Abstract
Pontin is a highly conserved DNA helicase/ATPase which is a component of several macromolecular complexes with functions that include DNA repair, telomere maintenance and tumor suppression. While Pontin is known to be essential in yeast, fruit flies and frogs, its physiological role in mammalian organisms remains to be determined. We here find that Pontin is highly expressed in embryonic stem cells and hematopoietic tissues. Through germline inactivation of Ruvbl1, the gene encoding Pontin, we found it to be essential for early embryogenesis, as Ruvbl1 null embryos could not be recovered beyond the blastocyst stage where proliferation of the pluripotent inner cell mass was impaired. Conditional ablation of Ruvbl1 in hematopoietic tissues led to bone marrow failure. Competitive repopulation experiments showed that this included the loss of hematopoietic stem cells through apopotosis. Pontin is, therefore, essential for the function of both embryonic pluripotent cells and adult hematopoietic stem cells.
Keywords: Pontin, regulation, hematopoietic stem cell, embryogenesis, adult
Introduction
Pontin and Reptin (also known as Tip49/Ruvbl1 and Tip48/Ruvbl2, respectively) are homologous ATPases with DNA helicase activity that have been identified as participants in macromolecular complexes carrying out key functions in trancription and gene regulation. These include DNA helicases (Ino80 complex),1 histone modification (Tip60 complex)2 and nucleosome remodeling (SWI/SNF complex). In addition, Pontin/Reptin participate in complexes important for SnoRNA biogenesis (R2TP complex) and telomere maintenance (Telomerase core complex).3 Finally, Pontin interacts directly with the TATA-binding protein,4 and acts as co-factor for specific transcription factors and oncoproteins, such as c-Myc5 and β-catenin,6 both of which play essential roles in regulating hematopoietic and other stem cells. These multiple interactions of Pontin/Reptin suggest an essential physiological role, and studies in S. Cerevisiae have demonstrated that yeast orthologs of both Reptin and Pontin are required for cell viability without overall shutdown of transcription.7,8 Essential deveopmental roles in Drosophila and Xenopus have also been observed.9,10 However, the only information currently available about their role in mammalian cells is that knockdown of Pontin in human diploid fibroblasts causes proliferation arrest,11 and that knockdown of Ruvbl1 (and other Tip60 complex components) in embryonic stem (ES) cells induces loss of pluripotency.12 We here use genetic ablation of the Ruvbl1 gene, encoding Pontin, in the mouse germline to demonstrate that this gene is essential for embryogenesis at an early stage. No Ruvbl1−/− embryos were retrieved post-implantation, and outgrowth of pluripotent cells from Ruvbl1−/− blastocysts was not achieved. To address the role of Pontin in hematopoietic stem cells (HSCs), we conditionally ablated Ruvbl1 from the hematopoietic system using the Mx1-Cre transgene. This led to complete hematopoietic failure, including apoptotic loss of hematopoietic stem cells. Pontin is, therefore, essential for both early embryogenesis and adult hematopoiesis.
Design and Methods
The Ruvbl1 gene was targeted by homologous recombination in E14.1 ES cells.13 Breeding to deleterFlp14 and deleterCre15 mice generated the conditional and null alleles, respectively. Genotyping of mice and embryos was as described in the Online Supplementary Design and Methods. Conditional gene inactivation was achieved by activating the Mx1-Cre transgene16 through polyIC injection.17 Bone marrow cells were counted from femur, tibia and ilium. Peripheral blood counts and flow cytometric analysis, as well as flow cytometric analysis of bone marrow were performed as previously described17,18 (antibodies and dilutions used are described in the Online Supplementary Design and Methods). Western blotting was performed on cell/tissue extracts (Online Supplementary Appendix) using Tip48, Tip4919, PCNA (Sigma) and β-tubulin (Sigma) antibodies as described.20
Results and Discussion
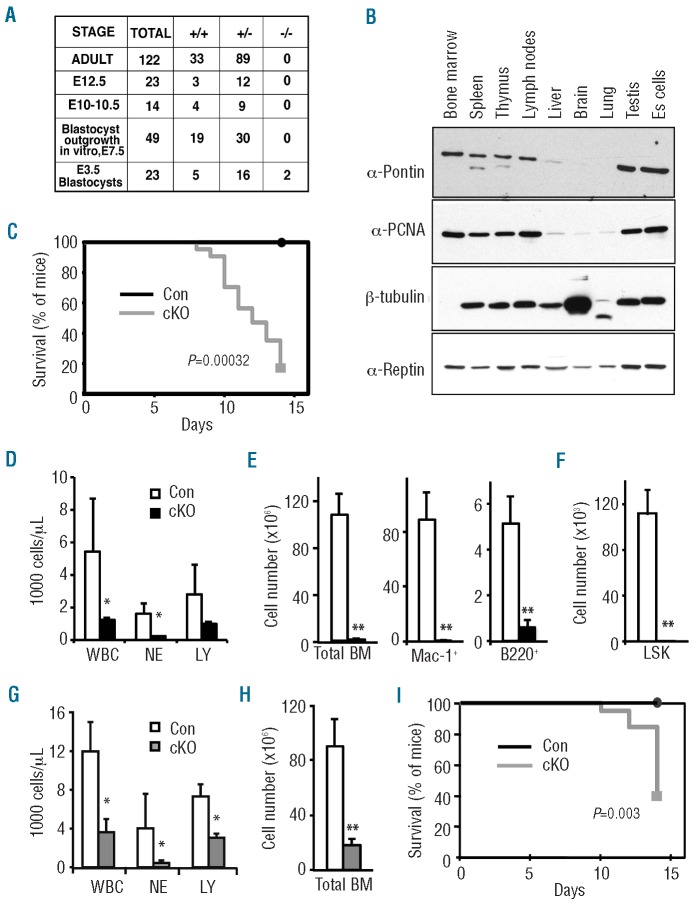
To address the physiological role of Pontin we first generated a Ruvbl1 null allele by targeting the mouse germline (Online Supplementary Figure S1). Intercrossing of Ruvbl1+/−mice did not generate any viable offspring, and no post-implantation Ruvbl1−/− embryos could be retrieved (Figure 1A). A few Ruvbl1−/− blastocysts were identified. However, upon culturing, which results in outgrowth of the pluripotent inner cell mass, no proliferating cultures were observed to be Ruvbl1−/−. We conclude from this that Pontin is required for embryogenesis at a very early stage, possibly involving the proliferation of pluripotent inner mass cells.
Figure 1.
Pontin is essential for mouse development and definitive hematopoiesis. (A) Ruvbl1+/− mice were intercrossed and litters and embryos of the indicated developmental stages were genotyped. Number of live animals/embryos with the 3 Ruvbl1 genotypes are shown for each developmental stage. Embryos were collected from at least 3 females at each stage. (B) Western blot of Pontin, Reptin and PCNA expression in various mouse tissues and E14.1 ES cells. The same filter was probed using antibodies specific for the indicated proteins, and an antibody against β-tubulin as a loading control. (C) Kaplan Meier plot of survival of PontincKO (n=14) and PontinCon (n=12) mice following injection of polyIC to induce Pontin deletion. The statistical significance of the difference in survival, determined by the log rank method, is indicated in the plot. Death was associated with pancytopenia. (D) Peripheral blood counts of live PontincKO (n=3) and PontinCon mice (n=3) at Day 8 after first polyIC injection. Mean ± SEM. *P<0.05. (E) Total bone marrow (BM) cell count, and number of Mac-1+ and B220+ bone marrow cells determined by flow cytometry in PontincKO (n=6) and PontinCon mice (n=8) mice on Day 8 after first polyIC injection, from 3 independent experiments. Mean ± SEM. **P<0.01. (F) Number of LSK cells in the bone marrow of PontincKO (n=3) and PontinCon mice (n=3) mice on Day 8 after first polyIC injection. Mean ± SEM. **P<0.01. (G) Peripheral blood counts of live recipeint mice transplanted with PontincKO (n=5) and PontinCon bone marrow (n=4) at Day 8 after first polyIC injection. PolyIC injections were initiated eight weeks after transplantation. Mean ± SEM. *P<0.05. (H) Total number of bone marrow cells in PontincKO (n=5) and PontinCon (n=4) non-competitively transplanted mice at Day 11 after first polyIC injection. Mean ± SEM. **P<0.01. (I) Kaplan-Meier plot of survival analysis of PontincKO (n=5) and PontinCon (n=4) non-competitively transplanted mice following polyIC injection. The statistical significance, determined by log rank test, of the difference in surivival is indicated in the plot. Death was associated with pancytopenia.
To address the role of Pontin after development, we analyzed the Pontin and Reptin expression patterns in the adult mouse and selected cell lines. Overall, the expression of Pontin, but not of Reptin, correlated with that of proliferating cell nuclear antigen (PCNA), consistent with Pontin playing a specific role in cell proliferation. In particular, we found Pontin to be highly expressed in hematopoietic tissues (bone marrow, spleen, thymus, lymph nodes), as well as pluripotent cells/tissues (ES cells, testis), with low levels in liver, brain and lung (Figure 1B). To address Pontin function in the hematopoietic system, a conditional Ruvbl1 allele (Online Supplementary Figure S1) was combined with the Mx1-Cre transgene, which deletes with high efficiency in hematopoietic tissues after induction with polyIC17. At two weeks after polyIC induction of Ruvbl1fl/fl;Mx-Cretg/+ mice (PontincKO mice) and Ruvbl1fl/fl;Mx-Cre+/+ controls (PontinCon mice), we observed specific lethality of PontincKO mice (Figure 1C), associated with a significant decrease in PontincKO peripheral blood cells (Figure 1D). Within eight days post-injection, surviving PontincKO mice displayed a massive loss of bone marrow cellularity (Figure 1E). This involved the coordinated loss of myeloid and lymphoid cells (Figure 1E) and was preceeded by the complete loss of Lin−Sca-1+c-Kit+ (LSK) HSCs (Figure 1F). To determine whether the observed effects could be attributed to an intrinsic hematopoietic requirement for Pontin, we transplanted CD45.2 PontincKO and PontinCon bone marrow non-competitively into irradiated CD45.1/2 recipients. Subsequent polyIC induction resulted in loss of hematopoietic cells (Figure 1G and H) and lethality (Figure 1I) similar to that observed in non-transplanted PontincKO mice.
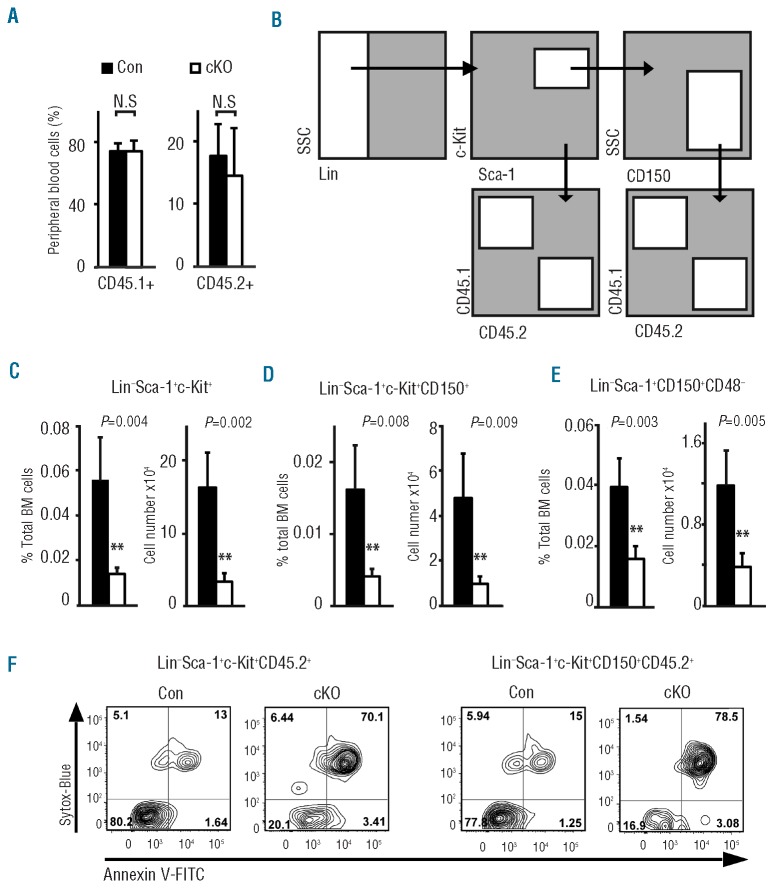
The severe phenotype made it difficult to assess the direct effects of Pontin loss on quiescent hematopoietic stem cells, since a strong depletion of mature cells and progenitors would activate HSCs, potentially rendering them more susceptible to the loss of Pontin. To circumvent this issue, we performed competitive transplantation of CD45.2 PontincKO and PontinCon bone marrow along with CD45.1 competitor into irradiated CD45.1/2 recipients, allowing wild-type CD45.1 hematopoietic cells to maintain hematopoiesis after deletion. To minimize indirect effects of Ruvbl1 deletion, we used a 3-fold excess of CD45.1 competitor bone marrow. We achieved similar levels of PontincKO and PontinCon CD45.2 chimerism prior to polyIC induction (Figure 2A). At four days after induction, the frequency of PontincKO HSCs (defined as either LSK, LSKCD150+ or Lin−Sca-1+CD48−CD150+) was significantly decreased compared to PontinCon-transplanted mice (Figure 2B-E), and a very high proportion of the remaining PontincKO HSCs were apoptotic, as measured by staining for AnnexinV and intracellular DNA (Figure 2F). From this we conclude that Pontin is required for HSC viability and that in its absence HSCs undergo apoptosis.
Figure 2.
Pontin is required for HSC survival. (A) Reconstitution levels of competitor (CD45.1) and experimental donor cells (CD45.2) in irradiated recipients reconstituted with 1,500,000 helper and 500,000 donor cells (either PontincKO or PontinCon). PontincKO: n=4; PontinCon: n=5. N.S.: no statistically significant difference. (B) Scheme of the gating for the flow cytometric analysis of HSC compartment in competitively transplanted mice. (C) Frequency (left panel) and cell number (right panel) of CD45.2+ LSK cells in BM of competitively transplanted mice at Day 4 after a single polyIC injection. Black bars: PontinCon (n=4); white bars: PontinCKo (n=4). Mean ± SEM. **P<0.01. (D) Analysis of the number of LSKCD150+ BM cells in the cohort from panel (C). Mean ± SEM. **P<0.01. (E) Analysis of the number of Lin−Sca-1+CD150+CD48− BM cells in the cohort from panel (C). Mean ± SEM. **P<0.01. (F) Analysis of apoptosis of PontincKO and PontinCon HSCs at Day 4 after polyIC injection by surface staining using AnnexinV and measurement of cell permeability through staining of intracellular DNA (Sytox-Blue dye). Contour plots show AnnexinV and Sytox-Blue staining of LSK and LSKCD150+ cells, gated on the experimental CD45.2 population. Four mice were analyzed for each condition and representative plots are shown.
These results demonstrate an essential role for Pontin in early mammalian development, consistent with its presence in multiple complexes carrying out essential cellular functions. In addition, the definitive hematopoietic system in general, and HSCs in particular, were critically dependent on Pontin for its maintenance, with HSC apoptosis and depletion being an immediate consequence of Ruvbl1 inactivation. This is in contrast to the proliferation arrest observed after in vitro Ruvbl1 knockdown.11 Conditional hematopoietic inactivation of both Myc and Mycn results in a very similar phenotype to loss of Pontin.21 Since Pontin was shown to directly interact with c-Myc and control its oncogenic activity,5 loss of Myc function may contribute significantly to the hematopoietic Ruvbl1 null phenotype. Embryonic lethality in the absence of Pontin occurred at a stage of development consistent with a defect in the pluripotent inner cell mass. This phenotype is consistent with Ruvbl1 being essential for ES cell maintenance, most likely due to Pontin being required for the function of the Tip60 complex.12 However, due to the pleiotropic functions of Pontin, other molecular interactions could prove critical for the developmental processes observed to be affected by its absence. Pontin and Reptin functionally interact with proteins playing prominent roles in cancer, such as β-catenin, c-Myc and telomerase. Furthermore, Pontin and/or Reptin overexpression was found in a number of solid tumors, as well as in several hematologic malignancies.22 This suggests that Pontin/Reptin proteins could be involved in tumorigenesis and may represent a potential target for cancer therapy. Based on our own and other published data, inhibition of Pontin expression or potentially its enzymatic activity represents a plausible way to impede tumor growth and induce apoptosis in proliferating cancer cells. Use of these mice in hemizygocity may provide an insight into the dosage-dependent requirent of Pontin in various types of cancer. Overall, our results establish Pontin as a critical regulator of both embryonic and adult hematopoietic stem cells.
Acknowledgements
The authors would like to thank Dr. Bruno Amati for supplying Tip48 and Tip49 antibodies.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding: the work was supported through the FP6 EuroStemCell and EuroCSC consortia, and by an MRC Program Grant (grant n. 93339) and MRC Strategic Award to CN. OB was the recipient of an HFSP postdoctoral fellowship, and is a recipient of a José Carreras Young Investigator Fellowship.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Shen X, Mizuguchi G, Hamiche A, Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature. 2000;406(6795):541–4. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 2.Jha S, Shibata E, Dutta A. Human Rvb1/Tip49 is required for the histone acetyltransferase activity of Tip60/NuA4 and for the downregulation of phosphorylation on H2AX after DNA damage. Mol Cell Biol. 2008;28(8):2690–700. doi: 10.1128/MCB.01983-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huen J, Kakihara Y, Ugwu F, Cheung KL, Ortega J, Houry WA. Rvb1-Rvb2: essential ATP-dependent helicases for critical complexes. Biochem Cell Biol. 2010;88(1):29–40. doi: 10.1139/o09-122. [DOI] [PubMed] [Google Scholar]
- 4.Kanemaki M, Makino Y, Yoshida T, Kishimoto T, Koga A, Yamamoto K, et al. Molecular cloning of a rat 49-kDa TBP-interacting protein (Pontin) that is highly homologous to the bacterial RuvB. Biochem Biophys Res Commun. 1997;235(1):64–8. doi: 10.1006/bbrc.1997.6729. [DOI] [PubMed] [Google Scholar]
- 5.Wood MA, McMahon SB, Cole MD. An ATPase/helicase complex is an essential cofactor for oncogenic transformation by c-Myc. Mol Cell. 2000;5(2):321–30. doi: 10.1016/s1097-2765(00)80427-x. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Lee N, Fearon ER. TIP49 regulates beta-catenin-mediated neoplastic transformation and T-cell factor target gene induction via effects on chromatin remodeling. Cancer Res. 2003;63(24):8726–34. [PubMed] [Google Scholar]
- 7.Qiu XB, Lin YL, Thome KC, Pian P, Schlegel BP, Weremowicz S, et al. An eukaryotic RuvB-like protein (RUVBL1) essential for growth. J Biol Chem. 1998;273(43):27786–93. doi: 10.1074/jbc.273.43.27786. [DOI] [PubMed] [Google Scholar]
- 8.Kanemaki M, Kurokawa Y, Matsuura T, Makino Y, Masani A, Okazaki K, et al. TIP49b, a new RuvB-like DNA helicase, is included in a complex together with another RuvB-like DNA helicase, TIP49a. J Biol Chem. 1999;274(32):22437–44. doi: 10.1074/jbc.274.32.22437. [DOI] [PubMed] [Google Scholar]
- 9.Bauer A, Chauvet S, Huber O, Usseglio F, Rothbacher U, Aragnol D, et al. Pontin52 and reptin52 function as antagonistic regulators of beta-catenin signalling activity. EMBO J. 2000;19(22):6121–30. doi: 10.1093/emboj/19.22.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etard C, Gradl D, Kunz M, Eilers M, Wedlich D. Pontin and Reptin regulate cell proliferation in early Xenopus embryos in collaboration with c-Myc and Miz-1. Mech Dev. 2005;122(4):545–56. doi: 10.1016/j.mod.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Chan HM, Narita M, Lowe SW, Livingston DM. The p400 E1A-associated protein is a novel component of the p53 --> p21 senescence pathway. Genes Dev. 2005;19(2):196–201. doi: 10.1101/gad.1280205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazzio TG, Huff JT, Panning B. An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell. 2008;134(1):162–74. doi: 10.1016/j.cell.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porse BT, Pedersen TA, Xu X, Lindberg B, Wewer UM, Friis-Hansen L, et al. E2F repression by C/EBPalpha is required for adipogenesis and granulopoiesis in vivo. Cell. 2001;107(2):247–58. doi: 10.1016/s0092-8674(01)00516-5. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25(2):139–40. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 15.Schwenk F, Baron U, Rajewsky K. A cretransgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23(24):5080–1. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269(5229):1427–9. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 17.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7(10):1048–56. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 18.Bereshchenko O, Mancini E, Moore S, Bilbao D, Mansson R, Luc S, et al. Hematopoietic stem cell expansion precedes the generation of committed myeloid leukemia-initiating cells in C/EBPalpha mutant AML. Cancer Cell. 2009;16(5):390–400. doi: 10.1016/j.ccr.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Frank SR, Parisi T, Taubert S, Fernandez P, Fuchs M, Chan HM, et al. MYC recruits the TIP60 histone acetyltransferase complex to chromatin. EMBO rep. 2003;4(6):575–80. doi: 10.1038/sj.embor.embor861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen TA, Bereshchenko O, Garcia-Silva S, Ermakova O, Kurz E, Mandrup S, et al. Distinct C/EBPalpha motifs regulate lipogenic and gluconeogenic gene expression in vivo. EMBO J. 2007;26(4):1081–93. doi: 10.1038/sj.emboj.7601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurenti E, Varnum-Finney B, Wilson A, Ferrero I, Blanco-Bose WE, Ehninger A, et al. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell. 2008;3(6):611–24. doi: 10.1016/j.stem.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigoletto A, Lestienne P, Rosenbaum J. The multifaceted proteins Reptin and Pontin as major players in cancer. Biochem Biophys Acta Rev Cancer. 2011;1815(2):147–57. doi: 10.1016/j.bbcan.2010.11.002. [DOI] [PubMed] [Google Scholar]