Abstract
Background:
Health-related quality-of-life (HRQL) measures have been correlated with lung function in patients with COPD and interstitial lung disease (ILD). However, different pathophysiologic mechanisms may influence how these distinct diseases affect HRQL, resulting in differing HRQL by pulmonary diagnosis among patients with similar severity of ventilatory impairment.
Methods:
The National Heart, Lung, and Blood Institute Lung Tissue Research Consortium provided data on well-characterized participants with COPD (n = 576) and ILD (n = 405) at four clinical sites. Using multiple linear regression, we examined the effects of FEV1 (% predicted) and diagnosis (ILD vs COPD) on HRQL scores, including total St. George Respiratory Questionnaire (SGRQ) scores and Short Form-12 (SF-12) physical component summary (PCS) and mental component summary (MCS) scores.
Results:
Participants with ILD had, on average, higher SGRQ scores (15.33 points; 95% CI, 12.46-18.19; P <.001) and lower SF-12 PCS scores ( − 4.73 points; 95% CI, − 6.31 to − 3.14; P <.001) compared with patients with COPD with similar FEV1 % predicted values, indicating worse HRQL. The specific diagnosis also modified the effect of FEV1 on the total SGRQ score (P = .003) and the SF-12 PCS score (P = .03). There was no relationship between lung function and SF-12 MCS scores.
Conclusions:
HRQL scores were worse for patients with ILD compared with patients with COPD with similar degrees of ventilatory impairment. Differences in dyspnea mechanism or in the rate of disease progression may account for these differences in HRQL.
Health status and health-related quality of life (HRQL) are common research outcomes in patients with chronic pulmonary diseases. The St. George Respiratory Questionnaire (SGRQ) is a widely used instrument that was originally designed to assess HRQL in patients with obstructive lung disease.1,2 Several studies have demonstrated an association between SGRQ scores and FEV1 in patients with COPD.3‐16 The SGRQ has been evaluated for use in patients with other pulmonary diseases, including interstitial lung disease (ILD), and a correlation between SGRQ scores and lung function has been established in patients with ILD as well.17‐19
Unlike the SGRQ, which is a respiratory disease-specific measure of HRQL, the Short Form-12 (SF-12) survey is a generic measure of overall health status. It was derived from the Medical Outcomes Study Short Form-36 survey.20 Correlation between SF-12 scores and pulmonary function has been demonstrated for patients with COPD,21‐23 but little has been previously published regarding the validity of SF-12 in patients with ILD.24
Both COPD and ILD cause ventilatory impairment, but because of substantial differences with respect to their pathophysiology, their impact on HRQL may be different for the same degree of ventilatory impairment. Because ILD tends to be more rapidly progressive than COPD and this may adversely affect tolerance of lung function decline, we hypothesized that patients with ILD would have worse HRQL scores compared with those with COPD with similar severity of ventilatory impairment.
To test this hypothesis, we used data from the Lung Tissue Research Consortium (LTRC) (to learn more about the LTRC, see www.ltrcpublic.com). This multicenter project has generated a database of patients with ILD and COPD who are well characterized with respect to pulmonary function, imaging, and histologic confirmation of their pulmonary diagnosis. We used FEV1 (% predicted) as the primary measure of ventilatory impairment, because FEV1 predicts ventilatory capacity on average.25 Correlation between FEV1 and maximal exercise ventilation and oxygen uptake has been demonstrated in both patients with COPD and patients with ILD.26 Moreover, FEV1 correlates with severity of dyspnea relative to exercise intensity in COPD and ILD.27
Materials and Methods
Study Design
Since September 2005, the LTRC has been enrolling patients with COPD or ILD from four clinical centers: Mayo Clinic (Rochester, Minnesota), University of Colorado, University of Michigan, and University of Pittsburgh. Inclusion criteria are age ≥ 21 years and (1) clinical indication of ILD leading to surgical lung biopsy, (2) diagnosis of COPD leading to treatment with lung volume reduction surgery, (3) diagnosis of ILD or COPD in patients listed for lung transplantation, or (4) lung nodule or mass requiring resection. All surgical procedures are selected using standard clinical indications by the medical providers caring for the patients. Exclusion criteria are (1) an active primary infectious process or (2) a diagnosis of cystic fibrosis, berylliosis, or pulmonary hypertension as the indication for transplantation. All LTRC participants provide informed consent, and local institutional review board approval was obtained at each institution.
In October 2009, 981 records were identified in the LTRC database for patients with a major clinical diagnosis of ILD (n = 405) or COPD (n = 576) for inclusion in our cross-sectional analysis. The clinical center’s principal investigator determined the final clinical diagnosis after reviewing local clinical and pathologic data and reports from the LTRC Tissue Core (University of Colorado) and the LTRC Radiology Core (Mayo Clinic).
Data Collection
Demographic information and medical history were acquired from the patients by interview. HRQL instruments (SGRQ and SF-12) were self-administered in written format. Spirometry was performed in accordance with American Thoracic Society/European Respiratory Society standards,28 and postbronchodilator spirometry was performed if the FEV1/FVC ratio was < 75%. All data were obtained prior to tissue collection.
Statistical Analyses
Characteristics of the diagnosis groups (ILD and COPD) were compared using the χ2 test and the Wilcoxon rank sum test. The primary response variable of interest was HRQL score, and three different HRQL scores were examined in separate analyses: total SGRQ score, SF-12 physical component summary (PCS) score, and SF-12 mental component summary (MCS) score. Lower SGRQ scores indicate better HRQL, whereas higher SF-12 scores correspond to better HRQL. The primary explanatory variable of interest was prebronchodilator FEV1 % predicted, and the relationships between HRQL scores and FEV1 % predicted values were explored using nonparametric methods, including boxplots and locally weighted scatterplot smoothing.
Multivariable linear regression analysis was performed to (1) adjust for potential confounders of the relationship between HRQL score and FEV1 % predicted, (2) evaluate the effect of diagnosis (ILD vs COPD) on HRQL score, and (3) test for interaction between FEV1 % predicted and diagnosis. Comorbidity data were unavailable for five patients, and these individuals were excluded from multivariable analyses. SF-12 scores were unavailable for 11 additional patients, so these individuals were only included in the SGRQ analysis.
Sensitivity analyses and resistant regression analyses were performed to assess the influence of outliers on regression coefficient estimates. The assumptions of linear regression were verified using residual plots, adjusted variables plots, and tests of normality for distribution of residuals. All analyses were performed using Stata, version 11 (StataCorp).
Results
Patient Characteristics
Compared with patients with COPD, patients with ILD were younger and had greater diversity with respect to race and ethnicity (Table 1). However, the majority of patients in both groups were Caucasian. A higher proportion of patients with ILD had diabetes, and more patients with COPD had a history of cancer.
Table 1.
—Comparison of LTRC Participants With ILD and COPD
| Characteristic | ILD | COPD | P Value |
| No. | 405 | 576 | … |
| Age, y | 62 (54-68) | 65 (58-71) | <.0001 |
| Sex | |||
| Male | 223 (55.1) | 296 (51.4) | .26 |
| Race and ethnicitya | .04 | ||
| Caucasian | 370 (91.4) | 550 (95.5) | |
| African American | 16 (4.0) | 18 (3.1) | |
| Hispanic | 10 (2.5) | 3 (0.5) | |
| Other | 9 (2.1) | 5 (0.9) | |
| BMI, kg/m2 | 29.9 (26.5-33.5) | 25.8 (22.7-29.3) | <.0001 |
| Comorbid diseaseb | |||
| Angina | 43 (10.6) | 64 (11.2) | .84 |
| Heart failure | 26 (6.4) | 42 (7.4) | .60 |
| Diabetes | 74 (18.3) | 59 (10.3) | <.001 |
| Renal failure | 7 (1.7) | 16 (2.8) | .28 |
| Cancer diagnosis | 39 (9.6) | 217 (37.7) | <.001 |
| Rheumatologic disease | 40 (9.9) | 47 (8.2) | .35 |
| FEV1 % predictedc | 70 (55-82) | 52 (25.5-73) | <.0001 |
| FVC % predicted | 66 (52-78) | 73 (56-88) | <.0001 |
| Dlco,d mL/min/mm Hg | 12 (9-15) | 13 (9-18) | .05 |
| Tissue collection | <.001 | ||
| Single lung explant | 45 (11.1) | 51 (8.9) | |
| Double lung explant | 22 (5.4) | 43 (7.5) | |
| Lobectomy/wedge resection | 41 (10.1) | 314 (54.5) | |
| Lung biopsy | 265 (65.4) | 34 (5.9) | |
| LVRS | 0 (0.0) | 84 (14.6) | |
| Not performed | 32 (7.9) | 50 (8.7) |
Data are expressed as No. (%) for categorical variables and median (IQR) for continuous variables. Dlco = diffusing capacity of the lung for carbon monoxide; ILD = interstitial lung disease; IQR = interquartile range; LTRC = Lung Tissue Research Consortium; LVRS = lung volume reduction surgery.
Race was self-reported by participants.
Data unavailable for comorbid diseases for five patients.
Prebronchodilator spirometry.
Data unavailable for Dlco for 114 patients.
Among patients with ILD, the most common diagnosis was idiopathic pulmonary fibrosis (IPF) (n = 239, 59.0%), followed by hypersensitivity pneumonitis (n = 45, 11.1%) and nonspecific interstitial pneumonia (n = 30, 7.4%). Other ILD diagnoses included respiratory bronchiolitis-ILD, desquamative interstitial pneumonia, and cryptogenic organizing pneumonia.
Relationship Between SGRQ Score and Diagnosis
SGRQ scores were on average 15.33 points (95% CI, 12.46-18.19) higher in patients with ILD compared with those with COPD, after adjusting for FEV1 % predicted and potential confounders (Table 2). This indicates worse HRQL in patients with ILD compared with those with COPD, with similar severity of ventilatory impairment.
Table 2.
—Relationship Between Chronic Lung Disease Diagnosis, Other Predictors, and Total SGRQ Score
| Variablesa | Unadjusted β Estimate | 95% CI | P Value | Adjusted β Estimateb | 95% CI | P Value |
| Diagnosis (ILD vs COPD) | 11.34 | 8.36 to 14.31 | <.001 | 15.33 | 12.46 to 18.19 | <.001 |
| FEV1 % predicted | − 0.43 | − 0.49 to − 0.38 | <.001 | − 0.46 | − 0.52 to − 0.40 | <.001 |
| Age, y | − 0.85 | − 0.99 to − 0.71 | <.001 | − 0.23 | − 0.37 to − 0.10 | <.001 |
| Sex (male vs female) | − 3.64 | − 6.65 to − 0.63 | .02 | − 2.54 | − 4.88 to − 0.20 | .03 |
| BMI, kg/m2 | 0.31 | 0.05 to 0.56 | .02 | 0.25 | 0.04 to 0.47 | .02 |
| Supplemental oxygen at rest (yes vs no) | 15.72 | 12.86 to 18.57 | <.001 | 7.32 | 4.88 to 9.77 | <.001 |
SGRQ = St. George Respiratory Questionnaire. See Table 1 legend for expansion of other abbreviation.
Continuous variables: FEV1 % predicted, age, BMI. Categorical variables (coding): diagnosis (ILD = 1, COPD = 0), sex (male = 1, female = 0), supplemental oxygen requirement at rest (yes = 1, no = 0), race (Caucasian = 1, non-Caucasian = 0), and individual comorbid diseases (1 = yes, 0 = no). In an additional regression model (results not shown), BMI was modeled as a categorical variable rather than a continuous variable, but this did not alter the regression coefficient estimates or their statistical significance.
Adjusted regression coefficient estimates are adjusted for all variables listed in the table as well as race and comorbidities, including angina, congestive heart failure, diabetes, renal failure, cancer diagnosis, and rheumatologic disease. This multiple linear regression analysis included 976 observations for which complete data were available (r2 = 0.42, σ = 18.24).
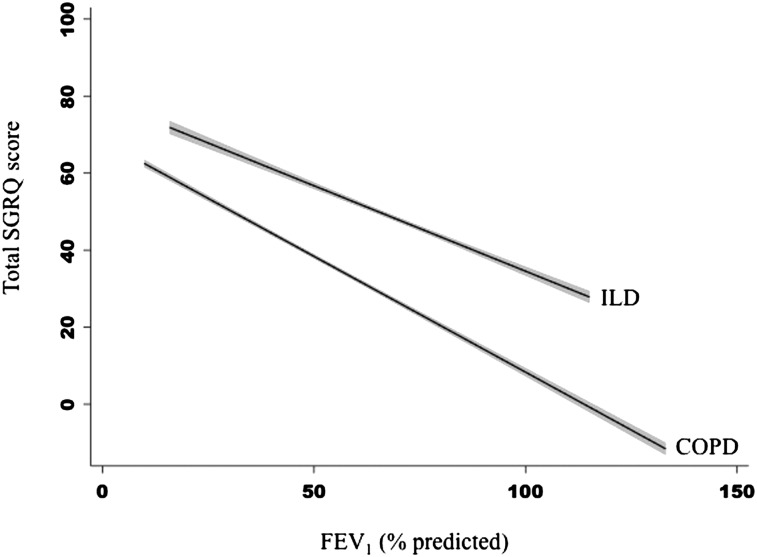
A significant interaction was identified between diagnosis (ILD vs COPD) and FEV1 % predicted that suggests the slope of the relationship between lung function and SGRQ differs by pulmonary diagnosis (P = .003). For a 10% difference in predicted FEV1, the SGRQ score differed on average by 5.0 points (95% CI, 4.4-5.6) among patients with COPD, whereas in ILD, it differed by 3.4 points (95% CI, 2.4-4.4) (Fig 1, Table 3).
Figure 1.
Slope of the adjusted relationship between FEV1 % predicted and SGRQ score varies by diagnosis. Participants with ILD on average had higher SGRQ scores, indicating worse HRQL compared with those with COPD with similar degrees of ventilatory impairment. However, the slope of the relationship between SGRQ score and FEV1 % predicted was steeper in COPD, indicating greater differences in HRQL for similar differences in FEV1 % predicted in COPD compared with ILD. The gray area around the fitted lines indicates the 95% CI around the estimate. HRQL = health-related quality of life; ILD = interstitial lung disease; SGRQ = St. George Respiratory Questionnaire.
Table 3.
—Relationship Between FEV1 % Predicted and HRQL Score Varies by Chronic Lung Disease Diagnosis
| Outcome | Diagnosis | Adjusted β Estimate for FEV1 % Predicteda | 95% CI | P Value for Interaction Term |
| Total SGRQ | ILD | − 0.34 | − 0.44 to − 0.24 | .003 |
| COPD | − 0.50 | − 0.56 to − 0.44 | … | |
| SF-12 PCS | ILD | 0.13 | 0.08 to 0.18 | .03 |
| COPD | 0.20 | 0.16 to 0.23 | … |
HRQL = health-related quality of life; PCS = physical component summary. See Table 1 for expansion of other abbreviations.
Regression coefficient estimates are adjusted for age, BMI, sex, race, requirement for supplemental oxygen, and comorbidities, including angina, congestive heart failure, diabetes, renal failure, cancer diagnosis, and rheumatologic disease.
A sensitivity analysis was performed that substituted FVC % predicted for FEV1 in our main model. Similar results were obtained with higher SGRQ scores among patients with ILD, and again a significant interaction between diagnosis and lung function was identified (P = .001) (e-Tables 1 (1,009.1KB, pdf) , 2 (1,009.1KB, pdf) ). We also performed an analysis that substituted postbronchodilator FEV1 % for prebronchodilator FEV1 % predicted for those patients with postbronchodilator spirometry available and found that this did not alter our results.
Relationship Between SF-12 PCS Score and Diagnosis
SF-12 PCS scores were on average 4.73 points (95% CI, 3.14-6.31) lower in ILD compared with COPD, after adjusting for FEV1 % predicted and potential confounders (Table 4). This again signifies worse HRQL in patients with ILD compared with patients with COPD with similar degrees of ventilatory impairment.
Table 4.
—Relationship Between Chronic Lung Disease Diagnosis, Other Predictors, and SF-12 PCS Score
| Variablesa | Unadjusted β Estimate | 95% CI | P Value | Adjusted β Estimateb | 95% CI | P Value |
| Diagnosis (ILD vs COPD) | − 3.60 | − 5.10 to − 2.11 | <.001 | − 4.73 | − 6.31 to − 3.14 | <.001 |
| FEV1 % predicted | 0.18 | 0.15 to 0.20 | <.001 | 0.18 | 0.15 to 0.21 | <.001 |
| Age, y | 0.30 | 0.23 to 0.38 | <.001 | 0.09 | 0.02 to 0.16 | .02 |
| Sex (male vs female) | 0.003 | − 1.49 to 1.49 | .99 | − 0.31 | − 1.61 to 0.98 | .64 |
| BMI, kg/m2 | − 0.16 | − 0.29 to − 0.04 | .01 | − 0.15 | − 0.27 to − 0.03 | .01 |
| Supplemental oxygen at rest (yes vs no) | − 6.82 | − 8.24 to − 5.39 | <.001 | − 3.64 | − 5.00 to − 2.29 | <.001 |
SF-12 = Short Form-12. See Table 1 legend for expansion of other abbreviation.
Continuous variables: FEV1 % predicted, age, BMI. Categorical variables (coding): diagnosis (ILD = 1, COPD = 0), sex (male = 1, female = 0), supplemental oxygen requirement at rest (yes = 1, no = 0), race (Caucasian = 1, non-Caucasian = 0), and individual comorbid diseases (1 = yes, 0 = no). In an additional regression model (results not shown), BMI was modeled as a categorical variable rather than a continuous variable, but this did not alter the regression coefficient estimates or their statistical significance.
Adjusted regression coefficient estimates are adjusted for all variables listed in the table as well as race and comorbidities, including angina, congestive heart failure, diabetes, renal failure, cancer diagnosis, and rheumatologic disease. This multiple linear regression analysis included 965 observations for which complete data were available (r2 = 0.28, σ = 10.05).
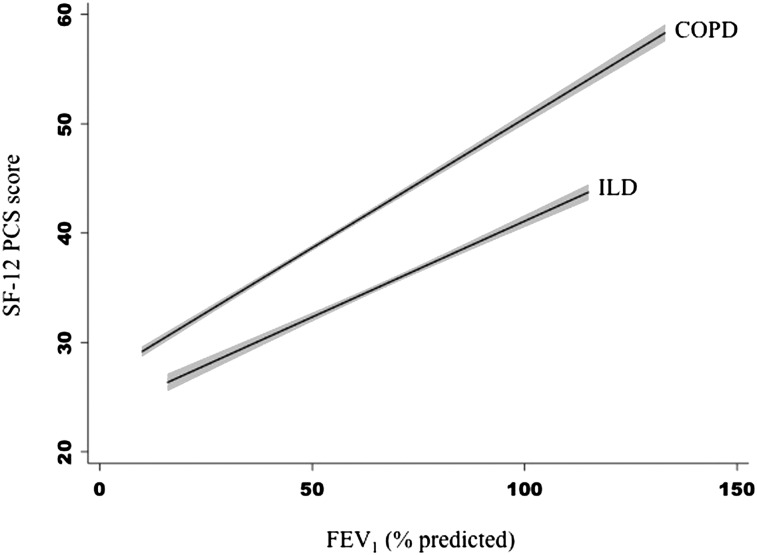
An interaction between diagnosis (ILD vs COPD) and FEV1 % predicted was also identified for the SF-12 PCS model (P = .03), suggesting the slope of the relationship between lung function and SF-12 PCS score is modified by diagnosis. Among patients with COPD, the SF-12 PCS score differed on average by 2.0 points (95% CI, 1.6-2.3) for a 10% difference in predicted FEV1, whereas in ILD, it differed on average by 1.3 points (95% CI, 0.8-1.8) (Fig 2, Table 3).
Figure 2.
Slope of the adjusted relationship between FEV1 % predicted and SF-12 PCS score varies by diagnosis. Participants with ILD on average had lower SF-12 PCS scores, indicating worse HRQL compared with those with COPD with similar degrees of ventilatory impairment. However, the slope of the relationship between SF-12 PCS score and FEV1 % predicted was steeper in COPD, indicating greater differences in HRQL for similar differences in FEV1 % in COPD compared with ILD. The gray area around the fitted lines indicates the 95% CI around the estimate. SF-12 = Short Form-12. See Figure 1 legend for expansion of other abbreviations.
A sensitivity analysis using FVC % predicted was also performed, but unlike with the SGRQ analyses, there was no difference in average SF-12 PCS score by diagnosis after adjusting for FVC and other confounders (e-Table 3 (1,009.1KB, pdf) ). However, there continued to be evidence of an interaction between lung function and diagnosis, with the slope of the relationship between FVC % predicted and SF-12 PCS score differing significantly by diagnosis (P = .005) (e-Table 2 (1,009.1KB, pdf) ). We again performed an analysis that substituted postbronchodilator FEV1 % predicted for prebronchodilator FEV1 % predicted and found that this did not alter our results.
Relationship Between SF-12 MCS Score and Diagnosis
There was no association between SF-12 mental component summary score and FEV1 % predicted after adjustment for age. These results are not shown.
Discussion
The main finding of this study is that patients with ILD have worse HRQL scores compared with those with COPD with similar severity of ventilatory impairment as assessed by FEV1. This was true for both a respiratory disease-specific measure of HRQL (SGRQ) and a generic measure of HRQL (SF-12 PCS). The mechanism causing this difference is unclear but may be related to differences in disease pathophysiology. Patients with ILD experience a concomitant decline in FEV1 and FVC as a result of decreased lung compliance, which increases the work of breathing both at rest and with exertion. In contrast, FEV1 decline in COPD occurs due to increased airways resistance and decreased elastic recoil, which increases the work of breathing predominantly during exertion because of dynamic hyperinflation.29
Other disease factors aside from ventilatory impairment may result in activity limitation, such as exercise desaturation. Oxygen desaturation with exertion is associated with a low diffusing capacity of the lung for carbon monoxide (Dlco), and Dlco may be decreased in both ILD and COPD. We performed a supplemental analysis adjusting for mean Dlco, which reduced the magnitude of the regression coefficient for diagnosis. However, HRQL scores still remained higher in ILD compared with COPD (e-Appendix 1 (1,009.1KB, pdf) , e-Tables 4 (1,009.1KB, pdf) -6 (1,009.1KB, pdf) ).
Another possible explanation for the difference in HRQL scores is that lung function decline is usually more rapid in patients with ILD compared with COPD. Slower decline in lung function may allow patients with COPD to gradually modify their activities and lifestyle such that they perceive less impact on HRQL. As patients with COPD become more sedentary, their activities may be limited by muscle fatigue rather than breathlessness. In a study of incremental exercise testing in patients with COPD and ILD, patients with COPD stopped exercise due to fatigue (46%) more than dyspnea (25%), whereas dyspnea was the reason for stopping exercise in the majority of patients with ILD (62%).27 Many SGRQ items ask about activity limitation due to breathlessness, but activity may be restricted by fatigue before dyspnea is limiting in patients with COPD. Hence, differences in activity limitation attributed to dyspnea may result in higher SGRQ scores for patients with ILD, but this would not explain the difference in SF-12 PCS scores that we observed.
Prior studies in patients with COPD and ILD have suggested dyspnea is an important factor influencing HRQL scores.11‐13,21,30‐34 In an effort to investigate whether dyspnea accounts for the difference in average total SGRQ score by diagnosis, we examined the individual SGRQ component scores (symptoms, activity, and impacts). Each SGRQ component score was higher for patients with ILD compared with COPD (e-Tables 7 (1,009.1KB, pdf) -9 (1,009.1KB, pdf) ). The symptoms domain includes questions about cough and sputum in addition to breathlessness, whereas the activity and impacts domains focus on how dyspnea, exercise limitation, and other disease factors affect physical and social activities and overall life satisfaction. Because the scores for ILD were higher across all domains, this suggests that factors other than dyspnea, such as cough and sputum, contribute to the total SGRQ difference by diagnosis.
In addition to finding that patients with ILD had worse HRQL scores compared with those with COPD with similar ventilatory impairment, our study showed that the slope of the relationship between FEV1 % predicted and HRQL score varies by diagnosis, with a steeper slope among patients with COPD. We do not know the reason for this, but a possible explanation is that patients with COPD with worse FEV1 may accumulate other conditions that negatively affect HRQL, such as more frequent exacerbations, drug effects, systemic manifestations, and comorbid diseases.
Traditionally, FEV1 has been used as a primary metric of severity for obstructive lung disease, whereas FVC has been used for restrictive lung disease. Our study findings support FEV1 as a meaningful measure of disease severity in ILD. However, the interpretation of FEV1 in ILD as a marker of disease severity is different than in COPD, in that patients with ILD have more disease impact for the same degree of ventilatory impairment.
The results of our study suggest that the relationship between FEV1 and HRQL score is linear and statistically significant for both SGRQ and SF-12 PCS in a population of patients with chronic lung disease. With regard to this association, we were surprised to find that there was no clear threshold effect. We had expected that there would be a threshold above which lung function would not affect HRQL measures, but using nonparametric methods, this was not observed (e-Figs 1 (1,009.1KB, pdf) -8 (1,009.1KB, pdf) ).
Using the original SGRQ instrument developed for use in obstructive lung disease, we found that the total SGRQ score is independently associated with FEV1 in both patients with COPD and patients with ILD. This was also true of for SF-12 PCS scores. The association we observed between lung function measures and HRQL scores in patients with ILD adds to the growing body of literature that both SGRQ and SF-12 are useful instruments in patients with ILD. It appears that lung function is a major predictor of HRQL as measured by SGRQ, regardless of the specific pulmonary diagnosis. Because our study was cross-sectional, we cannot comment on the responsivity of SGRQ to changes in disease state in ILD, but results from a recent longitudinal study of patients with IPF indicate that SGRQ may be a meaningful instrument for measuring changes in IPF disease severity over time.19
Recently, the SGRQ-I was developed, which is a subset of SGRQ items intended for specific use in IPF.35 Although we cannot infer how our results might have differed had we used the SGRQ-I, we note that particular items in the original SGRQ seem less relevant to ILD (such as questions about wheezing). We would have expected this to result in a higher average SGRQ score among patients with COPD, which was not observed in our study. Interestingly, in development of the SGRQ-I, some of those items without obvious face validity for IPF (eg, wheezing) were retained because of good fit with other important model components.35
In contrast to our results from the SGRQ and SF-12 PCS analyses, we did not find a significant association between FEV1 and SF-12 MCS scores after accounting for age. This is consistent with other studies that have demonstrated no association or relatively weak association between MCS scores and lung function in patients with COPD4,16,23 and ILD.18 Taken together, these findings indicate that differences in ventilatory impairment have minimal impact on the emotional and psychosocial aspects of HRQL.
The strengths of this study include the use of a well-characterized patient population with the detailed and consistent evaluation afforded by the LTRC using predefined protocols at multiple clinical centers. We recognize that patients were selected to participate in this study because of their potential for tissue donation, and, hence, the patients in this study may not be representative of the general population of patients with COPD and ILD. However, the LTRC allowed us to examine HRQL scores among patients with a wide range of lung function, from those undergoing thoracic surgery for nodule resection to those requiring lung transplantation. An additional strength of this study is that our findings were consistent across different measures of lung function and different HRQL scores.
In summary, patients with ILD have worse HRQL scores than those with COPD. This is related to factors other than severity of ventilatory impairment.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Berry is the guarantor of this manuscript and is responsible for this work as a whole, from inception to publication.
Dr Berry: contributed to conception, hypotheses delineation, study design, data analysis, results interpretation, and writing and revision of the manuscript.
Dr Drummond: contributed to conception, hypotheses delineation, study design, data analysis, results interpretation, and writing and revision of the manuscript.
Dr Han: contributed to the conception, study design, data acquisition, and revision of the manuscript.
Ms Li: contributed to the conception, study design, data acquisition, and approval of the manuscript.
Ms Fuller: contributed to the conception, study design, data acquisition, and approval of the manuscript.
Dr Limper: contributed to data acquisition and review and approval of the manuscript.
Dr Martinez: contributed to data acquisition and review and approval of the manuscript.
Dr Schwarz: contributed to data acquisition and revision of the manuscript.
Dr Sciurba: contributed to data acquisition and review and approval of the manuscript.
Dr Wise: contributed to conception, hypotheses delineation, study design, data analysis, results interpretation, and writing and revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Han has performed speaking for Boehringer Ingelheim GmbH; Pfizer, Inc; and GlaxoSmithKline. She has also consulted for Novartis AG; Genentech, Inc; GlaxoSmithKline; Pfizer, Inc; Boehringer Ingelheim GmbH; and MedImmune LLC. Dr Martinez has participated in advisory boards in COPD development for GlaxoSmithKline; MedImmune LLC; AstraZeneca; Merck & Co, Inc; Perl; Novartis AG; Forest Laboratories, Inc/Almirall, SA; Takeda Pharmaceuticals International GmbH; Schering-Plough Corp; Hoffmann-La Roche Inc; Bayer; Actelion Pharmaceuticals Ltd; Pfizer, Inc; Talecris; Health Learning Systems; Comgenix; FBCommunications; BoomComm; and Ikaria, Inc. He has been a member of a steering committee for COPD studies sponsored by GlaxoSmithKline, Actelion Pharmaceuticals Ltd, MPex, and Takeda Pharmaceuticals International GmbH. He has participated in US Food and Drug Administration mock panels for Boehringer Ingelheim GmbH and Forest Laboratories, Inc. He has been a member of advisory boards for IPF drug development for Elan Corp, plc; Quark; Genzyme Corp; Takeda Pharmaceuticals International GmbH; and Ikaria, Inc. He has been a member of steering committees for IPF studies sponsored by Actelion Pharmaceuticals, Ltd; Genzyme Corp; Gilead; and Johnson & Johnson/Janssen Biotech, Inc. The University of Michigan received funds from Boehringer Ingelheim GmbH for COPD study and from Actelion Pharmaceuticals Ltd for IPF study. Dr Martinez has served on speaker’s bureaus or in continuing medical education activities sponsored by GlaxoSmithKline; NACE; Med-Ed; Potomac; Pfizer, Inc; Boehringer Ingelheim GmbH; Schering-Plough Corp; Vox Medica, Inc; American Lung Association; WebMD; Epocrates; AstraZeneca; France Foundation; Altana Pharma/Nycomed; and UpToDate. He has received royalties from Associates in Medical Marketing and Castle Connolly. Dr Sciurba has received research funds from the National Institutes of Health; GlaxoSmithKline; Pfizer, Inc; Boehringer Ingelheim GmbH; and Forest Laboratories, Inc. He has also received consulting funds from GlaxoSmithKline, AstraZeneca, and PneumRx. Dr Wise has served as a consultant for AstraZeneca; Boehringer Ingelheim GmbH; GlaxoSmithKline; InterMune; Merck & Co, Inc; MedImmune LLC; Novartis AG; Pfizer, Inc; Sunovion Pharmaceuticals, Inc; and Spiration, Inc. He has received research grants from Boehringer-Ingelheim GmbH; Forest Laboratories, Inc; and GlaxoSmithKline. Drs Berry, Drummond, Limper, and Schwarz and Mss Li and Fuller have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the “Supplemental Materials” area of the online article.
Role of sponsors: The National Heart, Lung and Blood Institute Lung Tissue Research Consortium (LTRC) provided the data for this study. This publication was made possible by Grant 1KL2RR025006-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Abbreviations
- Dlco
diffusing capacity of the lung for carbon monoxide
- HRQL
health-related quality of life
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- LTRC
Lung Tissue Research Consortium
- MCS
mental component summary
- PCS
physical component summary
- SF-12
Short Form-12
- SGRQ
St. George Respiratory Questionnaire
Footnotes
Funding/Support: This study was supported by the National Institutes of Health [Grant 1KL2RR025006-01] and the National Heart, Lung, and Blood Institute [Grant NO1-HR-46164].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Jones PW, Quirk FH, Baveystock CM. The St. George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31 [DOI] [PubMed] [Google Scholar]
- 2.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321-1327 [DOI] [PubMed] [Google Scholar]
- 3.Weatherall M, Marsh S, Shirtcliffe P, Williams M, Travers J, Beasley R. Quality of life measured by the St George’s Respiratory Questionnaire and spirometry. Eur Respir J. 2009;33(5):1025-1030 [DOI] [PubMed] [Google Scholar]
- 4.Ståhl E, Lindberg A, Jansson SA, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes. 2005;356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira ED, Pinto R, Alcantara M, Medeiros M, Mota RM. Influence of respiratory function parameters on the quality of life of patients with COPD. J Bras Pneumol. 2009;35(8):730-736 [DOI] [PubMed] [Google Scholar]
- 6.Moy ML, Reilly JJ, Ries AL, et al. National Emphysema Treatment Trial Research Group Multivariate models of determinants of health-related quality of life in severe chronic obstructive pulmonary disease. J Rehabil Res Dev. 2009;46(5):643-654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez FF, Faganello MM, Tanni SE, Lucheta PA, Padovani CR, Godoy I. Relationship between disease severity and quality of life in patients with chronic obstructive pulmonary disease. Braz J Med Biol Res. 2008;41(10):860-865 [DOI] [PubMed] [Google Scholar]
- 8.Miravitlles M, Molina J, Naberan K, Cots JM, Ros F, Llor C; EVOCA study Factors determining the quality of life of patients with COPD in primary care. Ther Adv Respir Dis. 2007;1(2):85-92 [DOI] [PubMed] [Google Scholar]
- 9.Katsura H, Yamada K, Kida K. Both generic and disease specific health-related quality of life are deteriorated in patients with underweight COPD. Respir Med. 2005;99(5):624-630 [DOI] [PubMed] [Google Scholar]
- 10.Ferrer M, Villasante C, Alonso J, et al. Interpretation of quality of life scores from the St George’s Respiratory Questionnaire. Eur Respir J. 2002;19(3):405-413 [DOI] [PubMed] [Google Scholar]
- 11.Engström CP, Persson LO, Larsson S, Sullivan M. Health-related quality of life in COPD: why both disease-specific and generic measures should be used. Eur Respir J. 2001;18(1):69-76 [DOI] [PubMed] [Google Scholar]
- 12.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Comparison of discriminative properties among disease-specific questionnaires for measuring health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(3 pt 1):785-790 [DOI] [PubMed] [Google Scholar]
- 13.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T. Stages of disease severity and factors that affect the health status of patients with chronic obstructive pulmonary disease. Respir Med. 2000;94(9):841-846 [DOI] [PubMed] [Google Scholar]
- 14.Harper R, Brazier JE, Waterhouse JC, Walters SJ, Jones NM, Howard P. Comparison of outcome measures for patients with chronic obstructive pulmonary disease (COPD) in an outpatient setting. Thorax. 1997;52(10):879-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ketelaars CA, Schlösser MA, Mostert R, Huyer Abu-Saad H, Halfens RJ, Wouters EF. Determinants of health-related quality of life in patients with chronic obstructive pulmonary disease. Thorax. 1996;51(1):39-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickard AS, Yang Y, Lee TA. Comparison of health-related quality of life measures in chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2011;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beretta L, Santaniello A, Lemos A, Masciocchi M, Scorza R. Validity of the Saint George’s Respiratory Questionnaire in the evaluation of the health-related quality of life in patients with interstitial lung disease secondary to systemic sclerosis. Rheumatology (Oxford). 2007;46(2):296-301 [DOI] [PubMed] [Google Scholar]
- 18.Chang JA, Curtis JR, Patrick DL, Raghu G. Assessment of health-related quality of life in patients with interstitial lung disease. Chest. 1999;116(5):1175-1182 [DOI] [PubMed] [Google Scholar]
- 19.Swigris JJ, Brown KK, Behr J, et al. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med. 2010;104(2):296-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233 [DOI] [PubMed] [Google Scholar]
- 21.Voll-Aanerud M, Eagan TM, Wentzel-Larsen T, Gulsvik A, Bakke PS. Respiratory symptoms, COPD severity, and health related quality of life in a general population sample. Respir Med. 2008;102(3):399-406 [DOI] [PubMed] [Google Scholar]
- 22.Martín A, Rodríguez-González Moro JM, Izquierdo JL, Gobartt E, de Lucas P; VICE Study Group Health-related quality of life in outpatients with COPD in daily practice: the VICE Spanish Study. Int J Chron Obstruct Pulmon Dis. 2008;3(4):683-692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrasco Garrido P, de Miguel Díez J, Rejas Gutiérrez J, et al. Negative impact of chronic obstructive pulmonary disease on the health-related quality of life of patients. Results of the EPIDEPOC study. Health Qual Life Outcomes. 2006;4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han MK, Swigris J, Liu L, et al. Gender influences health-related quality of life in IPF. Respir Med. 2010;104(5):724-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sue DY. Evaluation of impairment and disability: the role of cardiopulmonary exercise testing. In: Weisman IM, Zeballos RJ, eds. Clinical Exercise Testing. Progr Respir Res Vol. 32.. Basel, Switzerland: Karger; 2002:217-320 [Google Scholar]
- 26.LoRusso TJ, Belman MJ, Elashoff JD, Koerner SK. Prediction of maximal exercise capacity in obstructive and restrictive pulmonary disease. Chest. 1993;104(6):1748-1754 [DOI] [PubMed] [Google Scholar]
- 27.Rampulla C, Baiocchi S, Dacosto E, Ambrosino N. Dyspnea on exercise: pathophysiologic mechanisms. Chest. 1992;101(suppl 5):248S-252S [PubMed] [Google Scholar]
- 28.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338 [DOI] [PubMed] [Google Scholar]
- 29.O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225-236 [DOI] [PubMed] [Google Scholar]
- 30.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T, Izumi T. A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPD. Chest. 1999;116(6):1632-1637 [DOI] [PubMed] [Google Scholar]
- 31.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(4):1185-1189 [DOI] [PubMed] [Google Scholar]
- 32.Nishiyama O, Taniguchi H, Kondoh Y, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor?. Respir Med. 2005;99(4):408-414 [DOI] [PubMed] [Google Scholar]
- 33.Baddini Martinez JA, Martinez TY, Lovetro Galhardo FP, de Castro Pereira CA. Dyspnea scales as a measure of health-related quality of life in patients with idiopathic pulmonary fibrosis. Med Sci Monit. 2002;8(6):CR405-CR410 [PubMed] [Google Scholar]
- 34.Mahler DA, Mackowiak JI. Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with COPD. Chest. 1995;107(6):1585-1589 [DOI] [PubMed] [Google Scholar]
- 35.Yorke J, Jones PW, Swigris JJ. Development and validity testing of an IPF-specific version of the St George’s Respiratory Questionnaire. Thorax. 2010;65(10):921-926 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement