Abstract
Neurons must develop complex structure to form proper connections in the nervous system. The initiation of axons in defined locations on the cell body and their extension to synaptic targets are critical steps in neuronal morphogenesis, yet the mechanisms controlling axon formation in vivo are poorly understood. The centrosome has been implicated in multiple aspects of neuronal morphogenesis; however, its function in axon development is under debate. Conflicting results from studies of centrosome function in axonogenesis suggest that its role is context dependent and underscore the importance of studying centrosome function as neurons develop in their natural environment. Using live imaging of zebrafish Rohon-Beard (RB) sensory neurons in vivo, we discovered a spatiotemporal relationship between centrosome position and the formation of RB peripheral, but not central, axons. We tested centrosome function by laser ablation and found that centrosome disruption inhibited peripheral axon outgrowth. In addition, we show that centrosome position and motility are regulated by LIM homeodomain transcription factor activity, which is specifically required for the development of RB peripheral axons. Furthermore, we show a correlation between centrosome mislocalization and ectopic axon formation in bashful (laminin alpha 1) mutants. Thus, both intrinsic transcription factor activity and extracellular cues can influence centrosome position and axon formation in vivo. This study presents the first positive association between the centrosome and axon formation in vivo and suggests that the centrosome is important for differential neurite formation in neurons with complex axonal morphologies.
Keywords: Neuronal polarity, Centrosome, LIM transcription factors, Axon formation
INTRODUCTION
Neurons must develop highly polarized and specific morphologies for proper nervous system function. Axon formation is a key step of neuronal morphogenesis and the site of axon initiation is important for establishing correct neuronal polarity in vivo (Barnes and Polleux, 2009; Polleux and Snider, 2010). This process is regulated by mechanisms that establish asymmetry within the neuron and lead to localized remodeling of the F-actin and microtubule (MT) cytoskeleton within the nascent axon (Stiess and Bradke, 2011). When isolated and cultured at an early stage, many neurons are capable of undergoing polarization (Barnes and Polleux, 2009; Randlett et al., 2011a), demonstrating that cell type-specific intrinsic programs can direct axon formation in vitro. However, the extent to which autonomous mechanisms contribute to neuronal polarization in vivo is unclear. In the embryo, external cues and the polarity of surrounding tissue undoubtedly influence neuronal polarity and axon position (Adler et al., 2006; Lerman et al., 2007; Polleux et al., 1998; Randlett et al., 2011b; Zolessi et al., 2006). To fully understand the mechanisms controlling neuronal morphogenesis, it is important to study the respective contributions of both intrinsic and extracellular signals as axons form within the context of the natural in vivo environment.
The centrosome functions as the main MT-organizing center for cells and has key roles in many biological processes (Vaughan and Dawe, 2011), including neurogenesis and neuronal migration (Higginbotham and Gleeson, 2007). However, conflicting evidence has come from studies of centrosome function in axon development. The centrosome and associated Golgi apparatus predict the site of axon formation in cultured mammalian cerebellar granule and hippocampal neurons (de Anda et al., 2005; Zmuda and Rivas, 1998) and in cortical slices (de Anda et al., 2010). Furthermore, disruption of centrosome function inhibits axon formation in cortical slices (de Anda et al., 2010) and in cultured Drosophila neurons (de Anda et al., 2005). However Sas-4 mutant flies, which lack the ability to replicate centrosomes, have grossly normal brain development and properly oriented axons in the developing eye disc (Basto et al., 2006), suggesting that the centrosome is dispensable for fly neuronal morphogenesis in vivo. In addition, in vivo imaging of the centrosome in developing zebrafish retinal ganglion cells (RGCs) and rhombic lip neurons, which initially have simple bipolar morphology, showed that the axon could form independently of centrosome proximity in these neuronal types (Zolessi et al., 2006; Distel et al., 2010). One explanation for the apparent contradiction between these studies is that the role of the centrosome is context dependent. For example, differences in the organization of the MT arrays of vertebrate and fly neurons (Baas and Lin, 2011; Nguyen et al., 2011; Rolls, 2011) could determine whether centrosomes are needed for polarization in one system and not the other. It is also plausible that the centrosome is more important for defining polarity of neurons with complex initial neurite patterns, such as cortical and hippocampal neurons, than for those with a simpler initial structure, such as RGCs and hindbrain neurons. Furthermore, the dynamic nature of centrosome positioning could prevent detection of a transient role in axon development. Indeed, transient centrosome localization to the axon formation site precedes axon specification in cortical neurons within slices (de Anda et al., 2010). Moreover, the organization of MT arrays shifts from primarily centrosome derived to centrosome independent during the development of hippocampal neurons in culture, and removal of the centrosome after axon specification, during axon extension or regeneration, does not affect these later processes (Stiess et al., 2010). On the whole, these studies suggest that the centrosome has divergent roles in axon formation and highlight the need to study diverse cell types using high-resolution spatiotemporal imaging to understand its function during neuronal morphogenesis in vivo.
Zebrafish Rohon-Beard (RB) neurons provide an excellent model with which to study centrosome dynamics during the development of complex neuronal morphology in vivo. RB neurons extend two types of axons: central axons that ascend and descend in the spinal cord, and a single peripheral axon that extends orthogonally to the central axons, exits the spinal cord and innervates the skin (Clarke et al., 1984; Kuwada et al., 1990). RB axon morphology is controlled in part by the intrinsic activity of LIM homeodomain (HD)-containing transcription factors (Becker et al., 2002; Segawa et al., 2001). Expression of a dominant-negative co-factor of LIM (DN-CLIM) specifically inhibits RB peripheral axon formation without affecting central axon trajectories (Becker et al., 2002). The mechanisms by which LIM-HD activity differentially regulates central versus peripheral RB axons are poorly understood. We previously characterized the effects of DN-CLIM on RB axon behaviors and F-actin dynamics and discovered that peripheral axon initiation, extension and branching were impaired (Andersen et al., 2011). This effect on multiple cell motility processes suggests that DN-CLIM affects cytoskeletal dynamics. However, F-actin accumulations and filopodial protrusions still form in these embryos, which led us to hypothesize that LIM-HDs might act through the regulation of MT dynamics and/or MT invasion into the peripheral axon.
Here, we used live imaging to determine the dynamics of centrosome positioning during morphogenesis of RB neurons in vivo. We found that centrosome proximity coincides with peripheral, but not central, axon formation. The centrosome consistently localizes to the base of the nascent peripheral neurite during its transition into an established axon. Laser disruption of the centrosome inhibits peripheral axon growth. Furthermore, we found that centrosomes are mispositioned and have reduced motility in DN-CLIM-expressing embryos, suggesting a mechanism by which LIM-HD activity may affect peripheral axon development. Centrosomes are also mislocalized in bashful (bal; laminin alpha 1) mutant embryos and this phenotype is associated with ectopic peripheral axons. Together, our results support a role for the centrosome in peripheral, but not central, RB axon development, and suggest that the centrosome functions in differential neurite development in neurons with complex axonal morphologies.
MATERIALS AND METHODS
Animals
Adult zebrafish (Danio rerio) were maintained in a laboratory breeding colony. Wild-type AB strain, Tg(–3.1ngn1:TagRFP-caax) (Andersen et al., 2011) and baluw1 (Paulus and Halloran, 2006; Semina et al., 2006) embryos were used. Embryos were raised at 23-28.5°C and staged as described (Kimmel et al., 1995). Animals were handled according to guidelines established by the NIH and Institutional Animal Care and Use Committee.
DNA constructs
DNA expression constructs were generated with the Multisite Gateway Cloning System (Invitrogen, Carlsbad, CA, USA) using the 5′ entry vector p5E-ngn(–koz) (Andersen et al., 2011) containing an RB neuron driver (Blader et al., 2003) and zebrafish-compatible Tol2 transposon vectors (Kawakami, 2004; Kwan et al., 2007; Villefranc et al., 2007). For centrosome labeling by DNA injection, a middle entry vector containing zebrafish centrin 2 with N-terminal GFP (pME-gfp-Zcentrin2; gift from M. Granato, University of Pennsylvania, Philadelphia, PA, USA) was used to generate pEXP-3.1ngn1:gfp-Zcentrin2 (GFP-Zcentrin).
RNA synthesis
5′-capped mRNA was synthesized using the mMessage mMachine Kit (Ambion, Austin, TX, USA). Xenopus GFP-Centrin and DN-CLIM RNAs were generated from plasmids pCS2+eGFP-Xcentrin (gift from K. Kwan, University of Utah, Salt Lake, UT, USA) and pCS2+dn-clim [gift from I. Bach (Becker et al., 2002)], respectively.
RB and centrosome labeling
For individual RB cell labeling, we injected 10-25 pg pEXP-3.1ngn1:tagrfp-caax (TagRFP-CAAX) DNA (Andersen et al., 2010) at the 1- to 2-cell stage, which resulted in transient mosaic expression in a few RB neurons per embryo. Embryos with labeled RB neurons were sorted between 15 and 24 hours postfertilization (hpf) using a Nikon AZ100 fluorescence dissecting microscope.
For centrosome labeling, embryos were injected at the 1- to 2-cell stage with either 10-45 pg eGFP-Xcentrin mRNA, which is expressed ubiquitously, or 10-25 pg GFP-Zcentrin DNA, which mosaically labels RB neurons.
Centrosome ablations
Tg(–3.1ngn1:TagRFP-caax) embryos were injected with GFP-Xcentrin mRNA at the 1-cell stage and immobilized in agarose at ∼15.5 hpf. A nitrogen dye pulsed laser (Photonics Instruments; 440 nm) attached to a BioRad confocal microscope was focused on the centrosome with a 63× (1.4 NA) objective and pulsed at 5 Hz for 2-4 seconds. Embryos were allowed to develop for 3-5 hours before imaging. Confocal z-stacks (0.5 μm slices) of living embryos were captured with an Olympus FV1000. Embryos were then fixed 1-3 hours after imaging (∼21-22 hpf) and immunolabeled with antibodies against γ-tubulin (1:1000; Sigma-Aldrich) and TagRFP (1:1000; Evrogen). Alexa Fluor 488-conjugated anti-mouse IgG and Alexa Fluor 568-conjugated anti-rabbit IgG secondary antibodies were used.
DN-CLIM expression
Approximately 150 pg DN-CLIM mRNA was co-injected with TagRFP-CAAX DNA and GFP-Xcentrin mRNA into wild-type embryos at the 1-cell stage. Embryos were sorted at ∼16 hpf, and those with DN-CLIM-associated eye and midbrain-hindbrain boundary phenotypes (Becker et al., 2002) were selected for further analysis.
bal analysis
Embryos from a bal+/uw1 incross were co-injected with TagRFP-CAAX DNA and GFP-Xcentrin mRNA at the 1- to 2-cell stage. baluw1/uw1 embryos were sorted at ∼24 hpf for gross morphological phenotypes. Siblings (bal+/uw1 or bal+/+) were used as controls.
Live embryo imaging
To analyze centrosome positioning during different stages of RB development and for time-lapse imaging, embryos with individually labeled RB neurons were raised to the appropriate age (15-24 hpf), anesthetized with 0.02% 3-amino benzoic acid ethylester (tricaine), and mounted in 1% low-melting-point agarose in 10 mM HEPES-buffered E3 embryo medium. Images were captured using an Olympus FV1000, IX81 confocal microscope with a 60× oil-immersion objective (NA 1.35). RB neurons in the central region of the trunk (somites 3-14) were selected for live imaging. Optical sections (0.5-1 μm) encompassing a total of ∼25-30 μm were taken to visualize the RB peripheral and central axon pathways. For time-lapse imaging, embryos ranged from 15-19 hpf at the beginning of the experiment and were imaged for 2-8.5 hours at 28°C, with z-stacks captured at 1- to 2-minute intervals.
Image processing
Images were processed using Volocity software (Perkin Elmer, Waltham, MA, USA), and figures assembled with Photoshop and Illustrator (Adobe Systems, San Jose, CA, USA). For time-lapse images, a single xy focal plane containing the centrosome (green) was superimposed onto a z-projection of the labeled RB neuron (red or black/white) at each time point. Thus, for embryos with centrosomes labeled by ubiquitous eGFP-Xcentrin expression, the centrosomes of other cells in the same focal plane can be seen. Certain images were cropped to remove centrosomes and axons of other cells that obscured visualization of RB morphology in Volocity and/or Photoshop. For all images, contrast, brightness, levels and noise reduction adjustments were made. Movies were made with Volocity and ImageJ (NIH) and played at 10 frames per second.
Data analysis
Images were analyzed using Volocity and ImageJ. Statistical and graphical analyses were performed using Excel (Microsoft) or Prism (GraphPad). Values are reported as mean ± 1 or 2 s.e.m.
Centrosome position with respect to the peripheral axon initiation site (PAS) was measured as the angle between lines drawn from the cell body centroid to the centrosome and from the centroid to the PAS. Distances were measured in xyz dimensions. For peripheral axons that formed as branches off a central axon, the central axon initiation site was used as the PAS. A 45° cut-off was defined as localization of the centrosome near the PAS.
Centrosome position along the apical-basal axis was calculated as the distance of the centrosome to the basal edge of the cell body divided by total apical-basal cell body length, and reported as percentage. For wild-type and DN-CLIM embryos, live embryos were analyzed. For bal embryos, fixed and live embryos were analyzed.
Plots of centrosome localization were generated in Microsoft Excel by measuring the distance between the centrosome and PAS and the distance between the cell body centroid and the furthest peripheral protrusion in the xy dimensions of a z-projection. The furthest peripheral protrusion was defined as the tip of the longest filopodium, neurite, or axon extended orthogonal to the anterior-posterior axis. Measurements were taken at 10-minute intervals over a minimum of 60 minutes per movie. A 5-μm cut-off was defined as localization of the centrosome near the PAS, as it is less than half the RB cell body diameter (mean diameter, 13.7±0.5 μm; mean radius, 6.9 μm; minimum radius, 5.5 μm; n=9). The onset of the initiation and extension phases were defined as the times when the peripheral neurite first becomes distinguishable and exits the spinal cord, respectively.
Centrosome movement was measured in xy dimensions from z-projection images. To compensate for drift during imaging, the cell body centroid was used as a positional landmark. We used the Manual Tracking plug-in in ImageJ to measure the positions of the centrosome and cell body centroid. Measurements were taken at 10-minute intervals over a 120-minute time window. Tracks were generated in Microsoft Excel by plotting the corrected centrosome position, calculated by subtracting the xy position of the centroid from the xy position of the centrosome, for each time point. The total distance of centrosome migration was calculated by summing the distance between the cell body centroid and centrosome.
Peripheral axon lengths after centrosome ablation were measured in xyz dimensions. Axons in centrosome-ablated cells or control-lased cells were compared using a paired t-test with axons of neighboring unlased cells within one somite distance.
RESULTS
Centrosome position correlates with peripheral, but not central, RB axon formation
To examine centrosome positioning within developing RB neurons, we used in vivo neuron and centrosome labeling techniques. To visualize axon morphology, we labeled individual RB neurons by injecting DNA encoding membrane-targeted TagRFP (TagRFP-CAAX) driven by a cis-regulatory element from the neurogenin 1 gene (–3.1ngn1) (Blader et al., 2003). Centrosomes were labeled either by injection of mRNA encoding Xenopus Centrin fused to GFP (GFP-Xcentrin), which labels all centrosomes, or DNA encoding zebrafish Centrin 2 fused to GFP and driven by –3.1ngn1 (GFP-Zcentrin), which labels centrosomes in RB neurons. GFP-Xcentrin was previously validated for centrosome labeling in zebrafish (Sepich et al., 2011). GFP-Zcentrin also labels centrosomes in zebrafish (Baye et al., 2011; Distel et al., 2010; Randlett et al., 2011b; Zolessi et al., 2006) and, at higher expression levels, allows simultaneous visualization of the cytoplasm. These methods allowed high-resolution spatiotemporal imaging of centrosomes and developing axons in vivo.
We first examined centrosome localization in fixed preparations at a stage when RB neurons have established axonal projections (24 hpf). Both central and peripheral RB axons emerge from the basal region of the cell (Andersen et al., 2011). At 24 hpf, the majority of neurons had centrosomes localized within the basal half and toward the lateral side of the cell body (Fig. 1A,B). The peripheral axon typically forms within a specific RB cell compartment, arising either from the basal-lateral cell body or as a branch off one of the central axons near the cell body (Andersen et al., 2011). To determine the relationship between centrosome position along the anterior-posterior (A-P) cell axis and the peripheral axon, we measured the angle between two lines drawn from the cell body centroid to the centrosome or to the peripheral axon initiation site (PAS) (Fig. 1C). We found that the centrosome was localized near the PAS in 80% (n=20) of RB neurons (Fig. 1D). Thus, in mature RB neurons the centrosome is located basally, where axons initiate, and its position along the A-P axis correlates with the location of the PAS. These results suggest that the centrosome might function in RB axon development and could have a role in positioning the site of peripheral axon formation.
Fig. 1.
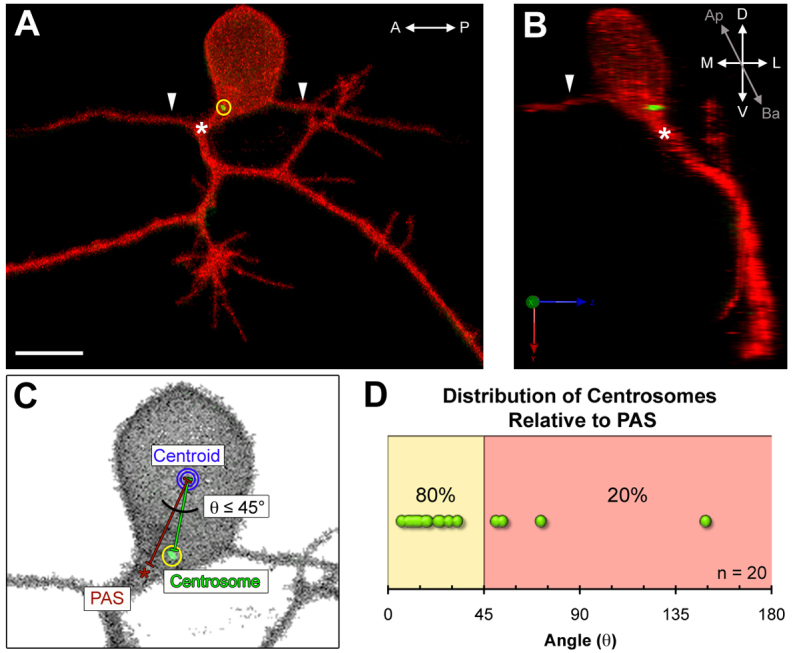
The centrosome is positioned near the peripheral axon initiation site in mature Rohon-Beard neurons. (A,B) Images of a mature Rohon-Beard (RB) neuron labeled by transient expression of TagRFP-CAAX in a wild-type zebrafish embryo expressing GFP-Xcentrin mRNA. (A) z-projection showing central axons extended along the anterior-posterior (A-P) axis (arrowheads) and a branched peripheral axon orthogonal to the A-P axis. The centrosome (yellow circle) is localized near the peripheral axon initiation site (PAS, asterisk). Scale bar: 10 μm. (B) Three-dimensional rendering of the neuron in A (yz view) showing the centrosome at the basal-lateral (Ba-L) cell body surface, near the PAS. (C) Schematic showing how centrosome position (yellow circle) was measured relative to the PAS and centroid (blue circle). (D) Distribution of angle (θ) measurements defined in C. The line between the yellow and red zones indicates 45° angle cut-off.
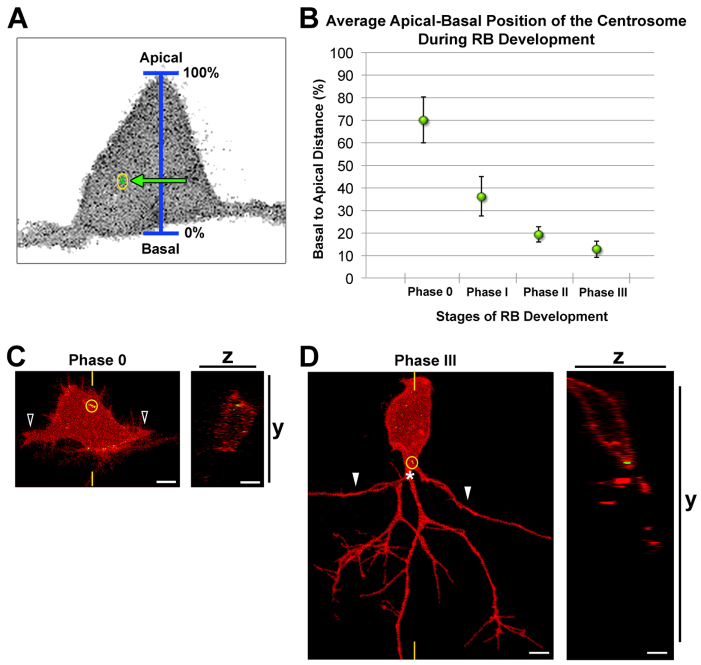
We next analyzed how centrosome position changes during RB morphogenesis and axonogenesis. During neurulation and prior to neuronal differentiation, the centrosome is apically localized in neuroepithelial cells (Hong et al., 2010), but little is known about how or whether its position changes during neuronal differentiation. We previously defined four stages of RB development: central axon initiation, which we refer to here as phase 0, and subsequent phases I-III (Andersen et al., 2011). Here we imaged living embryos during these stages (15-24 hpf) and analyzed centrosome positioning along the apical-basal axis (Fig. 2A). During phase 0, when central axons initiate outgrowth, the centrosome is localized apically within the RB cell body (Fig. 2B,C). In subsequent phases, its position becomes progressively more basal (Fig. 2B). During phase I, when central axons begin to extend, the centrosome is positioned slightly basal to the cell center. In phase II, when the peripheral axon initiates, and phase III, when the peripheral axon exits the spinal cord and arborizes in the epidermis, the centrosome occupies a basal position within the cell body (Fig. 2B,D). These results show that basal localization of the centrosome occurs after central axon initiation, but before and during peripheral axon formation, suggesting a potential role for the centrosome in this later process of RB morphogenesis.
Fig. 2.
The centrosome occupies a progressively basal position during RB development. (A) Schematic showing how centrosome position (yellow circle) was measured relative to the apical-basal axis. (B) Quantification of average centrosome position and its distribution about the mean (± 2 s.e.m.) during stages of RB development. Mean percentages are: phase 0, 70.0±10.2% (n=13); phase I, 36.1±8.6% (n=12); phase II, 19.3±3.4% (n=14); phase III, 12.7±3.6% (n=11). A total of 36 neurons were analyzed in live zebrafish embryos. (C,D) RB neurons labeled by transient expression of TagRFP-CAAX in GFP-Xcentrin mRNA-expressing wild-type embryos. Images shown are z-projections and optical cross-sections through the centrosome-containing region (indicated with yellow hatch marks). (C) Centrosome position during central axon initiation (phase 0). The open arrowheads indicate central axons. z-projection and xy view show centrosome (yellow circle) localized apically in the cell body. (D) Centrosome position during peripheral extension (phase III). White arrowheads indicate central axons. The centrosome is localized basally, near the PAS (asterisk). Scale bars: 5 μm.
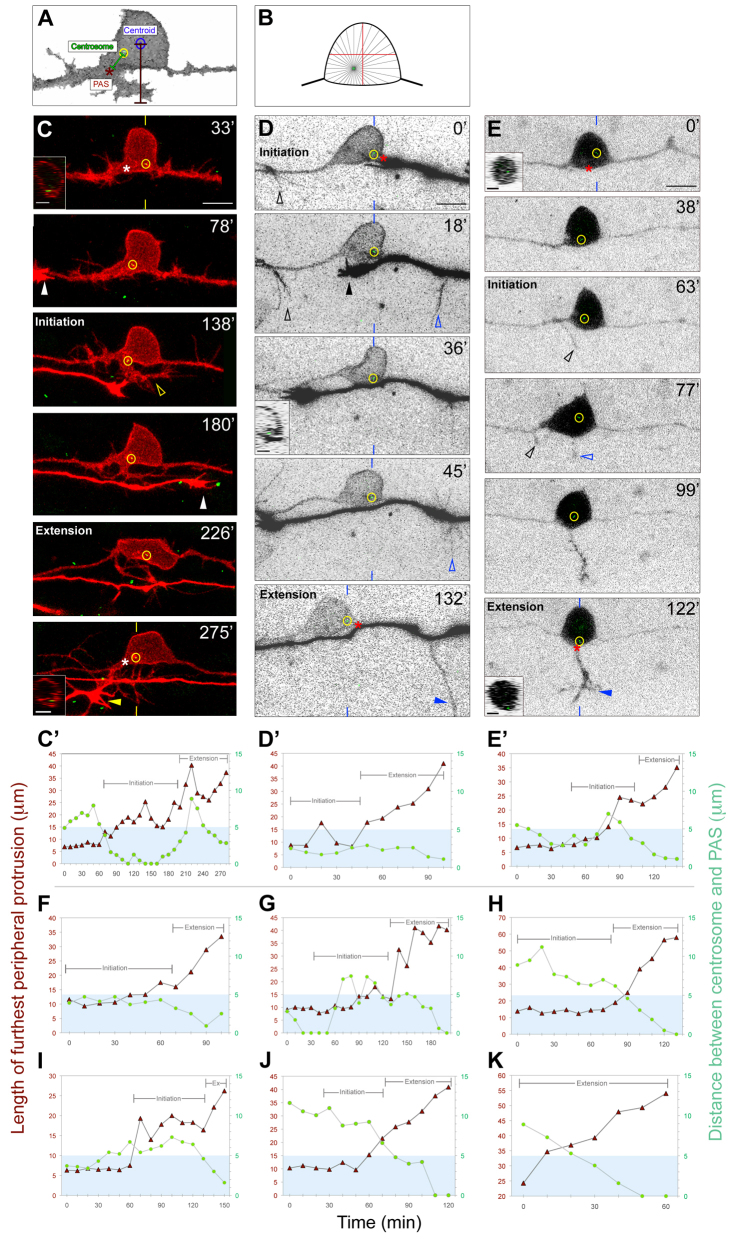
To further characterize centrosome dynamics and to examine whether the centrosome influences the A-P position of peripheral axon initiation, we imaged living embryos at 1- to 2-minute intervals during phases II and III. To compare centrosome positioning with peripheral outgrowth dynamics, we measured the distance between the centrosome and the PAS, along with the distance between the cell body centroid and the tip of the longest filopodium, neurite or axon (length of furthest peripheral protrusion) throughout peripheral axon development (Fig. 3A). If the centrosome produces MTs to an equal degree in all radial directions, it need only be positioned slightly off the cell center to deliver significantly more MTs to one edge of the cell versus another (Fig. 3B). Thus, we considered the centrosome to be biased near the site of peripheral axon formation when it was within 5 μm of the PAS, which is less than half the RB cell diameter (see Materials and methods). We defined two stages of peripheral axon growth: initiation, which occurs prior to spinal cord exit, and extension, which begins as axons exit the spinal cord. Peripheral axon formation is preceded by the formation of numerous filopodia along the central axons and cell body (Andersen et al., 2011). During initiation, this protrusive activity becomes concentrated in a particular location as a growth cone-tipped neurite emerges (Fig. 3C; supplementary material Movie 1). Upon exiting the spinal cord and entering the extension stage, the peripheral axon becomes established and extends more rapidly (Andersen et al., 2011).
Fig. 3.
The centrosome localizes near the RB PAS during axon formation. (A) Schematic showing how centrosome localization and peripheral axon length were measured for the plots in C′-E′ and F-K (see Materials and methods). (B) Diagram showing how off-center displacement of the centrosome could cause asymmetric microtubule (MT) delivery. (C-E) Individual RB neurons labeled by transient expression of either TagRFP-CAAX in GFP-Xcentrin mRNA-expressing zebrafish embryos (C,D) or of GFP-Zcentrin (E) alone. Dorsal-lateral views, anterior left. Images are z-projections of RB neuron (red or black-white inverted) overlaid with single xy planes (green) of the centrosome (yellow circles). Insets are optical cross-sections (medial is left) through the region of the cell containing the centrosome (indicated with yellow or blue hatch marks). (C′-E′,F-K) Plots of length of furthest peripheral protrusion (red triangles) and centrosome distance to PAS (green circles) versus time during peripheral axon initiation and extension (brackets). Blue box indicates region within 5 μm of the PAS. (C,C′) Time-lapse images and corresponding plot showing centrosome localization to the PAS during peripheral axon initiation (open arrowhead) and extension. Asterisks at 33′ and 275′ indicate future and formed PAS. White and yellow arrowheads at 78′ and 275′ indicate growth cones of neighboring central and peripheral axons entering the field of view. (D,D′) Time-lapse images and corresponding plot showing centrosome localization near the PAS during initiation and extension. A peripheral axon initiates off the ascending central axon (black open arrowhead), but retracts after a second peripheral initiates off the descending central axon (blue open arrowhead) and extends (blue arrowhead). Asterisks at 0′ and 132′ indicate position measured for PAS. Black arrowhead at 18′ indicates growth cone of neighboring central axon. (E,E′) Time-lapse images and corresponding plot showing centrosome localization to the PAS during peripheral axon initiation and extension. A peripheral axon initiates off the ascending central axon (black open arrowheads), but retracts after a second peripheral axon initiates off the cell body (blue open arrowhead) and extends into the periphery (blue arrowhead). Asterisks at 0′ and 122′ indicate future and formed PAS, respectively. Time is in minutes. Plots in F-K are from six additional movies. Scale bars: 10 μm; 5 μm in insets.
We generated spatiotemporal profiles of centrosome localization relative to peripheral axon outgrowth for each neuron imaged (n=9; Fig. 3C′-E′,F-K). In eight of our movies, we captured the peripheral axon initiation stage (Fig. 3C-J). In five of these, the centrosome localized to the future PAS, either throughout initiation (Fig. 3C′,D′,F; supplementary material Movie 1) or transiently (Fig. 3E′,G; supplementary material Movie 2). In the other three neurons, the centrosome did not localize to the PAS during initiation (Fig. 3H-J), but did undergo directed migration towards the PAS during, or shortly after, the transition between initiation and extension. In all neurons imaged, the centrosome was consistently localized to the PAS during the extension stage (Fig. 3C-K). These results suggest that the centrosome might be recruited to the PAS during initiation, where it could support or drive the transition of an initiating neurite into a peripheral axon.
This idea is further supported by cases in which we imaged more than one initiating peripheral axon. We previously showed that, in some RB neurons, neurites will initiate outgrowth from multiple locations along the A-P axis; however, only one of these is converted into the successful peripheral axon (Andersen et al., 2011). Here, we observed multiple initiating peripheral neurites in two RB neurons (Fig. 3D,E; supplementary material Movie 2). In both instances, the initiating neurite nearest the centrosome became the successful axon. These results suggest that the centrosome might support the preferential outgrowth of a particular neurite, thereby limiting the RB neuron to a single peripheral axon.
Centrosome disruption causes defects in peripheral axons
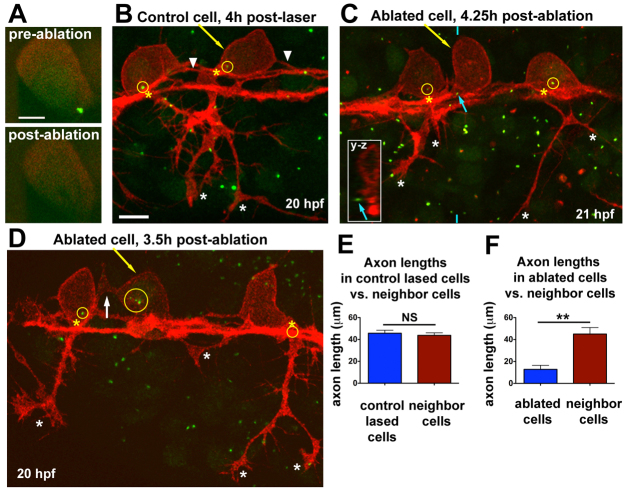
To test the necessity of the centrosome for peripheral axon formation, we disrupted it by ablation with a pulsed laser. This approach has been used to successfully ablate centrosomes in other organisms (Nguyen et al., 2011; von Dassow et al., 2009). Transgenic embryos with all RBs labeled red [Tg(–3.1ngn1:TagRFP-caax)] were injected with GFP-Xcentrin mRNA at the 1-cell stage. At 16-17 hpf (during phase I, before peripheral axon formation), centrosomes in individual neurons were ablated by focusing the laser and pulsing at 5 Hz for 2-4 seconds. This treatment was sufficient to eliminate the GFP-Xcentrin signal without causing damage to the neurons (Fig. 4A). Embryos were raised another 3-5 hours after ablations, then live imaged during stages when peripheral axons are normally extending (19-21 hpf). At the end of the experiment (∼22 hpf), embryos were fixed and immunolabeled with antibodies against γ-tubulin (to visualize the centrosome) and TagRFP (to visualize axons).
Fig. 4.
Centrosome ablation causes RB peripheral axon defects and ectopic protrusions. (A) Single optical section showing centrosome (labeled with GFP-Xcentrin) before and immediately after laser ablation. (B-D) z-projections of RB neurons (red) in Tg(–3.1ngn:tagrfp-caax) zebrafish embryos during phase III, overlaid with partial z-projections of GFP-Xcentrin (green) containing the z-planes spanning the cell body of interest. Yellow arrows indicate lased cells, yellow circles show centrosomes within neurons, and yellow asterisks indicate the PAS. (B) Control cell lased away from the centrosome shows normal cell morphology. White asterisks indicate peripheral axon branches of lased cell. Arrowheads indicate central axons. The central axons of neighboring cells are also in view. (C) RB neuron with ablated centrosome lacks a peripheral axon. Blue arrow indicates centrosome outside lased cell, shown in yz cross-section taken at the position of the blue hatch mark (inset). White asterisks indicate peripheral axon branches of non-lased cells. (D) RB neuron with fragmented centrosome lacks a peripheral axon. White arrow indicates an ectopic protrusion from the cell body. Peripheral axons of non-lased cells extend normally (white asterisks). The centrosome of the neuron on the right is obscured by other central axons at the PAS. (E) Mean (±s.e.m.) axon length: control-lased cells, 45.7±2.7 μm; neighbors, 43.8±2.3 μm; NS, not significant (P=0.64, paired t-test). (F) Mean axon length: ablated cells, 12.8±3.7 μm; neighbors, 45.1±5.9 μm; **P=0.0018, paired t-test. Axons from neighboring cells and control cells often extended out of the imaging field and thus their lengths are an underestimate. Control-lased and centrosome-ablated axon lengths are also significantly different from each other (P<0.0001, unpaired t-test). Scale bars: 5 μm in A; 10 μm in B-D.
Control cells were lased in a non-centrosomal region. These cells displayed normal morphology and peripheral axon extension (Fig. 4B,E; n=12). Ablated cells (n=10) either had no centrosome (e.g. Fig. 4C) or centrosome fragments (e.g. Fig. 4D), as visualized by GFP-Xcentrin and anti-γ-tubulin (data not shown) 3-6 hours after ablation. In eight out of ten ablated neurons, peripheral axon growth was markedly inhibited (Fig. 4C-E). These neurons had either no peripheral axon or short axons that were significantly delayed compared with neighboring cells (Fig. 4E). Three of these cells also displayed ectopic protrusions from the cell body (e.g. arrow in Fig. 4D). The remaining two neurons extended peripheral axons to the skin, but also showed ectopic apical protrusions from the cell body. In all neurons, the central axons had extended out of the field of view by the time of imaging. These results suggest that an intact centrosome is required for proper growth and positioning of the peripheral axon.
The centrosome may mediate peripheral axon formation downstream of LIM-HD activity
Increasing evidence shows that transcription factors have important roles in defining axon trajectories and neuronal polarity (de la Torre-Ubieta and Bonni, 2011; Polleux et al., 2007), yet the mechanisms by which they regulate axon guidance or specific neuronal compartments are not well understood. We previously found that DN-CLIM expression impaired peripheral axon initiation but did not prevent F-actin accumulations or the F-actin-based filopodia that precede peripheral axons (Andersen et al., 2011). We hypothesized that LIM-HD transcription factor activity regulates another aspect of axon formation, such as the invasion of filopodia by MTs, which is required for neuritogenesis in culture (Dehmelt et al., 2003; Dent et al., 2007). We also observed ectopic F-actin accumulation and neurite formation in apical locations of the RB cell body in DN-CLIM-expressing embryos (Andersen et al., 2011), suggesting that LIM-HD activity is required for the proper basal positioning of peripheral axons.
To investigate a potential relationship between LIM-HD activity and the centrosome, we measured the apical-basal position of the centrosome in developing DN-CLIM-expressing embryos (Fig. 5E). Similar to wild type, the centrosome is localized apically during central axon initiation (phase 0). However, the centrosome fails to migrate as far basally during phases I, II and III. We next used live imaging to characterize centrosome dynamics and RB axon behavior in DN-CLIM-expressing embryos. In seven of eight RBs imaged, the centrosome was mislocalized, either apically (Fig. 5A; data not shown) or medially (Fig. 5B) during the imaging period. Moreover, we found that centrosome motility was significantly reduced by DN-CLIM (Fig. 5A′-D). We quantified centrosome migration by measuring centrosome position relative to the cell body centroid at regular time intervals over a 2-hour time window when peripheral axons normally form (Fig. 5D). These defects in centrosome position and motility correlated with peripheral axon defects. In the majority of neurons (five of eight), no peripheral axon initiated outgrowth (data not shown). In the other three neurons, neurites initiated from the cell body but failed to extend out of the spinal cord. Two of these neurons displayed abnormal apical protrusions concomitant with an apically localized centrosome (Fig. 5A; supplementary material Movie 3; data not shown). The other, which initiated a neurite from a typical basal location of the cell body, had a basal but medially localized centrosome (Fig. 5B, inset). Together, these data suggest that the centrosome might play a role in peripheral axon formation downstream of LIM-HD activity.
Fig. 5.
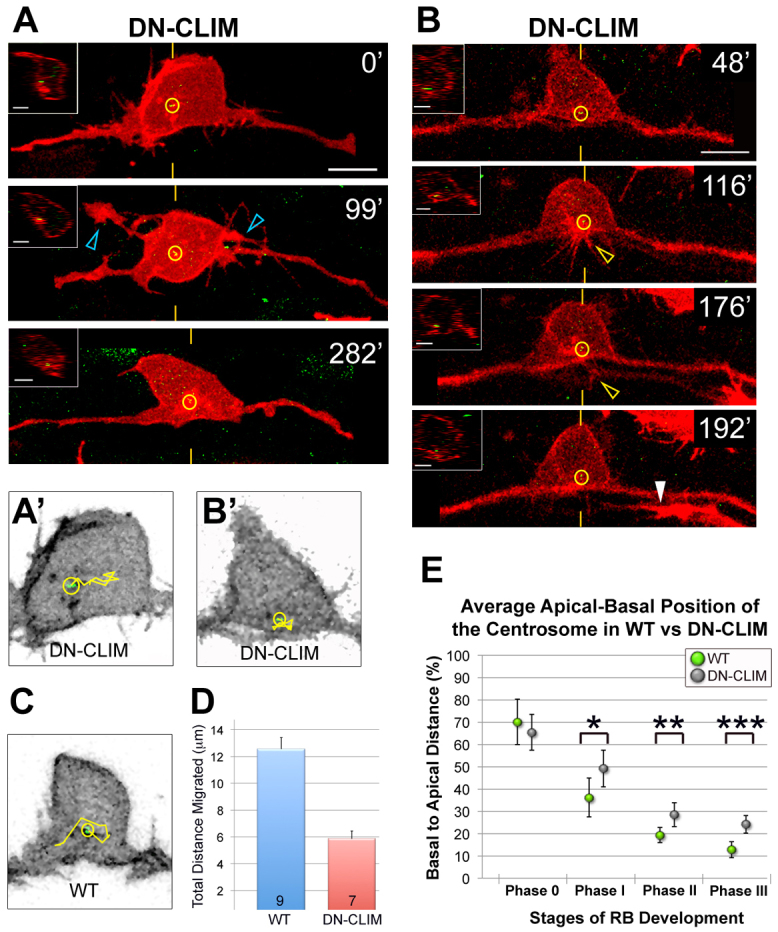
Disruption of LIM-HD transcription factor activity affects centrosome motility and positioning in RB neurons. (A,B) Individual RB neurons labeled by transient expression of TagRFP-CAAX in GFP-Xcentrin mRNA- and DN-CLIM mRNA-expressing zebrafish embryos. Dorsal-lateral views, anterior left. Images are z-projections of RB neuron (red) overlaid with single xy planes of the centrosome (green). Insets are optical cross-sections (medial is left) through the centrosome region (indicated with yellow hatch marks). (A) Time-lapse images show a relatively apical centrosome position (yellow circles) (compare with wild type, Fig. 3) during ectopic apical neurite formation (blue open arrowheads). (B) Time-lapse images show a normal basal but relatively medial centrosome localization (compare with wild type, Fig. 3) in a neuron that initiates then retracts a peripheral neurite (yellow open arrowhead). White arrowhead indicates a neighboring central axon entering the field. Scale bars: 10 μm; 5 μm in insets. (A′,B′,C) Tracks (yellow lines) of centrosome movement in neurons shown in A and B (DN-CLIM) and in Fig. 3C (wild type, WT). (D) Quantification of average total distance of centrosome migration during a 2-hour period. Error bars represent s.e.m. The number of neurons (n) is indicated in each bar. P<10–4, two-tailed t-test. (E) The average centrosome position and its distribution about the mean (± 2 s.e.m.) during developmental stages. For comparison, wild-type data (Fig. 2B) are included. For DN-CLIM, mean percentages are: phase 0, 65.4±8.0% (n=14); phase I, 49.2±8.3% (n=10); phase II, 28.5±5.5% (n=20); phase III, 24.1±3.9% (n=15). A total of 42 neurons were analyzed in live embryos. *P=0.0481, **P=0.0180, ***P=0.0004, two-tailed t-test.
bal mutant embryos have mispositioned centrosomes and misguided axons
Extracellular cues cooperate with intrinsic factors to orchestrate neuronal morphogenesis (Barnes and Polleux, 2009; Polleux and Snider, 2010; Randlett et al., 2011a). Laminin is an extracellular matrix molecule that can influence the position of the centrosome in cultured cerebellar granule cells (Gupta et al., 2010) or in zebrafish branchiomotor neurons or RGCs (Grant and Moens, 2010; Randlett et al., 2011b). Moreover, laminin has well-known effects on axon growth (e.g. Barros et al., 2011; Paulus and Halloran, 2006; Powell and Kleinman, 1997) and can direct the position of axon emergence in zebrafish RGCs (Randlett et al., 2011b). To determine whether Laminin-α1 influences centrosome localization or axon morphology in RB neurons in vivo, we examined bal mutant embryos at 24 hpf. We found that about half of labeled RB neurons (11/24) exhibited abnormal morphology, with ectopic apical and/or supernumerary axons (e.g. Fig. 6A,B). In an extreme example, an apical axon crossed the midline and innervated the contralateral trunk (Fig. 6A). We measured centrosome position and found a significant shift to an apical location in bal neurons with ectopic axons as compared with wild-type siblings (Fig. 6C). Of the bal neurons with abnormal axon morphology, most (10/11) had the centrosome displaced outside the basal third of the cell. These results suggest that laminin influences both centrosome position and RB polarity, and provide another correlation between the apical-basal position of peripheral axon formation and centrosome location.
Fig. 6.
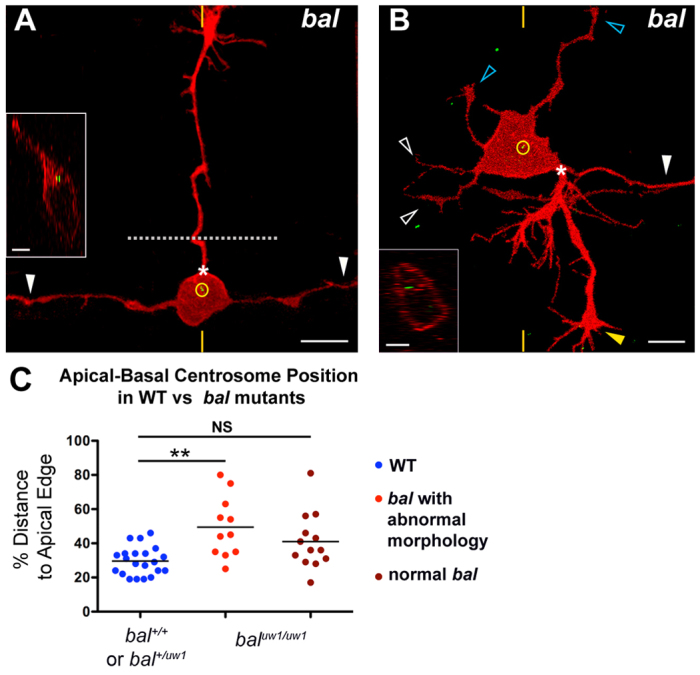
RB neurons have mislocalized centrosomes and aberrant morphology in bal mutant embryos. (A,B) Individual RB neurons labeled by transient expression of TagRFP-CAAX in GFP-Xcentrin mRNA-expressing bal mutant zebrafish embryos. Dorsal-lateral views, anterior left. Images are z-projections and insets are optical cross-sections through the centrosome region (indicated with yellow hatch marks). (A) Mature RB neuron in a bal embryo with normal central axons (white arrowheads) and an ectopic apical axon (PAS indicated by asterisk) that crossed the midline (dotted line) and grew to the contralateral trunk. The centrosome (yellow circle) is localized near the apical cell body edge. (B) Mature RB neuron in a bal embryo with supernumerary axons. Neuron extended a normal descending central axon (white arrowhead) and peripheral axon (yellow arrowhead) from the PAS (asterisk), but has truncated ascending central neurites (white open arrowheads) and ectopic apical neurites (blue open arrowheads). The centrosome (yellow circle) is localized halfway between the apical and basal cell surfaces. (C) Scatter plot of centrosome position. Mean ± s.e.m. for WT: 29.6±1.8 (n=21); abnormal bal, 49.5±5.4 (n=11); normal bal, 41.1±4.5. ANOVA with Tukey’s multiple comparison shows that abnormal bal is significantly different from WT (**P<0.05) and that normal bal is not significantly different (NS) from WT.
DISCUSSION
In this study, we imaged centrosome dynamics as individual neurons develop complex morphology in their natural in vivo environment. We used several approaches to manipulate centrosome position and found that centrosome proximity correlates with specific neurite formation. Our results provide new insight into centrosome function during axon development and into the role of LIM-HD transcription factors in controlling neuronal morphology.
The centrosome has been linked to several aspects of neuronal morphogenesis (Higginbotham and Gleeson, 2007), yet its function in axon development remains controversial. The centrosome is dispensable for extension of hippocampal axons in culture (Stiess et al., 2010) and for axon elaboration in the fly retina (Basto et al., 2006). Moreover, in vivo imaging of zebrafish neurons that initially extend a single axon and dendrite from opposite sides of the cell body show no correlation between the centrosome and axon position (Zolessi et al., 2006; Distel et al., 2010). Central RB axon formation might represent an analogous stage. These axons are the first to emerge from the RB cell body, when the neuron shows simple bipolar morphology, and, as we discovered here, can form without centrosome proximity. These observations suggest that the centrosome does not define axon position during initial bipolar morphology.
Instead, several lines of evidence from our study and others suggest that the centrosome might be important for specifying neurite identity during development of complex morphology. In RB neurons, centrosome position is variable as peripheral neurites initiate, but is consistently localized to the PAS as the peripheral axon exits the spinal cord and begins to grow rapidly. This behavior suggests that the centrosome is recruited to the initiating peripheral neurite, where it might be important for its transition into an axon. Our laser disruption experiments support this idea and suggest that the centrosome is required for this process. Moreover, in cases in which several peripheral neurites initiated, the position of the centrosome appeared to predict which neurite became the established axon. This suggests that the centrosome might also determine the A-P position of the peripheral axon and provides a potential mechanism to ensure that only one peripheral axon forms. Our data are consistent with a model in which neurites can initiate from varying positions, but transition into a successful peripheral axon requires centrosome proximity. A similar mechanism is proposed to function in cortical and hippocampal neurons, which undergo a transitory multipolar stage during development, extending several undifferentiated neurites, one of which later becomes specified as the axon (Barnes and Polleux, 2009). In these neurons, the centrosome transiently localizes to the future axon site at the multipolar stage (Zmuda and Rivas, 1998; de Anda et al., 2005; de Anda et al., 2010) and polarization is accompanied by cytoskeletal remodeling and the segregation of numerous proteins, lipids and organelles (Arimura and Kaibuchi, 2007; Namba et al., 2011; Stiess and Bradke, 2011; Tahirovic and Bradke, 2009). Similar events are likely to be required for RB morphogenesis, which involves the formation of distinct central and peripheral axon compartments. The centrosome, through its inherent MT-organizing activity and complex protein composition (Bornens, 2002; Doxsey, 2001), as well as its associations with other cellular organelles (Badano et al., 2005), could mediate multiple processes to influence axon formation or neurite identity in multipolar cells. The Golgi apparatus, which is crucial for directional protein transport (Sütterlin and Colanzi, 2010) and is a significant source of MTs in polarized migrating cells (Efimov et al., 2007), has been shown to colocalize with the centrosome (de Anda et al., 2010; de Anda et al., 2005; Hong et al., 2010; Pouthas et al., 2008; Zmuda and Rivas, 1998). Perhaps centrosome positioning at the base of a nascent axon is important not only for creating a polarized MT network, but also for regulating selective transport and trafficking of molecules that define axon identity. In this way, the centrosome could impart unique character to the RB peripheral axon and to axons versus dendrites in other neuronal cell types.
Our results also show that centrosome positioning is controlled in part by intrinsic LIM-HD transcription factor activity. Our previous finding that DN-CLIM does not affect F-actin protrusions (Andersen et al., 2011) led us to hypothesize that LIM-HD transcription factor activity might regulate MT invasion into filopodia, which is a key step in axon formation and branching (Dehmelt et al., 2003; Dent and Kalil, 2001; Dent et al., 2007). Here, we found that DN-CLIM causes apical mispositioning of the centrosome and reduces its motility. Interestingly, the centrosome not only regulates MT assembly and organization, but its position and motility are also controlled by MT dynamics (de Anda et al., 2010; Hong et al., 2010). LIM-HD activity could potentially regulate any of these processes. Moreover, because DN-CLIM specifically affects peripheral RB axons, these results provide another correlation between centrosome and peripheral axon defects, and further support a role for the centrosome in specifying neurite identity. In addition, our DN-CLIM results argue against a model in which the centrosome is passively pulled by growing axons, as central axons extend normally in DN-CLIM-expressing embryos and yet their growth is not sufficient to pull the centrosome to the normal basal position.
In addition to intrinsic signals, extracellular cues also influence neuronal polarization in vitro (Esch et al., 1999; Gupta et al., 2010; Mai et al., 2009; Ménager et al., 2004; Shelly et al., 2007) and are required for positioning the site of axon formation in vivo (Adler et al., 2006; Zolessi et al., 2006). Extracellular laminin can direct centrosome position and axon position in vitro and in vivo (Gupta et al., 2010; Randlett et al., 2011b). Here, we discovered that in bal (laminin alpha 1) mutant embryos, RB neurons have apically mislocalized centrosomes and ectopic apical axons. This result suggests that Laminin-α1 might define the basal-lateral position of the peripheral axon, and provides an additional correlation between the centrosome and the peripheral axon. Centrosome mispositioning in bal mutants could either cause, or be the result of, ectopic peripheral axon formation. Our finding that neurite initiation can sometimes precede centrosome translocation in wild-type embryos suggests that the first signal for peripheral axon formation might be extracellular. Whether Laminin-α1 is permissive or instructive to RB axons, centrosome recruitment may provide a link between extracellular growth-promoting signals and the intracellular growth machinery.
In summary, our data show that intrinsic transcription factor activity and external laminin coordinate to influence centrosome positioning and the specific site of peripheral RB axon formation. We propose a model whereby, during peripheral axon formation, multiple neurites may be induced to initiate outgrowth by cues in the extracellular environment, but only those with proximity to the centrosome can transition into an extending axon. We suggest that centrosome positioning, through its inherent ability to polarize the MT cytoskeleton (Doxsey, 2001; Bornens, 2002) and capacity to create cellular asymmetry through its interactions with protein complexes and organelles (Badano et al., 2005), has a central role in differential neurite formation in neurons with complex morphologies.
Supplementary Material
Acknowledgments
We thank Bill Bement for suggesting these experiments and for many helpful discussions; Dustin Tryggestad, John Irwin and Kyle Swiggum for fish care; Amanda Enders and Xuejiao Tian for technical assistance; Kristen Kwan, Chi-Bin Chien, Nathan Lawson, Michael Granato, Ingolf Bach and Uwe Strähle for DNA constructs; Tim Gomez, Namrata Asuri and Matt Clay for assistance with quantifications; and Erik Dent and Tim Gomez for comments on the manuscript.
Footnotes
Funding
The confocal microscope was acquired with a National Institutes of Health (NIH) shared instrumentation grant [S10RR023717]. This work was supported by the NIH [grant R01-NS042228 to M.C.H.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.081513/-/DC1
References
- Adler C. E., Fetter R. D., Bargmann C. I. (2006). UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat. Neurosci. 9, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen E., Asuri N., Clay M., Halloran M. (2010). Live imaging of cell motility and actin cytoskeleton of individual neurons and neural crest cells in zebrafish embryos. J. Vis. Exp. 3 1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen E. F., Asuri N. S., Halloran M. C. (2011). In vivo imaging of cell behaviors and F-actin reveals LIM-HD transcription factor regulation of peripheral versus central sensory axon development. Neural Dev. 6, 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura N., Kaibuchi K. (2007). Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 8, 194–205 [DOI] [PubMed] [Google Scholar]
- Baas P. W., Lin S. (2011). Hooks and comets: The story of microtubule polarity orientation in the neuron. Dev. Neurobiol. 71, 403–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano J. L., Teslovich T. M., Katsanis N. (2005). The centrosome in human genetic disease. Nat. Rev. Genet. 6, 194–205 [DOI] [PubMed] [Google Scholar]
- Barnes A. P., Polleux F. (2009). Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 32, 347–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros C. S., Franco S. J., Müller U. (2011). Extracellular matrix: functions in the nervous system. Cold Spring Harb. Perspect. Biol. 3, a005108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C. G., Khodjakov A., Raff J. W. (2006). Flies without centrioles. Cell 125, 1375–1386 [DOI] [PubMed] [Google Scholar]
- Baye L. M., Patrinostro X., Swaminathan S., Beck J. S., Zhang Y., Stone E. M., Sheffield V. C., Slusarski D. C. (2011). The N-terminal region of centrosomal protein 290 (CEP290) restores vision in a zebrafish model of human blindness. Hum. Mol. Genet. 20, 1467–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T., Ostendorff H. P., Bossenz M., Schlüter A., Becker C. G., Peirano R. I., Bach I. (2002). Multiple functions of LIM domain-binding CLIM/NLI/Ldb cofactors during zebrafish development. Mech. Dev. 117, 75–85 [DOI] [PubMed] [Google Scholar]
- Blader P., Plessy C., Strähle U. (2003). Multiple regulatory elements with spatially and temporally distinct activities control neurogenin1 expression in primary neurons of the zebrafish embryo. Mech. Dev. 120, 211–218 [DOI] [PubMed] [Google Scholar]
- Bornens M. (2002). Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25–34 [DOI] [PubMed] [Google Scholar]
- Clarke J. D., Hayes B. P., Hunt S. P., Roberts A. (1984). Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J. Physiol. 348, 511–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Anda F. C., Pollarolo G., Da Silva J. S., Camoletto P. G., Feiguin F., Dotti C. G. (2005). Centrosome localization determines neuronal polarity. Nature 436, 704–708 [DOI] [PubMed] [Google Scholar]
- de Anda F. C., Meletis K., Ge X., Rei D., Tsai L.-H. (2010). Centrosome motility is essential for initial axon formation in the neocortex. J. Neurosci. 30, 10391–10406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre-Ubieta L., Bonni A. (2011). Transcriptional regulation of neuronal polarity and morphogenesis in the mammalian brain. Neuron 72, 22–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L., Smart F. M., Ozer R. S., Halpain S. (2003). The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J. Neurosci. 23, 9479–9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E. W., Kalil K. (2001). Axon branching requires interactions between dynamic microtubules and actin filaments. J. Neurosci. 21, 9757–9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E. W., Kwiatkowski A. V., Mebane L. M., Philippar U., Barzik M., Rubinson D. A., Gupton S., Van Veen J. E., Furman C., Zhang J., et al. (2007). Filopodia are required for cortical neurite initiation. Nat. Cell Biol. 9, 1347–1359 [DOI] [PubMed] [Google Scholar]
- Distel M., Hocking J. C., Volkmann K., Köster R. W. (2010). The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J. Cell Biol. 191, 875–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S. (2001). Re-evaluating centrosome function. Nat. Rev. Mol. Cell Biol. 2, 688–698 [DOI] [PubMed] [Google Scholar]
- Efimov A., Kharitonov A., Efimova N., Loncarek J., Miller P. M., Andreyeva N., Gleeson P., Galjart N., Maia A. R. R., McLeod I. X., et al. (2007). Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12, 917–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch T., Lemmon V., Banker G. (1999). Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J. Neurosci. 19, 6417–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. K., Moens C. B. (2010). The neuroepithelial basement membrane serves as a boundary and a substrate for neuron migration in the zebrafish hindbrain. Neural Dev. 5, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Meiri K. F., Mahfooz K., Bharti U., Mani S. (2010). Coordination between extrinsic extracellular matrix cues and intrinsic responses to orient the centrosome in polarizing cerebellar granule neurons. J. Neurosci. 30, 2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H. R., Gleeson J. G. (2007). The centrosome in neuronal development. Trends Neurosci. 30, 276–283 [DOI] [PubMed] [Google Scholar]
- Hong E., Jayachandran P., Brewster R. (2010). The polarity protein Pard3 is required for centrosome positioning during neurulation. Dev. Biol. 341, 335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K. (2004). Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 77, 201–222 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- Kuwada J. Y., Bernhardt R. R., Nguyen N. (1990). Development of spinal neurons and tracts in the zebrafish embryo. J. Comp. Neurol. 302, 617–628 [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C.-B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 [DOI] [PubMed] [Google Scholar]
- Lerman O., Ben-Zvi A., Yagil Z., Behar O. (2007). Semaphorin3A accelerates neuronal polarity in vitro and in its absence the orientation of DRG neuronal polarity in vivo is distorted. Mol. Cell. Neurosci. 36, 222–234 [DOI] [PubMed] [Google Scholar]
- Mai J., Fok L., Gao H., Zhang X., Poo M. M. (2009). Axon initiation and growth cone turning on bound protein gradients. J. Neurosci. 29, 7450–7458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménager C., Arimura N., Fukata Y., Kaibuchi K. (2004). PIP3 is involved in neuronal polarization and axon formation. J. Neurochem. 89, 109–118 [DOI] [PubMed] [Google Scholar]
- Namba T., Nakamuta S., Funahashi Y., Kaibuchi K. (2011). The role of selective transport in neuronal polarization. Dev. Neurobiol. 71, 445–457 [DOI] [PubMed] [Google Scholar]
- Nguyen M. M., Stone M. C., Rolls M. M. (2011). Microtubules are organized independently of the centrosome in Drosophila neurons. Neural Dev. 6, 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus J. D., Halloran M. C. (2006). Zebrafish bashful/laminin-alpha 1 mutants exhibit multiple axon guidance defects. Dev. Dyn. 235, 213–224 [DOI] [PubMed] [Google Scholar]
- Polleux F., Snider W. (2010). Initiating and growing an axon. Cold SpringHarb. Perspect. Biol. 2, a001925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F., Giger R. J., Ginty D. D., Kolodkin A. L., Ghosh A. (1998). Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science 282, 1904–1906 [DOI] [PubMed] [Google Scholar]
- Polleux F., Ince-Dunn G., Ghosh A. (2007). Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat. Rev. Neurosci. 8, 331–340 [DOI] [PubMed] [Google Scholar]
- Pouthas F., Girard P., Lecaudey V., Ly T. B. N., Gilmour D., Boulin C., Pepperkok R., Reynaud E. G. (2008). In migrating cells, the Golgi complex and the position of the centrosome depend on geometrical constraints of the substratum. J. Cell Sci. 121, 2406–2414 [DOI] [PubMed] [Google Scholar]
- Powell S. K., Kleinman H. K. (1997). Neuronal laminins and their cellular receptors. Int. J. Biochem. Cell Biol. 29, 401–414 [DOI] [PubMed] [Google Scholar]
- Randlett O., Norden C., Harris W. A. (2011a). The vertebrate retina: a model for neuronal polarization in vivo. Dev. Neurobiol. 71, 567–583 [DOI] [PubMed] [Google Scholar]
- Randlett O., Poggi L., Zolessi F. R., Harris W. A. (2011b). The oriented emergence of axons from retinal ganglion cells is directed by laminin contact in vivo. Neuron 70, 266–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls M. M. (2011). Neuronal polarity in Drosophila: sorting out axons and dendrites. Dev. Neurobiol. 71, 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa H., Miyashita T., Hirate Y., Higashijima S., Chino N., Uyemura K., Kikuchi Y., Okamoto H. (2001). Functional repression of Islet-2 by disruption of complex with Ldb impairs peripheral axonal outgrowth in embryonic zebrafish. Neuron 30, 423–436 [DOI] [PubMed] [Google Scholar]
- Semina E. V., Bosenko D. V., Zinkevich N. C., Soules K. A., Hyde D. R., Vihtelic T. S., Willer G. B., Gregg R. G., Link B. A. (2006). Mutations in laminin alpha 1 result in complex, lens-independent ocular phenotypes in zebrafish. Dev. Biol. 299, 63–77 [DOI] [PubMed] [Google Scholar]
- Sepich D. S., Usmani M., Pawlicki S., Solnica-Krezel L. (2011). Wnt/PCP signaling controls intracellular position of MTOCs during gastrulation convergence and extension movements. Development 138, 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly M., Cancedda L., Heilshorn S., Sumbre G., Poo M. M. (2007). LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 129, 565–577 [DOI] [PubMed] [Google Scholar]
- Stiess M., Bradke F. (2011). Neuronal polarization: the cytoskeleton leads the way. Dev. Neurobiol. 71, 430–444 [DOI] [PubMed] [Google Scholar]
- Stiess M., Maghelli N., Kapitein L. C., Gomis-Rüth S., Wilsch-Bräuninger M., Hoogenraad C. C., Tolic-Nørrelykke I. M., Bradke F. (2010). Axon extension occurs independently of centrosomal microtubule nucleation. Science 327, 704–707 [DOI] [PubMed] [Google Scholar]
- Sütterlin C., Colanzi A. (2010). The Golgi and the centrosome: building a functional partnership. J. Cell Biol. 188, 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahirovic S., Bradke F. (2009). Neuronal polarity. Cold Spring Harb. Perspect. Biol. 1, a001644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan S., Dawe H. R. (2011). Common themes in centriole and centrosome movements. Trends Cell Biol. 21, 57–66 [DOI] [PubMed] [Google Scholar]
- Villefranc J. A., Amigo J., Lawson N. D. (2007). Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dassow G., Verbrugghe K. J., Miller A. L., Sider J. R., Bement W. M. (2009). Action at a distance during cytokinesis. J. Cell Biol. 187, 831–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmuda J. F., Rivas R. J. (1998). The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil. Cytoskeleton 41, 18–38 [DOI] [PubMed] [Google Scholar]
- Zolessi F. R., Poggi L., Wilkinson C. J., Chien C. B., Harris W. A. (2006). Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 1, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.