Abstract
Background:
Sleepiness is one of the most burdensome symptoms of sleep-disordered breathing (SDB). While caffeine is frequently used to avert sleepiness, the association between SDB and caffeine use has not been thoroughly explored. The current study examined whether SDB is associated with caffeine consumption and if factors such as sex, age, and daytime sleepiness explain or modify the association.
Methods:
Data from the Sleep Heart Health Study, a community-based study on the consequences of SDB, were used to characterize the association between SDB and caffeine intake. SDB was assessed with full-montage polysomnography. Caffeine use was quantified as the number of cans of soda or the cups of coffee or tea consumed daily. The Epworth Sleepiness Scale was used to assess daytime sleepiness. Multivariable negative binomial regression models were used to characterize the independent association between SDB and caffeine use.
Results:
Caffeinated soda, but not tea or coffee, intake was independently associated with SDB severity. Compared with participants without SDB, the relative ratios for caffeinated soda consumption in women with mild, moderate, and severe SDB were 1.20 (CI, 1.03-1.41), 1.46 (CI, 1.14-1.87), and 1.73 (CI, 1.23-2.42), respectively. For men, an association was only noted with severe SDB and caffeinated soda use. Age did not modify the SDB-caffeine association, and sleepiness could not explain the observed associations.
Conclusions:
SDB is independently associated with caffeinated soda use in the general community. Identifying excessive caffeine used in SDB has potential significance given the cardiovascular effects of caffeine and untreated SDB.
Sleep-disordered breathing (SDB) is a prevalent disorder associated with a multitude of symptoms as well as clinical sequelae including neurocognitive dysfunction,1 hypertension,2,3 cardiovascular disease,4 and mortality.5 Excessive sleepiness in SDB is the most prominent and troublesome symptom for many patients, often leading them to seek medical attention. Unfortunately, SDB frequently goes unrecognized by patients and physicians,6,7 and overt symptoms are commonly attributed to some other cause. Thus, many patients remain undiagnosed and may institute countermeasures to circumvent the sleepiness and fatigue associated with SDB. Of the many countermeasures employed, the use of caffeinated drinks such as coffee, tea, or sodas is perhaps the most pervasive given the well-known alerting effects of caffeine.8,9
Research on caffeine use in those affected with SDB is extremely limited.10‐13 In fact, there are no studies that have directly addressed the impact of SDB on caffeine intake. The few studies that have examined caffeine use in SDB have not assessed whether there is a dose-response association between the severity of SDB and the amount of caffeine consumed or whether the propensity for using caffeinated beverages is attributed to excessive sleepiness. Furthermore, it is not known whether factors such as age or sex modify the association between SDB and caffeine intake. Given the high prevalence of SDB in the general community, the present study sought to (1) determine whether the severity of SDB was associated with caffeine consumption in the form of coffee, tea, or soda, independent of confounding factors such as age, sex, race, education level, sleep duration, and smoking status; (2) assess whether factors such as sex and age modify the association between SDB and caffeine consumption; and (3) define whether self-reported sleepiness can account for the greater propensity to consume caffeinated drinks. It was hypothesized that, independent of potential confounders, SDB would be associated with caffeine consumption in a dose-dependent manner. Moreover, it was hypothesized that sex and age would modify the association between SDB and caffeine consumption and that self-reported sleepiness would explain the underlying association.
Materials and Methods
Study Sample
The sample for the current study consisted of the Sleep Heart Health Study (SHHS) cohort. Participants for the SHHS were recruited from the following studies: Framingham Offspring and Omni Cohort Studies, Atherosclerosis Risk in Communities Study, Cardiovascular Health Study, Strong Heart Study, Tucson Epidemiologic Study of Respiratory Disease, and New York City studies on hypertension, which included the New York Hospital, Harlem, and Work Site cohorts. Details regarding the design and methodology for recruiting and characterizing the sample have been previously described.14 Eligible participants were at least 40 years of age and were not being treated for SDB with positive pressure therapy, oral appliance, oxygen, or tracheostomy. A total of 6,441 participants completed the baseline examination and constitute the sample for the current analysis. Participants were required to provide written consent, and secondary analyses were approved by the Johns Hopkins University Institutional Review Boards (approval, NA_00010790).
Polysomnography
In-home polysomnography was conducted by trained technicians using a portable monitor (Compumedics P-series; Compumedics Limited). The recording montage consisted of continuous recordings of the following physiologic channels: C3-A2 and C4-A1 EEG, right and left electrooculogram, a single bipolar ECG, chin electromyogram, oxyhemoglobin saturation by pulse oximetry, chest and abdominal excursion by inductance plethysmography, airflow by an oronasal thermocouple, and body position by a mercury gauge.15 Recordings were stored in real time and shipped to a central reading facility for review and scoring. Sleep-stage scoring was performed according to criteria of Rechtschaffen and Kales.16 Apneas were identified if airflow was absent or nearly absent for at least 10 s. Apneas were further classified as obstructive if movement on either the chest or abdominal inductance channels was noted, or as central if no displacement was observed on both of these channels. Hypopneas were identified when there was at least 30% reduction in airflow or thoracoabdominal movement below baseline values for at least 10 s. No attempt was made to classify hypopneas as obstructive or central. The apnea-hypopnea index (AHI) was defined as the number of apneas and hypopneas, each associated with a 4% decrease in oxygen saturation, per hour of sleep.
Assessment of Caffeine Consumption and Other Covariates
Each participant completed several interviewer-administered questionnaires that included queries on age, sex, race, educational level, marital status, sleep duration, smoking history (current, former, never), and caffeine consumption. Race was classified as white, black, Native American, Hispanic, or other. Smoking status was based on responses to the following questions: “Have you ever smoked cigarettes (yes or no)?” and “If you smoked before, do you smoke now (yes or no)?” Smoking was categorized as never, former, and current. For current smokers, lifetime smoking exposure was quantified in pack-years, where 1 pack-year was equal to 20 cigarettes smoked per day for 1 year. Caffeine consumption was assessed with the following questions: (1) “On a typical day, how many cups of regular coffee (with caffeine) do you drink?” (2) “How many cups of regular tea (with caffeine) do you drink on a typical day?” and (3) “How many cans of cola or other soda with caffeine do you drink on a typical day?” Responses were recorded as integer values and constituted the dependent variables for the current study. Participants also completed the Epworth Sleepiness Scale (ESS), a self-administered survey of sleep tendency.17,18
Statistical Analysis
The dependent variables included the number of cups of caffeinated coffee and tea and cans of caffeinated soda, consumed daily. Because caffeine amounts vary in coffee, tea, and sodas, a composite measure of the total number of cups of caffeinated beverages consumed daily was not used. The primary independent variable was the severity of SDB as assessed by the AHI. The following commonly used clinical categories of SDB severity were used for classifying subjects into four groups: < 5.0 events/h (no SDB), 5.0-14.9 events/h (mild SDB), 15.0-29.9 events/h (moderate SDB), and ≥ 30.0 events/h (severe SDB).
Analysis of variance and χ2 statistics were initially used to assess differences in demographic (eg, age, sex, race) and covariate (eg, smoking status) data as a function of SDB severity. Coffee, tea, and soda consumption were also cross-tabulated by SDB severity. Given that the dependent variables represented count data, Poisson regression models were used to investigate the association between SDB severity and caffeine consumption. However, model diagnostics revealed overdispersion, making the Poisson distribution inappropriate for these data. Therefore, the negative binomial distribution, which can accommodate the overdispersion of count data, was used to construct multivariable models relating SDB severity to caffeine consumption. Inclusion of a particular covariate in the multivariable models was based on a priori hypotheses and findings from the bivariate analyses. Covariates considered included age, sex, race, educational level, smoking status, sleep duration, and BMI. The resulting regression coefficients represent the log of the ratio comparing a particular category of SDB severity to the reference category (no SDB) on the number of cups or cans consumed daily. Exponentiation of the regression coefficients provided the relative ratios comparing categories of SDB severity on the number of caffeinated drinks consumed daily. Analyses were stratified by sex given the differences between men and women in SDB severity and in the amount and type of caffeinated drink consumed. All analyses were conducted with the SAS 9.2 software package (SAS Institute Inc).
Results
The analysis sample with complete data consisted of 6,352 participants and included 3,359 women (52.9%) and 2,993 men (47.1%). The prevalence of SDB and disease severity significantly differed between men and women. In men, 41.9% of the sample did not have SDB, 33.0% had mild SDB, 15.9% had moderate SDB, and 9.3% had severe SDB. In women, the distribution was as follows: 64.3% (no SDB), 24.4% (mild), 7.9% (moderate), and 3.4% (severe). Bivariate analyses revealed that age, sex, race, BMI, and smoking status were associated with SDB severity (Table 1). Moreover, use of caffeinated sodas, but not tea or coffee, was also associated with SDB severity. Finally, cigarette smoking was associated with caffeinated coffee and soda, but not tea, consumption (Table 2).
Table 1.
—Characteristics of the Study Sample by Sleep-Disordered Breathing Severity
| Variable | AHI < 5.0 (n = 3,413) | AHI 5.0-14.9(n = 1,808) | AHI 15.0-29.9 (n = 739) | AHI ≥ 30.0(n = 392) |
| Age,a y | 61.3 (11.1) | 64.8 (10.6) | 65.1 (10.5) | 64.6 (10.7) |
| BMI,a kg/m2 | 27.0 (4.5) | 29.5 (5.3) | 30.7 (5.8) | 32.1 (6.1) |
| Sex,a % | ||||
| Women | 63.3 | 45.4 | 35.7 | 29.3 |
| Men | 36.7 | 54.6 | 63.3 | 70.7 |
| Race,a % | ||||
| White | 77.7 | 76.7 | 75.5 | 74.2 |
| African American | 8.1 | 6.9 | 8.1 | 9.2 |
| Native American | 7.9 | 11.2 | 12.0 | 12.2 |
| Hispanic | 4.7 | 3.7 | 3.8 | 3.6 |
| Other | 1.6 | 1.5 | 0.5 | 0.8 |
| Smoking status,a % | ||||
| Never | 47.7 | 43.4 | 44.5 | 42.4 |
| Former | 38.5 | 47.9 | 45.9 | 51.3 |
| Current | 13.9 | 8.7 | 9.6 | 6.4 |
| Caffeinated soda,a % | ||||
| None | 70.6 | 67.1 | 68.2 | 58.2 |
| 1 can/d | 18.2 | 20.3 | 17.9 | 22.3 |
| 2 cans/d | 6.6 | 7.2 | 8.3 | 10.0 |
| 3 cans/d | 2.5 | 3.1 | 2.8 | 4.1 |
| ≥ 4 cans/d | 2.1 | 2.3 | 2.8 | 5.4 |
| Caffeinated coffee, % | ||||
| None | 37.2 | 39.2 | 38.7 | 35.2 |
| 1 cup/d | 16.7 | 14.8 | 15.1 | 17.9 |
| 2 cups/d | 17.6 | 18.1 | 17.2 | 16.3 |
| 3 cups/d | 11.1 | 11.9 | 12.2 | 12.2 |
| ≥ 4 cups/d | 17.4 | 16.0 | 16.8 | 18.4 |
| Caffeinated tea, % | ||||
| None | 74.9 | 74.6 | 74.1 | 75.5 |
| 1 cup/d | 13.0 | 12.2 | 13.7 | 12.5 |
| 2 cups/d | 6.6 | 7.2 | 6.1 | 5.9 |
| 3 cups/d | 2.7 | 2.8 | 2.7 | 3.1 |
| ≥ 4 cups/d | 2.8 | 3.2 | 3.4 | 3.0 |
AHI = apnea-hypopnea index (events/h).
P < .001 for comparisons of age, sex, race, smoking status, BMI, and caffeinated soda intake across AHI categories.
Table 2.
—Consumption of Caffeinated Drinks and Smoking Status in Men and Women
| Variable | Current Smoker | Former Smoker | Never Smoker |
| Women | |||
| Sample size | 367 | 1,130 | 1,859 |
| Caffeinated soda,a % | |||
| None | 66.5 | 74.0 | 74.1 |
| 1 can/d | 16.3 | 16.5 | 16.1 |
| 2 cans/d | 9.3 | 5.8 | 6.3 |
| 3 cans/d | 3.8 | 2.0 | 2.4 |
| ≥ 4 cans/d | 4.1 | 1.7 | 1.1 |
| Caffeinated coffee,a % | |||
| None | 21.2 | 36.1 | 46.9 |
| 1 cup/d | 12.3 | 16.2 | 17.6 |
| 2 cups/d | 18.3 | 20.9 | 17.7 |
| 3 cups/d | 13.9 | 12.0 | 8.9 |
| ≥ 4 cups/d | 34.3 | 14.8 | 8.9 |
| Caffeinated tea, % | |||
| None | 74.1 | 75.0 | 72.0 |
| 1 cup/d | 11.4 | 11.3 | 14.6 |
| 2 cups/d | 6.5 | 7.1 | 7.6 |
| 3 cups/d | 4.1 | 3.2 | 3.3 |
| ≥ 4 cups/d | 3.8 | 3.5 | 2.5 |
| Men | |||
| Sample size | 359 | 1,585 | 1,044 |
| Caffeinated soda,a % | |||
| None | 50.4 | 66.0 | 63.4 |
| 1 can/d | 29.8 | 21.0 | 21.4 |
| 2 cans/d | 8.6 | 7.6 | 8.3 |
| 3 cans/d | 5.0 | 2.8 | 3.3 |
| ≥ 4 cans/d | 6.1 | 2.5 | 3.5 |
| Caffeinated coffee,a % | |||
| None | 15.9 | 33.4 | 43.3 |
| 1 cup/d | 11.7 | 14.7 | 17.9 |
| 2 cups/d | 13.3 | 18.4 | 14.2 |
| 3 cups/d | 13.4 | 13.6 | 11.1 |
| ≥ 4 cups/d | 45.7 | 19.9 | 13.5 |
| Caffeinated tea, % | |||
| None | 80.0 | 77.0 | 74.7 |
| 1 cup/d | 8.9 | 12.0 | 14.2 |
| 2 cups/d | 5.6 | 5.7 | 6.4 |
| 3 cups/d | 2.2 | 2.3 | 1.8 |
| ≥ 4 cups/d | 3.3 | 3.0 | 2.9 |
See Table 1 for expansion of abbreviation.
P < .001 for comparisons of caffeinated soda and coffee consumption across AHI categories. Values represent percentage of participants reporting drinking a specific number of cups or cans per day. No significant association was noted between consumption of caffeinated tea and AHI category.
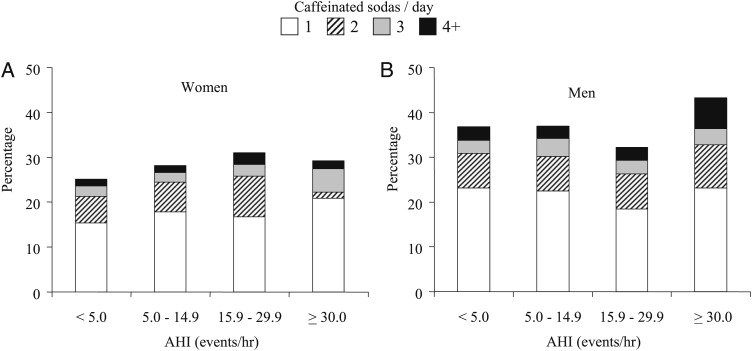
Given the notable differences in SDB severity between men and women, stratified analyses by sex were used to characterize the impact of SDB severity on caffeine use. Figure 1 displays the percentages of women and men using one, two, three, or four or more caffeinated sodas per day as a function of SDB severity. Compared with the reference group without SDB (AHI < 5 events/h), the percentage of women consuming at least one or more caffeinated sodas per day increased as the severity of SDB increased (P < .002 for linear trend). In contrast, only men with severe SDB (AHI ≥ 30 events/h) had a significantly higher percentage consuming at least one caffeinated soda daily when compared with men without SDB or even those with mild or moderate disease (P < .0013). No associations between SDB severity and coffee or tea consumption were noted in analyses stratified by sex, thereby confirming findings based on the full sample.
Figure 1.
Percentage of women and men drinking one to four or more cans of caffeinated soda per day as a function of the AHI. A, Women. B, Men. AHI = apnea-hypopnea index.
To delineate the independent association between SDB severity and caffeinated soda consumption, negative binomial regression models were used. Three nested models were used to assess incremental effects of several covariates. The base model included the AHI as a categorical variable with adjustments for age and race. The second model added smoking to the base model. The third model added sleep duration as the final covariate to the multivariable model. Analyses were also conducted with inclusion of educational level and BMI for additional adjustment. However, given that the regression coefficients relating SDB severity to caffeine use were materially unchanged, educational level and BMI were not included in any of the final models. Table 3 summarizes the results of multivariable models for caffeinated soda, coffee, and tea intake in women and men. As noted in the bivariate analyses, a dose-response association was noted between caffeinated sodas and SDB severity in women. Specifically, after adjusting for age, race, smoking, and habitual sleep duration, women with mild, moderate, and severe SDB reported consuming 20%, 46%, and 73% more cans of caffeinated soda than women with no SDB. In men, the effect of severe SDB on caffeinated soda use also remained, despite multivariable adjustments. Men with severe SDB consumed 44% more cans of caffeinated soda than men without SDB. Interestingly, men with mild and moderate SDB showed no significant differences in caffeinated soda intake compared with those without SDB. Finally, stratified analyses by age in women and men did not reveal any significant interactions between SDB severity and age (data not shown).
Table 3.
—Adjusted Relative Ratios for Consumption of Caffeinated Drinks as a Function of the AHI
| Outcome as Function of Sex and AHI (Events/h) | Model 1a | Model 2b | Model 3c |
| Caffeinated soda | |||
| Women | |||
| < 5.0 | 1.00 | 1.00 | 1.00 |
| 5.0-14.9 | 1.18 (1.01-1.40) | 1.20 (1.02-1.42) | 1.20 (1.03-1.41) |
| 15.0-29.9 | 1.45 (1.13-1.84) | 1.46 (1.15-1.86) | 1.46 (1.14-1.87) |
| ≥ 30.0 | 1.66 (1.19-2.33) | 1.71 (1.22-2.39) | 1.73 (1.23-2.42) |
| Men | |||
| < 5.0 | 1.00 | 1.00 | 1.00 |
| 5.0-14.9 | 1.13 (0.99-1.30) | 1.14 (0.99-1.31) | 1.13 (0.98-1.30) |
| 15.0-29.9 | 0.99 (0.83-1.19) | 1.00 (0.83-1.20) | 0.99 (0.83-1.19) |
| ≥ 30.0 | 1.45 (1.18-1.77) | 1.47 (1.20-1.80) | 1.44 (1.17-1.77) |
| Caffeinated coffee | |||
| Women | |||
| < 5.0 | 1.00 | 1.00 | 1.00 |
| 5.0-14.9 | 0.93 (0.84-1.02) | 0.97 (0.87-1.06) | 0.96 (0.87-1.06) |
| 15.0-29.9 | 0.98 (0.84-1.14) | 1.00 (0.86-1.16) | 1.00 (0.86-1.17) |
| ≥ 30.0 | 0.85 (0.67-1.07) | 0.92 (0.73-1.16) | 0.93 (0.74-1.17) |
| Men | |||
| < 5.0 | 1.00 | 1.00 | 1.00 |
| 5.0-14.9 | 0.91 (0.83-1.00) | 0.93 (0.85-1.02) | 0.93 (0.85-1.02) |
| 15.0-29.9 | 0.88 (0.78-1.00) | 0.90 (0.81-1.02) | 0.91 (0.81-1.03) |
| ≥ 30.0 | 1.00 (0.86-1.15) | 1.04 (0.90-1.19) | 1.04 (0.90-1.20) |
| Caffeinated tea | |||
| Women | |||
| < 5.0 | 1.00 | 1.00 | 1.00 |
| 5.0-14.9 | 1.04 (0.87-1.23) | 1.03 (0.86-1.22) | 1.02 (0.86-1.22) |
| 15.0-29.9 | 1.13 (0.87-1.49) | 1.13 (0.86-1.48) | 1.10 (0.84-1.44) |
| ≥ 30.0 | 0.94 (0.63-1.41) | 0.93 (0.62-1.40) | 0.96 (0.64-1.44) |
| Men | |||
| < 5.0 | 1.00 | 1.00 | 1.00 |
| 5.0-14.9 | 0.95 (0.77-1.16) | 0.94 (0.76-1.15) | 0.94 (0.77-1.16) |
| 15.0-29.9 | 0.90 (0.70-1.17) | 0.88 (0.68-1.14) | 0.89 (0.69-1.16) |
| ≥ 30.0 | 0.89 (0.65-1.22) | 0.87 (0.64-1.20) | 0.89 (0.65-1.22) |
See Table 1 for expansion of abbreviation.
Model 1: Adjusted for age (continuous) and race.
Model 2: Adjusted for covariates of model 1 and smoking status (never, former, current).
Model 3: Adjusted for covariates of model 2 and habitual sleep duration.
Additional analyses were conducted using the ESS score as the independent variable, and the number of caffeinated cups of tea or coffee and cans of caffeinated sodas as the dependent variables to determine whether caffeine consumption was explained with subjective sleep tendency. Surprisingly, no associations were noted between the ESS scores and consumption of caffeinated soda or coffee in unadjusted or adjusted analyses. The only significant finding was the association between caffeinated tea use and an ESS ≥ 11 in women, independent of factors such as age, race, smoking status, and AHI. Women with an ESS ≥ 11 consumed 22% (relative ratio, 1.22; 95% CI, 1.02-1.46) more cups of tea than women with an ESS < 11. Finally, analyses involving inclusion of the ESS score in multivariable models relating AHI to caffeine intake (soda, coffee, or tea) showed no attenuation of regression coefficients associated with AHI, indicating that self-reported sleep tendency did not explain the association between SDB and caffeine consumption.
Discussion
The current study provides several new findings on the patterns of caffeine use in a large community sample of middle-aged men and women with and without SDB. Women with SDB consumed more caffeinated sodas than women without SDB in a dose-dependent manner. In men, the association between caffeinated soda intake and SDB was only noted in those participants with severe SDB (AHI ≥ 30 events/h). Interestingly, an association between SDB severity and the most common dietary sources of caffeine, coffee and tea, was not identified. Perhaps most surprising was the finding that self-reported daytime sleepiness did not explain the observed association between SDB severity and caffeine use. Finally, the well-established association between cigarette smoking and caffeinated coffee and soda use was confirmed.
Despite the fact that excessive sleepiness and fatigue are common and potentially hazardous manifestations of SDB, there is a dearth of information regarding the use of countermeasures in patients with SDB. It is particularly notable that the impact of SDB on caffeine intake, the most universally used stimulant, has not been well investigated. The few studies that have assessed caffeine use in SDB have done so indirectly and often amid examining other outcomes.10‐13 For example, effect modification of BP by caffeine use in the setting of SDB has been previously investigated. Bardwell et al10 demonstrated that while caffeine levels were almost three times higher in those with SDB compared with those without, ambulatory BP was not associated with caffeine levels and effect modification was not present. Similarly, Robinson et al11 demonstrated that caffeine levels were not significantly different among patients with SDB that received therapeutic CPAP vs those who received subtherapeutic CPAP despite improvements in BP in the therapeutic group. Finally, previous research on SDB and neurocognitive function has shown that caffeine use is associated with less impairment in those that use more caffeine.13 While these studies have important implications, they do not directly assess the independent effects of SDB on caffeine use. The current study fills the existing gap by being the first to provide empirical evidence on patterns of caffeine use in a community sample with and without SDB and how these patterns vary as a function of disease severity.
There are several parallel practices that may account for the finding that only caffeinated soda use, but not coffee or tea use, was independently correlated with SDB severity. Coffee use is pervasive, with > 50% of the US population consuming coffee on a daily basis. Among those who habitually consume coffee, it has been estimated that, on average, three cups are consumed per day.19‐21 For many individuals, coffee drinking is not only a customary part of their postawakening routine, but it also provides a pretext for social and work-related interactions. The current study echoes this national trend of extensive coffee use with well over half of our study sample drinking at least one cup of coffee per day. Given that coffee is widely consumed, differences in patterns of consumption specifically attributable to SDB may be difficult to identify. In contrast, even though tea consumption overall is increasing, tea intake in the United States is still relatively uncommon compared with other countries in Europe and Asia.22 In many parts of the world, tea drinking, perceived as a healthier and more leisurely activity than coffee drinking, is even more pervasive than coffee use in the United States, and sets the background for social and business gatherings. It is most likely that the limited number of tea drinkers in the SHHS, especially those with moderate and severe SDB, precluded the ability to identify an association between SDB severity and caffeinated tea intake.
Over the last 2 decades, caffeinated soda use has become an increasing source of dietary caffeine for many in the United States. Data collected by the Department of Agriculture between 1994 and 1998 on a nationally representative sample showed that while coffee remains the primary source of caffeine, sodas had become the second leading source of daily caffeine consumption.21 Caffeinated sodas are affordable, require no preparation on the part of the consumer, and are extensively marketed at convenience stores. A shift in national caffeine usage is occurring with an increasing number opting for caffeinated sodas and high-energy drinks over other sources of caffeine. Although soda intake is not as ubiquitous as coffee, it clearly is a more popular beverage than tea. Thus, of the three types of caffeinated beverages assessed in the SHHS cohort, caffeinated soda seemed to best capture the association between caffeine use and SDB.
The observed differences in caffeine use noted between men and women are not surprising given that prior work has shown genetically and environmentally driven differences between men and women with regards to caffeine consumption quantity and preference. Overall, men have a predilection for coffee use, while tea use is more widespread among women.23 Given that SDB also manifests differently in men and women, stratification by sex was implemented to clarify the interaction between sex, SDB, and caffeine use. These analyses did in fact uncover a differential pattern of caffeinated soda consumption in men and women with SDB, a finding that was not expected. The observation that even mild to moderate SDB may lead to an increase in caffeinated soda consumption in women supports the notion that clinical manifestations of SDB are distinct in women when compared with men24‐26 and that women may institute countermeasures at a lower level of SDB severity.
The finding that caffeine consumption in SDB could not be attributed to self-reported sleepiness could be due to multiple reasons. First, it is certainly possible that subjects with SDB may have self-treated their sleepiness with increasing caffeine intake, thus masking the association between SDB severity and caffeine consumption. Second, a high ESS score indicates increased tendency for daytime sleepiness but does not necessarily denote a greater likelihood of implementing countermeasures to avert sleepiness. Thus, inferences regarding the association between caffeine use and daytime sleepiness are likely to be inexact. Moreover, if a person consumes a caffeinated beverage to help mitigate daytime sleepiness, it is generally due to their immediate circumstances. The ESS, however, asks about the likelihood of falling asleep over the recent past. Third, as previously discussed, social behaviors and not just subjective sleepiness frequently drive caffeine use, making it difficult to ascertain the true impact of SDB on caffeine consumption. Additional research is clearly needed on the impact of SDB on caffeine consumption to ascertain whether caffeinated drinks are being specifically consumed to mitigate symptoms of daytime sleepiness.
The current investigation has a number of strengths and limitations that warrant discussion. The large sample size allows for control of confounding factors, thereby making independent associations more apparent. Moreover, the study sample included the full range of SDB severity, subjective sleepiness, and caffeinated beverage usage. Utilizing a community sample rather than a clinic sample also makes the findings more germane for the general community. Limitations included a lack of precision in measuring caffeine intake which was based on the number of cups or cans consumed. Although the size of soda cans are for the most part standardized, there is considerable variability in sizes of coffee and tea cups as well as the caffeine content of an individual cup. The estimated caffeine content can range from 40 to 175 mg in a cup of coffee (8 oz), 30 to 80 mg in a cup of tea (8 oz), and 35 to 70 mg in a can of soda (12 oz).9 Availability of exact caffeine amounts in consumed beverages would have permitted more rigorous assessment of how SDB impacts caffeine consumption. Finally, it is important to recognize that the observed increase in caffeinated soda consumption in SDB may, in fact, not be due to the caffeine content. An alternative explanation is that an increase in body weight due to poor health habits, including consuming large amounts of caffeinated sodas, may have led to SDB. Given the cross-sectional design of the study, conclusions regarding directionality of cause-and-effect are not possible. However, sensitivity analyses that include BMI as a covariate showed no significant differences in the inferences regarding the association between SDB and caffeinated soda intake.
The implications of the current work are broad in scope. Given that untreated SDB27 and caffeine intake28,29 have been linked with hypertension, the absence of prior literature assessing caffeine use in SDB is surprising. While the current study delves into this largely unexplored topic and offers novel findings, equally importantly, it underscores the complexity of delineating caffeine use and highlights areas of future research. Examining whether caffeine use augments vascular risk associated with untreated SDB is of clinical value. Future studies relating SDB to cardiovascular outcomes should include a detailed assessment of caffeine intake as it may confound or modify the association of interest. Lastly, ascertaining whether the SDB treatment results in a decrease in caffeine use and alters SDB-related clinical consequences should be a topic of further study.
Acknowledgments
Author contributions: Dr Punjabi takes responsibility for the manuscript and the integrity of the data.
Dr Aurora: contributed to data analyses and the writing of the manuscript collectively with the other authors.
Dr Crainiceanu: contributed to data analyses and the writing of the manuscript collectively with the other authors.
Dr Caffo: contributed to data analyses and the writing of the manuscript collectively with the other authors.
Dr Punjabi: contributed to data analyses and the writing of the manuscript collectively with the other authors.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Crainiceanu has a consulting contract with Merck & Co, Inc, for statistical methods support for the analysis of EEG spectrogram data. The data analyzed and the scientific problems are unrelated to the topic of the current manuscript. Dr Crainiceanu is also consulting with On-X Life Technologies, Inc, on statistical methods for the design and analysis of adaptive clinical trials with application to mitral valve implant clinical trials. The data and scientific problems are unrelated to the current manuscript. Dr Caffo has provided consulting services to entities delivering products in this area. The consulting had no scientific overlap with the current work. Dr Punjabi has received research grant support from ResMed. Dr Aurora has reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: Dr Punjabi was the Principal Investigator of the Sleep Heart Health Study in Baltimore, Maryland, and directed all data collection operations at that site.
Abbreviations
- AHI
apnea-hypopnea index
- ESS
Epworth Sleepiness Scale
- SDB
sleep-disordered breathing
- SHHS
Sleep Heart Health Study
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Funding/Support: This work was supported by the National Institutes of Health [Grants HL075078 and HL086862].
References
- 1.Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep. 2007;7(2):161-166 [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378-1384 [DOI] [PubMed] [Google Scholar]
- 3.O’Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705-706 [DOI] [PubMed] [Google Scholar]
- 7.Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6(2):49-54 [DOI] [PubMed] [Google Scholar]
- 8.Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40(9):1243-1255 [DOI] [PubMed] [Google Scholar]
- 9.Roehrs T, Roth T. Caffeine: sleep and daytime sleepiness. Sleep Med Rev. 2008;12(2):153-162 [DOI] [PubMed] [Google Scholar]
- 10.Bardwell WA, Ziegler MG, Ancoli-Israel S, et al. Does caffeine confound relationships among adrenergic tone, blood pressure and sleep apnoea?. J Sleep Res. 2000;9(3):269-272 [DOI] [PubMed] [Google Scholar]
- 11.Robinson GV, Pepperell JC, Davies RJ, Stradling JR. Caffeine levels following treatment of obstructive sleep apnoea. Thorax. 2003;58(9):801-802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28(3):309-314 [PubMed] [Google Scholar]
- 13.Norman D, Bardwell WA, Loredo JS, Ancoli-Israel S, Heaton RK, Dimsdale JE. Caffeine intake is independently associated with neuropsychological performance in patients with obstructive sleep apnea. Sleep Breath. 2008;12(3):199-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077-1085 [PubMed] [Google Scholar]
- 15.Redline S, Sanders MH, Lind BK, et al. Sleep Heart Health Research Group Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21(7):759-767 [PubMed] [Google Scholar]
- 16.Rechtschaffen A, Kales A. Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: US Government Printing Office; 1968. NIH publication 204 [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545 [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381 [DOI] [PubMed] [Google Scholar]
- 19.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34(1):119-129 [DOI] [PubMed] [Google Scholar]
- 20.Knight CA, Knight I, Mitchell DC, Zepp JE. Beverage caffeine intake in US consumers and subpopulations of interest: estimates from the Share of Intake Panel survey. Food Chem Toxicol. 2004;42(12):1923-1930 [DOI] [PubMed] [Google Scholar]
- 21.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105(1):110-113 [DOI] [PubMed] [Google Scholar]
- 22.Song WO, Chun OK. Tea is the major source of flavan-3-ol and flavonol in the U.S. diet. J Nutr. 2008;138(8):1543S-1547S [DOI] [PubMed] [Google Scholar]
- 23.Luciano M, Kirk KM, Heath AC, Martin NG. The genetics of tea and coffee drinking and preference for source of caffeine in a large community sample of Australian twins. Addiction. 2005;100(10):1510-1517 [DOI] [PubMed] [Google Scholar]
- 24.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 1: clinical features. Sleep. 2002;25(4):412-419 [PubMed] [Google Scholar]
- 25.Jordan AS, McEvoy RD. Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev. 2003;7(5):377-389 [DOI] [PubMed] [Google Scholar]
- 26.Larsson LG, Lindberg A, Franklin KA, Lundbäck B. Gender differences in symptoms related to sleep apnea in a general population and in relation to referral to sleep clinic. Chest. 2003;124(1):204-211 [DOI] [PubMed] [Google Scholar]
- 27.Durán-Cantolla J, Aizpuru F, Martínez-Null C, Barbé-Illa F. Obstructive sleep apnea/hypopnea and systemic hypertension. Sleep Med Rev. 2009;13(5):323-331 [DOI] [PubMed] [Google Scholar]
- 28.Noordzij M, Uiterwaal CS, Arends LR, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. J Hypertens. 2005;23(5):921-928 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Hu G, Caballero B, Appel L, Chen L. Habitual coffee consumption and risk of hypertension: a systematic review and meta-analysis of prospective observational studies. Am J Clin Nutr. 2011;93(6):1212-1219 [DOI] [PubMed] [Google Scholar]