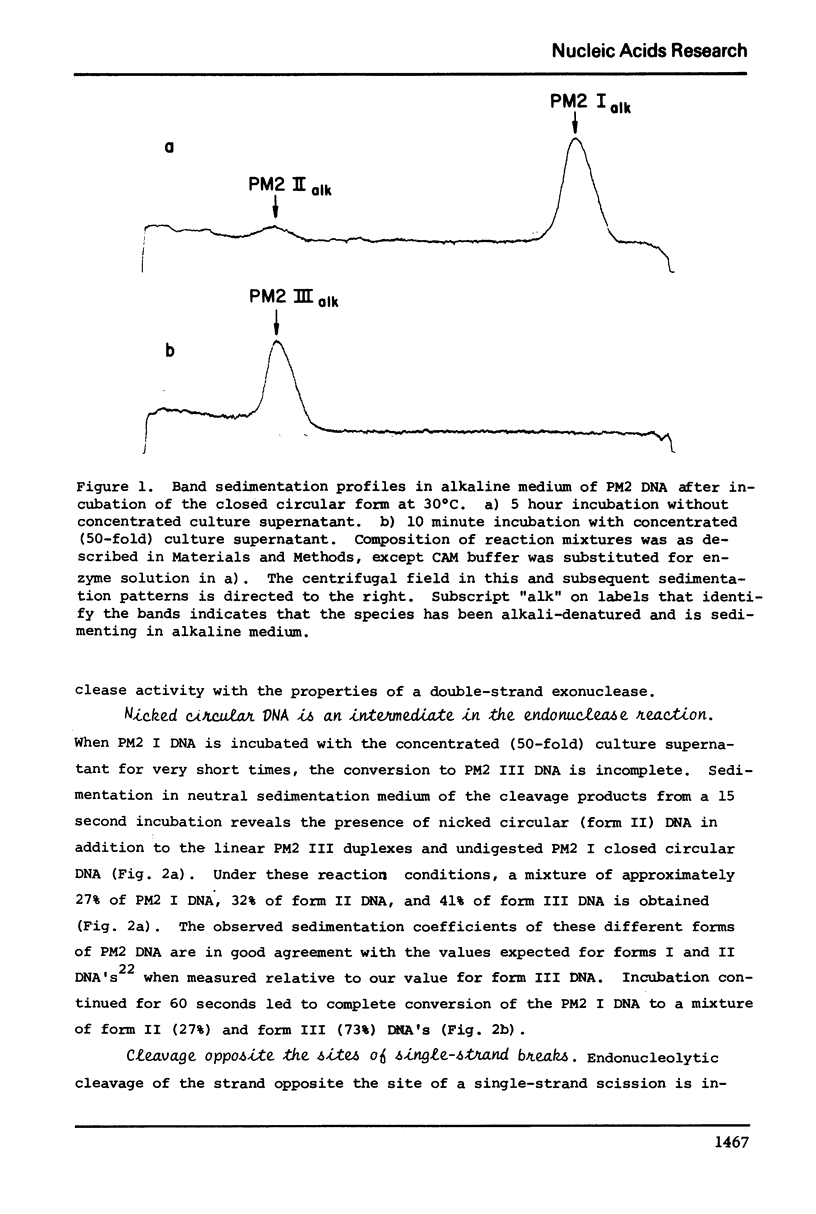
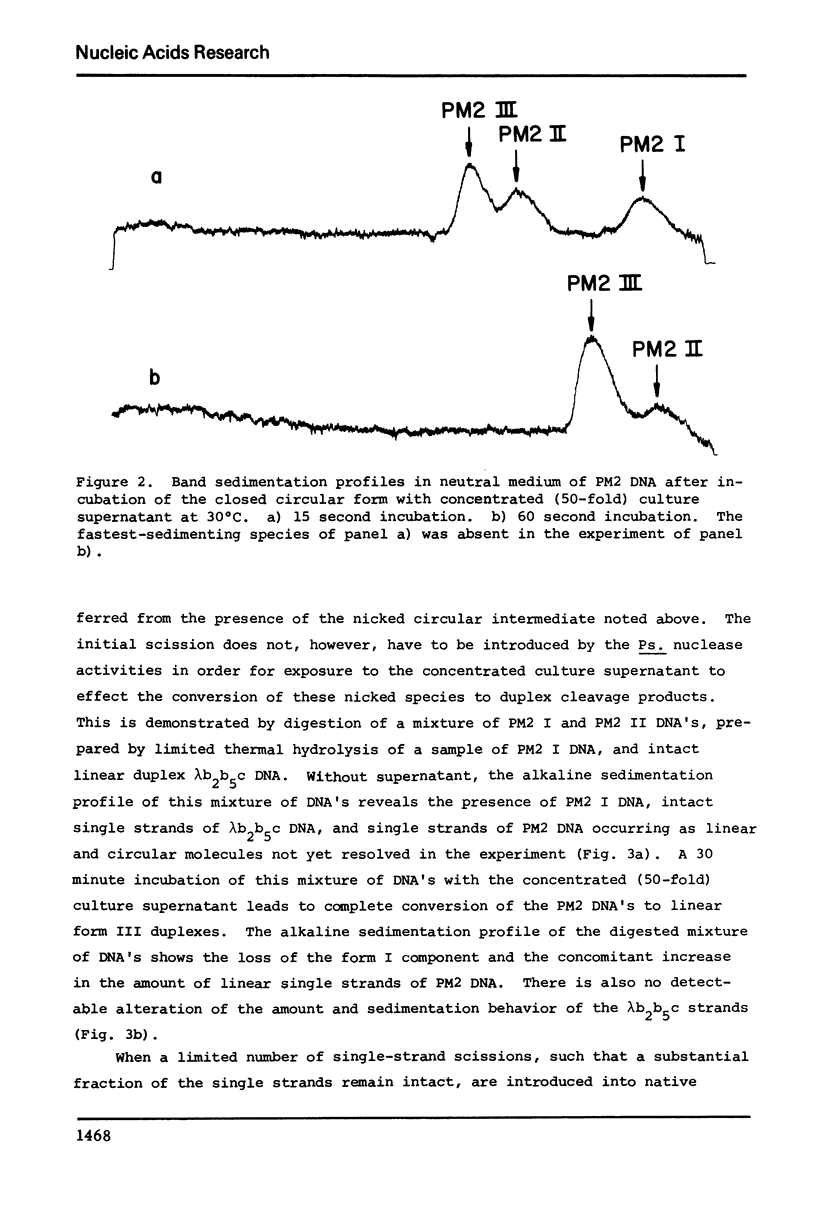
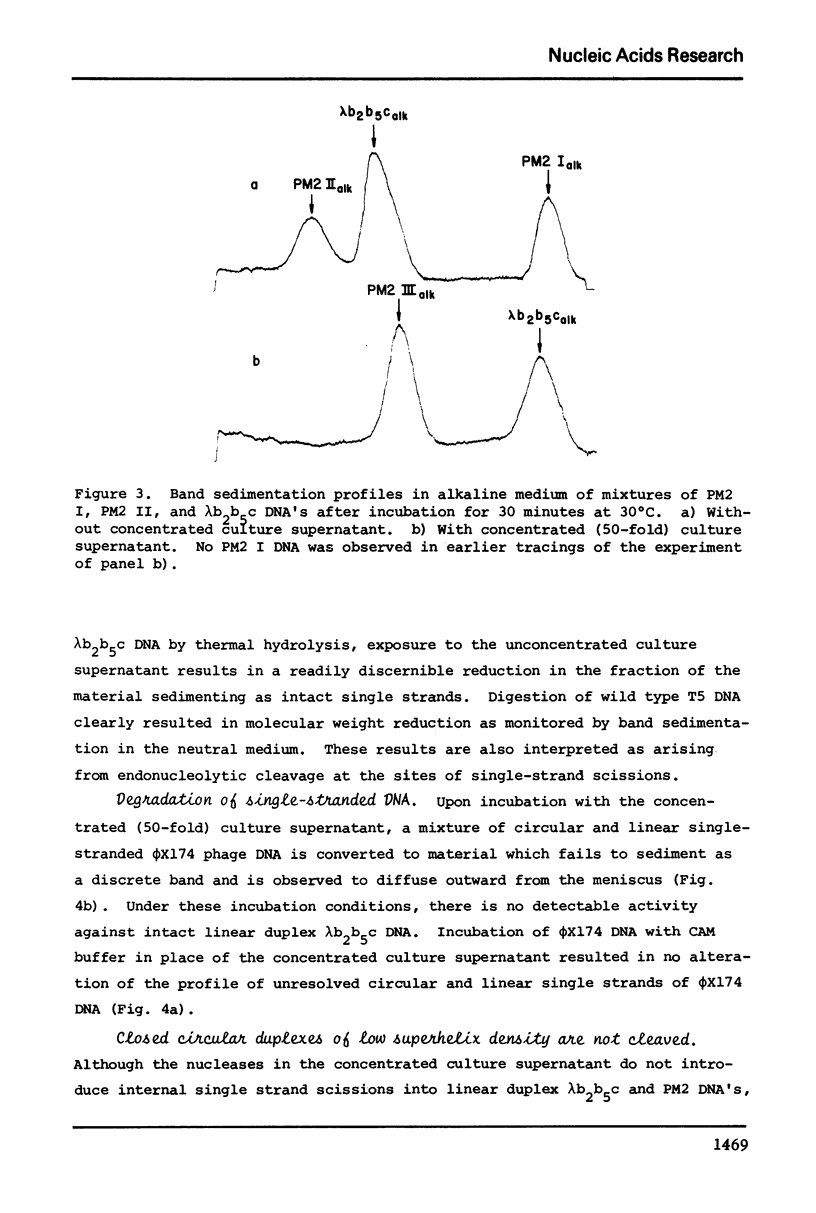
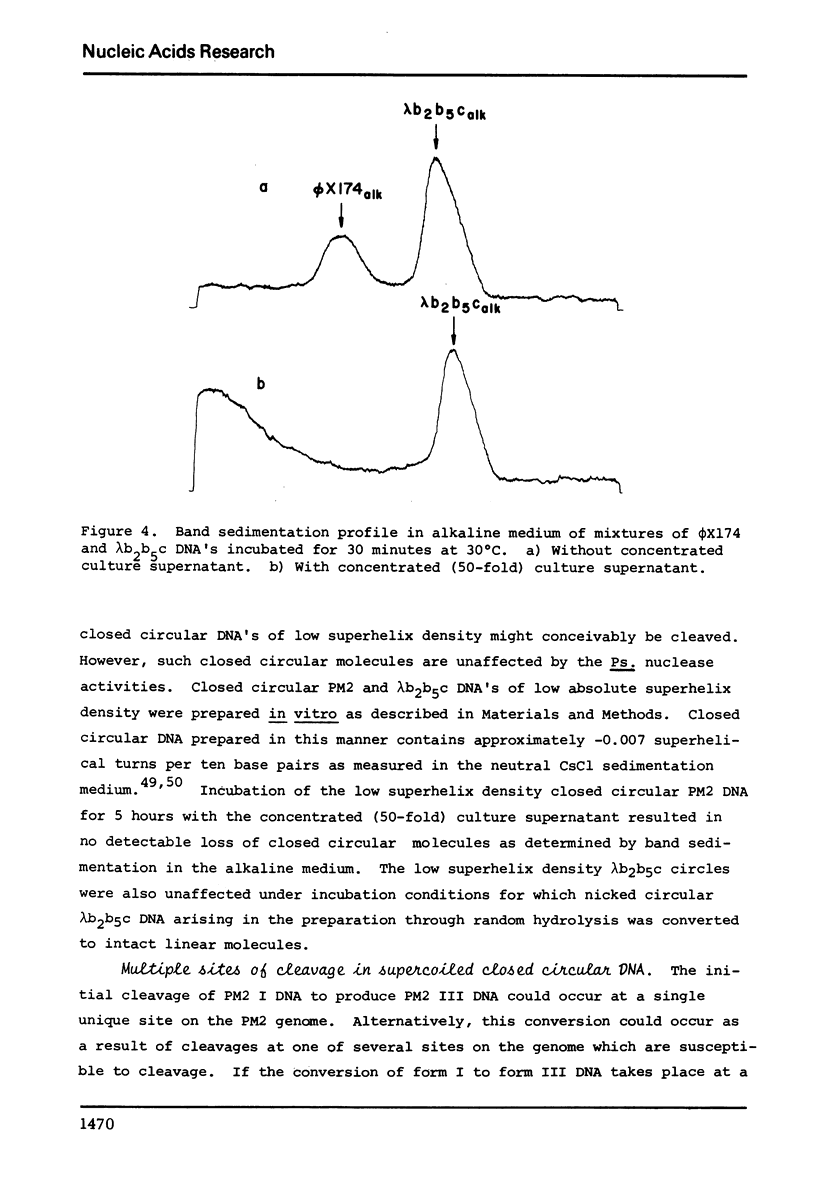
Abstract
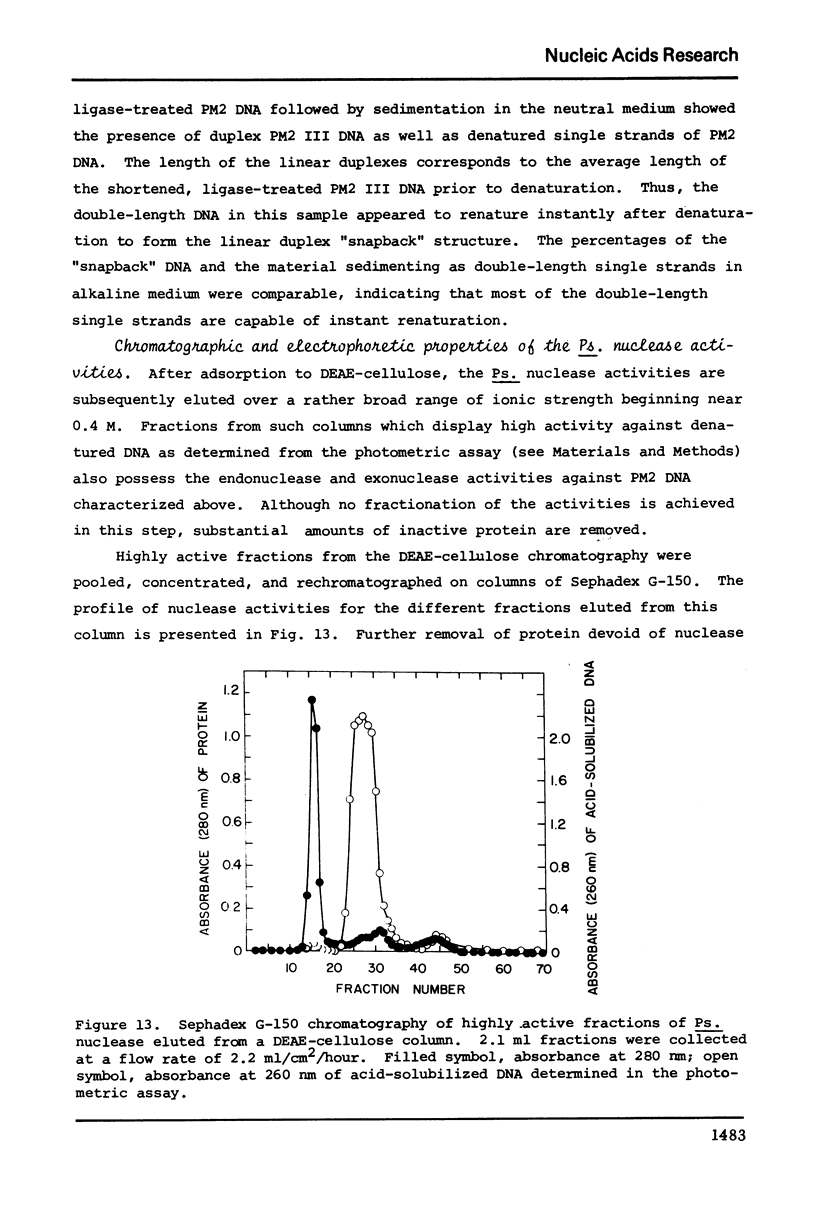
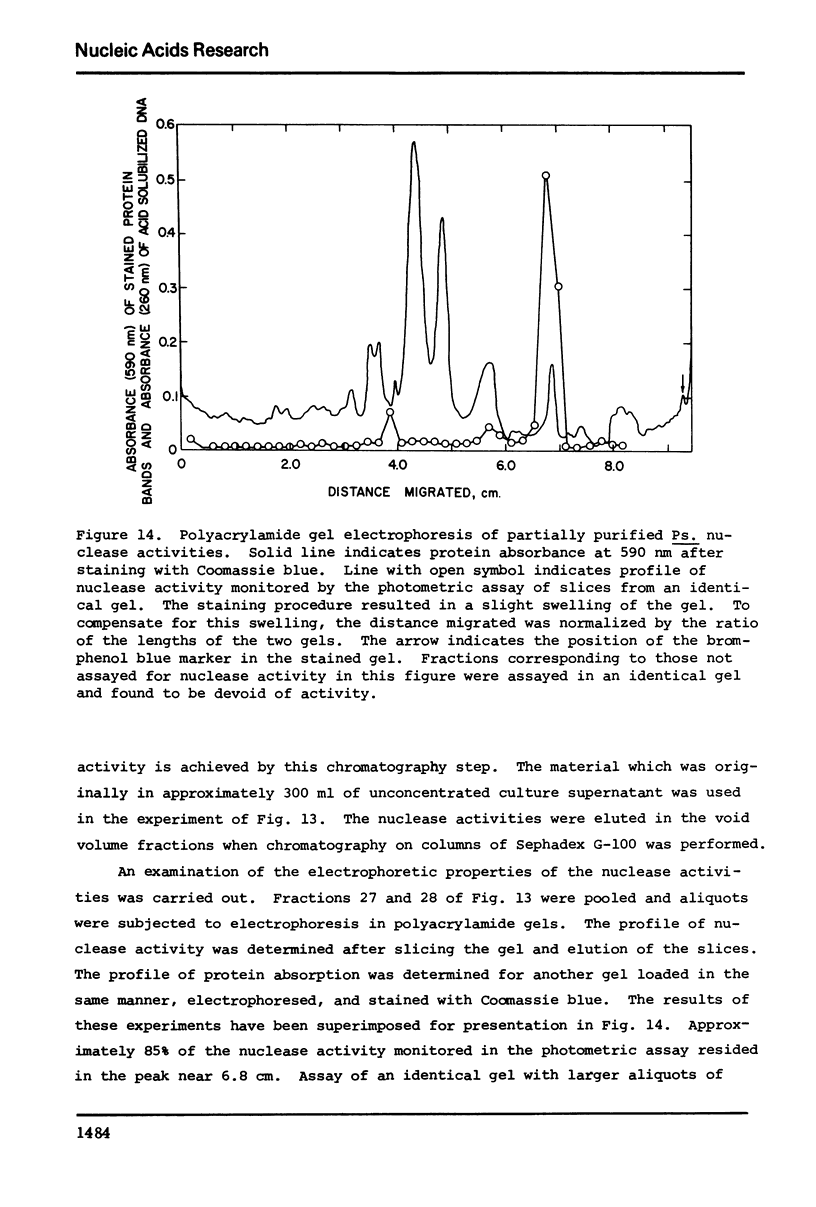
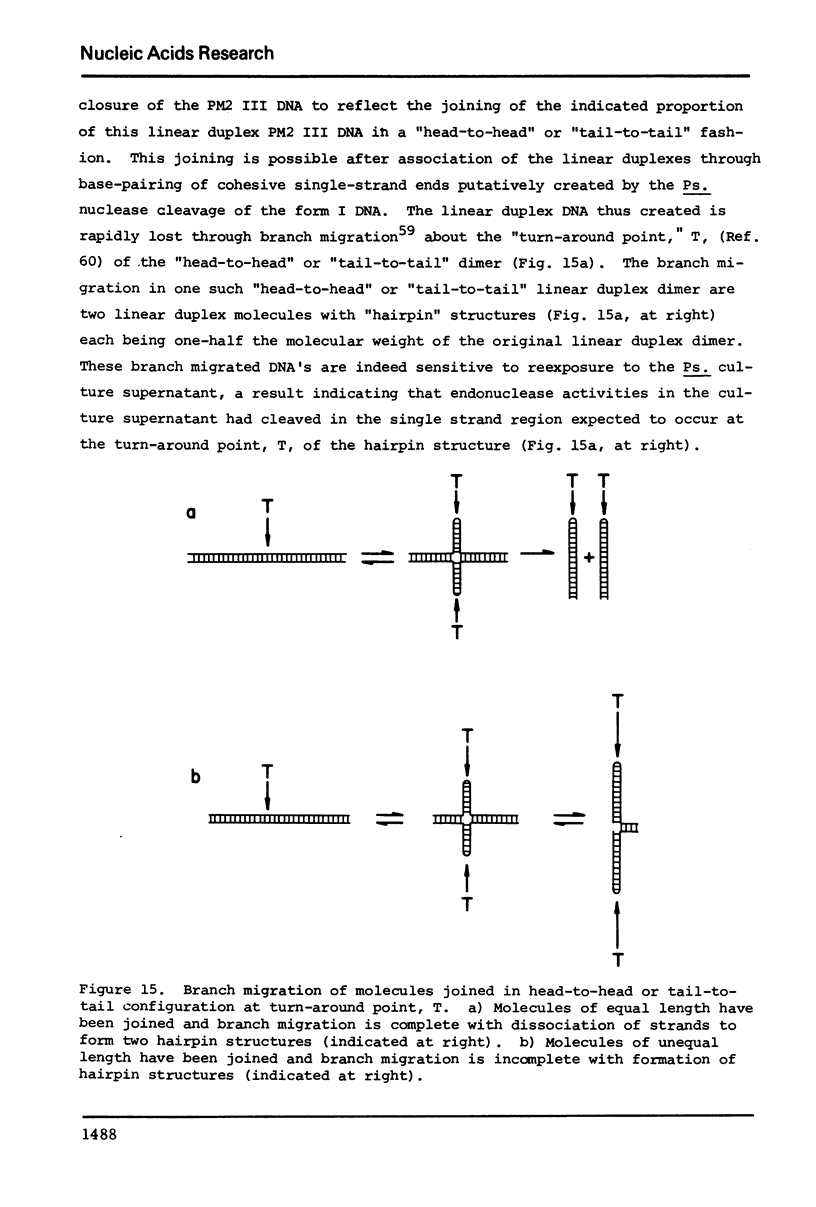
The culture medium of Pseudomonas BAL 31 contains endonuclease activities which are highly specific for single-stranged DNA and for the single-stranded or weakly hydrogen-bonded regions in supercoiled closed circular DNA. Exposure of nicked DNA to the culture medium results in cleavage of the strang opposite the sites of preexisting single-strand scissions. At least some of the linear duplex molecules derived by cleavage of supercoiled closed circular molecules contain short single-stranded ends. Single-strand scissions are not introduced into intact, linear duplex DNA or unsupercoiled covalently closed circular DNA. Under these same reaction conditions, 0X174 phage DNA is extensively degraded and PM2 form I DNA is quantitatively converted to PM2 form III linear duplexes. Prolonged exposure of this linear duplex DNA to the concentrated culture medium reveals the presence of a double-strand exonuclease activity that progressively reduces the average length of the linear duplex. These nuclease activities persist at ionic strengths up to 4 M and are not eliminated in the presence of 5% sodium dodecyl sulfate. Calcium and magnesium ion are both required for optimal activity. Although the absence of magnesium ion reduces the activities, the absence of calcium ion irreversibly eliminates all the activities.
Full text
PDF









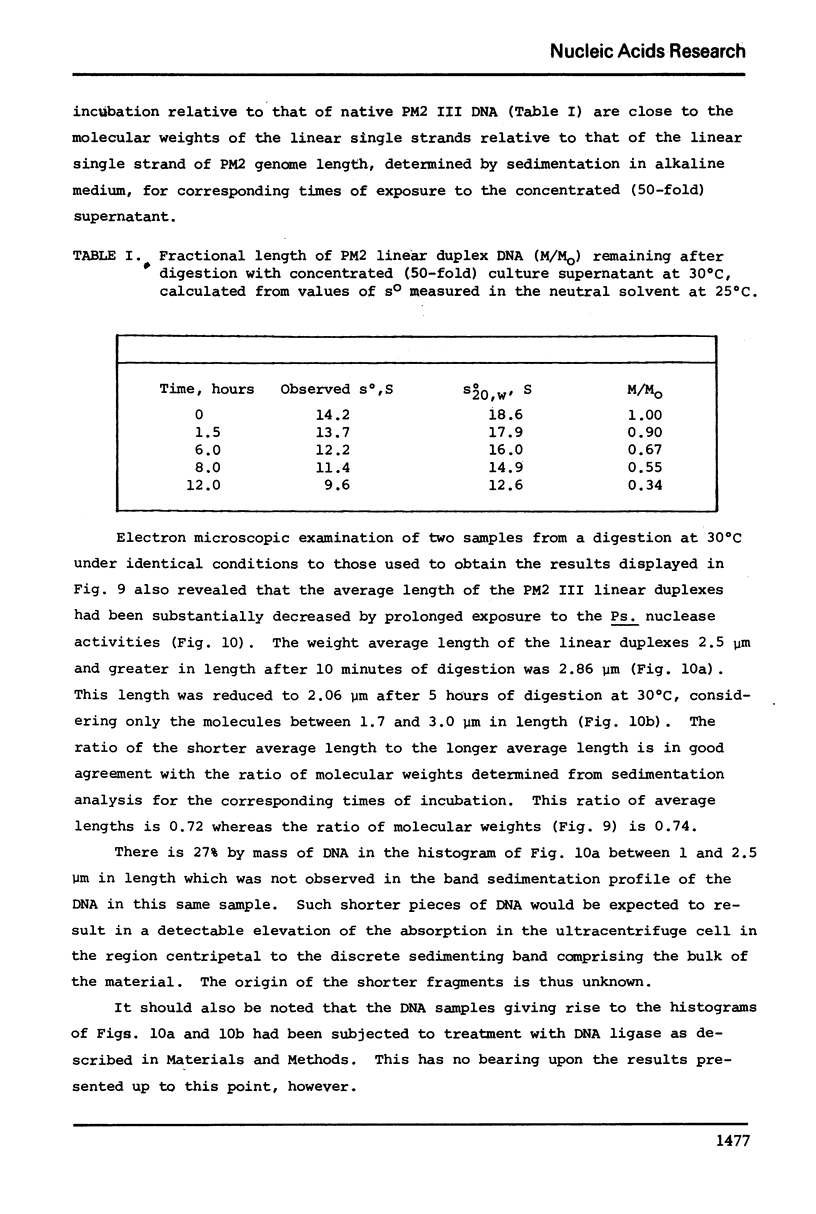
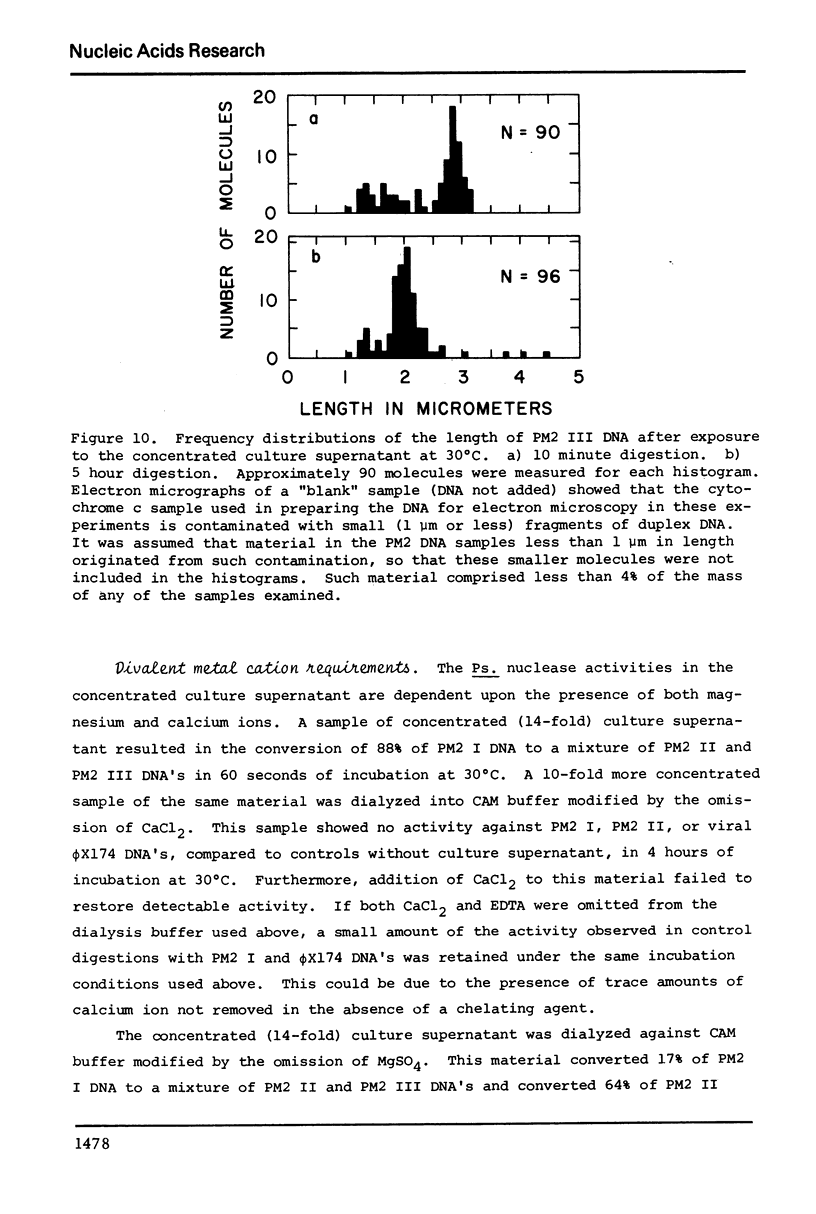

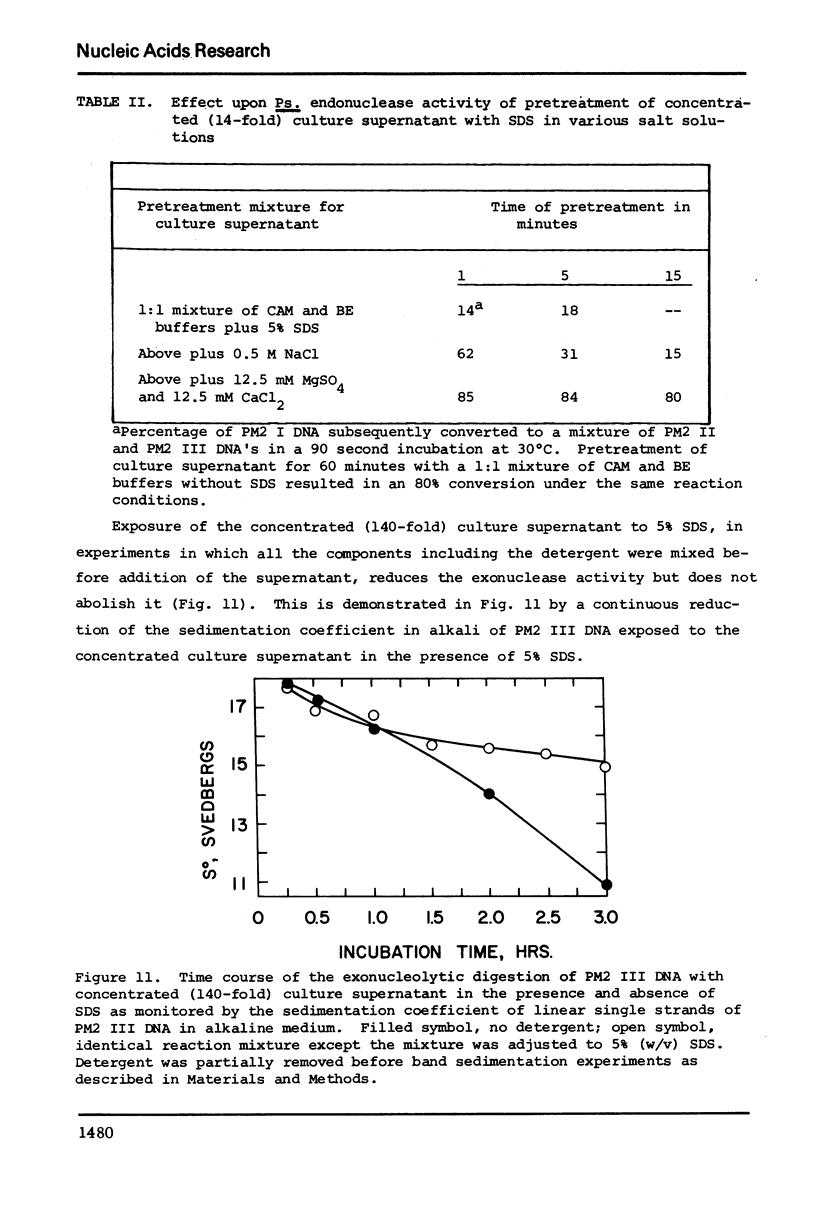

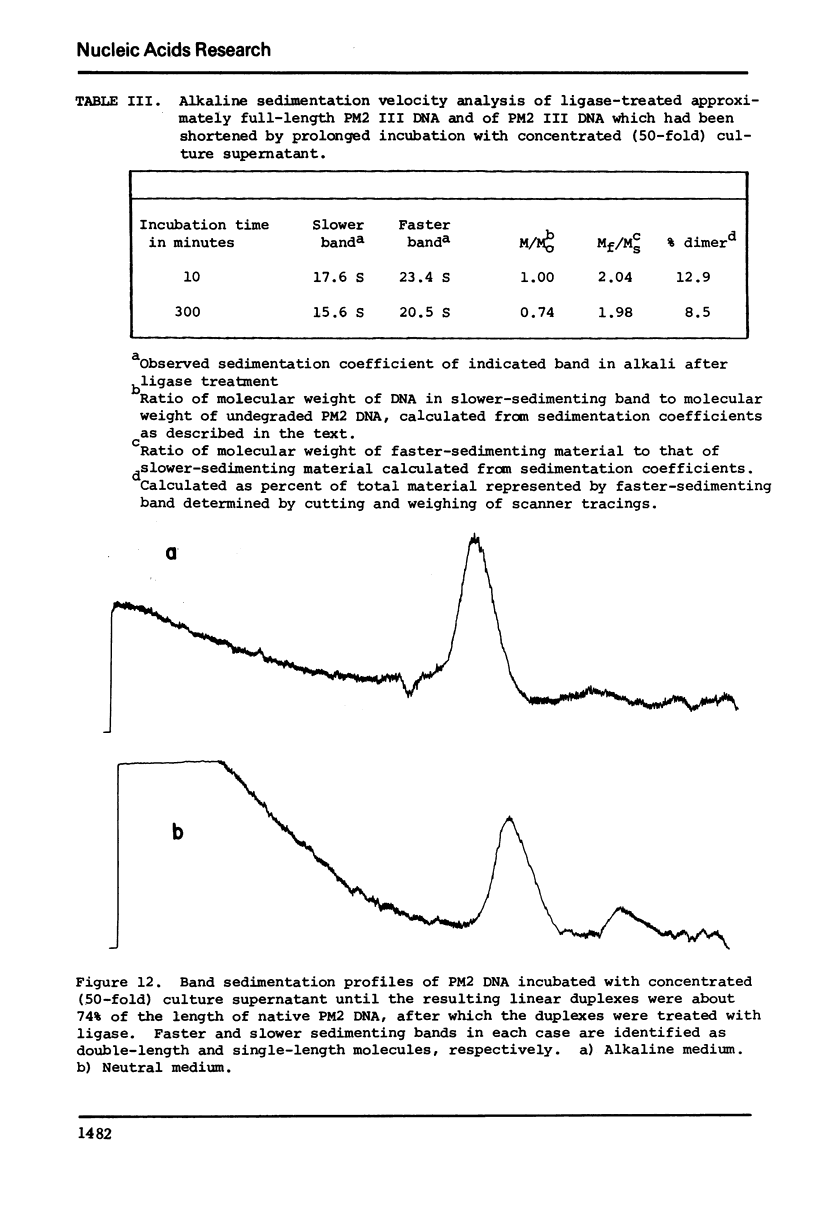







Images in this article
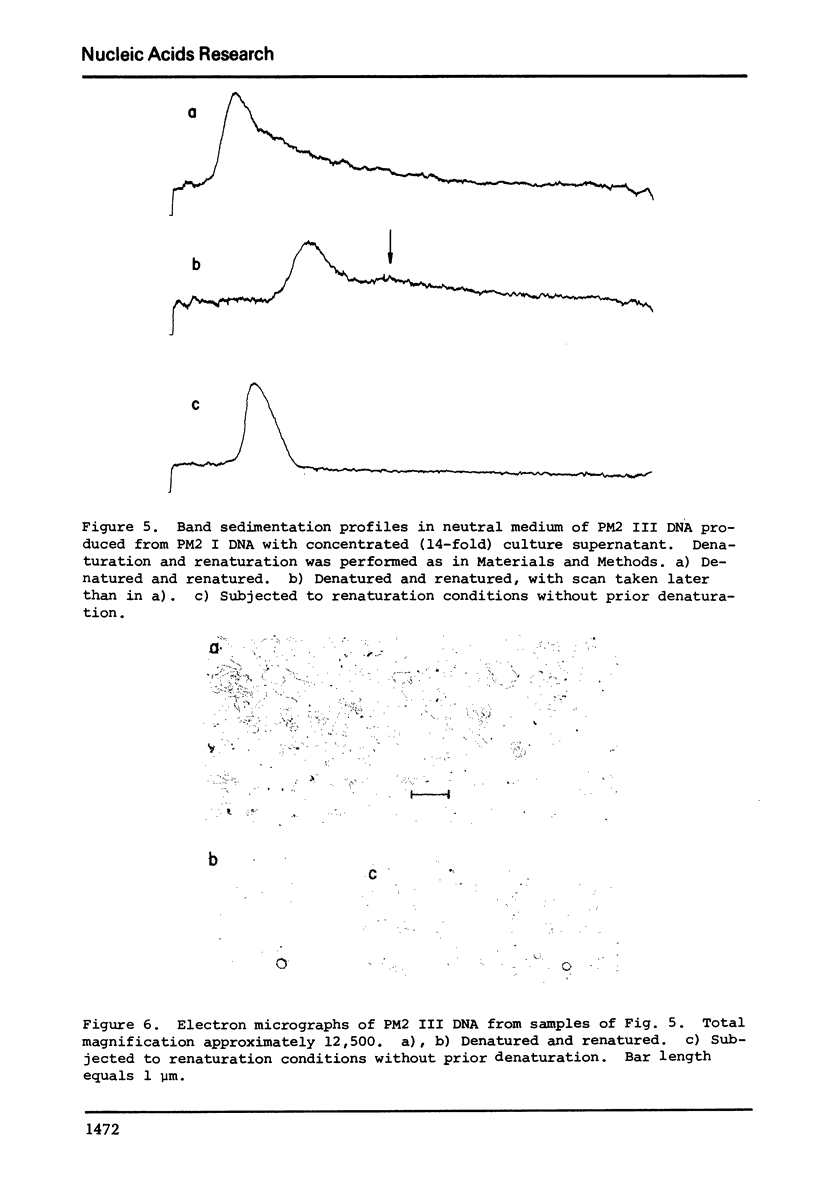
Selected References
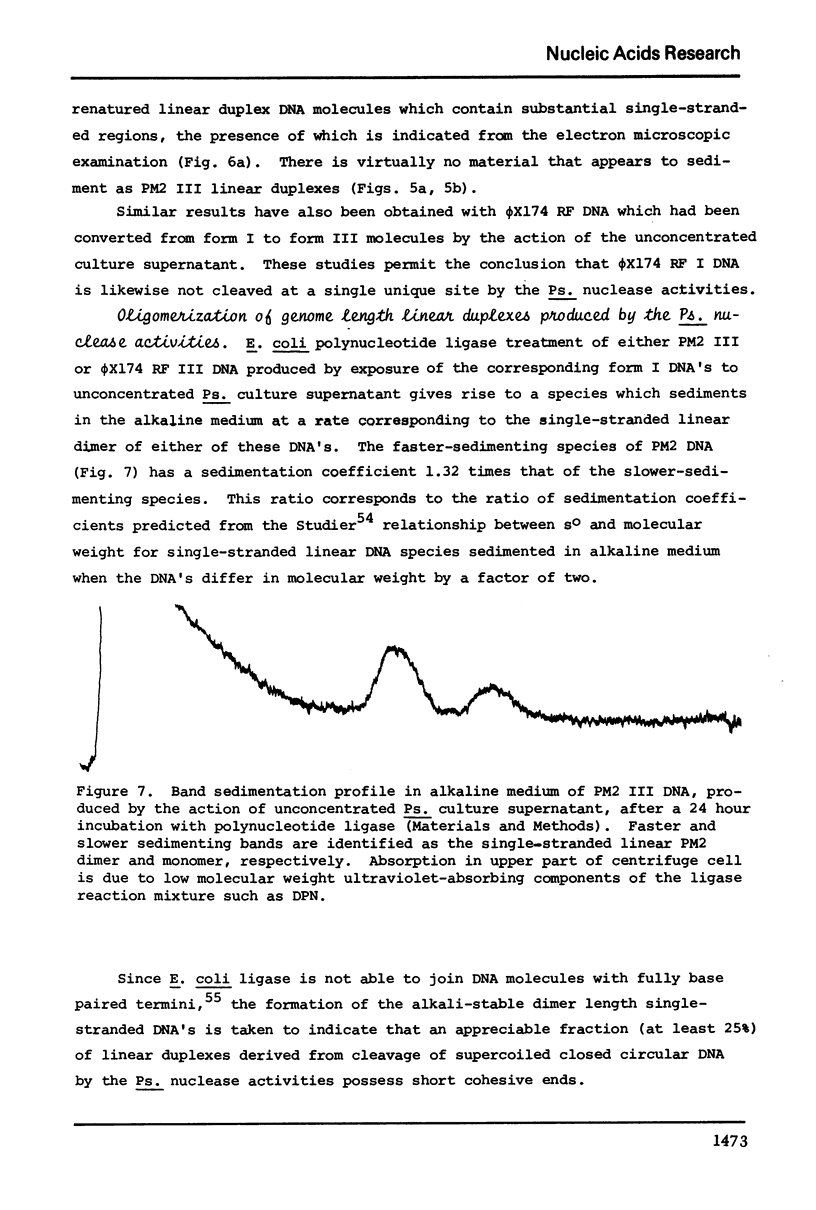
These references are in PubMed. This may not be the complete list of references from this article.
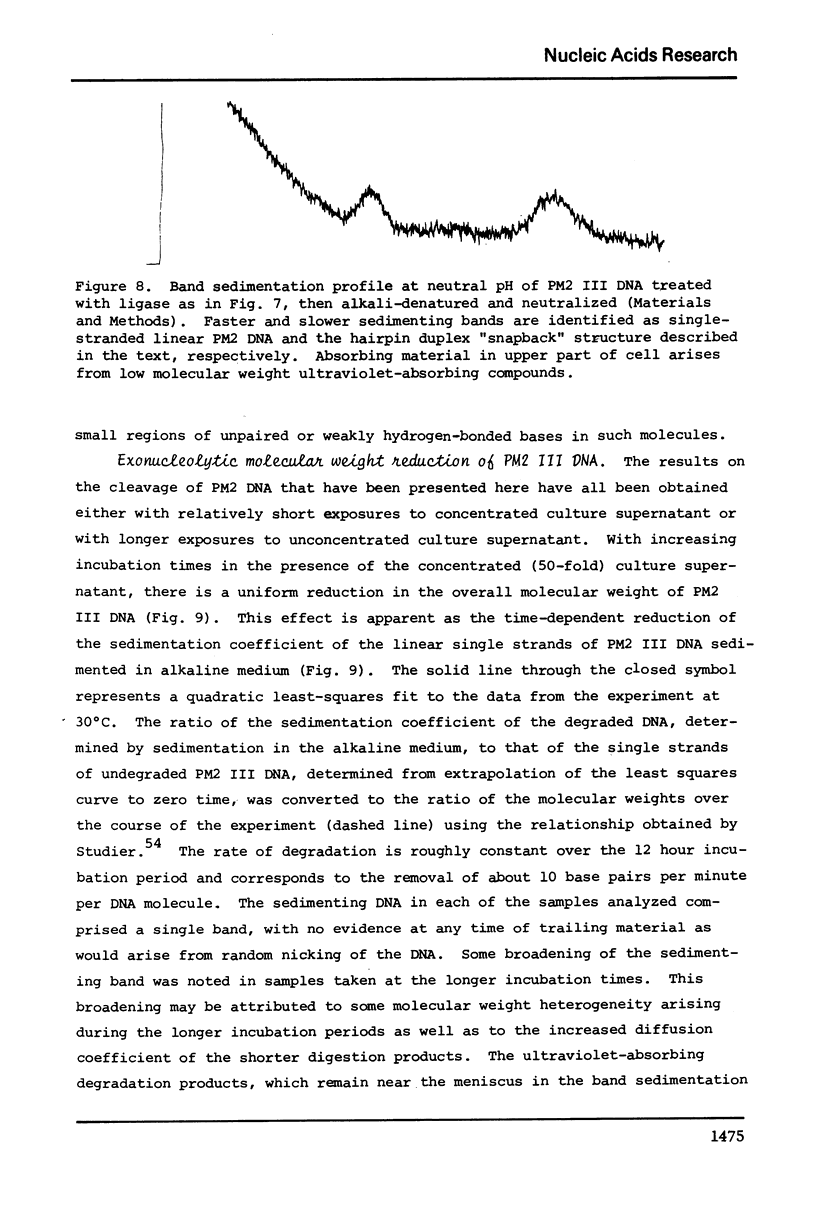
- Adler S. P., Nathans D. Studies of SV 40 DNA. V. Conversion of circular to linear SV 40 DNA by restriction endonuclease from Escherichia coli B. Biochim Biophys Acta. 1973 Mar 19;299(2):177–188. doi: 10.1016/0005-2787(73)90340-7. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Expression of the mitochondrial genome in HeLa cells. II. Evidence for complete transcription of mitochondrial DNA. J Mol Biol. 1971 Jan 28;55(2):251–267. doi: 10.1016/0022-2836(71)90195-1. [DOI] [PubMed] [Google Scholar]
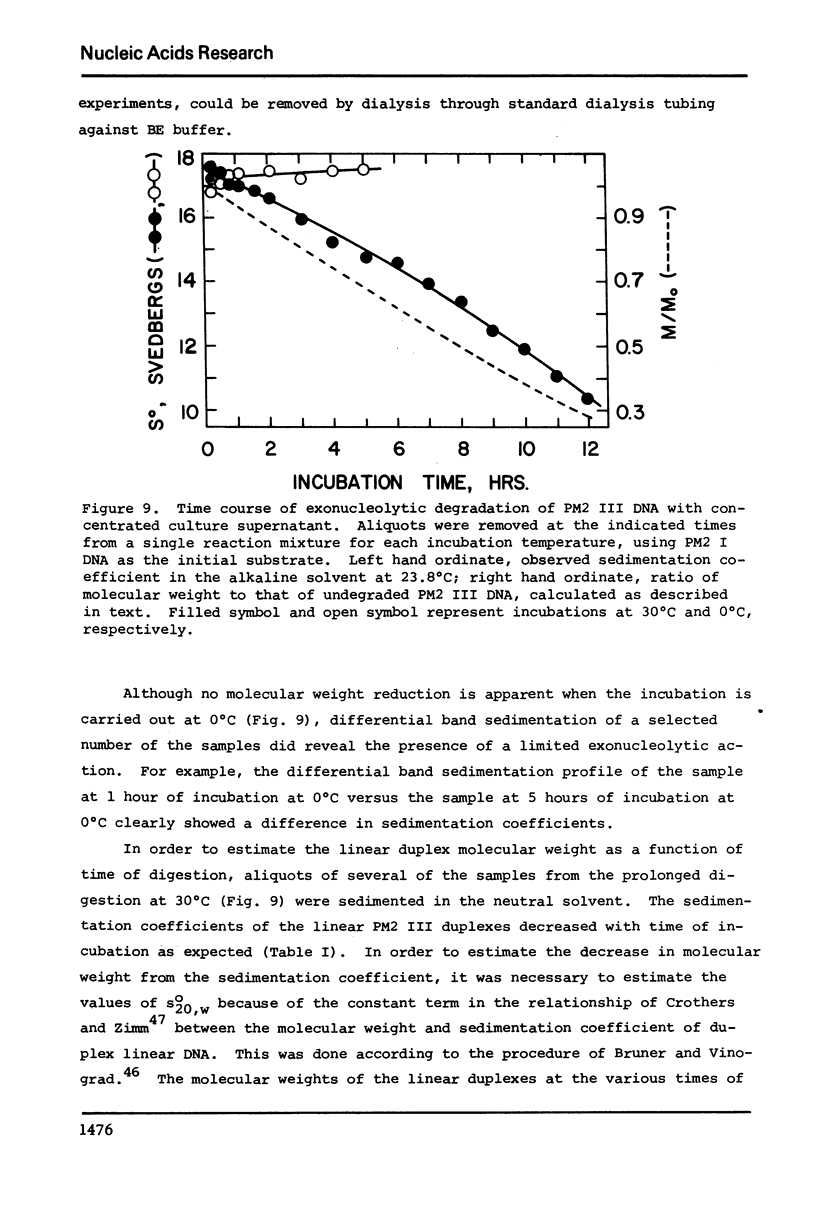
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner R., Vinograd J. The evaluation of standard sedimentation coefficients of sodium RNA and sodium DNA from sedimentation velocity data in concentrated NaCl and CsCl solutions. Biochim Biophys Acta. 1965 Sep 6;108(1):18–29. doi: 10.1016/0005-2787(65)90104-8. [DOI] [PubMed] [Google Scholar]
- Bujard H., Hendrickson H. E. Structure and function of the genome of coliphage T5. 1. The physical structure of the chromosome of T5 + . Eur J Biochem. 1973 Mar 15;33(3):517–528. doi: 10.1111/j.1432-1033.1973.tb02711.x. [DOI] [PubMed] [Google Scholar]
- Bujard H. Location of single-strand interruptions in the DNA of bacteriophage T5. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1167–1174. doi: 10.1073/pnas.62.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Purification and properties. J Biol Chem. 1974 Jul 25;249(14):4545–4552. [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers D. M., Zimm B. H. Viscosity and sedimentation of the DNA from bacteriophages T2 and T7 and the relation to molecular weight. J Mol Biol. 1965 Jul;12(3):525–536. doi: 10.1016/s0022-2836(65)80310-2. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Wolstenholme D. R. Renaturation and hybridization studies of mitochondrial DNA. Biophys J. 1968 Jan;8(1):65–81. doi: 10.1016/S0006-3495(68)86475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz A. A., Lubrano T. Separation and quantitation of lactic dehydrogenase isoenzymes by disc electrophoresis. Anal Biochem. 1967 Aug;20(2):246–257. doi: 10.1016/0003-2697(67)90030-9. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties and characterization of the host bacterium of bacteriophage PM2. J Bacteriol. 1968 May;95(5):1887–1891. doi: 10.1128/jb.95.5.1887-1891.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. Replication of bacteriophage PM2 deoxyribonucleic acid: a closed circular double-stranded molecule. J Mol Biol. 1971 Mar 28;56(3):597–621. doi: 10.1016/0022-2836(71)90404-9. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Vogt V. M., Hirt B. Characterization of the single-strand-specific nuclease S1 activity on double-stranded supercoiled polyoma DNA. Eur J Biochem. 1974 Apr 16;43(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03446.x. [DOI] [PubMed] [Google Scholar]
- Godson G. N. Action of the single-stranded DNA specific nuclease S1 on double-stranded DNA. Biochim Biophys Acta. 1973 Apr 21;308(7):59–67. doi: 10.1016/0005-2787(73)90122-6. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Gray H. B., Jr, Hearst J. E. Flexibility of native DNA from the sedimentation behavior as a function of molecular weight and temperature. J Mol Biol. 1968 Jul 14;35(1):111–129. doi: 10.1016/s0022-2836(68)80041-5. [DOI] [PubMed] [Google Scholar]
- Gray H. B., Jr, Upholt W. B., Vinograd J. A buoyant method for the determination of the superhelix density of closed circular DNA. J Mol Biol. 1971 Nov 28;62(1):1–19. doi: 10.1016/0022-2836(71)90127-6. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. II. Arrangement of the single-stranded DNA fragments in the T5 + and T5st(O) chromosomes. J Mol Biol. 1972 Feb 14;63(3):397–407. doi: 10.1016/0022-2836(72)90436-6. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Cleavage of bacteriophage fl DNA by the restriction enzyme of Escherichia coli B. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3220–3224. doi: 10.1073/pnas.69.11.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R. J., Lebowitz J., Kleinschmidt A. K. Locating interrupted hydrogen bonding in the secondary structure of PM2 circular DNA by comparative denaturation mapping. J Virol. 1974 Jun;13(6):1176–1185. doi: 10.1128/jvi.13.6.1176-1185.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin-Sablon A., Richardson C. C. Analysis of the interruptions in bacteriophage T5 DNA. J Mol Biol. 1970 Feb 14;47(3):477–493. doi: 10.1016/0022-2836(70)90316-5. [DOI] [PubMed] [Google Scholar]
- Kato A. C., Bartok K., Fraser M. J., Denhardt D. T. Sensitivity of superhelical DNA to a single-strand specific endonuclease. Biochim Biophys Acta. 1973 Apr 21;308(7):68–78. doi: 10.1016/0005-2787(73)90123-8. [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Young E. T., 2nd, Sinsheimer R. L. Purification and properties of intracellular lamba DNA rings. J Mol Biol. 1968 Apr 28;33(2):395–413. doi: 10.1016/0022-2836(68)90197-6. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval F. Endonuclease activity associated with purified PM2 bacteriophages. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4965–4969. doi: 10.1073/pnas.71.12.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- MacKay V., Linn S. The mechanism of degradation of duplex deoxyribonucleic acid by the recBC enzyme of Escherichia coli K-12. J Biol Chem. 1974 Jul 10;249(13):4286–4294. [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méchali M., de Recondo A. M., Girard M. Action of the S1 endonuclease from Aspergillus oryzae on simian virus 40 supercoiled component I DNA. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1306–1320. doi: 10.1016/0006-291x(73)91130-3. [DOI] [PubMed] [Google Scholar]
- Ostrander D. A., Gray H. B., Jr, Robberson D. L. Catenanes of closed circular intracellular PM2 phage DNA. Biochim Biophys Acta. 1974 May 31;349(3):296–304. doi: 10.1016/0005-2787(74)90117-8. [DOI] [PubMed] [Google Scholar]
- Ostrander D. A., Gray H. B., Jr Sedimentation and intrinsic viscosity behavior of PM2 bacteriophage DNA in alkaline solution. Biopolymers. 1973 Jun;12(6):1387–1419. doi: 10.1002/bip.1973.360120614. [DOI] [PubMed] [Google Scholar]
- Ostrander D. A., Gray H. B., Jr Superhelix density heterogeneity in closed circular intracellular PM2 DNA. Biopolymers. 1974 May;13(5):955–975. doi: 10.1002/bip.1974.360130511. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Hilbers C. W. Proton nuclear magnetic resonance investigations of fraying in double-stranded d-ApTpGpCpApT in H2O solution. Biochemistry. 1975 Jun 17;14(12):2651–2656. doi: 10.1021/bi00683a014. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Fried M. Sequence arrangements in clonal isolates of polyoma defective DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3497–3501. doi: 10.1073/pnas.71.9.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sgaramella V. Enzymatic oligomerization of bacteriophage P22 DNA and of linear Simian virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3389–3393. doi: 10.1073/pnas.69.11.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido K., Ando T. Site-specific fragmentation of bacteriophage T5 DNA by single-strand-specific S1 endonuclease. Biochim Biophys Acta. 1975 Apr 16;390(1):125–132. doi: 10.1016/0005-2787(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Gray H. B., Jr, Vinograd J. Sedimentation velocity behavior of closed circular SV40 DNA as a function of superhelix density, ionic strength, counterion and temperature. J Mol Biol. 1971 Nov 28;62(1):21–38. doi: 10.1016/0022-2836(71)90128-8. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interactions between twisted DNAs and enzymes: the effects of superhelical turns. J Mol Biol. 1974 Aug 25;87(4):797–816. doi: 10.1016/0022-2836(74)90085-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Palindromes in chromosomes. J Mol Biol. 1974 Mar 25;84(1):115–138. doi: 10.1016/0022-2836(74)90216-2. [DOI] [PubMed] [Google Scholar]




