Abstract
p53R2 is a small subunit of ribonucleotide reductase (RR) which has 80% homology to hRRM2 and metastasis-suppressing potential. Previous reports suggested that the expression of p53R2 is used as a prognostic factor and chemotherapy response indicator in several types of cancer. This study aimed to elucidate the association of p53R2 expression and the clinicopathological characteristics of early stage non-small cell lung cancer (NSCLC). Immunohistochemistry was conducted on a tissue array including 92 early stage NSCLC samples. Correlations between p53R2 and clinicopathological factors, recurrence/metastasis and outcomes were analyzed. The analyses showed that there was no correlation between p53R2 expression and the clinicopathological factors. Among disease-free patients during follow-up, patients with p53R2(+) had a better outcome than those with p53R2(−) (P=0.022). By using Cox multivariate regression analysis, p53R2 (risk factor 3.801; 95% CI 1.004–9.454; P=0.044) served as a prognostic biomarker in the prediction of the survival rate for NSCLC patients. Detection of the RR subunit p53R2 may therefore be a useful prognostic marker in early stage NSCLC.
Keywords: p53R2, ribonucleotide reductase, non-small cell lung cancer, early stage
Introduction
Tumor stage is the most potent and widely accepted parameter predictive of survival for patients with stage I–IV non-small cell lung cancer (NSCLC) (1). Although locoregional control of NSCLC can be achieved by curative resection, more than 70% of patients with stage I disease suffer either a locoregional relapse or distant metastasis. A number of prognostic molecular markers have been described for patients with NSCLC, but none are currently in use in treatment decision making (2).
Ribonucleotide reductase (RR) is a highly regulated rate-limiting enzyme in the conversion of ribonucleoside diphosphate to 2′-deoxyribonucleoside diphosphate, which is essential for DNA synthesis (3). In humans, one large subunit (M1) and two small subunits (hRRM2 and p53R2) of RR have been identified (4). Inhibition of RR activity has been tested as a potential treatment modality in anticancer settings (5). The two RR small subunits, p53R2 and hRRM2, have an 80% similarity in the protein sequence (4). An in vitro assay showed that recombinant p53R2 protein, as well as hRRM2, interacts with hRRM1 to form a holoenzyme with the ability to convert CDP to dCDP (6,7). Piao et al demonstrated that p53R2 negatively modulates serum-induced MEK-ERK activity and inhibits the MEK-ERK-mediated malignancy potential of human cancer cells (8). Overexpression of p53R2 is associated with clinical response in myelodysplatic syndrome/acute myelogenous leukemia (9). It has been suggested that the opposite regulation of hRRM2 and p53R2 in the invasion potential may play a critical role in determining the invasion and metastasis phenotype in cancer cells (10). The implications of p53R2 expression, a regulatory subunit of RR, have yet to be determined and deserve further investigation. Subsequently, an immunohistochemical method was evaluated to determine the association between p53R2 expression and the clinical outcome of early stage NSCLC.
Materials and methods
Patients and samples
From January 2000 to December 2006, a total of 92 consecutive patients underwent surgical treatment for NSCLC at the China Medical University Hospital in Taichung, Taiwan. Patients who had pre-operative chemotherapy or radiotherapy were excluded from this study. Prior to surgery, written informed consent for the use of paraffin-embedded tissues and for information regarding sociodemographic characteristics was obtained from each patient. The study protocol was approved by the Institutional Review Board of the China Medical University Hospital. All of the available paraffin blocks were reviewed by a thoracic pathologist.
The study population consisted of 69 men and 23 women (mean age 65.3 years; range 36–83). The procedures included sampling of hilar and mediastinal lymph nodes, and the pathology of the specimens confirmed stage I (T1-2N0M0) in 66 patients and stage II (T1N1, T2N1 and T3N0) in 26 patients. Histological classification and grade were assessed by light microscopy according to the World Health Organization criteria (1). Clinical data including gender, age (≤65 vs. >65 years), smoking habits, histopathology (squamous cell carcinoma vs. adenocarcinoma vs. others), tumor stage by TNM (T1 vs. T2), lymphovascular invasion and tumor differentiation were collected from the patient charts and are shown in Table I.
Table I.
Clinicopathological characteristics of the patients with early stage lung cancer.
| Parameters | Patient no. (%) |
|---|---|
| Age (years) | |
| ≤65 | 40 (43.5) |
| >65 | 52 (56.5) |
| Gender | |
| Female | 23 (25.0) |
| Male | 69 (75.0) |
| Smoking status | |
| Negative | 46 (50.0) |
| Positive | 46 (50.0) |
| Tumor type | |
| AD | 52 (56.5) |
| SCC | 40 (43.5) |
| Tumor stage | |
| I | 66 (71.7) |
| II | 26 (28.3) |
| Lymphovascular invasion | |
| Negative | 76 (82.6) |
| Positive | 16 (17.4) |
| Recurrence/metastasis | |
| Negative | 59 (64.1) |
| Positive | 32 (35.9) |
| Differentiation | |
| Well | 12 (13.0) |
| Moderate | 55 (59.8) |
| Poor | 25 (27.2) |
AD, adenocarcinoma; SCC, squamous cell carcinoma.
Postoperative follow-up visits were scheduled 1 and 2 months after surgery, every 3 months during the first 2 years and every 6 months following that, or more frequently if required. The median duration of follow-up after a curative resection was 4.8 years.
Tissue microarray and immunohistochemical staining
Formalin-fixed and paraffin-embedded specimens were sectioned at 3 μm. The sections were then deparaffinized in xylene, rehydrated through serial dilutions of alcohol and washed in phosphate-buffered saline (PBS) (pH 7.2), which was the buffer used for all subsequent washes. For p53R2 detection, sections were heated in a microwave oven twice for 5 min in citrate buffer (pH 6.0) and then incubated with a polyclonal anti-p53R2 (Santa Cruz, CA, USA) for 90 min at 25˚C. The conventional streptavidin peroxidase method (LSAB kit K675; Dako, Copenhagen, Denmark) was performed to develop signals, and the cells were counterstained with hematoxylin. Negative controls were obtained by omitting the primary antibody. The signal intensities were evaluated independently by three observers. Cases with 0–10% positive nuclei were defined as having negative immunostaining, and cases with >10% positive nuclei were exhibited positive immunostaining. The antibody dilution buffer was used to replace antibodies to serve as a negative control.
Statistical analysis
Data were collected using an MS-Excel spreadsheet. The data were analyzed using the JMP Statistical Discovery Software version 6.0 (SAS Institute, Cary, NC, USA). For categorical data, the Fisher’s exact test or the binomial test of proportions was employed. Survival rates were estimated using the Kaplan-Meier method, and statistical analysis was carried out using the log-rank test for equality of the survival curves. Multivariate survival analysis was carried out on variables that were found to be significant with univariate analysis using the Cox proportional hazards model. Results from this model are reported as relative risk with 95% CI. Statistical significance was set at P<0.05.
Results
Relationship of p53R2 expression with clinicopathological parameters
To elucidate the role of p53R2 in tumor progression, 92 patients with early stage lung cancer, including 66 with stage I and 26 with stage II, were enrolled in this study. p53R2 protein expression in lung tumors was analyzed by immunohistochemistry in a tissue array section. p53R2 was expressed in 32 (34.8%) patients. The p53R2 proteins were expressed in the cytoplasm of the lung tumor cells as shown in Fig. 1. Table II shows that no correlation occurred between p53R2 and the clinicopathological parameters. However, p53R2 expression in the well-differentiated tumor cells had a higher trend of expression than in the poorly differentiated tumor cells (P=0.051).
Figure 1.
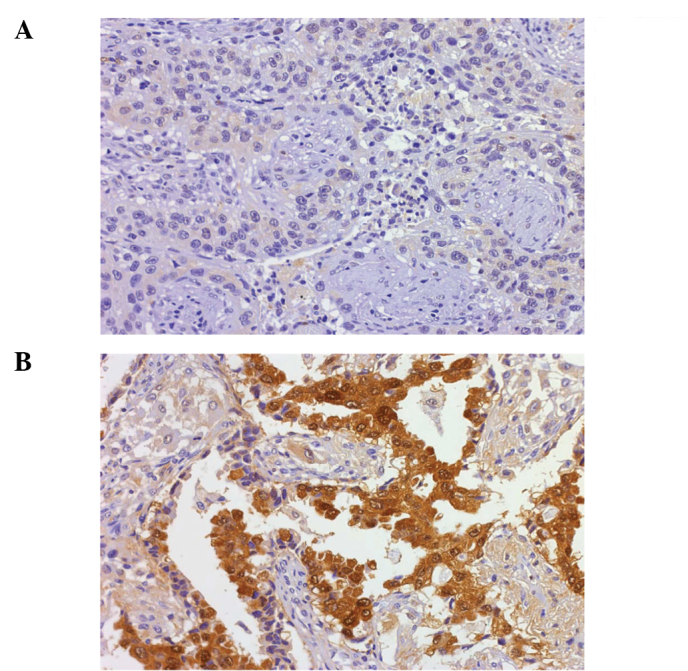
Immunohistochemical analysis of p53R2 protein in the lung tumors. (A) A negative result of immunostaining in the tumor cells (x100). (B) p53R2 protein expressed in the tumors (x400).
Table II.
Association between p53R2 protein expression and clinical characteristics in patients with early stage lung cancer.
| Parameters | p53R2 protein expression | P-value | |
|---|---|---|---|
|
|
|||
| Negative | Positive | ||
| Age (years) | |||
| ≤65 | 25 | 15 | |
| >65 | 25 | 17 | 0.207 |
| Gender | |||
| Female | 11 | 12 | |
| Male | 39 | 30 | 0.481 |
| Smoking status | |||
| Negative | 24 | 22 | |
| Positive | 26 | 20 | 0.834 |
| Tumor type | |||
| AD | 26 | 26 | |
| SCC | 24 | 16 | 0.401 |
| Tumor stage | |||
| I | 35 | 31 | |
| II | 15 | 11 | 0.817 |
| Lymphovascular invasion | |||
| Negative | 41 | 35 | |
| Positive | 9 | 7 | 1.000 |
| Recurrence/metastasis | |||
| Negative | 32 | 28 | |
| Positive | 18 | 14 | 0.829 |
| Differentiation | |||
| Well | 4 | 8a | |
| Moderate | 29 | 26 | |
| Poor | 17 | 8a | 0.121 |
AD, adenocarcinoma; SCC, squamous cell carcinoma. The Chi-square test was used for statistical analysis.
p53R2 expression in well-differentiated tumor cells had a higher trend of expression than in the poorly differentiated cells (P=0.051).
p53R2 is an unfavorable prognostic factor in early stage NSCLC
The effects of clinical characteristics on the clinical outcome of patients with early stage NSCLC were calculated by univariate analysis. Among the characteristics, p53R2 protein expression, tumor stage and tumor recurrence/metastasis were significant prognostic factors (Table III; P=0.022 for p53R2 protein, P<0.0001 for tumor stage, P<0.0001 for lymphovascular invasion, P=0.0003 for tumor recurrence/metastasis and P<0.0001 for tumor differentiation). Patients with stage I lung cancer (median survival 1,151 days) had longer survival rates than those with stage II of the disease (median survival 642 days). Patients with no lymphovascular invasion (median survival 1,103 days) had longer survival rates than those with lymphovascular invasion (median survival 555 days). Patients with no tumor recurrence/metastasis (median survival 1,082 days) had longer survival rates than those with recurrence/metastasis (median survival 868 days). Patients with p53R2 protein expression (median survival 900 days) also had longer survival rates than those without p53R2 expression (median survival 660 days) (Fig. 2). Moreover, Cox’s regression analysis showed that p53R2, tumor stage and recurrence/metastasis acted as significant independent prognostic factors (Table IV; P=0.044, 95% CI 1.004–9.454 for p53R2 protein; P=0.025, 95% CI 0.036–0.801 for tumor stage and P=0.020, 95% CI 0.094–0.816 for tumor recurrence/metastasis). Patients without p53R2 protein had a 3.483-fold higher risk than patients with p53R2. The results suggest that the presence of p53R2, tumor stage and recurrence/metastasis are similarly significant as unfavorable prognostic factors in early stage NSCLC.
Table III.
Univariate analysis of the influences of clinical characteristics on the overall survival of patients with early stage NSCLC.
| Prognostic factor | No. | Median survival (days) | 3-year survival (%) | Log-rank P-value |
|---|---|---|---|---|
| P53R2 | ||||
| Negative | 50 | 660 | 36.0 | |
| Positive | 42 | 900 | 40.1 | 0.0220 |
| Age (years) | ||||
| ≤65 | 40 | 1,074 | 36.0 | |
| >65 | 52 | 957 | 30.8 | 0.5750 |
| Gender | ||||
| Female | 23 | 1,033 | 30.5 | |
| Male | 69 | 999 | 36.3 | 0.6020 |
| Tumor type | ||||
| AD | 52 | 1,020 | 30.8 | |
| SCC | 40 | 991 | 40.0 | 0.8700 |
| Tumor stage | ||||
| I | 66 | 1,151 | 43.9 | |
| II | 29 | 642 | 11.5 | <0.0001 |
| Lymphovascular invasion | ||||
| Negative | 76 | 1,103 | 59.2 | |
| Positive | 16 | 555 | 6.25 | <0.0001 |
| Recurrence/metastasis | ||||
| Negative | 59 | 1,082 | 37.3 | |
| Positive | 32 | 868 | 31.2 | 0.0030 |
| Differentiation | ||||
| Well | 12 | 1,105 | 33.0 | |
| Moderate | 55 | 1,140 | 40.0 | |
| Poorly | 25 | 670 | 24.0 | <0.0001 |
AD, adenocarcinoma; SCC, squamous cell carcinoma. The Kaplan-Meier log-rank test was used for statistical analysis.
Figure 2.
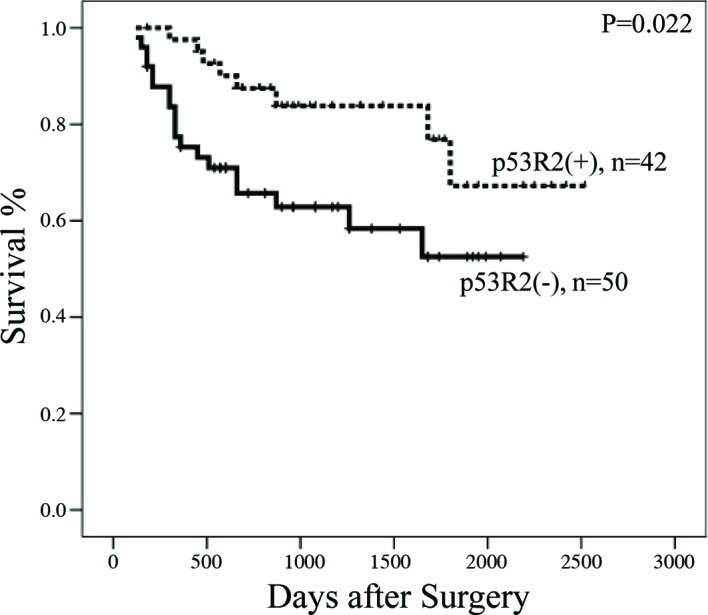
Kaplan-Meier survival curves in early stage NSCLC patients with or without p53R2 protein.
Table IV.
Cox-regression analysis of various potential prognostic factors in patients with early stage NSCLC with different p53R2 protein expression.
| Variable | RR | Unfavorable/favorable | 95% CI | P-value |
|---|---|---|---|---|
| P53R protein | 3.801 | Negative/positive | 1.004–9.454 | 0.044 |
| Tumor stage | 5.920 | II/I | 0.036–0.801 | 0.025 |
| Lymphovascular invasion | 1.360 | Positive/negative | 0.124–4.345 | 0.734 |
| Recurrence/metastasis | 3.610 | Positive/negative | 0.094–0.816 | 0.020 |
RR, ribonucleotide reductase. Cox-regression analysis was used for statistical analysis.
Discussion
Numerous reports discussed p53R2 expression in response to chemotherapy (9,11). The present study analyzed the correlation of p53R2 and RRM2 protein and their clinical significance in early stage lung cancer. A recent study concluded that the overexpression of RRM2 and p53R2, but not RRM1, in mice specifically induces lung neoplasms (12). p53R2 was also found to contain a malignancy-suppressing activity (10). The role of p53R2 expression in tumor formation has yet to be determined. Uramato et al reported that p53R2 was detected in 46.2% of lung cancer patients and was higher in patients with stage II–III, pathological T3-4 and N1-3 NSCLC (13). However, p53R2 expression could not be used as an independent prognostic marker in NSCLC (13). Additionally, previous reports showed that p53R2 expression is correlated with tumor invasion, lymph node metastasis and tumor size in esophageal and oral cancer (14,15). p53R2 expression in patients with late stage cancer was higher than that in patients with early stage esophageal cancer (14). The present study analyzed p53R2 protein expression in early stage lung cancer and found that only 45.6% (42 of 92) of the patients were positive. p53R2 protein expression was similar to previous reports (13). Additionally, we found that p53R2 protein expression was negatively correlated with tumor cell differentiation in early stage NSCLC (Table II). These findings appear to be inconsistent with a previous report (13). Therefore, we suggest that p53R2 expression in early and advanced stages of lung cancer plays a different role.
Previous reports showed that p53R2 protein expression correlates with tumorigenesis (15). However, it was found that p53R2 expression was negatively related to the metastasis of colon adenocarcinoma (16). The invasion-suppressing ability of p53R2 was also found in oropharyngeal, prostate, pancreatic and colon cancer cell lines (15,16). In addition, these authors determined that the invasion-suppressing ability of p53R2 was not related to RR enzymatic activity (10). Plao et al concluded that p53R2 negatively modulates serum-induced MEK-ERK activity and inhibits the MEK-ERK-mediated malignancy potential to suppress invasion of human lung cancer cells (8). The balance of R1 and R2 expression significantly modifies the transformation, tumorigenicity and metastatic potential (17,18). Altering the RR level may change the tumorigenicity (19). In this study, patients with p53R2(+) had a significantly higher median disease-free survival rate (900 days) compared to those with p53R2(−) (660 days) (P=0.022) (Fig. 2). Our previous study found that the disease-free survival rate was significantly higher for patients with RRM1(−) than that for patients with RRM1(+). Additionally, patients with p53R2(+)/hRRM2(−) had a significantly higher median survival rate (960 days) than that of the other three groups of patients: p53R2(−)/hRRM2(−), 810 days; p53R2(−)/hRRM2(+), 600 days and p53R2(+)/hRRM2(+), 840 days. Thus, the expression of p53R2 and RRM2 may correlate with patient survival. Further studies should be conducted to verify the RRM1 expression in the same patient group in order to understand the role of RR in lung cancer progression.
In conclusion, we showed that the p53R2 expression is negatively correlated with tumor cell differentiation, and the presence of p53R2 protein is a favorable prognostic factor in early stage lung cancer. Therefore, we propose that the expression of p53R2 is, not only a chemotherapy indicator in late stage lung cancer, but also a tumor progression marker for early stage lung cancer.
Acknowledgements
This research was supported by grants from the National Science Council (NSC 97-2314-B-040-010-MY3) of Taiwan, R.O.C.
References
- 1.Montain C. Revision in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 2.WHO. The World Health Organization histological typing of lung tumors. Am J Clin Pathol. 1982;77:123–136. doi: 10.1093/ajcp/77.2.123. [DOI] [PubMed] [Google Scholar]
- 3.Reichard P. Ribonucleotide reductases: the evolution of allosteric regulation. Arch Biochem Biophys. 2002;397:149–155. doi: 10.1006/abbi.2001.2637. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka H, Arakawa H, Yamaguchi T, et al. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404:42–49. doi: 10.1038/35003506. [DOI] [PubMed] [Google Scholar]
- 5.Cerqueira N, Pereira S, Fernandes PA, Ramos MJ. Overview of ribonucleotide reductase inhibitors: an appealing target in anti-tumour therapy. Curr Med Chem. 2005;12:1283–1294. doi: 10.2174/0929867054020981. [DOI] [PubMed] [Google Scholar]
- 6.Shao J, Zhou B, Zhu L, et al. In vitro characterization of enzymatic properties and inhibition of the p53R2 subunit of human ribonucleotide reductase. Cancer Res. 2004;64:1–6. doi: 10.1158/0008-5472.can-03-3048. [DOI] [PubMed] [Google Scholar]
- 7.Guittet O, Hakansson P, Voevodskaya N, et al. Mammalian p53R2 protein forms an active ribonucleotide reductase in vitro with the R1 protein, which is expressed both in resting cells in response to DNA damage and in proliferating cells. J Biol Chem. 2001;276:40647–4051. doi: 10.1074/jbc.M106088200. [DOI] [PubMed] [Google Scholar]
- 8.Piao C, Lin M, Kim HB, et al. Ribonucleotide reductase small subunit p53R2 suppresses MEK-ERK activity by binding to ERK kinase 2. Oncogene. 2009;28:2173–2184. doi: 10.1038/onc.2009.84. [DOI] [PubMed] [Google Scholar]
- 9.Link PA, Baer MR, James SR, et al. p53-inducible ribonucleotide reductase (p53R2/RRM2B) is a DNA hypomethylation-independent decitabine gene target that correlates with clinical response in myelodysplastic syndrome/acute myelogenous leukemia. Cancer Res. 2008;68:9358–9366. doi: 10.1158/0008-5472.CAN-08-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Zhou B, Xue L, et al. Metastasis-suppressing potential of ribonucleotide reductase small subunit p53R2 in human cancer cells. Clin Cancer Res. 2006;12:6337–6344. doi: 10.1158/1078-0432.CCR-06-0799. [DOI] [PubMed] [Google Scholar]
- 11.Furuta E, Okuda H, Aya K, Watabe K. Metabolic genes in cancer: the role in tumor progression and clinical implications. Biochim Biophys Acta. 2010;1805:141–152. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Page JL, Surtees JA, et al. Broad overexpression of ribonucleotide reductase genes in mice specifically induces lung neoplasms. Cancer Res. 2008;68:2652–2660. doi: 10.1158/0008-5472.CAN-07-5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uramoto H, Sugio K, Oyama T, et al. P53R2, p53 inducible ribonucleotide reductase gene, correlated with tumor progression of non-small cell lung cancer. Anticancer Res. 2006;25:983–988. [PubMed] [Google Scholar]
- 14.Okumura H, Natsugoe S, Yokomalura N, et al. Expression of p53R2 is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 2006;12:3740–3744. doi: 10.1158/1078-0432.CCR-05-2416. [DOI] [PubMed] [Google Scholar]
- 15.Yanamoto S, Kawasaki G, Yamada S, et al. Ribonucleotide reductase small subunit p53R2 promotes oral cancer invasion via the E-cadherin/beta-catenin pathway. Oral Oncol. 2009;45:521–525. doi: 10.1016/j.oraloncology.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Zhou B, Xue L, et al. Ribonucleotide reductase subunits M2 and p53R2 are potential biomarkers for metastasis of colon cancer. Clin Colorectal Cancer. 2007;6:374–381. doi: 10.3816/CCC.2007.n.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhou BS, Tsai P, Ker R, et al. Overexpression of transfected human ribonucleotide reductase M2 subunit in human cancer cells enhances their invasive potential. Clin Exp Metastasis. 1998;16:43–49. doi: 10.1023/a:1006559901771. [DOI] [PubMed] [Google Scholar]
- 18.Fan H, Villegas C, Wright JA. Ribonucleotide reductase R2 component is a novel malignancy determinant that cooperates with activated oncogenes to determine transformation and malignant potential. Proc Natl Acad Sci USA. 1996;93:14036–14040. doi: 10.1073/pnas.93.24.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan H, Huang A, Villegas C, Wright JA. The R1 component of mammalian ribonucleotide reductase has malignancy-suppressing activity as demonstrated by gene transfer experiments. Proc Natl Acad Sci USA. 1997;94:13181–13186. doi: 10.1073/pnas.94.24.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]


