Abstract
A novel line of mutant mice [monoamine oxidase A knockout (MAOAA863T KO)] harboring a spontaneous point nonsense mutation in exon 8 of the MAO A gene was serendipitously identified in a 129/SvEvTac colony. This mutation is analogous to the cause of a rare human disorder, Brunner syndrome, characterized by complete MAO A deficiency and impulsive aggressiveness. Concurrent with previous studies of MAO A KO mice generated by insertional mutagenesis (‘Tg8’), MAOAA863T KO lack MAO A enzyme activity and display enhanced aggression toward intruder mice. MAOAA863T KO, however, exhibited lower locomotor activity in a novel, inescapable open field and similar immobility during tail suspension compared with wild type, observations which differ from reports of Tg8. These findings consolidate evidence linking MAO A to aggression and highlight subtle yet distinctive phenotypical characteristics.
Keywords: Brunner syndrome, monoamine oxidase, nonsense mutation
Introduction
Monoamine oxidases (MAOs) are the major enzymes responsible for the degradation of biogenic amines and the deactivation of brain monoamine neurotransmitters. The two isoforms characterized to date, A and B, are encoded by different genes closely located on the X chromosome [1] and share 70% amino acid homology [1,2]. Despite these similarities, their substrate affinity profiles are conspicuously different: MAO A selectively metabolizes serotonin [5-hydroxytryptamine (5-HT)] and norepinephrine (NE), whereas MAO B prefers phenylethylamine [3]. Both catabolize dopamine [4]. Such a divergence is also reflected by their different function in behavioral regulation. In particular, we and other groups have provided cogent evidence that MAO A plays a key role in the modulation of impulsive and aggressive responses (for a review, see Ref. [5]).
In humans, a point nonsense mutation in exon 8 of the MAO A gene (maoa) causes Brunner syndrome, a recessive X-linked condition characterized by complete absence of MAO A activity in association with mild retardation and violent aggressiveness [6]. The low prevalence of the syndrome and the elusiveness of its nosographical description [7], however, hinder our understanding of its specific psychobiological determinants and limit our ability to characterize its specific features and distinguish it from other impulse-control disorders, such as the intermittent explosive disorder.
A useful tool to elucidate the phenotypical consequence of MAO A deficiency is afforded by MAO A knockout (KO) mice. A first line of MAO A KO mice (referred to as the ‘Tg8’ line) was generated by the inadvertent insertion of an IFN-β minigene into exon 2 of the MAO A coding region of one-cell embryos of C3H/HeJ mice [8]. Tg8 mice display several behavioral abnormalities, including increased intermale aggression [8]. Nevertheless, the translational value of this line is somewhat tempered by the different mutation site and the presence of the inserted cassette, which may influence the epigenetic regulation of MAO A-dependent behaviors.
Here, we describe the discovery and characterization of a novel, spontaneous point mutation in the eighth exon of the maoa gene in 129/SvEvTac mice. This murine line (MAOAA863T KO) shows enhanced aggression toward intruders, in addition to unique behavioral characteristics, such as a significant reduction in open field locomotor activity.
Materials and methods
Animals
Mice were housed in cages with free access to food and water on a 12-h light cycle in accordance with the protocol approved by the University of Southern California Institutional Animal Care and Use Committee. As our wild type (WT) and MAOAA863T KO are a congenic line on an essentially homogeneous genetic background, we bred WT mice by WT–WT pairs and MAOAA863T KO mice by KO–KO pairs.
Genotyping and sequencing
Mice were genotyped by polymerase chain reaction (PCR) using genomic DNA from tail samples as template and Taq polymerase (Invitrogen, Carlsbad, California, USA). Each sample was genotyped for MAO A using the following primers: MAO A forward: 5′-ACGCGCTCTTCTGGTGCAT-3′, MAO A reverse: 5′-AGCTTACTTCAGGGC-3′. MAO A PCR products were then processed with Dra1 (New England Biolabs, Ipswich, Massachusetts, USA), as the loss of this cutting site indicates the A→T point mutation. Undigested PCR products were cloned into a pCR4-topo cloning vector (Invitrogen) and sequencing results analyzed with Sequence Scanner v1.0. Each sample was genotyped for MAO B using the following primers: MAO B forward: 5′-CTACAAAGCAGATTGCCACGC-3′; MAO B reverse: 5′-TACCTGACATCAACTGGTCCC-3′.
MAO A and B catalytic activity assays
One-month-old male WT 129/SvEvTac (n=3) and MAOAA863T KO (n=3) mice were killed by cervical dislocation. Liver and brain regions (frontal cortex, hippocampus, cerebellum, and brain stem) were removed and homogenized in assay buffer (50 mM sodium phosphate buffer, pH 7.4). To control for the high level of MAO B in the liver, liver samples were preincubated in 10–6 M deprenyl (selective MAO B inhibitor) for 20 min at 37°C before measurement of MAO A catalytic activity. Catalytic activity for MAO A and B was measured as described previously [9].
Behavior tests
All behavioral experiments were conducted on male MAOAA863T KO mice (n=6) and age-matched male WT 129/SvEvTac mice (n=6), the MAOAA863T KO parental line. Experiments were performed between 13 : 00 and 15 : 00 h. All mice were at least 3 months of age and isolated for a minimum of 10 days before the initiation of behavioral experiments.
Open field
Locomotor activity was measured in a square arena 40 × 40 cm under low lighting for 5 min. The arena was divided into two virtual zones of equal area, one central and one peripheral. Data were collected by video camera and scored by computer interface (Ethovision, Noldus Inc., Wageningen, the Netherlands). The total distances traveled in the whole arena, center, and periphery (cm), the time spent in the center and periphery (s), the angular (cm/s) and vector (cm/s2) velocities in the whole arena, center, and periphery, as well as the number of entries into the center zone, were recorded.
Tail suspension
Mice were suspended vertically by the tail using medical tape for a total of 6 min without prior habituation to the test room. The duration of immobility (s) and duration of trunk motion (s) were recorded. Trunk motion was defined as whole-body upward movement toward the base of the tail, as opposed to paw movement or twitching. Data were scored using Behavior Tracker software (Greensburg, Pennsylvania, USA).
Resident intruder test
Each mouse was tested in its home cage after a 30-min habituation period in the testing room against an age-matched and weight-matched WT 129/SvEvTac male for a total of 5 min. A fighting bout was defined as a continuous series of behavioral interactions including offensive attack, wrestling, and biting. If 2 s or more elapsed between bouts of fighting, these were considered separate events. For each resident, the number of fighting bouts and latency to first offensive attack (s) were recorded. Resident male tail rattling was also recorded. If 1 s or more elapsed between tail rattles, these were considered separate events. Data were scored using Behavior Tracker.
Statistical procedures
All results are expressed as mean±SEM. Statistical differences between the two genotypes’ mean enzyme activity levels, mean duration of immobility and trunk motion during the tail suspension test, and mean latency to first attack and mean numbers of tail rattles and fighting bouts during the resident intruder test were performed using two-tailed, unpaired t-tests. Open field data were analyzed by one-way or two-way analysis of variance (ANOVA) as indicated. Statistical significance was accepted at the probability level of P<0.05.
Results
Identification and biochemical characterization of a line of mice harboring a point mutation in maoa
A line of mice with a spontaneous point mutation in maoa was discovered through genotyping in a colony of 129/SvEvTac mice. The unexpected PCR products in these mice revealed the abrogation of a Dra1 restriction enzyme cutting site in the MAO A PCR product, indicating a single point mutation in exon 8 (Fig. 1a) [10,11]; MAO B PCR products for the MAOAA863T KO mice are identical to WT (Fig. 1b).
Fig. 1.
Genotyping of MAOAA863T KO mice. MAOAA863T KO mice were first identified in a mixed colony of MAO B KO [10] and MAO AB KO [11] mice. Genotyping revealed that MAOAA863T KO mice are WT for maob but contain a single point mutation in maoa that eliminates a Dra1 restriction enzyme cutting site. (a) MAO A genotyping using primers specific for maoa exon 8 reveals identical bands for all three genotypes (top). The point mutation is identified following Dra1 digest (bottom). Lane 1, MAOAA863T KO male; lane 2, WT male; lane 3, heterozygous (+/–) MAOAA863T KO female. (b) MAOAA863T KO mice are WT for maob, as revealed by the lack of the ~1.2 kb insert in exon 6 which abrogates this isoenzyme [10]. Lane 1, MAOAA863T KO male; lane 2, MAO B KO male; lane 3, MAO B KO heterozygous (+/–) female.
Subsequent cloning and sequencing of the MAO A and B genotyping PCR products from the MAOAA863T KO line confirmed these results and revealed that the site of this point mutation is very closely located to the site of the point mutation in exon 8 of maoa harbored by humans with Brunner syndrome (Fig. 2). MAO A enzymatic activity is completely abrogated in the mutant MAOAA863T KO mice, whereas MAO B activity is similar to WT (Table 1).
Fig. 2.
Sites of spontaneous mutation in mouse and human MAO A, exon 8. Arrows indicate sites of mutation resulting in premature stop codons in mouse (top) corresponding to amino acid 284 (lysine) and in human (bottom) corresponding to amino acid 296 (glutamine). Nucleic acid numbering is according to mouse MAO A GenBank entry NM_173740 and human gene bank entry M68840.
Table 1.
Mean (±SEM) catalytic activity of MAO A and MAO B (nmol/20min/mg protein) in brain and liver samples from one-month-old male pups
| WT | MAOAA863TKO | |
|---|---|---|
| MAO A | ||
| Frontal cortex | 13.67 ± 0.24 | 0.00 |
| Hippocampus | 12.74 ± 0.40 | 0.00 |
| Cerebellum | 17755 ± 0.21 | 0.00 |
| Brainstem | 22.36 ± 0.36 | 0.00 |
| Liver | 7.23 ± 0.37 | 2.13 ± 0.13 |
| Liver + deprenyla | 1.62 ± 0.37 | 0 00 |
| MAO B | ||
| Frontal cortex | 15.74 ± 0.57 | 15.59 ± 0.68 |
| Hippocampus | 13.18 ± 1.03 | 11.42 ± 0 30 |
| Cerebellum | 10.73 ± 0.61 | 10.56 ± 0.43 |
| Brainstem | 15.89 ± 1.13 | 12.37 ± 0.27 |
| Liver | 153.00 ± 13.32 | 148.34 ± 2.06 |
Refer to Materials and methods.
Physical and behavioral characterization
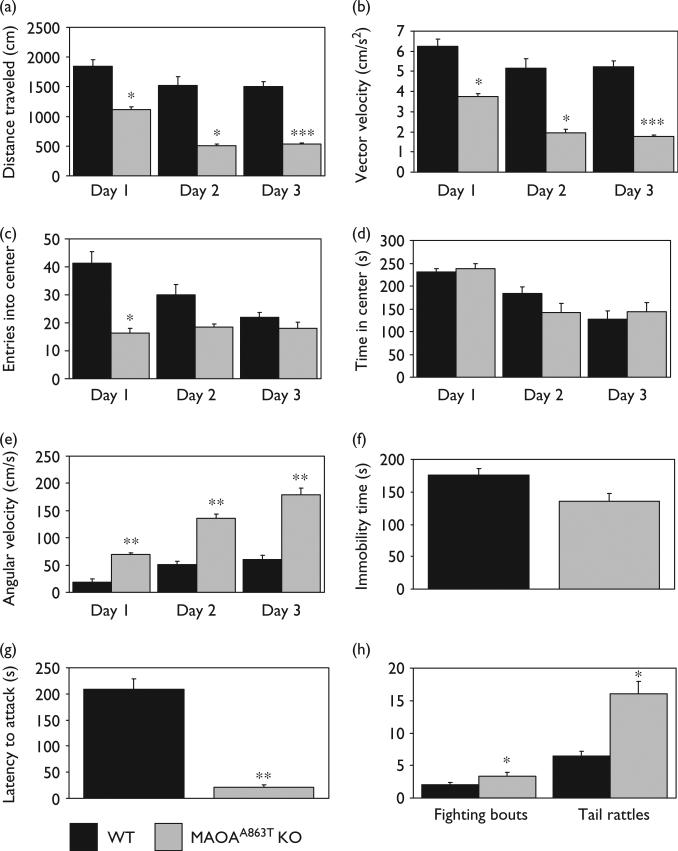
Postnatal MAOAA863T KO pups tend to be smaller in body weight than their WT littermates, but do not display any gross physical deformity or ambulatory impairment. Behavioral studies of the MAOAA863T KO line revealed a decrease in locomotor activity as compared with WT in a novel, inescapable open field (Fig. 3). Over 3 consecutive test days, MAOAA863T KO mice traveled less throughout the entire arena (two-way ANOVA, F1,10=14.43, P=0.003) and averaged lower vector velocity (two-way ANOVA, F1,10=13.77, P=0.004). Although MAOAA863T KO mice entered the center zone fewer times than WT on the first day (one-way ANOVA, F1,10=5.56, P=0.038) there was no difference in the time spent in the central zone between the two genotypes on either day 1 or over the 3-day test period (day 1, one-way ANOVA, F1,10=0.05, P=0.8261; all 3 days, two-way ANOVA, F1,10=0.01, P=0.916). MAOAA863T KO also showed higher angular velocity throughout the whole arena on all days (two-way ANOVA, F1,10=25.92, P=0.0007). Complete immobility time did not differ between the two genotypes during the tail suspension test (P=0.25), although MAOAA863T KO mice did spend significantly more time engaged in trunk motion than WT (P=0.007, data not shown). The resident intruder test revealed significantly higher aggression in the MAOAA863T KO mice, as indicated by a reduced latency to first attack from 275.5 s in WT to 30.5 s in MAOAA863T KO (P<0.01). In addition, the number of fighting bouts (P<0.05) and resident mouse tail rattles (P<0.05) were significantly increased in MAOAA863T KO.
Fig. 3.
Behavioral alterations in MAOAA863T KO mice. MAOAA863T KO mice showed decreased locomotor activity in a novel, inescapable open field, indicated by reduced distance traveled in the entire arena (a) and average vector velocity (cm/s2) (b) during the first 5 min in the field. Although MAOAA863T KO entered the center zone fewer times on day1 (c), they did not spend significantly less time in the center on any test day (d). MAOAA863T KO also demonstrated consistently higher angular velocity (cm/s) (e).MAOAA863T KO mice did not spend significantly less time immobile during 6 min.Tail suspension test (f). MAOAA863T KO showed increased aggression in the resident intruder paradigm, as measured by their reduced latency to first attack (s) (g), and increased number of tail rattles and bouts of fighting (h). *= P<0.05, **= P<0.01, ***= P<0.001.
Discussion
This study is the first report of a novel line of MAO A KO 129/SvEvTac mice, harboring a nonsense point mutation (adenine to thymine) in exon 8, position 863 of the MAO A gene. This mutation changes a lysine to a premature stop codon in exon 8, presumably leading to nonsense-mediated mRNA decay [12]. A crystal structure of the human MAO A protein has recently become available, revealing key differences in its quaternary structure and active site as compared with the previously elucidated human MAO B and rat MAO A structures [13]. These data may add to our understanding of these isoenzymes’ substrate and inhibitor affinities [3].
Different mutations in the same gene have been shown to lead to KO lines with distinct phenotypes [14,15]. Thus, the availability of different mutant lines for the same gene offers an interesting approach to elucidate the mechanisms of functional regulation of the targeted genes and proteins. We previously generated a different frameshift mutation of MAO A gene by accidental insertion of interferon b transgene in exons 2 and 3 in C3H mice (Tg8) [8]. These mice display marked levels of aggressiveness in the resident–intruder paradigm, and a number of other alterations in exploratory behavior [8,16].
Similarly to Tg8 mice, MAOAA863T KO resident mice exhibited a significant reduction in latency to attack as well as increased fighting behavior and tail rattling when exposed to WT intruders in the resident–intruder paradigm. This finding further confirms that MAO A deletion results in aggressiveness and impulsivity, behaviors likely mediated by increased 5-HT and NE. Indeed, genetic reduction of NE function reduces aggression in the resident–intruder paradigm [17], whereas altered 5-HT metabolism has been associated with aggression and poor impulse control in both humans and primates [6,18]. Although brain monoamine levels were not quantified for this study, we hypothesize that MAOAA863T KO have elevated 5-HT and NE.
In contrast to Tg8 mice [19], however, MAOAA863T KO displayed a markedly reduced locomotion in the open field. As MAOAA863T KO mice do not exhibit any apparent impairment in gait, this finding suggests that the reduction of locomotor activity may be a unique phenotype of this line. This difference may reflect a divergent environmental reactivity. Indeed, MAOAA863T KO failed to display significant changes in absolute immobility time in the tail suspension test, a dependable parameter for the measurement of depressive-like behaviors [20,21]. This evidence is at variance with previous findings on Tg8 mice, indicating reduced immobility duration in another validated model of depression, the forced swim test [8]. A possible interpretation for this apparent discrepancy is that Tg8 and MAOAA863T KO mice may display a different sensitivity to environmental stress. Different intrinsic variations between the two mutations or the two different background strains (129/SvEvTac and C3H/HeJ) may account for these divergent phenotypes.
Interestingly, a mutation identical to that harbored in the MAOAA863T KO mice was discovered in a colony of MAO B KO mice [10] (also from a 129/SvEvTac parental strain), producing MAO A/B double KO (MAO AB KO) mice which are deficient in both isoenzymes [11]. MAO AB KO mice display a phenotype that is distinctive from either Tg8 or MAO B KO mice, characterized by increased anxiety-like behavior [11].
It is worth noting that the nonsense point mutation in MAOAA863T KO mice is similar to the human mutation reported in Brunner syndrome. This resemblance highlights MAOAA863T KO mice as a highly reliable model to study the neurochemical and neurophysiological alterations associated to Brunner syndrome and related disorders.
Conclusion
MAOAA863T KO mice are the first murine line with a naturally occurring nonsense mutation of MAO A gene. The similarity of this mutation to the human condition makes this line of mice a valuable tool for translational research, and further exploration of the role of this enzyme on brain function and behavioral regulation.
Acknowledgements
The authors thank Sean Godar, Bin Qian, and Jack Liu for technical assistance. This study was supported by National Institute of Mental Health Grants R37 MH39085 (MERIT Award), R01 MH67968, the Pacific Rim Transdisciplinary Tobacco and Alcohol Use Research Center: Genes, Environment, and Tobacco and Alcohol Use Across Cultures Grant no. P50 CA84735, and the Boyd and Elsie Welin Professorship (to J.C.S.). The authors claim no conflicts of interest.
References
- 1.Lan NC, Heinzmann C, Gal A, Klisak I, Orth U, Lai E, et al. Human monoamine oxidase A and B genes map to Xp11.23 and are deleted in a patient with Norrie disease. Genomics. 1989;4:552–559. doi: 10.1016/0888-7543(89)90279-6. [DOI] [PubMed] [Google Scholar]
- 2.Bach AW, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan SW, et al. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci U S A. 1988;85:4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geha RM, Rebrin I, Chen K, Shih JC. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276:9877–9882. doi: 10.1074/jbc.M006972200. [DOI] [PubMed] [Google Scholar]
- 4.O'Carrol AM, Fowler CJ, Phillips JP, Tobbia I, Tipton KF. The deamination of dopamine by human brain monoamine oxidase: specificity for the two enzyme forms in seven brain regions. Naunyn-Schmiedeberg's Arch Pharmacol. 1983;332:198–202. doi: 10.1007/BF00500765. [DOI] [PubMed] [Google Scholar]
- 5.Shih JC, Thompson RF. Monoamine oxidase in neuropsychiatry and behavior. Am J Hum Genet. 1999;65:593–598. doi: 10.1086/302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- 7.Hebebrand J, Klug B. Specification of the phenotype required for men with monoamine oxidase type A deficiency. Hum Genet. 1995;96:372–376. doi: 10.1007/BF00210430. [DOI] [PubMed] [Google Scholar]
- 8.Cases A, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu HF, Chen K, Shih JC. Site-directed mutagenesis of monoamine oxidase A and B: role of cysteines. Mol Pharmacol. 1993;43:888–893. [PubMed] [Google Scholar]
- 10.Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams J, et al. Increased stress response and β-phenylethylamine in MAOB-deficient mice. Nat Genet. 1997;17:206–210. doi: 10.1038/ng1097-206. [DOI] [PubMed] [Google Scholar]
- 11.Chen K, Holschneider DP, Wu W, Rebrin I, Shih JC. A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. J Biochem. 2004;279:39645–39652. doi: 10.1074/jbc.M405550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He F, Peltz SW, Donahue JL, Rosbash M, Jacobson A. Stabilization and ribosome association of unspliced pre-mRNAs in a yeast upfl-mutant. Proc Natl Acad Sci U S A. 1993;90:7034–7038. doi: 10.1073/pnas.90.15.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Colibus L, Li M, Binda C, Lustig A, Edmondson DE, Mattevi A. Three-dimensional structure of human monoamine oxidase A (MAO A): relation to the structures of rat MAO A and human MAO B. Proc Natl Acad Sci U S A. 2005;102:12684–12689. doi: 10.1073/pnas.0505975102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming SM, Fernagut P-O, Chesselet M-F. Genetic mouse models of parkinsonism: strengths and limitations. J Am Soc Exp Neurother. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simoni M. Mutations of the G protein-coupled receptors of the hypothalamo-pituitary-gonadal axis. Where do we stand? Eur J Endocrinol. 1998;139:145–147. doi: 10.1530/eje.0.1390145. [DOI] [PubMed] [Google Scholar]
- 16.Popova NK, Skrinskaya YA, Amstislavskaya TG, Vishnivetskaya GB, Seif I, de Meier E. Behavioral characteristics of mice with genetic knockout of monoamine oxidase type A. Neurosci Behav Physiol. 2001;31:597–602. doi: 10.1023/a:1012364910091. [DOI] [PubMed] [Google Scholar]
- 17.Marino MD, Bourdélat-Parks BN, Liles LC, Weinshenker D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav Brain Res. 2005;161:197–203. doi: 10.1016/j.bbr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, et al. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry. 1994;151:1485–1491. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- 19.Agatsuma S, Lee M, Zhu H, Chen K, Shih JC, Seif I, Hiroi N. Monoamine oxidase A knockout mice exhibit impaired response to nicotine preference but normal responses to novel stimuli. Hum Mol Genet. 2006;15:2721–2731. doi: 10.1093/hmg/ddl206. [DOI] [PubMed] [Google Scholar]
- 20.El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, et al. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci U S A. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]