Abstract
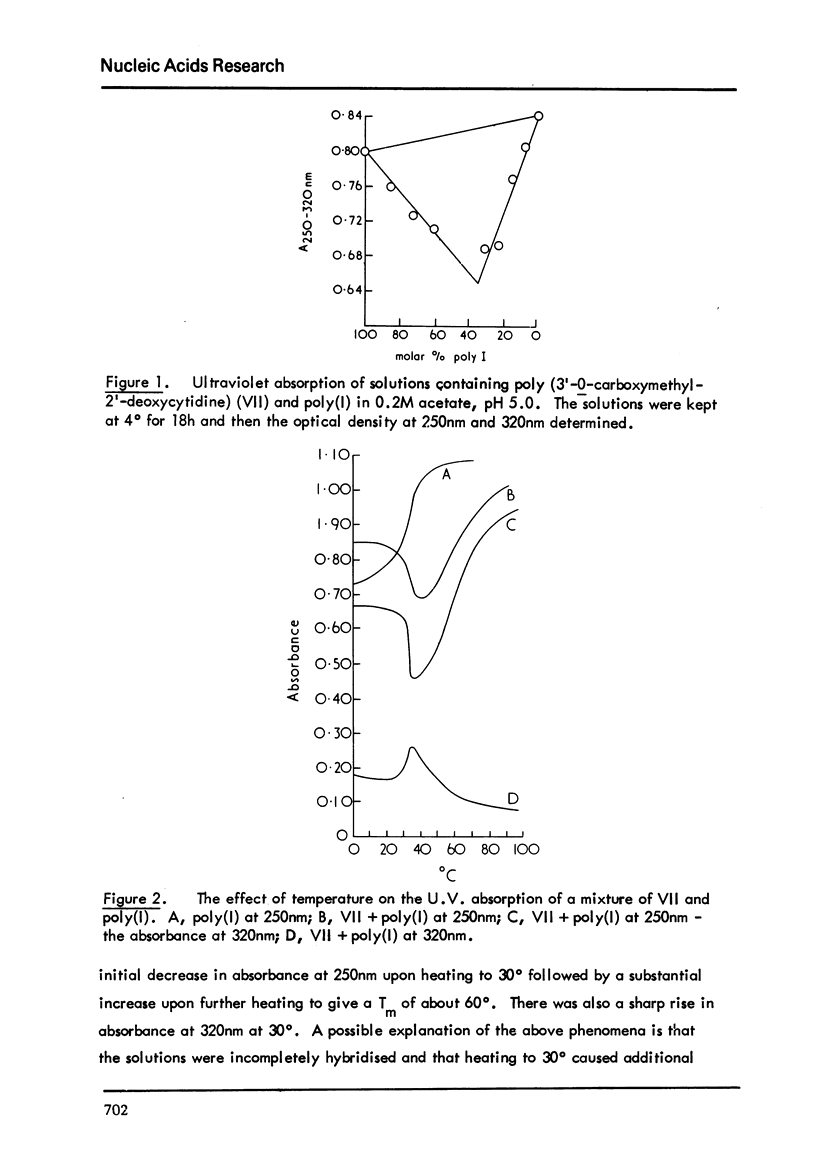
Poly (3'-O-carboxymethyl-2'-deoxyctidine) (VII) has been synthesised by the polymerisation of 3'-O-carboxymethyl-4-N-phenoxyacety-2'-deoxycytidine (V) and removal of the phenoxyacetyl groups under acidic conditions. V was obtained by the action of 2,4-dinitrophenyl phenylacetate on 3'-O-carboxymethyl-5'-O-triphenylmethyl-2'-deoxycytidine (III) followed by removal of the triphenylmethyl group under carefully controlled acidic conditions. The polymer, VII gave a hypochromic effect of about 20% at 250nm when mixed with poly (1) in 0.2Macetate, pH 5.0. It appeared, therefore, that a complex was formed. Upon heating a solution of this complex there was an initial decrease in optical density followed by a much larger increase to give a Tm of about 60 degrees. Attempts to form the 3'-O-carboxymethyl derivative of 4-N-phenoxyacetyl-5'-O-'triphenylmethyl-2'-deoxycytidine to give a shorter synthetic route to VII were not successful. 3'-O-Carboxymethyl-2'-deoxycytidine was obtained by removal of thetriphenylmethyl group from III. Attempts to polymerise this compound in concentrated aqueous solution with a water-soluble carbodiimide were not successful.
Full text
PDF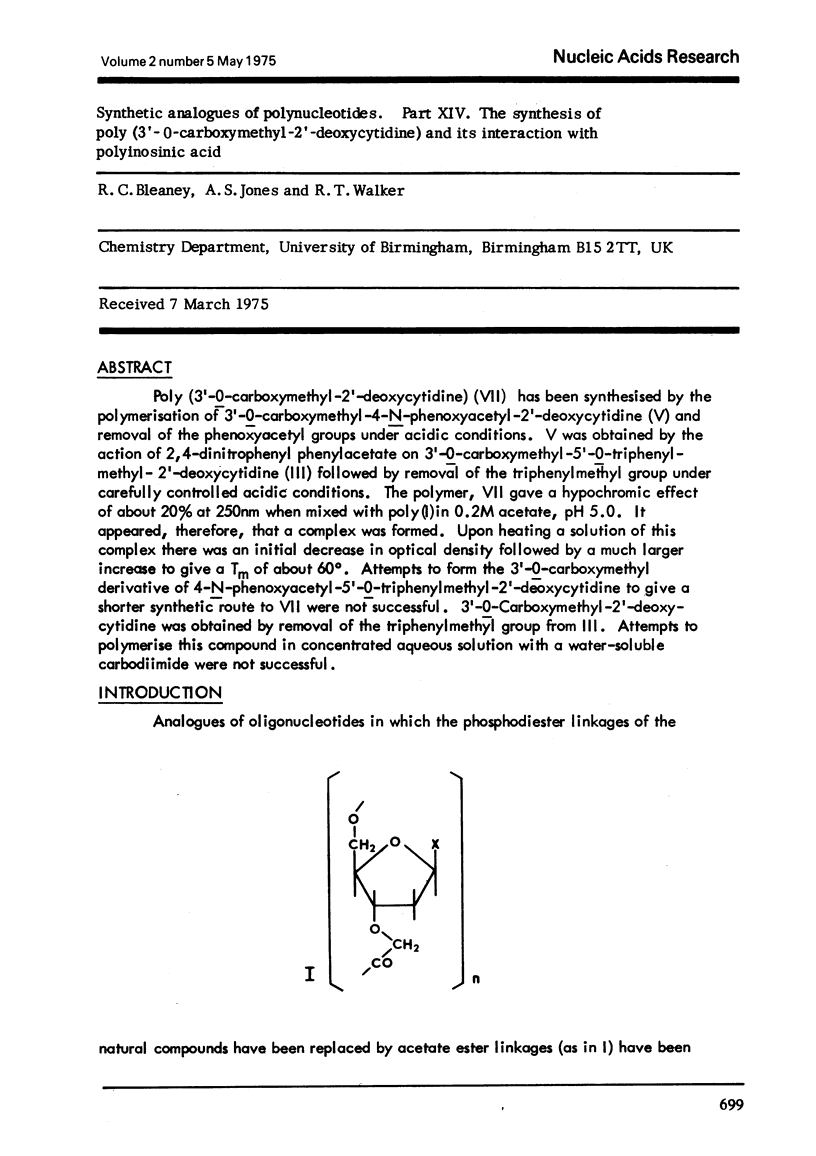





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cowling G. J., Jones A. S., Walker R. T. Polynucleotide analogue inhibition of messenger-stimulated transfer RNA binding to ribosomes. Biochim Biophys Acta. 1971 Dec 30;254(3):452–456. doi: 10.1016/0005-2787(71)90879-3. [DOI] [PubMed] [Google Scholar]
- Edge M. D., Hodgson A., Jones A. S., MacCoss M., Walker R. T. Synthetic analogues of polynucleotides. IX. Synthesis of 3'-O-carboxymethyl-2'-deoxyribonucleosides and their use in the synthesis of an analogue of 2'-deoxyadenylyl-(3' leads to 5'-)-thymidine 3'-phosphate. J Chem Soc Perkin 1. 1973;3:290–294. [PubMed] [Google Scholar]
- Edge M. D., Hodgson A., Jones A. S., Walker R. T. Synthetic analogues of polynucleotides. 8. Analogues of oligonucleotides containing carboxymethylthymidine. J Chem Soc Perkin 1. 1972;16:1991–1996. doi: 10.1039/p19720001991. [DOI] [PubMed] [Google Scholar]
- Edge M. D., Jones A. S. Synthetic analogues of polynucleotides. V. Analogues of trinucleoside diphosphates containing carboxymethylthymidine. J Chem Soc Perkin 1. 1971;10:1933–1939. doi: 10.1039/j39710001933. [DOI] [PubMed] [Google Scholar]
- Gait M. J., Jones A. S., Walker R. T. Synthetic-analogues of polynucleotides XII. Synthesis of thymidine derivatives containing an oxyacetamido- or an oxyformamido-linkage instead of a phosphodiester group. J Chem Soc Perkin 1. 1974;0(14):1684–1686. [PubMed] [Google Scholar]
- Halford M. H., Jones A. S. Synthetic analogues of polynucleotides. IV. Carboxymethyl derivatives of uridine and of thymidine. J Chem Soc Perkin 1. 1968;21:2667–2670. doi: 10.1039/j39680002667. [DOI] [PubMed] [Google Scholar]
- Jones A. S., MacCoss M., Walker R. T. Synthetic analogues of polynucleotides. X. The synthesis of poly-(3'-O-carboxymethyl-2'-deoxyadenosine) and its interaction with polynucleotides. Biochim Biophys Acta. 1973 Feb 4;294(1):365–377. [PubMed] [Google Scholar]
- Steward D. L., Herndon W. C., Jr, Schell K. R. Influence of 2'-O-acetylation on the antiviral activity of polyribonucleotides. Biochim Biophys Acta. 1972 Mar 14;262(2):227–232. doi: 10.1016/0005-2787(72)90237-7. [DOI] [PubMed] [Google Scholar]


