Background: The triggering receptor expressed on myeloid cell (TREM)-mediated signaling is essential for osteoclastogenesis.
Results: The alternative transcripts of triggering receptor expressed on myeloid cell-like transcript-1 (TLT-1s) inhibits osteoclast formation by counteracting the TREM-2 signaling pathway.
Conclusion: TLT-1s is a negative regulator of osteoclastogenesis, by constitutively associating with SHP-1 and SHIP-1 phosphatases, abrogating the TREM-2 signaling upon RANKL stimulation.
Significance: We discovered that an alternative transcript of TLT-1, namely TLT-1s, negatively regulates osteoclastogenesis.
Keywords: Akt, Calcium Signaling, NFAT Transcription Factor, Osteoblasts, Osteoclast, TREM-like Transcript-1, Triggering Receptor Expressed on Myeloid Cells
Abstract
Triggering receptor expressed on myeloid cells (TREM)-like transcript-1 (TLT-1) is an immunoreceptor tyrosine-based inhibitory motif (ITIM)-baring TREM family protein. In this study, we identified an alternative transcript form of TLT-1, namely TLT-1s, which has very short extracellular immunoglobulin domain consisting of only 202 amino acids. TLT-1s was mainly expressed in macrophages and osteoclast precursor cells. Upon receptor activator of nuclear factor-κB ligand stimulation, TLT-1s mRNA and protein levels were gradually decreased in BMMs. We also showed the TLT-1s is localized to the cytoplasmic membrane in osteoclast precursor cells. TLT-1s silencing strongly enhanced the formation and resorption activity of osteoclast. In addition, forced expression of TLT-1s showed reduced formation of osteoclast. Because ITIM-baring proteins inhibit immunoreceptor tyrosine-based activation motif (ITAM)-mediated receptor signaling, we tested whether TLT-1s physically interacted with TREM-2, the ITAM-associated co-stimulatory receptor essential for osteoclast differentiation. We showed that TLT-1s is associated with TREM-2 in osteoclast precursor cells. TLT-1s is also associated with tyrosine Src homology 2 domain-containing phosphatase-1 and SH2 domain-containing inositol phosphatase-1 and recruited them to the TREM2-ITAM signaling complex. In addition, knockdown of TLT-1s markedly elevated the intracellular calcium concentration and oscillation in osteoclast precursor cells. In addition, calcium-mediated induction of nuclear factor of activated T cells was also increased by TLT-1s silencing. Furthermore, TREM-2-mediated Akt activation and proliferation of osteoclast precursor cells were also enhanced in TLT-1s silenced cells. In this paper, we found the noble ITIM-baring inhibitory membrane protein; TLT-1s, which regulates ITAM-mediated signaling on osteoclastogenesis.
Introduction
Triggering receptor expressed on myeloid cells (TREM)2 is a member of the activating immunoreceptor expressed on monocytes, macrophages, microglia, and neutrophils (1–3). To date, 3 activating TREM genes have been identified, clustered on human chromosome 6 and mouse chromosome 17 (4). TREM is characterized by a single V-set immunoglobulin domain, short cytoplasmic domain, and transmembrane domain capable of interaction with an immunoreceptor tyrosine-based activation motif (ITAM)-baring protein, DNAX activating protein of 12 (DAP12) (5). In addition to 3 activating TREM receptors, the TREM gene cluster includes an inhibitory receptor, TREM-like transcript-1 (TLT-1). Unlike other TREMs, TLT-1 contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain capable of recruiting protein-tyrosine phosphatases (6). TLT-1 expression has been reported in α-granules of platelets and megakaryocytes, regulating platelet activation and inflammation (7). TLT-1 null mice exhibit deficiency in platelet aggregation and are susceptible to lipopolysaccharide-induced septic shock (8). Recently, two splice variants of TLT-1 with short cytoplasmic domains were reported (9), suggesting that isoforms of TLT-1 may have different cellular functions.
Osteoclasts are bone-resorbing cells differentiated from monocyte/macrophage lineage cells in the presence of receptor activator of nuclear factor-κB ligand (RANKL) and macrophage-colony stimulating factor (M-CSF) (9, 10). RANKL activates signaling pathways involving the induction of the nuclear factor of activated T cells (NFATc1) upstream of osteoclastogenic genes such as tartrate-resistant acid phosphatase (TRAP) and cathepsin K. In addition to RANKL, co-stimulatory receptors such as TREM-2 and osteoclast-associated receptor provide calcium signals required for the optimal NFATc1 activation (11). The ITAM bearing proteins DAP12 and FcRγ recruit SYK kinase that activates calcium signaling through phospholipase Cγ (PLCγ) (11–13). On the other hand, phosphatases such as tyrosine Src homology 2 (SH2) domain-containing phosphatase-1 (SHP-1) and SH2 domain-containing inositol phosphatase-1 (SHIP-1) binds to ITIMs to negate the ITAM-mediated signaling (14, 15). During osteoclast differentiation, LILRB and PIR-B down-regulates the osteoclastogenesis by recruiting SHP-1 via their ITIMs (16). In the present report, we discovered an alternative transcript form of TLT-1, namely TLT-1s, which has a very short extracellular Ig domain. Here we show that TLT-1s is a negative regulator of osteoclastogenesis, by constitutively associating with SHP-1 and SHIP-1 phosphatases, abrogating the TREM-2 signaling upon RANKL stimulation.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Anti-TLT-1 antibody was from Novous Biologicals (Littleton, CO). Anti-NFATc1, TREM-2, and osteoclast-associated receptor antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other antibodies were purchased from Cell Signaling Technology (Beverly, MA). Human soluble RANKL and M-CSF were from PeproTech (Rocky Hill, NJ). Lipofectamine 2000 was purchased from Invitrogen. Anti-actin antibody and all other chemicals were purchased from Sigma.
Bone Marrow-derived Macrophage Culture and Osteoclast Differentiation
Bone marrow-derived macrophages (BMMs) were generated as described previously (15, 16). In brief, isolated bone marrow cells from mouse femurs were cultured overnight in α-modified essential medium containing 10% FBS on culture dishes. Nonadherent cells were further cultured for 3 days in the presence of 5 ng/ml of M-CSF to generate BMMs. Osteoclasts were obtained by culturing cells in α-MEM containing 10% FBS, 30 ng/ml of M-CSF, and 200 ng/ml of RANKL for 3 days.
Preparation of Platelet
To harvest platelets, mice were anesthetized with isoflurane and 500 μl of blood was collected into a tube containing 3.8% sodium citrate (1/9, v/v) by cardiac puncture. Platelet-rich plasma was obtained by centrifugation at 200 × g for 7 min. The plasma and buffy coat were transferred to a fresh tube. Platelet was isolated by centrifugation at 850 × g for 7 min.
In Vitro Resorption Pit Formation Assay
BMMs were cultured on dentin slices for 5 days in the presence of RANKL and M-CSF. After removing the cells by sonication, dentin slices were stained with hematoxylin and observed under a light microscope. The bone resorption area was measured with image analysis software (Image Pro-Plus, Media Cybernetics).
Gene Knock-down by Small Interfering RNA Oligonucleotides
The 22-nucleotide small interfering RNA (siRNA) duplexes for TLT-1s and negative control were purchased from Invitrogen. The TLT-1s target sequence was 5′-ACATGTGGAATGTCCGAGGGTAGT-3′. BMMS were transfected with siRNA oligonucleotides using Lipofectamine 2000 following the manufacturer's instructions.
Retroviral Transduction
Mouse TLT-1s was cloned into pMX-IRES vector. Retroviral particles were packaged by transfecting Plat-E cells with DNA plasmids using Lipofectamine 2000 according to the manufacturer's instructions. After a 48-h culture in DMEM supplemented with 10% FBS, viral supernatants were collected and filtered through a 0.45-μm syringe filter. BMMs were infected with viral supernatants in the presence of Polybrene (10 μg/ml) and M-CSF for 12 h.
5′-Rapid Amplification of cDNA Ends (5′-RACE)
Total RNA was isolated from mouse bone marrow-derived macrophages using the PARISTM Kit (Ambion, TX). The 5′-RACE was performed using the System for Rapid Amplification of cDNA Ends, version 2 (Invitrogen).
Reverse Transcriptase-Polymerase Chain Reaction Analysis
Total RNA prepared using TRIzol (Invitrogen) were reverse transcribed using SuperScript II reverse transcriptase (Invitrogen). One μl of cDNA synthesized from 1 μg of total RNA was amplified with the specific primers.
Western Blotting
Cells were washed with ice-cold PBS, scraped with a rubber policeman, and lysed in RIPA buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.5 mm PMSF, 1 μg/ml of aprotinin, 1 μg/ml of leupeptin, and 1 μg/ml of pepstatin). After protein quantification with a protein assay kit (Bio-Rad), whole cell extracts were separated on polyacrylamide gels and transferred onto nitrocellulose membranes. After blocking for 1 h with 5% skim milk in Tris-buffered saline containing 0.1% Tween 20, membranes were incubated overnight at 4 °C with primary antibodies in TBST containing 2% skim milk. Membranes were washed, incubated with secondary antibodies conjugated with horseradish peroxidase, and developed using an enhanced chemiluminescence system.
TRAP Staining
Cells were fixed in 3.7% formaldehyde solution and permeabilized with 0.1% Triton X-100. After washing with PBS, cells were stained using the Leukocyte Acid Phosphatase Assay Kit (Sigma) following the manufacturer's instruction. TRAP-positive multinuclear osteoclasts containing three or more nuclei were counted under a light microscope and photographed.
Measurement of Intracellular Calcium Concentration and Oscillations
For measurement of total intracellular calcium concentration, cells were loaded with Fluo-4 NW dye (Molecular Probes, Eugene, OR) at 37 °C for 30 min followed by an additional incubation for 30 min at room temperature. The calcium-dependent fluorescence was measured in a CytoFluor plate reader (Applied Biosystems, Foster City, CA) with a 485/535 nm excitation/emission filter pair. Calcium oscillations were measured as described previously (17).
BrdU Incorporation Assay
Bromodeoxyuridine (BrdU) incorporation into cells was measured using the BrdU Cell Proliferation Assay (Calbiochem, San Diego, CA) following the manufacturer's instruction.
Cell Viability Assay
Cell viability was measured using the CCK-8 assay (Dogindo Laboratories) according to the manufacturer's protocol. Cells were incubated with the CCK-8 reagent for 1 h and optical density was determined at 450 nm.
Statistics
To determine the significance of results, Student's t test was used. Differences with p < 0.05 were regarded as significant.
RESULTS
Cloning and Characterization of the Alternative Transcript of Mouse TLT-1
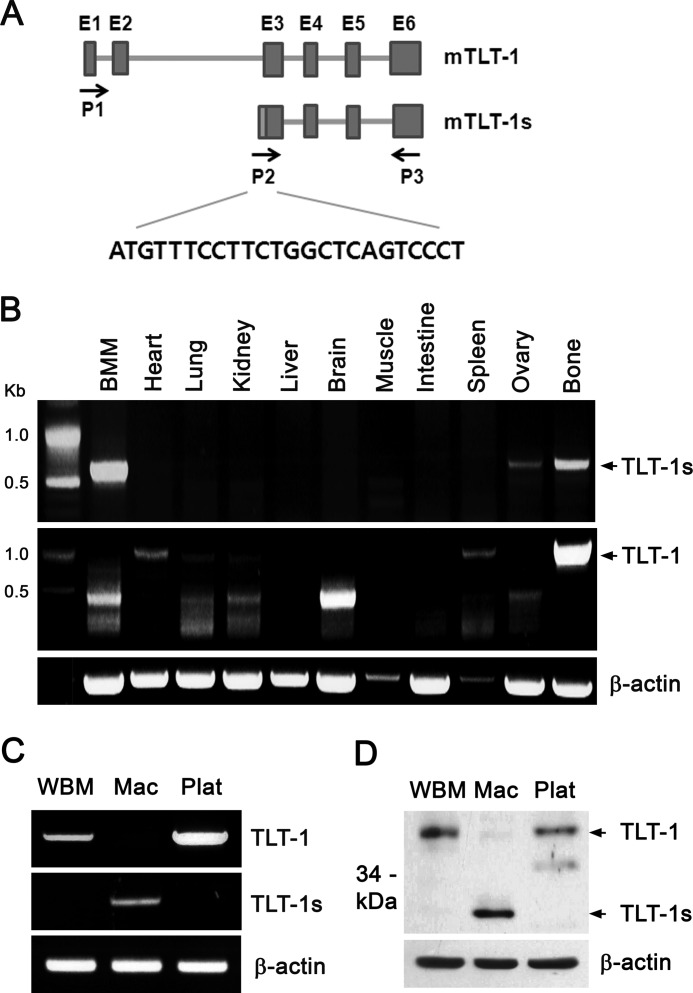
To discover new molecules that regulate osteoclast differentiation, we monitored gene expression profiles during osteoclast differentiation of human peripheral blood mononuclear cells (18). Microarray analysis revealed that the TLT-1 mRNA level negatively correlated with osteoclastogenesis. Interestingly, RT-PCR amplification in mouse BMMs failed to gain the full-length TLT-1 mRNA, resulting in only the C-terminal 400 base pairs of TLT-1. Both in BMMs and osteoclasts, the N-terminal region of TLT-1 could not be amplified using primer sets designed with the known TLT-1 sequences. So, we hypothesized that transcript variants of TLT-1 with alternative N-terminal sequences existed in osteoclast precursors. To test this, a 5′-RACE was carried out in BMMs. Indeed, a cDNA clone, designated as TLT-1s, containing a 5′-untranslated region (UTR) distinct from that of TLT-1 was identified. Comparison of the TLT-1s 5′-UTR sequence with the mouse TLT-1 gene (GenBankTM accession number AY078502) revealed that the TLT-1s 5′-UTR was derived from intron 2 of the TLT-1 gene (Fig. 1A). Exons 1 and 2 were totally missing in TLT-1s, starting with the intervening 16-bp sequence of intron 2 immediately before exon 3 of TLT-1. As a result, full-length TLT-1 consists of 322 amino acids, TLT-1s was predicted to have only 202 amino acids mostly lacking the N-terminal extracellular Ig domain of TLT-1. To gain further insights into the nature of TLT-1s, the tissue expression pattern was investigated by RT-PCR using specific primer sets. Strong TLT-1 mRNA expression was detected in bone, spleen, and to a lesser extent in heart (Fig. 1B). Notably, only TLT-1s was expressed in BMMs, although both TLT-1 and TLT-1s were detected in bone. Comparison of mRNA expression in whole bone marrow cells (WBMs), BMMs (Mac), and platelets (Plat) revealed that TLT-1s was expressed exclusively only in BMMs (Fig. 1C). On the other hand, TLT-1 expressed in WBMs seemed to disappear during the M-CSF-derived differentiation into macrophages. Western blotting using an antibody detecting a common C-terminal region confirmed that the 37-kDa TLT-1 was detected in WBMs and platelets, whereas 27-kDa TLT-1s was exclusively detected in BMMs (Fig. 1D).
FIGURE 1.
TLT-1s is an alternative transcript of TLT-1 in macrophages. A, schematic representation of TLT-1 and TLT-1s genes. The TLT-1 gene consists of 6 exons and 5 introns. Boxes represent individual exons. The primers used to amplify TLT-1 (P1 and P3) and TLT-1s (P2 and P3) were indicated by arrows. B, the expression of TLT-1 and TLT-1s in mouse tissues was assessed by RT-PCR. C, mouse BMMs were differentiated from WBM by incubation with 10 ng/ml of M-CSF for 3 days. The expression of TLT-1 and TLT-1s was detected by RT-PCR in WBMs, BMMs (Mac), and platelet (Plat). D, the protein levels of TLT-1 and TLT-1s were measured by Western blotting using an antibody against the C terminus of TLT-1.
Expression of TLT-1s during RANKL-mediated Osteoclastogenesis
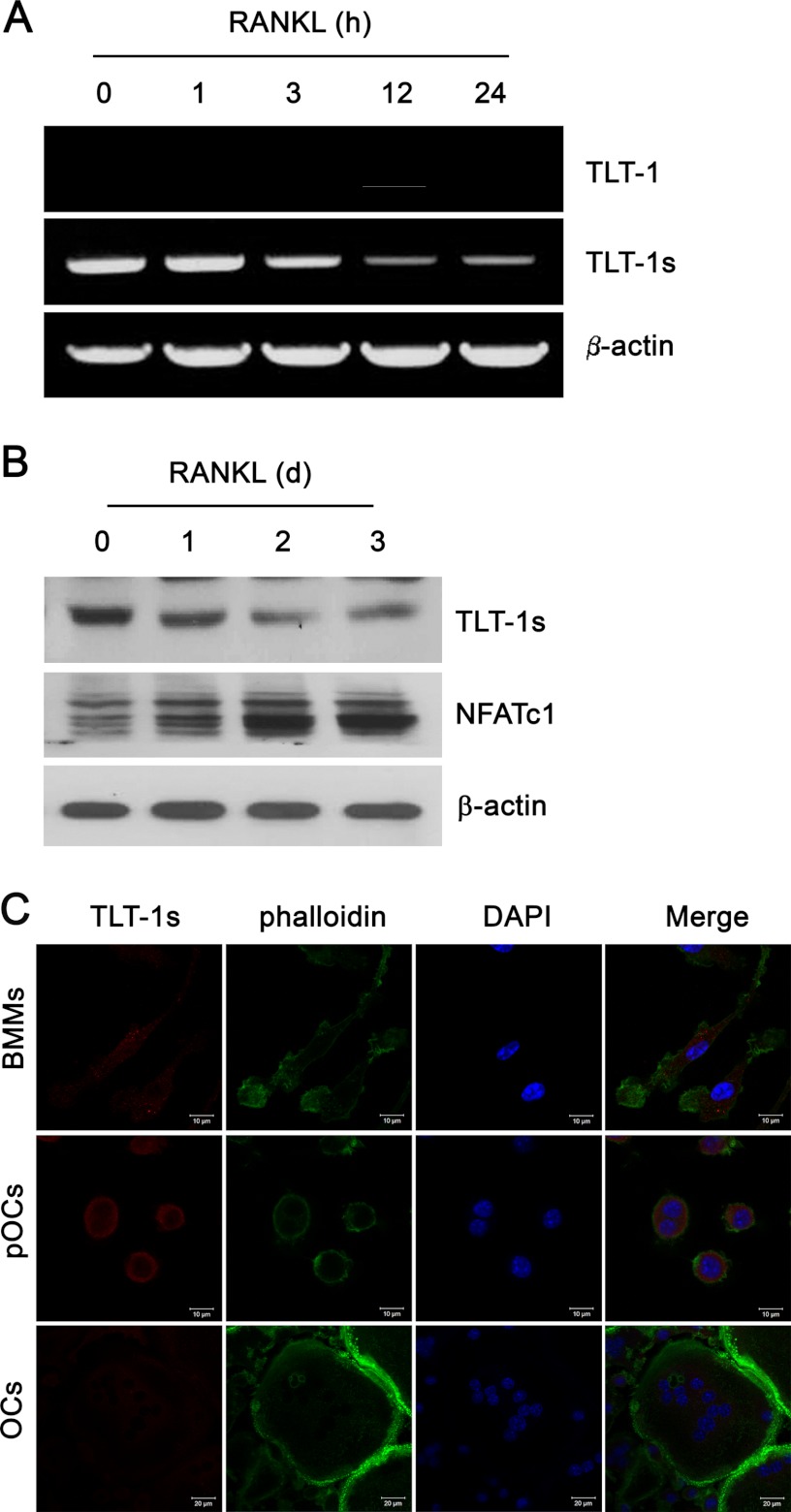
Because TLT-1s highly expressed in macrophages, the expression of TLT-1s during RANKL-induced osteoclastogenesis was investigated. TLT-1s mRNA levels gradually decreased during the 24-h treatment of RANKL (Fig. 2A). Full-length TLT-1 was not detected in BMMs before and after RANKL stimulation. TLT-1s expression was also down-regulated by RANKL in RAW 264.7 mouse macrophage cell lines (data not shown). Western blotting corroborated the decrease in TLT-1s expression during RANKL-dependent osteoclastogenesis (Fig. 2B). It was previously shown that TLT-1 moved to cytoplasmic membranes upon stimulation in platelets (9). The examination of subcellular localization of TLT-1s by confocal microscopy in Fig. 2C revealed that TLT-1s was dispersed in the cytoplasm in BMMs and localized in the plasma membrane region in pre-osteoclasts. TLT-1s was hardly detectable in the mature osteoclast.
FIGURE 2.
The TLT-1s expression was reduced by RANKL. A, BMMs were stimulated with 200 ng/ml of RANKL for the indicated times. The mRNA levels of TLT-1 and TLT-1s were determined by RT-PCR. B, BMMs were stimulated with 200 ng/ml of RANKL for the indicated days. The protein level of TLT-1s was determined by Western blotting using an antibody against the C terminus of TLT-1. NFATc1 expression was used as a marker for osteoclast differentiation. C, BMMs were cultured with 200 ng/ml of RANKL for 1 (pOC, pre-osteoclasts) or 3 days (OS, osteoclasts). Cells were immunostained using an antibody against the C terminus of TLT-1 followed by Cy3-conjugated secondary antibody. Nuclei and actin filaments were stained with DAPI and FITC-conjugated phalloidin.
Suppression of RANKL-mediated Osteoclastogenesis and Bone Resorption by TLT-1s
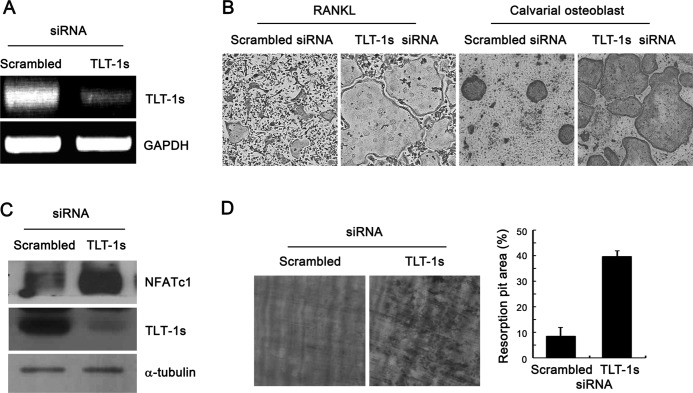
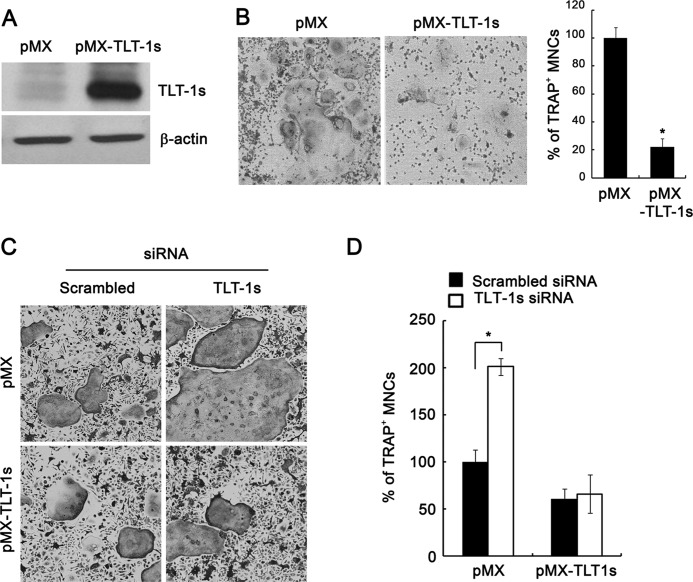
To determine the role of TLT-1s during osteoclast differentiation, TLT-1s knockdown was performed using siRNA oligonucleotides specific for TLT-1s that efficiently reduced the TLT-1s mRNA levels in BMMs (Fig. 3A). The knockdown of TLT-1s significantly increased the number of multinuclear osteoclasts upon RANKL stimulation of BMMs (Fig. 3B, left panel). Similarly, the silencing of TLT-1s in BMMs significantly enhanced osteoclastogenesis in BMM-osteoblast co-culture experiments (Fig. 3B, right panel). In accordance with enhanced osteoclast differentiation, TLT-1 knockdown markedly elevated NFATc1 expression in RANKL-stimulated BMMs (Fig. 3C). In addition, the bone resorption activity of osteoclasts cultured on dentin slices was also significantly increased by TLT-1s knockdown (Fig. 3D). To further clarify the role of TLT-1s on osteoclastogenesis, TLT-1s was ectopically expressed in BMMs by retrovirus-mediated gene transfer (Fig. 4A). As expected, the overexpression of TLT-1s significantly reduced the number of osteoclasts compared with control (Fig. 4B). We also showed the inhibitory effect of TLT-1 on osteoclastogenesis in the RAW 265.7 macrophage cell line (supplemental Fig. S1). These inhibitory effects of TLT-1s on osteoclast differentiation were eliminated by the addition of TLT-1s-siRNA oligonucleotides (Fig. 4, C and D), suggesting the specific role of TLT-1s in osteoclastogenesis.
FIGURE 3.
TLT-1s knockdown enhanced the formation of osteoclasts and resorption activity. A, BMMs were transfected with siRNA oligonucleotides specific for TLT-1s. At 2 days after transfection, the mRNA level of TLT-1s was determined by RT-PCR analysis. B, TLT-1s knockdown was performed in BMMs by siRNA transfection. Cells were further incubated in the presence of 20 ng/ml of M-CSF and 100 ng/ml of RANKL or co-cultured with calvarial osteoblasts for 4 days before TRAP-staining. C, TLT-1s knockdown was performed in BMMs by siRNA transfection. Cells were further cultured with RANKL for 2 days. The level of NFATc1 protein expression was measured by Western blotting. D, control or TLT-1s-silenced BMMs were plated on dentin slices and cultured in the presence of RANKL for 5 days. The resorption pits were visualized by hematoxylin staining after removing the cells. The resorbed area was measured by densitometry.
FIGURE 4.
Retroviral overexpression of TLT-1s reduced the formation of osteoclasts. A, BMMs were infected with viruses harboring control (pMX) or TLT-1s (pMX-TLT-1s) constructs. Cells were further cultured with RANKL for 2 days and the protein level of TLT-1s was detected by Western blotting. B, TLT-1s virus-infected BMMs were differentiated with RANKL for 4 days before TRAP staining. The number of TRAP-positive multinuclear osteoclasts was counted. C, BMMs were first transfected with siRNA oligonucleotides for TLT-1s knockdown. On the next day, cells were infected with pMX- or pMX-TLT-1s-containing viruses. BMMs were further cultured with RANKL for 4 days before TRAP staining. D, the number of TRAP-positive osteoclasts in C was counted. *, p < 0.05 versus control.
Recruitment of SHIP and SHP-1 to TREM-2 by TLT-1s
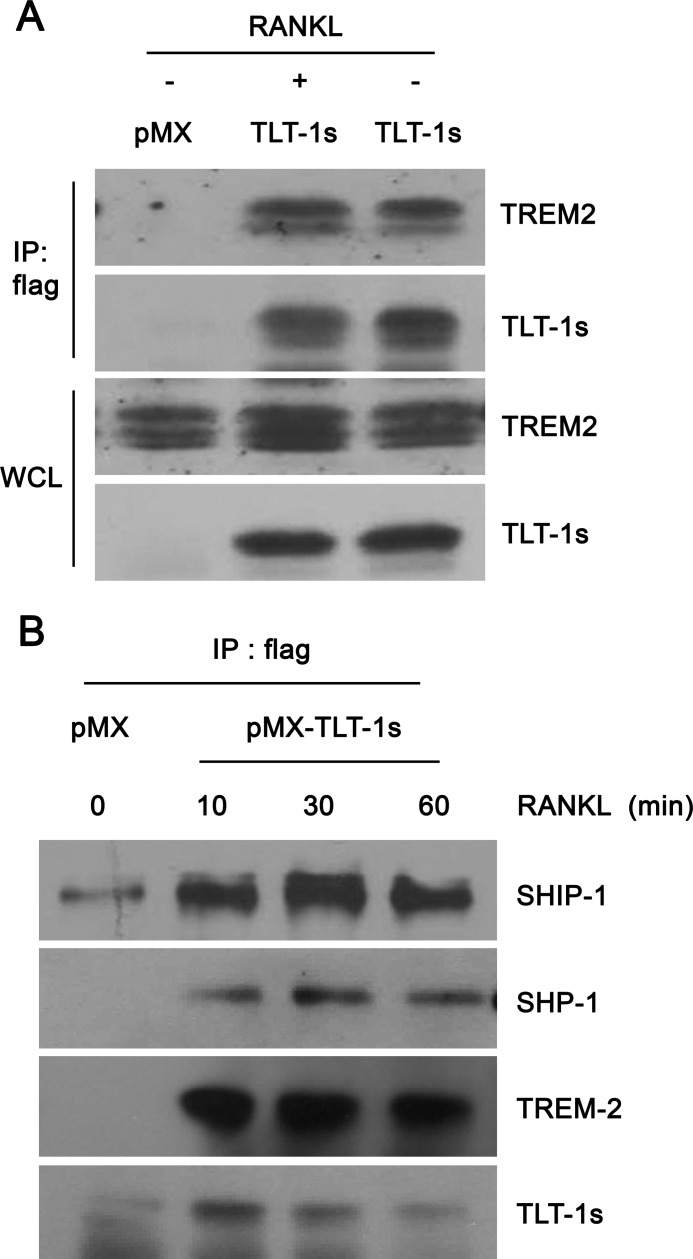
Co-stimulatory receptor signaling is critical for RANKL-mediated calcium oscillations and NFATc1 induction during osteoclastogenesis. Because TLT-1s contain ITIM, we asked whether TLT-1s inhibited osteoclast differentiation by affecting co-stimulatory receptor signaling in osteoclast precursors. Although TLT-1s did not associate with osteoclast-associated receptor (data not shown), FLAG-tagged TLT-1s co-immunoprecipitated with TREM-2 (Fig. 5A) independently of RANKL stimulation in BMMs. Notably, SHIP-1 and SHP-1 phosphatases co-immunoprecipitated with TLT-1s in association with TREM-2 (Fig. 5B) indicating that TLT-1s inhibited TREM-2-mediated signaling by recruiting SHP-1 and SHIP-1 to TREM-2 in osteoclast precursor cells.
FIGURE 5.
TLT-1s recruits SHP-1 and SHIP-1 phosphatases to TREM-2. A, BMMs were infected with viruses harboring empty vector (pMX) or FLAG-tagged TLT-1s (pMX-FLAG-TLT-1s) and further cultured with RANKL for 2 days. After serum starvation for 3 h, cells were stimulated with 200 ng/ml of RANKL for 30 min. The FLAG-tagged TLT-1s was immunoprecipitated using an anti-FLAG antibody. Immunoprecipitated (IP) proteins or whole cell lysates were subjected to Western blotting. B, control or TLT-1s-overexpressing BMMs were cultured with RANKL for 2 days. After serum starvation for 3 h, cells were stimulated with 200 ng/ml of RANKL for the indicated times. Whole cell lysates were subjected to immunoprecipitation using an anti-FLAG antibody followed by Western blotting.
Modulation of Calcium Oscillations by TLT-1s
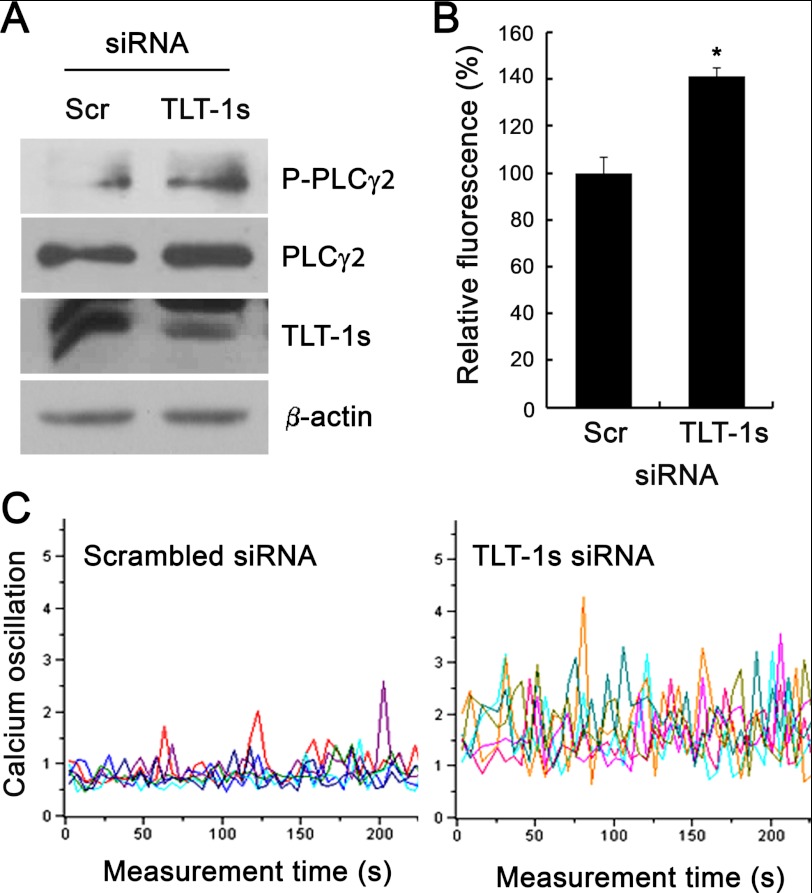
Because TREM-2 activation has been shown to facilitate PLCγ2-mediated calcium oscillations, the effect of TLT-1s knockdown on PLCγ2 activation and calcium oscillations was investigated. After TLT-1s knockdown, pre-osteoclasts were serum-starved and re-stimulated with RANKL. As shown in Fig. 6A, TLT-1s knockdown significantly elevated the RANKL-mediated PLCγ2 phosphorylation. In addition, the total intracellular calcium concentration was also elevated in TLT-1s-silenced pre-osteoclasts (Fig. 6B). Furthermore, the frequency and amplitude of RANKL-dependent calcium oscillations were also dramatically increased by TLT-1s knockdown (Fig. 6C). These results suggest that TLT-1s is a transmembrane adaptor molecule that regulates RANKL-mediated calcium oscillations.
FIGURE 6.
TLT-1s modulates calcium oscillations. BMMs were transfected with scrambled control siRNA or TLT-1s-siRNA. A, siRNA-transfected BMMs were incubated with RANKL for 2 days and the whole cell lysates were subjected to Western blotting. B, TLT-1s-silenced BMMs were seeded on 96-well plates, incubated with RANKL for 2 days, and loaded with Fluo-4 NW. The calcium-dependent fluorescence was measured using a plate reader. C, BMMs were plated on coverslips after TLT-1s knockdown. After incubation with RANKL for 2 day, cells were loaded with Fura-2/AM and the calcium oscillations were monitored using a confocal microscope. *, p < 0.05 versus control.
Modulation of Osteoclast Precursor Proliferation by TLT-1s
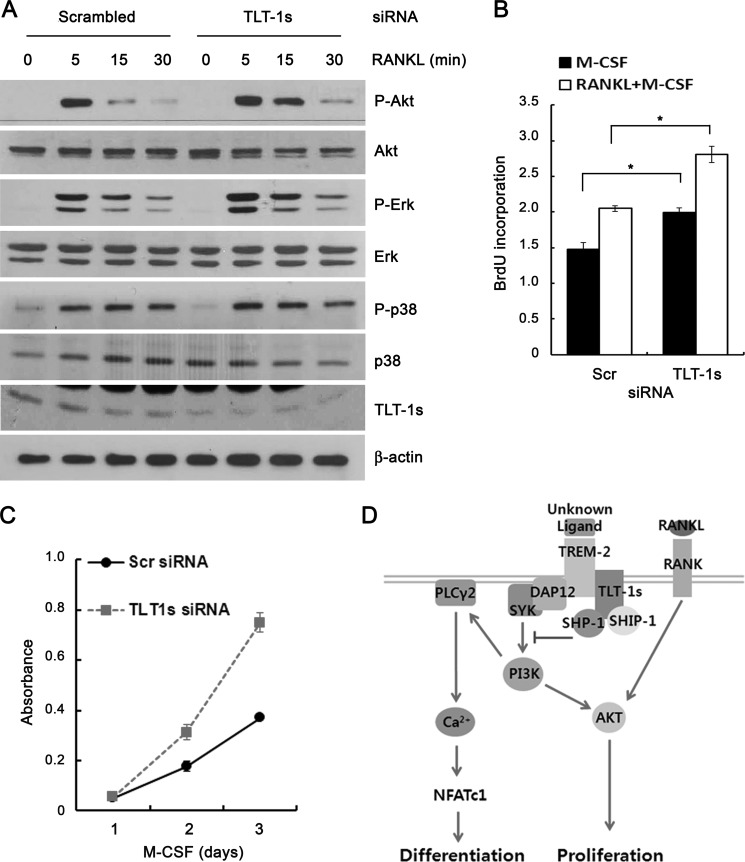
TREM-2 signaling has been shown to enhance the proliferation of osteoclast precursors via a phosphatidylinositol 3-kinase-mediated activation of Akt, the proliferation of osteoclast precursors was tested after TLT-1s knock-down. Fig. 7A showed that RANKL-induced Akt phosphorylation was significantly increased in TLT-1s-silenced BMMs. However, the phosphorylation of p38, ERK, and JNK was marginally altered by TLT-1s knockdown. The BrdU incorporation was significantly higher in BMMs treated with TLT-1s-siRNA following both M-CSF and M-CSF plus RANKL stimulation, compared with control cells treated with scrambled siRNA (Fig. 7B). The BrdU incorporation was consistently higher in TLT-1s-silenced BMMs over a 3-day culture with M-CSF stimulation (Fig. 7C). To summarize, we propose the following working model (Fig. 7D): RANKL and co-stimulatory receptor signaling evokes PLCγ2-mediated calcium oscillations, which induce NFATc1-dependent osteoclast differentiation. TLT-1s, an adaptor molecule that associates with TREM-2, inhibits osteoclastogenesis by recruiting SHP-1 and SHIP-1 phosphatases to the complex.
FIGURE 7.
TLT-1s inhibits the proliferation of osteoclast precursors. A, after TLT-1s-siRNA transfection, BMMs were serum starved for 3 h and stimulated with 200 ng/ml of RANKL for the indicated times. The phosphorylation of MAPKs was determined by Western blotting. Control cells were transfected with scrambled siRNA. B, after TLT-1s-siRNA transfection, BMMs were cultured with 30 ng/ml of M-CSF for 3 days. Cell proliferation was measured using CCK assay. C, after TLT-1s-siRNA transfection, BMMs were incubated with BrdU for 2 h. BrdU incorporation was determined by the ELISA method. D, upon ligation of RANK by RANKL, co-stimulatory receptors such as TREM-2 associate with ITAM-containing adaptor molecule including DAP12 to trigger intracellular calcium signaling that is critical for NFATc1 induction as well as proliferation signals through Akt activation. TLT-1s recruits SHP-1 and SHIP-1 phosphatases to TREM-2, thus negatively regulating osteoclast differentiation and proliferation. *, p < 0.05 versus control.
DISCUSSION
ITIM-containing receptors were identified by their ability to inhibit signaling by ITAM-bearing receptors. Such ITIM-bearing receptors have been known to regulate osteoclastogenesis via the recruitment of phosphatases to co-stimulatory receptors (16, 19). In the present report, we identified and characterized the novel ITIM-bearing alternative transcript TLT-1 designated as TLT-1s by 5′-RACE analysis in BMMs. TLT-1s has a distinct N-terminal sequence from intron 2 of the TLT-1 gene. TLT-1 and TLT-1s showed the different tissue distribution patterns. TLT-1 is expressed exclusively in platelets and megakaryocytes. Interestingly, the TLT-1s was not expressed in platelets. TLT-1s was expressed in macrophages and osteoclast only. Although further investigation is required, the possible use of an alternative promoter might have resulted in the tissue-specific expression of TLT-1s. Because primary calvarial osteoblasts expressed neither TLT-1 nor TLT-1s (data not shown), TLT-1s could be a specific therapeutic target for bone erosive disease such as osteoporosis induced by unregulated osteoclastogenesis.
TLT-1, mainly expressed in platelets and megakaryocytes, is the only inhibitory receptor of the TREM cluster. In contrast to the full-length TLT-1, TLT-1s has a very short extracellular Ig domain while containing an intact transmembrane domain and cytoplasmic ITIM domain. In this study, we found that TLT-1s inhibited TREM signaling in osteoclast precursor cells. Because the ITAM-mediated calcium signaling is crucial for osteoclastogenesis, mice deficient in both ITAM-signaling adaptors DAP12 and FcRγ are severely osteopetrotic because of the impaired formation of osteoclast (20). Both DAP12 and FcRγ associate with the surface receptor TREM-2 in the osteoclast, which is responsible for SYK kinase and PLCγ activation upstream of the calcium oscillations. On the other hand, ITIM-bearing adaptor molecules are known to inhibit ITAM signaling via a recruitment of phosphatases such as SHP-1, SHP-2, and SHIP proteins. SHP-1 and SHIP-1 knock-out mice are reported to exhibit severe osteoporosis because of a dramatically increased number of osteoclasts (21, 22). Because TLT-1s lacks the extracellular Ig domain compared with full-length TLT-1, we hypothesized that TLT-1s acts as a genuine adaptor molecule in a similar fashion with DAP12 and FcRγ that are also deficient in the extracellular domain. Immunoprecipitation experiments revealed that TLT-1s is associated with TREM-2, SHP-1, and SHIP-1. Indeed, the knockdown of TLT-1s induced strong calcium oscillations and subsequent NFATc1 induction. Interestingly, RANKL stimulation did not affect the binding affinity of TLT-1 and TREM-2, suggesting that TLT-1s regulates the basal TREM-2 signaling. TLT-1s seems to be regulated at the transcription level by RANKL. Both TLT-1s mRNA and protein expressions significantly decreased upon RANKL stimulation during the late stages of osteoclastogenesis, allowing TREM-2 signaling to play a key role in osteoclast differentiation.
In summary, we identified a novel ITIM-bearing membrane protein, TLT-1s. TLT-1s down-regulated the TREM-2-mediated ITAM signaling that is crucial for calcium oscillations by recruiting SHP-1 and SHIP-1 phosphatases to inhibit osteoclastogenesis. To our knowledge, this is the first report providing evidence for the regulation of osteoclastogenesis by TLT-1 family proteins. The TLT-1s-mediated negative regulatory mechanism of TREM-2 may be important to prevent excessive ITAM signaling, ultimately maintaining bone homeostasis.
Supplementary Material
This work was supported by 21C Frontier Functional Proteomics Project Grant FPR08B1-170 and Science Research Center Grant 2009-0063269.

This article contains supplemental Fig. S1.
- TREM
- triggering receptor expressed on myeloid cells
- ITAM
- immunoreceptor tyrosine-based activation motif
- DAP12
- DNAX activating protein of 12
- TLT-1
- TREM-like transcript-1
- ITIM
- immunoreceptor tyrosine-based inhibitory motif
- RANKL
- receptor activator of nuclear factor-κB ligand
- M-CSF
- macrophage-colony stimulating factor
- NFAT
- nuclear factor of activated T cell
- TRAP
- tartrate-resistant acid phosphatase
- PLC
- phospholipase C
- SH2
- Src homology 2
- SHP-1
- SH2 domain containing phosphatase-1
- SHIP-1
- SH2 domain-containing inositol phosphatase-1
- BMM
- bone marrow-derived macrophages
- 5′-RACE
- 5′-rapid amplification of cDNA ends
- WBM
- whole bone marrow cell.
REFERENCES
- 1. Bouchon A., Dietrich J., Colonna M. (2000) Cutting edge. Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995 [DOI] [PubMed] [Google Scholar]
- 2. Bleharski J. R., Kiessler V., Buonsanti C., Sieling P. A., Stenger S., Colonna M., Modlin R. L. (2003) A role for triggering receptor expressed on myeloid cells-1 in host defense during the early induced and adaptive phases of the immune response. J. Immunol. 170, 3812–3818 [DOI] [PubMed] [Google Scholar]
- 3. Chung D. H., Seaman W. E., Daws M. R. (2002) Characterization of TREM-3, an activating receptor on mouse macrophages. Definition of a family of single Ig domain receptors on mouse chromosome 17. Eur. J. Immunol. 32, 59–66 [DOI] [PubMed] [Google Scholar]
- 4. Klesney-Tait J., Turnbull I. R., Colonna M. (2006) The TREM receptor family and signal integration. Nat. Immunol. 7, 1266–1273 [DOI] [PubMed] [Google Scholar]
- 5. Colonna M. (2003) TREMs in the immune system and beyond. Nat. Rev. Immunol. 3, 445–453 [DOI] [PubMed] [Google Scholar]
- 6. Washington A. V., Quigley L., McVicar D. W. (2002) Initial characterization of TREM-like transcript (TLT)-1. A putative inhibitory receptor within the TREM cluster. Blood 100, 3822–3824 [DOI] [PubMed] [Google Scholar]
- 7. Washington A. V., Schubert R. L., Quigley L., Disipio T., Feltz R., Cho E. H., McVicar D. W. (2004) A TREM family member, TLT-1, is found exclusively in the α-granules of megakaryocytes and platelets. Blood 104, 1042–1047 [DOI] [PubMed] [Google Scholar]
- 8. Washington A. V., Gibot S., Acevedo I., Gattis J., Quigley L., Feltz R., De La Mota A., Schubert R. L., Gomez-Rodriguez J., Cheng J., Dutra A., Pak E., Chertov O., Rivera L., Morales J., Lubkowski J., Hunter R., Schwartzberg P. L., McVicar D. W. (2009) TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J. Clin. Invest. 119, 1489–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrow A. D., Astoul E., Floto A., Brooke G., Relou I. A., Jennings N. S., Smith K. G., Ouwehand W., Farndale R. W., Alexander D. R., Trowsdale J. (2004) Cutting edge. TREM-like transcript-1, a platelet immunoreceptor tyrosine-based inhibition motif encoding costimulatory immunoreceptor that enhances, rather than inhibits, calcium signaling via SHP-2. J. Immunol. 172, 5838–5842 [DOI] [PubMed] [Google Scholar]
- 10. Teitelbaum S. L. (2000) Bone resorption by osteoclasts. Science 289, 1504–1508 [DOI] [PubMed] [Google Scholar]
- 11. Koga T., Inui M., Inoue K., Kim S., Suematsu A., Kobayashi E., Iwata T., Ohnishi H., Matozaki T., Kodama T., Taniguchi T., Takayanagi H., Takai T. (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763 [DOI] [PubMed] [Google Scholar]
- 12. Mao D., Epple H., Uthgenannt B., Novack D. V., Faccio R. (2006) PLC-γ2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J. Clin. Invest. 116, 2869–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mócsai A., Humphrey M. B., Van Ziffle J. A., Hu Y., Burghardt A., Spusta S. C., Majumdar S., Lanier L. L., Lowell C. A., Nakamura M. C. (2004) The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. U.S.A. 101, 6158–6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barrow A. D., Trowsdale J. (2006) You say ITAM and I say ITIM, let's call the whole thing off. The ambiguity of immunoreceptor signaling. Eur. J. Immunol. 36, 1646–1653 [DOI] [PubMed] [Google Scholar]
- 15. Ravetch J. V., Lanier L. L. (2000) Immune inhibitory receptors. Science 290, 84–89 [DOI] [PubMed] [Google Scholar]
- 16. Mori Y., Tsuji S., Inui M., Sakamoto Y., Endo S., Ito Y., Fujimura S., Koga T., Nakamura A., Takayanagi H., Itoi E., Takai T. (2008) Inhibitory immunoglobulin-like receptors LILRB and PIR-B negatively regulate osteoclast development. J. Immunol. 181, 4742–4751 [DOI] [PubMed] [Google Scholar]
- 17. Kim H. J., Lee Y., Chang E. J., Kim H. M., Hong S. P., Lee Z. H., Ryu J., Kim H. H. (2007) Suppression of osteoclastogenesis by N,N-dimethyl-d-erythro-sphingosine. A sphingosine kinase inhibition-independent action. Mol. Pharmacol. 72, 418–428 [DOI] [PubMed] [Google Scholar]
- 18. Chang E. J., Ha J., Huang H., Kim H. J., Woo J. H., Lee Y., Lee Z. H., Kim J. H., Kim H. H. (2008) The JNK-dependent CaMK pathway restrains the reversion of committed cells during osteoclast differentiation. J. Cell Sci. 121, 2555–2564 [DOI] [PubMed] [Google Scholar]
- 19. Hayashi M., Nakashima T., Kodama T., Makrigiannis A. P., Toyama-Sorimachi N., Takayanagi H. (2010) Ly49Q, an ITIM-bearing NK receptor, positively regulates osteoclast differentiation. Biochem. Biophys. Res. Commun. 393, 432–438 [DOI] [PubMed] [Google Scholar]
- 20. Zou W., Zhu T., Craft C. S., Broekelmann T. J., Mecham R. P., Teitelbaum S. L. (2010) Cytoskeletal dysfunction dominates in DAP12-deficient osteoclasts. J. Cell Sci. 123, 2955–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bauler T. J., Kamiya N., Lapinski P. E., Langewisch E., Mishina Y., Wilkinson J. E., Feng G. S., King P. D. (2011) Development of severe skeletal defects in induced SHP-2-deficient adult mice. A model of skeletal malformation in humans with SHP-2 mutations. Dis. Model. Mech. 4, 228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aoki K., Didomenico E., Sims N. A., Mukhopadhyay K., Neff L., Houghton A., Amling M., Levy J. B., Horne W. C., Baron R. (1999) The tyrosine phosphatase SHP-1 is a negative regulator of osteoclastogenesis and osteoclast resorbing activity. Increased resorption and osteopenia in me(v)/me(v) mutant mice. Bone 25, 261–267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.