Abstract
Bird song, like human speech, is a learned vocal behavior that requires auditory feedback. Both as juveniles, while they learn to sing, and as adults, songbirds use auditory feedback to compare their own vocalizations with an internal model of a target song. Here we describe experiments that explore a role for the songbird anterior forebrain pathway (AFP), a basal ganglia-forebrain circuit, in evaluating song feedback and modifying vocal output. First, neural recordings in anesthetized, juvenile birds show that single AFP neurons are specialized to process the song stimuli that are compared during sensorimotor learning. AFP neurons are tuned to both the bird's own song and the tutor song, even when these stimuli are manipulated to be very different from each other. Second, behavioral experiments in adult birds demonstrate that lesions to the AFP block the deterioration of song that normally follows deafening. This observation suggests that deafening results in an instructive signal, indicating a mismatch between feedback and the internal song model, and that the AFP is involved in generating or transmitting this instructive signal. Finally, neural recordings from behaving birds reveal robust singing-related activity in the AFP. This activity is likely to originate from premotor areas and could be modulated by auditory feedback of the bird's own voice. One possibility is that this activity represents an efference copy, predicting the sensory consequences of motor commands. Overall, these studies illustrate that sensory and motor processes are highly interrelated in this circuit devoted to vocal learning, as is true for brain areas involved in speech.
Human speech and bird song share numerous features (1). Both are complex acoustic sequences, generated by coordinated actions of the vocal apparatus and the muscles of respiration. Most importantly, both speech and song are learned and are strongly influenced by hearing in early life and in adulthood: neither birds nor humans learn to vocalize normally in the absence of hearing, and as adults, both show deterioration of vocal output after hearing loss (2–6). Songbirds thus provide a promising model system for elucidating general neural mechanisms involved in vocal learning, including how the brain evaluates auditory feedback and uses it to modify vocal output.
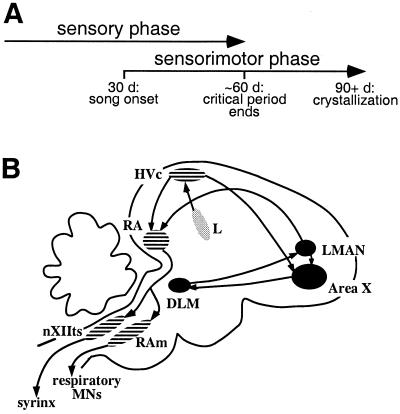
Experiments to investigate the neural basis of vocal learning in songbirds are aided by a wealth of information about the behavioral time course of learning (7–9) and its dependence on hearing (4, 6). Song learning occurs in two stages, called the sensory and sensorimotor phases (Fig. 1A). During the sensory phase, a young bird listens to and memorizes the song of an adult tutor, often the bird's father. This memory is called the template. The sensorimotor phase begins later, when the young bird begins to sing; during sensorimotor learning the juvenile uses auditory feedback to compare its own immature vocalizations (plastic song) to the tutor song template, and gradually refines and adapts its vocal output until it matches the template. Thus, auditory experience of both the tutor song and the bird's own song (BOS) is required during learning. In adulthood, elimination or alteration of auditory feedback of BOS induces gradual deterioration of adult song structure (5, 10). These behavioral observations suggest that there must be neural circuitry involved in memorization and evaluation of song. Specifically, there must be mechanisms that compare auditory feedback from vocal output to the internal song template and that generate signals to guide changes in vocal output.
Figure 1.
(A) Song learning occurs in two phases. For zebra finches, the sensory phase ends at ≈60 days of age and the sensorimotor phase begins when birds are ≈30 days old and continues until they are ≥90 days of age; thus the phases of learning overlap in this species. (B) Anatomy of the song system, which consists of two pathways. Motor pathway nuclei are striped, and AFP pathway nuclei are in black. The motor pathway, necessary for normal song production throughout life, includes HVc, the robust nucleus of the archistriatum (RA), and the tracheosyringeal portion of the hypoglossal nucleus (nXIIts). RA also projects to nuclei involved in control of respiration, such as nucleus retroambigualis (Ram). The AFP comprises Area X (X), the medial nucleus of the dorsolateral thalamus (DLM), and LMAN. The Field L complex and related areas (stippled) provide auditory input to the song system.
One candidate circuit for processing and evaluating these song experiences is the anterior forebrain pathway (AFP), a basal ganglia-forebrain circuit found within a system of interconnected nuclei dedicated to song learning and production (Fig. 1B; ref. 11). The AFP plays a special, but unclear, role during learning. Lesions of the AFP severely disrupt song learning in juveniles, whereas the same lesions do not affect song in normal adults (12–14). The output nucleus of the AFP, the lateral magnocellular nucleus of the anterior neostriatum (LMAN), projects to the motor pathway for song, which is necessary for normal song production throughout life (11). Thus, the AFP is well positioned to influence activity in the motor pathway and could drive changes in vocal output. We review here experiments that implicate AFP function in the sensory and sensorimotor phases of learning, as well as in the sensorimotor processes crucial to song maintenance in adulthood.
Song Learning in Juveniles
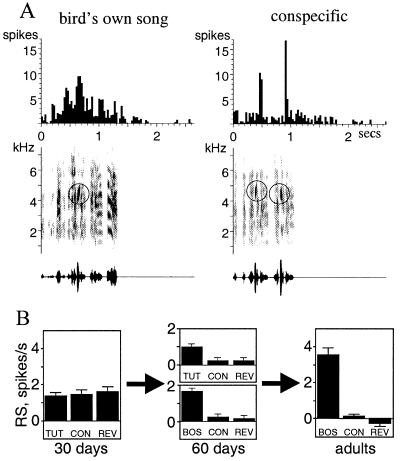
As might be expected of neural circuits that may be involved in mediating song learning, neurons in the AFP are responsive to song stimuli. In adult, anesthetized zebra finches, these neurons respond more strongly to BOS than to acoustically similar songs of other zebra finches (conspecific songs; Fig. 2) or BOS played in reverse (15). The properties of these neurons are very similar to those of song-selective neurons first described in the song nucleus HVc (Fig. 1B; refs. 16 and 17). Neurons that are sensitive to the complex spectral and temporal properties of song could be useful for processing song stimuli during learning. Moreover, this song selectivity emerges during the course of song learning: AFP neurons from birds early in the sensory learning phase (30 days of age) respond equally well to all song stimuli, and then over time increase their response to their own song while losing responsiveness to other stimuli (15, 18) (Fig. 2B). There is a striking parallel to this result in human speech development: human infants initially show sensory discrimination of phonemes from all human languages tested, but gradually lose their capacity to accurately discriminate sounds that they are not experiencing, and improve their discrimination of the sounds of the language spoken around them (19–21). In both cases, the initial broad sensitivity endows the young organism with the capacity to learn any language or species-specific song, but this sensitivity then is narrowed and shaped by experience.
Figure 2.
AFP neurons are song-selective. (A) A song-selective neuron from an adult zebra finch. Peristimulus time histograms (PSTHs) show the greater response of a single LMAN neuron to BOS than to conspecific song. Song is shown underneath each PSTH as a sonogram (plot of frequency versus time, with the energy of each frequency band indicated by the darkness of the trace). Song-selective neurons respond to multiple acoustic features of the BOS: the circles in the sonograms identify a feature that is shared between both songs shown here and appears to elicit a response, but the figure also illustrates that many other features of BOS must contribute to the overall response of this neuron to BOS. (B) AFP neurons develop selectivity for song during development. In zebra finches of 30 days of age, LMAN neurons exhibit equivalent response strengths (RS; mean stimulus-evoked response minus background) to tutor song (TUT), conspecific song (CON), and reverse tutor song (REV). By 60 days of age, these neurons respond significantly more to TUT than to CON or to REV. In addition, BOS also elicits a much stronger response than CON and reverse BOS (REV). In adults, LMAN neurons are extremely selective for BOS.
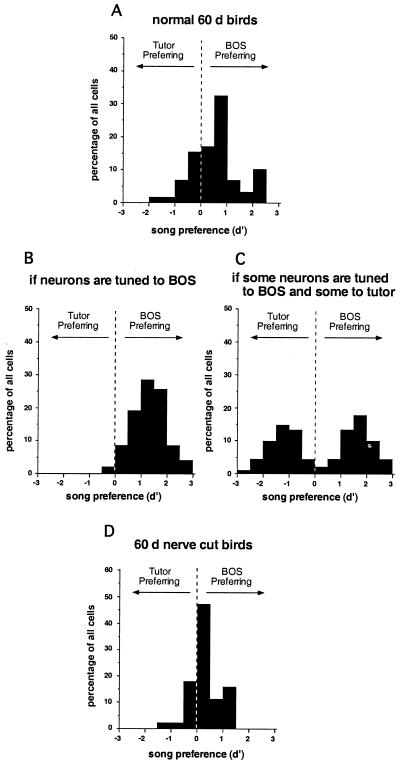
Song selectivity develops rapidly, because it is found in the AFP of zebra finches that have completed the sensory phase of learning (60 days of age: Figs. 1A and 2B; ref. 18). At this time zebra finches are also in the middle of the sensorimotor phase and have been producing plastic song for about a month. Thus, experience of either the BOS or the tutor song could have shaped the selectivity of these neurons. Knowing which experience is responsible for selectivity would inform our hypotheses about AFP function during song learning. For example, neurons tuned by BOS experience could provide information about the current state of BOS, whereas those tuned by tutor song could encode the tutor song memory. When we compared the neural responses to BOS and tutor song in 60-day-old birds, we found a range of preferences for one song over the other (Fig. 3A). Many neurons preferred BOS over tutor song, supporting a role for BOS experience in shaping selectivity. A few neurons preferred tutor over BOS, suggesting that they were tuned by tutor song experience. Finally, many neurons responded equally well to both songs. These neurons were clearly selective, because they did not respond as well to conspecific or reversed song stimuli. Thus, such neurons might reflect experiences of both BOS and tutor song.
Figure 3.
Preferences for BOS versus tutor song by single AF neurons. (A) Histograms show that in 60-day-old zebra finches, there is a range of preferences among LMAN neurons. The preference for each neuron is quantified with a d′ value (see refs. 18 and 25). When d′ ≥ 0.5, this indicates a strong preference for BOS over tutor song; when d′ ≤ −0.5, this indicates a strong preference for tutor song over BOS. Neurons with d′ values in between were considered to have equivalent responses to both song stimuli. (B and C) Predicted results of the manipulation of BOS. (B) If neurons with equivalent responses to BOS and tutor song are shaped by BOS during development but respond to both stimuli as a result of acoustic similarities between these two songs, this type of dually responsive neuron is not expected in birds with songs unlike their tutor song, and the distribution should reveal only BOS-tuned neurons. If both BOS and tutor song independently shape different neurons in the AFP, the distribution in birds with songs very different from their tutor songs is predicted to be bimodal, as shown by the histogram. (D) The observed distribution of song preferences from ts cut birds at 60 days of age. Neurons with equivalent responses to BOS and tutor song were maintained, even though these birds' songs did not resemble the tutor song.
Two important caveats exist with respect to the apparent shaping of AFP neurons by these two sensory experiences. First, although BOS selectivity initially might seem to reflect the bird's experience of its own song, it is also possible that it actually represents the template. If a bird memorized the tutor song poorly during sensory learning, then modeled its own song after this inaccurate template, BOS selectivity would be a better representation of the template than the tutor song. The question of whether BOS indeed reflects the bird's own vocalizations could be solved if the bird were made to sing something very different from its tutor by a manipulation of its peripheral vocal system. Because the bird would hear the highly abnormal BOS only as a result of its own singing, neurons tuned to the abnormal song would verify that it was the experience of BOS that was critical. Second, neurons tuned to both BOS and tutor song might not reflect the experience of both of these songs, but simply reflect acoustic similarities between these two stimuli. The bird is trying to model its own song after the tutor song, and by 60 days of age, plastic song often resembles the tutor song. This question also could be addressed if the acoustic similarity that normally develops between BOS and tutor song were minimized by inducing juvenile zebra finches to sing abnormal songs (22). If the neurons that respond equally well to BOS and tutor song actually are shaped by the experience of the bird's voice but respond to both stimuli because of acoustic similarities between these songs, then this kind of neuron should not exist in birds with song unlike their tutor song (Fig. 3B). Alternatively, if these neurons reflect independent contributions of both BOS and tutor song experience to selectivity, then they should persist in birds with song unlike their tutor song, perhaps as separate neural populations (Fig. 3C).
To induce abnormal song, we bilaterally transected the tracheosyringeal portion of the hypoglossal nerve (NXIIts) before song onset (≈25 days of age in zebra finches; Fig. 1A), thus denervating the muscles of the avian vocal organ (the syrinx). These juveniles therefore experienced a normal sensory phase with their tutor, but their entire experience of BOS was of the abnormal, nerve cut (ts cut) song. Song analyses demonstrated that this manipulation successfully minimized both the spectral and temporal similarity between BOS and tutor song.
Using ts cut song and tutor song as stimuli, we characterized neuronal selectivity in the AFP of ts cut birds at 60 days of age. Some neurons responded more strongly to the unique ts cut BOS (tsBOS) than to tutor song, clearly demonstrating a role for BOS experience in shaping neural selectivity. Strikingly, a sizable proportion of neurons still responded equally well to both tsBOS and tutor song, despite the acoustic differences between these two songs (Fig. 3D). These neurons were not simply immature, because they exhibited selectivity for tsBOS and tutor song over conspecific and reverse song. Thus, the presence of neurons with equivalent responses to tsBOS and tutor song in these ts cut birds suggests that both song experiences can shape the selectivity of single neurons.
How might these different types of song selectivity function in song learning? Because BOS selectivity reflects the bird's current vocal output, it might provide information about the state of plastic song to a neural circuit involved in comparing BOS to a tutor song template stored in sensory coordinates. The high selectivity for BOS also might provide a kind of filter or gating function, aiding the bird in distinguishing its own vocalizations from those of others. It also may reflect in some way the pattern of motor activation during singing. The function of this selectivity could be further investigated with experiments in which AFP selectivity was broadened during song learning, perhaps with pharmacological agents.
Tutor song selectivity could encode information about the tutor song and function during sensorimotor learning as the neural reference of tutor song for birds. That is, this selectivity would result from experience of the tutor song during the sensory phase of learning. During the sensorimotor phase, the level or pattern of firing of these neurons in response to BOS then would reflect the degree to which BOS resembles the tutor song. A role for the AFP in sensory learning of the template also is supported by behavioral experiments that demonstrate a need for normal LMAN activity specifically during tutor song exposure (23).
In addition, these experiments found that BOS selectivity often coexists with tutor song selectivity in the same individual AFP neurons. This dual selectivity may reflect a function for AFP neurons in the actual comparison of BOS and tutor song that is essential to learning. For example, auditory feedback from the bird's own vocalizations would elicit activity from BOS selective cells. If this auditory feedback of the bird's own voice also matched the tutor song, then this might elicit greater or different activity in neurons that were also tuned to the tutor song than in neurons tuned to BOS or tutor song alone. Thus, the extent to which BOS resembles the tutor might be reflected in activity of dually tuned neurons, which then could participate in the reinforcement of the motor pathway.
A further suggestion that song selectivity might not only be linked to evaluation of auditory feedback, but might actually be sensitive to how well that feedback matches the target, came from studies of adult birds that were experimentally prevented from ever producing a good copy of their tutor template. We found that when we let birds that experienced transections of the tracheosyringeal portion of the hypoglossal nerve before song onset grow to adulthood, they had abnormally low song selectivity in the AFP (24). Neurons were selective enough to discriminate BOS and tutor song from conspecific and reverse songs, but the degree of selectivity was less than that found in normal adults. This result suggests that selectivity is compromised by a chronic inability of birds to match their tutor song model. If true, then these neurons are not simply reflecting sensory experience, but are influenced by the degree of matching during sensorimotor learning. Similarly, LMAN selectivity is not apparent in adult birds raised in isolation, even though they have developed stereotyped songs.‡ Isolate birds do not have experience with a tutor, so they may experience a mismatch between auditory feedback of BOS and the template because they are missing this internal song model.
Despite the joint representation of BOS and tutor song in many AFP neurons, it seems likely that a pure sensory representation of tutor song is present somewhere in the brain. Although this could be encoded by an unidentified subset of neurons lying within other song system nuclei or even within the AFP, it seems equally plausible that such a representation lies elsewhere in the brain, perhaps in the earlier high-level auditory areas that also process songs of conspecifics (26, 27).
Song Maintenance in Adults
The striking selectivity for BOS and tutor song found in the AFP, along with the knowledge that lesions to this circuit disrupt song learning (12–14, 23), are consistent with a role for the AFP in evaluation of vocal output and adaptive modification of song during sensorimotor learning. An apparent problem for the proposed function for the AFP arose when it became clear that adult zebra finches that have been deafened or that experience persistently altered feedback of their own vocalizations exhibit gradual deterioration of their song structure (5, 10). In contrast, lesions of the AFP have no effect on normal adult song production (28). These results clearly indicate that lesions of the AFP are not equivalent to interrupting the neural encoding of auditory feedback as it enters the nervous system. They do not, however, rule out a role for the AFP in evaluating auditory feedback of song in adult birds (29).
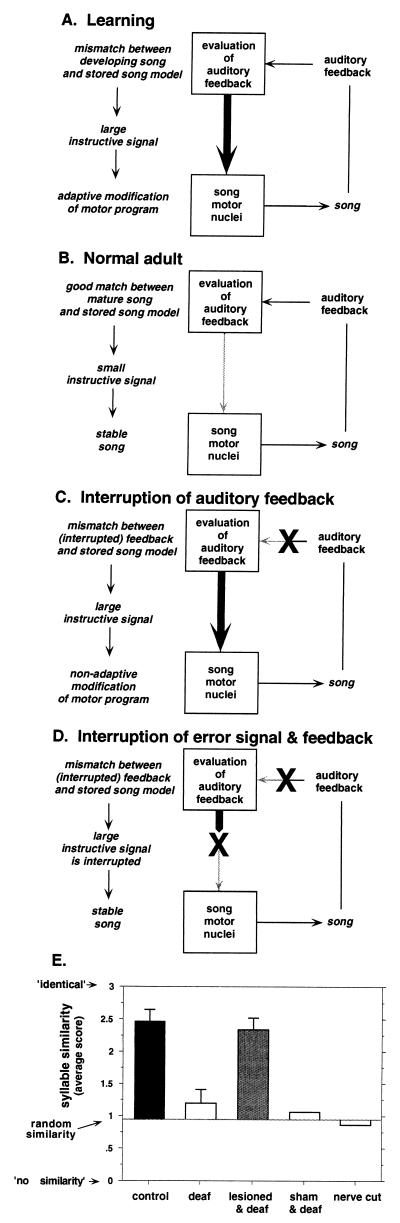
The distinction between encoding and evaluating auditory feedback of song is illustrated in Fig. 4 A–D, which traces the hypothetical flow of auditory feedback through the song system. Normally, during song learning, auditory feedback of BOS is first encoded by the nervous system, and then compared with an internal model of the song target, the template. To the extent that there is a mismatch between the bird's song and the template, the evaluation results in an instructive signal that drives adaptive changes in the song motor program (Fig. 4A). After the completion of learning, auditory feedback of adult song matches the template, and evaluation results in a small or stable signal that does not cause changes in song (Fig. 4B). By this model, the stability of adult song results from a match between vocal output and the internal target, rather than loss of sensitivity of the motor pathway to auditory experience. Subsequent removal of all auditory feedback, however, causes a large mismatch between expected and actual feedback, and would again generate an instructive signal that drives (now nonadaptive) changes in the song (Fig. 4C), such as those seen experimentally in deaf zebra finches (5). In contrast, if instead of interrupting auditory feedback before its evaluation, the output of the evaluation itself were interrupted (Fig. 4D), the consequences would be quite different. In this case, no instructive signal would reach the motor pathway and song would not change. Indeed, by this hypothesis, interrupting the instructive signal would occlude the effects of altering auditory feedback (Fig. 4D).
Figure 4.
Hypothetical flow of auditory feedback through the song system during sensorimotor processes and effect of AFP lesions. (A) During learning, auditory feedback from song is compared with an internal song model. Differences between the bird's own vocalizations and the model result in an instructive signal that drives adaptive modification of song. (B) In adulthood, the bird's song resembles the stored song model. Thus, there is little drive for vocal change. (C) Removal of auditory feedback leads to generation of a large instructive signal that, because of inappropriate feedback, leads to nonadaptive changes in song. (D) Interruption of the instructive signal removes the drive for change in song, even when auditory feedback is eliminated. (E) Comparison of syllable stability among adult birds that were deafened, those that were deafened and had also received LMAN lesions, and intact controls. Two additional controls included deaf birds that received sham lesions, and hearing birds with acute ts nerve transections, which eliminate syllable structure. The similarity of song syllables measured the degree to which syllables produced before experimental manipulations resembled those produced afterward (see ref. 29). Bars show means and standard errors for each group.
In the context of this model, the failure of LMAN lesions to cause deterioration in song (28) indicates that these lesions do not interrupt auditory feedback before its evaluation. We tested the alternate hypothesis, that the AFP participates in evaluating auditory feedback, by comparing changes in song that followed deafening with those that followed a combination of deafening and bilateral AFP lesions directed at nucleus LMAN. If LMAN has a role in guiding vocal adaptation based on auditory feedback, then we expected that LMAN lesions would block the effects of deafening.
In agreement with other studies (5, 6), the songs of intact adult zebra finches were stable over long periods of time, whereas the songs of birds deafened as adults gradually deteriorated over a period of weeks to months. This deterioration included severe changes in both syllable structure and temporal patterning of song. In contrast, the songs of birds that received LMAN lesions at the same time as they were deafened remained stable and essentially unchanged (Fig. 4E). In fact, their songs were as stable as those of intact adults and remained so for at least a year, indicating that lesions did not simply delay the effects of deafening. Thus, LMAN lesions blocked deafening-induced song deterioration, including changes to both syllable and temporal structure.
These results demonstrate that the deterioration of song after deafening is an active process, because it can be blocked by lesions of a particular brain area. Moreover, these findings indicate that LMAN is required for changes in adult song, consistent with the AFP either computing or conveying an instructive signal about the quality of song, which then drives changes in vocal output. This interpretation is consistent with two previously reported effects of adult lesions of LMAN: the prevention of incorporation of new syllables into songs of birds undergoing late learning (30), and the prevention of gradual changes to the abnormal songs of adult birds whose motor production has been disrupted by denervation of the syrinx (31). In both these cases LMAN lesions also may act by eliminating signals from the AFP to the motor pathway about the mismatch between sensory feedback and the stored song model, thereby eliminating any impetus for change in vocal output. Finally, our results are compatible with the AFP playing the same role in adults as hypothesized for juveniles: the auditory feedback-based evaluation and adaptive modification of the BOS. The difference between LMAN lesions in juveniles and those in adults does not reflect a changing function of LMAN during development; rather, it highlights the difference between the state of the motor pathway in plastic song and adult song. When vocal output is in a state of flux, as during learning, the sudden absence of an instructive signal leads to a failure of song progression. In contrast, when song is already stable, as in adults, the presumptive instructive signal for change is small, and its removal has no effect. The continuing function of the AFP in adult birds is revealed only when a mismatch between actual and expected feedback is experimentally generated. Presumably, in adult birds, the AFP normally participates in the correction of any alterations in song that result from small changes to the motor pathway. Such changes to the motor pathway must normally be remarkably small, because auditory feedback is not required (in birds with AFP lesions) for adult song to remain stable.
An alternate (and not mutually exclusive) hypothesis about the role of the AFP is that neural or trophic inputs from this circuit are permissive for plasticity in the motor pathway (32, 33). According to this hypothesis, the instructive signal driving changes in song might arise elsewhere, but without the integrity of the AFP would be unable to bring about changes in song. The question of whether AFP is instructive, permissive, or both in driving vocal plasticity requires further investigation, perhaps with experiments in which the pattern, but not the amount, of AFP activity is altered.
Exactly how this putative instructive signal would be encoded by neural activity is unclear. Its sign is unknown, and it could manifest itself as magnitude and/or patterns of activity. Experiments to address these issues could assess whether AFP neural activity changes when the normal match between what the bird intends to produce and what it actually hears is disrupted. Manipulations in which auditory feedback is consistently altered in a way to which the bird gradually could adapt by altering its vocalizations would be especially informative, because changes in the instructive signal during altered feedback could be associated with subsequent changes in vocal output. For example, an initial change in auditory feedback of the song (for instance an upward shift in frequency) should result in a change in activity in neurons that compute an instructive signal, signifying a mismatch between auditory feedback and the internal song target. After a period of this altered feedback, the bird might gradually change its vocal output (in this instance lowering the frequency of its song), so that the auditory feedback once again resembles the internal song model. During this adaptation, the activity of an instructive signal gradually should return to its original condition. Psychophysical experiments in humans show just such rapid adaptive changes in vocal behavior in response to altered feedback (34), but the song system provides the opportunity to record not only the vocal output but also the neural signals in the AFP and motor pathways during such experiments.
Singing-Related Activity in the AFP
Because the auditory feedback most relevant to song learning and maintenance occurs when the bird actually sings, it was clearly critical to record AFP activity during singing. To characterize signals present in the AFP of normal adult birds, we recorded single-unit and multiunit activity in LMAN during singing in adult zebra finches (35, 36). LMAN neurons fired vigorously throughout singing in adult birds (Fig. 5), despite the fact that this nucleus is not required for normal song production. Moreover, excitation began before song output, indicating that at least some of the activity is independent of auditory feedback of the bird's own voice. On average there was a consistent pattern of activity related to individual song elements, and peaks of activity tended to precede syllables. This activity resembles that reported in previous studies of singing-related premotor activity in the song control nucleus HVc (37, 38). This raises the possibility that much of the AFP activity during singing originates from the song motor circuit and may represent in part a version of the premotor signals also sent to the motor output pathway.
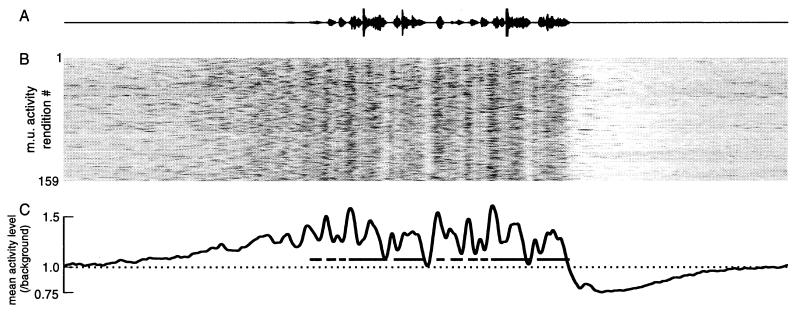
Figure 5.
LMAN neurons exhibit strong, singing-related activity. The oscillogram shows the song produced by the bird; the mean level of LMAN multiunit activity recorded in this bird before, during, and after each of 159 renditions of the song is shown below, aligned to the song. Activity level is represented by a color scale, where black indicates high neural activity, and white low activity (see ref. 36). The bottom trace shows the mean of activity during all of the renditions above, illustrating the onset of AFP activity before sound, and the peaks of activity related to syllables, which are indicated by the black bars. The duration of the entire panel is 4.5 sec.
The properties of this singing-related activity raised the question of whether any of it is related to sensory feedback. In playback experiments, song-selective responses to auditory stimuli like those studied in anesthetized birds were apparent in LMAN of awake birds. However, they were variable from trial to trial and between birds, and it remains to be determined whether they are present in the same neurons that show singing-related activity. Moreover, the level of activity elicited by playback of auditory stimuli was low relative to singing-related activity, making it possible that small auditory feedback signals are embedded within the robust singing-related AFP activity. As an initial step to see whether AFP activity during singing contains both sensory and motor activity, we recorded multiunit activity from LMAN before and 1–3 days after deafening. Neural activity during singing was very similar predeafening and postdeafening, indicating that much of the activity during singing is not dramatically altered by an acute loss of auditory feedback.
Although selective responses to playback of BOS had suggested that sensorimotor learning influences the AFP, the marked AFP activity during singing demonstrates very directly that this circuit is not a pure sensory pathway, but instead, a sensorimotor circuit. Its function during singing may be clarified with studies that determine whether activity in individual neurons or across a population of neurons is a mixture of motor and sensory signals, and if so, how these relate to each other. In addition, recording LMAN activity in response to altered rather than absent feedback could be an important approach to studying these neurons; this would allow multiple interleaved recordings of song-related activity with and without altered feedback, which could be useful for detecting small sensory feedback signals.
The singing-related activity in the AFP might represent an efference copy, perhaps predicting the sensory consequences of motor commands. The properties of AFP neurons are consistent with this hypothesis. Because efference copy signals are triggered by motor commands, neurons with such signals would be expected to be active during singing, even in the absence of auditory feedback. Furthermore, if these neurons encode an internally generated prediction of the sensory outcome of a motor command to sing, then they might exhibit BOS selectivity when probed with song stimuli in playback experiments. Efference copies often are seen in sensorimotor systems (39–41) and can be useful for providing information about intended motor activity to multiple areas of the brain and for comparing motor instructions with the consequences of these instructions. The utility of an efference copy signal during sensorimotor learning has been explored in a computational model (42, 43). In this model, premotor activity in HVc gradually becomes associated with the resulting auditory feedback. This creates an internal prediction of the auditory feedback expected after a particular motor command is elicited. Thus, this efference copy is learned, and the role of auditory feedback is to maintain an accurate efference copy. The AFP then evaluates this sensory prediction, rather than the actual feedback. One advantage of this scheme is that it greatly shortens the normal delay between motor activity and auditory feedback, which otherwise might cause feedback evaluation signals to arrive during the motor commands for the next vocal gesture.
If a sensory prediction is learned as described above, then the considerable time it takes for altered auditory feedback to result in vocal change in adult birds might reflect the time necessary to revise the efference copy signal. An instructive signal for change would emerge only after consistently altered feedback changed the pattern of association of auditory feedback and motor commands in HVc. Alternatively, the time course for vocal change after deafening could reflect the time necessary for an instructive signal to take effect within the motor pathway. In this scheme, altered auditory feedback would immediately result in an instructive signal; however, a change in vocal output would not occur until the instructive signal was maintained over a certain period. Simultaneous recordings in the AFP and the motor pathway during singing and especially during song learning should help clarify the relationship of AFP activity to motor output and sensory feedback. Presentation of incorrect feedback again might be a useful manipulation, because altered feedback should provide a more potent signal for altering the association between motor commands and feedback in the putative efference copy than the complete absence of sound. In humans, delayed or altered auditory feedback changes vocal output much more rapidly than deafness (3, 34, 44).
Conclusions
The studies here used a combination of neurophysiological and behavioral studies to investigate the function of the AFP, in each case investigating not only normal birds but also animals in which the normal relationship between vocal motor output and sensory input had been in some way disrupted. The results revealed that AFP neurons develop selectivity during learning for both BOS and tutor song, are required for changes in vocal output throughout the bird's life, and are strongly active during singing, even in deaf birds. This suggests that AFP neurons reflect multiple sensory and motor aspects of song, and that these processes are almost inextricably entangled, even at the level of single neurons. In this respect, bird song is reminiscent of human speech (1): electrical stimulation of a single language area can affect both production and perception of speech (45), and some cortical neurons respond differently to the same word depending on whether it was spoken by the subject or by someone else (46). Perhaps this entanglement indicates that the primary task assigned to the song system, and to many speech areas as well, is not sensory learning, but rather the sensorimotor learning required to produce a vocal imitation. Memorizing a tutor song is crucial to learning, but can be very rapid (47–49). In contrast, developing a learned song, like other motor skills, is a protracted process, involving a series of interactions between motor acts, feedback from those acts, and gradual convergence onto an internalized song model. Thus, sensorimotor learning of song alone may be sufficient to have created the need for the song system, and to have specialized it for sensorimotor processing, with much of the initial sensory processing and memorizing of songs taking place elsewhere in the brain.
It is also relevant to the results here that the AFP is a cortical-basal ganglia circuit (50, 51). Such basal ganglia circuits are well conserved evolutionarily and generally are implicated in motor and reinforcement learning, functions critical to sensorimotor learning of song. In primates, striatal neurons have predictive information related to movement and reward and might participate in comparisons of motor output to internal models (52–54). AFP neurons could similarly receive or even compute reinforcement signals and transfer them to the motor pathway. Moreover, the covert contribution of the AFP to adult plasticity is reminiscent of mammalian systems, where damage to cortical-basal ganglia circuits can impair procedural learning while having little effect on previously learned performance (55–57). Because the AFP is a discrete basal ganglia-forebrain circuit specialized for one well-defined behavior, it may prove a particularly tractable system for elucidating the neural signals present in these structures and their function in the learning and modification of sequenced motor acts.
Acknowledgments
The work described here was supported by the National Institutes of Health (Grants MH55987 and NS34835 to A.J.D. and Grant NS00913 to N.A.H.), a National Sciences Foundation graduate fellowship (M.M.S.), a Burroughs Wellcome Fund fellowship of the Life Sciences Research Foundation (M.S.B.), and the John Merck Fund and the EJLB Foundation (A.J.D.).
Abbreviations
- AFP
anterior forebrain pathway
- BOS
bird's own song
- LMAN
lateral magnocellular nucleus of the anterior neostriatum
Footnotes
This paper was presented at the National Academy of Sciences colloquium “Auditory Neuroscience: Development, Transduction, and Integration,” held May 19–21, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
‡ Maekawa, M. (1998) Soc. Neurosci. Abstr. 24, 190.
References
- 1.Doupe A J, Kuhl P K. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 2.Waldstein R S. J Acoust Soc Am. 1989;88:2099–2114. doi: 10.1121/1.400107. [DOI] [PubMed] [Google Scholar]
- 3.Cowie R, Douglas-Cowie E. In: Postlingually Acquired Deafness: Speech Deterioration and the Wider Consequences. Winter W, editor. Berlin: de Gruyter; 1992. [Google Scholar]
- 4.Konishi M. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- 5.Nordeen K W, Nordeen E J. Behav Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- 6.Price P H. J Comp Physiol Psychol. 1979;93:268–277. [Google Scholar]
- 7.Marler P. J Comp Physiol Psychol. 1970;71:1–25. [Google Scholar]
- 8.Immelmann K. In: Bird Vocalizations. Hinde R A, editor. London: Cambridge Univ. Press; 1969. pp. 61–74. [Google Scholar]
- 9.Eales L A. Anim Behav. 1985;33:1293–1300. [Google Scholar]
- 10.Leonardo A, Konishi M. Nature (London) 1999;399:466–470. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- 11.Nottebohm F, Stokes T M, Leonard C M. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 12.Bottjer S W, Miesner E A, Arnold A P. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 13.Sohrabji F, Nordeen E J, Nordeen K W. Behav Neurol Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 14.Scharff C, Nottebohm F. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doupe A J. J Neurosci. 1997;17:1147–1167. doi: 10.1523/JNEUROSCI.17-03-01147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCasland J S, Konishi M. Proc Natl Acad Sci USA. 1981;78:7815–7819. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margoliash D. J Neurosci. 1983;3:1039–1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solis M M, Doupe A J. J Neurosci. 1997;17:6447–6462. doi: 10.1523/JNEUROSCI.17-16-06447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eimas P D, Miller J L, Jusczyk P W. In: Categorical Perception. Harnard S, editor. New York: Cambridge Univ. Press; 1987. pp. 161–195. [Google Scholar]
- 20.Kuhl P K. Curr Opin Neurobiol. 1994;4:812–822. doi: 10.1016/0959-4388(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 21.Werker J F, Tees R C. Annu Rev Neurosci. 1992;15:377–402. doi: 10.1146/annurev.ne.15.030192.002113. [DOI] [PubMed] [Google Scholar]
- 22.Solis M M, Doupe A J. J Neurosci. 1999;19:4559–4584. doi: 10.1523/JNEUROSCI.19-11-04559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basham M E, Nordeen E J, Nordeen K W. Neurobiol Learning Mem. 1996;66:295–304. doi: 10.1006/nlme.1996.0071. [DOI] [PubMed] [Google Scholar]
- 24.Solis M M, Doupe A J. Neuron. 2000;25:109–121. doi: 10.1016/s0896-6273(00)80875-2. [DOI] [PubMed] [Google Scholar]
- 25.Theunissen F E, Doupe A J. J Neurosci. 1998;18:3786–3802. doi: 10.1523/JNEUROSCI.18-10-03786.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mello C V, Vicario D S, Clayton D F. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolhuis J J, Zijlstra G G O, den Boer-Visser A M, Van der Zee E A. Proc Natl Acad Sci USA. 2000;97:2282–2285. doi: 10.1073/pnas.030539097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordeen K W, Nordeen E J. Behav Neural Biol. 1993;59:79–82. doi: 10.1016/0163-1047(93)91215-9. [DOI] [PubMed] [Google Scholar]
- 29.Brainard M S, Doupe A J. Nature (London) 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- 30.Morrison R G, Nottebohm F. J Neurobiol. 1993;24:1045–1064. doi: 10.1002/neu.480240805. [DOI] [PubMed] [Google Scholar]
- 31.Williams H, Mehta N. J Neurobiol. 1999;39:14–28. [PubMed] [Google Scholar]
- 32.Johnson F, Bottjer S W. Development (Cambridge, UK) 1994;120:13–24. doi: 10.1242/dev.120.1.13. [DOI] [PubMed] [Google Scholar]
- 33.Akutagawa E, Konishi M. Proc Natl Acad Sci USA. 1994;91:12413–12417. doi: 10.1073/pnas.91.26.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houde J F, Jordan M I. Science. 1998;36:1213–1216. doi: 10.1126/science.279.5354.1213. [DOI] [PubMed] [Google Scholar]
- 35.Hessler N A, Doupe A J. Nat Neurosci. 1999;2:209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- 36.Hessler N A, Doupe A J. J Neurosci. 1999;19:10461–10481. doi: 10.1523/JNEUROSCI.19-23-10461.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCasland J S. J Neurosci. 1987;7:23–39. doi: 10.1523/JNEUROSCI.07-01-00023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu A C, Margoliash D. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- 39.von Holst E, Mittelstaedt H. Naturwissenshaften. 1950;37:464–476. [Google Scholar]
- 40.Bell C. J Exp Biol. 1989;146:229–253. doi: 10.1242/jeb.146.1.229. [DOI] [PubMed] [Google Scholar]
- 41.Bridgeman B. Ann Biomed Eng. 1995;23:409–422. doi: 10.1007/BF02584441. [DOI] [PubMed] [Google Scholar]
- 42.Troyer T, Doupe A J. J Neurophysiol. 2000;84:1204–1223. doi: 10.1152/jn.2000.84.3.1204. [DOI] [PubMed] [Google Scholar]
- 43.Troyer T, Doupe A J. J Neurophysiol. 2000;84:1224–1239. doi: 10.1152/jn.2000.84.3.1224. [DOI] [PubMed] [Google Scholar]
- 44.Lee B S. J Acoust Soc Am. 1950;22:824–826. [Google Scholar]
- 45.Ojemann G A. J Neurosci. 1991;11:2281–2287. doi: 10.1523/JNEUROSCI.11-08-02281.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Creutzfeldt O, Ojemann G, Lettich E. Exp Brain Res. 1989;77:451–489. doi: 10.1007/BF00249600. [DOI] [PubMed] [Google Scholar]
- 47.Todt D, Hultsch H, Heike D. Z Tierpsychol. 1979;51:23–35. [Google Scholar]
- 48.Peters S, Marler P, Nowicki S. Condor. 1992;94:1016–1019. [Google Scholar]
- 49.Tchernichovski O, Lints T, Mitra P P, Nottebohm F. Proc Natl Acad Sci USA. 1999;96:12901–12904. doi: 10.1073/pnas.96.22.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bottjer S W, Johnson F. J Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 51.Luo M, Perkel D J. J Neurosci. 1999;19:6700–6711. doi: 10.1523/JNEUROSCI.19-15-06700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hikosaka O, Sakamoto M, Usui S. J Neurophysiol. 1989;61:814–832. doi: 10.1152/jn.1989.61.4.814. [DOI] [PubMed] [Google Scholar]
- 53.Hollerman J R, Tremblay L, Schultz W. J Neurophysiol. 1998;80:947–963. doi: 10.1152/jn.1998.80.2.947. [DOI] [PubMed] [Google Scholar]
- 54.Tremblay L, Hollerman J R, Schultz W. J Neurophysiol. 1998;80:964–977. doi: 10.1152/jn.1998.80.2.964. [DOI] [PubMed] [Google Scholar]
- 55.Graybiel A M, Aosaki T, Flaherty A W, Kimura M. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 56.Knowlton B J, Mangels J A, Squire L R. Science. 1996;6:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura K, Sakai K, Hikosaka O. J Neurophysiol. 1999;82:1063–1068. doi: 10.1152/jn.1999.82.2.1063. [DOI] [PubMed] [Google Scholar]