Abstract
BACKGROUND
This study sought to determine whether [18F]fluorothymidine (FLT) positron emission tomography (PET)/computed tomography (CT) imaging allows assessment of tumor viability and proliferation in patients with soft tissue sarcomas who are treated with neoadjuvant therapy.
METHODS
Twenty patients with biopsy-proven, resectable, high-grade soft tissue sarcoma underwent [18F]FLT PET/CT imaging before and after neoadjuvant therapy. Histologic subtypes included sarcomas not otherwise specified (n = 5), malignant peripheral nerve sheath tumors (n = 3), gastrointestinal stromal tumors (n = 3), leiomyosarcomas (n = 3), angiosarcomas (n = 2), and others (n = 4). Changes in [18F]FLT peak standardized uptake value (SUVpeak) were correlated with percent necrosis in excised tissue, whereas posttreatment [18F]FLT tumor uptake was correlated with thymidine kinase 1 (TK1) expression and Ki-67 staining indices in excised tumor tissue.
RESULTS
Tumor FLT SUVpeak averaged 7.1 ± 3.7 g/mL (range, 1.9–16.1 g/mL) at baseline and decreased significantly to 2.7 ± 1.6 g/mL (range, 0.8–6.0 g/mL) at follow-up (P < .001); however, marked reductions in SUV were not specific for histopathological response. The posttreatment SUVpeak did not correlate with TK1 (P = .27) or Ki-67 expression (P = .21).
CONCLUSIONS
Marked reductions in [18F]FLT tumor uptake in response to neoadjuvant treatment were observed in most patients with sarcoma. However, these reductions were not specific for histopathologic response to neoadjuvant therapy. Furthermore, posttreatment [18F]FLT tumor uptake was unrelated to tumor proliferation by Ki-67 and TK1 staining. These results question the value of [18F]FLT PET imaging for treatment response assessments in patients with soft tissue sarcoma.
Keywords: FLT, PET/CT, sarcoma, Ki-67, TK1
[18F]Fludeoxyglucose (FDG) positron emission tomography (PET) imaging in patients with high-grade soft tissue sarcoma identifies histopathologic treatment responders with a high sensitivity after a single cycle and at the end of neo-adjuvant therapy.1,2 However, a significant fraction of patients who were classified as responders according to FDG-PET analysis were found to be histopathologic nonresponders. A noninvasive imaging approach that allows for more accurate response predictions would therefore be desirable.
Proliferative tumor activity provides important diagnostic and prognostic information and can be obtained from biopsy samples or excised tumor tissue using an antibody against the human protein Ki-67, which is expressed in actively dividing but not in resting cells (ie, those in the G0 phase).3
[3H]Methyl or [14C]thymidine have also been used extensively to determine tumor proliferation in vitro (reviewed in Bading and Shields4). Thymidine nucleotides are derived from de novo DNA synthesis via demethylation of deoxyuridine monophosphate by thymidilate synthase or through the salvage pathway via phosphorylation of thymidine by thymidine kinase 1 (TK1).
[11C]Thymidine,5 developed for imaging of tissue proliferative activity in humans, is incorporated into DNA and is therefore a noninvasive marker of tumor cell proliferation. However, due to its metabolic instability and its short physical half-life, it did not gain widespread use for imaging cancer patients (reviewed in Bading and Shields4).
Subsequently, a metabolically stable thymidine analogue, [18F]fluorothymidine (FLT), was synthesized.6,7 It is transported into cells via nucleoside transporters and is phosphorylated in the cytosol to [18F]FLT monophosphate (FLT-MP) by the cell cycle–dependent cytosolic enzyme TK1. [18F]FLT-MP is a very poor substrate for thymidylate kinase, the second kinase in the thymidine salvage pathway, and very little FLT becomes incorporated into the DNA.8 Nevertheless, the degree of [18F]FLT tumor uptake correlates with Ki-67 labeling index in most9–13 but not in all untreated tumor types,14 because TK1 activity is regulated by the cell cycle, and the highest activity is observed during S-phase.
Several preclinical reports15–18 proposed [18F]FLT as a promising marker for the noninvasive assessment of antiproliferative drug efficacy. Clinical studies addressing the correlation of [18F]FLT tumor uptake with histopathologic and clinical response assessment or patient prognosis have yielded variable results, ranging from good to poor predictability.19–30
Many therapies have a direct or indirect influence on thymidine metabolism. It is therefore unknown whether the predictive value of [18F]FLT imaging after treatment is dependent on tumor, treatment, or both and whether a correlation between [18F]FLT uptake and tissue markers of proliferation is maintained.
[18F]FLT uptake in high-grade soft tissue sarcoma is sufficient to detect tumors, with good sensitivity, and the degree of [18F]FLT uptake is significantly correlated with tumor grade.31,32 However, posttreatment [18F]FLT uptake in sarcoma has not been correlated with TK1 activity or tumor proliferative activity by Ki-67 immunostaining. The motivation behind the current study was to investigate whether posttreatment [18F]FLT uptake in sarcoma could be used to predict histopathological responses and if it correlates with tissue TK1 and Ki-67 expression.
MATERIALS AND METHODS
Adult patients (≥18 years) with resectable, biopsy-proven, high-grade soft tissue sarcoma who did not receive any treatments within 6 months of imaging were eligible for the study. Exclusion criteria included active second malignancy and unresectable disease. Furthermore, patients were excluded if performance status prevented initiation of neo-adjuvant therapy. Patients with baseline tumor [18F]FLT maximum standardized uptake value (SUVmax) of <2.5 g/ mL were excluded, because changes in response to therapy are difficult to measure in these tumors. The protocol was reviewed and approved by the Institutional Review Board for Human Subjects at UCLA. All patients provided written, informed consent after the details of the study were explained by a study physician.
[18F]FLT PET/Computed Tomography Imaging
Whole-body [18F]FLT images were obtained before the initiation (baseline scan) and after completion (follow-up scan) of neoadjuvant therapy. [18F]FLT scans were performed on an integrated PET/computed tomography (CT) system (Siemens Biograph 64 TruePoint PET/CT). Patients were instructed to fast for at least 6 hours before imaging.
[18F]FLT was injected intravenously at an average dose of 6.64 ± 0.50 mCi (range, 5.7–8.1 mCi) approximately 60 minutes before image acquisition. The CT portion of the study was performed with intravenous contrast (Omnipaque; Novaplus) in all but 4 patients (contrast allergies, n = 2; borderline creatinine levels, n = 2). The use of intravenous contrast results in only minor changes of tumor SUV.33 The CT acquisition parameters were: 120 kV peak, 170 mA·s, 0.5-s tube rotation, 5-mm slice collimation, and a pitch of 0.8 mm.
To minimize misregistration between the CT and PET images, patients were instructed to use shallow breathing during the image acquisition.34 The CT images were reconstructed using filtered back-projection, at 3.4-mm axial intervals to match the slice separation of the PET data. PET images were reconstructed using an iterative algorithm (ordered subset expectation maximization [OSEM], 2 iterations, 8 subsets). To correct for photon attenuation, a previously published CT-based algorithm was applied.35 The time interval between baseline PET/ CT and the initiation of neoadjuvant therapy was 7 ± 8.7 days, and 13.6 ± 9.3 days between end of treatment and follow-up [18F]FLT-PET/CT. All patients were taken into surgery for planned tumor resection at an average of 5 ± 2.9 days after follow-up [18F]FLT PET/CT imaging.
PET/CT Image Analysis
[18F]FLT tumor uptake was measured by determination of the peak SUV (SUVpeak) as described.36 Regions of interest (ROI) were drawn around the area of abnormal tracer uptake, whereby care was taken to exclude areas of physiological tracer uptake. The software program (Mirada; REVEAL-MVS; CTI Mirada Solutions) automatically determined the pixel with the highest tracer accumulation (SUVmax) in these regions. A ROI with a diameter of 1.5 cm was drawn around the SUVmax, and the average uptake in this ROI (SUVpeak) was defined as the SUVpeak.36 The maximum tumor diameters were measured on baseline and follow-up CT scans.
Histopathology
Pathology specimens were reviewed by a pathologist with expertise in sarcoma pathology; imaging results were not available to the pathologist at the time of histopathologic review. Posttreatment tumor necrosis and/or fibrosis was assessed as described.2 Histopathologic response was classified by determining the fraction of necrotic or fibrotic tissue of the entire excised tumor. On the basis of previous studies, patients whose tumors showed ≥95% necrosis or fibrosis were classified as histopathologic responders.37
Posttreatment proliferative activity was assessed in all tumors by Ki-67 and TK1 immunohistochemical staining of those formalin-fixed, paraffin-embedded tissue sections that showed the greatest viability and mitotic activity by standard light microscopy. Full tissue sections were selected from viable sections of the tumor. These sections averaged 1.5 cm in width and 2 to 3 cm in length. Given that entire tissue sections were used and that the Ki-67 scoring was based on all viable tumor cells within that tissue section, we believe this to reasonably reflect tissue proliferative activity within a significant tumor block.
Sections were deparaffinized in xylene and rehydrated in graded alcohol series. Endogenous peroxidase activity was blocked with 3% H2O2. Antigen retrieval was performed by boiling the sections in 0.01 M citric acid buffer (pH 6.0) for 15 minutes. Sections were first blocked with 5% normal donkey serum, and then incubated with primary antibody against Ki-67 (VP-RM04, 1:500; Vector Laboratories) and TK1 (1:100, catalog no. 2563; Epitomics) overnight at 4°C. Sections were then incubated with biotinylated secondary antibody (1:500; Jackson ImmunoResearch Laboratories) for 1 hour at room temperature. Antibody binding was assessed with Vectastain ABC Elite Kit (PK-6100; Vector Laboratories) and was visualized by incubation with 3,3′-diaminobenzidine (Vector Laboratories). Sections were counterstained in Gill’s hematoxylin. Negative and positive control slides were included. Proliferative activity and TK1 expression were defined as the percentage of positively stained tumor cells relative to total tumor cells within the section, and were reviewed independently by 2 pathologists.
Statistical Analysis
The Spearman rank correlation was used to assess the relationship between 2 quantitative variables. The Wilcoxon signed rank test was used to compare changes in FLT uptake from baseline to follow-up. The Fisher exact test was used to correlate metabolic and histopathologic response. SPSS software for Windows (version 14.0, SPSS, Inc., Chicago, Ill) and Statistica, version 8.0 for Windows (StatSoft, Inc., Tulsa, Okla) were used for the statistical analysis. All quantitative parameters are expressed as mean ± standard deviation, median, and range. P < .05 was considered statistically significant.
RESULTS
From October 2008 to July 2009, 26 patients were prospectively enrolled. Six were excluded after they underwent baseline [18F]FLT-PET/CT imaging. Two of these (sarcoma not otherwise specified [NOS] and myxofibrosarcoma) were excluded due to low baseline tumor [18F]FLT uptake (SUVpeak, 1.2 and 1.3 g/mL, respectively). Further diagnostic workup after the [18F]FLT scan revealed unresectable disease in 2 patients with leio-myosarcoma and in 1 patient with liposarcoma who had a second malignancy (hepatocellular carcinoma). One patient declined to undergo the follow-up [18F]FLT-PET/CT scan. Therefore, 20 patients completed this imaging study.
The patient characteristics are listed in Table 1. In brief, there were 11 females and 9 males with a mean age of 57 ± 18 years (median, 62 years; range, 26–94 years). Seventeen (85%) had primary disease, and 3 (15%) presented with recurrent disease.
Table 1.
Clinical, Pathologic, and Treatment Characteristics (n = 20)
| Characteristics | n (%) |
|---|---|
| Age, y | |
| Median (range) | 62 (26–94) |
| Sex | |
| Male | 9 (45) |
| Female | 11 (55) |
| Site | |
| Extremity | 10 (50) |
| Chest/trunk | 6 (30) |
| Retroperitoneal/Abdominal | 4 (20) |
| Presentation status | |
| Primary | 17 (85) |
| Recurrent | 3 (15) |
| Tumor size | |
| <5 cm | 2 (10) |
| 5–10 cm | 12 (60) |
| >10 cm | 6 (30) |
| Histology | |
| NOS | 5 (25) |
| MPNST | 3 (15) |
| GIST | 3 (15) |
| Leiomyosarcoma | 3 (15) |
| Angiosarcoma | 2 (10) |
| Others | 4 (20) |
| Grade | |
| High | 20 (100) |
| Neoadjuvant therapy | |
| Chemoradiation | 9 (45) |
| Chemotherapy | 6 (30) |
| Gleevec | 3 (15) |
| Radiation | 2 (10) |
| Pathologic | |
| Responder | 3 (15) |
| Nonresponder | 17 (85) |
Abbreviations: GIST, gastrointestinal stromal tumor; MPNST, malignant peripheral nerve sheath tumor; NOS, sarcoma not otherwise specified.
All patients underwent neoadjuvant therapy followed by complete surgical resection. Nine patients (45%) underwent neoadjuvant ifosfamide-based treatments, whereas 4 patients (20%) had gemcitabine-based therapy. Standard ifosfamide-based chemotherapy consisted of two 28-day cycles of ifosfamide (14 g/m2) given via a continuous venous infusion 24 hours per day over a 7-day period. The ifosfamide therapy was often followed by a single dose of doxorubicin (60–90 mg/m2) given concurrently with radiation. Standard gemcitabine-based chemotherapy consisted of 2 cycles of gemcitabine (900 mg/m2 through a 10 mg/m2/minute infusion on day 1 and 8 of a 21-day cycle) and docetaxel (75–100 mg/m2 on day 8 of a 21-day cycle).
One patient underwent treatment with Adriamycin (doxorubicin; 75 mg/m2) and another patient was treated with Taxol (paclitaxel; 175 mg/m2) and bevacizumab. Gastrointestinal stromal tumors (GISTs; n = 3; 15%) were treated with imatinib at a dose of 400 mg per os daily. Two patients (10%) received neoadjuvant external beam radiation only. Nine patients (45%) underwent neoadjuvant chemoradiation therapy.
Tumor Size
The baseline tumor diameter averaged 8.3 ± 4.3 cm (median, 6.4 cm; range, 1.2–18.0 cm). It decreased to 6.9 ± 3.4 cm (median, 6.1 cm; range, 1.7–14.5 cm) at follow-up (P = .02).
Tumor FLT Uptake
The SUVpeak averaged 7.1 ± 3.7 g/mL (median, 7.1 g/ mL; range, 1.9–16.1 g/mL) at baseline and decreased significantly to 2.7 ± 1.6 g/mL (median, 2.5 g/mL; range, 0.8–6.0 g/mL) at follow-up (P < .001). Spearman correlation revealed a poor but significant correlation between changes in tumor [18F]FLT uptake and changes in tumor size (r = 0.52; P = .02).
Histopathologic and [18F]FLT Response
The extent of necrosis in excised tumor tissue as an index of tumor response averaged 52% ± 33% (median, 55%; range, 0%–99%). Three patients (15%) were classified as histopathologic responders (≥95% necrosis/fibrosis in the excised tumor). Histopathological responses occurred in 1 patient with angiosarcoma, 1 patient with sarcoma NOS, and 1 patient with malignant peripheral nerve sheath tumor.
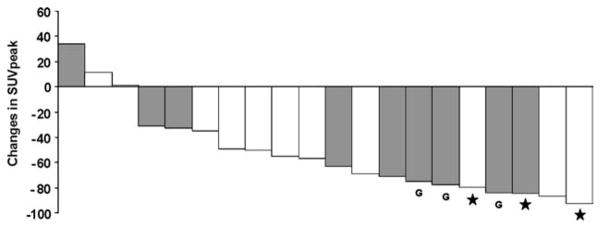
Changes in [18F]FLT uptake were significantly correlated with the extent of tumor necrosis (r = −0.68; P = .001) (Fig. 1). However, prominent decreases in [18F]FLT uptake were also observed in histopathologic nonresponders (Figs. 1 and 2). The relationship between extent of tumor necrosis and changes in SUVpeak is shown in a waterfall diagram (Fig. 3).
Figure 1.
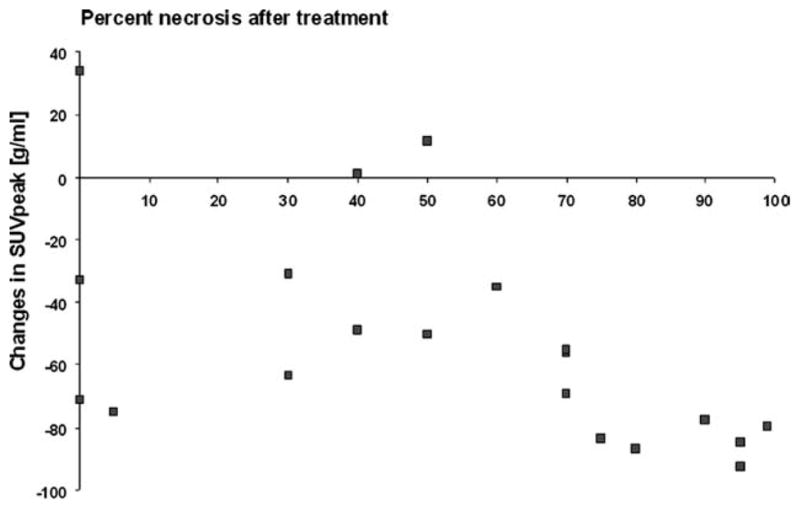
Changes in peak standardized uptake value (SUV-peak) from baseline to follow-up are poorly but significantly correlated with percent of histopathologic necrosis in the excised tumor tissue (r = −0.68; P = .001).
Figure 2.
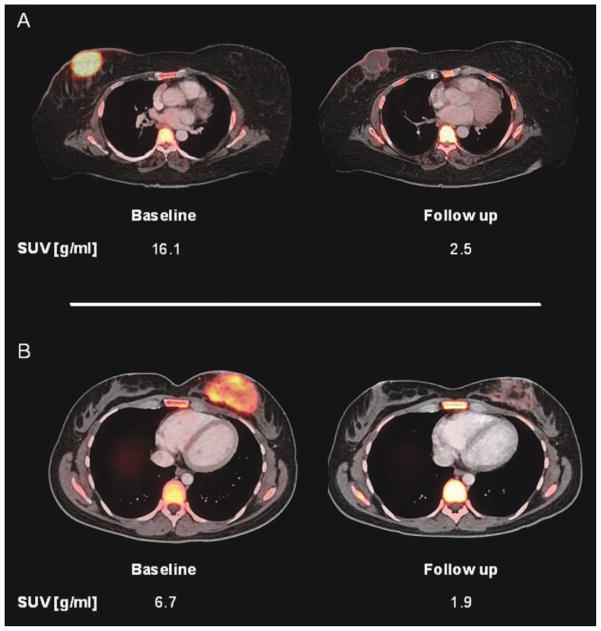
Baseline and follow-up [18F]fluorothymidine positron emission tomography/computed tomography images are shown of 2 patients with angiosarcoma of the breast. The patient shown in (A) exhibited >95% tissue necrosis after neoadjuvant treatment and was classified as a histopathologic responder. The patient in (B) was a histopathologic nonresponder with <5% tissue necrosis after treatment. However, comparable decreases in fluorothymidine uptake of 85% and 71%, respectively, were seen. Therefore, the patient in (B) was misclassified as a metabolic responder by fluorothymidine positron emission tomography analysis. SUVpeak indicates peak standardized uptake value.
Figure 3.
Changes in peak standardized uptake value (SUV-peak) after completion of chemotherapy are shown. Patients who received chemotherapy or Gleevec only are depicted in gray. Patients with gastrointestinal stromal tumors are indicated by letter “G”. Three patients, indicated by stars (★), were classified as histopathologic responders.
[18F]FLT Uptake and Tumor Tissue Proliferation Markers
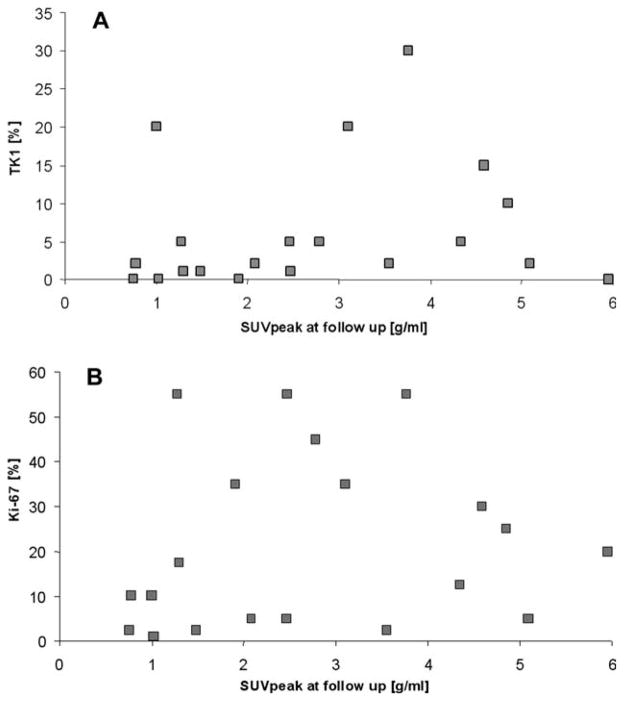
The TK1 and Ki-67 staining index in excised tumor tissue averaged 6.6% ± 8.5% (median, 2.0%; range, 0.0%–30.0%) and 21.4 ± 19.3% (median, 15.0%; range, 1.0%–55.0%), respectively. Spearman rank correlation revealed a poor but significant correlation between TK1 and Ki-67 expression after neoadjuvant treatment (r = 0.53; P = .02). However, neither posttreatment TK1 (r = 0.26; P = .27; Fig. 4A) nor Ki-67 activity (r = 0.29; P = .21; Fig. 4B) correlated with posttreatment tumor [18F]FLT uptake.
Figure 4.
Lack of correlation is shown between (A) posttreatment peak standardized uptake value (SUVpeak) and thymidine kinase 1 (TK1) (r = 0.26; P = .27) and (B) posttreatment SUVpeak and Ki-67 (r = 0.29; P = .21).
To account for the heterogeneous study population, a subset analysis in 14 patients was performed that excluded those with GIST (n = 3), those undergoing radiotherapy only (n = 2), as well as those who received Avastin (bevacizumab; n = 1). Again, no significant correlation between tumor [18F]FLT uptake and TK1 or Ki-67 was found. (TK1: r = 0.23, P = .43; Ki-67: r = −0.082, P = .78).
To account for disease heterogeneity, we conducted an analysis that only included the largest sarcoma subgroup (sarcomas NOS, n = 5). In this group, TK1 expression but not Ki-67 expression was significantly correlated with posttreatment tumor [18F]FLT uptake (r = 0.90; P = .04 and r = 0.21; P = .74, respectively).
DISCUSSION
In this study, we explored whether PET imaging of tumor proliferative activity using [18F]FLT can provide specific treatment response assessments in patients with soft tissue sarcoma. Furthermore, we investigated whether imaging findings reflected tumor proliferative activity as determined by immunohistochemical assays.
We found that tumor [18F]FLT uptake after treatment was poorly but significantly correlated with the extent of posttreatment tumor necrosis, but was unrelated to TK1 expression and tumor proliferative activity by Ki-67 staining. The [18F]FLT uptake decreased by more than 20% in 17 of 20 patients (Fig. 3) and by more than 60% in 10 of 20 patients after completion of neoadjuvant therapy. However, only 3 of these 10 patients were classified as histopathologic responders. Changes in tumor uptake of [18F]FLT were poorly but significantly correlated with histopathologic response (Figs. 1 and 2). Although there is no reason to expect, biologically, that a measure of cellular proliferation would correlate with cell death, it is reasonable to test this correlation from a clinical standpoint, because earlier studies have shown that the degree of tissue necrosis after completion of neoadjuvant sarcoma therapy has prognostic value for long-term outcome.37
Furthermore, FLT uptake was unrelated to TK1 and Ki-67 expression in excised tissue (Fig. 4). These observations suggest that measurements of [18F]FLT tumor uptake after neoadjuvant chemotherapy and/or radiation therapy are unlikely to improve treatment response assessment as compared with [18F]FDG-PET.
The current study population was heterogeneous with regards to tumor histology and treatment approaches. This problem is not unique to sarcomas, because many other cancers also have many subtypes with varying biological characteristics and differing treatment approaches. However, to account for treatment heterogeneity, we conducted a subgroup analysis that excluded patients with GIST, those undergoing radiotherapy only, as well as those who received an angiogenesis inhibitor. No correlations between posttreatment tumor [18F]FLT uptake and Ki-67 or TK1 expression were found in this subgroup. Therefore, treatment heterogeneity did not appear to have an effect on the correlation between post-treatment imaging findings and immunohistochemistry.
To address the issue of disease heterogeneity, we conducted another analysis that only included the largest sarcoma subgroup (sarcomas NOS, n = 5). Here, TK1 expression but not Ki-67 expression was significantly correlated with posttreatment tumor [18F]FLT uptake. Thus, posttreatment [18F]FLT uptake might follow the expected correlation with TK1 in some sarcoma subtypes.
Imaging tests that are useful for response assessment only in subgroups of soft tissue sarcomas (or any other cancers) are, in our view, of limited clinical value. Any imaging biomarker proposed or used for therapy response assessments must be applicable across a wide spectrum of cancers, and within cancers, across a variety of genomic alterations and therapeutic approaches. This wide applicability has made [18F]FDG imaging successful for therapeutic response assessments.
We show in the current study that most sarcomas have a strong [18F]FLT phenotype at baseline (ie, are “routinely [18F]FLT avid”). If [18F]FLT should prove useful for 1) providing readouts of proliferative activity and 2) changes in proliferative activity in response to treatment, the underlying disease subtype as well as the treatment strategy should be irrelevant.
[18F]FLT was introduced as an indirect marker of tumor cell proliferative activity, the target enzyme being the S-phase–dependent TK1. In other words, [18F]FLT imaging is supposed to image the proliferative tumor phenotype regardless of underlying tumor category. Because tumor [18F]FLT uptake correlates with pretreatment Ki-67 expression, its use for monitoring the therapeutic effects on proliferative activity has been promoted. In the current study, we used an immunohistochemical approach to stain for TK1 protein. This approach is justified because TK1 protein levels are correlated with tumor proliferative activity38 and TK1 enzyme activity.39 However, even though TK1 activity is considered to be the key determinant of [18F]FLT uptake, other factors such as up-or down-regulation of the equilibrative nucleoside transporter 1 have been shown to significantly alter [18F]FLT uptake in vivo.40,41 Such altered equilibrative nucleoside transporter 1 expression and/or activity might have contributed to the lack of correlation between tumor FLT uptake and TK1 or Ki-67 expression.
Because ineffective treatment approaches can be modified, early therapy response assessments are important for managing patients with cancer. Radiation and chemotherapy, if successful, decrease tumor cell proliferation rates and/or kill tumor cells rapidly.42 These changes precede reductions in tumor size as determined by the Response Evaluation Criteria In Solid Tumors (RECIST) criteria,43 and it is clinically important to detect such changes as early as possible after the start of treatment.
Most clinical studies reported a significant correlation between Ki-67 expression and [18F]FLT tumor uptake across many types of cancers in patients who were not recently treated.9–13 The relationship between TK1 protein levels or enzymatic activity and [18F]FLT uptake has not been investigated extensively. However, in a study of 17 patients with oral cancer, pretreatment [18F]FLT tumor uptake did not correlate with tumor TK1 antibody staining.24 Nevertheless, the lack of correlation between [18F]FLT uptake in sarcoma and TK1 and Ki-67 expression in the current study was surprising. Animal experimental studies have shown that [18F]FLT tumor uptake decreases rapidly in response to a variety of therapeutic interventions,15–17,44 and some have reported earlier response predictions with [18F]FLT than with [18F]FDG.15,17
Most clinical studies have shown marked decreases in tumor [18F]FLT uptake in response to a variety of treatments. However, the predictive value of these changes was inconsistent. In patients with glioblastoma, the degree of reductions in [18F]FLT uptake at 1 to 2 weeks after start of treatment was predictive of long-term survival.19 In breast cancer, reductions in [18F]FLT uptake after 1 cycle of chemotherapy correlated with late changes in tumor markers and tumor size.20 Reductions in [18F]FLT uptake 1 week after start of treatment were predictive of progression-free survival in patients with lung cancer who were treated with gefitinib.21 Variable [18F]FLT responses with increased tumor uptake (flare) in 1 of 5 patients was observed in response to radiation treatment in patients with lung cancer.22
Less encouraging findings were reported in patients with rectal cancer who were imaged with [18F]FLT 2 weeks after the start of neoadjuvant chemoradiation treatment.23 In this study, histopathological responders and nonresponders, who are defined by the fraction of necrotic tissue in excised tumor tissue, showed comparable decreases in [18F]FLT tumor uptake. A uniform and therefore possibly nonspecific decrease in [18F]FLT uptake was also observed in response to radiation therapy in patients with head and neck cancer24 and in patients with lymphoma early after start of chemotherapy.25 In another study of patients with lymphoma, posttreatment [18F]FLT tumor uptake did not provide more accurate prognostic information than [18F]FDG imaging.26 Finally, neither changes in [18F]FLT nor [18F]FDG uptake in response to treatment predicted histopathological responses in patients with metastatic germ cell tumors.27
Several factors might account for the variable predictive value of [18F]FLT imaging. First, the magnitude of changes in [18F]FLT uptake might be dependent on treatment and/or tumor factors, as discussed above. In the current study, 13 of the 20 patients received ifosfamide-or gemcitabine-based chemotherapies.45 Both drugs exert complex effects on cellular deoxyribonucleotide pools, which, by feedback mechanisms, can affect TK1 enzymatic activity and/or transporter mechanisms that affect tumor [18F]FLT uptake.
Second, 11 patients (55%) received radiation treatment that is known to reduce tumor vascularization and perfusion,46 which could also reduce tumor [18F]FLT uptake. Another potential explanation considers leakage of TK1 and/or thymidine in the tumor microenvironment, which may also interfere with tumor [18F]FLT uptake.47,48 In addition, efflux pumps associated with chemotherapy resistance might also affect tumor [18F]FLT uptake after treatment.49 Moreover, [18F]FLT-MP dephosphorylation by cytoplasmic nucleotidases might reduce [18F]FLT retention by the tumor.
Finally, the lack of correlation might be unrelated to altered [18F]FLT kinetics in response to treatment. Ki-67 may not be an ideal marker of tumor proliferation after therapy. This protein is expressed throughout the cell cycle except during the G0 phase. Therefore, cell cycle arrest in any other phase in response to treatment should leave tumor cells positive for Ki-67.
Several tumors had a level of Ki-67 labeling close to 0%, but tumor SUVs as high as >5 g/mL were observed (Fig. 4B). This is in contrast to the studies in untreated tumors where there was generally very little [18F]FLT uptake in tumors with a Ki-67 labeling index of less than ~20.9,31 One could speculate that this indicates DNA repair in the absence of proliferation, as recently reported.50 High [18F]FLT uptake was observed in some tumors without measurable TK1 expression. In fact, the highest 2 SUVs (~5.1 and 6) were measured in lesions with no or very few TK1-positive cells. This might indicate that TK1 staining is insensitive to detecting active TK1. Alternatively, and despite our attempts to identify by light microscopy the tumor sections that showed greatest viability and mitotic activity, sampling errors cannot be ruled out with certainty. Regardless of the underlying mechanisms that cannot be uncovered from the current data, the lack of relationship between tissue markers of proliferation and tumor [18F]FLT uptake after treatment raises questions about the usefulness of this approach in patients with soft tissue sarcoma.
This study has several limitations. First, measurement inaccuracies of TK1 and Ki-67 activity cannot be ruled out. However, the significant correlation between TK1 and Ki-67 staining index argues against such error. To accurately correlate [18F]FLT tumor uptake to immunohistochemical results, we conducted the imaging studies as closely as possible to the date of surgery (average time interval, 5 ± 2.9 days). Therefore, the time from end of treatment to follow-up imaging was more variable and averaged 13.6 ± 9.3 days.
Second, as described above, the study population was heterogeneous and included patients with various sarcoma subtypes, who underwent various treatments.
Third, the study population included only 20 patients with soft tissue sarcoma. Therefore, we cannot exclude a weak correlation between [18F]FLT uptake and Ki-67 labeling if more patients had been enrolled in this study. However, for [18F]FLT-PET to emerge as a clinically useful test for response assessments, a close correlation between [18F]FLT SUVs and histopathologic response and/or proliferation would be necessary. On the basis of our data, this appears to be very unlikely.
Fourth, we used SUVs to quantify [18F]FLT uptake. This decision was based on a series of articles which demonstrated 1) that [18F]FLT SUVs and influx constants (Ki) show a close correlation in untreated tumors, 2) that [18F]FLT SUVs correlate well with histopathologic markers of tumor cell proliferation, and 3) that [18F]FLT SUVs are easier to measure clinically than tracer kinetic parameters. Further studies are needed to investigate whether tracer kinetic analysis51,52 of [18F]FLT uptake in treated tumors allows more accurate assessments of tumor proliferation in soft tissue sarcoma than do SUV measurements.
Fifth, Eilber et al37 have shown that a large extent of tissue necrosis following neoadjuvant treatment of patients with sarcoma is associated with improved patient outcome. However, to the best of our knowledge, this has not been proven for patients with GISTs.
In summary, in patients with high-grade soft tissue sarcoma, [18F]FLT PET/CT imaging does not reliably predict histopathological response to neoadjuvant therapy, and [18F]FLT uptake is unrelated to TK1 and Ki-67 expression. These findings suggest that response assessments based on [18F]FLT-PET analysis do not provide an advantage over [18F]FDG-based response assessments in patients with soft tissue sarcoma. Moreover, the reasons for the uncoupling between [18F]FLT retention and TK1 and Ki-67 activity in sarcoma warrants further studies. Given the limited value of [18F]FLT-PET in predicting therapeutic responses in rectal cancer,23 germ cell tumors,27 and now in soft tissue sarcoma, further studies in other cancers are needed to better understand the altered [18F]FLT kinetics in patients after treatment.
Acknowledgments
FUNDING SOURCES
Grant support was provided by a Jonsson Comprehensive Cancer Center Collaboration Grant, Sarcoma Foundation of America, University of California, Cancer Research Coordinating Committee, University of California at Los Angeles In Vivo Cellular and Molecular Imaging Centers, grant P50 CA086306.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors made no disclosure.
References
- 1.Evilevitch V, Weber WA, Tap W, et al. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2008;14:715–720. doi: 10.1158/1078-0432.CCR-07-1762. [DOI] [PubMed] [Google Scholar]
- 2.Benz M, Czernin J, Allen-Auerbach M, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2009;15:2856–2863. doi: 10.1158/1078-0432.CCR-08-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Bading JR, Shields AF. Imaging of cell proliferation: status and prospects. J Nucl Med. 2008;49(suppl 2):64S–80S. doi: 10.2967/jnumed.107.046391. [DOI] [PubMed] [Google Scholar]
- 5.Christman D, Crawford EJ, Friedkin M, Wolf AP. Detection of DNA synthesis in intact organisms with positron-emitting [methyl-11C]thymidine. Proc Natl Acad Sci U S A. 1972;69:988–992. doi: 10.1073/pnas.69.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grierson J, Shields A, Eary J. Development of a radiosynthesis for 3′-[F-18]fluoro-3′-deoxynucleosides. J Labelled Comp Radiopharm. 1997;40:60–62. [Google Scholar]
- 7.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 8.Kong XB, Zhu QY, Vidal PM, et al. Comparisons of anti-human immunodeficiency virus activities, cellular transport, and plasma and intracellular pharmacokinetics of 3′-fluoro-3′-deoxythymidine and 3′-azido-3′-deoxythymidine. Antimicrob Agents Chemother. 1992;36:808–818. doi: 10.1128/aac.36.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck AK, Schirrmeister H, Hetzel M, et al. 3-Deoxy-3-[18F]fluorothymidine-positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res. 2002;62:3331–3334. [PubMed] [Google Scholar]
- 10.Vesselle H, Grierson J, Muzi M, et al. In vivo validation of 3′deoxy-3′-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–3323. [PubMed] [Google Scholar]
- 11.Yamamoto Y, Nishiyama Y, Ishikawa S, et al. Correlation of 18F-FLT and 18F-FDG uptake on PET with Ki-67 immunohistochemistry in non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007;34:1610–1616. doi: 10.1007/s00259-007-0449-7. [DOI] [PubMed] [Google Scholar]
- 12.Yap CS, Czernin J, Fishbein MC, et al. Evaluation of thoracic tumors with 18F-fluorothymidine and 18F-fluorodeoxyglucose-positron emission tomography. Chest. 2006;129:393–401. doi: 10.1378/chest.129.2.393. [DOI] [PubMed] [Google Scholar]
- 13.Kenny LM, Vigushin DM, Al-Nahhas A, et al. Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18F]fluorothymidine-positron emission tomography imaging: evaluation of analytical methods. Cancer Res. 2005;65:10104–10112. doi: 10.1158/0008-5472.CAN-04-4297. [DOI] [PubMed] [Google Scholar]
- 14.van Westreenen HL, Cobben DCP, Jager PL, et al. Comparison of 18F-FLT PET and 18F-FDG PET in esophageal cancer. J Nucl Med. 2005;46:400–404. [PubMed] [Google Scholar]
- 15.Barthel H, Cleij M, Collingridge D, et al. 3′-Deoxy-3′-[18F]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer Res. 2003;63:3791–3798. [PubMed] [Google Scholar]
- 16.Sugiyama M, Sakahara H, Sato K, et al. Evaluation of 3′-deoxy-3′-18F-fluorothymidine for monitoring tumor response to radiotherapy and photodynamic therapy in mice. J Nucl Med. 2004;45:1754–1758. [PubMed] [Google Scholar]
- 17.Waldherr C, Mellinghoff IK, Tran C, et al. Monitoring anti-proliferative responses to kinase inhibitor therapy in mice with 3′-deoxy-3′-18F-fluorothymidine PET. J Nucl Med. 2005;46:114–120. [PubMed] [Google Scholar]
- 18.Wei LH, Su H, Hildebrandt IJ, Phelps ME, Czernin J, Weber WA. Changes in tumor metabolism as readout for Mammalian target of rapamycin kinase inhibition by rapamycin in glioblastoma. Clin Cancer Res. 2008;14:3416–3426. doi: 10.1158/1078-0432.CCR-07-1824. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluoro-thymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 20.Pio BS, Park CK, Pietras R, et al. Usefulness of 3′-[F-18]fluoro-3′-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol Imaging Biol. 2006;8:36–42. doi: 10.1007/s11307-005-0029-9. [DOI] [PubMed] [Google Scholar]
- 21.Sohn HJ, Yang YJ, Ryu JS, et al. [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adeno-carcinoma of the lung. Clin Cancer Res. 2008;15:7423–7429. doi: 10.1158/1078-0432.CCR-08-0312. [DOI] [PubMed] [Google Scholar]
- 22.Everitt S, Hicks RJ, Ball D, et al. Imaging cellular proliferation during chemo-radiotherapy: a pilot study of serial 18F-FLT positron emission tomography/computed tomography imaging for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;75:1098–1104. doi: 10.1016/j.ijrobp.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Wieder HA, Geinitz H, Rosenberg R, et al. PET imaging with [18F]3′-deoxy-3′-fluorothymidine for prediction of response to neoadjuvant treatment in patients with rectal cancer. Eur J Nucl Med Mol Imaging. 2007;34:878–883. doi: 10.1007/s00259-006-0292-2. [DOI] [PubMed] [Google Scholar]
- 24.Troost EG, Bussink J, Hoffmann AL, Boerman OC, Oyen WJ, Kaanders JH. 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med. 2010;51:866–874. doi: 10.2967/jnumed.109.069310. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann K, Wieder HA, Buck AK, et al. Early response assessment using 3′-deoxy-3′-[18F]fluorothymidine-positron emission tomography in high-grade non-Hodgkin’s lymphoma. Clin Cancer Res. 2007;13:3552–3558. doi: 10.1158/1078-0432.CCR-06-3025. [DOI] [PubMed] [Google Scholar]
- 26.Kasper B, Egerer G, Gronkowski M, et al. Functional diagnosis of residual lymphomas after radiochemotherapy with positron emission tomography comparing FDG- and FLT-PET. Leuk Lymphoma. 2007;48:746–753. doi: 10.1080/10428190601113568. [DOI] [PubMed] [Google Scholar]
- 27.Pfannenberg C, Aschoff P, Dittmann H, et al. PET/CT with 18F-FLT: does it improve the therapeutic management of metastatic germ cell tumors? J Nucl Med. 2010;51:845–853. doi: 10.2967/jnumed.109.070425. [DOI] [PubMed] [Google Scholar]
- 28.Been LB, Suurmeijer AJ, Elsinga PH, Jager PL, van Ginkel RJ, Hoekstra HJ. 18F-Fluorodeoxythymidine PET for evaluating the response to hyperthermic isolated limb perfusion for locally advanced soft-tissue sarcomas. J Nucl Med. 2007;48:367–372. [PubMed] [Google Scholar]
- 29.Kenny L, Coombes RC, Vigushin D, Al-Nahhas A, Shousha S, Aboagye EO. Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur J Nucl Med Mol Imaging. 2007;34:1339–1347. doi: 10.1007/s00259-007-0379-4. [DOI] [PubMed] [Google Scholar]
- 30.Yue J, Chen L, Cabrera AR, et al. Measuring tumor cell proliferation with 18F-FLT PET during radiotherapy of esophageal squamous cell carcinoma: a pilot clinical study. J Nucl Med. 2010;51:528–534. doi: 10.2967/jnumed.109.072124. [DOI] [PubMed] [Google Scholar]
- 31.Buck AK, Herrmann K, Meyer zum Büschenfelde CM, et al. Imaging bone and soft tissue tumors with the proliferation marker [18F]fluorodeoxythymidine. Clin Cancer Res. 2008;14:2970–2977. doi: 10.1158/1078-0432.CCR-07-4294. [DOI] [PubMed] [Google Scholar]
- 32.Cobben DC, Elsinga PH, Suurmeijer AJ, et al. Detection and grading of soft tissue sarcomas of the extremities with (18)F-3′-fluoro-3′-deoxy-L-thymidine. Clin Cancer Res. 2004;10:1685–1690. doi: 10.1158/1078-0432.ccr-03-0040. [DOI] [PubMed] [Google Scholar]
- 33.Mawlawi O, Erasmus JJ, Munden RF, et al. Quantifying the effect of IV contrast media on integrated PET/CT: clinical evaluation. AJR Am J Roentgenol. 2006;186:308–319. doi: 10.2214/AJR.04.1740. [DOI] [PubMed] [Google Scholar]
- 34.Beyer T, Antoch G, Müller S, et al. Acquisition protocol considerations for combined PET/CT Imaging. J Nucl Med. 2004;45(1 suppl):25S–35S. [PubMed] [Google Scholar]
- 35.Kinahan PE, Townsend DW, Beyer T, Sashin D. Attenuation correction for a combined 3D PET/CT scanner. Med Phys. 1998;25:2046–2053. doi: 10.1118/1.598392. [DOI] [PubMed] [Google Scholar]
- 36.Benz MR, Evilevitch V, Allen-Auerbach MS, et al. Treatment monitoring by 18F-FDG PET/CT in patients with sarcomas: interobserver variability of quantitative parameters in treatment-induced changes in histopathologically responding and nonresponding tumors. J Nucl Med. 2008;49:1038–1046. doi: 10.2967/jnumed.107.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203–3209. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]
- 38.Wang N, He Q, Skog S, Eriksson S, Tribukait B. Investigation on cell proliferation with a new antibody against thymidine kinase 1. Anal Cell Pathol. 2001;23:11–19. doi: 10.1155/2001/658312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherley JL, Kelly TJ. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988;263:8350–8358. [PubMed] [Google Scholar]
- 40.Paproski RJ, Wuest M, Jans HS, et al. Biodistribution and uptake of 3′-deoxy-3′-fluorothymidine in ENT1-knockout mice and in an ENT1-knockdown tumor model. J Nucl Med. 2010;51:1447–1455. doi: 10.2967/jnumed.110.076356. [DOI] [PubMed] [Google Scholar]
- 41.Plotnik DA, Emerick LE, Krohn KA, Unadkat JD, Schwartz JL. Different modes of transport for 3H-thymidine, 3H-FLT, and 3H-FMAU in proliferating and nonproliferating human tumor cells. J Nucl Med. 2010;51:1464–1471. doi: 10.2967/jnumed.110.076794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skipper HE, Perry S. Kinetics of normal and leukemic leukocyte populations and relevance to chemotherapy. Cancer Res. 1970;30:1883–1897. [PubMed] [Google Scholar]
- 43.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 44.Apisarnthanarax S, Alauddin MM, Mourtada F, et al. Early detection of chemoradioresponse in esophageal carcinoma by 3′-deoxy-3′-3H-fluorothymidine using preclinical tumor models. Clin Cancer Res. 2006;12:4590–4597. doi: 10.1158/1078-0432.CCR-05-2720. [DOI] [PubMed] [Google Scholar]
- 45.Jordheim LP, Dumontet C. Review of recent studies on resistance to cytotoxic deoxynucleoside analogues. Biochim Biophys Acta. 2007;1776:138–159. doi: 10.1016/j.bbcan.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Johansson M, Bergenheim AT, Widmark A, Henriksson R. Effects of radiotherapy and estramustine on the microvasculature in malignant glioma. Br J Cancer. 1999;80:142–148. doi: 10.1038/sj.bjc.6690333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellims PH, Eng Gan T, Medley G, Van Der Weyden MB. Prognostic relevance of thymidine kinase isozymes in adult non-Hodgkin’s lymphoma. Blood. 1981;58:926–930. [PubMed] [Google Scholar]
- 48.Nisman B, Yutkin V, Nechushtan H, et al. Circulating tumor M2 pyruvate kinase and thymidine kinase 1 are potential predictors for disease recurrence in renal cell carcinoma after nephrectomy. Urology. 2010;76:513. doi: 10.1016/j.urology.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 49.Guo Y, Kotova E, Chen ZS, et al. MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003;278:29509–29514. doi: 10.1074/jbc.M304059200. [DOI] [PubMed] [Google Scholar]
- 50.Juweid M, Syrbu S, Buck A. Error-prone DNA repair underlying somatic hypermutation/class-switch recombination (SHM/CSR) identified as major contributor of 18F-fluorothymidine (FLT) in low-grade follicular lymphoma (FL) [abstract] J Nucl Med. 2010;51(suppl 2):558. [Google Scholar]
- 51.Muzi M, Spence AM, O’sullivan F, et al. Kinetic analysis of 3′-deoxy-3′-18F-fluorothymidine in patients with gliomas. J Nucl Med. 2006;47:1612–1621. [PubMed] [Google Scholar]
- 52.Schiepers C, Dahlbom M, Chen W, et al. Kinetics of 3′-deoxy-3′-18F-fluorothymidine during treatment monitoring of recurrent high-grade glioma. J Nucl Med. 2010;51:720–727. doi: 10.2967/jnumed.109.068361. [DOI] [PubMed] [Google Scholar]