Background: Autophagy is associated to human caspase-10-induced cell death in yeast.
Results: Caspase-10-induced cell death in yeast is a specific process that depends on an intact MAPK pathway, the Factor-arrest (Far) protein family, and the autophagy machinery.
Conclusion: Far11 coordinates a death-promoting signal and regulates both autophagy and the DNA damage response.
Significance: New insights into the function of Far11 in both autophagy and cell-cycle regulation.
Keywords: Autophagy, Cell Death, DNA Damage Response, MAP Kinases (MAPKs), Phosphatase, Yeast, Caspase-10, Far11
Abstract
The heterologous expression of human caspase-10 in Saccharomyces cerevisiae induces a lethal phenotype, which includes some hallmarks of apoptosis and autophagy, alterations in the intra-S checkpoint, and cell death. To determine the cellular processes and pathways that are responsible of the caspase-10-induced cell death we have designed a loss-of-function screening system to identify genes that are essential for the lethal phenotype. We observed that the ER-Golgi-localized family of proteins Far, MAPK signaling, the autophagy machinery, and several kinases and phosphatases are essential for caspase-10 toxicity. We also found that the expression of caspase-10 elicits a simultaneous activation of the MAP kinases Fus3, Kss1, and Slt2. Furthermore, the protein Far11, which is a target of MAP kinases, is essential for the dephosphorylation of Atg13 and, consequently, for the induction of autophagy. In addition, Far11 participates in the regulation of the DNA damage response through the dephosphorylation of Rad53. Finally, we have also demonstrated that Far11 is able to physically interact with the phosphatases Pph21, Pph22, and Pph3. Overall, our results indicate that the expression of human caspase-10 in S. cerevisiae activates an intracellular death signal that depends on the Far protein complex and that Far11 may function as a regulator subunit of phosphatases in different processes, thus representing a mechanistic link between them.
Introduction
Apoptosis (self-killing) and autophagy (self-eating) constitute two essential biological processes aimed at preserving the integrity of multicellular organisms, and defects in these processes have been linked to several pathologies such as cancer and age-related diseases (1, 2). Apoptosis is considered to be a principal form of programmed cell death (type I cell death), although numerous apoptotic effectors also play major roles in development, proliferation, and responses to stress (3). Several orthologues of mammalian genes participating in apoptosis such as the metacaspase (MCA1), AIF1, Omi (NMA111), a BH3-only protein (YBH3), and EndoG (NUC1) have also been identified in S. cerevisiae (4–8), thus indicating that apoptosis is an evolutionary conserved process from lower unicellular eukaryotes to mammals. Autophagy is a catabolic mechanism of cellular turnover responsible for recycling long-lived proteins, damaged organelles, and superfluous fractions of the cytosol during nutrient deprivation and stress. However, under certain conditions macroautophagy (hereafter referred to as autophagy) can also trigger cell death and is therefore considered as another form of programmed cell death (type II cell death) that plays an important role in mammalian development and homeostasis (1, 9). Our understanding of autophagy genes and pathways has mostly been based in genetic screenings in S. cerevisiae, where, to date, at least 35 autophagy-related genes (ATG)5 have been found (10, 11). The process of autophagy begins with the formation of a double-membrane vesicle (autophagosome), which engulfs autophagic cargos at the phagophore assembly site (PAS), and this process is followed by the expansion, closure, and fusion of the autophagosome with the lysosome/vacuole (10, 12, 13). The regulation of autophagy is complex and largely unknown. It is induced by different stimuli through several signaling pathways, such as nutrient deprivation through the TOR and RAS-PKA pathways, and ER stress through the unfolded protein response pathway (10–12). In yeast, the interaction and activation of the Atg1-Atg13-Atg17 kinase complex is essential for the induction of autophagy. Under normal conditions autophagy is inhibited by TOR and PKA kinases, which hyperphosphorylate Atg13, hindering the interaction with Atg1. Nutrient starvation or rapamycin treatment lead to the inactivation of both TOR and PKA, causing the dephosphorylation of Atg13 and the association between Atg1 and Atg13, which constitutes the initial step in autophagy (10, 14–17). In mammals there are two orthologs of ATG1 (ULK1 and ULK2), and the ULK complex is also composed of the mammalian homolog of Atg13 (mAtg13), together with two other interacting proteins (FIP200 and Atg101) (12, 18).
The cross-talk between apoptosis and autophagy is also relatively unknown, but recent studies have revealed that both cellular processes share common pathways and effectors, and that certain stimuli may trigger mixed phenotypes of apoptosis and autophagy at the cellular level (11, 19). For example, human ATG5, an inducer of autophagy, is able to activate the intrinsic apoptotic pathway after removal of its C terminus by calpains (20). In addition, it has been reported that the autophagic degradation of caspase-8, a pro-apoptotic protein, inhibits the pro-cell death response in mammalian cells (21).
The use of both loss-of-function and overexpression screenings in yeast allows novel functions and regulation mechanisms of human genes, with or without fungal counterparts (22), to be unraveled. An example of such an approach has been shown in studies addressing the mammalian Bcl-2 family of proteins in humanized yeast models, which have identified important new human genes, such as the Bax inhibitor-1, which suppresses the apoptosis induced by Bax, and the bifunctional apoptosis regulator (BAR), which constitutes a link between Bcl-2 proteins and caspases (23–25).
Human initiator caspase-8 and caspase-10 are also toxic in yeast, and their expression induces a lethal phenotype with most of the principal hallmarks of apoptosis and autophagy, which include the production of ROS, chromatin condensation, phosphatidylserine externalization, and increased vacuolization (26). In addition, the expression of caspase-10 in yeast completely abrogates the intra-S checkpoint in response to genotoxic agents (26). Thus, the identification of gene-specific deletions that suppress caspase-10 toxicity might be useful for unveiling novel levels of regulation that intersect the cell cycle, apoptosis, and autophagy.
In the present study we carried out a genetic screening for suppressors of caspase-10 toxicity using the yeast haploid knock-out collection. Our results indicate that the pheromone MAPK signaling and autophagy pathways are essential for caspase-10-induced lethality. In particular, we observed that Far11, and probably other members of the Far family that have previously been implicated in the maintenance of the cell-cycle arrest in response to the pheromone (27), also participate in the activation of both the intra-S checkpoint of the cell cycle and the autophagic process through the regulation of the phosphorylation state of their downstream effectors Rad53 and Atg13.
EXPERIMENTAL PROCEDURES
S. cerevisiae Strains, Culture Media, and Plasmids
The nonessential haploid MATa yeast knockout collection (YKO) derived from the parent strain BY4741 (MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0) was obtained from Euroscarf (Frankfurt, Germany). Other S. cerevisiae strains used in this study are listed in supplemental Table S1. Cells were routinely grown at 28 °C in synthetic complete medium lacking either uracil (SC-Ura) or uracil/leucine (SC-Ura-Leu) and containing either 2% glucose (noninducing conditions, SD media) or 2% galactose plus 1% raffinose (inducing conditions, SG medium) as carbon sources. Growth on liquid cultures was monitored spectrophotometrically at an A600 nm. The pESC-URA/CASP10 plasmid was used to express the human CASP10 under control of the GAL1 promoter (26). Other plasmids used in this study are listed in supplemental Table S2.
Loss-of-function Screening
To perform this screening, the pESC-URA/CASP10 plasmid was transformed en masse into all strains of the BY4741 deletion set. The YKO collection, which comprises ∼4500 mutant strains, was split into 10 pools of about 450 strains each to facilitate the transformation of all the strains (22). To avoid competition among strains with different growth rates, we grouped the fast-growing strains in nine pools and the slow-growing strains in the 10th pool. All the pools were distributed in aliquots with rich YPD media containing 5% DMSO and stored at −80 °C. These aliquots were used to inoculate 50 ml of YPD media plus G418 (150 mg liter−1; Invitrogen) and the yeast cells were transformed following the lithium acetate (LiAc) TRAFO method (28). The transformants were selected in noninducing conditions (SC-Ura), at transformation efficiencies between 103 and 104, depending on the pool. Additionally, the transformations were also plated onto SG-Ura plates (inducing conditions) to recover suppressors of caspase-10 toxicity directly. The colonies of the SC-Ura plates were replicated onto SG-Ura plates to induce the expression of caspase-10 and the transformants that were able to grow under the inducing conditions were restreaked on SG-Ura plates for their confirmation as suppressors. All pools were transformed in three independent experiments. The identification of the specific gene that was deleted in each suppressor strain was carried out by PCR amplification and the sequencing of molecular tags (Uptag and Downtag), as previously described (29). To further verify the results, all the mutant strains identified as suppressors of caspase-10 toxicity were transformed individually with the pESC-URA/CASP10 plasmid and the ability to grow under inducing conditions was confirmed for four different clones.
Clonogenic Assays, Cell Viability Analyses, and ROS Production Assays
For the clonogenic assay, S. cerevisiae cells transformed with either the pESC-URA empty vector or the pESC-URA/CASP10 vector were grown in noninducing conditions (glucose) to an A600 nm of 0.1. The cultures were harvested, washed, and resuspended in SC-Ura media with either glucose (noninducing conditions) or galactose/raffinose (inducing conditions) as carbon sources. After induction of caspase-10 expression at different time points the cells were plated onto SC-Ura media with glucose as a carbon source. The number of surviving colonies was counted and the results are shown as a percentage of the number of colonies in induced cultures with respect to the noninduced cultures. The cell viability of S. cerevisiae expressing human caspase-10 was assessed using FUN-1 staining (Molecular Probes), which allows the determination of metabolically active cells (living cells), marked with red fluorescent intravacuolar structures; in contrast, dead cells exhibit a diffuse green cytoplasmic fluorescence. The FUN-1 staining was carried out as described (26). Intracellular free radicals were detected with 2′,7′-dichlorodihydrofluorescein diacetate (Sigma).
Cloning and Expression of the S. cerevisiae FAR11 and Human FAM40A and FAM40B Genes
The ORF and the promoter region (323 bp upstream of ATG) corresponding to the yeast FAR11 gene were amplified from BY4741 genomic DNA using the primers listed in supplemental Table S3 and were cloned into the NcoI-BamHI sites of the yeast centromeric expression vector YCpLac111. The ORFs encoding human Fam40A and Fam40B were amplified from a Jurkat cell cDNA library by PCR using the primers listed in supplemental Table S3. The ORFs corresponding to human Fam40A and Fam40B were cloned into the SphI restriction site of the YCpLac111 vector under control of the FAR11 promoter.
Preparation of Protein Extracts and Western Blotting
A TCA precipitation protocol was used to prepare the protein extracts for Western blotting. Briefly, cells were treated with 20% TCA and cell lysis was performed with glass beads in a FastPrep instrument. Cell lysates were washed twice with 5% TCA and centrifuged at 1000 × g for 5 min. The precipitated proteins were solubilized in 100 μl of Laemmli buffer (1.5 times) and 50 μl of 1.5 m Tris-HCl. The samples were then denatured at 65 °C, centrifuged at 1000 × g, and the supernatants were used for SDS-PAGE. After electrophoresis, the protein samples were transferred to PVDF membranes (Immobilon-P, Millipore) and probed with the appropriate primary and secondary antibodies. Immunodetection was carried out using the ECL Western blotting detection kit (Amershan Biosciences). The primary antibodies were anti-p44/42 (E10, Cell Signaling), anti-HA (12AC5, Roche Applied Science), anti-GFP (JL-8, Clontech), and anti-Rad53 (yC-19, Santa Cruz Biotechnology).
Transcription Analyses by Quantitative Real-time PCR
Quantitative real-time PCR was performed with a LightCyler 480 real-time PCR instrument (Roche Applied Science), using SYBR Green I master mix (Roche Applied Science) and following the manufacturer's instructions. Total RNA samples were obtained using a hot-phenol protocol, as previously described (30), and the cDNA samples were prepared using the Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science). The primer sequences are indicated in supplemental Table S3. All real-time PCR were performed in duplicate in at least two independent experiments. Quantitative analyses were carried out using LightCycler 480 software.
Analyses of DNA Contents
Yeast cells were grown in synthetic complete medium with either glucose or galactose/raffinose to an A600 nm of 0.1. For hydroxyurea (HU) treatment, the cultures were incubated with 0.2 m HU for 2 h. Aliquots were taken at the indicated time points, harvested, and fixed in 70% ethanol overnight at 4 °C. The samples were resuspended in 400 μl of 50 mm sodium citrate plus 0.2 mg/ml of RNase, and incubated overnight at 37 °C. Then, 200 μl of 50 mm sodium citrate, 55 mm HCl, and 5 mg/ml of pepsin were added to each sample and the mixture was incubated for 15 min at 37 °C. Finally, 400 μl of 50 mm sodium citrate containing 10 μg/ml of propidium iodide was added and the samples were sonicated briefly to avoid agglutination. Samples were analyzed by flow cytometry using a FACS SORT (BD Biosciences) and Cell Quest software.
Split Ubiquitin System (SUS)
This method is based on the ability of N-terminal (Nub) and C-terminal (Cub) ubiquitin polypeptides to reconstitute a functional ubiquitin. NubG is a mutant with very low affinity for Cub; functional ubiquitin can only be reconstituted when NubG and Cub are in close vicinity due to fusion with proteins that interact (31). The Cub is fused to the synthetic transcription factor PLV, which activates expression of the reporter genes (LACZ, HIS3, ADE2) in the THY.AP4 yeast strain (see supplemental Table S1). The NubG fragment was fused to the proteins of interest (Far3, Tpd3, Pph21, Pph22, and Pph3) in the pXNgate vector, and the Far11 protein was fused to the Cub-PLV in the pMetYCgate. The construct with the Far11-Cub-PLV fusion was introduced into the THY.AP4 strain and the NubG constructs were transformed into the THY.AP5 strain (see supplemental Table S1). The THY.AP4/Far11-Cub-PLV and THY.AP5/NubG derivatives were crossed, and the ability of the proteins to interact in the resulting diploid strains was tested in the corresponding media. When an interaction occurs, the PLV transcription factor can be released from Far11-Cub by ubiquitin-specific proteases and expression of the reporter genes is activated. The internal positive control was a diploid strain with a wild-type Nub and the internal negative control was a diploid strain with an empty pXNgate vector, as previously described (31).
Bimolecular Fluorescence Complementation (BiFC)
This method was used for the visualization of protein-protein interactions in vivo, as previously described (32). Briefly, the two nonfluorescent halves of the yellow fluorescent protein (YFP) were fused separately to the proteins of interest. In this case, Far11 was fused to the C-terminal portion of the YFP (VC-YFP) and the other proteins (Far3, Tpd3, Pph21, Pph22, and Pph3) were fused to the N-terminal portion of the YFP (VN-YFP). The interaction of two fusion proteins leads to the reconstitution of the YFP, and hence fluorescence can be observed. A Nikon Eclipse 90i instrument was used for fluorescence microscopy.
RESULTS
Genetic Screening for Suppressors of Caspase-10 Toxicity in S. cerevisiae
We have previously described that human caspase-10 induces death phenotypes in S. cerevisiae, including some hallmarks of apoptosis, autophagy, and alterations in the intra-S checkpoint of the cell cycle (26). To define the cellular pathways that are responsible of such phenotypes, we performed a genetic screening to identify knockout mutations that abolish the toxicity of caspase-10 expression in yeast. A galactose-inducible pESC-URA/CASP10 expression plasmid was used to transform the YKO collection (see “Experimental Procedures” for details).
Few transformants grew under the condition of caspase-10 expression, and the number of transformants varied considerably among the different pools. The mutants that were able to grow on SG-Ura plates contained deletions of the specific genes that were essential for the toxicity of caspase-10 in S. cerevisiae. These knockout mutants were considered to be suppressors of the caspase-10 toxicity, and the specific gene deletion of each mutant could be identified by sequencing the molecular barcodes of each strain (29). The 10 pools covering the complete set of YKO strains in the BY4741 background were transformed in three independent experiments and the ability of the suppressors to grow after CASP10 expression was confirmed in both solid and liquid media containing galactose/raffinose.
In the screenings we identified 46 suppressors, including a mutant in the GAL4 gene that lacks the galactose-dependent expression of caspase-10 (Table 1 and supplemental Table S4). Interestingly, we found six genes of the same family (FAR3, FAR7, FAR8, FAR9, FAR10, and FAR11); five genes encoding phosphatases (PPH21, PPH22, PPH3, PTC2, and PTC3); three genes encoding catalytic subunits of PKA kinase (TPK1, TPK2, and TPK3), and several genes involved in the following biological processes: autophagy (ATG1, ATG4, ATG6, ATG8, and ATG13) and MAPK signaling (GPA1, STE7, STE11, STE20, FUS3, KSS1, and SLT2). These results demonstrate that caspase-10-induced toxicity is a genetically specified death process and also indicate the cellular pathways involved in the caspase-10-derived phenotypes.
TABLE 1.
List of the genes identified that are essential for caspase-10-induced cell death in S. cerevisiae
| MAPK pathway | Phosphatases | Kinases | Far family | Autophagy | Stress response | Other | |
|---|---|---|---|---|---|---|---|
| GPA1 | PPH21 | TPK1 | FAR3 | ATG1 | MSN2 | GAL4 | RPL21B |
| STE7 | PPH22 | TPK2 | FAR7 | ATG4 | GLN3 | SSN2 | MDM30 |
| STE11 | PPH3 | TPK3 | FAR8 | ATG6 | ASG1 | PMT5 | BFR1 |
| STE20 | PTC2 | FAR9 | ATG8 | NTH1 | MPH1 | ORT1 | |
| FUS3 | PTC3 | FAR10 | ATG13 | MBR1 | SSM4 | CWP1 | |
| KSS1 | FAR11 | ITT1 | AIM9 | ||||
| SLT2 | LDB18 | CUP9 | |||||
| WHI3 | |||||||
Caspase-10 Expression in S. cerevisiae Promotes the Activation of MAPK Pathways
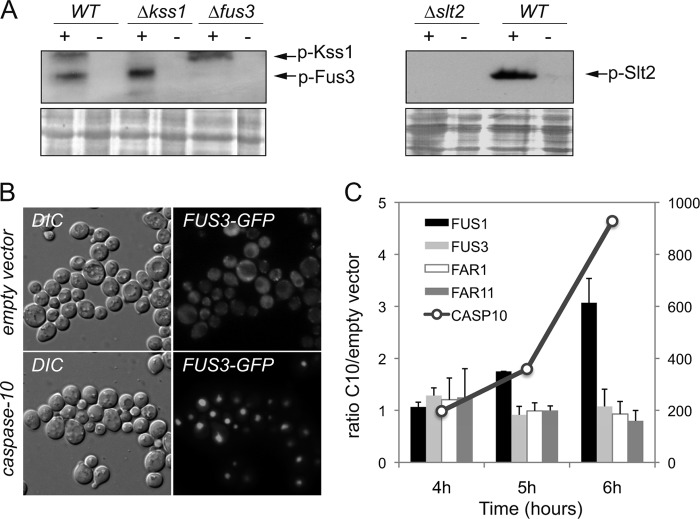
Our screenings revealed that several genes of the MAPK pathways (GPA1, STE7, STE11, STE20, FUS3, KSS1, and SLT2) are essential for caspase-10 toxicity in S. cerevisiae, suggesting that the activation of these signaling pathways may be important for the cell death phenotypes induced by the expression of caspase-10. Accordingly, we checked whether the expression of human caspase-10 might trigger the activation of the MAPK module that eventually determines the phosphorylation of Fus3, Kss1, and Slt2 MAPKs. Western blot analysis revealed that the kinases Fus3, Kss1, and Slt2 were efficiently phosphorylated after the expression of human caspase-10; the immunospecificity of the phosphoproteins was confirmed by the absence of signal in the lanes corresponding to the knockout strains (Fig. 1A). Additionally, we investigated the effect of human caspase-10 on both the subcellular localization of Fus3 and the transcriptional activation of the Fus3-dependent gene FUS1. A Fus3-GFP fusion protein was imported into the nucleus upon caspase-10 expression and transcription of the Fus3 target gene FUS1 was significantly increased at 6 h after the induction of caspase-10 expression in galactose-containing media. However, the expression of other related effectors of the pheromone response pathway, such as FAR1 and FAR11, remained unaltered (Fig. 1, B and C). Overall, our results indicate that the expression of caspase-10 in S. cerevisiae causes the simultaneous activation of MAP kinases Fus3, Kss1, and Slt2.
FIGURE 1.
The MAP kinases Fus3, Kss1, and Slt2 are activated after the expression of caspase-10. A, Western blot of protein extracts from yeast strains BY4741 (WT), kss1Δ, fus3Δ, and slt2Δ transformed with either the pESC-URA/CASP10 (+) or the pESC-URA empty vector (−). Cells were grown in SG-Ura medium for 6 h before the cultures were harvested. The anti-p44/42 (E10, Cell Signaling), which detects the phosphorylated forms of Fus3, Kss1, and Slt2, was used as primary antibody. Ponceau red staining of the blot was used as a protein loading control. B, fus3Δ cells co-transformed with a YCplac22/FUS3-GFP plasmid and either the pESC-URA empty vector or the pESC-URA/CASP10 vector were grown in galactose-containing medium for 6 h. The subcellular localization of the Fus3-GFP fusion was analyzed by fluorescence microscopy. C, relative transcription levels of FUS1, FUS3, FAR1, FAR11, and CASP10 in BY4741 cells transformed with either the pESC-URA empty vector or the pESC-URA/CASP10 plasmid. Total mRNA was obtained from yeast cells at different time points (4, 5, and 6 h) after the induction of caspase-10 expression in galactose-containing medium. Transcription levels were normalized using the ACT1 gene as a reference. Relative quantitative analyses were performed using LightCycler 480 software. The results are the average of two independent experiments and are expressed as a ratio of the cDNA abundance of the target genes in BY4741/pESC-URA/CASP10 cells with respect to BY4741/pESC-URA cells. CASP10 transcription levels are indicated on the scale at the right of the graph.
Far11 Is a Golgi-associated Protein That Is Essential for Caspase-10-induced Toxicity
The Far (Factor arrest) family comprises 7 proteins that are essential for the cell cycle arrest induced by the pheromone in S. cerevisiae. In addition, it has been reported that both Far1 and Far11 are phosphorylated by the MAPK Fus3 (33). Far1 participates in the induction of the G1 arrest through the inactivation of Cdc28 after pheromone treatment (34). In contrast, a complex formed by Far3, Far7, Far8, Far9, Far10, and Far11 has been implicated in the maintenance of G1 arrest after pheromone treatment, thus preventing premature recovery of the cell cycle arrest. However, the precise mechanism underlying this function remains unknown (27).
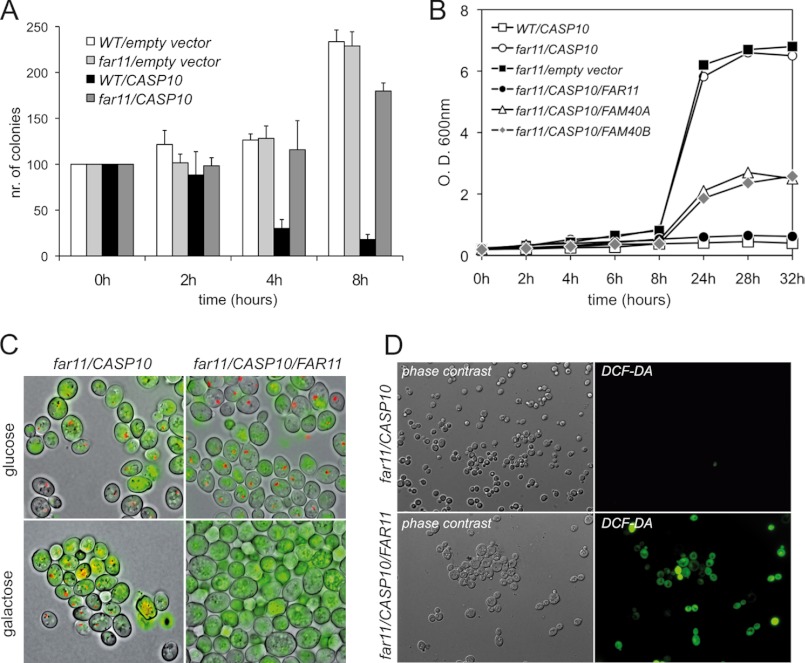
In our screenings, we found that all the FAR genes, except FAR1, were essential for the lethality induced after the expression of caspase-10 in S. cerevisiae and we therefore decided to study the function of the FAR family in cell death processes. In particular, we focused our attention on the FAR11 gene, whose deletion was identified in the highest number of suppressors (see supplemental Table S4) and, interestingly, has two human orthologs of unknown function (35). A clonogenic plating assay showed that the knockout mutant far11Δ was able to rescue the toxic effect of the expression of caspase-10 on colony formation (Fig. 2A). Additionally, an episomic copy of the FAR11 gene was able to restore the lethal phenotype of caspase-10 expression in a far11Δ strain (Fig. 2, B and C). Also, the expression both of the human FAR11 orthologs FAM40A and FAM40B partially complemented the far11Δ mutation (Fig. 2B). Furthermore, the production of ROS induced by caspase-10 expression was completely abolished in the far11Δ mutant and, in agreement with the previous results, the presence of an episomic copy of FAR11 restored the wild-type phenotype of oxidative stress (Fig. 2D). These results indicate that FAR11 is essential for the cell-death phenotypes induced by the expression of human caspase-10 in S. cerevisiae.
FIGURE 2.
Far11 is essential for caspase-10 toxicity in yeast. A, clonogenic assay of the yeast strains expressing human CASP10. Data are presented as percentages of surviving colonies as referred to noninducing conditions at time 0 h. Negative controls of the yeast strains transformed with the pESC-URA empty vector are included. B, growth curves of the caspase-expressing strains performed in SG-Ura-Leu containing 2% galactose plus 1% raffinose as carbon sources. C, FUN1 staining of far11Δ cells expressing either caspase-10 alone or the CASP10 and FAR11 genes. The cells were grown in either noninducing conditions (glucose) or inducing conditions (galactose). Metabolically active cells show red fluorescent intravacuolar structures. D, CASP10-expressing far11Δ cells (lower panels, coexpressing an episomic copy of FAR11) were stained with DCF-DA to assess ROS production and yeast cells were visualized under fluorescence microscopy.
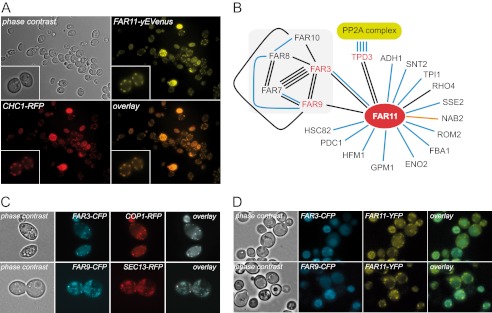
We next examined the subcellular localization of a Far11-yEVenus fusion protein by fluorescence microscopy to gain further insight into the cellular function of Far11. Our results showed that a Far11-yEVenus fusion protein was mainly located at the cytosolic foci at the cellular periphery. This Far11-yEVenus fluorescence was clearly co-localized with a Chc1-RFP fusion, which is a marker of late Golgi vesicles (Fig. 3A).
FIGURE 3.
Subcellular localization of Far proteins. A, subcellular localization of the Far11-yEVenus and Chc1-RFP fusion proteins. A magnification of two cells is shown in the inset. B, schematic representation of the physical interaction network of Far11 according to the information deposited at SGD. The number of lines indicates the number of experiments reported and the color of the lines indicates the type of experiment described: black lines represent two-hybrid analyses; blue lines indicate affinity-capture-MS experiments; and the red line corresponds to an affinity-capture RNA experiment. C, subcellular localization of: Far3-CFP and Cop1-RFP (upper panels); Far9-CFP and Sec13-RFP (lower panels). D, colocalization of Far11-yEVenus with either Far3-CFP (upper panels) or Far9-CFP (lower panels).
As mentioned above, it has been described that Far3, -7, -8, -9, -10, and -11 can form a protein complex and the specific interactions between the different members of the Far family have also been determined (27). Far11 is able to interact physically with Far3 and Far9, among others (Fig. 3B). Therefore, we also wished to analyze the subcellular localization of both Far3-CFP and Far9-CFP fusion proteins. As shown in Fig. 3C, the Far3-CFP protein was mainly colocalized with a Cop1-RFP fusion and the Far9-CFP largely colocalized with a Sec13-RFP, indicating that at least a fraction of the proteins, Far3-CFP and Far9-CFP, are localized in early Golgi vesicles and in the ER-Golgi, respectively. In addition, both Far3-CFP and Far9-CFP partially colocalized with the Far11-yEVenus fusion protein in the cytosolic compartments corresponding to the Golgi vesicles (Fig. 3D).
Autophagy Induced by Caspase-10 Expression Depends on the Yeast Protein Far11
We have previously described that the expression of caspase-10 triggers several autophagy-related phenotypes such as high vacuolization, ER alterations, and organelle disorganization (26). In addition, in our screenings we found that some members of the autophagic machinery were essential for capase-10 lethality (ATG1, ATG4, ATG6, ATG8, and ATG13).
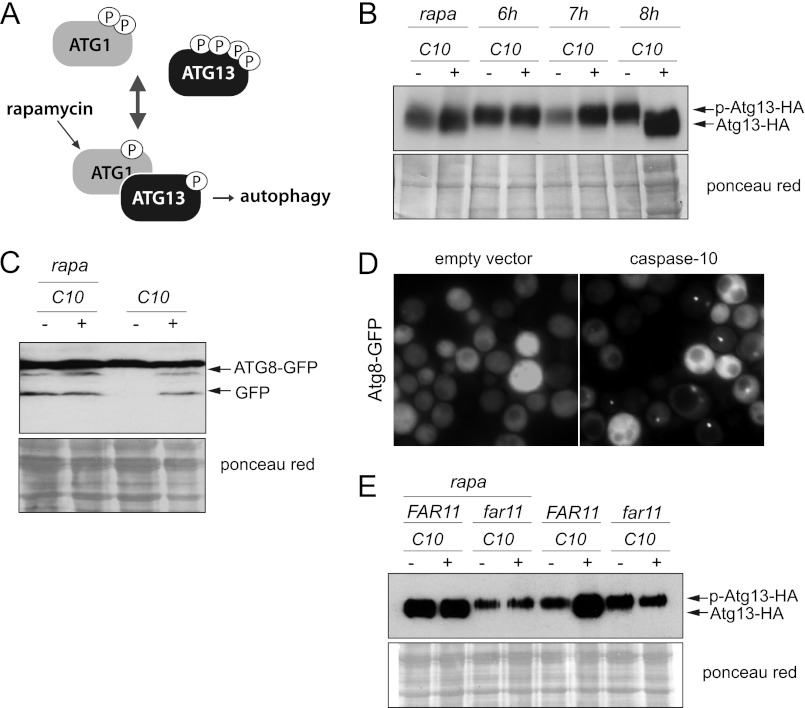
Induction of the autophagic processes is triggered by the dephosphorylation of Atg13, thus allowing its interaction with Atg1 and the formation of the Atg1-Atg13-Atg17 complex (Fig. 4A). Consequently, we analyzed the phosphorylation state of an Atg13-HA protein in caspase-10-expressing yeast cells at different times after induction of the expression of caspase-10 in galactose-containing medium. We found that Atg13 was clearly dephosphorylated after 8 h of growth under inducing conditions, as also occurred after the addition of rapamycin to the cultures (Fig. 4B). We also confirmed that the induction of autophagy by rapamycin treatment did not cause a decrease in the clonogenicity of yeast cells (supplemental Fig. S1). The progression of autophagy after the expression of caspase-10 was also reflected in both the proteolytic degradation of a GFP-Atg8 fusion and the accumulation of the GFP-Atg8 fluorescence mainly in cytosolic foci, which also occurred 8 h after the shift of the cultures to the galactose-containing media (Fig. 4, C and D).
FIGURE 4.
The autophagy induced by the expression of caspase-10 depends on Far11. A, simplified current mechanism of regulation for the induction of autophagy. B, Western blot of protein extracts from yeast cells expressing a HA-tagged version of Atg13 and transformed with either the pESC-URA/CASP10 (+) or the pESC-URA empty vector (−). Cells were grown in SG-Ura media and harvested at the indicated time points. The induction of autophagy by treatment with rapamycin was used as a positive control (rapa). The blot was probed with an anti-HA antibody. Ponceau red staining of the blot was used as a protein loading control. C, Western blot of protein extracts from atg8Δ cells expressing an GFP-Atg8 fusion and transformed with either the pESC-URA/CASP10 (+) or the pESC-URA empty vector (−). Cells were grown in SG-Ura medium for 8 h. Rapamycin (rapa) was used to induce autophagy. An anti-GFP (JL-8) was used as primary antibody. Ponceau red staining of the blot was used as a protein loading control. D, GFP-Atg8 foci are detected after the expression of caspase-10 for 8 h. E, far11Δ cells expressing a HA-tagged version of Atg13 were co-transformed with either the pESC-URA/CASP10 and YCplac111/FAR11 or the corresponding empty vectors, as indicated. Phosphorylation of the Atg13-HA protein was analyzed by Western blotting of cell lysates from cultures grown under inducing conditions. The induction of autophagy with rapamycin was used as a positive control (rapa). Ponceau red staining of the blot was used as a protein loading control.
We next wished to investigate whether Far11, which was shown to be an endomembrane-associated protein, might be involved in the induction of autophagy triggered by the expression of caspase-10. We therefore analyzed phosphorylation of the Atg13-HA protein in a far11Δ strain after the induction of autophagy. We observed that Atg13 invariably remained hyperphosporylated in the far11Δ mutant both after the expression of caspase-10 and after the induction of autophagy either by the addition of rapamycin to the cultures (Fig. 4E) or nitrogen starvation (supplemental Fig. S2), thereby indicating that Far11 must be essential for the dephosphorylation of Atg13 and hence for the induction of autophagy.
Far11 Participates in the Activation of the DNA Damage Intra-S Checkpoint
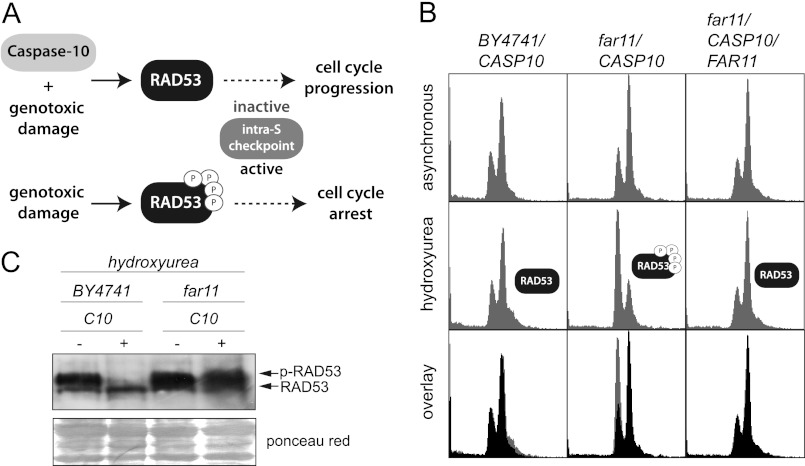
DNA damaging agents such as HU, which did not decrease clonogenicity of yeast cells (supplemental Fig. S3), induce the activation of the intra-S checkpoint. The expression of human caspase-10 in S. cerevisiae leads to the inactivation of the intra-S checkpoint (26). Accordingly, cells expressing caspase-10 are not able to respond to DNA damaging agents by arresting their cell cycle (Fig. 5A).
FIGURE 5.
The inactivation of the intra-S checkpoint after caspase-10 expression is dependent on Far11. A, scheme of the regulation of the intra-S checkpoint. B, yeast strains BY4741/pESC-URA/CASP10, far11Δ/pESC-URA/CASP10, and far11Δ/pESC-URA/CASP10/YCplac111/FAR11 were grown in galactose-containing medium, and the DNA content was analyzed by FACS in both asynchronous and HU-treated cultures. The predicted phosphorylation state of Rad53 is indicated. C, Western blot of protein extracts from the yeast strains BY4741 and far11Δ transformed with either pESC-URA/CASP10 or the empty vector. Cells were grown in galactose-containing medium and treated with HU. The blot was probed with an anti-Rad53 antibody (yC-19). Ponceau red staining of the blot was used as a protein loading control.
To check whether Far11 is also involved in this phenotype, we analyzed the DNA content of far11Δ cells expressing caspase-10 after the addition of the genotoxic agent HU. We found that far11Δ cells that express caspase-10 underwent G1-S arrest after HU treatment, indicating that these cells were able to activate the DNA damage checkpoint; in contrast, the expression of caspase-10 abrogated the cell cycle arrest induced by HU treatment in both a wild-type strain and in a far11Δ mutant expressing an episomic copy of FAR11 (Fig. 5B). These results indicate that Far11 is involved in the activation of the intra-S checkpoint.
The activation of the intra-S checkpoint depends on the phosphorylation of Rad53 by the upstream kinases Mec1 and Tel1 (36). We therefore investigated the phosphorylation state of Rad53 in our system after the expression of caspase-10. As expected, we found that in wild-type cells caspase-10 either prevented the phosphorylation of Rad53 or increased its dephosphorylation, which, in any case, resulted in the inactivation of the intra-S checkpoint (Fig. 5C). In contrast, we found that Rad53 was efficiently phosphorylated after treatment with HU in a far11Δ mutant expressing caspase-10, thus indicating that FAR11 must be required for the dephosphorylation of Rad53 (Fig. 5C).
Far11 Interacts with Different Subunits of Phosphatases
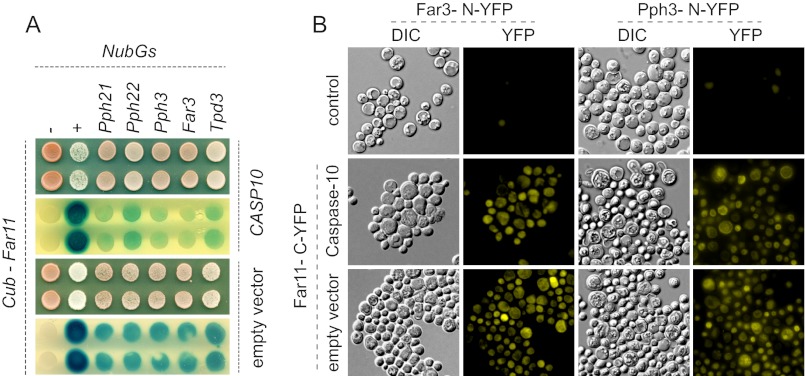
Our results suggested that Far11 is involved in the dephosphorylation of the effectors Atg13 and Rad53, which regulate the induction of autophagy and the DNA damage response, respectively. Furthermore, we show that the catalytic subunits of phosphatases Pph21, Pph22, and Pph3 are essential for caspase-10 toxicity, indicating that the activity of these phosphatases must be critical for caspase-10-dependent phenotypes. Additionally, it has been reported that Tpd3, which is a regulatory subunit of the protein phosphatase PP2A, interacts physically with Far11 (37, 38). Thus, we surmised that Far11 could be a novel cofactor of phosphatase complexes. Accordingly, we were next prompted to investigate the interactions between Far11 and phosphatases Pph21, Pph22, and Pph3. We used two different in vivo methods to study these protein interactions: the ubiquitin-based split-protein sensor, also termed the SUS, and the BiFC, which have been used previously to analyze in vivo interactions of membrane-associated proteins (31, 32). In the split-ubiquitin system, the protein interactions are confirmed by the transcriptional activation of both the ADE2 and LacZ reporter genes; in contrast, the BiFC method is based on the reconstitution of a YFP functional protein, which can be detected by fluorescence microscopy (see ”Experimental Procedures“ for details). We detected a clear association between Far11 and phosphatases Pph21, Pph22, and Pph3, which determined both the growth in SC-Ade medium (not shown) and activation of the LacZ gene (Fig. 6A). As positive controls of the experiment we used the proteins Far3 and Tpd3, which have previously been reported to interact with Far11 (27, 38). We performed the experiment before and after the expression of caspase-10, but failed to find any differences among the different treatments. These results were confirmed using the BiFC method, because a Far11-C-YFP fusion was able to reconstitute YFP fluorescence through interaction with Pph21-N-YFP, Pph22-N-YFP, or Pph3-N-YFP (Fig. 6B; only shown for Pph3). Once again, the expression of caspase-10 did not influence the interaction between Far11 and the proteins analyzed. Overall, these results show that Far11 interacts with different subunits of phosphatase complexes: at least Tpd3, Pph21, Pph22, and Pph3.
FIGURE 6.
In vivo assays of the physical interaction between Far11 and phosphatases Pph21, Pph22, and Pph3. A, SUS assay of the interaction between Far11 and phosphatases Pph21, Pph22, and Pph3 (see ”Experimental Procedures“ for details). The positive interactions are confirmed by the activation of the LACZ reporter. Far3 and Tpd3 were used as positive controls. Additional negative (−) and positive (+) internal controls were included as described (31). The SUS experiments were carried out with yeast strains transformed with either pESC-URA/CASP10 (upper panels) or the empty vector (lower panels) in galactose-containing medium. B, BiFC assay of Far11-VC-YFP and Pph3-VN-YFP. A Far3-VN-YFP was included as a positive control. The corresponding haploid strains harboring the VN-YFP fusions were used as negative controls (upper panels). The protein interactions were confirmed by the reconstitution of a functional YFP protein. The experiments were also carried out with the diploid strains transformed with either pESC-URA/CASP10 (middle panels) or the empty vector (lower panels) in galactose-containing medium.
DISCUSSION
The regulation of cell death processes and cytotoxic responses is crucial for the survival of living organisms. However, the molecular mechanisms underlying these regulatory networks are highly intricate and relatively unknown, especially with regard to the cross-talk that has been demonstrated for these cellular processes (11, 19).
In recent years, humanized yeasts have proved to be useful tools for the study of the conserved evolutionary processes that occur in eukaryotes, such as cell death-related processes, which are involved in important pathologies such as cancer and neurodegenerative diseases (23, 39). We have recently described that the expression of the human initiator caspase-10 induces a type of cell death in S. cerevisiae that is associated with apoptotic and autophagic phenotypes (26), suggesting that our humanized yeast model might serve for the study of these cell death mechanisms. With a view to uncovering novel regulatory mechanisms of the cell death processes, we performed a loss-of-function screening using the haploid YKO collection to identify genes that are essential for the toxicity of the caspase-10 in S. cerevisiae. We found 46 knockout mutations that suppressed caspase-10 lethality, which clearly support the idea that caspase-10-induced cell death is a gene-specific process and cannot be attributed to unspecific protease activity of the caspase-10.
In this regard, some of the phenotypes induced by caspase-10 are also observed after the expression of both caspase-8 (26) and caspase-3 (not shown). These caspases are also toxic for S. cerevisiae and their effect rely on their proteolytic activity. In addition, none of them cause DNA degradation and their effects are independent of the yeast apoptotic proteins Mca1 and Aif1 (26, 40). However, we think that there must be a differential signaling among different caspases because far11Δ mutation does not suppress the lethal effect of caspase-8 (not shown).
Among those 46 knockout mutations, we focused on the FAR11 gene, which has two human orthologs of unknown function that partially complemented the absence of a functional Far11. This partial complementation of the human orthologs might be explained either by yeast codon usage bias or by some functional divergence among the yeast and human orthologs. The yeast Far11 protein has been reported to be involved in the maintenance of the cell cycle arrest that is induced after treatment with the pheromone; however, there is no evidence concerning either the precise molecular mechanism involved or the downstream effectors of Far11 (27).
Here, we demonstrate that activation of MAPK signaling, in particular the simultaneous phosphorylation of kinases Fus3, Kss1, and Slt2, is necessary for caspase-10-induced cell death. Accordingly, it was previously reported that the simultaneous activation of Fus3, Kss1, and Slt2 is associated with growth impairment after the expression in S. cerevisiae of the guanine nucleotide exchange factor SopE2 from Salmonella typhimurium (41). These results suggest a possible mode of action of caspase-10 in yeast through the activation of different MAP kinases and a latter phosphorylation of Far proteins, because it has been described that both Far1 and Far11 are phosphorylated by the MAP kinase Fus3 upon activation of the pheromone MAPK pathway (33). Indeed, the Far11 protein has seven serine residues that can be phosphorylated under certain conditions (33, 36, 42, 43). In addition, the activation of MAPK signaling upon caspase-10 expression indicates that the primary target of caspase-10 in yeast might be an upstream effector of the MAPK pathways that would be essential for induced cell death, such as Gpa1 or Ste20. In this sense, Mst1, which is encoded by the mammalian ortholog of STE20, can be recognized and proteolyzed by caspase-3 (44). However, neither Gpa1 nor Ste20 are degraded by human caspase-10 in yeast (not shown), suggesting that the link between caspase-10 and the MAPK pathways may not be well defined. These results, together with our previous findings regarding the harmless effect of the overexpression of mutant alleles of caspase-10 (26), rule out the possibility that the phenotypes observed after the expression of human CASP10 could result from activation of the unfolded protein response pathway.
As mentioned above, the expression of caspase-10 in yeast induces autophagy, which is an endomembrane-associated mechanism involved in both cell survival and cell death (45). We characterized the subcellular localization of Far3, Far9, and Far11, which are essential for caspase-10-induced lethality, and found that they were all associated with endomembranes (ER and/or Golgi complex). The localization of these Far proteins may provide some clues that could help to understand their molecular functions, because the endomembrane system is very important for cellular dynamics and autophagy (12). Indeed, Far9 is also known as Vps64 and it participates in vesicle transport to the vacuole (46). More importantly, our results show that Far11 is essential for the activation of the autophagy inducer Atg13 through modulation of its phosphorylation state. Atg13 remains highly phosphorylated, thus preventing the induction of autophagy, in the absence of Far11 even when the autophagy process is triggered either by treatment with rapamycin or caspase-10 expression, indicating that Far11 must be involved, either directly or indirectly, in the dephosphorylation of Atg13. This finding supports the notion that the localization of Atg13 at the PAS, whose formation is associated with endomembranes, may play an important role in the local regulation of autophagy at the PAS. In this regard, it has been reported that PKA signaling strongly affects the localization of Atg13 at the PAS and that the inactivation of PKA activity induces an autophagic response (47, 48). Therefore, the hyperphosphorylation of Atg13 by the nutrient signaling kinases TOR and PKA could be completely blocked after caspase-10 expression through two different mechanisms in which Far11 might be involved. First, Far11 might be responsible for an increased Far11-dependent dephosphorylation of Atg13, which finally elicits the induction of autophagy and, second, Far11 might inhibit the TOR/PKA kinase activities that prevent the induction of autophagy through the phosphorylation of Atg13. Thus, we found that the catalytic subunits of the PKA kinase Tpk1, Tpk2, and Tpk3 were also essential for the lethal phenotype induced by the expression of caspase-10.
The nutrient-sensing pathways and the autophagy process are involved in the regulation of lifespan (49, 50). It has been described that mutants in the autophagy pathway have a shortened lifespan and in the same study the authors also reported that knockout mutants far3Δ and far11Δ exhibit a long-life phenotype (49), which could be attributed to the alteration of either the autophagy process or TOR/PKA signaling in such far mutants, thus supporting our hypothesis regarding the involvement of the Far family in the regulation of autophagy.
We have also observed that Far11 is involved in the activation of the intra-S checkpoint in response to genotoxic damage, suggesting that Far11 must play a more general role in cellular physiology. We found that Far11 was essential for the dephosphorylation of Rad53, which is required for cell-cycle arrest in response to DNA damage (36). Although it cannot be ruled out that Far11 might affect the activity of protein kinases, such a result suggests that Far11 would be a novel regulator of protein phosphatase complexes that could be involved in the dephosphorylation of different effectors. Moreover, it has been described that phosphatases Ptc2, Ptc3, and Pph3 are involved in the dephosphorylation of Rad53 (51) and that those phosphatases, besides Pph21 and Pph22, are essential for caspase-10-induced cell death. We also found that Far11 was able to interact physically with Pph21, Pph22, and Pph3, probably to modulate the activity of these phosphatases locally at the ER-Golgi and, therefore, to regulate the activity of Atg13 and Rad53, among other effectors that we have not yet identified. In this regard, we have confirmed that Far11 functions upstream of both Atg13 and Pph3, because an episomic copy of FAR11 did not restore the caspase-10-induced lethality in the double mutants far11Δ,atg13Δ or far11Δ,pph3Δ (supplemental Fig. S4).
Furthermore, supporting the idea about the regulatory connection between Far11 and PP2A phosphatase complexes, it has been reported that both the homolog of FAR11 from Neurospora crassa HAM2 and the PP2A complex are required for cell-to-cell fusion in a crucial event of the sexual cycle (52). Other homologs of Far11 from different filamentous fungi, such as Sordaria macrospora and Ashbya gossypi, are involved in hypha development and septum formation (53), which are processes regulated by protein phosphorylation.
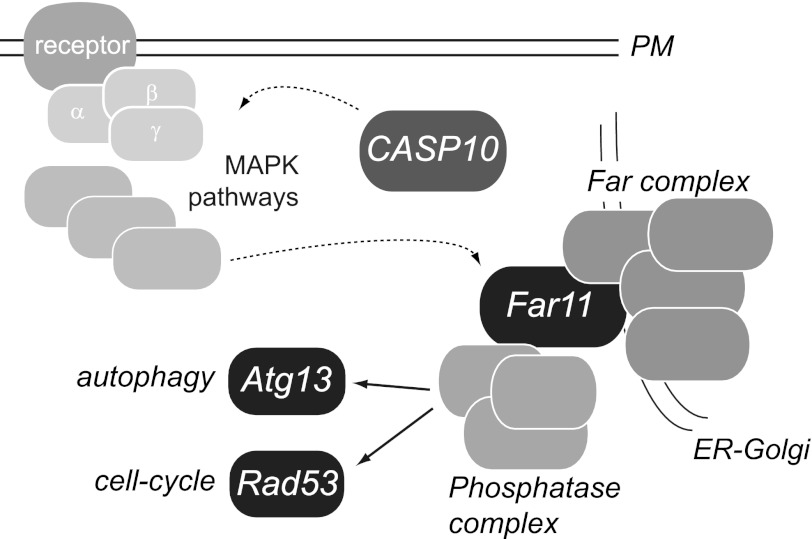
Reversible protein phosphorylation is estimated to affect one-third of the proteome and constitutes one of the most important post-translational modifications that regulate many cellular responses and basic physiological processes, such as cell division, cell death, and development (54, 55). Here we propose that Far11 participates in the regulation of two important cellular processes (i.e. autophagy and the DNA damage response) likely through an interaction with phosphatases (Fig. 7). The localization of the Far family at the ER-Golgi suggests a novel mechanism of intracellular signaling that might place the endomembranous system within the regulatory landscape of cell physiology. Indeed, the activity, localization, and substrate specificity of many phosphatase holoenzymes (protein serine/threonine phosphatases) is regulated by combinatorial interactions with a large number of regulatory subunits (56). Finally, it has been reported that the human orthologs of Far11 (Fam40A and Fam40B) can form a protein complex named STRIPAK with kinase and phosphatase activities (35), suggesting that the molecular function of Far11 might have been conserved along evolution.
FIGURE 7.
Scheme of the proposed mechanism of action of human caspase-10 in yeast. The expression of human caspase-10 triggers the activation of the MAPK signaling that might eventually elicit activation of the Far complex, which is located at the ER-Golgi. Thus, Far11 may physically interact and regulate the activity of phosphatase complexes that finally modulate the phosphorylation of both Atg13 and Rad53. Discontinuous lines indicate mechanisms not fully known. PM, plasma membrane.
Supplementary Material
Acknowledgments
We thank D. Klionsky, D. E. Stone, P. Pryciak, P. Orbdlik, and F. Madeo for providing strains and plasmids. We also thank M. D. Sánchez for excellent technical help and N. Skinner for correcting the manuscript.
This work was supported in part by Junta de Castilla y León Grants SA008B08 (to A. J.) and GR147 (to J. L. R.) and Ministerio de Ciencia y Innovación Grant BIO2008-00194.

This article contains supplemental data Figs. S1–S4 and Tables S1–S4.
- ATG
- autophagy related gene/protein
- CASP10
- caspase-10
- Far
- factor arrest
- HU
- hydroxyurea
- SUS
- split ubiquitin system
- BiFC
- bimolecular fluorescence complementation
- PAS
- phagophore assembly site
- Nub
- N-terminal ubiquitin
- Cub
- C-terminal ubiquitin.
REFERENCES
- 1. Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008) Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vicencio J. M., Galluzzi L., Tajeddine N., Ortiz C., Criollo A., Tasdemir E., Morselli E., Ben Younes A., Maiuri M. C., Lavandero S., Kroemer G. (2008) Senescence, apoptosis, or autophagy? When a damaged cell must decide its path, a minireview. Gerontology 54, 92–99 [DOI] [PubMed] [Google Scholar]
- 3. Galluzzi L., Joza N., Tasdemir E., Maiuri M. C., Hengartner M., Abrams J. M., Tavernarakis N., Penninger J., Madeo F., Kroemer G. (2008) No death without life. Vital functions of apoptotic effectors. Cell Death Differ. 15, 1113–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Büttner S., Eisenberg T., Carmona-Gutierrez D., Ruli D., Knauer H., Ruckenstuhl C., Sigrist C., Wissing S., Kollroser M., Fröhlich K. U., Sigrist S., Madeo F. (2007) Endonuclease G regulates budding yeast life and death. Mol. Cell 25, 233–246 [DOI] [PubMed] [Google Scholar]
- 5. Büttner S., Ruli D., Vögtle F. N., Galluzzi L., Moitzi B., Eisenberg T., Kepp O., Habernig L., Carmona-Gutierrez D., Rockenfeller P., Laun P., Breitenbach M., Khoury C., Fröhlich K. U., Rechberger G., Meisinger C., Kroemer G., Madeo F. (2011) A yeast BH3-only protein mediates the mitochondrial pathway of apoptosis. EMBO J. 30, 2779–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fahrenkrog B., Sauder U., Aebi U. (2004) The S. cerevisiae HtrA-like protein Nma111p is a nuclear serine protease that mediates yeast apoptosis. J. Cell Sci. 117, 115–126 [DOI] [PubMed] [Google Scholar]
- 7. Madeo F., Herker E., Maldener C., Wissing S., Lächelt S., Herlan M., Fehr M., Lauber K., Sigrist S. J., Wesselborg S., Fröhlich K. U. (2002) A caspase-related protease regulates apoptosis in yeast. Mol. cell 9, 911–917 [DOI] [PubMed] [Google Scholar]
- 8. Wissing S., Ludovico P., Herker E., Büttner S., Engelhardt S. M., Decker T., Link A., Proksch A., Rodrigues F., Corte-Real M., Fröhlich K. U., Manns J., Candé C., Sigrist S. J., Kroemer G., Madeo F. (2004) An AIF orthologue regulates apoptosis in yeast. J. Cell Biol. 166, 969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cecconi F., Levine B. (2008) The role of autophagy in mammalian development. Cell makeover rather than cell death. Dev. Cell 15, 344–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inoue Y., Klionsky D. J. (2010) Regulation of macroautophagy in Saccharomyces cerevisiae. Semin. Cell Dev. Biol. 21, 664–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y., Klionsky D. J. (2011) The regulation of autophagy, unanswered questions. J. Cell Sci. 124, 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He C., Klionsky D. J. (2009) Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet 43, 67–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Z., Klionsky D. J. (2010) Eaten alive. A history of macroautophagy. Nat. Cell Biol. 12, 814–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheong H., Nair U., Geng J., Klionsky D. J. (2008) The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell 19, 668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kabeya Y., Kamada Y., Baba M., Takikawa H., Sasaki M., Ohsumi Y. (2005) Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy. Mol. Biol. Cell 16, 2544–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamada Y., Yoshino K., Kondo C., Kawamata T., Oshiro N., Yonezawa K., Ohsumi Y. (2010) Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol. Cell Biol. 30, 1049–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cebollero E., Reggiori F. (2009) Regulation of autophagy in yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1793, 1413–1421 [DOI] [PubMed] [Google Scholar]
- 18. Mizushima N. (2010) The role of the Atg1-ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol. 22, 132–139 [DOI] [PubMed] [Google Scholar]
- 19. Maiuri M. C., Zalckvar E., Kimchi A., Kroemer G. (2007) Self-eating and self-killing. Cross-talk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8, 741–752 [DOI] [PubMed] [Google Scholar]
- 20. Yousefi S., Perozzo R., Schmid I., Ziemiecki A., Schaffner T., Scapozza L., Brunner T., Simon H. U. (2006) Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 8, 1124–1132 [DOI] [PubMed] [Google Scholar]
- 21. Hou W., Han J., Lu C., Goldstein L. A., Rabinowich H. (2010) Autophagic degradation of active caspase-8. A cross-talk mechanism between autophagy and apoptosis. Autophagy 6, 891–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giorgini F., Muchowski P. J. (2006) Screening for genetic modifiers of amyloid toxicity in yeast. Methods Enzymol. 412, 201–222 [DOI] [PubMed] [Google Scholar]
- 23. Greenwood M. T., Ludovico P. (2010) Expressing and functional analysis of mammalian apoptotic regulators in yeast. Cell Death Differ. 17, 737–745 [DOI] [PubMed] [Google Scholar]
- 24. Xu Q., Reed J. C. (1998) Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol. Cell 1, 337–346 [DOI] [PubMed] [Google Scholar]
- 25. Zhang H., Xu Q., Krajewski S., Krajewska M., Xie Z., Fuess S., Kitada S., Pawlowski K., Godzik A., Reed J. C. (2000) BAR, an apoptosis regulator at the intersection of caspases and Bcl-2 family proteins. Proc. Natl. Acad. Sci. U.S.A. 97, 2597–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lisa-Santamaría P., Neiman A. M., Cuesta-Marbán A., Mollinedo F., Revuelta J. L., Jiménez A. (2009) Human initiator caspases trigger apoptotic and autophagic phenotypes in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1793, 561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kemp H. A., Sprague G. F., Jr. (2003) Far3 and five interacting proteins prevent premature recovery from pheromone arrest in the budding yeast Saccharomyces cerevisiae. Mol. Cell Biol. 23, 1750–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gietz R. D., Woods R. A. (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350, 87–96 [DOI] [PubMed] [Google Scholar]
- 29. Shoemaker D. D., Lashkari D. A., Morris D., Mittmann M., Davis R. W. (1996) Quantitative phenotypic analysis of yeast deletion mutants using a highly parallel molecular bar-coding strategy. Nat. Genet. 14, 450–456 [DOI] [PubMed] [Google Scholar]
- 30. Jiménez A., Mateos L., Pedrajas J. R., Miranda-Vizuete A., Revuelta J. L. (2007) The txl1+ gene from Schizosaccharomyces pombe encodes a new thioredoxin-like 1 protein that participates in the antioxidant defense against tert-butyl hydroperoxide. Yeast 24, 481–490 [DOI] [PubMed] [Google Scholar]
- 31. Obrdlik P., El-Bakkoury M., Hamacher T., Cappellaro C., Vilarino C., Fleischer C., Ellerbrok H., Kamuzinzi R., Ledent V., Blaudez D., Sanders D., Revuelta J. L., Boles E., André B., Frommer W. B. (2004) K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc. Natl. Acad. Sci. U.S.A. 101, 12242–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu C. D., Chinenov Y., Kerppola T. K. (2002) Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9, 789–798 [DOI] [PubMed] [Google Scholar]
- 33. Gruhler A., Olsen J. V., Mohammed S., Mortensen P., Faergeman N. J., Mann M., Jensen O. N. (2005) Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway. Mol. Cell. Proteomics 4, 310–327 [DOI] [PubMed] [Google Scholar]
- 34. Chang F., Herskowitz I. (1990) Identification of a gene necessary for cell cycle arrest by a negative growth factor of yeast. FAR1 is an inhibitor of a G1 cyclin, CLN2. Cell 63, 999–1011 [DOI] [PubMed] [Google Scholar]
- 35. Goudreault M., D'Ambrosio L. M., Kean M. J., Mullin M. J., Larsen B. G., Sanchez A., Chaudhry S., Chen G. I., Sicheri F., Nesvizhskii A. I., Aebersold R., Raught B., Gingras A. C. (2009) A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol. Cell. Proteomics 8, 157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smolka M. B., Albuquerque C. P., Chen S. H., Zhou H. (2007) Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc. Natl. Acad. Sci. U.S.A. 104, 10364–10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uetz P., Giot L., Cagney G., Mansfield T. A., Judson R. S., Knight J. R., Lockshon D., Narayan V., Srinivasan M., Pochart P., Qureshi-Emili A., Li Y., Godwin B., Conover D., Kalbfleisch T., Vijayadamodar G., Yang M., Johnston M., Fields S., Rothberg J. M. (2000) A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403, 623–627 [DOI] [PubMed] [Google Scholar]
- 38. Yu H., Braun P., Yildirim M. A., Lemmens I., Venkatesan K., Sahalie J., Hirozane-Kishikawa T., Gebreab F., Li N., Simonis N., Hao T., Rual J. F., Dricot A., Vazquez A., Murray R. R., Simon C., Tardivo L., Tam S., Svrzikapa N., Fan C., de Smet A. S., Motyl A., Hudson M. E., Park J., Xin X., Cusick M. E., Moore T., Boone C., Snyder M., Roth F. P., Barabási A. L., Tavernier J., Hill D. E., Vidal M. (2008) High-quality binary protein interaction map of the yeast interactome network. Science 322, 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braun R. J., Büttner S., Ring J., Kroemer G., Madeo F. (2010) Nervous yeast. Modeling neurotoxic cell death. Trends Biochem. Sci. 35, 135–144 [DOI] [PubMed] [Google Scholar]
- 40. Puryer M. A., Hawkins C. J. (2006) Human, insect, and nematode caspases kill Saccharomyces cerevisiae independently of YCA1 and Aif1p. Apoptosis 11, 509–517 [DOI] [PubMed] [Google Scholar]
- 41. Rodríguez-Pachón J. M., Martín H., North G., Rotger R., Nombela C., Molina M. (2002) A novel connection between the yeast Cdc42 GTPase and the Slt2-mediated cell integrity pathway identified through the effect of secreted Salmonella GTPase modulators. J. Biol. Chem. 277, 27094–27102 [DOI] [PubMed] [Google Scholar]
- 42. Albuquerque C. P., Smolka M. B., Payne S. H., Bafna V., Eng J., Zhou H. (2008) A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol. Cell Proteomics 7, 1389–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li X., Gerber S. A., Rudner A. D., Beausoleil S. A., Haas W., Villén J., Elias J. E., Gygi S. P. (2007) Large-scale phosphorylation analysis of α-factor-arrested Saccharomyces cerevisiae. J. Proteome Res. 6, 1190–1197 [DOI] [PubMed] [Google Scholar]
- 44. Graves J. D., Gotoh Y., Draves K. E., Ambrose D., Han D. K., Wright M., Chernoff J., Clark E. A., Krebs E. G. (1998) Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 17, 2224–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mizushima N. (2009) Physiological functions of autophagy. Curr. Top. Microbiol. Immunol. 335, 71–84 [DOI] [PubMed] [Google Scholar]
- 46. Bonangelino C. J., Chavez E. M., Bonifacino J. S. (2002) Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 2486–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stephan J. S., Yeh Y. Y., Ramachandran V., Deminoff S. J., Herman P. K. (2010) The Tor and cAMP-dependent protein kinase signaling pathways coordinately control autophagy in Saccharomyces cerevisiae. Autophagy 6, 294–295 [DOI] [PubMed] [Google Scholar]
- 48. Stephan J. S., Yeh Y. Y., Ramachandran V., Deminoff S. J., Herman P. K. (2009) The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc. Natl. Acad. Sci. U.S.A. 106, 17049–17054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fabrizio P., Hoon S., Shamalnasab M., Galbani A., Wei M., Giaever G., Nislow C., Longo V. D. (2010) Genome-wide screen in Saccharomyces cerevisiae identifies vacuolar protein sorting, autophagy, biosynthetic, and tRNA methylation genes involved in lifespan regulation. PLoS Genet. 6, e1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Madeo F., Tavernarakis N., Kroemer G. (2010) Can autophagy promote longevity? Nat. Cell Biol. 12, 842–846 [DOI] [PubMed] [Google Scholar]
- 51. Travesa A., Duch A., Quintana D. G. (2008) Distinct phosphatases mediate the deactivation of the DNA damage checkpoint kinase Rad53. J. Biol. Chem. 283, 17123–17130 [DOI] [PubMed] [Google Scholar]
- 52. Fu C., Iyer P., Herkal A., Abdullah J., Stout A., Free S. J. (2011) Identification and characterization of genes required for cell-to-cell fusion in Neurospora crassa. Eukaryot. Cell 10, 1100–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bloemendal S., Lord K. M., Rech C., Hoff B., Engh I., Read N. D., Kück U. (2010) A mutant defective in sexual development produces aseptate ascogonia. Eukaryot. Cell 9, 1856–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cohen P. (2002) The origins of protein phosphorylation. Nat. Cell Biol. 4, E127–130 [DOI] [PubMed] [Google Scholar]
- 55. Ptacek J., Devgan G., Michaud G., Zhu H., Zhu X., Fasolo J., Guo H., Jona G., Breitkreutz A., Sopko R., McCartney R. R., Schmidt M. C., Rachidi N., Lee S. J., Mah A. S., Meng L., Stark M. J., Stern D. F., De Virgilio C., Tyers M., Andrews B., Gerstein M., Schweitzer B., Predki P. F., Snyder M. (2005) Global analysis of protein phosphorylation in yeast. Nature 438, 679–684 [DOI] [PubMed] [Google Scholar]
- 56. Shi Y. (2009) Serine/threonine phosphatases. Mechanism through structure. Cell 139, 468–484 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.