Background: The Escherichia coli ArfA peptide mediates alternative ribosome rescue.
Results: Proteobacterial arfA transcripts lack in-frame stop codons, leading to ssrA tagging and degradation of ArfA peptides.
Conclusion: ArfA synthesis from non-stop mRNA is conserved, ensuring that alternative rescue is regulated by tmRNA.
Significance: The alternative ribosome rescue pathway acts as a failsafe to the tmRNA system in bacteria that contain arfA homologues.
Keywords: Bacteria, RNA Synthesis, RNA Turnover, tmRNA, Translation Control, ArfA, RNase III, Ribosome Rescue, ssrA, tmRNA
Abstract
The translation of non-stop mRNA (which lack in-frame stop codons) represents a significant quality control problem for all organisms. In eubacteria, the transfer-messenger RNA (tmRNA) system facilitates recycling of stalled ribosomes from non-stop mRNA in a process termed trans-translation or ribosome rescue. During rescue, the nascent chain is tagged with the tmRNA-encoded ssrA peptide, which promotes polypeptide degradation after release from the stalled ribosome. Escherichia coli possesses an additional ribosome rescue pathway mediated by the ArfA peptide. The E. coli arfA message contains a hairpin structure that is cleaved by RNase III to produce a non-stop transcript. Therefore, ArfA levels are controlled by tmRNA through ssrA-peptide tagging and proteolysis. Here, we examine whether ArfA homologues from other bacteria are also regulated by RNase III and tmRNA. We searched 431 arfA coding sequences for mRNA secondary structures and found that 82.8% of the transcripts contain predicted hairpins in their 3′-coding regions. The arfA hairpins from Haemophilus influenzae, Proteus mirabilis, Vibrio fischeri, and Pasteurella multocida are all cleaved by RNase III as predicted, whereas the hairpin from Neisseria gonorrhoeae functions as an intrinsic transcription terminator to generate non-stop mRNA. Each ArfA homologue is ssrA-tagged and degraded when expressed in wild-type E. coli cells, but accumulates in mutants lacking tmRNA. Together, these findings show that ArfA synthesis from non-stop mRNA is a conserved mechanism to regulate the alternative ribosome rescue pathway. This strategy ensures that ArfA homologues are only deployed when the tmRNA system is incapacitated or overwhelmed by stalled ribosomes.
Introduction
Messenger RNAs that lack in-frame stop codons represent a significant molecular quality control problem for all organisms (1–4). Oftentimes, these “non-stop” messages are truncated transcripts produced by ribonuclease activity or premature transcription termination. Translation of non-stop mRNA leads to ribosome arrest at the 3′-end of the transcript because translation termination and ribosome recycling require an A-site stop codon. All eubacteria use the tmRNA·SmpB quality control system to deal with the consequences of non-stop mRNA translation (5–7). The core of the system is transfer-messenger RNA (tmRNA),2 which acts as both transfer RNA (tRNA) and messenger RNA (mRNA) to facilitate the recycling of stalled ribosomes (8). SmpB is a tmRNA-binding protein that coordinates mRNA function and is required for delivery to the ribosome (9–13). The tmRNA·SmpB complex binds the A site of stalled ribosomes by virtue of an alanine-charged tRNA-like domain within tmRNA. The nascent peptide chain is transferred to tmRNA and the truncated message is released from the ribosome. The ribosome then resumes translation using a small open reading frame found within tmRNA. This process results in the co-translational addition of the tmRNA-encoded ssrA peptide tag to the C terminus of the nascent chain. The ssrA coding sequence is terminated by a stop codon, allowing for normal translation termination and ribosome recycling. Once released from the ribosome, ssrA-tagged proteins are rapidly degraded by a variety of proteases (8, 14–16). Thus, the tmRNA system facilitates recycling of stalled ribosomes and promotes degradation of the associated incomplete nascent chains.
Although the tmRNA·SmpB system is completely conserved in the eubacteria, the ssrA gene encoding tmRNA is not essential for the viability of several species including Escherichia coli, Bacillus subtilis, and Caulobacter crescentus (17–19). These observations suggest that some bacteria possess an additional ribosome rescue system(s) that can compensate for the loss of tmRNA·SmpB activity. Indeed, Abo and colleagues (20) have recently discovered an alternative ribosome rescue system in E. coli. They reasoned that inactivation of a parallel ribosome rescue system would be synthetically lethal in a ΔssrA background and used a genetic screen to identify a mutation in the yhdL gene (20). The peptide encoded by yhdL promotes nascent chain release in S30 translation reactions, and based on this activity the gene has been renamed arfA for alternative ribosome rescue factor A (20). The discovery of alternative ribosome rescue explains why the tmRNA·SmpB system is not essential in E. coli, but recognizable arfA homologues are largely limited to a subset of β- and γ-proteobacteria. Presumably, other bacteria must also possess similar systems that mediate ribosome rescue in the absence of tmRNA·SmpB activity.
We have recently reported that ArfA synthesis is regulated by the tmRNA·SmpB system in E. coli (21). The 3′-coding region of the arfA transcript contains an extensive hairpin structure, which is cleaved efficiently by RNase III to generate non-stop mRNA. Translating ribosomes stall at the ends of the cleaved arfA transcripts, and ArfA nascent chains are ssrA-tagged by tmRNA·SmpB. Because the ssrA-tagged ArfA chains are rapidly degraded, the alternative rescue pathway is effectively suppressed. However, if the tmRNA·SmpB system is overwhelmed with stalled ribosomes, or if the ssrA (or smpB) gene is disrupted, then truncated ArfA chains are released from the ribosome and accumulate in the cell. Thus, the synthesis of ArfA from non-stop mRNA provides a mechanism to rapidly induce the alternative rescue pathway when the tmRNA·SmpB system is incapacitated in E. coli.
Here, we examine arfA sequences from selected β- and γ-proteobacteria to determine whether these homologues are also regulated by tmRNA·SmpB. Most arfA messages contain predicted hairpin structures in their 3′-coding regions, suggesting that these transcripts may be cleaved by RNase III. The arfA hairpins from Haemophilus influenzae (Rd), Pasteurella multocida (Pm70), Proteus mirabilis (HI4320), and Vibrio fischeri (ATCC 700601) are all cleaved in an RNase III-dependent manner when overproduced in E. coli cells. In contrast, the Neisseria gonorrhoeae (PID1) arfA hairpin is not cleaved by RNase III, but instead functions as an intrinsic transcription terminator to generate non-stop mRNA. Therefore, all of the examined ArfA peptides are synthesized from non-stop mRNA and as a consequence are tagged for degradation by the tmRNA·SmpB system. These findings suggest that ArfA synthesis is regulated by tmRNA·SmpB in most bacteria that contain the alternative ribosome rescue system.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Plasmids
The E. coli strains used in this study are presented in Table 1. The ΔarfA::kan (ΔyhdL::kan) gene deletion was obtained from the Keio collection and the Δrnc-38::kan allele was provided by Sydney Kushner (University of Georgia). Gene disruptions were transferred into derivatives of E. coli strain X90 using phage P1-mediated transduction. All E. coli strains were cultured at 37 °C with aeration in LB medium supplemented with the appropriate antibiotics (150 μg ml−1 of ampicillin and/or 12 μg ml−1 of tetracycline) to maintain plasmids. Cell cultures were seeded at an optical density at 600 nm (A600) of 0.05. After growth to A600 ∼ 0.6, plasmid-borne expression constructs were induced by addition of either isopropyl β-d-1-thiogalactopyranoside (1.5 mm final concentration) or l-arabinose (0.2% w/v). For the in vivo RNase III experiments, the rnc(Eco) and rnc(Ngo) constructs were induced with arabinose for 30 min prior to induction of arfA expression with isopropyl β-d-1-thiogalactopyranoside. All cells were collected after 30 min of induction and RNA or protein were extracted for analysis.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or descriptiona | Reference |
|---|---|---|
| Strains | ||
| X90 | F′ lacIq lac′ pro′/ara Δ(lac-pro) nal1 argE(amb) rifr thi-1 | 42 |
| CH12 | X90 (DE3) | 35 |
| CH113 | X90 (DE3) ssrA::cat, CmR | 35 |
| CH2016 | CH12 Δrna ΔslyD::kan, KanR | 43 |
| CH2017 | CH113 ΔslyD::kan, KanR | 21 |
| CH3574 | CH113 Δrnc-38::kan, KanR | 21 |
| CH4514 | CH12 ΔslyD::kan, KanR | 21 |
| CH4516 | X90 (DE3) ssrA (his6) ΔslyD::kan, Kan R | 21 |
| CH7189 | CH12 ΔarfA::kan, KanR | 21 |
| CH7330 | X90 (DE3) ΔarfA::kan ssrA::cat pCH450-arfA(Eco), KanR CmR TetR | This study |
| CH8230 | CH12 Δrnc-38::kan, KanR | 21 |
| CH8674 | CH113 ΔarfA::kan pCH450-arfA(Ngo), CmR KanR TetR | This study |
| CH8676 | CH113 ΔarfA::kan pCH450-arfA(Hin), CmR KanR TetR | This study |
| Plasmids | ||
| pCH201 | pACYC184 derivative that expresses tmRNA (His6), TetR | 33 |
| pET-his6-arfA | pET21 derivative that overexpresses E. coli his6-arfA, AmpR | 21 |
| pCH450-arfA | Arabinose-inducible expression of E. coli ArfA under control of PBAD promoter, TetR | 21 |
| pET-his6-arfA(Ngo) | pET21 derivative that overexpresses E. coli his6-arfA, AmpR | This study |
| pCH450-arfA(Ngo) | Arabinose-inducible expression of N. gonorrhoeae ArfA under control of PBAD promoter, TetR | This study |
| pCH450-arfA(Hin) | Arabinose-inducible expression of H. influenzae ArfA under control of PBAD promoter, TetR | This study |
| pFG21-trxA-arfA′(Eco) | pET21 derivative that overexpresses flag-trxA fused to codons Gly49--Ser69 of E. coli arfA, AmpR | This study |
| pFG21-trxA-arfA′(recode) | pET21 derivative that overexpresses flag-trxA fused to synonymously recoded Gly49--Ser69 of E. coli arfA, AmpR | This study |
| pFG21-trxA-arfA′(Vfi) | pET21 derivative that overexpresses flag-trxA fused to codons Lys58--Thr65 (and 38 nt from the 3′-UTR) of V. fischeri arfA, AmpR | This study |
| pFG21-trxA-arfA′(Pmu) | pET21 derivative over-expresses flag-trxA fused to codons Glu61--Leu79 of P. multocida arfA, AmpR | This study |
| pFG21-trxA-arfA(Eco) | pET21 derivative that overexpresses flag-trxA fused to codons Ser2--Cys72 of E. coli arfA, AmpR | This study |
| pFG21-trxA-arfA(Ngo) | pET21 derivative that overexpresses flag-trxA fused to codons Gly2--Leu73 of N. gonorrhoeae arfA, AmpR | This study |
| pFG21-trxA-arfA(Hin) | pET21 derivative that over-expresses flag-trxA fused to codons Arg2--Phe69 of H. influenzae arfA, AmpR | This study |
| pFG21-trxA-arfA(Pmi) | pET21 derivative that overexpresses flag-trxA fused to codons Ser2--Phe67 of P. mirabilis arfA, AmpR | This study |
| pCH450-flag-arfA | Arabinose-inducible expression of FLAG-ArfA(Eco) under control of PBAD promoter, TetR | 21 |
| pCH450-flag-arfA(Ngo) | Arabinose-inducible expression of FLAG-ArfA(Ngo) under control of PBAD promoter, TetR | This study |
| pCH450-flag-arfA(Ngo-fs) | Arabinose-inducible expression of FLAG-ArfA(Ngo) from gene with a frameshift in the hairpin coding sequence, TetR | This study |
| pFG501-trxA-arfA(Eco) | IPTG-inducible expression of FLAG-TrxA-ArfA(Eco) under control of Ptrc promoter, AmpR | This study |
| pFG501-trxA-arfA(Ngo) | IPTG-inducible expression of FLAG-TrxA-ArfA(Ngo) under control of Ptrc promoter, AmpR | This study |
| pFG501-trxA-arfA(Ngo-fs) | IPTG-inducible expression of FLAG-TrxA-ArfA(Ngo) from gene with a frameshift in the hairpin coding sequence, AmpR | This study |
| pTrc99A-flag-arfA(Eco) | IPTG-inducible expression of FLAG-ArfA(Eco) under control of Ptrc promoter, AmpR | This study |
| pTrc99A-flag-arfA(recode) | IPTG-inducible expression of FLAG-ArfA(Eco) from recoded construct under control of Ptrc promoter, AmpR | This study |
| pTrc99A-flag-arfA(Ngo) | IPTG-inducible expression of FLAG-ArfA(Ngo) under control of Ptrc promoter, AmpR | This study |
| pET21P-his6-rnc(Eco) | pET21 derivative for overproduction of E. coli RNase III carrying an N-terminal His6 tag, AmpR | This study |
| pET21P-his6-rnc(Ngo) | pET21 derivative for overproduction of N. gonorrhoeae RNase III carrying an N-terminal His6 tag, AmpR | This study |
| pCH450-his6-rnc(Ngo) | Arabinose-inducible expression of N. gonorrhoeae RNase III carrying an N-terminal His6 tag, TetR | This study |
a The abbreviations used are: AmpR, ampicillin resistant; CmR, chloramphenicol resistant; KanR, kanamycin resistant; TetR, tetracycline resistant; UTR, untranslated region.
The plasmids used in this study are presented in Table 1. The rnc(Ngo), arfA(Ngo), and arfA(Hin) coding sequences were synthesized as pUC57 clones with 5′-sequences that encode His6 peptide epitopes (GenScript, sequences available upon request). The his6-arfA(Ngo) coding sequence was excised from pUC57-his6-arfA(Ngo) by NcoI/XhoI digestion and ligated to NcoI/XhoI-digested pFG21b (22) to generate plasmid pET-his6-arfA(Ngo). The arfA(Ngo) gene (lacking the his6 sequence) was amplified using Ngo-yhdL-Nco-for (5′-ACA CCA TGG GCG GCA AAG CGC AGC) and Ngo-yhdL-Xho-rev (5′-AAA CTC GAG GAA GGT TTC AAA T), followed by digestion with NcoI/XhoI (restriction endonuclease sites underlined) and ligation to plasmid pCH450 to generate an l-arabinose inducible expression construct. Similarly, the arfA(Hin) gene was amplified with Hin-yhdL-Eco-for (5′-GGA GGA ATT CAC TAT GAG AAA GAA ACA AAA AAG TGC) and Hin-yhdL-Xho-rev (5′-AAA CTC GAG AAA AAC CCG ATT), and ligated to plasmid pCH450 using EcoI and XhoI restriction sites. The flag-trxA fusion to the E. coli arfA hairpin was amplified from plasmid pFG501-trxA-(hairpin)-trxA (21) using primers lacI-Spe (5′-TAT CCC GCC GTT AAC TAG TAT CAA ACA GGA TTT TCG C) and arfA′-Xho-rev (5′-GCT CTC GAG ATT CCC TTA ATT TGA AAG CAG AAG ACC), and the product was digested with NcoI/XhoI and ligated into pFG21P to generate plasmid pFG21-trxA-arfA′(Eco). Similarly, the flag-trxA fusion to recoded arfA(Eco) was amplified from plasmid pFG501-trxA-(recode)-trxA (21) using primers lacI-Spe and arfA′(recode)-Xho-rev (5′-GCT CTC GAG ATT CCC TTA ATT ACT CAA CAA CAA CC), followed by digestion with NcoI/XhoI and ligation into pFG21P to generate plasmid pFG21-trxA-arfA′(recode). Fusions of the arfA(Vfi) and arfA(Pmu) hairpin sequences to flag-trxA were generated by sequential PCR. Plasmid pFG21-trxA-arfA′(Eco) was used as a template for PCR with primer pET-Sph/Pst (5′-CAA GGA ATG GTG CAT GCC TGC AGA TGG CGC CC) in conjunction with either Vibr-arfA-BamI (5′-CTC AAC ATT CTT ATG TTG CTT TTG AAT AGG GCT CTT TTT GGA TCC CCG CCA GG) or Past-arfA-BamI (5′-AAT CTT TCA AAT CAA AAA TCT TAT AAT CGG GCT TTT CTT GGA TCC CCG CCA GG). The resulting PCR products were used as templates for subsequent amplifications using pET-Sph/Pst and Vibr-arfA-Xho2 (5′-GTG CTC GAG ATT CCC TTA AAA AAA AGC CCT ATT CAA ACT CAA CAT TCT TAT GT) or Past-arfA-Xho2 (5′-GTG CTC GAG ATT CCC TTA TAA GAA AAA CCC GAT TAT AAA ATC TTT CAA ATC AA). The final products were digested with NcoI/XhoI and ligated to pFG21b to generate plasmids pFG21-trxA-arfA′(Vfi) and pFG21-trxA-arfA′(Pmu).
The full-length arfA coding sequences from E. coli, H. influenzae, P. mirabilis, and N. gonorrhoeae were amplified using primer pairs Eco-arfA-Bam-for (5′-TTT GGA TCC AAA GTC GAT ATC AGC ATA CTA A)/arfA-Xho (5′-AAA CTC GAG ATT TGC TGA AAG AGC AG), Hin-arfA-Bam-for (5′-TTT GGA TCC AAA GAA AGA AAC AAA AAA GTG C)/Hin-yhdL-Xho-rev (5′-AAA CTC GAG AAA AAC CCG ATT), Pmir-arfA-Bam-for (5′-TTT GGA TCC AAT CTA GCA AAT ATC AGC ACC)/Pmira-yhdL-Xho (5′-AGA AAC TCG AGA TCT ATT AAA AAA TCA GC), and Ngo-arfA-Bam-for (5′-TTT GGA TCC AAG GCG GCA AAG CGC AGC ACA A)/Ngo-yhdL-Xho-rev (5′-AAA CTC GAG GAA GGT TTC AAA T), and the resulting products were ligated to plasmid pFG21-trxA-arfA′(Eco) using the BamHI and XhoI restriction sites. The arabinose-inducible FLAG-ArfA(Ngo) expression construct was generated through subcloning the SpeI/XhoI fragment from pUC57-arfA(Ngo) into SpeI/XhoI-digested plasmid pCH450-flag-arfA (21). The flag-arfA(Eco), flag-arfA(recode), and flag-arfA(Ngo) coding sequences were also subcloned into plasmid pTrc99A (GE Healthcare) using NcoI and PstI restriction sites to produce templates for E. coli RNA polymerase transcription reactions. Nucleotide A131 (in codon Glu44) was deleted from the arfA(Ngo) coding sequence by megaprimer PCR using oligonucleotide Ngo-arfA-fs (5′-GCT ACA ACA GGC AGG AGC GAA AAA AC) to generate a construct with a frameshifted coding sequence in the hairpin structure. The rnc(Ngo) coding sequence was removed from plasmid pUC57-rnc(Ngo) and subcloned into SpeI/XhoI-digested plasmid pET-his6-arfA (21) to generate plasmid pET-his6-rnc(Ngo). The his6-rnc(Ngo) coding sequence was then subcloned into plasmid pCH450 using NcoI/XhoI digestion to generate the arabinose-inducible pCH450-his6-rnc(Ngo) construct. The E. coli rnc gene was amplified using rnc-Spe-for (5′-TGG ACT AGT ATG AAC CCC ATC GTA ATT AAT CGG C)/rnc-Xho (5′-AAT CTC GAG TAA CTT TTA TCG ATG CTC ATT CCA GC), followed by digestion with SpeI/XhoI and ligation to SpeI/XhoI-digested pET-his6-arfA.
Computational Analysis of arfA Structures
431 predicted ArfA homologues were selected based on their annotation as Pfam PF03889/DUF331 family members in the Sanger Institute Pfam data base. The corresponding coding sequences were collected from GenBankTM together with 50 nucleotides of downstream 3′-noncoding sequence. Each arfA sequence was scanned for stable secondary structures using UNAfold (23) with a sliding 50-nucleotide test window. Adjacent windows containing predicted structures of ΔG <−20 kcal/mol were selected and consolidated for output. Sequences without apparent structures were re-analyzed with a less stringent energy threshold of <−18 kcal/mol.
RNA Analysis
RNA isolation, Northern blot analyses, and T7 RNA polymerase transcription reactions were performed as described previously (24). Oligonucleotides pET-rbs (5′-GTA TAT CTC CTT CTT AAA GTT AAA C), pBAD-probe, (5′-GAA TTC CTC CTG CTA GCC CA AAA AAC G), and pTrc-SD (5′-CAT GGT CTG TTT CCT GTG TGA AAT TG) were radiolabeled using T4 polynucleotide kinase and used as Northern blot hybridization probes for arfA-containing transcripts expressed from pET, pBAD, and pTrc-based plasmids, respectively. Additionally, oligonucleotides arfA(Ngo) L61-S69-probe (5′-GCT GCG GCG AGA GCT TCC ACG TTT AAG) and arfA(Ngo) S39-K46-probe (5′-TTT CGC TTC CTG CCT GTT GTA GCT), which hybridize to the Leu61–Ser69 and Ser39–Lys46 coding sequences of arfA(Ngo), respectively, were used to estimate the 3′-end of the arfA(Ngo) transcript produced from PBAD. In vitro T7 transcription templates for the arfA(Eco) and arfA(Ngo) messages were amplified from plasmids pET-his6-arfA and pET-his6-arfA(Ngo), respectively, using primer pET-Sph/Pst in conjunction with arfA-Xho (5′-AAA CTC GAG ATT TGC TGA AAG AGC AG) and Ngo-yhdL-Xho-rev (5′-AAA CTC GAG GAA GGT TTC AAA T). The flag-arfA(Eco), flag-arfA(recode), and flag-arfA(Ngo) templates were amplified from pTrc plasmid clones with primers lacI-Spe and Trc-rev seq (5′-CGT TCT GAT TTA ATC TGT ATC AGG C), and the resulting products were used for in vitro transcription reactions with E. coli RNA polymerase holoenzyme (Epicenter). Purified template DNA (0.5 μg) was transcribed with 2.0 units of E. coli holoenzyme in 40 mm Tris-HCl (pH 7.6), 150 mm KCl, 10 mm MgCl2, 0.01% TritonTM X-100, 1 mm rNTPs at 37 °C for 1 h. Reactions were quenched and extracted with guanidine isothiocyanate/phenol as described (25), and analyzed by Northern blot hybridization using radiolabeled oligonucleotide pTrc-SD as a probe. The percentage of terminated transcripts was determined from phosphorimager data using the Quantity One (Bio-Rad) software package.
Protein Analysis
After induction for 30–90 min, cells were collected by centrifugation and frozen at −80 °C. Frozen cell pellets were resuspended in urea lysis buffer (8 m urea, 150 mm NaCl, 10 mm Tris-HCl (pH 8.0)) and subjected to an additional freeze-thaw cycle to facilitate cell lysis. Whole cell lysates were clarified by centrifugation and analyzed by Western blot using monoclonal anti-FLAG antibody as described (26). SsrA(His6)-tagged ArfA fusion proteins were extracted from frozen E. coli ssrA(his6) cell pellets with guanidine lysis buffer (6 m guanidinium HCl, 10 mm Tris-HCl (pH 8.0)), and purified by Ni2+-nitrilotriacetic acid (NTA)-agarose affinity chromatography. SsrA(His6)-tagged proteins were purified further by reverse-phase high-performance liquid chromatography (HPLC) as described (25). HPLC fractions were then concentrated by SpeedVac, reconstituted in aqueous 50% acetonitrile, 1% formic acid and injected directly into a Waters Q-TOF IITM mass spectrometer. Mass data were processed using MassLynx analytical software.
RNase III Purification and in Vitro Activity Assays
N-terminal His6-tagged RNase III from E. coli and N. gonorrhoeae was overproduced in strain CH2016 using plasmids pET21P-his-rnc(Eco) and pET21P-his-rnc(Ngo), respectively. Cells were grown A600 ∼ 1.0 in LB medium supplemented with 150 μg ml−1 of ampicillin, RNase III synthesis was induced with 1.5 mm isopropyl β-d-1-thiogalactopyranoside, and cells were incubated for an additional 2 h. Cells were then harvested over ice, collected by centrifugation, and frozen at −80 °C. Frozen cell pellets were resuspended in urea lysis buffer (8 m urea, 150 mm NaCl, 10 mm Tris-HCl (pH 8.0)) and subjected to one freeze-thaw cycle to facilitate cell lysis. Cell lysates were clarified by centrifugation at 30,000 × g for 15 min, and the supernatants were applied to a Ni2+-NTA-agarose column. The column was washed with 10 volumes of urea lysis buffer supplemented with 20 mm imidazole, and His6-RNase III was eluted in urea lysis buffer supplemented with 250 mm imidazole. Fractions were pooled and dialyzed against RNase III storage buffer (1 m NaCl, 60 mm Tris-HCl (pH 7.9), 1 mm EDTA, 1 mm DTT). The dialysates were adjusted to 50% glycerol and stored at −20 °C. The his6-arfA(Eco) and his6-arfA(Ngo) transcripts (2.0 μg) were digested for 30 min at 37 °C with purified RNase III (50 nm) in reaction buffer (30 mm Tris-HCl (pH 8.0), 160 mm NaCl, 10 mm MgCl2, 0.1 mm EDTA, 0.1 mm DTT) supplemented with 10 μg of total E. coli RNA. Reactions were quenched and extracted with guanidine isothiocyanate/phenol, then analyzed by Northern blot hybridization using radiolabeled oligonucleotide pET-rbs as a probe.
RESULTS
ArfA Peptides from H. influenzae and N. gonorrhoeae Mediate Alternative Ribosome Rescue
H. influenzae and N. gonorrhoeae contain recognizable arfA homologues, yet the tmRNA·SmpB system is essential for the viability of both species (27, 28). These observations suggest that the alternative ribosome rescue pathway may be defective in these bacteria. To determine whether the arfA(Hin) and arfA(Ngo) genes (from H. influenzae and N. gonorrhoeae, respectively) function in ribosome rescue, we tested their ability to complement an arfA disruption in E. coli. Chadani et al. (20) have shown that loss of both ssrA (encoding tmRNA) and arfA is synthetically lethal to E. coli cells. Therefore, we cloned the arfA(Eco), arfA(Hin), and arfA(Ngo) open reading frames under control of the arabinose-inducible PBAD promoter and introduced the plasmids into E. coli ssrA::cat cells. The resulting strains were then transduced with the ΔarfA::kan gene disruption in the presence of l-arabinose to induce plasmid-borne arfA expression. Each transduction produced several dozen kanamycin-resistant clones (data not shown), suggesting that the arfA(Hin) and arfA(Ngo) genes can rescue the viability of E. coli ssrA arfA double mutants. Serial dilution of the transductants onto LB-agar supplemented with either l-arabinose or d-glucose showed that cell growth was related to induction of the arfA genes (Fig. 1). E. coli ssrA::cat ΔarfA::kan cells carrying the arfA(Ngo) gene required l-arabinose for growth, whereas the strains carrying the arfA(Eco) and arfA(Hin) genes showed some growth in the presence of d-glucose (Fig. 1). Presumably, leaky expression from the PBAD promoter allowed limited growth in the latter instances. These results indicate that ArfA(Hin) and ArfA(Ngo) support alternative ribosome rescue in E. coli cells, suggesting that the peptides perform the same function in H. influenzae and N. gonorrhoeae.
FIGURE 1.
The arfA(Ngo) and arfA(Hin) genes support alternative ribosome rescue in E. coli. E. coli ΔarfA ssrA::cat cells carrying plasmid-borne arfA genes from E. coli K-12 (Eco), N. gonorrhoeae PID1 (Ngo), or H. influenzae Rd (Hin) under control of an arabinose-inducible promoter were spotted onto selective media containing l-arabinose to induce or d-glucose to repress arfA expression. Cells were adjusted to an optical density at 600 nm of 1.0 and then plated as a 10-fold dilution series.
Most arfA Genes Contain Predicted Hairpin Structures in their 3′-Coding Sequences
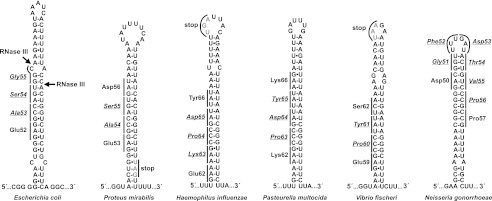
Like E. coli, the arfA messages from Salmonella enterica LT2 and Dickeya dadantii 3937 also contain hairpin structures that are cleaved by RNase III (21). To determine whether hairpins are a general feature of arfA transcripts, we used the UNAfold algorithm to search the coding sequences of 431 putative ArfA homologs (annotated as Pfam PF03889/DUF331 family members) for local secondary structure elements. 406 (94.2%) of the arfA sequences contain at least one predicted structure with ΔG <−20 kcal/mol (supplemental Table S1 and Fig. S1). Notably, structures were only detected in a subset of arfA(Hin) sequences from H. influenzae strains. Therefore, we repeated the UNAfold search with a higher energy threshold (ΔG <−18 kcal/mol) and identified structures in the remaining eight arfA(Hin) sequences. These latter arfA(Hin) genes contain a thymidylate nucleotide insertion that expands the hairpin loop and increases the ΔG from −21.8 to −18.8 kcal/mol (supplemental Table S1 and Fig. S1). This insertion also creates a UGA stop codon, resulting in a predicted ArfA peptide of 69 residues rather than 81 residues in the other H. influenzae strains (supplemental Table S1). The lower stringency search also identified structures in five additional arfA sequences, bringing the total number of transcripts with predicted structural elements to 419 of 431 (97.2%).
Although RNase III generally acts upon double-stranded RNAs of ∼20 bp, cleavage sites cannot be predicted with certainty because there is no consensus recognition sequence (29, 30). Our previous work shows that RNase III cleaves arfA hairpins with as few as 18 bp, and that the enzyme tolerates single-stranded bulges in the helix (21). Therefore, we identified possible RNase III substrates by screening the predicted secondary structures for hairpins with co-axial helices of at least 18 bp and no more than two bulges. 357 of the 463 predicted structures contain hairpins that meet these criteria, but conspicuously, the structures from most Pseudomonas and Shewanella species fail to pass the screen (supplemental Table S2). Notably, no arfA transcript contains more than one putative RNase III cleavage site (supplemental Table S2), and all of the hairpins are located just downstream of the sequence coding for the conserved KGKGS(Y/F)XR peptide motif (supplemental Fig. S2). Thus, 82.8% of the examined arfA transcripts contain a possible RNase III cleavage site at the same relative position, but the nucleotide and corresponding peptide sequence of this region is not conserved compared with the upstream coding region (Fig. 2 and supplemental Fig. S2). These observations suggest that maintenance of a stable secondary structure in the arfA 3′-coding region is more important than the encoded peptide sequence.
FIGURE 2.
Predicted arfA hairpin structures. The predicted hairpins found in the 3′-coding region of arfA genes from E. coli K-12, P. mirabilis HI4320, H. influenzae Rd, P. multocida Pm70, V. fischeri ATCC 700601, and N. gonorrhoeae PID1 are depicted. All structures were determined using UNAfold. The stop codons for P. multocida, H. influenzae, and V. fischeri arfA transcripts are indicated and rendered in gray. The stop codons for the other messages are found downstream of the predicted hairpin structures. The underlined/italicized amino acid residues are ssrA-tagged by the tmRNA·SmpB system. Arrows indicate the RNase III cleavage sites identified within the arfA(Eco) hairpin (21).
RNase III Cleaves arfA Transcripts from γ-Proteobacteria
The analysis described above suggests that many arfA transcripts are cleaved by RNase III to generate non-stop mRNA. Therefore, we tested a subset of arfA sequences for RNase III cleavage. Each arfA sequence was fused in-frame to a flag-trxA expression construct, and flag-trxA-arfA transcripts produced in E. coli cells lacking RNase III (Δrnc) were compared with transcripts produced in wild-type cells (rnc+). As a positive control, we first examined a flag-trxA fusion to the arfA′(Eco) hairpin sequence (Fig. 3A), which encodes residues Gly49–Ser69 of the E. coli ArfA peptide. Northern blot analysis showed that truncated flag-trxA-arfA′(Eco) message was the main product in rnc+ cells, whereas full-length transcripts accumulated in the Δrnc background (Fig. 3B). These results are consistent with RNase III-mediated cleavage within the arfA(Eco) hairpin. Disruption of the ssrA gene (encoding tmRNA) appeared to stabilize truncated mRNA in the rnc+ background (Fig. 3B), presumably because translating ribosomes stall at the 3′-ends of the cleaved transcripts thereby protecting them from exoribonuclease activity (24, 31). Additionally, a negative control construct containing a synonymously recoded arfA′(recode) sequence produced full-length transcripts in both rnc+ and Δrnc backgrounds (Fig. 3B), demonstrating that the hairpin structure is required for cleavage (21). We then extended this analysis to arfA hairpins from V. fischeri (Vfi) and P. multocida (Pmu) (Fig. 2) and found that truncated arfA′(Pmu) and arfA′(Vfi) fusion transcripts accumulated in E. coli rnc+ cells, but the full-length message predominated in the Δrnc backgrounds (Fig. 3B). These findings strongly suggest that RNase III cleaves the arfA′(Eco), arfA′(Pmu), and arfA′(Vfi) sequences in the same manner.
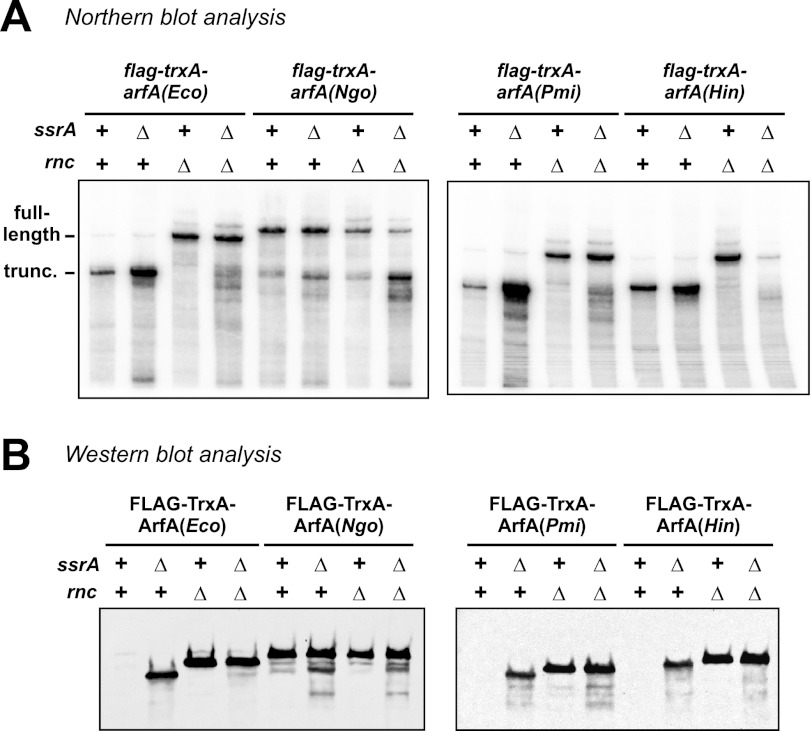
FIGURE 3.
arfA transcripts are truncated in an rnc-dependent manner. A, schematic for flag-trxA-arfA′ expression constructs. Sequences encoding the predicted arfA hairpin structures from E. coli (Eco), V. fischeri (Vfi), and P. multocida (Pmu) were fused in-frame to a flag-trxA gene under control of the bacteriophage T7 promoter. The arfA′(recode) construct is synonymously recoded to disrupt the predicted hairpin structure. The binding site for the Northern blot hybridization probe and the position of the T7 transcription terminator are indicated. B, Northern blot analysis of flag-trxA-arfA′ transcripts. Each expression construct was induced in the indicated rnc and ssrA backgrounds and total RNA analyzed by Northern blot using an oligonucleotide probe that hybridizes to the 5′-noncoding region of each transcript. The gel migration positions of full-length and truncated transcripts are indicated. C, Western blot analysis of FLAG-TrxA-ArfA′ proteins. FLAG-TrxA-ArfA′ fusion proteins produced in the indicated rnc and ssrA genetic backgrounds were analyzed by immunoblot using monoclonal antibodies to the N-terminal FLAG peptide epitope.
RNase III cleavage within the arfA′ hairpins should produce non-stop mRNA, and consequently the encoded fusion proteins should be ssrA-tagged and degraded in wild-type cells. We examined each fusion protein by immunoblot analysis against the N-terminal FLAG epitope to determine whether tmRNA·SmpB activity influences their levels. As predicted, the FLAG-TrxA-ArfA′(Eco) fusion was not detected in rnc+ ssrA+ cells but accumulated to high levels in ssrA mutant cells (Fig. 3C), presumably because untagged nascent chains are released from the ribosome by the ArfA pathway in the absence of tmRNA·SmpB (8, 21). Moreover, full-length fusion protein was produced in the Δrnc backgrounds (Fig. 3C), consistent with the translation of intact coding sequences in these cells. Additionally, full-length FLAG-TrxA-ArfA′(Eco) protein accumulated to similar levels in all genetic backgrounds when produced from the recoded construct lacking the hairpin structure (Fig. 3C). Immunoblot analysis of the FLAG-TrxA-ArfA′(Vfi) and FLAG-TrxA-ArfA′(Pmu) proteins showed essentially the same results as the E. coli fusion (Fig. 3C), suggesting that each protein is efficiently tagged by tmRNA·SmpB for degradation.
We also analyzed arfA genes from N. gonorrhoeae (Ngo), P. mirabilis (Pmi), and H. influenzae (Hin). In these experiments, we fused the entire arfA coding sequence in-frame to the flag-trxA module. As expected, fusion transcripts containing arfA(Pmi) and arfA(Hin) were cleaved in an rnc-dependent manner like the control transcript containing the full arfA(Eco) coding sequence (Fig. 4A). However, the arfA(Ngo) construct produced similar transcripts in rnc+ and Δrnc backgrounds (Fig. 4A), suggesting this message is not cleaved efficiently by RNase III. Immunoblot analysis of the corresponding fusion proteins showed that FLAG-TrxA-ArfA(Pmi) and FLAG-TrxA-ArfA(Hin) levels are regulated in the same manner as FLAG-TrxA-ArfA(Eco); however, the full-length FLAG-TrxA-ArfA(Ngo) protein was produced at approximately the same levels in all backgrounds (Fig. 4B). Together, these results indicate that the examined arfA transcripts from γ-proteobacteria are converted into non-stop mRNA by RNase III, whereas the β-proteobacterial arfA(Ngo) message is resistant to this cleavage.
FIGURE 4.
Full-length arfA(Ngo) transcript accumulates in rnc+ cells. A, Northern blot analysis of flag-trxA-arfA transcripts. Fusion constructs carrying the full-length arfA coding sequences from E. coli (Eco), N. gonorrhoeae (Ngo), P. mirabilis (Pmi), and H. influenzae (Hin) were induced from T7 promoters in the indicated rnc and ssrA genetic backgrounds, and total RNA was analyzed by Northern blot using an oligonucleotide probe that hybridizes to the 5′-noncoding region of each transcript. The gel migration positions of full-length and truncated transcripts are indicated. B, Western blot analysis of FLAG-TrxA-ArfA proteins. FLAG-TrxA-ArfA fusion proteins produced in the indicated rnc and ssrA genetic backgrounds were analyzed by Western blot using monoclonal antibodies to the N-terminal FLAG peptide epitope.
ArfA Fusion Proteins Are ssrA-tagged at Positions Encoded within the Hairpin Structure
Presumably, the γ-proteobacterial ArfA fusion proteins do not accumulate in wild-type (rnc+ ssrA+) cells because they are tagged with ssrA peptides and rapidly degraded. Therefore, we sought to identify the predicted ssrA tagging sites by overproducing ArfA fusions in E. coli cells that express tmRNA(His6). tmRNA(His6) encodes the modified ssrA(His6) peptide tag, which is resistant to proteolysis and allows rapid purification of tagged proteins (25, 32, 33). SsrA(His6)-tagged proteins were purified by Ni2+-affinity chromatography and the peptide-tagging sites were identified by mass spectrometry. As predicted, each fusion protein was tagged at positions encoded by the ascending stem of the arfA hairpin structure (Table 2 and Fig. 2). Moreover, we were unable to detect tagging at these sites in ArfA fusions purified from E. coli Δrnc ssrA(his6) cells (data not shown), strongly suggesting that RNase III is required for this tagging activity. We also analyzed the FLAG-TrxA-ArfA(Ngo) fusion and found that it was tagged, although at much lower efficiency than the γ-proteobacterial ArfA fusions (data not shown). In contrast to the other fusion proteins, FLAG-TrxA-ArfA(Ngo) was ssrA(His6) tagged at positions encoded by the loop and descending stem of the arfA(Ngo) hairpin (Table 2 and Fig. 2). These findings support a model in which the accumulation of γ-proteobacterial ArfA is suppressed by tmRNA·SmpB-mediated peptide tagging activity.
TABLE 2.
Identification of ssrA(His6) peptide tagging sites
FLAG-TrxA-ArfA fusion proteins were overproduced in E. coli rnc+ ssrA(his6) cells, and ssrA(His6)-tagged proteins purified for identification by electrospray ionization-mass spectrometry.
| Fusion construct | ssrA(His6)-tagged ArfA residuea | Observed mass | Predicted mass |
|---|---|---|---|
| Da | |||
| flag-trxA-arfA′(Eco) | Ala53 | 15,234 | 15,235.11 |
| Ser54 | 15,321 | 15,322.19 | |
| Gly55 | 15,378 | 15,379.24 | |
| flag-trxA-arfA′(Vfi) | Pro60 | 15,031 | 15,031.96 |
| Tyr61 | 15,194 | 15,195.13 | |
| Ala64 | 15,480 | 15,481.46 | |
| Thr65 | 15,582 | 15,582.57 | |
| flag-trxA-arfA′(Pmu) | Pro63 | 15,031 | 15,031.96 |
| Asp64 | 15,147 | 15,147.05 | |
| Tyr65 | 15,308 | 15,310.22 | |
| flag-trxA-arfA(Hin) | Glu62 | 21,754 | 21,755.86 |
| Lys63 | 21,882 | 21,884.04 | |
| Pro64 | 21,979 | 21,981.15 | |
| Asp65 | 22,094 | 22,096.24 | |
| flag-trxA-arfA(Pmi) | Ala54 | 20,808 | 20,809.62 |
| Ser55 | 20,895 | 20,896.69 | |
| flag-trxA-arfA(Eco)b | Ala53 | 20,737 | 20,737.43 |
| Ser54 | 20,823 | 20,824.5 | |
| flag-trxA-arfA(Ngo)b | Gly51 | 20,416 | 20,419.17 |
| Phe52 | 20,563 | 20,566.35 | |
| Asp53 | 20,679 | 20,681.43 | |
| Val55 | 20,881 | 20,881.67 | |
| Pro56 | 20,978 | 20,978.79 | |
a Residue numbers correspond to their positions in the native ArfA proteins and residues in bold represent the major species detected by mass spectrometry.
b These constructs were transcribed by E. coli RNA polymerase from the Ptrc promoter.
RNase III Does Not Cleave the arfA(Ngo) Transcript
The results presented thus far suggest that the arfA(Ngo) transcript is not an RNase III substrate. However, the preceding experiments were performed in E. coli cells, raising the question of whether Neisserial RNase III is required for arfA(Ngo) cleavage. Although RNase III is highly conserved, alignment of the N. gonorrhoeae and N. meningitidis enzyme sequences with those from γ-proteobacteria shows some sequence divergence in the C-terminal double-stranded RNA binding domain (supplemental Fig. S3). Additionally, Neisserial RNase III carries a lysine-rich C-terminal tail that is not present in the γ-proteobacterial enzymes (supplemental Fig. S3). Therefore, we asked whether RNase III from N. gonorrhoeae is able to cleave the arfA(Ngo) transcript. We cloned the rnc(Ngo) gene under the control of the PBAD promoter and assayed the cleavage of arfA transcripts produced from a compatible plasmid. We first produced RNase III(Ngo) in E. coli Δrnc cells and found that the Neisserial enzyme readily cleaved flag-trxA-arfA(Eco) mRNA to produce truncated transcripts that were indistinguishable from those produced in E. coli rnc+ cells (Fig. 5A). This result demonstrates that RNase III(Ngo) is functional in E. coli and recognizes the arfA(Eco) hairpin structure. We next tested whether RNase III(Ngo) is able to cleave the arfA(Ngo) hairpin in E. coli cells. Although Northern blot analysis revealed a prominent truncated flag-trxA-arfA(Ngo) transcript in all genetic backgrounds, its levels were not appreciably affected by the induction of RNase III(Ngo) (Fig. 5B). Thus, the production of this truncated transcript is not dependent on RNase III activity. We also purified N-terminal His6-tagged versions of RNase III from both E. coli and N. gonorrhoeae and tested their activities on arfA(Eco) and arfA(Ngo) transcripts. Both RNase III(Eco) and RNase III(Ngo) enzymes readily cleaved the arfA(Eco) transcript in vitro (Fig. 6). However, the arfA(Ngo) transcript was only partially cleaved by RNase III(Eco) and appeared to be even more resistant to RNase III(Ngo) activity (Fig. 6). Taken together, these data indicate that RNase III from N. gonorrhoeae and E. coli have similar specificities, and that the arfA(Ngo) hairpin is not a substrate for either enzyme.
FIGURE 5.
RNase III activity on arfA transcripts in vivo. A, Northern blot analysis of his6-arfA(Eco) transcripts. The his6-arfA(Eco) construct was induced the T7 promoter in the indicated rnc and ssrA genetic backgrounds, and total RNA was analyzed by Northern blot using an oligonucleotide probe that hybridizes to the 5′-noncoding region. All cell lines also carried a plasmid-borne copy of the rnc(Ngo) gene under control of the arabinose-inducible PBAD promoter. Induction of rnc(Ngo) expression with arabinose is indicated by plus (+) symbols. The gel migration positions of full-length and truncated his6-arfA(Eco) transcripts are indicated. B, Northern blot analysis of his6-arfA(Ngo) transcripts. The his6-arfA(Ngo) construct was induced in the same cell lines described in panel A, and total RNA was analyzed by Northern blot using an oligonucleotide probe that hybridizes to the 5′-noncoding region. Control in vitro transcripts were also run on the gel to mark the migration positions of full-length message and transcript truncated after the codon for Ser66 of ArfA(Ngo).
FIGURE 6.
The arfA(Ngo) transcript is resistant to RNase III cleavage in vitro. The arfA(Eco) and arfA(Ngo) transcripts were synthesized by in vitro transcription using phage T7 RNA polymerase, mixed with total RNA from E. coli, and digested with purified RNase III from E. coli or N. gonorrhoeae. arfA transcripts were detected by Northern hybridization using an oligonucleotide probe that hybridizes to a common sequence in the 5′-noncoding region of both transcripts.
Synthesis of ArfA(Ngo) Is Regulated by tmRNA·SmpB
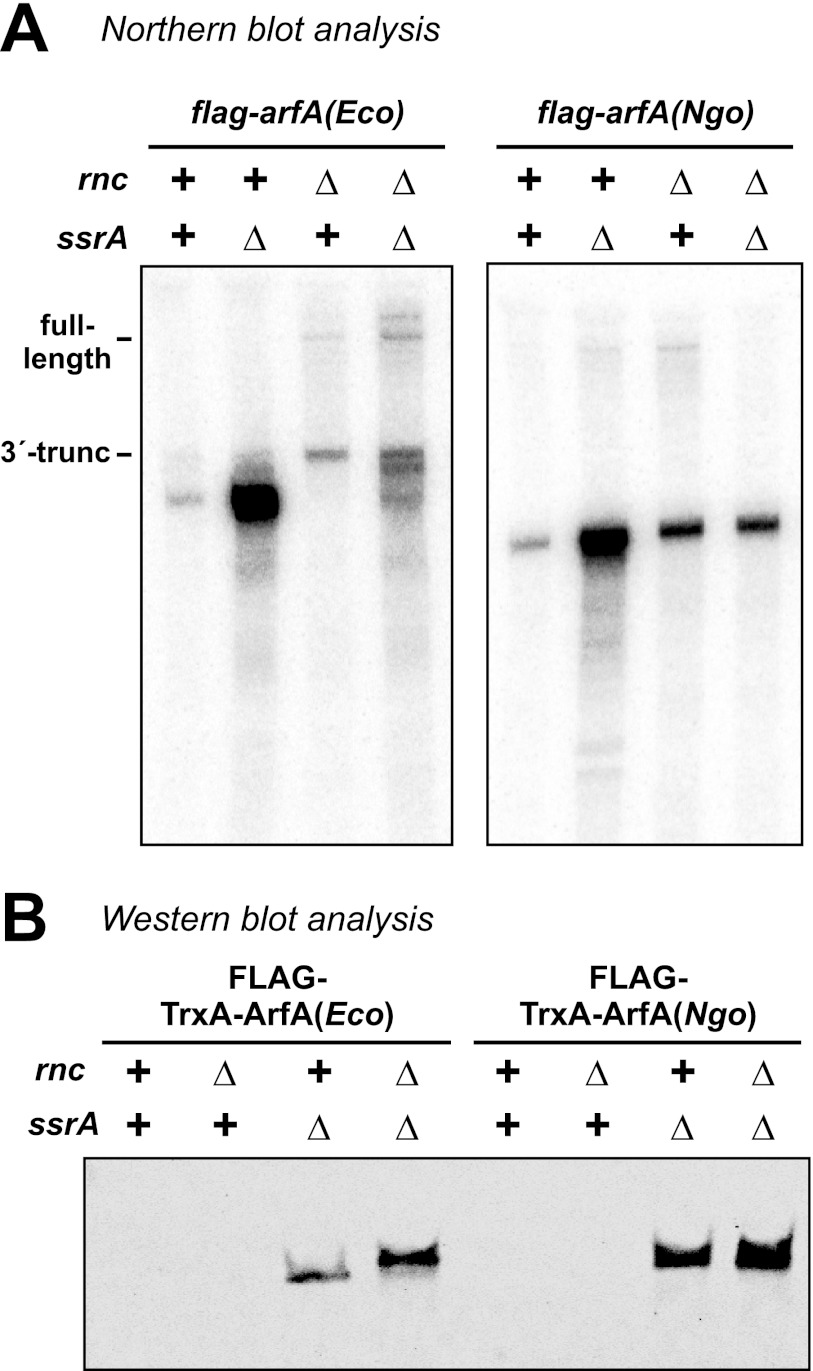
Because the arfA(Ngo) message is not cleaved by RNase III, ArfA(Ngo) synthesis is probably not regulated in precisely the same manner as the γ-proteobacterial peptides. However, a fraction of flag-trxA-arfA(Ngo) message is truncated in an RNase III-independent manner (Figs. 4A and 5B), and the FLAG-TrxA-ArfA(Ngo) protein is ssrA-tagged at a low but detectable level (Table 2). We hypothesized that the arfA(Ngo) hairpin could function as an intrinsic transcription terminator to generate low levels of non-stop mRNA. We reasoned that this termination activity was inefficient in the previously described experiments because the transcripts were produced by phage T7 RNA polymerase (RNAP), which transcribes through intrinsic terminators more readily than E. coli RNAP (34). Therefore, we re-examined flag-arfA(Eco) and flag-arfA(Ngo) transcripts synthesized by E. coli RNAP from the PBAD promoter. As expected, larger flag-arfA(Eco) transcripts accumulated in the Δrnc background compared with rnc+ cells (Fig. 7A). Although some full-length flag-arfA(Eco) transcript was detected in Δrnc cells, most products were truncated at the 3′-end of the arfA(Eco) hairpin structure (Fig. 7A). These results are consistent with our previous work showing that the arfA(Eco) transcript is degraded to the base of the hairpin by 3′ to 5′ exoribonucleases (21). In contrast, the same sized flag-arfA(Ngo) transcript was produced by E. coli RNAP regardless of rnc and/or ssrA background (Fig. 7A and supplemental Fig. S4B). This product is significantly smaller than the predicted full-length transcript, and Northern analysis using a probe to the Leu61–Ser69 coding region immediately downstream of the hairpin failed to detect this transcript (supplemental Fig. S4B). Together, these data indicate that non-stop flag-arfA(Ngo) message is produced by E. coli RNAP, but RNase III is not required to generate this truncated transcript.
FIGURE 7.
ArfA(Ngo) synthesis is regulated by tmRNA·SmpB activity. A, Northern blot analysis of flag-arfA transcripts produced by E. coli RNAP. The flag-arfA(Eco) and flag-arfA(Ngo) constructs were induced from the PBAD promoter in the indicated rnc and ssrA genetic backgrounds, and total RNA was analyzed by Northern blot using an oligonucleotide probe that hybridizes to a common 5′-noncoding region. The migration positions of full-length and 3′-truncated flag-arfA(Eco) transcripts are indicated. B, Western blot analysis of FLAG-TrxA-ArfA proteins. FLAG-TrxA-ArfA production from the Ptrc promoter and proteins were detected by immunoblot using monoclonal antibodies specific for the N-terminal FLAG peptide epitope.
If E. coli RNAP produces non-stop arfA(Ngo) transcripts, then the encoded ArfA(Ngo) peptides should be tagged and degraded in ssrA+ cells. We used immunoblot analysis against the N-terminal FLAG epitope to examine FLAG-TrxA-ArfA(Eco) and FLAG-TrxA-ArfA(Ngo) fusion protein levels when expressed from the Ptrc promoter in rnc and/or ssrA backgrounds. As previously reported (21), we were unable to detect FLAG-TrxA-ArfA(Eco) expressed from E. coli RNAP in ssrA+ cells regardless of rnc background (Fig. 7B). Presumably, the processing of flag-arfA(Eco) transcripts by 3′ to 5′ exoribonucleases provides an RNase III-independent mechanism to generate non-stop message (21). However, RNase III activity on the arfA(Eco) hairpin could be detected indirectly in ΔssrA cells, because a larger FLAG-TrxA-ArfA(Eco) protein is produced in the Δrnc background compared with rnc+ (Fig. 7B). In accord with the production of non-stop arfA(Ngo) transcripts by E. coli RNAP, we found that FLAG-TrxA-ArfA(Ngo) was also undetectable in ssrA+ cells (Fig. 7B), suggesting these nascent chains were tagged by tmRNA·SmpB for degradation. However, similar sized FLAG-TrxA-ArfA(Ngo) proteins were produced regardless of rnc background (Fig. 7B). Taken together, these results indicate that ArfA(Ngo) peptide levels are regulated by tmRNA·SmpB activity in an RNase III independent manner.
We note that the ArfA(Ngo) peptide contains a Pro-Pro sequence near the ssrA(His6) tagging sites (Fig. 2 and Table 2). Under some circumstances, Pro-Pro nascent peptide sequences can pause translation and induce mRNA cleavage (24, 35), so we tested whether this motif plays a role in arfA(Ngo) transcript cleavage. We deleted an adenylate residue from codon Glu44 of arfA(Ngo) to produce a modified transcript that retains the predicted hairpin structure but encodes an alternative, frameshifted peptide sequence (supplemental Fig. S4A). This frameshift construct produced transcripts that were indistinguishable from the wild-type construct (supplemental Fig. S4C), indicating that the translational reading frame does not influence transcript processing. Moreover, the FLAG-TrxA-ArfA(Ngo) protein produced from a frameshift construct only accumulated in cells lacking tmRNA and was the same size as protein produced from the wild-type construct (supplemental Fig. S4D). Together, these results indicate that the Pro-Pro peptide motif is not required for ssrA tagging of ArfA(Ngo).
The arfA(Ngo) Hairpin Functions as an Intrinsic Terminator
Finally, to determine whether the arfA(Ngo) hairpin does indeed function as an intrinsic transcription terminator to generate non-stop mRNA, we performed in vitro transcription experiments using E. coli RNAP holoenzyme. Control reactions programmed with an arfA(Eco) template produced a prominent termination product (54% of total transcript), whereas the recoded arfA(Eco) template produced a predominately full-length transcript (11% truncated) (Fig. 8). This high level of in vitro termination activity is somewhat surprising because the arfA(Eco) hairpin does not terminate transcription when expressed from its chromosomal locus in vivo (21). Termination was even more efficient (77% truncated product) in reactions programmed with the arfA(Ngo) template (Fig. 8). Comparison of the truncated arfA(Ngo) message with control transcripts synthesized with phage T7 RNAP indicated that termination occurs upstream of the codon for Ser66 (Fig. 8), confirming that the transcript is truncated within the coding sequence and hence lacks an in-frame stop codon.
FIGURE 8.
The arfA(Ngo) hairpin is an intrinsic transcription terminator. E. coli RNA polymerase holoenzyme in vitro transcription reactions were programmed with linear templates containing arfA genes under control of the Ptrc promoter. Reactions were analyzed by Northern blot hybridization using an oligonucleotide probe that hybridizes to a common sequence in the 5′-noncoding region of all transcripts. Transcription termination efficiency was ∼77, 54, and 11% for arfA(Ngo), arfA(Eco), and arfA(recode) templates, respectively. Control arfA(Ngo) transcripts were also produced with phage T7 RNA polymerase to indicate the gel migration positions of full-length and truncated (at codon Ser66) message.
DISCUSSION
Although the tmRNA·SmpB ribosome rescue system is ubiquitous in the eubacteria, the alternative rescue pathway is narrowly distributed with most recognizable arfA homologues found in a subset of β- and γ-proteobacteria (Neisseriales, Enterobacteriales, Pasteurellales, Vibrionales, Pseudomonadales, and Alteromonadales). There are also isolated instances of arfA genes in firmicutes (Clostridium carboxidivorans, Bacillus thuringiensis, and Paenibacillus polymyxa) and an α-proteobacterium (Candidatus pelagibacter), but the functional importance of these homologues is unclear because closely related bacteria lack these genes. The presence of arfA in H. influenzae and N. gonorrhoeae is also somewhat surprising because tmRNA·SmpB is essential for the viability of these species (27, 28) implying the absence of alternative ribosome rescue pathways. Our results indicate that ArfA(Hin) and ArfA(Ngo) mediate ribosome rescue in E. coli cells, so presumably these peptides are functional when expressed in their native backgrounds. One possible explanation for these observations is that H. influenzae and N. gonorrhoeae contain stalled ribosome complexes that are resistant to ArfA-mediated rescue. In accord with this hypothesis, we have found that E. coli ArfA is unable to release nascent chains from ribosomes stalled on full-length mRNAs.3 We also note that the recently described YaeJ ribosome rescue system is not found in either H. influenzae or N. gonorrhoeae. YaeJ is a truncated class I release factor that lacks the domain responsible for stop codon recognition (36). Nameki and Abo (37, 38) have independently shown that YaeJ releases nascent chains from stalled ribosomes in E. coli, so perhaps the absence of YaeJ accounts for the inviability of H. influenzae and N. gonorrhoeae ssrA mutants.
Each of the ArfA homologues examined here is translated from non-stop mRNA, suggesting that the regulation of ArfA levels by tmRNA·SmpB is conserved. The arfA transcripts from γ-proteobacteria (E. coli, P. mirabilis, P. multocida, H. influenzae, and V. fischeri) are all cleaved by RNase III to generate non-stop mRNA. RNase III only acts on double-stranded RNA, and accordingly each of these arfA messages contains a predicted hairpin structure of ∼22 bp in the 3′-coding region. The arfA hairpins exhibit only limited primary sequence conservation and the peptides encoded by these structures are divergent (21). This variation is consistent with the lack of a defined consensus site for RNase III cleavage (30), and suggests selection for double-stranded RNA structure rather than a particular peptide sequence. Indeed, our results show that the 3′-coding regions of arfA genes are not translated in wild-type cells, so these peptide sequences have no opportunity to exert biochemical activities. Thus, it appears that RNase III cleavage sites are retained solely to ensure that ArfA peptides are synthesized from non-stop mRNA. This arrangement subjects the alternative ribosome rescue pathway to direct regulation by tmRNA·SmpB and provides a mechanism to rapidly deploy ArfA in response to decreased tmRNA·SmpB rescue activity.
The synthesis of N. gonorrhoeae ArfA is also regulated by tmRNA·SmpB, but the corresponding transcript is not cleaved by RNase III. Instead, the arfA(Ngo) hairpin structure functions as an intrinsic transcription terminator to generate non-stop mRNA. This same strategy was exploited by Keiler et al. (8) to first demonstrate the trans-translation model for tmRNA function. In that work, the E. coli trp attenuator-terminator sequence was fused to a reporter gene to produce transcripts with no in-frame stop codons (8). The only other known example of a natural non-stop transcript produced by an intrinsic terminator sequence is the kinA mRNA from B. subtilis ATCC 6051 (39). However, the physiological significance of this transcript is unclear because the ancestral stop codon in kinA has clearly acquired a point mutation that is not found in other B. subtilis strains. In contrast, the arfA(Ngo) terminator appears to play a critical role regulating protein synthesis, making it the first biologically relevant non-stop transcription unit to be described. Given that intrinsic terminator sequences are sufficient to produce non-stop mRNA, it is not clear why the γ-proteobacterial arfA transcripts have retained RNase III cleavage sites. In fact, the E. coli arfA hairpin is able to terminate E. coli RNAP transcription in vitro, although not as efficiently as the arfA(Ngo) structure. However, we have shown previously that the hairpin does not terminate transcription of the chromosomal arfA locus in E. coli cells (21). This apparent discrepancy may be due to transcription elongation factors, which promote RNA synthesis through transcription pause sites (40, 41), but are missing from the in vitro transcription reactions. Moreover, RNase III is not strictly required to produce non-stop arfA transcripts in E. coli. In the absence of RNase III, 3′ to 5′ exoribonucleases degrade the arfA message to the 3′-end of the hairpin stem, thereby removing the stop codon (21). Perhaps the selective advantage of regulated ArfA expression has led to the evolution of multiple, redundant mechanisms to generate non-stop arfA transcripts. From this perspective, the maintenance of RNase III cleavage sites may be a low-cost and effective strategy to produce truncated mRNA. This reasoning also suggests that other unidentified ribosome rescue systems will be regulated by tmRNA·SmpB in some fashion. If this hypothesis is correct, then it should be possible to identify new ribosome rescue systems by searching for genes that contain either RNase III cleavage sites or transcription terminators within their coding regions.
Supplementary Material
Acknowledgments
We thank Sarah Abdul-Wajid for performing preliminary experiments for this study. Mass spectrometry was performed at the Mass Spectrometry Facility in the Department of Chemistry and Biochemistry, University of California, Santa Barbara.
This work was supported, in whole or in part, by National Institutes of Health Grant GM078634.

This article contains supplemental Figs. S1–S4 and Tables S1 and S2.
F. Garza-Sánchez and C. S. Hayes, unpublished results.
- tmRNA
- transfer-messenger RNA
- Ni2+-NTA
- nickel-nitrilotriacetic acid
- A600
- optical density at 600 nm
- RNAP
- RNA polymerase.
REFERENCES
- 1. Doma M. K., Parker R. (2006) Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440, 561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hayes C. S., Keiler K. C. (2010) Beyond ribosome rescue. TmRNA and co-translational processes. FEBS Lett. 584, 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pisareva V. P., Skabkin M. A., Hellen C. U., Pestova T. V., Pisarev A. V. (2011) Dissociation by Pelota, Hbs1, and ABCE1 of mammalian vacant 80 S ribosomes and stalled elongation complexes. EMBO J. 30, 1804–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shoemaker C. J., Eyler D. E., Green R. (2010) Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science 330, 369–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore S. D., Sauer R. T. (2007) The tmRNA system for translational surveillance and ribosome rescue. Annu. Rev. Biochem. 76, 101–124 [DOI] [PubMed] [Google Scholar]
- 6. Barends S., Kraal B., van Wezel G. P. (2011) The tmRNA-tagging mechanism and the control of gene expression. A review. Wiley Interdiscip. Rev. RNA 2, 233–246 [DOI] [PubMed] [Google Scholar]
- 7. Janssen B. D., Hayes C. S. (2012) The tmRNA ribosome-rescue system. Adv. Protein Chem. Struct. Biol. 86, 151–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keiler K. C., Waller P. R., Sauer R. T. (1996) Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science 271, 990–993 [DOI] [PubMed] [Google Scholar]
- 9. Karzai A. W., Susskind M. M., Sauer R. T. (1999) SmpB, a unique RNA-binding protein essential for the peptide-tagging activity of SsrA (tmRNA). EMBO J. 18, 3793–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sundermeier T. R., Dulebohn D. P., Cho H. J., Karzai A. W. (2005) A previously uncharacterized role for small protein B (SmpB) in transfer messenger RNA-mediated trans-translation. Proc. Natl. Acad. Sci. U.S.A. 102, 2316–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sundermeier T. R., Karzai A. W. (2007) Functional SmpB-ribosome interactions require tmRNA. J. Biol. Chem. 282, 34779–34786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacob Y., Sharkady S. M., Bhardwaj K., Sanda A., Williams K. P. (2005) Function of the SmpB tail in transfer-messenger RNA translation revealed by a nucleus-encoded form. J. Biol. Chem. 280, 5503–5509 [DOI] [PubMed] [Google Scholar]
- 13. Felden B., Gillet R. (2011) SmpB as the handyman of tmRNA during trans-translation. RNA Biol. 8, 440–449 [DOI] [PubMed] [Google Scholar]
- 14. Choy J. S., Aung L. L., Karzai A. W. (2007) Lon protease degrades transfer-messenger RNA-tagged proteins. J. Bacteriol. 189, 6564–6571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gottesman S., Roche E., Zhou Y., Sauer R. T. (1998) The ClpXP and ClpAP proteases degrade proteins with carboxyl-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 12, 1338–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herman C., Thévenet D., Bouloc P., Walker G. C., D'Ari R. (1998) Degradation of carboxyl-terminal-tagged cytoplasmic proteins by the Escherichia coli protease HflB (FtsH). Genes Dev. 12, 1348–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oh B. K., Apirion D. (1991) 10Sa RNA, a small stable RNA of Escherichia coli, is functional. Mol. Gen. Genet. 229, 52–56 [DOI] [PubMed] [Google Scholar]
- 18. Wiegert T., Schumann W. (2001) SsrA-mediated tagging in Bacillus subtilis. J. Bacteriol. 183, 3885–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keiler K. C., Shapiro L. (2003) TmRNA is required for correct timing of DNA replication in Caulobacter crescentus. J. Bacteriol. 185, 573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chadani Y., Ono K., Ozawa S., Takahashi Y., Takai K., Nanamiya H., Tozawa Y., Kutsukake K., Abo T. (2010) Ribosome rescue by Escherichia coli ArfA (YhdL) in the absence of the trans-translation system. Mol. Microbiol. 78, 796–808 [DOI] [PubMed] [Google Scholar]
- 21. Garza-Sánchez F., Schaub R. E., Janssen B. D., Hayes C. S. (2011) TmRNA regulates synthesis of the ArfA ribosome rescue factor. Mol. Microbiol. 80, 1204–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garza-Sánchez F., Gin J. G., Hayes C. S. (2008) Amino acid starvation and colicin D treatment induce A-site mRNA cleavage in Escherichia coli. J. Mol. Biol. 378, 505–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Markham N. R., Zuker M. (2008) UNAFold, software for nucleic acid folding and hybridization. Methods Mol. Biol. 453, 3–31 [DOI] [PubMed] [Google Scholar]
- 24. Hayes C. S., Sauer R. T. (2003) Cleavage of the A site mRNA codon during ribosome pausing provides a mechanism for translational quality control. Mol. Cell 12, 903–911 [DOI] [PubMed] [Google Scholar]
- 25. Garza-Sánchez F., Janssen B. D., Hayes C. S. (2006) Prolyl-tRNA(Pro) in the A-site of SecM-arrested ribosomes inhibits the recruitment of transfer-messenger RNA. J. Biol. Chem. 281, 34258–34268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janssen B. D., Hayes C. S. (2009) Kinetics of paused ribosome recycling in Escherichia coli. J. Mol. Biol. 394, 251–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang C., Wolfgang M. C., Withey J., Koomey M., Friedman D. I. (2000) Charged tmRNA but not tmRNA-mediated proteolysis is essential for Neisseria gonorrhoeae viability. EMBO J. 19, 1098–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akerley B. J., Rubin E. J., Novick V. L., Amaya K., Judson N., Mekalanos J. J. (2002) A genome-scale analysis for identification of genes required for growth or survival of Haemophilus influenzae. Proc. Natl. Acad. Sci. U.S.A. 99, 966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pertzev A. V., Nicholson A. W. (2006) Characterization of RNA sequence determinants and antideterminants of processing reactivity for a minimal substrate of Escherichia coli ribonuclease III. Nucleic Acids Res. 34, 3708–3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang K., Nicholson A. W. (1997) Regulation of ribonuclease III processing by double-helical sequence antideterminants. Proc. Natl. Acad. Sci. U.S.A. 94, 13437–13441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamamoto Y., Sunohara T., Jojima K., Inada T., Aiba H. (2003) SsrA-mediated trans-translation plays a role in mRNA quality control by facilitating degradation of truncated mRNAs. RNA 9, 408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roche E. D., Sauer R. T. (2001) Identification of endogenous SsrA-tagged proteins reveals tagging at positions corresponding to stop codons. J. Biol. Chem. 276, 28509–28515 [DOI] [PubMed] [Google Scholar]
- 33. Hayes C. S., Bose B., Sauer R. T. (2002) Stop codons preceded by rare arginine codons are efficient determinants of SsrA tagging in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 99, 3440–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Studier F. W., Moffatt B. A. (1986) Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189, 113–130 [DOI] [PubMed] [Google Scholar]
- 35. Hayes C. S., Bose B., Sauer R. T. (2002) Proline residues at the C terminus of nascent chains induce SsrA tagging during translation termination. J. Biol. Chem. 277, 33825–33832 [DOI] [PubMed] [Google Scholar]
- 36. Baranov P. V., Vestergaard B., Hamelryck T., Gesteland R. F., Nyborg J., Atkins J. F. (2006) Diverse bacterial genomes encode an operon of two genes, one of which is an unusual class-I release factor that potentially recognizes atypical mRNA signals other than normal stop codons. Biol. Direct 1, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chadani Y., Ono K., Kutsukake K., Abo T. (2011) Escherichia coli YaeJ protein mediates a novel ribosome-rescue pathway distinct from SsrA- and ArfA-mediated pathways. Mol. Microbiol. 80, 772–785 [DOI] [PubMed] [Google Scholar]
- 38. Handa Y., Inaho N., Nameki N. (2011) YaeJ is a novel ribosome-associated protein in Escherichia coli that can hydrolyze peptidyl-tRNA on stalled ribosomes. Nucleic Acids Res. 39, 1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobayashi K., Kuwana R., Takamatsu H. (2008) kinA mRNA is missing a stop codon in the undomesticated Bacillus subtilis strain ATCC 6051. Microbiology 154, 54–63 [DOI] [PubMed] [Google Scholar]
- 40. Marr M. T., Roberts J. W. (2000) Function of transcription cleavage factors GreA and GreB at a regulatory pause site. Mol. Cell 6, 1275–1285 [DOI] [PubMed] [Google Scholar]
- 41. Toulmé F., Mosrin-Huaman C., Sparkowski J., Das A., Leng M., Rahmouni A. R. (2000) GreA and GreB proteins revive backtracked RNA polymerase in vivo by promoting transcript trimming. EMBO J. 19, 6853–6859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beckwith J. R., Signer E. R. (1966) Transposition of the lac region of Escherichia coli. I. Inversion of the lac operon and transduction of lac by phi80. J. Mol. Biol. 19, 254–265 [DOI] [PubMed] [Google Scholar]
- 43. Garza-Sánchez F., Shoji S., Fredrick K., Hayes C. S. (2009) RNase II is important for A-site mRNA cleavage during ribosome pausing. Mol. Microbiol. 73, 882–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.