Abstract
Background
Myelodysplastic syndromes are a heterogeneous group of clonal hematopoietic stem cell disorders characterized by ineffective hematopoiesis. Survivin is a member of the inhibitor of apoptosis family and suppresses apoptosis. Survivin also functions as a subunit of the chromosomal passenger complex for regulating mitosis with Aurora-B. Survivin and Aurora-B play an important role in maintaining genome stability. The aim of this study was to determine the role of Survivin and Aurora-B kinase in disease progression and prognosis of myelodysplastic syndromes.
Design and Methods
We evaluated the expression levels of these two genes in CD34+ cells prepared from 64 patients with myelodysplastic syndrome or leukemic blasts from 50 patients with de novo acute myeloid leukemia using quantitative real-time PCR.
Results
Survivin and Aurora-B expression levels were highly correlated with the type of myelodysplastic syndrome, were much higher in refractory anemia with excess blasts-1, refractory anemia with excess blasts-2, and secondary acute myeloid leukemia following myelodysplastic syndrome than in normal control, and increased during disease progression. There was a significant correlation between these expression levels and the International Prognostic Scoring System. Interestingly, these levels were remarkably higher in patients with secondary acute myeloid leukemia following myelodysplastic syndromes than in those with de novo acute myeloid leukemia.
Conclusions
This is the first report showing that high levels of Survivin and Aurora-B kinase expression in CD34+ cells are distinctive molecular features of high-risk myelodysplastic syndromes and secondary acute myeloid leukemia following myelodysplastic syndrome. Marked upregulation of Survivin and Aurora-B kinase may contribute to genetic instability and disease progression of myelodysplastic syndromes. Our data may explain why patients with high-risk myelodysplastic syndromes frequently show complex chromosomal abnormality.
Keywords: Survivin, Aurora-B kinase, myelodysplastic syndromes, MDS
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal hematopoietic stem cell disorders characterized by peripheral cytopenias despite normal or hypercellular bone marrow (BM)1 which could evolve into an overt acute myeloid leukemia (AML). Underlying excessive apoptosis or an apoptosis-associated phenotype of BM cells, including clonal CD34 precursors, have been found to be a possible explanation for the ineffective hematopoiesis, especially in early cases of myelodysplastic syndrome.2–5 Several prognostic factors have been reported to be involved in disease progression of MDS.6–9 However, specific genes that contribute to MDS progression are still not completely understood.10
Deregulation of programmed cell death contributes to leukemogenesis and to blast cell survival. Cells resistant to apoptosis are prone to accumulate genetic aberrations, acquire the capacity to survive independently from growth factor stimulation and escape from immune system control.11 Survivin, a member of the inhibitor of apoptosis (IAP) family, is a bifunctional protein that acts as a suppressor of apoptosis12 and plays a central role in mitosis.13,14 Survivin is transiently expressed during embryonic development but is barely detectable in normal, differentiated adult tissue.12 However, Survivin is over-expressed in a number of different tumor tissues indicating that it has a role in carcinogenesis.12,15 A high level of Survivin expression correlates with poor outcome in a variety of solid tumors.16–18 Similarly, in malignant hematologic diseases, overexpression of Survivin correlates with reduced overall survival in patients with T-cell leukemia19 as well as those with diffuse large B-cell lymphoma.20 However, it remains unclear whether Survivin expression plays an important role during MDS progression.
Besides its role as an IAP, Survivin functions as a subunit of the chromosomal passenger complex (CPC) and together with the other CPC subunits such as Aurora-B, INCENP, and Borealin regulates cell division.13,21–24 The activity of Aurora-B kinase is stimulated by Survivin binding and phosphorylation.23 Aurora-B is the enzymatic core of the complex, whereas Survivin and INCENP dictate the timing and localization of the kinase activity.13 CPC corrects attachment errors between chromosomes and the mitotic spindle, regulates the quality-control of mitotic spindle checkpoint, and ensures the correct completion of cytokinesis.25,26 In solid tumor cells, high expression of Aurora-B and INCENP, as well as that of Survivin, has been observed.27 However, no studies have reported the impact of abnormal expression of Survivin and Aurora-B kinase in CD34+ cells during the clinical course of MDS. The aim of our study was to determine the clinical significance of Survivin and Aurora-B kinase expression in MDS and s-AML. We have demonstrated for the first time that high levels of Survivin and Aurora-B kinase expression are distinctive molecular features of high-risk MDS and s-AML. Marked upregulations of Survivin and Aurora-B Kinase may contribute to genetic instability and may be involved in disease progression of MDS.
Design and Methods
Patients
After obtaining informed consent from patients and approval from the institutional review board of The University of Fukui Hospital, bone marrow (BM) samples from 114 patients (64 MDS, 50 de novo AML) were tested for Survivin and Aurora-B kinase expression. Of the 64 MDS patients, 36 were male and 28 female; median age was 71 years (range 33–87 years); there were 11 patients with refractory anemia (RA), 3 with RA with ringed sideroblasts (RARS), 9 with refractory cytopenia with multilineage dysplasia (RCMD), 2 with RCMD with ringed sideroblasts (RCMD-RS), 10 with refractory anemia with excess of blasts-1 (RAEB-1), 11 with RAEB-2, and 18 with secondary AML (s-AML) that had evolved from MDS. Patients with de novo AML were classified as M0 (n=4), M1 (n=7), M2 (n=12), M3 (n=7), M4 (n=8), M5 (n=6), M6 (n=4) and M7 (n=2). Cytogenetic analysis identified the karyotype of t(8;21) (n=5), t(15;17) (n=7), inv(16) (n=4), −5/del(5) (n=2), −7/del(7) (n=3), +21(2) and normal (n=11). Complex karyotypes were found by cytogenetic analysis in 15% of patients with de novo AML. Eight samples from age-matched healthy volunteers were used as controls.
Sample collection and CD34+ cell enrichment
Bone marrow mononuclear cells were isolated by density gradient centrifugation using HISTOPAQUE 1077 (Sigma). Cells were washed twice in magnetic-activated cell separation (MACS) buffer (phosphate-buffered saline supplemented with 0.5% bovine serum albumin and 2 mM ethylenediaminete-traacetic acid, EDTA). After incubation with immunoglobulin (Ig) Fc receptor blocking reagent and hapten-conjugated anti-CD34 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 min at 4°C, cells were washed twice and resuspended in 500 μL MACS buffer. Magnetically labeled cells were passed through a positive selection column (LS column; Miltenyi Biotec) in a magnetic field. After 2 column washes, the retained cells were eluted with 1 mL MACS buffer. The eluate was passed through a fresh column, washed twice, and eluted in 500 μL MACS buffer. Average number of isolated CD34+ cells from low-risk MDS was 2.4×105. Purity of CD34 cells was determined by flow cytometry and was 85–99% with no difference in purity between samples from different patient and control groups. Representative FACS plot for CD34 expression are shown in Online Supplementary Figure S1.
Real-time quantitative polymerase chain reaction (RQ-PCR)
Total RNA was isolated using BIO ROBOT EZ1 (Qiagen, Hilden, Germany). The amount of RNA was measured by photometry. Reverse transcription of total RNA was performed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitative PCR was performed using TaqMan real-time PCR methods. The StepOne Plus PCR System and TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) were used for the quantification of all genes according to the manufacturer’s instructions. The assay IDs were: BIRC5 (Survivin) Hs03043574_m1; Aurora-B, Hs00177782_m1. GAPDH, Hs02786624_g1. The relative mRNA expression of Survivin or Auroa-B kinase was calculated using the comparative threshold method (Ct-method) with GAPDH for normalization.28 Human leukemia HL-60 cells were used as positive controls for Survivin and Aurora-B kinase expression. The Survivin or Aurora-B kinase expression level in normal CD34 cells from 7 healthy donors was investigated. Measurable amounts of Survivin or Aurora-B kinase were found in all CD34 samples isolated from healthy controls. Mean value of expression level of Survivin or Aurora-B kinase in normal CD34 cells was defined as 1. All experiments were performed in triplicate.
Statistical methods
Mann-Whitney U tests were used to estimate the statistical significance of the differences observed between groups. P<0.05 was considered statistically significant. The correlation between Survivin or Aurora-B kinase expression and IPSS score was investigated by means of Spearman’s correlation coefficient.
Results
Survivin and Aurora-B kinase expression in refractory anemia and refractory cytopenia with multilineage dysplasia
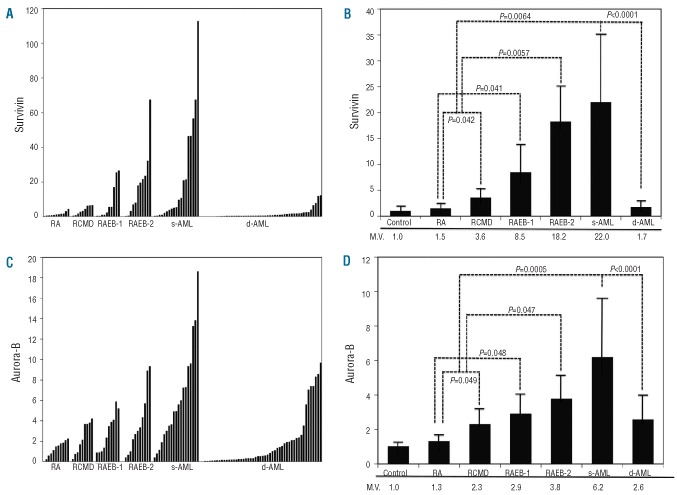
The degree of Survivin expression was higher in patients with RA and RCMD than in controls (Figure 1A and B). The median expression of Survivin was 1.5-fold higher in CD34 cells isolated from MDS RA patients than in normal CD34 cells (Figure 1B). The RCMD patients have significantly higher levels of Survivin transcript than RA patients (P=0.042) (Figure 1B). This value is significantly higher not only than the value in RA patients, but also than that in normal controls (P=0.011). In addition, we investigated the expression of Aurora-B kinase in RA and RCMD (Figure 1C and D). RA patients show 1.3-fold higher levels of Aurora-B kinase transcript than normal controls. The RCMD patients showed an even higher expression (2.3-fold) of Aurora-B kinase (Figure 1D). The median value of expression (2.3-fold) of Aurora-B kinase in RCMD patients is significantly higher not only than the value in normal controls (P=0.031) but also than that in RA patients (P=0.049) (Figure 1D). In addition, patients with RARS (n=3) and RCMD-RS (n=2) show increased expression of Survivin (1.7-fold and 3.3-fold, respectively) and Aurora-B kinase (1.5-fold and 2.4-fold, respectively) compared to that in normal controls. However, our study included only a small number of patients with RARS with RCMD-RS, and more samples are required to confirm this.
Figure 1.
Expression of Survivin and Aurora-B kinase in MDS, s-AML and de novo AML (d-AML). (A) Survivin expression evaluated by real-time quantitative polymerase chain reaction (RQ-PCR) in the samples from patients with RA, RCMD, RAEB-1, RAEB-2, secondary acute myeloid leukemia (s-AML) and de novo AML (d-AML). CD34 cells were isolated using a Mac bead column as described in Design and Methods. (B) Mean values of Survivin expression evaluated by RQ-PCR in the samples from normal volunteers, RA, RCMD, RAEB-1, RAEB-2, s-AML and de novo AML (d-AML). M.V.: mean expression value. (C) Aurora-B kinase expression evaluated by RQ-PCR in the samples from patients with RA, RCMD, RAEB-1, RAEB-2, s-AML and de novo AML (d-AML). CD34 cells were isolated using a Mac bead column as described in Design and Methods section. (D) Mean values of Aurora-B expression evaluated by RQ-PCR in the samples from normal volunteers, RA, RCMD, RAEB-1, RAEB-2, s-AML and de novo AML (d-AML). M.V.: mean expression value.
Survivin and Aurora-B kinase expression in RAEB-1, RAEB-2 and in s-AML
Survivin expression was markedly increased in the samples obtained from patients with RAEB-1 and RAEB-2 (Figure 1A). The median expression of Survivin mRNA was 8.5-fold higher in CD34 cells from RAEB-1 patients and 18.2-fold higher in CD34 cells from RAEB-2 patients than in normal CD34 cells (P=0.038 and P=0.0046, respectively) (Figure 1B). These values are significantly higher not only than the values in normal controls but also than those in RA and RCMD patients (Figure 1B). Furthermore, we examined the levels of Survivin in the samples isolated from 18 patients with s-AML. The median expression of Survivin mRNA was 22-fold higher in leukemic blast cells from s-AML patients than in normal controls (P=0.0057) (Figure 1B). These values are significantly higher than those in the combined group with RA and RCMD (P=0.0064) (Figure 1B). In addition, we determined the expression of Aurora-B kinase in RAEB-1, RAEB-2, and s-AML. Aurora-B kinase showed higher expression preferentially in patients with RAEB-1, RAEB-2, and s-AML than in patients with RA and RCMD (Figure 1C). These differences in Aurora-B kinase expression were statistically significant (Figure 1D). The expression profiles of Aurora-B kinase were similar to those of Survivin.
Cytogenetic analysis revealed complex karyotypes in 39% of patients in the combined group of RAEB-1, RAEB-2, and s-AML. In these patients, mean values of expression of Survivin and Aurora-B kinase were significantly higher (3.6-fold and 2.3-fold, respectively) than those in patients with normal karyotype (P<0.003 and P<0.01).
Correlations between the expression levels of Survivin or Aurora-B kinase and the IPSS Score in myelodysplastic syndromes
Spearman’s correlation coefficient analysis in all MDS patients showed that there was a strong correlation between the expression levels of Survivin and the risk categories of the patients defined according to the IPSS (P<0.0001) (Figure 2A). Expression levels of Aurora-B kinase in MDS patients were also strongly correlated with the IPSS risk categories (P=0.0002) (Figure 2B).
Figure 2.
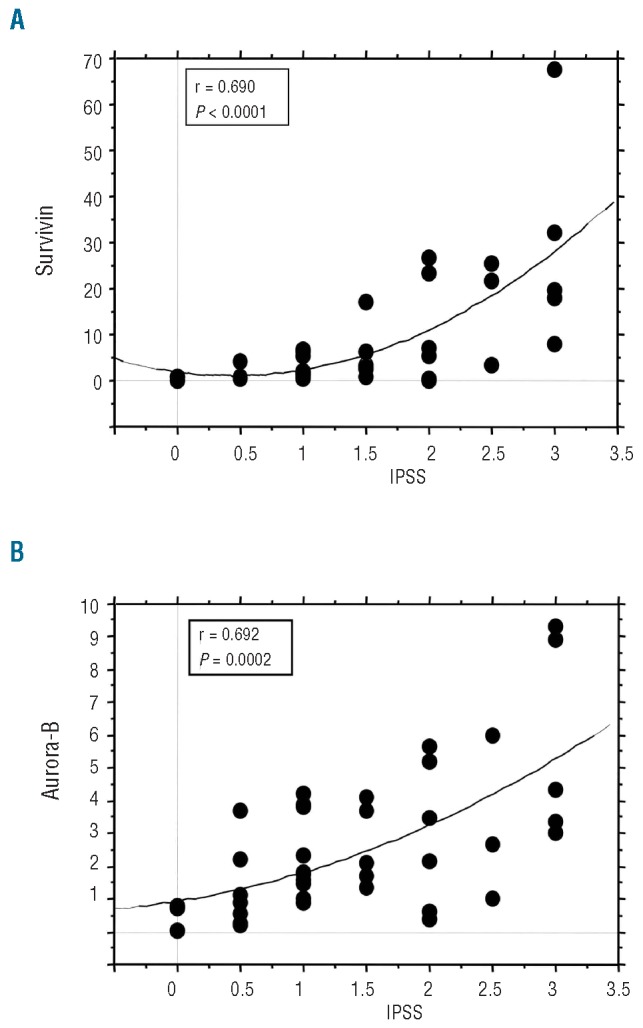
Correlations between the levels of Survivin or Aurora-B kinase expression and the IPSS Score in MDS. (A) Survivin expression was correlated with IPSS score in patients with MDS. R = 0.690 and P<0.0001 indicates a significant correlation between the 2 parameters (Survivin expression and IPSS score). (B) Aurora-B kinase expression was correlated with IPSS score in patients with MDS. R = 0.692 and P=0.0002 indicates a significant correlation between the 2 parameters (Aurora-B kinase expression and IPSS score). The correlation between the Survivin or Aurora-B kinase expression and IPSS was evaluated by the Spearman’s correlation coefficient.
Survivin and Aurora-B kinase expression in de novo acute myeloid leukemia
We investigated the expression of Survivin in blast cells isolated from 50 patients with de novo AML. Unexpectedly, the level of Survivin expression in de novo AML was lower than that in MDS (Figure 1A and B). The median expression of Survivin mRNA was 1.7-fold higher than that in normal CD34 cells (Figure 1B); however, this is not statistically significant. Median expression of Survivin in de novo AML was significantly lower than that in s-AML (P<0.0001) (Figure 1B). Comparison of Survivin expression between patients with normal karyotype and those with complex karyotypes showed no statistical difference. In addition, no significant association was observed between Survivin expression and specific chromosomal abnormalities, including t(8;21), t(15;17), inv16, +21, −5/del(5q) and −7/del(7q). There was no statistical difference in Survivin expression levels between French-American-British (FAB) classified subgroups. Median expression of Aurora-B kinase in de novo AML was significantly lower than that in s-AML (P<0.0001) (Figure 1D). No correlation was observed between Aurora-B expression and specific cytogenetics. No significant difference was observed in Aurora-B expression among various FAB groups.
Correlation between expression levels of Survivin and Aurora-B kinase in patients with myelodysplastic syndromes and s-AML
There may be some correlation between the expression levels of Survivin and Aurora-B kinase in each patient with MDS and s-AML (Online Supplementary Figure S2). Both Survivin and Aurora-B kinase are subunits of the chromosomal passenger complex (CPC) which plays an important role during mitosis as the mitotic spindle checkpoint.25,26,29 We performed regression analysis to examine whether there is any correlation between the expression levels of Survivin and Aurora-B kinase. Our results showed that expression of Aurora-B kinase was significantly correlated with Survivin expression in MDS and s-AML (Figure 3). However, there was no such correlation in patients with de novo AML (data not shown).
Figure 3.
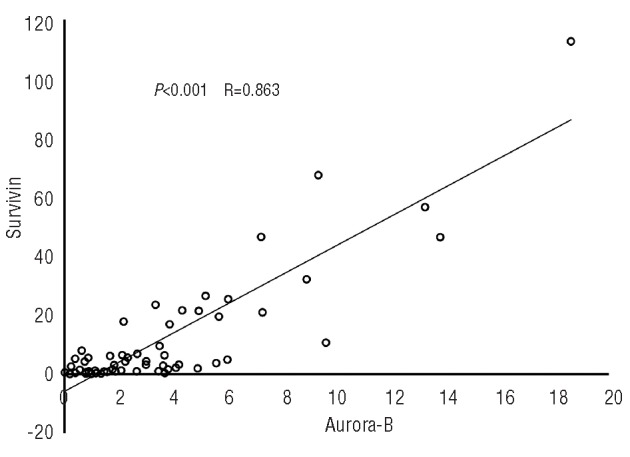
Correlation between the expression levels of Survivin and Aurora-B kinase in patients with MDS and s-AML. Regression analysis performed between Survivin and Aurora-B kinase expression in patients with MDS and s-AML R value = 0.863 indicates a good correlation between the two parameters considered.
Follow up of Survivin and Aurora-B kinase expression during myelodysplastic syndromes progression
Survivin and Aurora-B kinase expression levels were evaluated in 4 MDS patients at different time points during clinical follow up. These 4 patients included one patient with RA, one patient with RCMD and 2 patients with RAEB-1 (Figure 4 and Table 1). Patient 1 with RCMD and Patient 2 with RA were treated with only supportive therapies such as transfusion. These patients subsequently developed acute leukemia. These 2 patients showed a marked increase in the levels of Survivin transcript expression at disease progression. At first observation, Patient 2 showed del(7)(q22q36) as a cytogenetic abnormality (Table 1). At the time of leukemic progression, a marked increase (58-fold) in Survivin expression was observed (Figure 4) with additional cytogenetic abnormalities such as del(5)(q23q34) and der(17)t(5;17) (Table 1). Patient 3 with RAEB-1 was treated with low-dose cytarabine. However, no improvement was observed. This patient developed acute leukemia with an apparent increase in expression of Survivin and Aurora-B (Figure 4). Patient 4 with RAEB-1 developed acute leukemia. At that time, this patient was treated with combination chemotherapy including cytarabine and aclarubicin. Survivin and Aurora-B kinase returned to normal levels in one s-AML patient who achieved a complete remission; however, after five months this patient relapsed. High levels of Survivin were subsequently detected (Figure 4). These data indicate that longitudinal monitoring of Survivin and Aurora-B kinase levels may reflect disease status in MDS patients.
Figure 4.
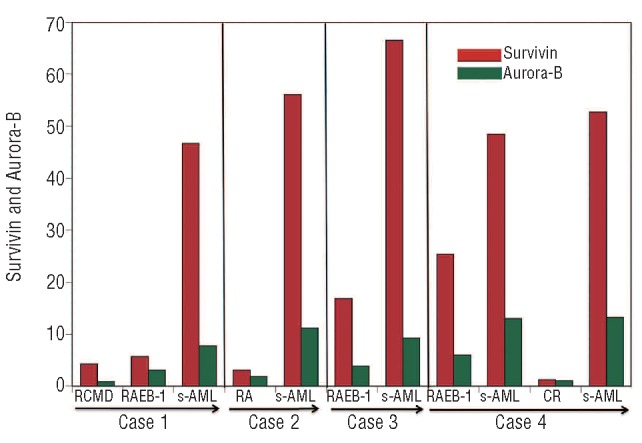
Survivin and Aurora-B kinase expression levels during follow up of 4 patients with MDS. In all cases, a marked increase in Survivin and Aurora-B kinase expression was noted in parallel with disease progression. Survivin or Aurora-B kinase expression was evaluated by RQ-PCR as described in Design and Methods.
Table 1.
Cytogenetic data for 4 patients with MDS. Data for these 4 patients were identical to those of patients with clinical follow up (Figure 4).

Discussion
To date there have been no studies that have examined whether expression of Survivin and Aurora-B kinase is increased in CD34+ cells isolated from MDS patients during disease progression. In this study, we measured Survivin and Aurora-B kinase expression in a series of MDS patients and de novo AML patients using a sensitive RQ-PCR method. Our data show that expression of Survivin and Aurora-B kinase are directly correlated with the type of MDS, being higher in a statistically significant manner in RA with respect to RAEB-2 and to s-AML. In addition, we found that expression of Survivin or Aurora-B kinase is strongly correlated with IPSS, the risk scoring system for MDS. Interestingly, we found that levels of Survivin and Aurora-B kinase were markedly higher in patients with s-AML than patients with de novo AML.
It is well known that Survivin overexpression is associated with a poor outcome in various cancers.26 The fact that Survivin acts as an inhibitor of effector caspases 3/7 and blocks the mutual downstream events of apoptosis pathways30 means that Survivin is a key factor in the response to chemotherapy. Patients with high-risk MDS and secondary AML are particularly resistant to standard chemotherapy compared to patients with de novo AML, suggesting that these entities are biologically distinct from de novo AML. However, the specific gene responsible for the pathological differences between secondary leukemia following MDS and de novo AML has still not been clarified. In the present study, we found that marked upregulation of Survivin was associated with disease progression of MDS. In particular, patients with high-risk MDS and secondary AML showed a significant increase in Survivin expression, which may contribute to the anti-cancer drug resistance and may explain why high-risk MDS and s-AML patients have a poor prognosis.
Only 2 studies on expression of Survivin in a series of adult patients with de novo AML have been reported.31,32 Adida et al.31 reported that Survivin was detected in 75 (60%) out of 125 de novo AML patients with data available from immunohistochemical analysis. No significant difference was observed in complete remission rate or overall survival between Survivin-positive and Survivin-negative AML patients.31 In addition, Wagner et al.32 reported that the expression of Survivin did not correlate with complete remission rate or overall survival in adult AML patients. In our present study, we observed that the median expression of mRNA of Survivin in AML patients was 1.7-fold higher than that in normal CD34 cells; however, this is not statistically significant. Most de novo AML patients do not show significant Survivin upregulation. Gianelli et al.33 reported that MDS patients belonging to the low or INT1 (IPSS) risk groups had higher levels of Survivin mRNA than those belonging to INT2 or high IPSS. Their results are not consistent with our data. However, Gianelli et al. did not examine the expression of this gene using isolated CD34+ cells. They used BM mononuclear cells. Therefore, it is difficult to make a comparison between their results and ours. Invernizzi et al.34 analyzed the expression of Survivin by immunocytochemistry in bone marrow cells from patients with chronic myelomonocytic leukemia (CMML). They found that Survivin levels were higher in patients with CMML than in MDS and AML (P<0.0001) but were similar to those found in MPD.34 In the present study, we did not examine Survivin expression in CMML because of the extremely small number of patients in this category.
Aurora-B kinase is over-expressed in various solid tumors.29,35,36 This kinase plays an integral part in the mitotic spindle checkpoint and monitors the biorientation in the spindle-kinetochord attachment needed for precise separation of chromosomes during metaphase.25,29 Disruption of Aurora-B function at the protein or gene level has been shown to impair mitotic spindle checkpoint.29 Nguyen et al. reported that a stable mutant of Aurora-B induces tetraploidy and aneuploidy in normal murine epithelial cells.37 In addition, they found that overexpression of Aurora-B kinase induces premature chromosome separation and generation of tetraploid and aneuploid cells which, in turn, facilitates genomic instability and tumor development in a xenograft model.38 This is particularly interesting because patients with MDS, especially high-risk patients, frequently show aneuploidy and genetic instability.39 In the present study, we demonstrated for the first time that Aurora-B kinase expression was increased during disease progression of MDS. It is intriguing to consider that increased expression of Aurora-B kinase may be involved in genetic instability and aneuploidy in MDS.
Our results indicate that high levels of Survivin and Aurora-B kinase expression may be associated with complex karyotypic abonormalities in MDS. Survivin expression increased when the patients had additional chromosomal abnormalities during disease progression (Table 1). For example, Patient 3 had a complex karyotype including ring chromosome and del(5) during progression to overt leukemia. At that time, a clear increase in Survivin expression was observed. Both Survivin and Aurora-B kinase genes are located on chromosome 17. However, abnormalities of chromosome 17 are not always associated with increased Survivin and Aurora-B expression in MDS. Hoffman et al.40 demonstrated that introduction of the wild-type p53 gene into p53-null human lung cancer cells resulted in a significant decrease in Survivin gene expression. They also found that wild-type p53 repressed promoter activity in the Survivin gene by binding to this promoter.40 In contrast, mutations of the p53 gene may be involved in the upregulation of Survivin.41,42 Mutations of the p53 tumor suppressor gene can be detected in high-risk MDS.43 Patients carrying p53 mutations frequently have a complex karyotype.44,45 Therefore, it could be hypothesized that a high level of Survivin expression may be caused by mutant p53 in patients with high-risk MDS. In the present study, however, we did not examine the status of the p53 gene in each MDS patient and further studies are required to explore this hypothesis.
Several in vitro studies on the functional correlation between Survivin and Aurora-B have been reported. However, there has been no study into the expression of Survivin and Aurora-B in clinical hematologic malignancies. Here, we compared the expression of Survivin with that of a subunit of CPC complex, Aurora-B. Our data clearly indicate that the amount of Aurora-B kinase was significantly correlated with Survivin expression in MDS and s-AML, but not in de novo AML. Aurora-B kinase activity is stimulated by binding and phosphorylation of Survivin.24 These findings lead to the hypothesis that interaction between Survivin and Aurora-B may be involved in development of a malignant tumor. However, the patho-physiological meaning of the upregulation of Survivin and Aurora-B kinase expression in high-risk MDS remains unclear. Further investigation will be required to clarify this phenomenon.
Currently, both Survivin and Aurora-B kinase are considered attractive molecular targets for cancer treatment. Our present data clearly indicate that high levels of Survivin and Aurora-B kinase expression are distinctive molecular features of high-risk MDS and s-AML, emphasizing the potential of these genes as molecular targets in their treatment.
Acknowledgments
We thank Dr. Toru Geshi for his excellent advice on statistical analysis. We thank Ms Saki Tanaka for her excellent technical assistance.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding: grant-in-aid for Scientific Research (C:21591196) from Japan Society for Promotion of Science. Grant-in-aid for Translational Research from University of Fukui, 2009.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.San Miguel JF, Sanz GF, Vallespi T, del Canizo MC, Sanz MA. Myelodysplastic syndromes. Crit Rev Oncol Hematol. 1996;23(1):57–93. doi: 10.1016/1040-8428(96)00197-7. [DOI] [PubMed] [Google Scholar]
- 2.Delia D, Aiello A, Soligo D, Fontanella E, Melani C, Pezzella F, et al. bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood. 1992;79(5):1291–8. [PubMed] [Google Scholar]
- 3.Rajapaksa R, Ginzton N, Rott LS, Greenberg PL. Altered oncoprotein expression and apoptosis in myelodysplastic syndrome marrow cells. Blood. 1996;88(11):4275–87. [PubMed] [Google Scholar]
- 4.Tsoplou P, Kouraklis-Symeonidis A, Thanopoulou E, Zikos P, Orphanos V, Zoumbos NC. Apoptosis in patients with myelodysplastic syndromes: differential involvement of marrow cells in ‘good’ versus ‘poor’ prognosis patients and correlation with apoptosis-related genes. Leukemia. 1999;13(10):1554–63. doi: 10.1038/sj.leu.2401538. [DOI] [PubMed] [Google Scholar]
- 5.Parker JE, Mufti GJ, Rasool F, Mijovic A, Devereux S, Pagliuca A. The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood. 2000;96(12):3932–8. [PubMed] [Google Scholar]
- 6.Thol F, Friesen I, Damm F, Yun H, Weissinger EM, Krauter J, et al. Prognostic significance of ASXL1 mutations in patients with myelodysplastic syndromes. J Clin Oncol. 2011;29(18):2499–506. doi: 10.1200/JCO.2010.33.4938. [DOI] [PubMed] [Google Scholar]
- 7.Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 8.Malcovati L. Impact of transfusion dependency and secondary iron overload on the survival of patients with myelodysplastic syndromes. Leuk Res. 2007;31(Suppl 3):S2–6. doi: 10.1016/S0145-2126(07)70459-9. [DOI] [PubMed] [Google Scholar]
- 9.Schlegelberger B, Göhring G, Thol F, Heuser M. Update on cytogenetic and molecular changes in myelodysplastic syndromes. Leuk Lymphoma. 2012;53(4):525–36. doi: 10.3109/10428194.2011.618235. [DOI] [PubMed] [Google Scholar]
- 10.Theilgaard-Monch K, Boultwood J, Ferrari S, Giannopoulos K, Hernandez-Rivas JM, Kohlmann A, et al. Gene expression profiling in MDS and AML: potential and future avenues. Leukemia. 2011;25(6):909–20. doi: 10.1038/leu.2011.48. [DOI] [PubMed] [Google Scholar]
- 11.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–21. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 13.Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Curr Opin Cell Biol. 2006;18(6):616–22. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131(2):271–85. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Velculescu VE, Madden SL, Zhang L, Lash AE, Yu J, Rago C, et al. Analysis of human transcriptomes. Nat Genet. 1999;23(4):387–8. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58(22):5071–4. [PubMed] [Google Scholar]
- 17.Monzo M, Rosell R, Felip E, Astudillo J, Sanchez JJ, Maestre J, et al. A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J Clin Oncol. 1999;17(7):2100–4. doi: 10.1200/JCO.1999.17.7.2100. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6(1):127–34. [PubMed] [Google Scholar]
- 19.Nakayama K, Kamihira S. Survivin an important determinant for prognosis in adult T-cell leukemia: a novel biomarker in practical hemato-oncology. Leuk Lymphoma. 2002;43(12):2249–55. doi: 10.1080/1042819021000039956. [DOI] [PubMed] [Google Scholar]
- 20.Adida C, Haioun C, Gaulard P, Lepage E, Morel P, Briere J, et al. Prognostic significance of survivin expression in diffuse large B-cell lymphomas. Blood. 2000;96(5):1921–5. [PubMed] [Google Scholar]
- 21.Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, et al. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166(2):179–91. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14(8):3325–41. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolton MA, Lan W, Powers SE, McCleland ML, Kuang J, Stukenberg PT. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol Biol Cell. 2002;13(9):3064–77. doi: 10.1091/mbc.E02-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beardmore VA, Ahonen LJ, Gorbsky GJ, Kallio MJ. Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J Cell Sci. 2004;117(Pt 18):4033–42. doi: 10.1242/jcs.01242. [DOI] [PubMed] [Google Scholar]
- 25.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8(10):798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 26.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18(6):609–15. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Adams RR, Eckley DM, Vagnarelli P, Wheatley SP, Gerloff DL, Mackay AM, et al. Human INCENP colocalizes with the Aurora-B/AIRK2 kinase on chromosomes and is overexpressed in tumour cells. Chromosoma. 2001;110(2):65–74. doi: 10.1007/s004120100130. [DOI] [PubMed] [Google Scholar]
- 28.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–8. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen HG, Ravid K. Tetraploidy/aneuploidy and stem cells in cancer promotion: The role of chromosome passenger proteins. J Cell Physiol. 2006;208(1):12–22. doi: 10.1002/jcp.20565. [DOI] [PubMed] [Google Scholar]
- 30.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58(23):5315–20. [PubMed] [Google Scholar]
- 31.Adida C, Recher C, Raffoux E, Daniel MT, Taksin AL, Rousselot P, et al. Expression and prognostic significance of survivin in de novo acute myeloid leukaemia. Br J Haematol. 2000;111(1):196–203. doi: 10.1046/j.1365-2141.2000.02328.x. [DOI] [PubMed] [Google Scholar]
- 32.Wagner M, Schmelz K, Wuchter C, Ludwig WD, Dorken B, Tamm I. In vivo expression of survivin and its splice variant survivin-2B: impact on clinical outcome in acute myeloid leukemia. Int J Cancer. 2006;119(6):1291–7. doi: 10.1002/ijc.21995. [DOI] [PubMed] [Google Scholar]
- 33.Gianelli U, Fracchiolla NS, Cortelezzi A, Pellegrini C, Savi F, Moro A, et al. Survivin expression in “low-risk” and “high-risk” myelodysplastic syndromes. Ann Hematol. 2007;86(3):185–9. doi: 10.1007/s00277-006-0215-0. [DOI] [PubMed] [Google Scholar]
- 34.Invernizzi R, Travaglino E, Benatti C, Malcovati L, Della Porta M, Cazzola M, et al. Survivin expression, apoptosis and proliferation in chronic myelomonocytic leukemia. Eur J Haematol. 2006;76(6):494–501. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2588.x. [DOI] [PubMed] [Google Scholar]
- 35.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15(5):241–50. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22(4):451–64. doi: 10.1023/a:1023789416385. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen HG, Chinnappan D, Urano T, Ravid K. Mechanism of Aurora-B degradation and its dependency on intact KEN and A-boxes: identification of an aneuploidy-promoting property. Mol Cell Biol. 2005;25(12):4977–92. doi: 10.1128/MCB.25.12.4977-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen HG, Makitalo M, Yang D, Chinnappan D, St Hilaire C, Ravid K. Deregulated Aurora-B induced tetraploidy promotes tumorigenesis. FASEB J. 2009;23(8):2741–8. doi: 10.1096/fj.09-130963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lange K, Holm L, Vang Nielsen K, Hahn A, Hofmann W, Kreipe H, et al. Telomere shortening and chromosomal instability in myelodysplastic syndromes. Genes Chromosomes Cancer. 2010;49(3):260–9. doi: 10.1002/gcc.20737. [DOI] [PubMed] [Google Scholar]
- 40.Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277(5):3247–57. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- 41.Vegran F, Boidot R, Oudin C, Defrain C, Rebucci M, Lizard-Nacol S. Association of p53 gene alterations with the expression of antiapoptotic survivin splice variants in breast cancer. Oncogene. 2007;26(2):290–7. doi: 10.1038/sj.onc.1209784. [DOI] [PubMed] [Google Scholar]
- 42.Tsuji N, Furuse K, Asanuma K, Furuya M, Kondoh K, Kamagata C, et al. Mutations of the p53 gene and loss of heterozygosity at chromosome 17p13.1 are associated with increased survivin expression in breast cancer. Breast Cancer Res Treat. 2004;87(1):23–31. doi: 10.1023/B:BREA.0000041575.73262.aa. [DOI] [PubMed] [Google Scholar]
- 43.Kita-Sasai Y, Horiike S, Misawa S, Kaneko H, Kobayashi M, Nakao M, et al. International prognostic scoring system and TP53 mutations are independent prognostic indicators for patients with myelodysplastic syndrome. Br J Haematol. 2001;115(2):309–12. doi: 10.1046/j.1365-2141.2001.03073.x. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Ramirez A, Urioste M, Alvarez S, Vizmanos JL, Calasanz MJ, Cigudosa JC, et al. Cytogenetic profile of myelodysplastic syndromes with complex karyotypes: an analysis using spectral karyotyping. Cancer Genet Cytogenet. 2004;153(1):39–47. doi: 10.1016/j.cancergencyto.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Kaneko H, Misawa S, Horiike S, Nakai H, Kashima K. TP53 mutations emerge at early phase of myelodysplastic syndrome and are associated with complex chromosomal abnormalities. Blood. 1995;85(8):2189–93. [PubMed] [Google Scholar]