A/K35A and PentaK-A (K4A/K5A/K6A/K9A/K35A) demonstrate that the positive electrostatic surface represented by conserved lysine residues of cyclophilin B seen in B is indispensable for cyclophilin B-chaperone interactions. Glutathione eluates were analyzed by SDS-PAGE and colloidal Coomassie staining (CB) in the left panel and in the right panel by SDS-PAGE and Western blotting (WB) with α-GRp94, α-ERp72, and α-calreticulin (CALR) antibodies. The fold of each cyclophilin B mutant was verified by one-dimensional NMR. (See also supplemental Fig. S9.)
The Electrostatic Surface of Cyclophilin B Is a General Binding Site for ER Chaperones
Having discovered that a positive electrostatic surface of cyclophilin B mediates its interaction with ERp72, we set out to determine whether this same surface is a general binding site for ER partners of cyclophilin B. We noted that each cyclophilin B partner (GRp94, BiP, PDI, calreticulin, and P5) contains a polyacidic sequence similar to the one located at the N terminus of ERp72. We synthesized the corresponding peptide of GRp94 and used it to titrate 15N-labeled cyclophilin B. We observed a similar pattern of chemical shift changes in response to the GRp94 peptide as in response to the ERp72 peptide (compare Fig. 5A, left and right panels), but the GRp94 peptide showed ∼10-fold weaker affinity. The lysines involved in binding the ERp72 and GRp94 peptides (Lys6, Lys9, and Lys35) of cyclophilin B are identical from worm to human (Fig. 5B, left panel) and are part of the surface (Fig. 5B, right panel) involved in binding to the tip of the P-domain of calnexin (29).
To determine the importance of individual residues within the positive electrostatic surface of cyclophilin B for partner binding, we prepared point mutants K6A and K35A, a double mutant K6A/K35A, and a pentuple Lys → Ala mutant with five lysines mutated to alanine (Lys4, Lys5, Lys6, Lys9, and Lys35). We previously determined that the individual K9A mutation did not significantly alter the interaction of cyclophilin B with calreticulin (29), so we focused here on the Lys6, Lys35, and combined mutations. We verified that the K6A/K35A and PentaK-A mutants are folded like wild-type cyclophilin B by one-dimensional NMR (supplemental Fig. S9). We then performed affinity purifications with wild-type and mutated cyclophilin B (Fig. 5C, left panel). The single K6A and K35A cyclophilin B point mutants could still affinity purify three distinctive chaperone bands from rat liver ER like wild-type cyclophilin B, but the K6A/K35A mutant and PentaK-A mutants had lost the ability to bind these chaperones (Fig. 5C, left panel). Immunoblotting eluates with antibodies against the chaperones (Fig. 5C, right panel) confirmed that the K6A/K35A and PentaK-A mutants of cyclophilin B had lost their ability to bind ERp72, GRp94, and calreticulin (Fig. 5C, right panel). These results indicate that Lys6 and Lys35 are individually dispensable for binding to ERp72, GRp94, and calreticulin in extracts of total rat ER, but either Lys6 or Lys35 must be present for binding to occur.
DISCUSSION
We have identified novel interactions between ER chaperones and foldases using different methods tailored for the ER. The affinity capture and tagged bait experiments detect complexes whereas ER-MYTHS defines binary interactions. Using these methods, we have developed a high quality map that is rich in new interactions between ER lumenal proteins, and we have defined in detail one type of physical and functional interaction.
A remarkable finding was the interaction of six different PDI family members with PPIs. Interaction between PDIs and PPIs that catalyze rate-limiting steps of protein folding could allow their activities to be concentrated simultaneously on a folding substrate protein. Functional PDI-PPI interactions have been investigated in vitro (51, 52), with one study demonstrating that an interaction between bovine PDI and cyclophilin B modestly enhanced the chaperone activity of PDI (53). Although we observed no effect of cyclophilin B on the oxidoreductase activity of ERp72 in vitro, it is possible that PDIs and PPIs directly modulate the activity of one another in vivo. Such effects have been observed in plants where the activity of chloroplast cyclophilin B is modulated by thioredoxin (54).
A previous study identified cyclophilin B as a binding partner of bovine PDI by affinity purifications from bovine liver microsomes (53). The authors measured the affinity of the PDI/cyclophilin B interaction in the presence of cyclosporine A using surface plasmon resonance (Kd = 3.67 μm). This affinity is identical to the affinity we measured by isothermal titration calorimetry (Kd = 3.6 ± 0.2 μm). These similar results suggest that PDI probably binds to the same surface of cyclophilin B bound by ERp72, far removed from the cyclosporine A-binding site. Binding of PDI to the same site seems likely, because PDI contains an acidic sequence at its C terminus that resembles the N terminus of ERp72. It will be of interest to determine the minimal acidic sequence that binds to cyclophilin B and/or any other requisites for these interactions.
During the course of our studies, a specific function for cyclophilin B in the folding and assembly of IgG was described (6), and ERp72 was shown to form mixed disulfides with IgG (11). We found that ERp72 and cyclophilin B together could enhance the rate of assembly of the IgG CH1/CL heterodimer in vitro. We observed cyclophilin B catalyzed rates of CH1/CL assembly (half-time = ∼64 min) that were significantly slower than those reported (half-time = 31 ± 5 min) (6), and this could be due to subtle differences in the assay conditions used, although the approximate rate we observed for the ERp72/cyclophilin B catalyzed assembly was still faster (half-time = ∼21 min) than that reported for cyclophilin B-catalyzed assembly. Together these data suggest an in vivo cooperation between ERp72 and cyclophilin B in IgG folding. However, this suggestion must be verified by in vivo studies that will be complicated by the redundancy of PDIs in the ER.
A novel protein called pERp1 was recently identified in activated plasma cells secreting IgG (55). It was shown to play a significant role in assembly of both IgG and IgM by knockdown and overexpression studies (55, 56). Although pERp1 bears no significant homology to any other ER protein, it was suggested to be involved in disulfide bonding of IgG because it contains a CXXC motif (CDAC). However, although it was found to form mixed disulfides with IgG, it appears to be a chaperone with limited redox function, because mutations within the active site sequence did not significantly alter its enhancement of IgG maturation (55). Given the complexity of IgG assembly, involvement of more than one chaperone and PDI in its folding would not be surprising. The enhancement of IgG assembly we observed with ERp72 and cyclophilin B suggests that these two can cooperate to assemble IgG, but the circumstances (including other chaperones involved) will be different in vivo. Clearly the PDI P5 is also involved, because it is preferentially recruited to BiP substrates (57). Further work is necessary to more precisely define the chaperone complexes that mediate IgG folding, but a role for the ERp72-CypB complex is likely.
A general observation from our studies is that abundant calcium-binding chaperones can engage cyclophilin B. Although the implications of these interactions in vivo are unknown, it is clear that cyclophilin B can interact with different chaperones using the same interaction mechanism. Recent studies illustrated that the activity of cytosolic FKBP12 on various substrates was greatly enhanced when fused to the chaperone domain of SlyD (58). Such PPI-chaperone cooperation allows recruitment of the PPI to a broad range of substrates. It will be of interest to determine how cyclophilin B substrate specificity and activity is modulated by its interactions with ER chaperones. Having identified the atomic determinants of the interaction between cyclophilin B and ERp72, future dissection of the in vivo importance of this and other cyclophilin B interactions should be possible with site-directed mutagenesis studies.
Overexpression of cyclophilin B protects against thapsigargin or tunicamycin-induced cell death, attenuating calcium release from the ER (59). Interestingly, an activity dead cyclophilin B/R62A mutant exacerbated calcium release and cell death. Using GST pulldowns and a Gal4 two-hybrid system, the authors identified interactions of cyclophilin B with GRp94 and BiP, two proteins we also identified in affinity purifications with cyclophilin B. It appears that cyclophilin B serves a protective role and can regulate ER calcium stores through its PPI activity that is directed to the major calcium binding chaperones of the ER.
When addressing the ER multiprotein complex using ER-MAP, we discovered a far more interconnected ER than we anticipated, with a fuzzy distinction between the calnexin cycle and BiP chaperones. Although calreticulin, calnexin, and ERp57 clearly do not directly interact with BiP, only a single degree of separation exists between the systems, because many partners of BiP do interact with these three prominent calnexin cycle proteins. The ability of so many proteins within the ER to interact with multiple partners supports the observation that chaperones are in dynamic flux and are rarely forever captive to any single partner (18) (with the possible exception of the very stable interaction between UGGT and SEP15). The interwoven interactions between the different ER chaperone systems suggest a more versatile folding environment not bound by strict enzyme-substrate interactions but rather an open box of available chaperone/foldase tools. This finding could explain why the phenotypes of knockouts of many foldases are difficult to clearly define and why the functional overlap between for example the PDIs is so pronounced (60). However, despite the many interconnections, specific partnerships do exist and should be further defined to provide a better understanding of how these folding tools work together.
Not surprisingly, BiP binds to many partners, perhaps to accommodate the different substrates recruited to it by co-chaperones like ERdj3. Substrates bound to different specialized chaperones or foldases may associate with BiP to form either anterograde-competent or ERAD-competent complexes. For example, although the ERdj3-BiP and DnaJc3-BiP complexes participate in the folding of proteins, the opposite is true for ERdj5-BiP that reduces terminally misfolded proteins and passes them to BiP for retrotranslocation (12). BiP partners may constantly compete to bind BiP, adjusting its partner preferences to accommodate different folding needs in the ER. We found that cyclophilin B bait can readily affinity purify BiP, whereas levels of cyclophilin B purified by BiP bait were below our identity threshold, indicating that more abundant binding partners of BiP such as GRp170, DnaJC3, and P5 were probably occupying its cyclophilin B-binding site. The many partners of BiP might also reflect its involvement in many roles including gating the translocon, regulating the unfolded protein response, targeting proteins for degradation, and stabilizing folding intermediates (9).
Our search for interacting partners of the EDEM proteins, known to contribute significantly to glycoprotein ERAD (61), revealed interactions with several PDIs. This finding is in agreement with studies in yeast that identified an intermolecular disulfide bond between the C-terminal domain of the EDEM homolog Htm1 and PDI (62). This interaction was further shown to be critical for the ERAD enhancing activity of Htm1 (62). It is unclear whether similar intermolecular disulfide linkages occur between EDEM1, 2, and 3 and the different PDI partners we identified, although one such interaction has been reported between ERp46 and EDEM3 (57). Overexpression of each of the EDEMs inhibits the formation of higher order disulfide-linked structures and more rapid ERAD (63), and this suggests close cooperation between EDEMs and PDIs.
Although we confirmed 22 known interactions, we did not detect 6 published interactions, presumably because of the different techniques employed in each study. For example, use of the endogenous low expression level IRE1 promoter potentially increases the number of false negatives, despite reducing false positives that result from artifacts of overexpression. Similarly, setting a high stringency for mass spectrometry also increases the number of false negatives while reducing false positives. Still, our method could identify the majority (79%) of known interactions. One caveat of the affinity purification method is that it does not detect very tight affinity interactions that do not readily exchange with exogenously supplied bait. Another limitation is that in the affinity method used at neutral pH, disulfide-linked complexes could dissociate unless exceptionally stable, and this may have been the case for the PRDX4-EDEM and PRDX4-ERp46 interactions that are sensitive to reducing conditions (57). We also found that using Western blotting was critical to detect some interactions not detected by mass spectrometry, (ERp72-SEP15 and ERdj5-EDEM1, 2, and 3), although in some cases mass spectrometry proved more sensitive than Western blotting (CypB-PDI and ERp57-PDIr), most likely because of the quality of antibodies used, highlighting the importance of using more than one detection method for increased sensitivity. The overlap between the ER-MYTHS and the affinity purification techniques, although very limited, was primarily due to differences in amenability of specific baits to each technique (supplemental Table SIV and Fig. S6). Indeed, the different results obtained from our own ER-MYTHS and affinity purification methods underline the benefit of using different methods to get a more complete survey of interactions as has been observed with other two-hybrid and affinity approaches. Using these combined methods to study chaperones and foldases, we have detected 53 novel ER protein-protein interactions, uncovering a pattern of protein partnerships in the ER and a unique molecular mechanism of protein-protein interaction.
The new interactions identified provide the basis for further structure/function analysis of ER complexes. Significance of the interactions will only be understood when they have been further characterized in vivo. Targeting of cyclophilin B to abundant calcium-binding foldases and chaperones provides an example of how these novel ER protein-protein interactions provide new avenues for functional characterization of the ER protein folding machinery.
Supplementary Material
Acknowledgments
We thank Elaine Tan for clones of ERp19 and ERp29, Ali Fazel (McGill University) for kindly donating rat liver LDMs, Isabelle Landrieu (Université de Lille, Lille, France) for cyclophilin B chemical shift assignments and solution structure coordinates used in Fig. 5 (A and B), Johannes Buchner and Matthias Feige (University of Munich, Munich, Germany) for purified CH1 and CL fragments of IgG, and Linda Hendershot, Michael Green, Chris Nicchita, and Daniel Tessier for antibodies.
Footnotes
* This work was supported by grants from the Canadian Institutes of Health Research (to D. Y. T. and K. G.) and a Canadian Institutes of Health Research Canada Graduate Scholarships Doctoral Award and Fonds de la Recherche en Santé du Quebec Bourse de Doctorate (to P. M.).
 This article contains supplemental material. Mass spectrometry raw data have been deposited at PeptideAtlas (ISB, Seattle, USA) and are available at ftp://PASS00063:ZD4933z@ftp.peptideatlas.org/ and ftp://PASS00064:DW4775yo@ftp.peptideatlas.org/.
This article contains supplemental material. Mass spectrometry raw data have been deposited at PeptideAtlas (ISB, Seattle, USA) and are available at ftp://PASS00063:ZD4933z@ftp.peptideatlas.org/ and ftp://PASS00064:DW4775yo@ftp.peptideatlas.org/.
1 The abbreviations used are:
- ER
- endoplasmic reticulum
- ERAD
- ER-associated degradation
- PDI
- protein-disulfide isomerase
- PPI
- peptidyl-prolyl cis-trans-isomerase
- NMR
- nuclear magnetic resonance
- IgG
- immunoglobulin G
- ER-MYTHS
- ER membrane yeast two-hybrid system
- ER-MAP
- ER membrane yeast two-hybrid system and affinity purification.
REFERENCES
- 1. Wiseman R. L., Powers E. T., Buxbaum J. N., Kelly J. W., Balch W. E. (2007) An adaptable standard for protein export from the endoplasmic reticulum. Cell 131, 809–821 [DOI] [PubMed] [Google Scholar]
- 2. Määttänen P., Gehring K., Bergeron J. J., Thomas D. Y. (2010) Protein quality control in the ER: The recognition of misfolded proteins. Semin. Cell Dev. Biol. 21, 500–511 [DOI] [PubMed] [Google Scholar]
- 3. Hebert D. N., Molinari M. (2007) In and out of the ER: Protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 87, 1377–1408 [DOI] [PubMed] [Google Scholar]
- 4. Nagradova N. (2007) Enzymes catalyzing protein folding and their cellular functions. Curr. Protein Pept. Sci. 8, 273–282 [DOI] [PubMed] [Google Scholar]
- 5. Hatahet F., Ruddock L. W. (2009) Protein disulfide isomerase: A critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal. 11, 2807–2850 [DOI] [PubMed] [Google Scholar]
- 6. Feige M. J., Groscurth S., Marcinowski M., Shimizu Y., Kessler H., Hendershot L. M., Buchner J. (2009) An unfolded CH1 domain controls the assembly and secretion of IgG antibodies. Mol. Cell 34, 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernasconi R., Soldà T., Galli C., Pertel T., Luban J., Molinari M. (2010) Cyclosporine A-sensitive, cyclophilin B-dependent endoplasmic reticulum-associated degradation. PLoS One 5, e13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimizu Y., Hendershot L. M. (2007) Organization of the functions and components of the endoplasmic reticulum. Adv. Exp. Med. Biol. 594, 37–46 [DOI] [PubMed] [Google Scholar]
- 9. Braakman I., Bulleid N. J. (2011) Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 80, 71–99 [DOI] [PubMed] [Google Scholar]
- 10. Zapun A., Darby N. J., Tessier D. C., Michalak M., Bergeron J. J., Thomas D. Y. (1998) Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J. Biol. Chem. 273, 6009–6012 [DOI] [PubMed] [Google Scholar]
- 11. Jessop C. E., Tavender T. J., Watkins R. H., Chambers J. E., Bulleid N. J. (2009) Substrate specificity of the oxidoreductase ERp57 is determined primarily by its interaction with calnexin and calreticulin. J. Biol. Chem. 284, 2194–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ushioda R., Hoseki J., Araki K., Jansen G., Thomas D. Y., Nagata K. (2008) ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science 321, 569–572 [DOI] [PubMed] [Google Scholar]
- 13. Hagiwara M., Maegawa K., Suzuki M., Ushioda R., Araki K., Matsumoto Y., Hoseki J., Nagata K., Inaba K. (2011) Structural basis of an ERAD pathway mediated by the ER-resident protein disulfide reductase ERdj5. Mol. Cell 41, 432–444 [DOI] [PubMed] [Google Scholar]
- 14. Jin Y., Awad W., Petrova K., Hendershot L. M. (2008) Regulated release of ERdj3 from unfolded proteins by BiP. EMBO J. 27, 2873–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen Y., Hendershot L. M. (2005) ERdj3, a stress-inducible endoplasmic reticulum DnaJ homologue, serves as a cofactor for BiP's interactions with unfolded substrates. Mol. Biol. Cell 16, 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Molinari M. (2007) N-Glycan structure dictates extension of protein folding or onset of disposal. Nat. Chem. Biol. 3, 313–320 [DOI] [PubMed] [Google Scholar]
- 17. Vembar S. S., Brodsky J. L. (2008) One step at a time: Endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 9, 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snapp E. L., Sharma A., Lippincott-Schwartz J., Hegde R. S. (2006) Monitoring chaperone engagement of substrates in the endoplasmic reticulum of live cells. Proc. Natl. Acad. Sci. U.S.A. 103, 6536–6541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salwinski L., Licata L., Winter A., Thorneycroft D., Khadake J., Ceol A., Aryamontri A. C., Oughtred R., Livstone M., Boucher L., Botstein D., Dolinski K., Berardini T., Huala E., Tyers M., Eisenberg D., Cesareni G., Hermjakob H. (2009) Recurated protein interaction datasets. Nat. Methods 6, 860–861 [DOI] [PubMed] [Google Scholar]
- 20. Stark C., Breitkreutz B. J., Chatr-Aryamontri A., Boucher L., Oughtred R., Livstone M. S., Nixon J., Van Auken K., Wang X., Shi X., Reguly T., Rust J. M., Winter A., Dolinski K., Tyers M. (2011) The BioGRID Interaction Database: 2011 update. Nucleic Acids Res. 39, D698–D704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corbett E. F., Oikawa K., Francois P., Tessier D. C., Kay C., Bergeron J. J., Thomas D. Y., Krause K. H., Michalak M. (1999) Ca2+ regulation of interactions between endoplasmic reticulum chaperones. J. Biol. Chem. 274, 6203–6211 [DOI] [PubMed] [Google Scholar]
- 22. Zhang X., Wang Y., Li H., Zhang W., Wu D., Mi H. (2004) The mouse FKBP23 binds to BiP in ER and the binding of C-terminal domain is interrelated with Ca2+ concentration. FEBS Lett. 559, 57–60 [DOI] [PubMed] [Google Scholar]
- 23. Chen C., Ma H., Wang Y., Mi H. (2007) Binding of FKBP23 to BiP in ER shown by gel filtration chromatography. Z. Naturforsch C 62, 133–137 [DOI] [PubMed] [Google Scholar]
- 24. Liu C. Y., Xu Z., Kaufman R. J. (2003) Structure and intermolecular interactions of the luminal dimerization domain of human IRE1α. J. Biol. Chem. 278, 17680–17687 [DOI] [PubMed] [Google Scholar]
- 25. Otsu M., Bertoli G., Fagioli C., Guerini-Rocco E., Nerini-Molteni S., Ruffato E., Sitia R. (2006) Dynamic retention of Ero1α and Ero1β in the endoplasmic reticulum by interactions with PDI and ERp44. Antioxid. Redox Signal. 8, 274–282 [DOI] [PubMed] [Google Scholar]
- 26. Tavender T. J., Sheppard A. M., Bulleid N. J. (2008) Peroxiredoxin IV is an endoplasmic reticulum-localized enzyme forming oligomeric complexes in human cells. Biochem. J. 411, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pollock S., Kozlov G., Pelletier M. F., Trempe J. F., Jansen G., Sitnikov D., Bergeron J. J., Gehring K., Ekiel I., Thomas D. Y. (2004) Specific interaction of ERp57 and calnexin determined by NMR spectroscopy and an ER two-hybrid system. EMBO J. 23, 1020–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nyfeler B., Hauri H. P. (2007) Visualization of protein interactions inside the secretory pathway. Biochem. Soc. Trans. 35, 970–973 [DOI] [PubMed] [Google Scholar]
- 29. Kozlov G., Bastos-Aristizabal S., Määttänen P., Rosenauer A., Zheng F., Killikelly A., Trempe J. F., Thomas D. Y., Gehring K. (2010) Structural basis of cyclophilin B binding by the calnexin/calreticulin P-domain. J. Biol. Chem. 285, 35551–35557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nigam S. K., Goldberg A. L., Ho S., Rohde M. F., Bush K. T., Sherman M. (1994) A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca2+-binding proteins and members of the thioredoxin superfamily. J. Biol. Chem. 269, 1744–1749 [PubMed] [Google Scholar]
- 31. Meunier L., Usherwood Y. K., Chung K. T., Hendershot L. M. (2002) A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol. Biol. Cell 13, 4456–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang J., Herscovitz H. (2003) Nascent lipidated apolipoprotein B is transported to the Golgi as an incompletely folded intermediate as probed by its association with network of endoplasmic reticulum molecular chaperones, GRP94, ERp72, BiP, calreticulin, and cyclophilin B. J. Biol. Chem. 278, 7459–7468 [DOI] [PubMed] [Google Scholar]
- 33. Vandenbroeck K., Martens E., Alloza I. (2006) Multi-chaperone complexes regulate the folding of interferon-γ in the endoplasmic reticulum. Cytokine 33, 264–273 [DOI] [PubMed] [Google Scholar]
- 34. Ferguson A. D., Labunskyy V. M., Fomenko D. E., Araç D., Chelliah Y., Amezcua C. A., Rizo J., Gladyshev V. N., Deisenhofer J. (2006) NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of a new thioredoxin-like family. J. Biol. Chem. 281, 3536–3543 [DOI] [PubMed] [Google Scholar]
- 35. Chung K. T., Shen Y., Hendershot L. M. (2002) BAP, a mammalian BiP-associated protein, is a nucleotide exchange factor that regulates the ATPase activity of BiP. J. Biol. Chem. 277, 47557–47563 [DOI] [PubMed] [Google Scholar]
- 36. Knarr G., Modrow S., Todd A., Gething M. J., Buchner J. (1999) BiP-binding sequences in HIV gp160. Implications for the binding specificity of bip. J. Biol. Chem. 274, 29850–29857 [DOI] [PubMed] [Google Scholar]
- 37. Noiva R. (1994) Enzymatic catalysis of disulfide formation. Protein Expr. Purif. 5, 1–13 [DOI] [PubMed] [Google Scholar]
- 38. Kozlov G., Määttänen P., Schrag J. D., Hura G. L., Gabrielli L., Cygler M., Thomas D. Y., Gehring K. (2009) Structure of the noncatalytic domains and global fold of the protein disulfide isomerase ERp72. Structure 17, 651–659 [DOI] [PubMed] [Google Scholar]
- 39. Zheng J., Liu X., Yan X., Dai L., Ji C. (2006) Purification and structural characterization of human ERp29. Protein Pept. Lett. 13, 753–759 [DOI] [PubMed] [Google Scholar]
- 40. Jeong W., Lee D. Y., Park S., Rhee S. G. (2008) ERp16, an endoplasmic reticulum-resident thiol-disulfide oxidoreductase: biochemical properties and role in apoptosis induced by endoplasmic reticulum stress. J. Biol. Chem. 283, 25557–25566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Horibe T., Gomi M., Iguchi D., Ito H., Kitamura Y., Masuoka T., Tsujimoto I., Kimura T., Kikuchi M. (2004) Different contributions of the three CXXC motifs of human protein-disulfide isomerase-related protein to isomerase activity and oxidative refolding. J. Biol. Chem. 279, 4604–4611 [DOI] [PubMed] [Google Scholar]
- 42. Lavoie C., Lanoix J., Kan F. W., Paiement J. (1996) Cell-free assembly of rough and smooth endoplasmic reticulum. J. Cell Sci. 109, 1415–1425 [DOI] [PubMed] [Google Scholar]
- 43. Rainey-Barger E. K., Mkrtchian S., Tsai B. (2007) Dimerization of ERp29, a PDI-like protein, is essential for its diverse functions. Mol. Biol. Cell 18, 1253–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., Dephoure N., O'Shea E. K., Weissman J. S. (2003) Global analysis of protein expression in yeast. Nature 425, 737–741 [DOI] [PubMed] [Google Scholar]
- 45. Zhao R., Davey M., Hsu Y. C., Kaplanek P., Tong A., Parsons A. B., Krogan N., Cagney G., Mai D., Greenblatt J., Boone C., Emili A., Houry W. A. (2005) Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120, 715–727 [DOI] [PubMed] [Google Scholar]
- 46. Van P. N., Peter F., Söling H. D. (1989) Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J. Biol. Chem. 264, 17494–17501 [PubMed] [Google Scholar]
- 47. Van P. N., Rupp K., Lampen A., Söling H. D. (1993) CaBP2 is a rat homolog of ERp72 with proteindisulfide isomerase activity. Eur. J. Biochem. 213, 789–795 [DOI] [PubMed] [Google Scholar]
- 48. Ashby M. C., Tepikin A. V. (2001) ER calcium and the functions of intracellular organelles. Semin. Cell Dev. Biol. 12, 11–17 [DOI] [PubMed] [Google Scholar]
- 49. Hanoulle X., Melchior A., Sibille N., Parent B., Denys A., Wieruszeski J. M., Horvath D., Allain F., Lippens G., Landrieu I. (2007) Structural and functional characterization of the interaction between cyclophilin B and a heparin-derived oligosaccharide. J. Biol. Chem. 282, 34148–34158 [DOI] [PubMed] [Google Scholar]
- 50. Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 51. Schönbrunner E. R., Schmid F. X. (1992) Peptidyl-prolyl cis-trans isomerase improves the efficiency of protein disulfide isomerase as a catalyst of protein folding. Proc. Natl. Acad. Sci. U.S.A. 89, 4510–4513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rupp K., Birnbach U., Lundström J., Van P. N., Söling H. D. (1994) Effects of CaBP2, the rat analog of ERp72, and of CaBP1 on the refolding of denatured reduced proteins. Comparison with protein disulfide isomerase. J. Biol. Chem. 269, 2501–2507 [PubMed] [Google Scholar]
- 53. Horibe T., Yosho C., Okada S., Tsukamoto M., Nagai H., Hagiwara Y., Tujimoto Y., Kikuchi M. (2002) The chaperone activity of protein disulfide isomerase is affected by cyclophilin B and cyclosporin A in vitro. J. Biochem. 132, 401–407 [DOI] [PubMed] [Google Scholar]
- 54. Motohashi K., Koyama F., Nakanishi Y., Ueoka-Nakanishi H., Hisabori T. (2003) Chloroplast cyclophilin is a target protein of thioredoxin: Thiol modulation of the peptidyl-prolyl cis-trans isomerase activity. J. Biol. Chem. 278, 31848–31852 [DOI] [PubMed] [Google Scholar]
- 55. Shimizu Y., Meunier L., Hendershot L. M. (2009) pERp1 is significantly up-regulated during plasma cell differentiation and contributes to the oxidative folding of immunoglobulin. Proc. Natl. Acad. Sci. U.S.A. 106, 17013–17018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Anken E., Pena F., Hafkemeijer N., Christis C., Romijn E. P., Grauschopf U., Oorschot V. M., Pertel T., Engels S., Ora A., Lástun V., Glockshuber R., Klumperman J., Heck A. J., Luban J., Braakman I. (2009) Efficient IgM assembly and secretion require the plasma cell induced endoplasmic reticulum protein pERp1. Proc. Natl. Acad. Sci. U.S.A. 106, 17019–17024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jessop C. E., Watkins R. H., Simmons J. J., Tasab M., Bulleid N. J. (2009) Protein disulphide isomerase family members show distinct substrate specificity: P5 is targeted to BiP client proteins. J. Cell Sci. 122, 4287–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Knappe T. A., Eckert B., Schaarschmidt P., Scholz C., Schmid F. X. (2007) Insertion of a chaperone domain converts FKBP12 into a powerful catalyst of protein folding. J. Mol. Biol. 368, 1458–1468 [DOI] [PubMed] [Google Scholar]
- 59. Kim J., Choi T. G., Ding Y., Kim Y., Ha K. S., Lee K. H., Kang I., Ha J., Kaufman R. J., Lee J., Choe W., Kim S. S. (2008) Overexpressed cyclophilin B suppresses apoptosis associated with ROS and Ca2+ homeostasis after ER stress. J. Cell Sci. 121, 3636–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rutkevich L. A., Cohen-Doyle M. F., Brockmeier U., Williams D. B. (2010) Functional relationship between protein disulfide isomerase family members during the oxidative folding of human secretory proteins. Mol. Biol. Cell 21, 3093–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kanehara K., Kawaguchi S., Ng D. T. (2007) The EDEM and Yos9p families of lectin-like ERAD factors. Semin. Cell Dev. Biol. 18, 743–750 [DOI] [PubMed] [Google Scholar]
- 62. Sakoh-Nakatogawa M., Nishikawa S., Endo T. (2009) Roles of protein-disulfide isomerase-mediated disulfide bond formation of yeast Mnl1p in endoplasmic reticulum-associated degradation. J. Biol. Chem. 284, 11815–11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Olivari S., Molinari M. (2007) Glycoprotein folding and the role of EDEM1, EDEM2 and EDEM3 in degradation of folding-defective glycoproteins. FEBS Lett. 581, 3658–3664 [DOI] [PubMed] [Google Scholar]
- 64. Jansen G., Wu C., Schade B., Thomas D. Y., Whiteway M. (2005) Drag&Drop cloning in yeast. Gene 344, 43–51 [DOI] [PubMed] [Google Scholar]
- 65. Wilson C. M., Bulleid N. J. (2003) Investigation of folding and degradation of mutant proteins synthesized in semipermeabilized cells. Methods Mol. Biol. 232, 295–312 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.