Abstract
Global change is challenging plant and animal populations with novel environmental conditions, including increased atmospheric CO2 concentrations, warmer temperatures, and altered precipitation regimes. In some cases, contemporary or “rapid” evolution can ameliorate the effects of global change. However, the direction and magnitude of evolutionary responses may be contingent upon interactions with other community members that also are experiencing novel environmental conditions. Here, we examine plant adaptation to drought stress in a multigeneration experiment that manipulated aboveground–belowground feedbacks between plants and soil microbial communities. Although drought stress reduced plant growth and accelerated plant phenologies, surprisingly, plant evolutionary responses to drought were relatively weak. In contrast, plant fitness in both drought and nondrought environments was linked strongly to the rapid responses of soil microbial community structure to moisture manipulations. Specifically, plants were most fit when their contemporary environmental conditions (wet vs. dry soil) matched the historical environmental conditions (wet vs. dry soil) of their associated microbial community. Together, our findings suggest that, when faced with environmental change, plants may not be limited to “adapt or migrate” strategies; instead, they also may benefit from association with interacting species, especially diverse soil microbial communities, that respond rapidly to environmental change.
Keywords: plant–microbe interaction, contemporary evolution, climate change, phenology, species interaction
Climate change is a major threat to biodiversity (1–3). Some species may cope with climate change through ecological strategies, including phenotypic plasticity, behavioral modifications, or migration to more favorable locations (4–8). Alternatively, species may require rapid evolutionary adaptation to persist under novel environmental conditions (9–12). Rapid evolution has been documented in response to global-change scenarios such as climate warming and altered precipitation patterns (e.g., refs. 13 and 14; reviewed in refs. 15 and 16). However, it is unknown whether such evolutionary changes occur rapidly enough to rescue populations from the negative consequences of global change.
Often, evolutionary and ecological responses to global change involve interactions with other species (17–20, reviewed in ref. 21). The myriad of biotic interactions that occur in natural communities can be important mediators of adaptation to global change for several reasons. First, the responses of nontarget taxa to environmental change can mitigate or magnify the demographic consequences of global change for focal populations. For example, the catastrophic effects of global warming on coral reef communities are greatly diminished when corals are colonized by particular clades of thermal-tolerant zooxanthellae symbionts (22). Second, tradeoffs between traits mediating biotic interactions and traits underlying adaptation to global change may hinder (or in some cases facilitate) evolutionary responses (23, 24). For example, insect herbivores appear to inhibit adaptive responses of native plants to biological invasions, likely because of genetic tradeoffs between traits mediating interactions with herbivores and exotic plant competitors (23, 24). These complex species interactions in natural communities can make the evolutionary consequences of global change difficult to predict, but understanding adaptation in a community context is necessary for assessing species’ responses to global change and identifying factors that contribute to adaptive responses to novel environments.
Natural plant populations interact with a diverse community of belowground microorganisms. Many of the global-change drivers that affect plant populations, such as rising CO2 concentrations, global warming, and altered precipitation regimes, simultaneously influence the abundance and composition of microbial communities (25). Several studies have shown that plant adaptation to certain stressors (e.g., salt, temperature, and heavy metal contamination) is facilitated by genetic changes in populations of closely associated microbial symbionts (e.g., fungal endophytes or mycorrhiza) (e.g., refs. 26, 27, reviewed in ref. 28). If microorganisms commonly influence plant fitness responses to the types of novel stressors associated with global change, then it is possible that diverse, belowground microbial communities may help maintain plant fitness in rapidly changing environments. This traditionally overlooked process of adaptive plant responses to global change is potentially important because microorganisms often are considered to be less dispersal-limited and more evolutionarily labile than their plant hosts (29, 30). However, the relative importance of microorganisms to adaptation, compared with genetic changes in the plants themselves, is yet to be determined.
Global climate models predict changes in precipitation (31, 32) that are likely to affect plant populations and their belowground microorganisms simultaneously and interactively (33). Here, we report on a multigeneration selection experiment that manipulated the soil-moisture environment of replicated plant populations and associated microbial communities for three plant generations and possibly for hundreds of microbial generations. Subsequently, we conducted a reciprocal transplant experiment to disentangle how plant evolutionary history and microbe history independently or interactively influenced plant growth and fitness in contemporary (wet vs. dry) soil environments. Our main experimental goals were (i) to determine whether rapid evolutionary changes in plant populations are important mediators of plant fitness responses to novel soil-moisture environments, (ii) to assess how biotic interactions with belowground microbial communities influence plant ecological and evolutionary responses to drought stress, and (iii) to evaluate evidence for plant–microbe coadaptation to a common global-change stressor. The results from our experiments provide insight into adaptive plant responses to global change by identifying the relative importance of rapid evolution of plant populations vs. changes in the genetic or community composition of the associated belowground microbial community.
Results and Discussion
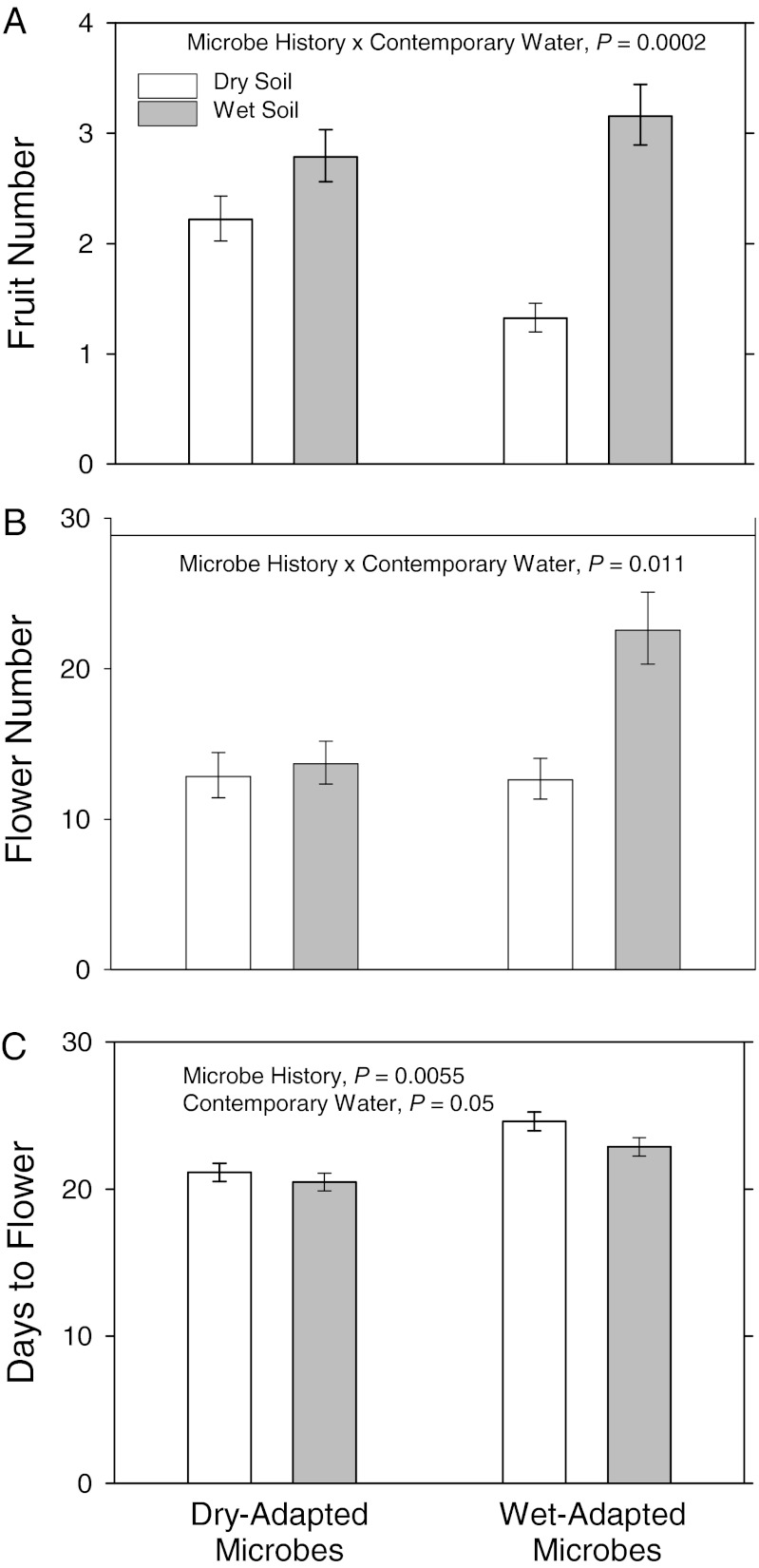
Plant fitness responses to drought stress were governed more by rapid changes in belowground microbial communities than by the rapid evolution of plant traits. Belowground microbial communities responded to multigeneration soil-moisture treatments in ways that increased plant growth and fitness. Both male and female components of plant fitness (flower and fruit production, respectively) increased when plants were grown in association with microorganisms that were adapted to the contemporary soil-moisture environment (Fig. 1 A and B). Surprisingly, plant evolutionary history (i.e., multigenerational exposure to moisture treatments) had no significant effect on fitness response to drought (fruit number: P > 0.39; flower number: P > 0.84), indicating that the adaptive plant responses observed in our experiment were driven primarily by changes in the soil microbial community rather than by genetic changes in the plants themselves.
Fig. 1.
Effects of microbe history (dry- or wet-adapted) and contemporary soil moisture (dry or wet soil) on plant fruit number (A), flower number (B), and flowering date (C). Error bars indicate back-transformed least squares means ± 1 SEM.
Effects of a Novel Stressor on Plant Fitness.
The predicted changes in precipitation and expected increases in drought stress in many regions throughout the world are likely to reduce the productivity of plant communities and the fitness of focal plant taxa (34). Altered precipitation regimes also have been shown to influence the structure and function of microbial communities (25, 35). In our study, both fruit and flower production (i.e., female and male fitness components) were lower in contemporary dry soil treatments than in contemporary wet soil treatments (Fig. 1 A and B), consistent with the negative fitness effects that commonly are observed under drought conditions. However, the magnitude of this effect was contingent upon microbe history (microbe history × contemporary soil moisture interaction on fruit number, F1,254 = 14.55, P = 0.0002, and on flower number, F1,284 = 6.51, P = 0.011), and rapid changes in microbial communities mitigated the negative effects of drought on plant fitness. Drought caused a 58% decrease in fruit production when plants were grown in association with a wet-adapted microbial community but only a 20% decrease in fruit number when plants were grown in association with a dry-adapted microbial community (Fig. 1A). Similarly, growing in the presence of wet-adapted microbes allowed plants to respond more positively to well-watered environmental conditions; specifically, plants increased flower production in wet environments but only when grown in association with microbes adapted to wet environmental conditions (Fig. 1B). In sum, plants were more fit when they were grown in the presence of a microbial community adapted to contemporary environmental conditions.
Changes in belowground microbial communities were the largest driver of adaptive plant responses to drought stress observed in our study. In contrast to the strong and consistent effects of microbe history on plant fitness components, plant history did not affect plant fruit or flower number responses to drought (fruit number: P > 0.39; flower number: P > 0.84), even though several traits showed that evolutionary responses to three generations of selection in different soil-moisture treatments were possible (significant plant history × microbe history interactions on biomass and fruit number; see below). Combined, the strong effects of microbe history and weak effects of plant history show that adaptive plant responses to drought stress are driven more by changes in the belowground microbial community than by rapid evolutionary changes in the plant populations. These findings illustrate a potentially widespread means for plant populations to maintain high fitness in the face of novel stressors.
Effects of Global Change on Plant Phenological Traits.
By shifting the timing of important developmental stages, plants can mitigate the negative consequences of global change (14). For example, accelerated flowering is a common plastic (36) and evolved (14, 37, 38) response to drought stress that allows plants to avoid the most serious consequences of drought by flowering early and escaping the periods of lowest water availability. However, much of the drought-induced acceleration of flowering time that we observed can be attributed to changes in the belowground microbial community rather than to plant evolutionary responses to drought stress or even plastic responses to contemporary soil-moisture conditions. We detected no evolutionary shift in flowering time between plant populations that had experienced three generations of wet vs. dry conditions (F1,10 = 2.61, P = 0.14) and only minimal effects of contemporary soil-moisture treatments on flowering time: Contemporary drought delayed flowering by 1 d (F1,10 = 4.93, P = 0.05). Instead, flowering was accelerated by 3 d when plants were grown in association with dry-adapted microbial communities, regardless of contemporary environmental conditions (F1,6 = 17.82, P = 0.0055; Fig. 1C).
Microbe Responses to Drought.
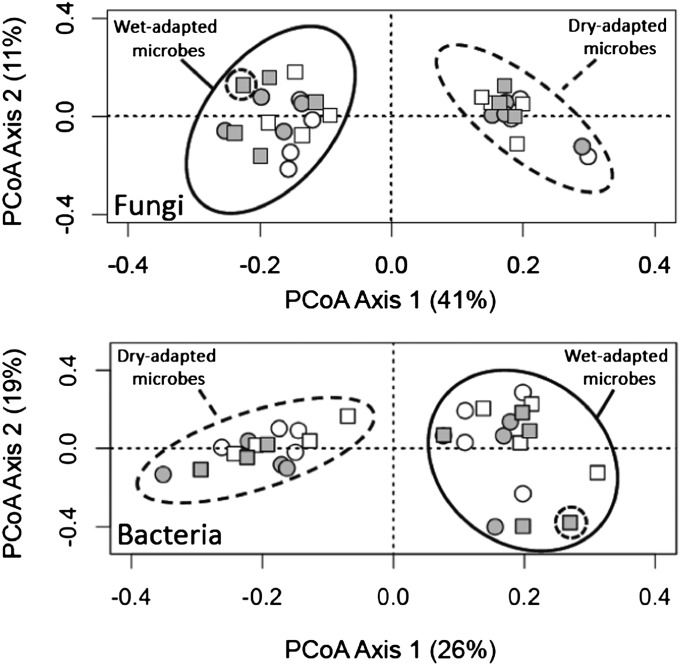
Global environmental changes influence the structure and function of microbial communities (25). For example, the diversity and composition of soil microorganisms shift along precipitation gradients and are sensitive to drought events (35, 39–41). Similarly, we found that prolonged drought stress affected soil microbial communities in a number of ways. Microbial communities from historically wet treatments had higher bacterial richness [means ± 1 SE: dry 80.4 ± 1.92 operational taxonomic units (OTUs), wet 87.2 ± 1.92 OTUs; F1,24 = 6.31, P = 0.019], and historical exposure to contrasting soil-moisture regimes (dry vs. wet) altered the composition of bacterial [permutation-based multivariate ANOVA (PERMANOVA), F1,24 = 7.42, P = 0.001] and fungal communities (PERMANOVA, F1,24 = 14.56, P = 0.001), explaining 20% and 33% of the variation in the relative abundances of bacterial and fungal OTUs, respectively (Fig. 2). These changes may be the result of the direct physiological effects of soil moisture on belowground microbial communities. For example, in the absence of plants, soil bacteria and fungi exhibit interspecific variation for optimum water potential and tolerance to drought stress, two components of the soil-moisture niche axis. As a result, some specialized taxa are likely to be restricted to high soil-moisture environments, whereas other taxa are habitat generalists capable of surviving at much lower soil water potentials (42). Alternatively, changes in microbial structure between moisture treatments may reflect plant-mediated indirect effects. For example, soil microorganisms may respond to altered carbon (C) inputs (via litter or root exudates) when plants are drought stressed (43). Although we did not detect significant effects of the long-term soil-moisture treatments on soil C (F1,22 = 2.06, P = 0.17), other types of global change (e.g., elevated atmospheric CO2 concentrations) have been shown to alter plant–soil feedbacks in ways that influence microbial communities (44). Regardless of whether the drought effects were direct or indirect, the microbial communities that developed under prolonged moisture treatments were rather resistant to changes in watering regimes during the reciprocal transplant experiment: Contemporary soil-moisture treatments explained only 6% (PERMANOVA, F1,24 = 2.04, P = 0.039) and 4% (PERMANOVA, F1,24 = 1.84, P = 0.097) of the variation in bacterial and fungal community composition, respectively.
Fig. 2.
Multivariate ordination showing the effects of microbe history, plant history, and contemporary soil moisture on microbial community composition. Microbe history affected the composition of soil fungi (Upper) and bacteria (Lower), as indicated by the strong separation of samples along PCoA axis 1. Dashed ellipses contain dry-adapted microbial assemblages, and solid ellipses include wet-adapted microbial assemblages. PERMANOVA confirmed the effect of the microbe-history treatment on both fungal and bacterial composition (P = 0.001). Although not as strong as microbe history, contemporary soil moisture (white symbols, dry; gray symbols, wet) significantly affected the composition of bacteria (P = 0.039) and marginally affected the composition of fungi (P = 0.096). In contrast, plant history (dry plants, circles; wet plants, squares) had no effect on fungal or bacterial composition (P = 0.55 and P = 0.22, respectively). Ordinations were created with the output of PCoA, and the percent variation explained by each PCoA axis is presented in parentheses in each axis label.
Microbe-Mediated Indirect Effects on Plants.
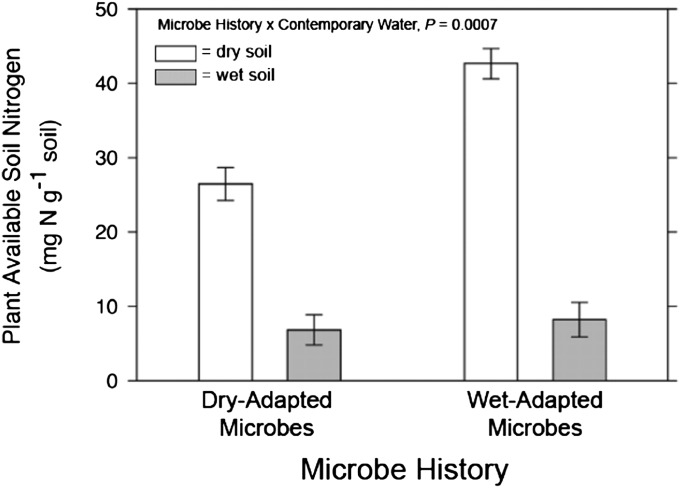
The moisture-mediated shifts in belowground microbial communities may have affected plant fitness responses to drought stress in two main ways. First, shifts in microbial community composition and bacterial diversity could be linked to changes in biogeochemical processes that influence the availability of resources, such as nitrogen (N), that commonly limit plant growth and fitness. For example, when challenged with drought conditions, N availability was 60% higher in soils with wet-adapted microbial communities than in soils with dry-adapted microbial communities (Fig. 3, microbe history × contemporary moisture, F1,8 = 29.04, P = 0.0007; also see Figs. S1 and S2), possibly because prolonged drought stress reduced the abundance and activity of certain guilds of soil microorganisms that are responsible for important N transformations (45, 46). Second, given that drought stress altered the composition of both bacterial and fungal communities, drought stress may have changed the relative abundances of mutualists and pathogens and also may have affected the fitness benefits of associating with mutualists (47) and susceptibility to pathogens (48).
Fig. 3.
Microbe history and contemporary soil moisture (dry, white bars; wet, gray bars) altered plant-available soil N (NH4+ and NO3−). Plant-available N was higher in dry contemporary soil-moisture treatments than in wet contemporary soil-moisture treatments, especially for soils containing a wet-adapted microbial community (microbe history × contemporary moisture, F1,8 = 29.04, P = 0.0007). Error bars indicate least squares means ± 1 SEM.
Plant–Microbe Coadaptation to Drought Stress.
Plant population responses to global change may depend on evolutionary responses of plants, ecological and/or evolutionary responses of associated microorganisms, or interactions between plant and microbial responses. Although much of the adaptive plant response to drought stress observed in our experiment can be attributed to changes in the belowground microbial community, we also detected some evidence for interactions between plant history and microbial history on plant growth and fitness traits. Plants produced slightly more fruits and biomass when there was a mismatch between plant history and microbe history, independent of the contemporary soil-moisture environment [plant history × microbe history interactions on fruit number (F1,252 = 8.40, P = 0.0041), and biomass (F1,94 = 5.27, P = 0.024)]. These interactions between plant evolutionary history and microbe history are most likely the result of the microbially mediated expression of genetic differences in plant traits.
Conclusion.
The ability of natural plant populations to maintain high fitness in the face of rapid anthropogenic environmental change is crucial to their long-term persistence. However, fitness responses to global change can be influenced strongly by interactions with other community members. Here we show that adaptive plant responses to drought stress were governed largely by the responses of belowground microbial communities. Associating with a microbial community adapted to contemporary environmental conditions—whether wet or dry—increased plant fitness, and even evolutionarily naive plant populations maintained high fitness under drought stress when grown in association with a drought-adapted microbial community. Our findings highlight that rapid adaptation to novel environments occurs in a community context and demonstrate that plant responses to novel stressors can be influenced heavily by the response of closely associated microbial communities that simultaneously are experiencing novel environmental conditions. These results suggest that plants may not be limited to “adapt or migrate” strategies (49) for persisting in the face of anthropogenic environmental change and instead may be facilitated by rapid responses of the surrounding biotic community.
Materials and Methods
Selection Experiment.
To investigate how both plant populations and belowground microbial communities respond to soil moisture, we conducted a “selection in a controlled environment experiment” (50). We planted replicate Brassica rapa populations into wet (high soil moisture) and dry (low soil moisture) mesocosms (n = 4 mesocosms per soil-moisture treatment). Each mesocosm consisted of a large (76-L) pot filled with steam-sterilized (121 °C, 15 psi, 16 h) potting medium (one part Baccto High Porosity Mix (Michigan Peat Company): one part perlite: one part vermiculate). We used potting medium for this experiment, rather than field soil, so that we could maintain relatively consistent abiotic soil conditions across generations. Each mesocosm was inoculated with 3 L of intact field soil at the beginning of the experiment to provide each mesocosm with a natural soil microbial community (see ref. 51 for details). We then sowed 128 B. rapa seeds into each mesocosm at 4-cm spacing. All mesocosms were watered until seeds germinated, after which we ceased watering the mesocosms in the dry soil-moisture treatments but continued to water the wet soil-moisture treatments (ca. 1–1.5 L per mesocosm every other day). As plants flowered, they were hand-pollinated by using a soft paintbrush to collect pollen from other flowering individuals in the same mesocosm and depositing pollen on all receptive stigmas. We harvested plants as they senesced but before silique dehiscence (fruit opening).
To begin each subsequent generation of selection, we counted all seeds and randomly selected 128 seeds from each mesocosm for use in the next generation of the experiment. We reestablished each mesocosm by removing half the existing soil and mixing in an equal volume of freshly sterilized potting medium. In this way we were able to maintain relatively intact soil microbial communities in each mesocosm while minimizing nutrient drawdown resulting from differences in plant growth between the wet and dry soil-moisture treatments. We then sowed each population of seeds back into the mesocosm from which they were collected. This process was repeated for three plant generations.
After three plant generations, a randomly selected subset of 125 seeds per mesocosm was propagated in a common garden environment to reduce maternal environmental effects. Individual seeds were sown into 164-mL Cone-tainers (Ray Leach Cone-tainers; Stuewe & Sons Inc.) filled with LP5 potting medium (Sungro Horticulture Canada Ltd.). All plants were watered as needed to ensure that each planted seed reproduced successfully to reduce selection during the common garden generation. As above, plants were outcrossed with other individuals from the same population. Seeds obtained from the common garden generation were used in the reciprocal transplant experiment described below. During the common garden generation, a fourth plant generation was planted into the mesocosms so that the microbial community in each mesocosm experienced continual exposure to the same plant population and soil-moisture environment for ca. 16 mo.
Reciprocal Transplant Experiment.
To test for plant adaptation to soil-moisture environments and to determine whether microbial communities changed in response to soil moisture in ways that affected plant growth, we performed a full reciprocal transplant experiment. Offspring from plant populations that had experienced wet or dry environments for three generations were grown in association with microbial communities from wet or dry environments under either wet or dry contemporary environmental conditions. We filled 0.72-L pots with soil from one of the eight fourth-generation mesocosms (n = 10 pots per mesocosm, 80 pots total). One randomly selected seed from each of four different populations (two dry adapted and two wet adapted) was planted into each pot. Half the pots were assigned to dry contemporary soil-moisture treatments, and half were assigned to wet contemporary soil-moisture treatments. Pots assigned to wet treatments were kept consistently moist. Pots assigned to dry treatments were watered only when plants began to show signs of drought stress.
We measured plant phenological, growth, and fitness traits, including flowering date and plant height. We harvested each plant when it had ceased flowering and fruits were ripe. After harvest, we weighed aboveground vegetative biomass (after drying for 2 d at 65 °C) and counted flower, fruit, and seed numbers. We also weighed all seeds to calculate mean seed mass.
Microbial Responses.
Because we were interested in feedbacks between plants and microbes, we planted an additional set of pots for microbial and soil nutrient analysis. Each pot was filled with soil from one of the eight mesocosms; all seeds in a given pot were of a single plant history (wet or dry), and soil-moisture conditions were kept wet or dry (n = 160 pots total). To determine how microbial communities had changed in response to treatments, we collected soil samples from each pot after plant senescence. Genomic DNA was extracted from 1 g of each soil sample using an UltraClean Soil DNA Isolation Kit (Mo Bio Laboratories, Inc.). This DNA was used as a template in quantitative PCR assays to estimate total bacterial and fungal abundance. For fungi we used ITS1-F (forward) and 5.8S (reverse) primers, and for bacteria we used Eub338 (forward) and Eub518 (reverse) primers (52). To assess treatment effects on microbial community composition, we fingerprinted the soil microbial community using terminal restriction length polymorphism (T-RFLP). For fungi, we PCR-amplified DNA using a fluorescently (FAM-6) labeled ITS1-F forward primer, an unlabeled ITS4 reverse primer, and the thermal cycler pattern described elsewhere (53). For bacteria, we amplified DNA using a fluorescently (FAM-6) labeled 8F forward primer, an unlabeled 1492R reverse primer, and the thermal cycler pattern described elsewhere (54). We then digested the fluorescently labeled product and quantified the size of fluorescently labeled fragments in our samples by comparison with an internal ROX-labeled size standard (50–2,000 bp). Then OTU richness for bacterial and fungal samples was calculated by summing of peaks that were present in the fragment profiles. For additional details see ref. 45.
To determine whether observed differences in microbial communities (Results) were accompanied by changes in N availability, we estimated plant-available soil N (NH4+ and NO3−) from a subset of samples (n = 29) with KCl extractions followed by analysis on a Flow Solution IV analyzer (OI Analytical). We consider the sum of soil NH4+ and NO3− to be an estimate of plant-available N. Similarly, to determine whether long-term soil-moisture treatments altered total soil organic C and N, we estimated percent C, percent N, and C:N ratios on a subset of soil samples (n = 30 soil samples; three subsamples were analyzed per soil sample) with a Costech Model 4010 Elemental Combustion analyzer (Costech Analytical Technologies Inc.).
Statistical Analyses.
We used mixed-model ANOVA (Proc MIXED, SAS Institute 2001) to test for effects of plant history, microbe history, and contemporary soil-moisture conditions on plant and microbial response variables and soil characteristics. Significant plant-history effects indicate that plant populations had evolved in response to three generations of selection in wet or dry environments. Significant microbe-history effects indicate that changes in microbial community composition and/or genetic changes in microbial populations occurred in response to selection in wet or dry environments. Plant growth, phenological, and/or fitness traits were included as response variables; plant history (wet or dry), microbe history (wet or dry), contemporary soil-moisture environment (wet or dry), and all interactions were included as fixed factors. Microbe mesocosm (i.e., the mesocosm from which the soil and microbial communities were collected) nested within microbe history, plant population nested within plant history, and pot nested within microbe mesocosm × contemporary soil moisture interaction were included as random factors. Interactions between random factors and between random and fixed factors also were included in the model. Flower number and fruit number were natural log transformed to meet the assumptions of ANOVA.
Similar analyses were conducted on soil characteristics (plant-available N, percent C, and C:N ratios) and univariate microbial response variables, including the fungal-to-bacterial ratio, fungal OTU richness, and bacterial OTU richness. Also, we characterized the multivariate composition data generated from T-RFLP using principal coordinates analysis (PCoA) on Bray–Curtis distance matrices derived from relativized fluorescence data. In addition to visualizing the data via ordination plots, we used PERMANOVA with the adonis command in the vegan package of R (55). Using 1,000 permutations, we tested for the main effects and interactions among the plant history, microbe history, and contemporary soil-moisture treatments on relative OTU abundances.
Supplementary Material
Acknowledgments
We thank Sandy Erwin, Mark Hammond, Robert Snyder, and Brent Lehmkuhl for greenhouse and laboratory assistance. Sarah Evans, members of the J.A.L. laboratory, and two anonymous reviewers provided helpful comments on earlier versions of this manuscript. Financial support was provided by the Rackham Research Endowment and the Michigan Agricultural Experiment Station. This project was also supported by National Research Initiative Grants 2008-35107-04481 and 2011-67019-30225 (both to J.T.L.) from the US Department of Agriculture National Institute of Food and Agriculture. This is Kellogg Biological Station publication no.1596.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Dryad Repository, http://datadryad.org (DOI no. 10.5061/dryad.qc537).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202319109/-/DCSupplemental.
References
- 1.Maclean IMD, Wilson RJ. Recent ecological responses to climate change support predictions of high extinction risk. Proc Natl Acad Sci USA. 2011;108:12337–12342. doi: 10.1073/pnas.1017352108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimm SL. Climate disruption and biodiversity. Curr Biol. 2009;19:R595–R601. doi: 10.1016/j.cub.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 3.Sala OE, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 4.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- 5.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 6.Post E, et al. Ecological dynamics across the Arctic associated with recent climate change. Science. 2009;325:1355–1358. doi: 10.1126/science.1173113. [DOI] [PubMed] [Google Scholar]
- 7.Root TL, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 8.Sih A, Ferrari MCO, Harris DJ. Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl. 2011;4:367–387. doi: 10.1111/j.1752-4571.2010.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis MB, Shaw RG, Etterson JR. Evolutionary responses to changing climate. Ecology. 2005;86:1704–1714. [Google Scholar]
- 10.Ellner SP, Geber MA, Hairston NG., Jr Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecol Lett. 2011;14:603–614. doi: 10.1111/j.1461-0248.2011.01616.x. [DOI] [PubMed] [Google Scholar]
- 11.Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: Contemporary evolution and the dynamics of persistence. Funct Ecol. 2007;21:444–454. [Google Scholar]
- 12.Schoener TW. The newest synthesis: Understanding the interplay of evolutionary and ecological dynamics. Science. 2011;331:426–429. doi: 10.1126/science.1193954. [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw WE, Holzapfel CM. Genetic shift in photoperiodic response correlated with global warming. Proc Natl Acad Sci USA. 2001;98:14509–14511. doi: 10.1073/pnas.241391498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franks SJ, Sim S, Weis AE. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc Natl Acad Sci USA. 2007;104:1278–1282. doi: 10.1073/pnas.0608379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradshaw WE, Holzapfel CM. Climate change. Evolutionary response to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann AA, Sgrò CM. Climate change and evolutionary adaptation. Nature. 2011;470:479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- 17.de Mazancourt C, Johnson E, Barraclough TG. Biodiversity inhibits species’ evolutionary responses to changing environments. Ecol Lett. 2008;11:380–388. doi: 10.1111/j.1461-0248.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- 18.Harmon JP, Moran NA, Ives AR. Species response to environmental change: Impacts of food web interactions and evolution. Science. 2009;323:1347–1350. doi: 10.1126/science.1167396. [DOI] [PubMed] [Google Scholar]
- 19.Lau JA, Shaw RG, Reich PB, Tiffin P. Species interactions in a changing environment: Elevated CO2 alters the ecological and potential evolutionary consequences of competition. Evol Ecol Res. 2010;12:435–455. [Google Scholar]
- 20.van der Putten WH, Macel M, Visser ME. Predicting species distribution and abundance responses to climate change: Why it is essential to include biotic interactions across trophic levels. Philos Trans R Soc B Biol Sci. 2010;365:2025–2034. doi: 10.1098/rstb.2010.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. Global change and species interactions in terrestrial ecosystems. Ecol Lett. 2008;11:1351–1363. doi: 10.1111/j.1461-0248.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 22.Baker AC, Starger CJ, McClanahan TR, Glynn PW. Coral reefs: Corals’ adaptive response to climate change. Nature. 2004;430:741–741. doi: 10.1038/430741a. [DOI] [PubMed] [Google Scholar]
- 23.Lau JA. Beyond the ecological: Biological invasions alter natural selection on a native plant species. Ecology. 2008;89:1023–1031. doi: 10.1890/06-1999.1. [DOI] [PubMed] [Google Scholar]
- 24.Lau JA. Evolutionary responses of native plants to novel community members. Evolution. 2006;60:56–63. [PubMed] [Google Scholar]
- 25.Blankinship JC, Niklaus PA, Hungate BA. A meta-analysis of responses of soil biota to global change. Oecologia. 2011;165:553–565. doi: 10.1007/s00442-011-1909-0. [DOI] [PubMed] [Google Scholar]
- 26.Adriaensen K, Vrålstad T, Noben JP, Vangronsveld J, Colpaert JV. Copper-adapted Suillus luteus, a symbiotic solution for pines colonizing Cu mine spoils. Appl Environ Microbiol. 2005;71:7279–7284. doi: 10.1128/AEM.71.11.7279-7284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez RJ, et al. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008;2:404–416. doi: 10.1038/ismej.2007.106. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez R, Redman R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J Exp Bot. 2008;59:1109–1114. doi: 10.1093/jxb/erm342. [DOI] [PubMed] [Google Scholar]
- 29.Elena SF, Lenski RE. Evolution experiments with microorganisms: The dynamics and genetic bases of adaptation. Nat Rev Genet. 2003;4:457–469. doi: 10.1038/nrg1088. [DOI] [PubMed] [Google Scholar]
- 30.Finlay BJ. Global dispersal of free-living microbial eukaryote species. Science. 2002;296:1061–1063. doi: 10.1126/science.1070710. [DOI] [PubMed] [Google Scholar]
- 31.Weltzin JF, et al. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience. 2003;53:941–952. [Google Scholar]
- 32.IPCC . Climate Change 2007: The Physical Science Basis. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 33.Wardle DA, et al. Ecological linkages between aboveground and belowground biota. Science. 2004;304:1629–1633. doi: 10.1126/science.1094875. [DOI] [PubMed] [Google Scholar]
- 34.Zhao MS, Running SW. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science. 2010;329:940–943. doi: 10.1126/science.1192666. [DOI] [PubMed] [Google Scholar]
- 35.Sheik CS, et al. Effect of warming and drought on grassland microbial communities. ISME J. 2011;5:1692–1700. doi: 10.1038/ismej.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernal M, Estiarte M, Peñuelas J. Drought advances spring growth phenology of the Mediterranean shrub Erica multiflora. Plant Biol (Stuttg) 2011;13:252–257. doi: 10.1111/j.1438-8677.2010.00358.x. [DOI] [PubMed] [Google Scholar]
- 37.Franks SJ. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol. 2011;190:249–257. doi: 10.1111/j.1469-8137.2010.03603.x. [DOI] [PubMed] [Google Scholar]
- 38.Sherrard ME, Maherali H. The adaptive significance of drought escape in Avena barbata, an annual grass. Evolution. 2006;60:2478–2489. [PubMed] [Google Scholar]
- 39.Angel R, Soares MIM, Ungar ED, Gillor O. Biogeography of soil archaea and bacteria along a steep precipitation gradient. ISME J. 2010;4:553–563. doi: 10.1038/ismej.2009.136. [DOI] [PubMed] [Google Scholar]
- 40.Bachar A, et al. Soil microbial abundance and diversity along a low precipitation gradient. Microb Ecol. 2010;60:453–461. doi: 10.1007/s00248-010-9727-1. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz E, Adair KL, Schuur EA. Bacterial community structure correlates with decomposition parameters along a Hawaiian precipitation gradient. Soil Biol Biochem. 2007;39:2164–2167. [Google Scholar]
- 42.Lennon JT, Aanderud Z, Lehmkuhl B, Schoolmaster DR. Mapping the niche space of soil microorganisms using taxonomy and traits. Ecology. 2012;93:1867–1879. doi: 10.1890/11-1745.1. [DOI] [PubMed] [Google Scholar]
- 43.Palta JA, Gregory PJ. Drought affects the fluxes of carbon to roots and soil in C-13 pulse-labelled plants of wheat. Soil Biol Biochem. 1997;29:1395–1403. [Google Scholar]
- 44.Bardgett RD, Freeman C, Ostle NJ. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2008;2:805–814. doi: 10.1038/ismej.2008.58. [DOI] [PubMed] [Google Scholar]
- 45.Hastings RC, Butler C, Singleton I, Saunders JR, McCarthy AJ. Analysis of ammonia-oxidizing bacteria populations in acid forest soil during conditions of moisture limitation. Lett Appl Microbiol. 2000;30:14–18. doi: 10.1046/j.1472-765x.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 46.Szukics U, et al. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol Ecol. 2010;72:395–406. doi: 10.1111/j.1574-6941.2010.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Kloepper JW, Ryu CM. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14:1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Garrett KA, Dendy SP, Frank EE, Rouse MN, Travers SE. Climate change effects on plant disease: Genomes to ecosystems. Annu Rev Phytopathol. 2006;44:489–509. doi: 10.1146/annurev.phyto.44.070505.143420. [DOI] [PubMed] [Google Scholar]
- 49.Aitken SN, Yeaman S, Holliday JA, Wang TL, Curtis-McLane S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol Appl. 2008;1:95–111. doi: 10.1111/j.1752-4571.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conner JK. Artificial selection: A powerful tool for ecologists. Ecology. 2003;84:1650–1660. [Google Scholar]
- 51.Lau JA, Lennon JT. Evolutionary ecology of plant-microbe interactions: Soil microbial structure alters selection on plant traits. New Phytol. 2011;192:215–224. doi: 10.1111/j.1469-8137.2011.03790.x. [DOI] [PubMed] [Google Scholar]
- 52.Fierer N, Jackson JA, Vilgalys R, Jackson RB. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol. 2005;71:4117–4120. doi: 10.1128/AEM.71.7.4117-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avis PG, Dickie IA, Mueller GM. A ‘dirty’ business: Testing the limitations of terminal restriction fragment length polymorphism (TRFLP) analysis of soil fungi. Mol Ecol. 2006;15:873–882. doi: 10.1111/j.1365-294X.2005.02842.x. [DOI] [PubMed] [Google Scholar]
- 54.Lennon JT, Martiny JBH. Rapid evolution buffers ecosystem impacts of viruses in a microbial food web. Ecol Lett. 2008;11:1178–1188. doi: 10.1111/j.1461-0248.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 55.R Core Development Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.