Abstract
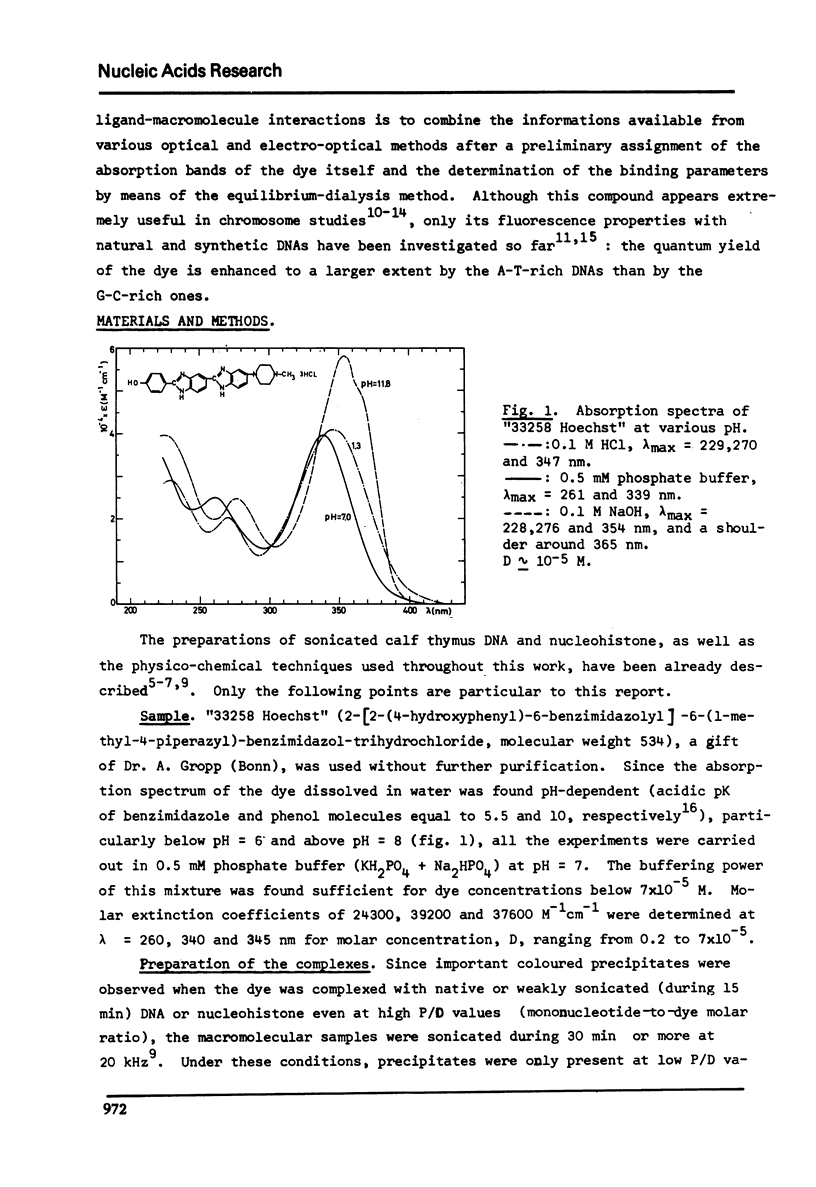
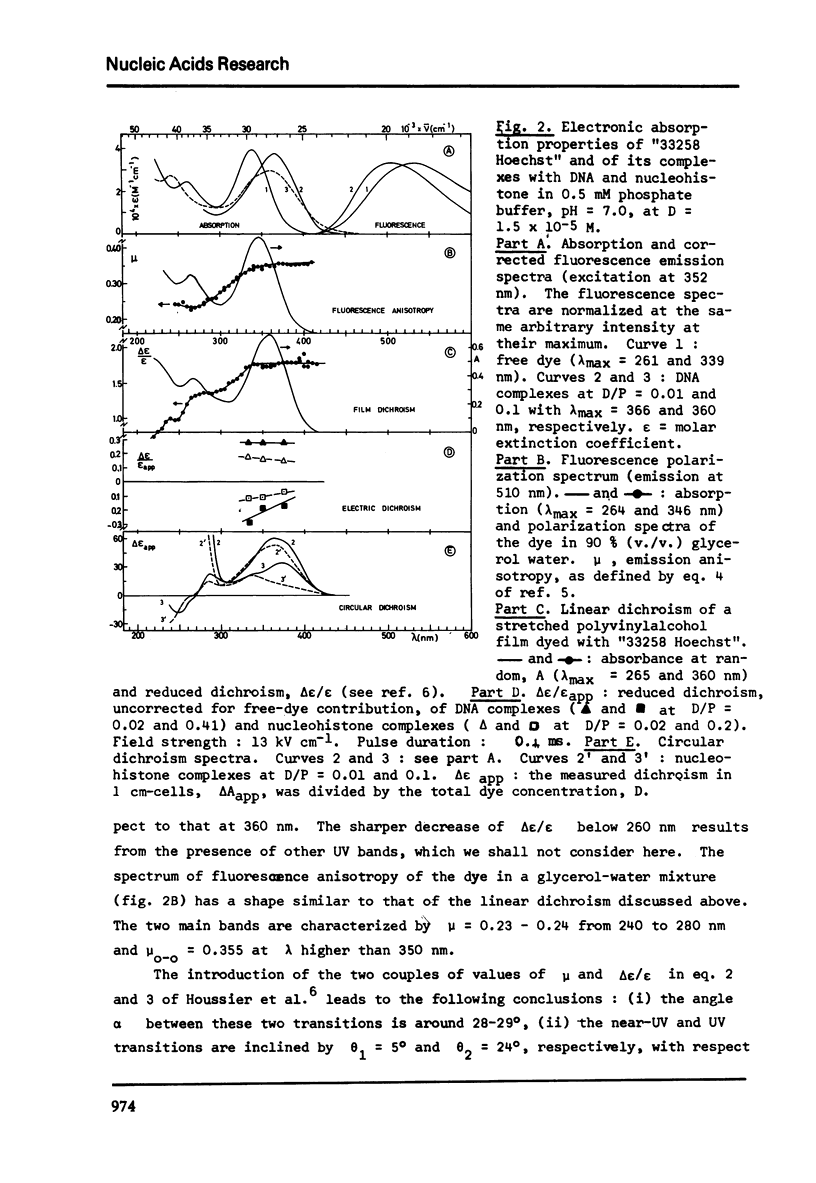
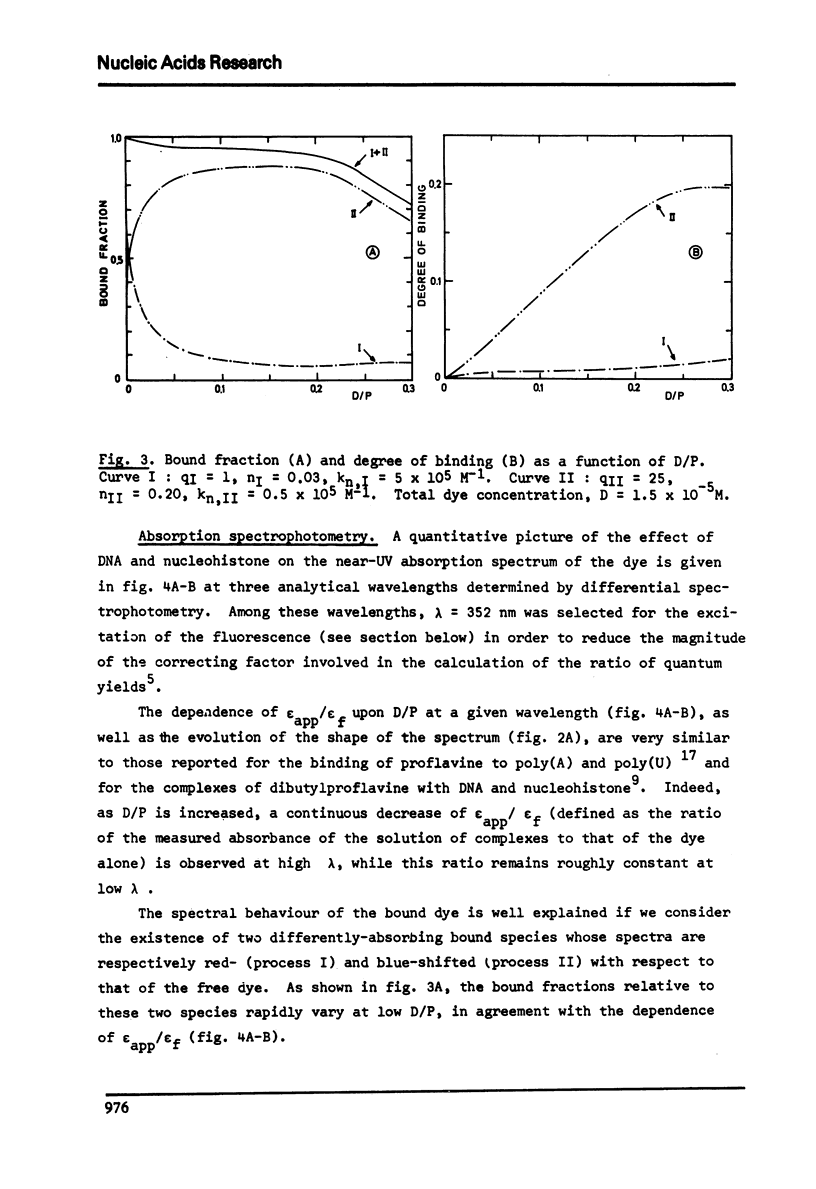
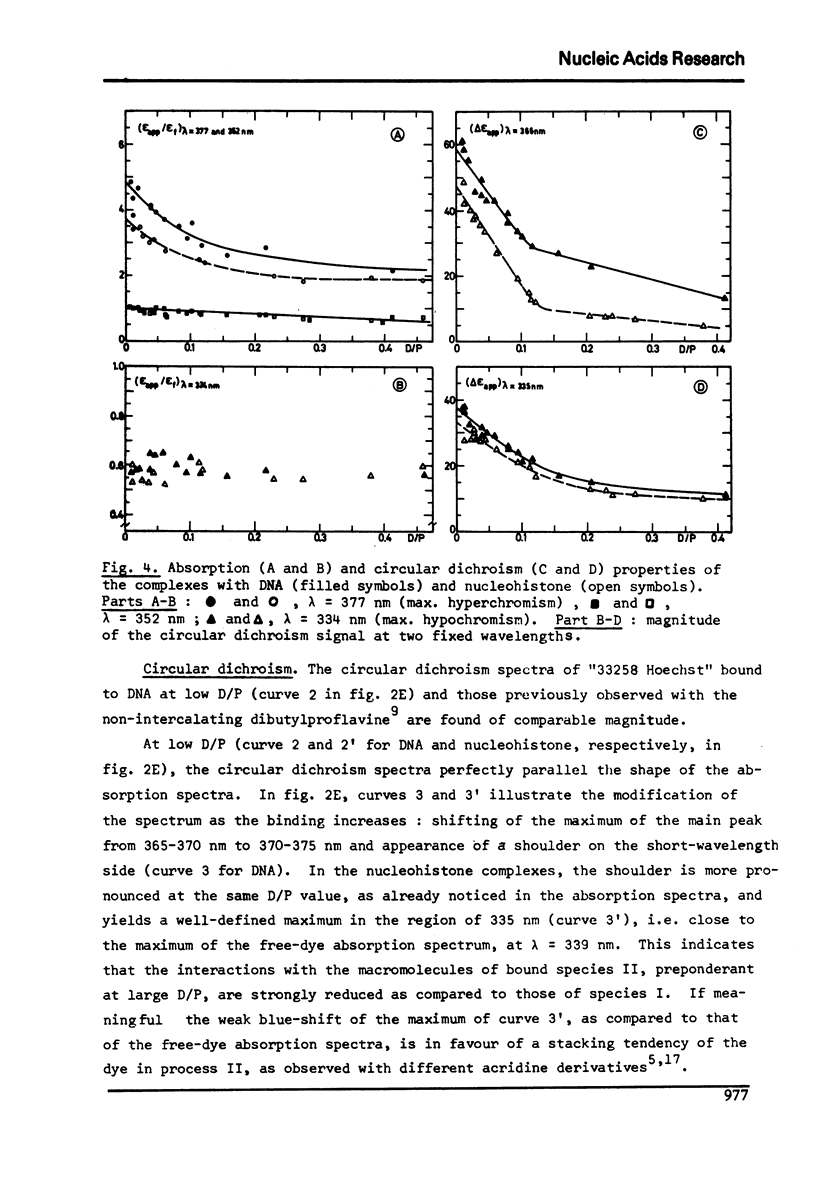
The degree of binding of "33258 Hoechst" to DNA and nucleohistone has been determined by equilibrium dialysis and the properties of the complexes have been followed by different optical and electro-optical methods, after determining the orientation of the main transition moments within the dye molecule. The binding isotherm was found composed of a Langmuir-type and of a strongly cooperative component. The existence of two bound species yielded a continuous variation of most of the properties of the complexes studied as the amount of binding increased, while the hydrodynamic properties of the macromolecules were not affected. At low binding, the strongly bound dye molecules appeared to bind to highly fluorescent sites with their long axis oriented at 45 degree to the helix axis. As the binding proceeds, less fluorescent sites are cooperatively occupied and the inclination of these ligand molecules becomes closer to that of the base planes. These results are compatible with the formation of two external complexes with the double helical structure.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Georghiou S., Moudrianakis E. N. Studies on the structure of deoxyribonucleoproteins. Spectroscopic characterization of the ethidium bromide binding sites. Biochemistry. 1974 Mar 12;13(6):1075–1082. doi: 10.1021/bi00703a003. [DOI] [PubMed] [Google Scholar]
- Bontemps J., Fredericq E. Comparative binding study of the interaction of quinacrine and ethidium bromide with DNA and nucleohistone. Biophys Chem. 1974 Jun;2(1):1–22. doi: 10.1016/0301-4622(74)80019-0. [DOI] [PubMed] [Google Scholar]
- Bontemps J., Houssier C., Fredericq E. Optical and electro-optical properties of the complexes of dibutylproflavine with DNA and nucleohistone. Biophys Chem. 1974 Dec;2(4):301–315. doi: 10.1016/0301-4622(74)80057-8. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Farber S., Foley G. E., Kudynowski J., Modest E. J., Simonsson E., Wagh U., Zech L. Chemical differentiation along metaphase chromosomes. Exp Cell Res. 1968 Jan;49(1):219–222. doi: 10.1016/0014-4827(68)90538-7. [DOI] [PubMed] [Google Scholar]
- Dalgleish D. G., Fujita H., Peacocke A. R. Circular dichroism of aminoacridines bound to DNA. Biopolymers. 1969;8(5):633–645. doi: 10.1002/bip.1969.360080506. [DOI] [PubMed] [Google Scholar]
- Dalgleish D. G., Peacocke A. R., Acheson R. M., Harvey C. W. The interaction of (+)- and (-)-9-sec-butylaminoacridines with DNA as measured by CD spectroscopy. Biopolymers. 1972;11(12):2389–2392. doi: 10.1002/bip.1972.360111202. [DOI] [PubMed] [Google Scholar]
- Distèche C., Bontemps J. Chromosome regions containing DNAs of known base composition, specifically evidenced by 2,7-di-t-butyl proflavine. Comparison with the Q-banding and relation to dye-DNA interactions. Chromosoma. 1974;47(3):263–281. doi: 10.1007/BF00328861. [DOI] [PubMed] [Google Scholar]
- Dourlent M., Hélène C. A quantitative analysis of proflavine binding to polyadenylic acid, polyuridylic acid, and transfer RNA. Eur J Biochem. 1971 Nov 11;23(1):86–95. doi: 10.1111/j.1432-1033.1971.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Festy B., Daune M. Hydroxystilbamidine. A nonintercalating drug as a probe of nucleic acid conformation. Biochemistry. 1973 Nov 20;12(24):4827–4834. doi: 10.1021/bi00748a003. [DOI] [PubMed] [Google Scholar]
- Fredericq E., Houssier C. Study of the interaction of DNA and acridine orange by various optical methods. Biopolymers. 1972;11(11):2281–2308. doi: 10.1002/bip.1972.360111108. [DOI] [PubMed] [Google Scholar]
- Glaubiger D., Kohn K. W., Charney E. The reaction of anthramycin with DNA. 3. Properties of the complex. Biochim Biophys Acta. 1974 Sep 13;361(3):303–311. doi: 10.1016/0005-2787(74)90373-6. [DOI] [PubMed] [Google Scholar]
- Hilwig I., Gropp A. Staining of constitutive heterochromatin in mammalian chromosomes with a new fluorochrome. Exp Cell Res. 1972 Nov;75(1):122–126. doi: 10.1016/0014-4827(72)90527-7. [DOI] [PubMed] [Google Scholar]
- Houssier C., Hardy B., Fredericq E. Interaction of ethidium bromide with DNA. Optical and electrooptical study. Biopolymers. 1974 Jun;13(6):1141–1160. doi: 10.1002/bip.1974.360130607. [DOI] [PubMed] [Google Scholar]
- Klásterská I., Natarajan A. T., Ramel C. Heterochromatin distribution and chiasma localization in the grasshopper Btyodema tuberculata (Fabr.) (Acrididae). Chromosoma. 1974 Jan 29;44(4):393–404. doi: 10.1007/BF00284899. [DOI] [PubMed] [Google Scholar]
- Kodama M., Tagashira Y., Nagata C. The interaction of pinacyanol with nucleic acids. Biochim Biophys Acta. 1966 Dec 21;129(3):638–640. doi: 10.1016/0005-2787(66)90083-9. [DOI] [PubMed] [Google Scholar]
- Kundu N. G., Heidelberger C. Cyclopenta(f)isoquinoline derivatives designed to bind specifically to native deoxyribonucleic acid. 3. Interaction of 6-carbamylmethyl-8-methyl-7H-cyclopenta(f)isoquinolin-3(2H)-one with deoxyribonucleic acids and polydeoxyribonucleotides. Biochem Biophys Res Commun. 1974 Sep 23;60(2):561–568. doi: 10.1016/0006-291x(74)90277-0. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3395–3399. doi: 10.1073/pnas.70.12.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. S., Latt S. A., Davidson R. L. Microfluorometric detection of asymmetry in the centromeric region of mouse chromosomes. Exp Cell Res. 1974 Jun;86(2):392–395. doi: 10.1016/0014-4827(74)90727-7. [DOI] [PubMed] [Google Scholar]
- Löber G., Schütz H., Kleinwächter V. Effect of organic solvents on the properties of the complexes of DNA with proflavine and similar compounds. Biopolymers. 1972;11(12):2439–2459. doi: 10.1002/bip.1972.360111206. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Monny C., Kovoor A. Action of quinacrine mustard on polynucleotides. Biochimie. 1972;54(9):1129–1136. doi: 10.1016/s0300-9084(72)80017-8. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M., Waring M. J. A non-intercalating proflavine derivative. Eur J Biochem. 1973 Nov 1;39(1):223–234. doi: 10.1111/j.1432-1033.1973.tb03120.x. [DOI] [PubMed] [Google Scholar]
- Nagata C., Kodama M., Tagashira Y., Imamura A. Interaction of polynuclear aromatic hydrocarbons, 4-nitroquinoline 1-oxides, and various dyes with DNA. Biopolymers. 1966 Apr-May;4(4):409–427. doi: 10.1002/bip.1966.360040403. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Raposa T., Natarajan A. T. Fluorescence banding pattern of human and mouse chromosomes with a benzimidazol derivative (Hoechst 33258). Humangenetik. 1974;21(3):221–226. doi: 10.1007/BF00279016. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Cooperative binding to linear biopolymers. 1. Fundamental static and dynamic properties. Eur J Biochem. 1970 Feb;12(3):442–453. doi: 10.1111/j.1432-1033.1970.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Selander R. K. The binding of quinacrine mustard to nucleic acids. Acta Chem Scand B. 1974;28(1):45–55. doi: 10.3891/acta.chem.scand.28b-0045. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Polacow I. Protein-DNA interactions in extended and condensed chromatin. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1078–1084. doi: 10.1016/s0006-291x(73)80005-1. [DOI] [PubMed] [Google Scholar]
- Utakoji T., Matsukuma S. Fluorescent staining of L cell centromeres and chromocenters with 1-dimethylaminonaphthalene-5-sulfonyl chloride and G-bandings. Exp Cell Res. 1974 Jul;87(1):111–119. doi: 10.1016/0014-4827(74)90531-x. [DOI] [PubMed] [Google Scholar]
- Van Holde K. E., Sahasrabuddhe C. G., Shaw B. R. A model for particulate structure in chromatin. Nucleic Acids Res. 1974 Nov;1(11):1579–1586. doi: 10.1093/nar/1.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Haenssler E. Fluorometric properties of the bibenzimidazole derivative Hoechst 33258, a fluorescent probe specific for AT concentration in chromosomal DNA. Chromosoma. 1974;46(3):255–260. doi: 10.1007/BF00284881. [DOI] [PubMed] [Google Scholar]


