Abstract
Aging is associated with loss of quality control in protein turnover. The ubiquitin-proteasome pathway is critical to this quality control process as it degrades mutated and damaged proteins. We identified a unique aging-dependent mechanism that contributes to proteasome dysfunction in Drosophila melanogaster. Our studies are the first to show that the major proteasome form in old (43–47 days old) female and male flies is the weakly active 20S core particle, while in younger (1–32 days old) flies highly active 26S proteasomes are preponderant. Old (43–47 days) flies of both genders also exhibit a decline (~50%) in ATP levels, which is relevant to 26S proteasomes, as their assembly is ATP-dependent. The steep declines in 26S proteasome and ATP levels were observed at an age (43–47 days) when the flies exhibited a marked drop in locomotor performance, attesting that these are “old age” events. Remarkably, treatment with a proteasome inhibitor increases ubiquitinated protein levels and shortens the life span of old but not young flies. In conclusion, our data reveal a previously unknown mechanism that perturbs proteasome activity in “old-age” female and male Drosophila most likely depriving them of the ability to effectively cope with proteotoxic damages caused by environmental and/or genetic factors.
Keywords: ubiquitin, protein degradation, 26S/20S proteasomes, ATP depletion, fly aging
In the last decade, molecular geneticists tried to identify genes that regulate longevity in yeast, worms, flies, and mice [reviewed in (1)]. At least four mutations were shown to extend longevity in Drosophila melanogaster: methuselah, encoding a putative G-protein coupled receptor, Indy, encoding a sodium dicarboxylate cotransporter, chico, encoding an insulin receptor substrate and InR encoding the insulin receptor [reviewed in (2)]. The majority of genes that are consistently up-regulated in the extended longevity fly mutants are those encoding stress resistance proteins that protect against oxidative stress and thermal damage. Because these conditions are associated with damaged proteins and the ubiquitin-proteasome pathway (UPP) is the major pathway that degrades intracellular damaged proteins, it is likely that UPP impairment plays a critical role in the aging process.
A fundamental characteristic of aging (3) and age-related neurodegenerative disorders (4, 5) is the accumulation and aggregation of ubiquitinated proteins in abnormal neuronal inclusions, such as neurofibrillary tangles in Alzheimer’s disease and Lewy bodies in Parkinson’s disease. The mechanisms causing the aggregation of ubiquitinated proteins and its role in aging and age-related neurodegeneration remain elusive. No apparent changes in the levels of ubiquitin or ubiquitinating enzymes (E1, E2, and E3) with aging are reported in the literature [reviewed in (3)]. However, one must keep in mind that because of the vast numbers and widely divergent substrates of E2 and E3 classes of enzymes, age-dependent changes in many of these enzymes remain to be assessed (3). Conversely, a decline in proteasome activity with the aging process has been shown in a variety of mammalian organs and tissues [reviewed in (6)]. The loss of proteasome activity with aging has been associated with decreased subunit expression, alterations and/or replacement of proteasome subunits and formation of inhibitory cross-linked proteins [reviewed in (7, 8)]. Food restriction, which is currently the only experimental paradigm that halts the aging process, prevents the age-dependent changes in proteasome function and structure in mice and rats, further supporting the notion that the proteasome plays a role in the aging process [reviewed in (9)]. Proteasome dysfunction thus provides a link between environmental and genetic factors associated with aging and aging-related neurodegeneration.
Herein, we demonstrate a significant decline in proteasome activity in female and male old (43–47 days of age) flies compared to younger (1 to 32 days of age) flies. Notably, we found that the major proteasome form in old flies is the 20S core particle, while in younger flies the 26S holoenzyme is the preponderant proteasome form. These findings establish that autoinhibited 20S proteasomes prevail in old flies, whereas the fully-assembled 26S proteasome is highly active in young flies. Our results support the view that an aging-dependent disassembly of the 26S proteasome is an important risk factor in aging. Assembly of the 26S proteasome is an ATP-dependent process. We also established that ATP steady-state levels decline by 50% in old (43–47 days) vs. younger (1–32 days) flies of both genders. Other studies also demonstrated a decline in ATP levels with aging in Drosophila (10). The observed ATP-depletion will perturb 26S proteasome assembly and have a negative impact on normal protein turnover and on the ability of old flies to eliminate abnormal proteins resulting from mutations and environmental damage. Remarkably, the steep reduction in ATP and 26S proteasome levels in old flies is observed when there is a major drop in their climbing performance, which might indicate that these events are milestones of the aging process.
MATERIALS AND METHODS
Flies
Wild-type Oregon R flies were reared on standard corn-meal agar medium (11). Flies were passed to fresh vials every 4–6 days and maintained in humidified, temperature-controlled environmental chambers at 25°C and 60% relative humidity throughout the experiments. Flies were sorted and collected under CO2. Samples containing a mixed population of females and males or separate groups of females and males for each age group were analyzed. In some experiments, two main age groups of flies were compared: flies 1–2 days of age, referred to as “young” flies, and flies 43–47 days of age, referred to as “old” flies throughout the article. Where indicated, three additional age groups were analyzed: 10–12, 18–20, and 30–32 days of age.
Peptidase activities in fly extracts
Mixed populations of females and males for each age group were analyzed. Whole fly extracts (30 flies per group) were prepared on ice by homogenization in 0.01 M Tris-EDTA, pH 7.5 buffer. The lysates were cleared by a 15-min centrifugation at 19,000 g at 4°C. The cleared samples were normalized for protein concentration determined by a bicinchoninic acid assay kit (Pierce, Rockford, IL, USA). Peptidase activities were assayed colorimetrically in 20 µg of protein/sample after 24 h incubations at 37°C as described by Wilk and Orlowski (12). The chymotrypsin-like activity was measured with the substrate Suc-LLVY-AMC, the trypsin-like activity with Z-GGR-NA and the caspase-like activity with Z-LLE-NA. All substrates were from BACHEM Bioscience (King of Prussia, PA, USA).
In-gel proteasome activity and detection
Mixed or separate populations of females and males for each age group were analyzed. Flies were harvested with buffer A [50 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 5 mM ATP (grade 1; Sigma, St. Louis, MO, USA), 1 mM DTT and 10% glycerol], which preserves 26S proteasome assembly (13). Following homogenization on ice with a Teflon pestle for microcentrifuge tubes (100 up and down strokes), sonication on ice (2 × 10 s with a 5-s interval), and centrifugation (19,000 g, 15 min at 4°C) the protein content of the cleared supernatants was determined with the Bradford assay (Bio-Rad, Hercules, CA, USA). The cleared supernatants were resolved by nondenaturing PAGE using a modification of the method described in (14).
To assess residual proteasome immunoreactivity in the pellet fraction, the latter was resuspended in buffer A, sonicated on ice (2 × 10 s with a 5 s interval) and resolved by nondenaturing PAGE just as the supernatant fraction. We established that the cleared supernatant fraction contains almost all of the proteasome immunoreactivity present in flies, with hardly any being detected in the residual pellet fraction.
We used a three-step gradient gel with approximately similar amounts of (from bottom up) 5%, 4%, and 3% polyacrylamide containing Rhinohide polyacrylamide strengthener (Invitrogen-Molecular Probes, Carlsbad, CA, USA). Bromophenol blue was added to the samples before loading. Nondenaturing minigels were run at 125 V for 3 h.
For detection of proteasome activity, the gels were incubated on a rocker for 10 to 30 min (depending on protein amount loaded) at 37°C with 15 ml of 0.4 mM Suc-LLVY-AMC in buffer B (buffer A modified to contain 1 mM ATP). Proteasome bands were visualized on exposure to UV light (360 nm) and were photographed with a Nikon Cool Pix 8700 camera with a 3–4219 fluorescent green filter (Peca Products, Beloit, WI, USA). Semiquantitative analysis of the bands corresponding to proteasome activity was performed by image analysis with the ImageJ program (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/, 1997–2006).
Proteins on the native gels were then transferred (110 mA) for 2.5 h onto PVDF membranes. Western blot analysis was carried-out for detection of the 26S and 20S proteasomes with our anti-dβ5 affinity purified antibody (1:4,000; BioSynthesis, College Station, TX, USA). The peptide NH2-(GC)DSGYH-WDLEDKEAQE-COOH was used to produce the antidβ5-specific antibody and corresponds to amino acids 213–227 of the Drosophila dβ5 subunit. The anti-dβ5 antibody reacts with a core particle subunit (dβ5), thus detecting both the 26S and 20S proteasomes. Upon incubation with the secondary antibody, antigens were visualized by a chemiluminescent horseradish peroxidase standard method with the ECL reagent.
To determine the protein pattern of young and old flies, parallel native gels were stained with Coomassie blue after assessment of proteasome activity with Suc-LLVY-AMC.
ATP measurements
ATP levels were assessed in separate populations of females or males for each of the five age groups. Steady-state ATP content was measured with a kit using the sensitive luciferin/luciferase system (Invitrogen-Molecular Probes, Carlsbad, CA, USA). This assay is based on the fact that luciferase requires ATP for light production using luciferin as a substrate. Flies (15 per trial) were harvested with 75 µl of 4% TCA followed by homogenization on ice with a Teflon pestle for microcentrifuge tubes (100 up and down strokes) and centrifugation (11,500 g, 15 min at 4°C). ATP steady-state levels were determined in cleared supernatants upon neutralizing the samples with 1 M Tris-HCl, pH 8.0 (1:10 dilution). Samples were then added to the reaction buffer containing luciferin and assayed using a Luminoskan Ascent (Thermo Electron Corporation, Waltham, MA, USA) microplate luminometer. Protein concentration was determined with the bicinchoninic acid assay kit (Pierce, Rockford, IL, USA) upon neutralizing total fly lysates with 10 mM Tris, pH 10.3. ATP levels were normalized for protein concentration. Similar protocols were previously used to measure ATP levels in Drosophila (10, 15).
Climbing performance
We used a slight modification of a climbing assay previously established as being reliable to evaluate locomotor performance in Drosophila (16). Females or males of the five age groups (10 flies per trial) were placed in an empty graduated 100 ml cylinder with a line drawn at the 66 ml (2/3) mark. Flies were gently tapped to the bottom of the cylinder after a 15 min recovery period from anesthesia. The number of flies that climbed above the 66 ml mark after 20 s was recorded. Three trials were carried out for each group and the results were averaged.
Glycerol density gradient centrifugation
Mixed populations of females and males for each age group were analyzed by glycerol gradient centrifugation. Flies (~350) were harvested in 25 mM Tris HCl, pH 7.5, 2 mM ATP, and 1 mM DTT. After homogenization and sonication, the lysates were centrifuged for 10 min at 19,000 g at 4°C. The cleared supernatants (4.5 mg of protein/sample) were subjected to centrifugation at 83,000 g for 24 h in a Beckman SW41 rotor in a 10–40% glycerol gradient (fractions 14 to 1) made in the same lysis buffer. After centrifugation, 14 fractions (800 µl each) were collected and analyzed. Aliquots (50 µl) of each fraction were assayed for chymotrypsin-like activity with the substrate Suc-LLVY-AMC colorimetrically after 24 h incubations at 37°C, as described in (12). The chymotrypsin-like activity was assessed in the presence and absence of the proteasome inhibitor PSI (40 µM in 0.5% DMSO).
Proteins were precipitated with acetone from 700 µl of each fraction and subjected to Western blot analysis (12% gels). The dβ5 proteasome subunit was detected with our anti-dβ5 antibody (1:4000; BioSynthesis) and the S5a subunit of the 19S regulatory particle of the 26S proteasome was detected with the anti-S5a antibody (1:1,000, Abcam, Cambridge, MA, USA). Upon incubation with the secondary antibody, antigens were visualized by a chemiluminescent horseradish peroxidase standard method with the ECL reagent.
Fly treatment
Mixed populations of both females and males for each age group were analyzed. Flies were kept in narrow Drosophila vials which were lined with 3 mm filter paper. Flies were starved for 6 h and then fed a solution of 5% (weight/vol) sucrose with either vehicle (0.5% DMSO, control) or with the proteasome inhibitor PSI (10 µM, in DMSO) for 48 h.
Western blot analysis of fly extracts
Mixed populations of females and males for each age group were analyzed.
Ubiquitinated proteins
Following the indicated treatments, flies were homogenized at 4°C in PBS with 1% Triton X-100 (TX) and a protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Following centrifugation (19,000 g, 15 min, 4°C), supernatants were collected (TX-soluble fraction). Pellets were resuspended in a buffer containing 50 mM Tris-HCl, pH 7.5, 2% SDS and protease inhibitor cocktail, briefly sonicated, and centrifuged (19,000 g, 15 min, RT), and the supernatants were collected (TX-insoluble fraction). TX-soluble and insoluble fractions were subjected to a 5 min boil at 100°C followed by a brief sonication. After determination of the protein concentration with a bicinchoninic acid assay kit (Pierce, Rockford, IL), the following was added to each sample: β-mercaptoethanol (358 mM), bromophenol blue (0.005%), glycerol (20%), and SDS (4%) in stacking gel buffer (0.1M Tris-Cl, pH 6.8). Fifty micrograms of protein/lane were analyzed by SDS-PAGE on polyacrylamide gels followed by Western blot analysis. Ubiquitinated proteins (8% gels) were detected in the TX-insoluble fractions with the anti-ubiquitin antibody (1:1,500, DAKO Corp., Carpinteria, CA). No ubiquitinated proteins were detected in the TX-soluble fraction (not shown).
Proteasome dβ5 subunit
Flies were harvested in a buffer containing 50 mM Tris-HCl, pH 7.5, 20% glycerol, 1% Nonidet P-40, 1 mM EDTA, 2 mM EGTA, 274 mM sodium chloride, 50 mM sodium fluoride, 2.5 mM sodium pyrophosphate, 1 mM sodium vanadate, 1 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail (Sigma, St. Louis, MO, USA). After a brief sonication, lysates were centrifuged (19,000 g, 15 min, RT). Supernatants were collected and subjected to a 5 min boil at 100°C. After determination of the protein concentration with a bicinchoninic acid assay kit (Pierce, Rockford, IL, USA) samples were processed as described above. The dβ5 proteasome subunit (12% gels) was detected with our anti-dβ5 affinity purified antibody (1:4000, BioSynthesis). Upon incubation with the secondary antibody, antigens were visualized by a standard chemiluminescent horseradish peroxidase method with the ECL reagent.
Fly survival
Mixed populations of females and males for each age group were analyzed. Survival curves were generated by counting the number of dead flies at specific time intervals as indicated.
Statistical analysis
Statistical comparisons were performed with the unpaired t test (for two groups) or Tukey-Kramer (for more than two groups) with Instat 2.0 GraphPad Software (San Diego, CA, USA).
RESULTS
The peptidase activities of the proteasome are lower in old (43–47 days) than in young (1–2 days) flies
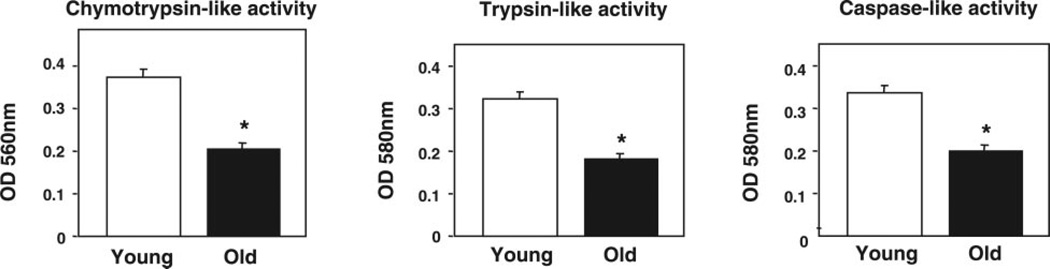
We assessed the effect of aging on the peptidase activities of the proteasome in fly lysates as described in Materials and Methods. Mixed populations of females and males from each age group were analyzed. The proteasome chymotrypsin-like activity was assayed with Suc-LLVY-AMC, the trypsin-like activity was assayed with Z-GGR-NA, and the caspase-like activity was assayed with Z-LLE-NA. The three peptidase activities of the proteasome were consistently and significantly (P≤0.0001) lower (≤60%) in old than in young flies (Fig. 1). The chromogenic substrates used to assess proteasome activity in total fly lysates may also be cleaved by other nonproteasomal proteases and thus may not specifically reflect proteasome activity in total lysates. To overcome this difficulty, we also assessed proteasome activity by an in-gel assay (Figs. 3, 4) and following glycerol density gradient centrifugation (Fig. 7 and 8).
Figure 1.
Proteasome activities in young (1–2 days) and old (43–47 days) flies. Mixed populations of females and males for each age group were analyzed. Proteasome activities from young (open bars) and old (solid bars) flies were measured in cleared supernatants obtained from total fly homogenates (20 µg of protein/sample). Peptidase activities were assayed colorimetrically after 24 h incubations at 37°C as described in Materials and Methods. The chymotrypsin-like activity was measured with Suc-LLVY-AMC, the caspase-like activity with Z-LLE-NA and the trypsin-like activity with Z-GGR-NA. Data represent the mean ± sem from five experiments. *Values in old flies significantly different (P≤0.0001) from young flies.
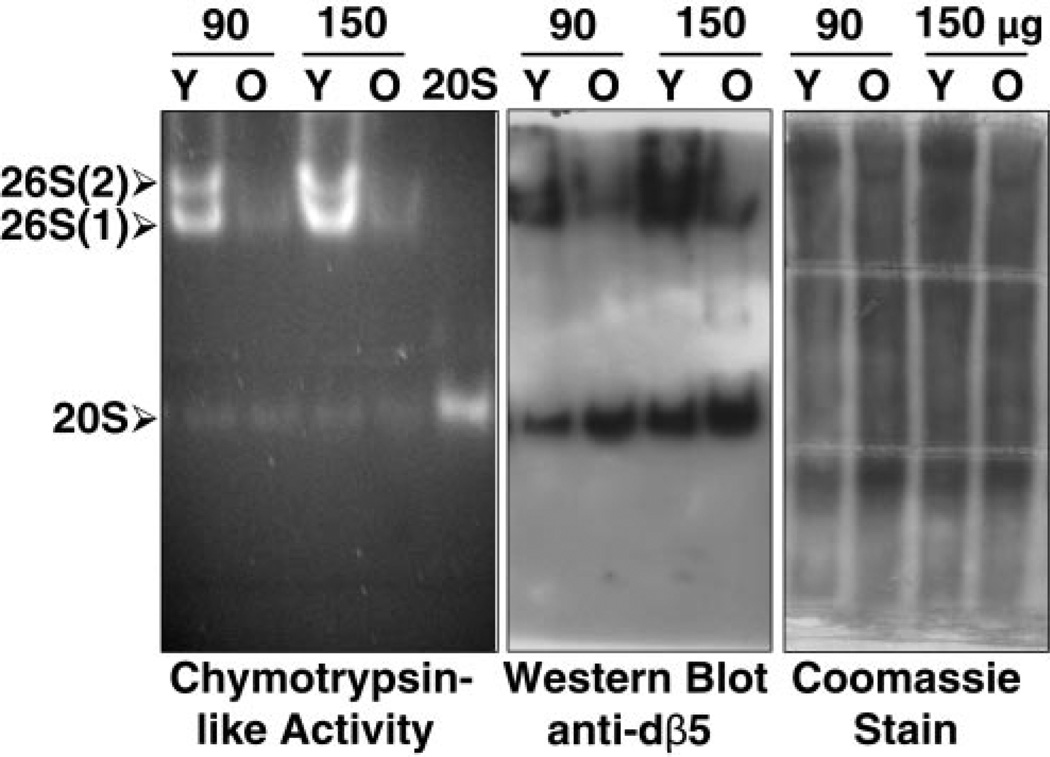
Figure 3.
Proteasome activity and levels in young (Y, 1–2 days) and old (O, 43–47 days) flies. Cleared fly lysates and 20S proteasomes partially purified from rabbit reticulocyte lysates (20S, as a marker) were subjected to nondenaturing gel electrophoresis, as described in Materials and Methods. Lysates from mixed populations of females and males of each age group were analyzed. The chymotrypsin-like activity was assessed with Suc-LLVY-AMC by the in-gel assay (left). 26S and 20S proteasomes were detected by immunobloting with our antibody that reacts with dβ5, a subunit of the core proteasome particle (middle). As indicated on the left, this antibody recognizes the symmetric [26S (2), two caps] and asymmetric [26S (1), one cap] 26S holoenzymes, as well the 20S core particle. Total protein pattern was established by Coomassie blue staining of native gels (right) following assessment of proteasome activity with Suc-LLVY-AMC. Similar results were obtained in at least quadruplicate experiments.
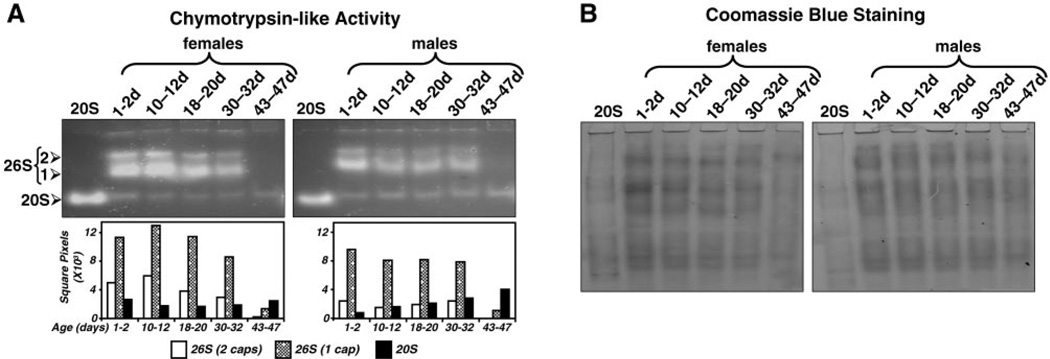
Figure 4.
Proteasome activity in female and male flies across different ages. Separate groups of females or males of different ages (1–2, 10–12, 18–20, 30–32 and 43–47 days) were analyzed. Cleared lysates, as well as 20S proteasomes, partially purified from rabbit reticulocyte lysates (20S, as a marker) were subjected to nondenaturing gel electrophoresis as described in Materials and Methods. Each lane with fly samples was loaded with an equal amount of protein (50 µg). A) The chymotrypsin-like activity was assessed with Suc-LLVY-AMC by an in-gel assay. The symmetric [26S (2), two caps] and asymmetric [26S (1), one cap] 26S holoenzymes, as well the 20S core particle are indicated on the left. Activity bands were semiquantified by densitometry as described in Materials and Methods (graph: open bars, 26S, two caps; stippled bars, 26S, one cap; solid bars, 20S). B) Protein pattern was established by Coomassie blue staining following assessment of proteasome activity with Suc-LLVY-AMC. Only the top halves of the activity gels are shown in A, as no signals were detected on the bottom halves. The entire Coomasie blue-stained gels are shown in B. Similar results were obtained in duplicate experiments.
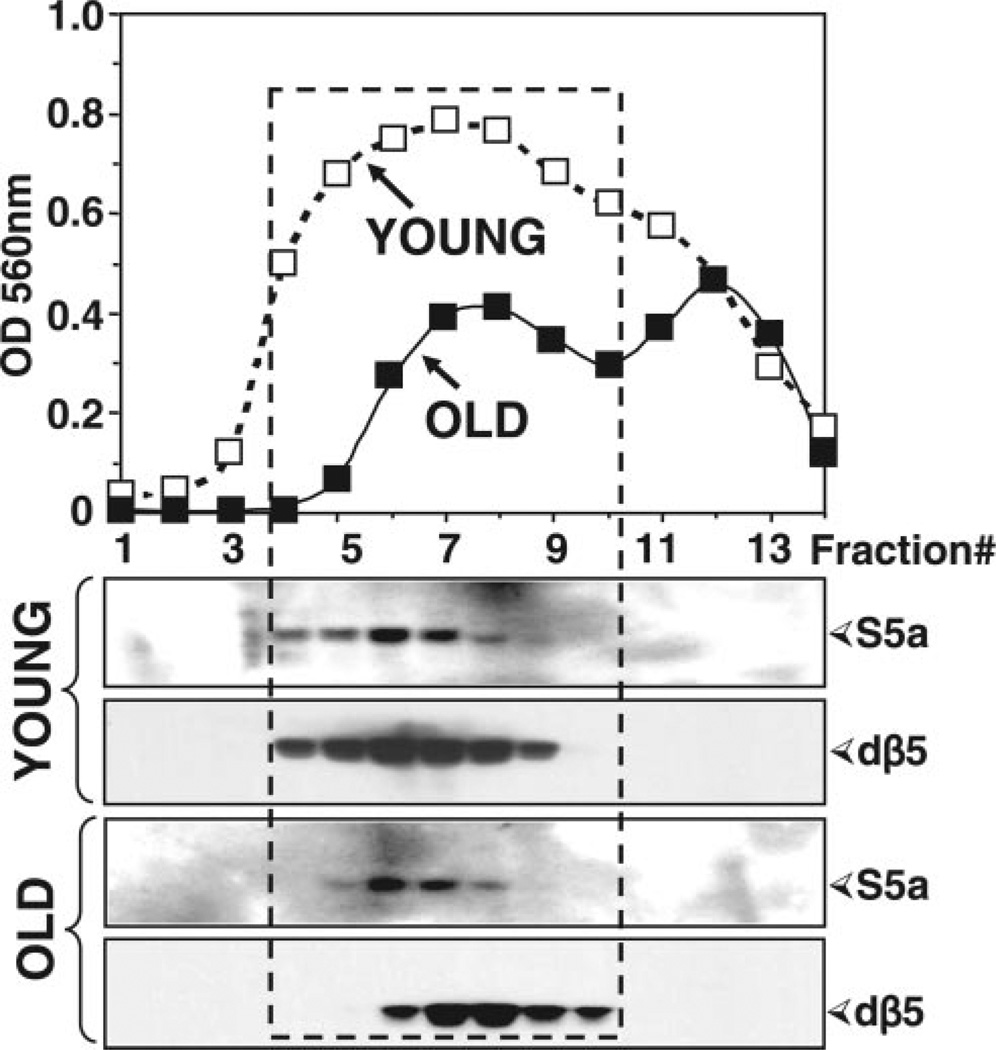
Figure 7.
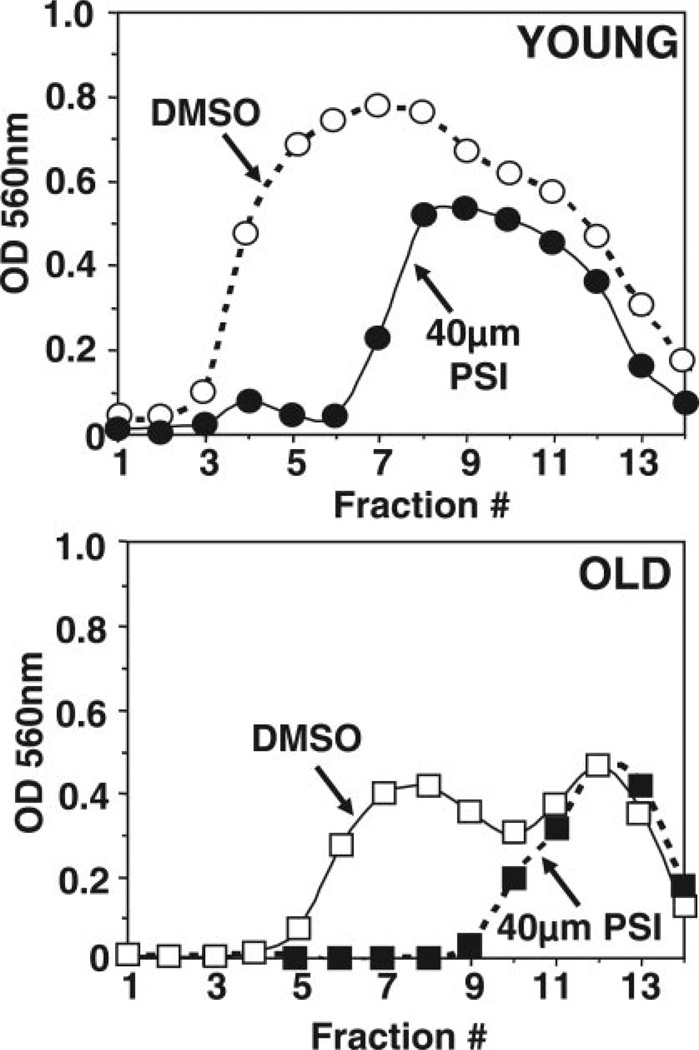
Sedimentation velocity analysis of proteasomes from young (1–2 days) and old (43–47 days) flies. Mixed populations of females and males for each age group were analyzed. Lysates (4.5 mg protein) were fractionated by glycerol density gradient centrifugation (10–40% glycerol corresponding to fractions 14 to 1). Top: Aliquots (50 µl) of each fraction obtained from young (open squares) and old (solid squares) flies were assayed for chymotrypsin-like activity with Suc-LLVY-AMC. Bottom: Immunoblot analysis of each fraction probed with our anti-dβ5 (core particle) and the anti-S5a (19S regulatory particle, Rpn10) antibodies. Proteins were precipitated with acetone from 700 µl of each fraction. The box indicates the elution of proteasome fractions. Similar results were obtained in duplicate experiments.
Figure 8.
Sedimentation velocity analysis of the chymotrypsin-like activity from young (1–2 days) and old (43–47 days) flies assayed in the absence and presence of the proteasome inhibitor PSI (40 µM). Mixed populations of females and males for each age group were analyzed. Lysates (4.5 mg protein) were fractionated by glycerol density gradient centrifugation (10–40% glycerol, fractions 14 to 1). Aliquots (50 µl) of each fraction from young (top, circles) and old (bottom, squares) flies were assayed for chymotrypsin-like activity with Suc-LLVY-AMC in the absence (open symbols) and presence (solid symbols) of the proteasome inhibitor PSI (40 µM). Similar results were obtained in duplicate experiments.
26S Proteasome assembly and activity are impaired in old (43–47 days) flies
We postulated that the decline in proteasome activity observed in old flies could be due to impaired proteasome assembly. To test this premise, we compared proteasome activity in flies of different age groups. Proteasome activity in cleared supernatants was assessed by an in-gel assay as described in Materials and Methods. No proteasome activity could be detected in the residual pellet fraction (not shown). As a marker, an aliquot of partially purified 20S proteasomes from rabbit reticulocyte lysates (20S) was run in a parallel lane.
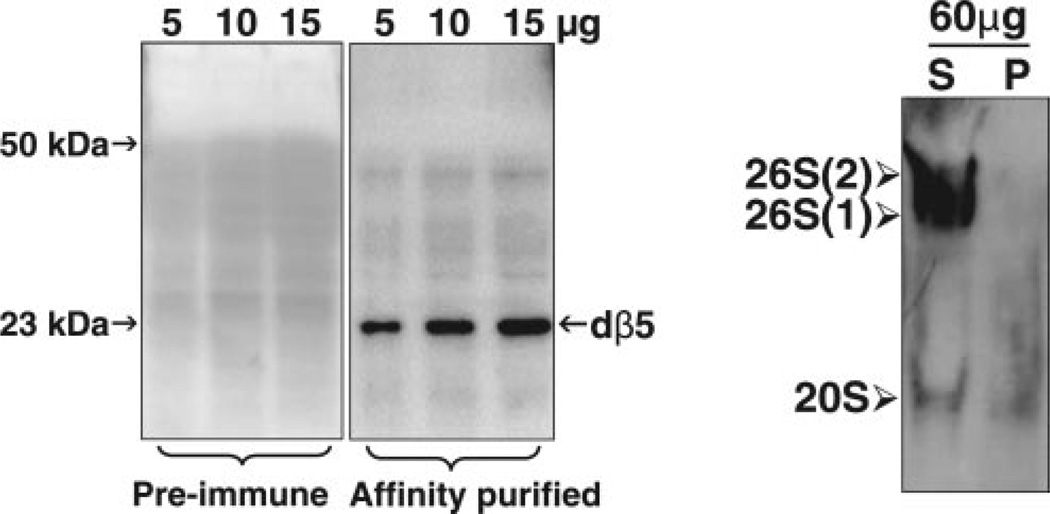
We also assessed proteasome levels with our peptide generated anti-dβ5 specific antibody (BioSynthesis). This antibody reacts with one band (23 kDa) corresponding to the mature dβ5 subunit of the proteasome core particle, when cleared supernatants were analyzed by Western blot analysis under denaturing conditions (Fig. 2, left). Proteasome immunoreactivity levels in the supernatant and pellet fractions obtained from total fly homogenates were assessed by Western blot analysis under native conditions, as described in Materials and Methods. As shown in Fig. 2 (right), the cleared supernatant fraction contained almost all of the proteasome immunoreactivity with very little being detected in the residual pellet. The latter contains mostly insoluble debris and chitin.
Figure 2.
Assessing proteasome levels in flies. Mixed populations of young females and males were analyzed. Left: The specificity of our anti-dβ5 antibody was assessed by Western blot analysis under denaturing conditions, as described under Materials and Methods. Cleared supernatants from total fly homogenates were analyzed with preimmune serum (left) or an antidβ5 (right) affinity purified antibody (1:4000) generated at BioSynthesis, as described in Materials and Methods. The dβ5 proteasome subunit migrates as a ~23-kDa band. Right: Proteasome levels were assessed by Western blot analysis under nondenaturing conditions as described under Materials and Methods. Crude extracts were homogenized, sonicated, and centrifuged. Cleared supernatant (S) and pellet (P) fractions (60 µg of protein per lane) were subjected to nondenaturing gel electrophoresis and Western blot analysis as described under Materials and Methods. 26S and 20S proteasomes in fly extracts were detected by immunobloting with our anti-dβ5 antibody. This antibody reacts with a subunit of the core proteasome particle, thus recognizing both the 20S and 26S proteasome forms. Symmetric [26S (2), two caps] and asymmetric [26S (1), one cap] 26S holoenzymes and the 20S core particle are indicated on the left.
Proteasome levels and activity (Fig. 3) from mixed populations of females and males were compared between two age groups: young (1–2 days) and old (43–47 days). In young flies, most of the proteasome activity assessed with the short substrate Suc-LLVY-AMC coincided with the 26S holoenzyme (not the 20S) form of the proteasome (Fig. 3, left). The ingel chymotrypsin-like activity assay revealed that the proteasome activity in young flies (Y) coincided almost exclusively with the 26S holoenzyme, in its symmetrical (two capped) and asymmetrical (one capped) forms. Only extremely low levels of chymotrypsin-like activity were associated with the 20S proteasome, demonstrating that the 26S holoenzyme is the most active in vivo form of the proteasome in young flies. In contrast, the chymotrypsin-like activity of both the 26S and the 20S proteasomes was low in old flies (O).
Immunoblot analysis of the native gels with our antid-β5 antibody revealed that, in young flies, proteasomes were detected as the 26S holoenzyme in its symmetric (two caps) and asymmetric (one cap) forms, as well as the 20S proteasome (Fig. 3, middle). In contrast, in old flies, proteasomes were found almost exclusively as 20S particles (Fig. 3, middle). These findings establish that low-activity 20S proteasomes prevail in old flies, while the assembled 26S holoenzyme is highly active in young flies.
Following assessment of proteasome activity with Suc-LLVY-AMC, native gels were stained with Coomassie blue (Fig. 3, right). The protein pattern of young and old flies was slightly but consistently different. This difference was not caused by postharvesting protein degradation, as similar patterns were observed when flies were harvested with or without a protease inhibitor cocktail (not shown). In addition, it is clear from the Western blot analysis that 20S proteasome levels are higher in old than in young flies (Fig. 3, middle) attesting that the difference in Coomassie blue staining is not due to less protein loading for old flies.
26S Proteasome activity declines sharply in 43- to 47-day-old female and male flies
We assessed proteasome activity with the in-gel assay in separate populations of females or males across five different ages: 1–2, 10–12, 18–20, 30–32 and 43–47 days (Fig. 4A). Each lane with fly samples was loaded with an equal amount of protein (50 µg). These studies clearly demonstrate that a sharp decline in 26S proteasome activity (by ≥89%) was observed in 43–47-day-old flies compared to young 1–2-day-old flies. This major decline in 26S proteasome activity was observed in both female (Fig. 4A, left) and male (Fig. 4A, right) 43–47-day-old flies. On the contrary, the activity of the 20S proteasome was similar or slightly increased across the age groups in both genders (Fig. 4A and semiquantification shown in the graph). Protein pattern was determined by Coomassie blue staining of the native gels following proteasome activity assessment. As shown in Fig. 4B, the protein pattern of the 43–47-day-old fly group is different from that of the other age groups. This pattern was consistently observed in all experiments. It is clear that the 20S proteasome activity band is similar or slightly stronger in the 43–47-day-old flies than in the other age groups (Fig. 4A), attesting that the difference in Coomassie blue staining is not due to less protein loading for old flies.
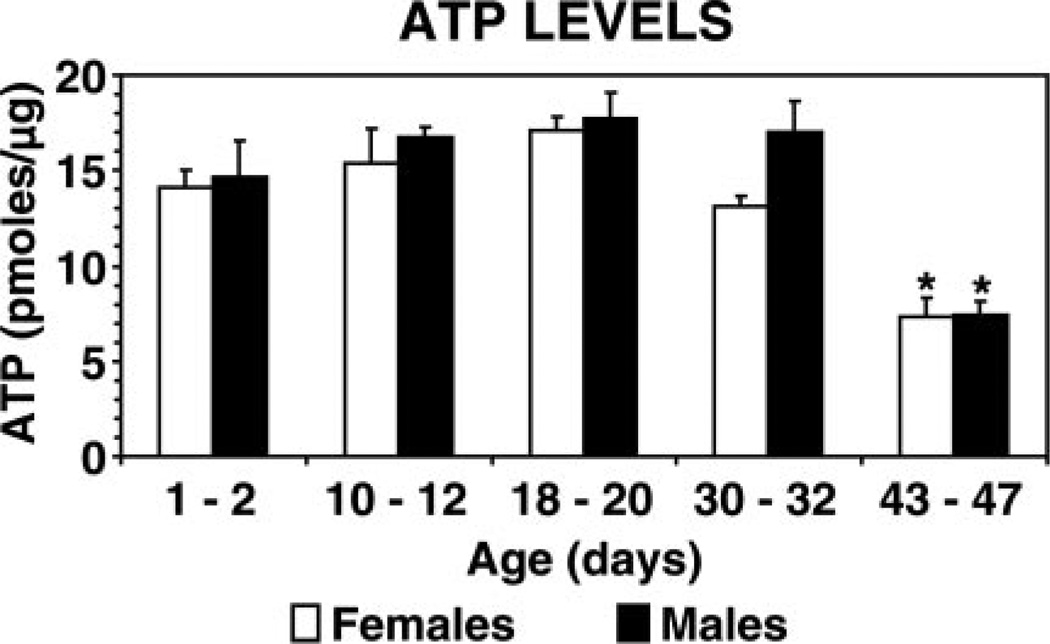
ATP steady state levels decrease by ~50% in 43–47-day-old female and male flies
The assembly of the 26S proteasome is known to be ATP-dependent (17); thus we assessed ATP steady-state levels in the flies. ATP levels were analyzed in separate populations of female or male flies at five different ages: 1–2, 10–12, 18–20, 30–32 and 43–47 days. ATP levels were maintained at an average of 15.7 pmol/µg of protein in flies of both genders up to the age of 30–32 days (Fig. 5). Remarkably, in female and male 43–47-day-old flies, ATP levels decreased by more than 50% to 7.4 pmol/µg protein.
Figure 5.
ATP steady-state levels in female and male flies across different ages. Separate groups of females (open bars) and males (solid bars) of different ages (1–2, 10–12, 18–20, 30–32 and 43–47 days) were analyzed. ATP concentrations (pmoles/µg of protein) in cleared supernatants were determined as described in Materials and Methods. Data represent the mean ± sem from four trials (15 flies per trial) per age group. *Values that are significantly different (P<0.05) from young (1–2 days of age) flies.
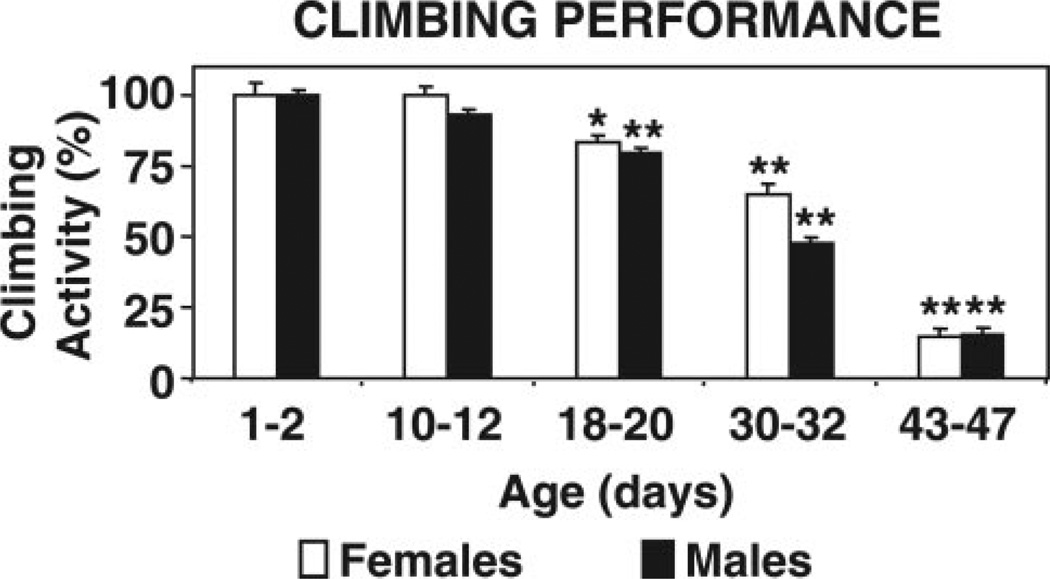
Climbing performance drops markedly in 43–47-day-old female and male flies
Generally, as flies get older they manifest locomotor dysfunction (16). We assessed locomotor performance in flies of different ages to compare the age at which they display locomotor dysfunction and proteasome impairment. Locomotor performance was measured with the climbing assay. Drosophila display a strong negative geotactic response by quickly rising to the top of a vial upon being tapped to its bottom. When tested for their climbing ability, 43–47-day-old females and males exhibited a marked (P<0.001) decline in climbing performance compared to young (1–2 days of age) flies (Fig. 6). The oldest flies (43–47 days) made short, abortive climbs and fell back to the bottom. Flies between 18 and 32 days of age also displayed a reduction in climbing ability, albeit not as steep as the oldest 43–47-day-old flies.
Figure 6.
Climbing performance of female and male flies across different ages. Separate groups of females (open bars) and males (solid bars) of different ages (1–2, 10–12, 18–20, 30–32, and 43–47 days) were analyzed. Locomotor performance was assessed with a climbing assay as described in Materials and Methods. Data represent the mean ± sem from three trials (10 flies per trial) per age group. *Values that are significantly different (*P<0.05, **P<0.001) from young (1–2 days of age) flies.
Different fractionation pattern of proteasomes from young (1–2 days) and old (43–47 days) flies
To corroborate that the proteasomal chymotrypsin-like activity was lower in old than in young flies, total extracts from each age group (4.5 mg/sample) were fractionated by glycerol density gradient centrifugation. These studies focused on the two age groups that exhibited the greatest change: young (1–2 days) and old (43–47 days) flies. We also analyzed mixed populations of female and male flies, since in the above described studies, no obvious difference was detected between genders.
Fractions were analyzed for chymotrypsin-like activity with Suc-LLVY-AMC. As shown in Fig. 7 (top), the chymotrypsin-like activity of old flies (solid squares) was significantly reduced when compared to young flies (open squares), particularly in the fractions corresponding to the proteasome elution position (Fig. 7, box, fractions 4–10).
To establish which fractions correspond to the proteasome, aliquots from each fraction were subjected to Western blot analysis with our anti-dβ5 antibody, which reacts with a subunit of the core particle, and with the anti-S5a antibody, which reacts with a non-ATPase subunit (Rpn10) of the 19S particle (Fig. 7, bottom). Immunodetection of the dβ5 subunit revealed a pronounced shift of the dβ5 peak in old flies toward lower molecular weight fractions corresponding to the 20S proteasome. Furthermore, the levels of the S5a subunit are clearly lower in the old flies than in the young ones. From these experiments, we can conclude that in old flies, the 20S is the predominant proteasome form.
Notably, we observed an additional peak of chymotrypsin-like activity that did not correspond to fractions containing proteasome subunits. This peak exhibited lower molecular weight than the proteasome-containing fractions (Fig. 7). That these fractions are not related to the proteasome is further demonstrated by their lack of sensitivity to PSI, a proteasome inhibitor (Fig. 8). This finding is not surprising, since it is well established that the substrate Suc-LLVY-AMC is cleaved not only by the proteasome (18) but also by other chymotrypsin-like proteases, as well as by calpains (19). In contrast to proteasome activity, the nonproteasomal chymotrypsin-like activity does not appear to be affected by aging (Fig. 7).
Old (43–47 days) flies are more sensitive to the proteasome inhibitor PSI than young (1–2 days) flies
Mixed populations of females and males from each age group were tested. We compared the in vivo sensitivity of young and old flies to the proteasome inhibitor PSI by feeding them a sucrose/PSI (10 µM) diet as described in Materials and Methods. At this concentration, we previously showed that PSI significantly inhibits the chymotrypsin-like activity of the proteasome in mammalian cell cultures (20).
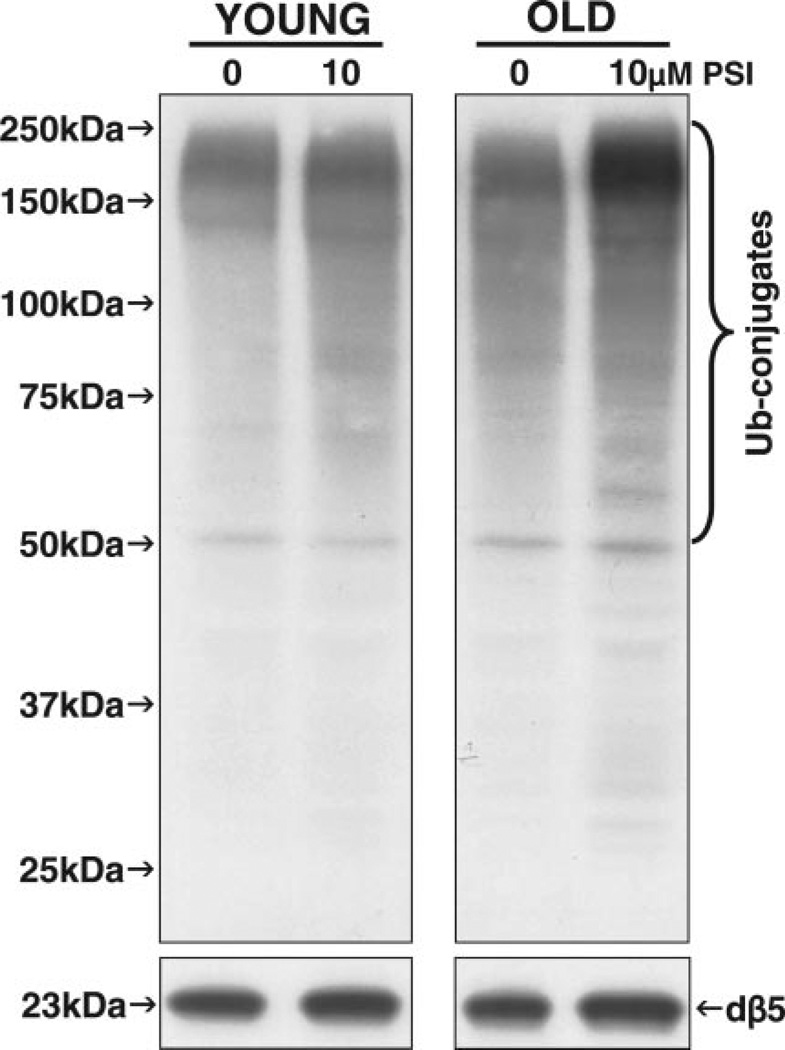
The decrease in proteasome activity did not induce the accumulation of ubiquitinated proteins in young flies, as observed by Western blot analysis with a specific antibody that detects polyubiquitinated proteins (Fig. 9, top left). However, the in vivo administration of PSI induced the accumulation of ubiquitinated proteins in old flies (Fig. 9, top right). In addition, no apparent changes in the levels of the proteasome subunit dβ5 were detected in young vs. old flies following PSI administration (Fig. 8, bottom). The latter subunit, dβ5, accounts for the chymotrypsin-like activity, which carries out the rate-limiting step in protein degradation by the proteasome (21).
Figure 9.
Polyubiquitinated protein and proteasome dβ5 subunit levels in young (1–2 days) and old (43–47 days) flies. Mixed populations of females and males for each age group were analyzed. Flies were fed 5% sucrose with DMSO (0, vehicle, control, 0.5%) or with a proteasome inhibitor (PSI, 10 µM in 0.5% DMSO) for 48 h after being starved for 6 h. The levels of polyubiquitin-protein conjugates (Ub-conjugates) and of a proteasome subunit (dβ5) were detected by Western blot analysis as described in Materials and Methods. Fifty micrograms of protein were loaded per lane. Similar results were obtained in duplicate experiments.
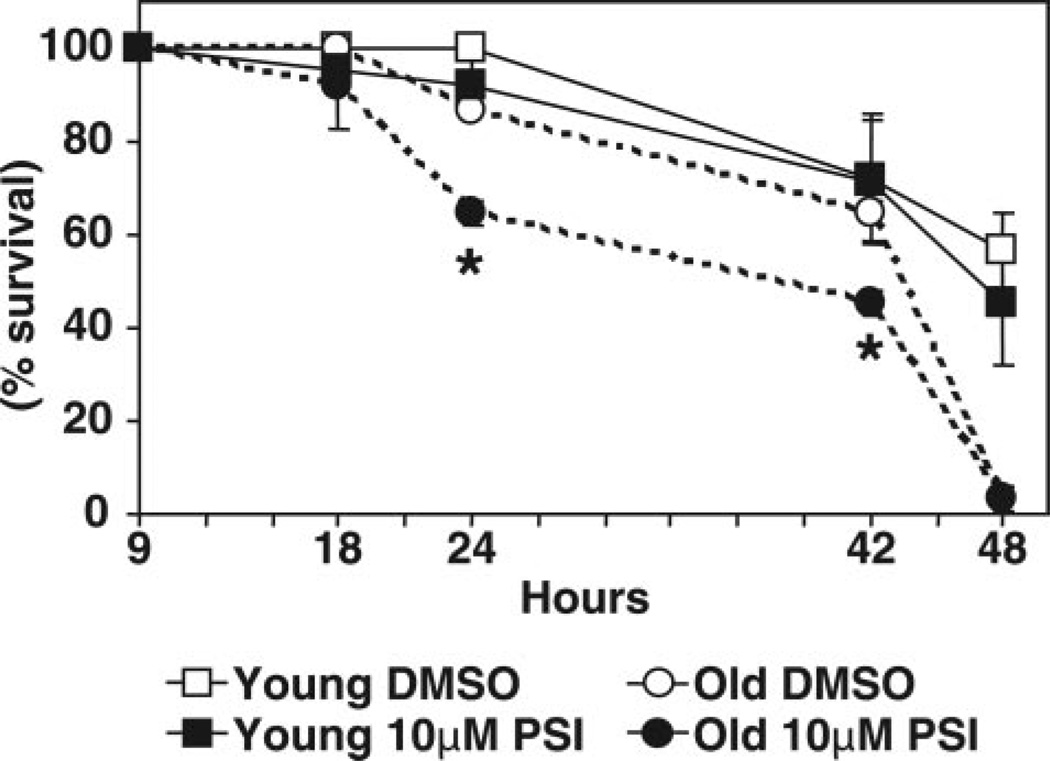
A significant shortening (20%) of life span was observed in old flies upon the in vivo administration of PSI for 24 h and 42 h (Fig. 10). In contrast, the life span of young flies was not altered by feeding them the proteasome inhibitor.
Figure 10.
Survival curves for young (1–2 days) and old (43–47 days) flies. Mixed populations of both females and males for each age group were analyzed. Flies were kept in narrow Drosophila vials, which were lined with 3 mm filter paper. Flies were starved for 6 h and then fed a solution of 5% (weight/vol) sucrose with either vehicle (0.5% DMSO, control) or with the proteasome inhibitor PSI (10 µM, in DMSO) for 48 h. Dead flies were counted after the time periods indicated (n=30 flies for each treatment). Data represent the mean ± sem from 3 experiments. *Values that are significantly different (P<0.05) between control and PSI-treated flies for each time point within each age group.
DISCUSSION
In this paper, we show for the first time that the aging process is associated with the disassembly of the 26S proteasome with a clear loss of its activity. The decline in 26S holoenzyme levels in old flies (43–47 days of age) coincides with increased levels of the 20S proteasome. Clearly, the activity of the 20S proteasome failed to decline. However, our studies demonstrate that the free 20S core particle is nearly inactive in all flies independently of their age group. Accordingly, the majority of the proteasome chymotrypsin-like activity coincides with the 26S proteasome, the levels of which decline sharply in 43–47-day-old female and male flies. That the 20S proteasome is almost inactive is not particular to flies, as a similar phenomenon was observed in yeast (22). It is well established that in the outer rings of the 20S core particle, the conformation of the α subunits is such that it seals the entrance into the catalytic chamber (23). Activation of the 20S proteasome thus requires disruption of the inflexible and passive barrier provided by the outer α rings. The opening of these gates to the catalytic chamber is triggered by regulatory complexes, such as the 19S and the 11S regulatory particles [reviewed in (24)]. In vitro studies with purified 20S proteasomes demonstrated that the closed-gate conformation is also destabilized by the binding of some hydrophobic peptides to noncatalytic sites on the core particle (25) or by certain proteins, such as p21 and α-synuclein (26). The latter mechanism for activation of the 20S core particle has not yet been demonstrated to occur in vivo. However, it was proposed that because proteasomes diffuse rapidly in the cytoplasm and nucleus of mammalian cells, they may continuously collide with some of their substrates (27).
The assembly of the 26S proteasome from its 20S core and 19S regulatory particles is an ATP-dependent process (17, 28). The concentration of ATP required for half-maximal 26S proteasome assembly was estimated to be 5 µM (29). Although the mechanism of assembly of the 26S proteasome is not completely understood, it is known that the molecular chaperone Hsp90 plays a role in this process (30) and that the 20S and 19S particles undergo a structural rearrangement to produce the optimal conformation of the full complex (31). ATP hydrolysis is also required for the actual degradation process of polyubiquitinated proteins, with an estimated Km of 12 µM (29). ATP hydrolysis is known to promote substrate unfolding and protein translocation through the 26S holoenzyme with an apparent 300–400 ATP molecules being hydrolyzed during the degradation of one molecule of globular or unfolded substrate (32). Recent studies suggest that ATP hydrolysis is also necessary for the rapid dissociation of the 26S proteasome into the 19S and 20S particles (33). This dissociation coincides with release of the products of degradation. In conclusion, ATP hydrolysis is a pivotal requirement not only for the assembly-disassembly cycle of the 26S holoenzyme but also for its function, i.e., to degrade polyubiquitinated proteins.
Our current data show that in the oldest flies tested (43–47 days of age) ATP steady state levels are reduced by more than 50%. A previous study with Drosophila also reported a decline (15%) in ATP levels with age, although only males were included in these experiments (10). Furthermore, a microarray analysis with Drosophila indicated that its metabolism is severely affected by aging (34). Accordingly, genes linked to oxidative phosphorylation, TCA cycle, and ATP production were found to be down-regulated in 40-day-old compared to 3-day-old flies. The decline in ATP steady-state levels together with our findings described above, strongly support the notion that ATP depletion associated with the aging process may have a critical impact on 26S proteasome activity by impairing, for example, its assembly. The distribution of ATP in cells seems to be compartmentalized with different ATP pools sustaining different cell functions. For example, while ATP produced by oxidative phosphorylation sustains contractility in smooth muscle cells, ATP generated from anaerobic glycolysis supports plasma membrane proton pumps (35, 36). Which ATP pool supports 26S proteasome activity is currently undetermined.
Changes in total cellular ATP caused by different metabolic conditions are not reflected uniformly in all intracellular compartments (37). In cultured cells, when both glycolysis in the cytosol and oxidative phosphorylation in mitochondria were active, mitochondrial ATP was double that of other cellular compartments tested, i.e., cytosol, subplasma membrane region, and nucleus. When oxidative phosphorylation was impaired, ATP levels were equilibrated in all four compartments, suggesting that the bulk of ATP was produced by glycolysis. When oxidative phosphorylation was the sole source of ATP, its levels were normal in the cytosol and subplasma membrane region but collapsed in mitochondria and nucleus (37).
The aging process is known to affect mitochondrial function and to be associated with mitochondrial genome changes, including point mutations and modifications, as well as deletions [reviewed in (38)]. Interestingly, aging in Drosophila seems to be associated with a decrease in the levels of mitochondrial RNA (39, 40). Furthermore, a decline in skeletal muscle mitochondrial function was observed with aging in humans (41, 42), and premature aging was induced in mice expressing defective mitochondrial DNA polymerase (43). On the other hand, caloric restriction, the only experimental paradigm known to extend life span in organisms ranging from yeast to mammals, was shown to promote mitochondrial biogenesis in mice (44). In addition, calorie restriction limits the generation but not the progression of mitochondrial abnormalities in aging skeletal muscle (45). Dietary restriction also seems to prevent a state of persistent glycolysis associated with ad libitum-fed animals [reviewed in (46)]. Most glycolytic intermediates are potentially toxic because they are able to glycate proteins and other macromolecules nonenzymatically [reviewed in (46)]. In conclusion, suppression of the aging process requires, among other factors, maintenance of mitochondrial function and a reduction in persistent glycolysis. Our studies uncover a potential novel factor, that is, maintenance of 26S proteasome assembly and ensuing activity, which may be intricately related to the other two. Accordingly, 26S proteasome assembly is ATP dependent (28), and glycated-proteins may escape proteasomal degradation (47).
Interestingly, we established that the oldest group of flies tested (43–47 days of age) exhibited a marked decline in locomotor activity assessed with a climbing assay. These data demonstrate that the steep decrease in 26S proteasome activity and ATP levels in flies is an “old-age” event that is observed when flies exhibit a major decline in locomotor performance.
Our studies also revealed that old flies are significantly more sensitive to the peptide aldehyde PSI, which is a proteasome inhibitor. The increased sensitivity was manifested by accumulation of ubiquitinated proteins and a shorter life span in old flies but not in young ones. Interestingly, yeast cells continue to grow when 70 –80% of their proteasome activity is inhibited by peptide aldehydes or β-lactone (48). This finding suggests that the capacity of the proteasome in yeast exceeds the required activity under homeostatic conditions. Thus, a 20–30% proteasome capacity is sufficient for yeast cell survival and growth under homeostatic conditions. It is possible that in young flies, there is also a surplus of proteasome activity. Therefore, feeding young flies sublethal concentrations of PSI has no apparent effect on survival or the levels of polyubiquitinated proteins. On the other hand, in old flies the observed disassembled state of the 26S proteasome severely diminishes its activity, therefore rendering old flies exceptionally sensitive to proteasome inhibitors.
Our finding that the disassembly of the 26S proteasome is an “old-age” event could explain the presence of protein deposits containing ubiquitinated proteins and oxidatively modified proteins in nonpathologic aging (3), as well as in a variety of aging-related neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases and amyotrophic lateral sclerosis, to name a few (4). The reduction in assembled 26S proteasomes concurrent with ATP depletion not only will affect normal protein turnover but also most likely deprives old flies of the ability to effectively cope with proteotoxic damages caused by lifelong environmental and/or genetic factors.
Acknowledgments
We would like to dedicate this manuscript to the memory of Dr. Cecile Pickart, an outstanding scientist who will always be missed in the years to come. This work was supported by the National Institutes of Health (NIGMS-GM60654) to M.E.F.-P and T.S.-G., NINDS-NS41073 (SNRP), M.E.F.-P head of subproject and NCRR-RR03037 to Hunter College of CUNY), and the City University of New York.
REFERENCES
- 1.Longo VD. Search for methuselah genes heats up (Online) Sci. Aging. Knowledge. Environ. 2004;2004:e6. doi: 10.1126/sageke.2004.6.pe6. [DOI] [PubMed] [Google Scholar]
- 2.Aigaki T, Seong KH, Matsuo T. Longevity determination genes in Drosophila melanogaster. Mech. Ageing. Dev. 2002;123:1531–1541. doi: 10.1016/s0047-6374(02)00089-1. [DOI] [PubMed] [Google Scholar]
- 3.Gray DA, Tsirigotis M, Woulfe J. Ubiquitin, proteasomes, and the aging brain (Online) Sci. Aging Knowledge Environ. 2003;2003:RE6. doi: 10.1126/sageke.2003.34.re6. [DOI] [PubMed] [Google Scholar]
- 4.Lowe J, Blanchard A, Morrell K, Lennox G, Reynolds L, Billett M, Landon M, Mayer RJ. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson’s disease, Pick’s disease, and Alzheimer’s disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and mallory bodies in alcoholic liver disease. J. Pathol. 1988;155:9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- 5.Alves-Rodrigues A, Gregori L, Figueiredo-Pereira ME. Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci. 1998;21:516–520. doi: 10.1016/s0166-2236(98)01276-4. [DOI] [PubMed] [Google Scholar]
- 6.Chondrogianni N, Gonos ES. Proteasome dysfunction in mammalian aging: steps and factors involved. Exp. Gerontol. 2005;40:931–938. doi: 10.1016/j.exger.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Carrard G, Bulteau A, Petropoulos I, Friguet B. Impairment of proteasome structure and function in aging. Int. J. Biochem. Cell Biol. 2002;34:1461–1474. doi: 10.1016/s1357-2725(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 8.Keller JN, Gee J, Ding Q. The proteasome in brain aging. Ageing Res. Rev. 2002;1:279–293. doi: 10.1016/s1568-1637(01)00006-x. [DOI] [PubMed] [Google Scholar]
- 9.Gaczynska M, Osmulski PA, Ward WF. Caretaker or undertaker? The role of the proteasome in aging. Mech. Ageing Dev. 2001;122:235–254. doi: 10.1016/s0047-6374(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 10.Schwarze SR, Weindruch R, Aiken JM. Oxidative stress and aging reduce COX I RNA and cytochrome oxidase activity in Drosophila. Free Radic. Biol. Med. 1998;25:740–747. doi: 10.1016/s0891-5849(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 11.Ashburner M. Drosophila: A Laboratory Handbook. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1989. [Google Scholar]
- 12.Wilk S, Orlowski M. Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex. J. Neurochem. 1983;40:842–849. doi: 10.1111/j.1471-4159.1983.tb08056.x. [DOI] [PubMed] [Google Scholar]
- 13.Elsasser S, Schmidt M, Finley D. Characterization of the proteasome using native gel electrophoresis. Methods Enzymol. 2005;398:353–363. doi: 10.1016/S0076-6879(05)98029-4. [DOI] [PubMed] [Google Scholar]
- 14.Glickman MH, Rubin DM, Fried VA, Finley D. The regulatory particle of the Saccharomyces cerevisiae proteasome. Mol. Cell. Biol. 1998;18:3149–3162. doi: 10.1128/mcb.18.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Blanco A, Fridell YW, Helfand SL. Involvement of Drosophila uncoupling protein 5 in metabolism and aging. Genetics. 2006;172:1699–1710. doi: 10.1534/genetics.105.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orso G, Martinuzzi A, Rossetto MG, Sartori E, Feany M, Daga A. Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J. Clin. Invest. 2005;115:3026–3034. doi: 10.1172/JCI24694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu CW, Li X, Thompson D, Wooding K, Chang TL, Tang Z, Yu H, Thomas PJ, DeMartino GN. ATP binding and ATP hydrolysis play distinct roles in the function of 26S proteasome. Mol. Cell. 2006;24:39–50. doi: 10.1016/j.molcel.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein RL, Melandri F, Dick L. Kinetic characterization of the chymotryptic activity of the 20S proteasome. Biochemistry. 1996;35:3899–3908. doi: 10.1021/bi952262x. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki T, Kikuchi T, Yumoto N, Yoshimura N, Murachi T. Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J. Biol. Chem. 1984;259:12489–12494. [PubMed] [Google Scholar]
- 20.Figueiredo-Pereira ME, Berg KA, Wilk S. A new inhibitor of the chymotrypsin-like activity of the multicatalytic proteinase complex (20S proteasome) induces accumulation of ubiquitin- protein conjugates in a neuronal cell. J. Neurochem. 1994;63:1578–1581. doi: 10.1046/j.1471-4159.1994.63041578.x. [DOI] [PubMed] [Google Scholar]
- 21.Groll M, Heinemeyer W, Jager S, Ullrich T, Bochtler M, Wolf DH, Huber R. The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10976–10983. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajorek M, Finley D, Glickman MH. Proteasome disassembly and downregulation is correlated with viability during stationary phase. Curr. Biol. 2003;13:1140–1144. doi: 10.1016/s0960-9822(03)00417-2. [DOI] [PubMed] [Google Scholar]
- 23.Groll M, Bajorek M, Kohler A, Moroder L, Rubin DM, Huber R, Glickman MH, Finley D. A gated channel into the proteasome core particle. Nat. Struct. Biol. 2000;7:1062–1067. doi: 10.1038/80992. [DOI] [PubMed] [Google Scholar]
- 24.Forster A, Hill CP. Proteasome degradation: enter the substrate. Trends Cell Biol. 2003;13:550–553. doi: 10.1016/j.tcb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Kisselev AF, Kaganovich D, Goldberg AL. Binding of hydrophobic peptides to several non-catalytic sites promotes peptide hydrolysis by all active sites of 20 S proteasomes. Evidence for peptide-induced channel opening in the alpha-rings. J. Biol. Chem. 2002;277:22260–22270. doi: 10.1074/jbc.M112360200. [DOI] [PubMed] [Google Scholar]
- 26.Liu CW, Corboy MJ, DeMartino GN, Thomas PJ. Endoproteolytic activity of the proteasome. Science. 2003;299:408–411. doi: 10.1126/science.1079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reits EAJ, Benham AM, Plougastel B, Neefjes J, Trowsdale J. Dynamics of proteasome distribution in living cells. EMBO J. 1997;16:6087–6094. doi: 10.1093/emboj/16.20.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eytan E, Ganoth D, Armon T, Hershko A. ATP-dependent incorporation of 20S protease into the 26S complex that degrades proteins conjugated to ubiquitin. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7751–7755. doi: 10.1073/pnas.86.20.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman L, Rechsteiner M. Effects of nucleotides on assembly of the 26S proteasome and degradation of ubiquitin conjugates. Mol. Biol. Rep. 1997;24:13–16. doi: 10.1023/a:1006892220996. [DOI] [PubMed] [Google Scholar]
- 30.Imai J, Maruya M, Yashiroda H, Yahara I, Tanaka K. The molecular chaperone Hsp90 plays a role in the assembly and maintenance of the 26S proteasome. EMBO J. 2003;22:3557–3567. doi: 10.1093/emboj/cdg349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurucz E, Ando I, Sumegi M, Holzl H, Kapelari B, Baumeister W, Udvardy A. Assembly of the Drosophila 26 S proteasome is accompanied by extensive subunit rearrangements. Biochem. J. 2002;365:527–536. doi: 10.1042/BJ20011520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benaroudj N, Zwickl P, Seemuller E, Baumeister W, Goldberg AL. ATP hydrolysis by the proteasome regulatory complex PAN serves multiple functions in protein degradation. Mol. Cell. 2003;11:69–78. doi: 10.1016/s1097-2765(02)00775-x. [DOI] [PubMed] [Google Scholar]
- 33.Babbitt SE, Kiss A, Deffenbaugh AE, Chang YH, Bailly E, Erdjument-Bromage H, Tempst P, Buranda T, Sklar LA, et al. ATP hydrolysis-dependent disassembly of the 26S proteasome is part of the catalytic cycle. Cell. 2005;121:553–565. doi: 10.1016/j.cell.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Girardot F, Lasbleiz C, Monnier V, Tricoire H. Specific age-related signatures in Drosophila body parts transcriptome. BMC Genomics. 2006;7:69. doi: 10.1186/1471-2164-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saks VA, Khuchua ZA, Vasilyeva EV, Belikova OY, Kuznetsov AV. Metabolic compartmentation and substrate channelling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration—a synthesis. Mol. Cell. Biochem. 1994;133–134:155–192. doi: 10.1007/BF01267954. [DOI] [PubMed] [Google Scholar]
- 36.Ishida Y, Riesinger I, Wallimann T, Paul RJ. Compartmentation of ATP synthesis and utilization in smooth muscle: roles of aerobic glycolysis and creatine kinase. Mol. Cell. Biochem. 1994;133–134:39–50. doi: 10.1007/BF01267946. [DOI] [PubMed] [Google Scholar]
- 37.Gajewski CD, Yang L, Schon EA, Manfredi G. New insights into the bioenergetics of mitochondrial disorders using intracellular ATP reporters. Mol. Biol. Cell. 2003;14:3628–3635. doi: 10.1091/mbc.E02-12-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CM, Weindruch R, Aiken JM. Age-associated alterations of the mitochondrial genome. Free Radic. Biol. Med. 1997;22:1259–1269. doi: 10.1016/s0891-5849(96)00546-1. [DOI] [PubMed] [Google Scholar]
- 39.Calleja M, Pena P, Ugalde C, Ferreiro C, Marco R, Garesse R. Mitochondrial DNA remains intact during Drosophila aging, but the levels of mitochondrial transcripts are significantly reduced. J. Biol. Chem. 1993;268:18891–18897. [PubMed] [Google Scholar]
- 40.Schwarze SR, Weindruch R, Aiken JM. Decreased mitochondrial RNA levels without accumulation of mitochondrial DNA deletions in aging Drosophila melanogaster. Mutat. Res. 1998;382:99–107. doi: 10.1016/s1383-5726(97)00013-7. [DOI] [PubMed] [Google Scholar]
- 41.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, Aiken JM. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am. J. Hum. Genet. 2006;79:469–480. doi: 10.1086/507132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly Y, Gidlof S, Oldfors A, Wibom R, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 44.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 45.Bua E, McKiernan SH, Aiken JM. Calorie restriction limits the generation but not the progression of mitochondrial abnormalities in aging skeletal muscle. FASEB J. 2004;18:582–584. doi: 10.1096/fj.03-0668fje. [DOI] [PubMed] [Google Scholar]
- 46.Hipkiss AR. On the mechanisms of ageing suppression by dietary restriction-is persistent glycolysis the problem? Mech. Ageing Dev. 2006;127:8–15. doi: 10.1016/j.mad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Bulteau AL, Verbeke P, Petropoulos I, Chaffotte AF, Friguet B. Proteasome inhibition in glyoxal-treated fibroblasts and resistance of glycated glucose-6-phosphate dehydrogenase to 20 S proteasome degradation in vitro. J. Biol. Chem. 2001;276:45662–45668. doi: 10.1074/jbc.M105374200. [DOI] [PubMed] [Google Scholar]
- 48.Lee DH, Goldberg AL. Selective inhibitors of the proteasome-dependent and vacuolar pathways of protein degradation in Saccharomyces cerevisiae. J. Biol. Chem. 1996;271:27280–27284. doi: 10.1074/jbc.271.44.27280. [DOI] [PubMed] [Google Scholar]