Abstract
The intestinal mucosa is densely packed with antibody-secreting B cells, the majority of which produce IgA. Mucosal antibodies have traditionally been thought of as neutralizing antibodies that exclude antigens, but they also function in antigen sampling, allowing for selective transcytosis of antigens from the intestinal lumen. IgE-mediated antigen uptake can facilitate the development of allergic reactions to foods, but emerging evidence indicates that IgG-mediated antigen uptake may also play an important role in the development of immune tolerance to foods, particularly in the neonate. This review will focus on the role of intestinal immunoglobulins in the development of clinical tolerance and allergy to food antigens.
Keywords: epithelium, transcytosis, sensitization, tolerance, food allergy
Introduction
The average dietary protein intake in North America is in the range of 50 g for young children to greater than 90 g for adults [1]. The vast majority of this is digested and absorbed as amino acids or non-immunogenic di- and tri-peptides. Despite the efficiency of this protein digestion, intact food antigens can be detected in the systemic circulation after a meal [2]. The immune system is not ignorant of these dietary antigens, and food-specific antibodies in circulation are commonly found despite a state of clinical tolerance to the foods [3]. Much of what we know about the development of sensitization or tolerance to foods comes from animal studies in which a novel antigen is given to a naïve mouse, but in humans there is repeated exposure to food antigens over time. The existing immune response to food antigens is likely to influence the response to subsequent exposures, and emerging data highlights the role of mucosal immunoglobulins in shaping the immune response to food antigens. Furthermore, antibodies in breast milk may influence the development of tolerance in the neonate. This is not merely due to immune exclusion, but due to modifying antigen presentation and the resulting cellular and humoral immune response to food antigens. Understanding how antibodies influence the response to dietary antigens has important implications for therapies aimed at re-establishing tolerance to food allergens.
Gastrointestinal antigen sampling
The gastrointestinal tract is exposed to a significant antigen burden derived from food. Much of what is known about antigen uptake in the gastrointestinal tract is focused on the small intestine. Because the small intestine is the site of nutrient absorption, and is the least resistant by electrophysiology measurement, it has been thought to be the most relevant site of antigenic exposure as well. However, similar to the stratified epithelium of the skin, there is a dense network of antigen presenting cells within the stratified epithelium of the mouth [4,5] and esophagus [6,7], suggesting a readiness to absorb and present antigens at these sites. The contribution of the mouth or esophagus to tolerance or sensitization to food antigens remains unclear, although sublingual routes can be effective for both vaccination and tolerance induction [8,9]. This review will focus on antigen uptake in the small intestine.
Soluble and particulate antigens are handled by distinct mechanisms in the small intestine. Particulate antigens, such as viruses and bacteria, are preferentially taken up into the gut-associated lymphoid tissue including Peyer’s patches and isolated lymphoid follicles. This is due to the presence of a specialized subset of epithelial cells termed M cells in the follicle-associated epithelium overlying the Peyer’s patches and isolated lymphoid follicles. M cells are flattened cells with a minimal cytoplasm and sparse glycocalyx, and their lack of microvilli makes them visible by scanning electron microscopy. Antigens taken up by M cells are rapidly transported to the organized lymphoid tissue below, including a network of subepithelial DCs in the Peyer’s patches. M cells have also been identified as scattered cells between enterocytes of the villus [10].
Soluble antigens including many food antigens are not preferentially sampled by the M cells, and are taken up primarily across enterocytes lining the intestinal villus. Under normal conditions, uptake of intact macromolecules occurs by a transcellular transport mechanism depositing antigenic material across the basolateral surface of the epithelium. This results in an immunologically significant quantity of food antigen reaching the systemic circulation intact after a meal [11]. Another mechanism of uptake of antigens includes direct sampling by resident phagocytes that extend dendrites between enterocytes and can directly engulf bacteria from the intestinal lumen [12]. The dendrite-extending cells are CD11c+, but they are functionally closer to macrophages than dendritic cells and do not migrate to the draining lymph node under steady state conditions [13]. They are efficient at capturing antigen [14], and there is evidence that these resident CD11c+ macrophages play a role in the expansion of antigen-specific regulatory T cells during development of immune tolerance to fed antigens [15].
The route of uptake of food allergens has a significant influence on the fate of the response to that antigen. We studied the three major allergens in milk with respect to antigen uptake in a mouse model of food allergy. The whey proteins α-lactalbumin (ALA) and β-lactoglobulin (BLG) are soluble and readily taken up across villus enterocytes where they can be visualized in the lamina propria [16]. In contrast, the allergen casein is found in micelles, is excluded from uptake by villus enterocytes, and is preferentially taken up into Peyer’s patches. Heating of ALA and BLG renders them insoluble, and they are preferentially taken up into Peyer’s patches like casein. We found that delivery into the Peyer’s patches resulted in a more robust sensitization (elevated antibody and cytokine responses), but if these antigens were unable to traffic across the villus enterocytes they were unable to elicit anaphylactic symptoms when mice were orally challenged. We also showed that heating of egg allergens reduced their ability to traffic across human epithelial cells in vitro, and abolished their ability to trigger reactions by the oral route in sensitized mice [17]. Clearly routes of antigen uptake in the intestinal mucosa can influence immune responsiveness to that antigen. Factors such as the food matrix also influence the digestion and absorption of intact food allergens for presentation to the mucosal immune system [18,19].
Studies on intestinal antigen uptake have primarily been performed in animals that are naïve to the antigen. But it has been known for many years that prior immunization or sensitization can modify the uptake of antigens in the intestine [20,21]. Prior exposure may lead to immune exclusion through the production of neutralizing antibodies, but there is a growing body of literature showing that mucosal antibodies can promote selective uptake of antigens across the epithelial barrier. This antibody-facilitated uptake leads to an alteration in the response of the mucosal immune system to that antigen, as will be reviewed below.
Mucosal antibody production
Immunostaining studies of human intestine provide striking visual evidence of the density of antibody secreting cells in the gastrointestinal mucosa under homeostatic conditions [22]. 80% of the body’s plasma cells are found in the gut. In the small intestine these are primarily polymeric IgA (pIgA) producing cells (~80%), followed by polymeric IgM (pIgM) secreting cells (15–20%), and IgG-secreting cells (3–4%). IgD-secreting cells can be found but are rare compared to the nasal mucosa or salivary glands. B cells in the mucosa highly express joining or “J” chain that is covalently bound to IgA and IgM to form immunoglobulin dimers and pentamers, respectively. J chain facilitates binding to the polymeric immunoglobulin receptor (pIgR) that exports immunoglobulins across the epithelial layer. In contrast to other isotypes, IgE-secreting B cells have not been demonstrated in the normal human gastrointestinal lamina propria.
Mucosal IgA- and IgG-secreting plasma cells are induced in the gut associated lymphoid tissue and mesenteric lymph nodes, followed by homing to the effector sites of the small intestinal lamina propria through expression of gut homing molecules α4β7 and the chemokine receptor CCR9 [23]. Isolated lymphoid follicles are also a substantial source of IgA, and are thought to play a critical role in fine-tuning the microbial load in the distal small intestine [24]. Local IgA class-switching has also been shown to occur within the lamina propria under the regulation of epithelial-derived cytokines [25,26]. There are two sub-types of IgA in humans, IgA1 and IgA2. IgA2 is resistant to bacterial proteases that cleave IgA1, and may therefore be more stable in the intestinal lumen. Serum IgA is predominantly IgA1, while mucosal IgA includes a substantial fraction of IgA2, which becomes the dominant subclass in the colon. Immunization with protein antigens such as tetanus preferentially induces the IgA1 subclass, while microbial polysaccharide antigens such as Haemophilus influenzae type b or Streptococcus pneumoniae capsular polysaccharides preferentially induce IgA2 antibodies [27].
In the absence of IgA, either in IgA-deficient humans or IgA-knockout mice, there is a compensatory increase in the secretion of IgM and IgG (or the presence of their secreting cells) that can contribute to protection against pathogenic insults [28]. IgA-deficiency can be found in otherwise healthy individuals, although IgA-deficiency is associated with higher levels of systemic antibody responses to foods antigens [29,30]. Systemic sensitization to foods may reflect a lack of appropriate compartmentalization of food antigens. For example, when compartmentalization of the intestinal microbial contents fails, a systemic antibody response to the commensal flora is generated that preserves the health of the organism, but when both local and systemic defenses are disrupted experimentally, a failure to thrive is observed in mice [31]. Therefore there are many layers of immunity that serve to keep microbes contained; failed mucosal compartmentalization of food antigens may play a role in the development of allergic sensitization to foods. The role of IgA as a key component of this mucosal compartmentalization will be discussed in further detail below.
Epithelial Expression of Fc Receptors
The intestinal epithelium is formed by a single layer of columnar epithelial cells that are connected at the apical pole by tight junctions that prevent the passive diffusion of macromolecules [32]. This not only limits antigens in the intestinal lumen from entry into the body, it limits immunoglobulins from reaching the intestinal lumen by diffusion. Intestinal immunoglobulin receptors are therefore needed to actively transport immunoglobulins across the epithelium and into the intestinal lumen. This system is best understood for the transport of secretory IgA (SIgA) and IgM (SIgM) [33]. SIgA was initially found to contain a glycoprotein called “secretory component” (SC) that was produced by the epithelium, not the plasma cell generating the pIgA [34]. SC is a proteolytic fragment of the IgA receptor pIgR that is cleaved to release pIgA containing J chain as well as SC. The binding of SC to pIgA provides enhanced stability in the intestinal lumen. pIgR binds to the J chain [35], found within both dimeric IgA and pentameric IgM. Transport of IgA by pIgR is uni-directional, since the ligand-binding portion of the receptor is cleaved at the apical surface to release SIgA. In mice that are genetically deficient for pIgR, there is a significant loss of secreted IgA and a significant increase in serum IgA [36]. A second receptor for IgA has been reported on M cells within the dome epithelium of Peyer’s patches. sIgA and sIgA-antigen complexes bind specifically to M cells, and this interaction is not inhibited by antibodies against Fcα1 [37]. This receptor would allow for uptake of IgA and antigen, and will be discussed in further detail below.
In addition to receptors for pIgA and pIgM, the intestinal epithelium also expresses a receptor for IgG, known as the neonatal Fc receptor or FcRn. This was first isolated from neonatal rat intestine, and is an MHC Class I-like molecule that forms a heterodimer with β2-microglobulin [38]. FcRn expression is lost post-weaning in rats, but in humans FcRn is expressed into adulthood [39]. Human FcRn has been shown to be a bi-directional transporter of IgG [40]. The receptor is best recognized as facilitating the uptake of maternal milk-derived immunoglobulins and therefore playing an important role in neonatal immunity. IgG has not been typically considered a secretory immunoglobulin since levels are low compared to IgA and IgM, but as will be discussed further, the presence of antigen-specific IgG in the intestinal lumen can have significant influence on immunity to food and flora.
IgE has not been described to be present in intestinal secretions, saliva, or nasal secretions under normal conditions [41], although it can be found in secreted form under the conditions of allergy and helminth infection [41–44]. Like IgA and IgG, this is associated with an epithelial receptor for IgE. The low-affinity IgE receptor CD23 was first described by Kaiserlian et al as being expressed and up-regulated on human intestinal epithelial cells in the context of intestinal inflammation [45]. Subsequently it was identified on rat and mouse epithelial cells in the context of allergic sensitization [46,47]. We have found that there is constitutive expression of CD23 by human intestinal epithelial cells, as measured by quantitative PCR and immunostaining ([48] and unpublished observations), and that these levels are not altered in the context of inflammation. The high-affinity IgE receptor FcεRI has also been reported on human intestinal epithelial cells [49].
Regulation of food allergic responses by IgA
IgA is the prototypic mucosal immunoglobulin, and is thought to contribute to mucosal homeostasis by limiting the uptake of antigens from the gut lumen. Binding of antigens by IgA in the lumen prevents their uptake, and if antigens penetrate the epithelial barrier they can be bound to IgA and secreted back out into the lumen with the IgA. As a neutralizing antibody, IgA has been hypothesized to play a protective role in the context of food allergy.
IgA responses were examined experimentally in mice sensitized or tolerized to the milk allergen BLG [50]. Intestinal IgA responses were elevated in tolerized mice compared to naïve or sensitized mice, but in contrast serum IgA responses were elevated in sensitized mice compared to naïve or tolerized mice. These data support the concept that local sIgA is protective against food allergy, but this was not directly tested. Strait et al addressed the protective effect of IgA against anaphylaxis by using a passive transfer approach [51]. They found that systemic transfer of antigen-specific IgA was protective against anaphylaxis triggered by the oral route. This was not dependent on pIgR or secretion into the lumen, and surprisingly IgA had no protective effect when it was administered together with the antigen intra-luminally. These data clearly show that IgA has protective effects at the systemic level, but do not rule out a protective role for IgA at the mucosa surface. Administration of IgA monomers intra-luminally may not reflect the normal situation in which dimers containing J chain are produced locally and secreted with SC in a form that is resistant to acid digestion.
In humans, IgA-deficiency in children has been reported to be associated with elevated frequency of food allergy [52]. Furthermore, antigen-specific and total IgA levels in maternal milk were found to be significantly lower in mothers whose children developed cow’s milk allergy, indicating that IgA may play a critical role in preventing early sensitization to food allergens [53]. From a therapeutic approach, sublingual immunotherapy with peanut for the treatment of peanut allergy in humans resulted in an increase in peanut-specific IgA levels in saliva, and levels of IgA correlated with the degree of clinical protection [54]. Together with the mouse data, these results support the concept that antigen-specific IgA is protective against primary sensitization or triggering of allergic reactions by food antigens. Factors that contribute to the generation of IgA-secreting plasma cells such as retinoic acid [55] may be useful adjuvant therapy together with immunotherapy for the generation of clinical tolerance. It should be noted that retinoic acid is not uniformly regulatory in function and in an IL-15-rich milieu can promote inflammatory responses to food antigens [56].
As mentioned previously, a second receptor for IgA has been described on M cells overlying Peyer’s patches although the identity of this receptor remains unknown [37]. Rather than participating in immune exclusion, this receptor appears to play a role in antigen sampling from the luminal to serosal direction [57]. There is selective uptake of SIgA-antigen complexes by the M cell. Binding of antigen to SIgA was shown to alter its conformational structure and enhance binding to known IgA receptors [58], suggesting a mechanism by which SIgA-antigen complexes could compete with free SIgA for uptake by M cells. Having crossed the M cell, SIgA-antigen complexes can interact with sub-epithelial dendritic cells. The interaction of SIgA with dendritic cells is mediated by the C-type lectin DC-SIGN [59]. Antigen presented in the context of IgA generates a different immune response than that of antigen presented alone. This was shown experimentally by orally administering mouse pIgA containing human SC, which generated a robust IgA and IgG1 response against human SC, similar to the response of orally administering human SC together with the mucosal adjuvant cholera toxin [60]. Administration of human SC alone did not elicit any significant antibody response. These experiments were done in naïve animals to address the immunomodulatory role of IgA, and may be representative of the neonatal situation when the infant is provided with maternal IgA and antigens via breast milk. These data suggest that maternal IgA may facilitate presentation of antigens to the neonatal mucosal immune system by selective uptake into Peyer’s patches and shape the subsequent immune response. In the context of an existing IgA response, when adaptive immunity to an antigen has already been established, the impact of IgA-facilitated presentation through the gut is not known.
IgG-facilitated antigen sampling: role in tolerance
In human intestinal epithelial cells FcRn is expressed into adulthood [39], beyond a time-point when it could have any role in passive transfer of immunity from breast milk. The discovery that FcRn on human intestinal epithelial cells was a bi-directional transporter of IgG led to the hypothesis that FcRn could play a role in immune surveillance [40]. The capacity of epithelial-expressed FcRn to function in IgG-facilitated systemic delivery of antigen was first shown using a fusion protein combining a mouse IgG1 Fc portion with human erythropoietin EPO [61]. Uptake of intact EPO was monitored by reticulocyte count. In neonatal intestine and adult lung of mice, this fusion protein induced the uptake of intact and functional EPO. Mutations in the FcRn binding domain prevented this uptake. These data show functional uptake of antigen coupled to IgG across mucosal barriers in vivo. IgG-mediated uptake was also demonstrated in lung of non-human primates [62]. In order to generate an experimental system that would mimic the human expression of FcRn in the intestine, mice were generated that expressed human FcRn and human β2-microglobulin on the background of an FcRn knockout mouse. These mice express FcRn into adulthood. Administration of antigen orally and human IgG systemically led to the formation of antigen-IgG complexes in the intestinal lumen and facilitated uptake of antigen across the epithelial barrier [63]. The end result was increased presentation by mucosal dendritic cells to T cells. These data show that IgG-facilitated antigen uptake can alter the resulting immune response to the sampled antigen. The result of that enhanced immune response, whether inflammatory or tolerogenic, may depend on the context of antigen presentation and presence of other immuno-modulatory factors.
FcRn has been shown to contribute to immune tolerance, host defense, and immune pathology in the gastrointestinal tract, as shown schematically in Figure 1. In the context of experimental inflammatory bowel disease, FcRn can amplify inflammation. Antibodies against bacterial flagellin are found in Crohn’s disease [64], and in experimental mouse models of colitis. In a model of dextran sodium sulfate-induced colitis, an anti-flagellin IgG response was generated during the course of colitis, and FcRn knockout mice had reduced severity of colitis. Passive transfer with anti-flagellin antibodies or immunization with flagellin prior to colitis worsened disease in an FcRn-dependent manner [65]. FcRn-mediated antigen uptake also contributes to bacterial clearance as shown using infection with Citrobacter rodentium [66]. FcRn is expressed on dendritic cells as well as epithelial cells, and participates in antibody-facilitated antigen uptake in these cells [67]. IgG-antigen complexes are presented more efficiently by dendritic cells and macrophages than soluble antigen, and activate CD4+ T cells in an FcRn-dependent manner [68]. FcRn was found to primarily influence antigen processing within endosomal compartments and presentation, [68]. Although it has been reported that FcRn-mediated presentation of IgG-antigen complexes does not influence the CD8 T cell response [68], others have found that cross-presentation of IgG-antigen complexes to CD8+ T cells is highly dependent on FcRn in the CD8− CD11b+ dendritic cell subset [69]. In contrast, soluble antigens are most efficiently cross-presented by CD8+ CD11b− dendritic cells. FcRn may therefore shape the mucosal immune response not only by facilitating epithelial uptake of antigen, but also by influencing antigen presentation by professional antigen presenting cells.
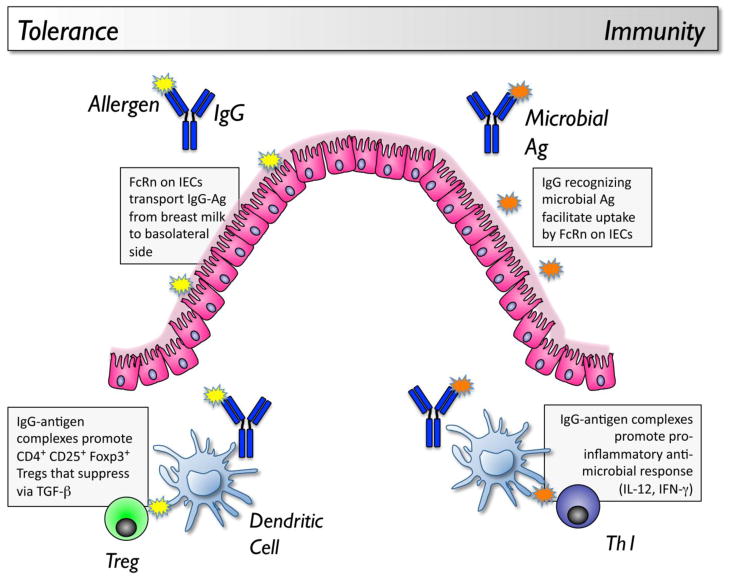
Figure 1. Tolerogenic and immunogenic Roles of FcRn in mucosal immunity.
In the neonate, IgG and antigens derived from the breast milk can be selectively taken up by an FcRn-dependent mechanism in the gastrointestinal tract. The immune consequence is active tolerance, mediated by CD4+ CD25+ Foxp3+ regulatory T cells that are induced by DCs exposed to IgG-antigen immune complexes. In the adult intestine, host production of antimicrobial IgG antibodies can facilitate the uptake of luminal microbial antigens through an FcRn-dependent mechanism. IgG-antigen complexes are taken up by dendritic cells, and drive a response that can be host-protective, resulting in pathogen clearance, or pathogenic, resulting in inflammation.
In the neonatal gut, FcRn has been proposed to have an immuno-regulatory role through its uptake of IgG-antigen complexes derived from maternal milk. Neonatal exposure to an antigen through breast milk induces antigen-specific tolerance and protection from asthma or peanut allergy in mice [70,71]. This was due to TGF-β present in milk, and was mediated by CD4+ T cells whose regulatory function was also dependent on TGF-β[70]. Tolerance induced by mothers who were sensitized to the antigen was more profound than that induced by mothers who were naïve to the antigen [72]. In a separate study, mothers immunized with a Th1 adjuvant were found to induce a greater level of tolerance in their offspring compared to mothers immunized with a Th2 adjuvant [73]. Mothers immunized to a bystander antigen did not transmit tolerance to their offspring, indicating that there was antigen specificity to the tolerance response. This tolerance in sensitized or immunized mice was subsequently found to be due to the presence of IgG-antigen complexes in the breast milk, and tolerance induction was dependent on FcRn [74,72]. Dendritic cells exposed to antigen in the context of immune complexes with IgG preferentially induced CD4+ CD25+ Foxp3+ regulatory T cells [72]. Although human mothers with food allergy would be avoiding the food and antigen would not be transmitted to the breastfeeding infant, the presence of food-specific IgG antibodies is common in healthy subjects [3], and if transferred in milk to the neonate together with the food allergen, could facilitate the development of immune tolerance to food in an FcRn-dependent manner. Delivery of antigen directly conjugated to IgG as immunotherapy delivered through mucosal routes could potentially improve tolerance induction by acting on FcRn.
IgE-facilitated antigen sampling: role in food allergy
The low-affinity IgE receptor CD23 (FcεRII) is constitutively expressed by human intestinal epithelial cells [45,48]. This is somewhat surprising as IgE-secreting cells have not been found in the normal gut [75]. Using a jejunal perfusate system in human subjects, a low but detectable level of IgE was found in intestinal secretions [76]. In children with gastrointestinal disease (unrelated to food allergy), milk-specific IgE could be detected in the duodenal secretions of 8 of 13 subjects [77]. In healthy adults, IgE was present in duodenal secretions of only 1 of 8 subjects at baseline conditions, but 4 of 8 subjects after stimulation with pancreozymin-cholecystokinin, suggesting a potential pancreatic source of IgE antibodies [77]. IgE in duodenal secretions was found to be increased approximately 2-fold in subjects with food allergy compared to controls [42]. In salivary secretions, IgE has not been found at detectable levels in either healthy controls or allergic subjects [78,41]. However, nasal washings from atopic individuals but not healthy controls have detectable IgE, and at a higher ratio of IgE to protein than is found in the serum [41], leading the authors to conclude that IgE could be considered to be a secretory immunoglobulin in nasal mucosa.
In the context of parasite infection (Trichinella spiralis) in mice, IgE was reported at high levels in intestinal secretions, and at a higher level than that observed in serum when the half life of IgE in the intestinal lumen was accounted for, suggesting local production of IgE within the intestinal mucosa or active transport into the gut lumen [44]. IgE-secreting B cells within the intestinal mucosa were also identified after T. spiralis infection [79]. Antigen-specific IgE can also be detected in intestinal lavage of mice with experimental food allergy [43]. The recent development of IgE-reporter mice [80,81] provides a powerful tool that will greatly contribute to our understanding of the mucosal regulation of IgE in health and disease.
CD23 on B cells functions as an antigen focusing mechanism, such that uptake and presentation of allergen is enhanced in the presence of IgE [82,83]. This led us and others to ask if CD23 had a similar uptake function in epithelial cells. Human intestinal epithelial cells were found to transport IgE through a CD23-dependent mechanism from both basal-to-apical and apical-to-basal directions [48,84,85]. Antigen-IgE complexes were preferentially transported in the apical-to-basal direction, and were released into the basolateral media in a form capable of degranulating rat basophil leukemia cells transfected with the human FcεRI receptor [48]. This bi-directional transport of IgE by CD23 has also been demonstrated in polarized human airway epithelial cells [86]. In addition to shuttling antigen-IgE complexes across the epithelial barrier, we showed that ligation of CD23 can activate pro-inflammatory pathways in human intestinal epithelial cells and lead to the release of chemokines including CCL20 [87]. CCL20 acts on the receptor CCR6 that is expressed by memory T cells, B cells, and immature dendritic cells. Supernatants from IgE-antigen stimulated intestinal epithelial cells were able to chemoattract human dendritic cells in a pertussis toxin-sensitive and CCL20-dependent manner, and silencing CD23 on the epithelial cells suppressed this response [87]. We hypothesized that this recruitment of dendritic cells and other effector cells could play a role in the generation of late-phase allergic inflammation in the gastrointestinal tract. Consistent with this, CCL20 is necessary for allergic inflammation and allergen-induced diarrhea in mice [88], but we have not yet established a link between epithelial CD23 activation and CCL20 expression in vivo. The concept that CD23 may serve as a mechanism of antigen delivery to dendritic cells has been suggested by work from Heyman and colleagues [89]. There are longstanding observations that IgE can facilitate the presentation of antigen to T cells in vivo and in vitro [90]. This facilitated presentation by IgE is CD23-dependent [91,92]. Recently it was demonstrated that although IgE-facilitated presentation is dependent on CD23-expressing B cells, antigen presentation to T cells is performed by dendritic cells rather than B cells in vivo [89]. Therefore it was suggested that B cells are acquiring antigen through CD23, but acting as a conduit to deliver antigen to the dendritic cell. Our in vitro studies with human intestinal epithelial cells also suggest that CD23 may facilitate the hand-off of antigen to dendritic cells by recruiting them to the proximity of the epithelial cell.
The high affinity IgE receptor FcεRI was also identified on human intestinal epithelial cells, and found to bind IgE [49], but the functional role of this receptor on IgE transcytosis, antigen uptake, or other epithelial functions has not yet been explored.
Conclusions
The presence of specific antibodies in the intestinal lumen has a significant effect on the way that antigen is handled by the intestinal mucosa. The prototypic mucosal immunoglobulin IgA functions in antigen exclusion, and specific IgA levels inversely correlate with clinical reactivity to food and aeroallergens. Therefore strategies to increase antigen-specific IgA may promote clinical tolerance and be useful therapeutically. IgG-mediated antigen uptake through FcRn in the neonatal intestine is tolerogenic, and suggests that antigen exposure through breast milk would be a helpful preventative strategy, particularly when the mother has existing IgG antibodies to that antigen. Once allergic sensitization has been established, it is not clear if IgG-facilitated antigen uptake through FcRn would amplify existing pro-allergic adaptive immune responses or promote active immune tolerance. Studies are needed to address the influence of FcRn on responses to food antigens in the sensitized state. In contrast to IgA and IgG, there is no evidence that IgE-mediated antigen sampling via CD23 is tolerogenic. IgE-facilitated antigen uptake results in increased delivery of antigen to allergic effector cells, activates pro-inflammatory pathways in intestinal epithelial cells, and enhances delivery of antigen to dendritic cells. IgE-facilitated antigen uptake by B cells can also have an adjuvant-like effect on the resulting adaptive immune response. Targeting of IgE-facilitated antigen presentation may be helpful for the establishment of tolerance during immunotherapy. Current trials utilizing anti-IgE therapy (with Omalizumab) together with oral immunotherapy for food allergy will establish if there is benefit in targeting IgE during allergen administration in the establishment of clinical or immune tolerance. A schematic showing the contributions of IgA, IgG, and IgE to mucosal immunity is provided in Figure 2. An understanding of these pathways may allow us to utilize antibody-facilitated antigen uptake to promote tolerance induction to foods.
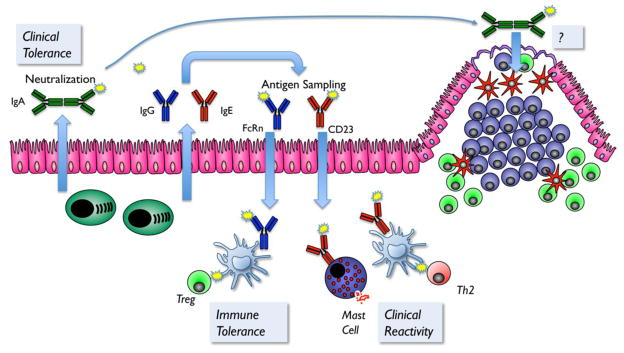
Figure 2. Mucosal antibodies in the regulation of tolerance and allergy to foods.
IgA secreted across the epithelium can bind and neutralize food allergens, and contribute to clinical tolerance to foods by preventing entry and interaction with mucosal immune cells. Secretory IgA bound to antigen can also be selectively taken up across the Peyer’s patch, but the consequence of that uptake in the context of food allergy has not yet been addressed. IgG and IgE can be secreted across the epithelium by FcRn and CD23, respectively. IgG-facilitated antigen uptake via FcRn contributes to the development of active immune tolerance in the neonate. In contrast, IgE-facilitated antigen uptake via CD23 contributes to clinical reactivity by enhancing the delivery of IgE-antigen complexes to allergic effector cells. Therapeutic approaches that promote food-specific IgA or IgG production in the gut, or inhibit antigen uptake via CD23, may be beneficial for the development of food-specific tolerance.
Abbreviations
- ALA
α-lactalbmin
- BLG
β-lactoglobulin
- FcRn
neonatal Fc receptor
- pIgA
polymeric IgA
- pIgR
polymeric immunoglobulin receptor
- SIgA
secretory IgA
Footnotes
This article is published as part of the Special Issue on Food Allergy [34:6]
References
- 1.Fulgoni VL., 3rd Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87(5):1554S–1557S. doi: 10.1093/ajcn/87.5.1554S. 87/5/1554S [pii] [DOI] [PubMed] [Google Scholar]
- 2.Husby S, Jensenius JC, Svehag SE. Passage of undegraded dietary antigen into the blood of healthy adults. Further characterization of the kinetics of uptake and the size distribution of the antigen. Scand J Immunol. 1986;24 (4):447–455. doi: 10.1111/j.1365-3083.1986.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 3.Husby S, Oxelius VA, Teisner B, Jensenius JC, Svehag SE. Humoral immunity to dietary antigens in healthy adults. Occurrence, isotype and IgG subclass distribution of serum antibodies to protein antigens. Int Arch Allergy Appl Immunol. 1985;77(4):416–422. doi: 10.1159/000233819. [DOI] [PubMed] [Google Scholar]
- 4.Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, Hofman P, Holmgren J, Anjuere F, Czerkinsky C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;25(51):8598–8610. doi: 10.1016/j.vaccine.2007.09.073. S0264-410X(07)01109-7 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Allam JP, Stojanovski G, Friedrichs N, Peng W, Bieber T, Wenzel J, Novak N. Distribution of Langerhans cells and mast cells within the human oral mucosa: new application sites of allergens in sublingual immunotherapy? Allergy. 2008;63(6):720–727. doi: 10.1111/j.1398-9995.2007.01611.x. ALL1611 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Yen EH, Hornick JL, Dehlink E, Dokter M, Baker A, Fiebiger E, Nurko S. Comparative analysis of FcepsilonRI expression patterns in patients with eosinophilic and refluxe sophagitis. J Pediatr Gastroenterol Nutr. 2010;51(5):584–592. doi: 10.1097/MPG.0b013e3181de7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucendo AJ, Navarro M, Comas C, Pascual JM, Burgos E, Santamaria L, Larrauri J. Immunophenotypic characterization and quantification of the epithelial inflammatory infiltrate in eosinophilic esophagitis through stereology: an analysis of the cellular mechanisms of the disease and the immunologic capacity of the esophagus. Am J Surg Pathol. 2007;31 (4):598–606. doi: 10.1097/01.pas.0000213392.49698.8c. [DOI] [PubMed] [Google Scholar]
- 8.Mascarell L, Saint-Lu N, Moussu H, Zimmer A, Louise A, Lone Y, Ladant D, Leclerc C, Tourdot S, Van Overtvelt L, Moingeon P. Oral macrophage-like cells play a key role in tolerance induction following sublingual immunotherapy of asthmatic mice. Mucosal Immunol. 2011;4(6):638–647. doi: 10.1038/mi.2011.28. mi201128 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Czerkinsky C, Cuburu N, Kweon MN, Anjuere F, Holmgren J. Sublingual vaccination. Hum Vaccin. 2011;7(1):110–114. doi: 10.4161/hv.7.1.13739. 13739 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD, Hiroi T, Tamagawa H, Iijima H, Kunisawa J, Yuki Y, Kiyono H. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci U S A. 2004;101(16):6110–6115. doi: 10.1073/pnas.0400969101. 0400969101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husby S, Jensenius JC, Svehag SE. Passage of undegraded dietary antigen into the blood of healthy adults. Quantification, estimation of size distribution, and relation of uptake to levels of specific antibodies. Scand J Immunol. 1985;22 (1):83–92. doi: 10.1111/j.1365-3083.1985.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 12.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2 (4):361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 13.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31(3):513–525. doi: 10.1016/j.immuni.2009.08.010. S1074-7613(09)00368-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206(13):3101–3114. doi: 10.1084/jem.20091925. jem.20091925 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34(2):237–246. doi: 10.1016/j.immuni.2011.01.016. S1074-7613(11)00037-9 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, Jensen-Jarolim E, Mayer L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy. 2008;63 (7):882–890. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 17.Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, Nowak-Wegrzyn A. Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol. 2011;127(4):990–997. e992. doi: 10.1016/j.jaci.2011.01.057. S0091-6749(11)00251-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulten V, Lauer I, Scheurer S, Thalhammer T, Bohle B. A food matrix reduces digestion and absorption of food allergens in vivo. Mol Nutr Food Res. 2011;55(10):1484–1491. doi: 10.1002/mnfr.201100234. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Ghoshal S, Ward M, de Villiers W, Woodward J, Eckhardt E. Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice. PLoS One. 2009;4(12):e8442. doi: 10.1371/journal.pone.0008442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker WA, Isselbacher KJ, Bloch KJ. Intestinal uptake of macromolecules. II. Effect of parenteral immunization. J Immunol. 1973;111 (1):221–226. [PubMed] [Google Scholar]
- 21.Berin MC, Kiliaan AJ, Yang PC, Groot JA, Taminiau JA, Perdue MH. Rapid transepithelial antigen transport in rat jejunum: impact of sensitization and the hypersensitivity reaction. Gastroenterology. 1997;113 (3):856–864. doi: 10.1016/s0016-5085(97)70180-x. [DOI] [PubMed] [Google Scholar]
- 22.Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. IMR283 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3(10):822–829. doi: 10.1038/nri1203 nri1203 [pii]. [DOI] [PubMed] [Google Scholar]
- 24.Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2009;2(6):478–485. doi: 10.1038/mi.2009.114. mi2009114 [pii] [DOI] [PubMed] [Google Scholar]
- 25.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26(6):812–826. doi: 10.1016/j.immuni.2007.04.014. S1074-7613(07)00287-7 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, Knowles DM, Santini PA, Cerutti A. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8(3):294–303. doi: 10.1038/ni1434. ni1434 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Tarkowski A, Lue C, Moldoveanu Z, Kiyono H, McGhee JR, Mestecky J. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgA2-subclass antibody responses. J Immunol. 1990;144 (10):3770–3778. [PubMed] [Google Scholar]
- 28.Micol R, Kayal S, Mahlaoui N, Beaute J, Brosselin P, Dudoit Y, Obenga G, Barlogis V, Aladjidi N, Kebaili K, Thomas C, Dulieu F, Monpoux F, Nove-Josserand R, Pellier I, Lambotte O, Salmon A, Masseau A, Galanaud P, Oksenhendler E, Tabone MD, Teira P, Coignard-Biehler H, Lanternier F, Join-Lambert O, Mouillot G, Theodorou I, Lecron JC, Alyanakian MA, Picard C, Blanche S, Hermine O, Suarez F, Debre M, Lecuit M, Lortholary O, Durandy A, Fischer A. Protective effect of IgM against colonization of the respiratory tract by nontypeable Haemophilus influenzae in patients with hypogammaglobulinemia. J Allergy Clin Immunol. 2012;129(3):770–777. doi: 10.1016/j.jaci.2011.09.047. S0091-6749(11)01659-9 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Cunningham-Rundles C, Brandeis WE, Good RA, Day NK. Milk precipitins, circulating immune complexes, and IgA deficiency. Proc Natl Acad Sci U S A. 1978;75 (7):3387–3389. doi: 10.1073/pnas.75.7.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husby S, Oxelius VA, Svehag SE. IgG subclass antibodies to dietary antigens in IgA deficiency quantification and correlation with serum IgG subclass levels. Clin Immunol Immunopathol. 1992;62 (1 Pt 1):85–90. doi: 10.1016/0090-1229(92)90026-k. [DOI] [PubMed] [Google Scholar]
- 31.Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, Bercik P, Verdu EF, McCoy KD, Macpherson AJ. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325(5940):617–620. doi: 10.1126/science.1172747. 325/5940/617 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. nri2653 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011;4(6):598–602. doi: 10.1038/mi.2011.37. mi201137 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mostov KE, Blobel G. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J Biol Chem. 1982;257 (19):11816–11821. [PubMed] [Google Scholar]
- 35.Johansen FE, Braathen R, Brandtzaeg P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J Immunol. 2001;167 (9):5185–5192. doi: 10.4049/jimmunol.167.9.5185. [DOI] [PubMed] [Google Scholar]
- 36.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, Betsholtz C, Brandtzaeg P. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190 (7):915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer’s patch M cells: evidence for a novel IgA receptor. J Immunol. 2002;169 (4):1844–1851. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 38.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 39.Israel EJ, Taylor S, Wu Z, Mizoguchi E, Blumberg RS, Bhan A, Simister NE. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92 (1):69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, Blumberg RS, Lencer WI. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104 (7):903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakajima S, Gillespie DN, Gleich GJ. Differences between IgA and IgE as secretory proteins. Clin Exp Immunol. 1975;21 (2):306–317. [PMC free article] [PubMed] [Google Scholar]
- 42.Belut D, Moneret-Vautrin DA, Nicolas JP, Grilliat JP. IgE levels in intestinal juice. Dig Dis Sci. 1980;25 (5):323–332. doi: 10.1007/BF01308055. [DOI] [PubMed] [Google Scholar]
- 43.Knight AK, Blazquez AB, Zhang S, Mayer L, Sampson HA, Berin MC. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1234–1243. doi: 10.1152/ajpgi.00323.2007. 00323.2007 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Negrao-Correa D, Adams LS, Bell RG. Intestinal transport and catabolism of IgE: a major blood-independent pathway of IgE dissemination during a Trichinella spiralis infection of rats. J Immunol. 1996;157 (9):4037–4044. [PubMed] [Google Scholar]
- 45.Kaiserlian D, Lachaux A, Grosjean I, Graber P, Bonnefoy JY. Intestinal epithelial cells express the CD23/Fc epsilon RII molecule: enhanced expression in enteropathies. Immunology. 1993;80 (1):90–95. [PMC free article] [PubMed] [Google Scholar]
- 46.Yu LC, Yang PC, Berin MC, Di Leo V, Conrad DH, McKay DM, Satoskar AR, Perdue MH. Enhanced transepithelial antigen transport in intestine of allergic mice is mediated by IgE/CD23 and regulated by interleukin-4. Gastroenterology. 2001;121 (2):370–381. doi: 10.1053/gast.2001.26470. [DOI] [PubMed] [Google Scholar]
- 47.Yang PC, Berin MC, Yu LC, Conrad DH, Perdue MH. Enhanced intestinal transepithelial antigen transport in allergic rats is mediated by IgE and CD23 (FcepsilonRII) J Clin Invest. 2000;106 (7):879–886. doi: 10.1172/JCI9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Nowak-Wegrzyn A, Charlop-Powers Z, Shreffler W, Chehade M, Thomas S, Roda G, Dahan S, Sperber K, Berin MC. Transcytosis of IgE-antigen complexes by CD23a in human intestinal epithelial cells and its role in food allergy. Gastroenterology. 2006;131 (1):47–58. doi: 10.1053/j.gastro.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 49.Untersmayr E, Bises G, Starkl P, Bevins CL, Scheiner O, Boltz-Nitulescu G, Wrba F, Jensen-Jarolim E. The high affinity IgE receptor Fc epsilonRI is expressed by human intestinal epithelial cells. PLoS One. 2010;5(2):e9023. doi: 10.1371/journal.pone.0009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frossard CP, Hauser C, Eigenmann PA. Antigen-specific secretory IgA antibodies in the gut are decreased in a mouse model of food allergy. J Allergy Clin Immunol. 2004;114 (2):377–382. doi: 10.1016/j.jaci.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 51.Strait RT, Mahler A, Hogan S, Khodoun M, Shibuya A, Finkelman FD. Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. J Allergy Clin Immunol. 2011;127(4):982–989. e981. doi: 10.1016/j.jaci.2011.01.034. S0091-6749(11)00123-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janzi M, Kull I, Sjoberg R, Wan J, Melen E, Bayat N, Ostblom E, Pan-Hammarstrom Q, Nilsson P, Hammarstrom L. Selective IgA deficiency in early life: association to infections and allergic diseases during childhood. Clin Immunol. 2009;133(1):78–85. doi: 10.1016/j.clim.2009.05.014. S1521-6616(09)00695-0 [pii] [DOI] [PubMed] [Google Scholar]
- 53.Jarvinen KM, Laine ST, Jarvenpaa AL, Suomalainen HK. Does low IgA in human milk predispose the infant to development of cow’s milk allergy? Pediatr Res. 2000;48 (4):457–462. doi: 10.1203/00006450-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, Staats H, Burks AW. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2011.11.045. S0091-6749(11)01910-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314 (5802):1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 56.DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, Semrad C, Kupfer SS, Belkaid Y, Guandalini S, Jabri B. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471(7337):220–224. doi: 10.1038/nature09849. nature09849 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4(6):603–611. doi: 10.1038/mi.2011.41. mi201141 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duc M, Johansen FE, Corthesy B. Antigen binding to secretory immunoglobulin A results in decreased sensitivity to intestinal proteases and increased binding to cellular Fc receptors. J Biol Chem. 2010;285(2):953–960. doi: 10.1074/jbc.M109.059220. M109.059220 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baumann J, Park CG, Mantis NJ. Recognition of secretory IgA by DC-SIGN: implications for immune surveillance in the intestine. Immunol Lett. 2010;131(1):59–66. doi: 10.1016/j.imlet.2010.03.005. S0165-2478(10)00110-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Favre L, Spertini F, Corthesy B. Secretory IgA possesses intrinsic modulatory properties stimulating mucosal and systemic immune responses. J Immunol. 2005;175(5):2793–2800. doi: 10.4049/jimmunol.175.5.2793. 175/5/2793 [pii] [DOI] [PubMed] [Google Scholar]
- 61.Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196 (3):303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bitonti AJ, Dumont JA, Low SC, Peters RT, Kropp KE, Palombella VJ, Stattel JM, Lu Y, Tan CA, Song JJ, Garcia AM, Simister NE, Spiekermann GM, Lencer WI, Blumberg RS. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc Natl Acad Sci U S A. 2004;101 (26):9763–9768. doi: 10.1073/pnas.0403235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20 (6):769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113 (9):1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobayashi K, Qiao SW, Yoshida M, Baker K, Lencer WI, Blumberg RS. An FcRn-dependent role for anti-flagellin immunoglobulin G in pathogenesis of colitis in mice. Gastroenterology. 2009;137(5):1746–1756. e1741. doi: 10.1053/j.gastro.2009.07.059. S0016-5085(09)01382-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, Wakatsuki Y, Roopenian DC, Mizoguchi A, Lencer WI, Blumberg RS. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116 (8):2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, Roopenian DC, Lencer WI, Blumberg RS. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci U S A. 2008;105(27):9337–9342. doi: 10.1073/pnas.0801717105. 0801717105 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu X, Lu L, Yang Z, Palaniyandi S, Zeng R, Gao LY, Mosser DM, Roopenian DC, Zhu X. The neonatal FcR-mediated presentation of immune-complexed antigen is associated with endosomal and phagosomal pH and antigen stability in macrophages and dendritic cells. J Immunol. 2011;186(8):4674–4686. doi: 10.4049/jimmunol.1003584. jimmunol.1003584 [pii] [DOI] [PubMed] [Google Scholar]
- 69.Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, Andersen JT, Sandlie I, Chen Z, de Haar C, Lencer WI, Fiebiger E, Blumberg RS. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc Natl Acad Sci U S A. 2011;108(24):9927–9932. doi: 10.1073/pnas.1019037108. 1019037108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N, Julia V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14(2):170–175. doi: 10.1038/nm1718. nm1718 [pii] [DOI] [PubMed] [Google Scholar]
- 71.Lopez-Exposito I, Song Y, Jarvinen KM, Srivastava K, Li XM. Maternal peanut exposure during pregnancy and lactation reduces peanut allergy risk in offspring. J Allergy Clin Immunol. 2009;124(5):1039–1046. doi: 10.1016/j.jaci.2009.08.024. S0091-6749(09)01266-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mosconi E, Rekima A, Seitz-Polski B, Kanda A, Fleury S, Tissandie E, Monteiro R, Dombrowicz DD, Julia V, Glaichenhaus N, Verhasselt V. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 2010;3(5):461–474. doi: 10.1038/mi.2010.23. mi201023 [pii] [DOI] [PubMed] [Google Scholar]
- 73.Matson AP, Zhu L, Lingenheld EG, Schramm CM, Clark RB, Selander DM, Thrall RS, Breen E, Puddington L. Maternal transmission of resistance to development of allergic airway disease. J Immunol. 2007;179(2):1282–1291. doi: 10.4049/jimmunol.179.2.1282. 179/2/1282 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matson AP, Thrall RS, Rafti E, Lingenheld EG, Puddington L. IgG transmitted from allergic mothers decreases allergic sensitization in breastfed offspring. Clin Mol Allergy. 2010;8:9. doi: 10.1186/1476-7961-8-9. 1476-7961-8-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandtzaeg P, Baklien K. Inconclusive immunohistochemistry of human IgE in mucosal pathology. Lancet. 1976;1(7972):1297–1298. doi: 10.1016/s0140-6736(76)91767-0. S0140-6736(76)91767-0 [pii] [DOI] [PubMed] [Google Scholar]
- 76.Jonard PP, Rambaud JC, Dive C, Vaerman JP, Galian A, Delacroix DL. Secretion of immunoglobulins and plasma proteins from the jejunal mucosa. Transport rate and origin of polymeric immunoglobulin A. J Clin Invest. 1984;74(2):525–535. doi: 10.1172/JCI111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freier S, Lebenthal E, Freier M, Shah PC, Park BH, Lee PC. IgE and IgD antibodies to cow milk and soy protein in duodenal fluid: effects of pancreozymin and secretin. Immunology. 1983;49 (1):69–75. [PMC free article] [PubMed] [Google Scholar]
- 78.Miranda DO, Silva DA, Fernandes JF, Queiros MG, Chiba HF, Ynoue LH, Resende RO, Pena JD, Sung SS, Segundo GR, Taketomi EA. Serum and salivary IgE, IgA, and IgG4 antibodies to Dermatophagoides pteronyssinus and its major allergens, Der p1 and Der p2, in allergic and nonallergic children. Clin Dev Immunol. 2011;2011:302739. doi: 10.1155/2011/302739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang CH, Richards EM, Bell RG. Rapid anti-helminthic response of B lymphocytes in the intestinal mucosal tissues of rats. Cell Immunol. 1999;193(1):59–70. doi: 10.1006/cimm.1999.1474. S0008-8749(99)91474-0 [pii] [DOI] [PubMed] [Google Scholar]
- 80.Yang Z, Sullivan BM, Allen CD. Fluorescent In Vivo Detection Reveals that IgE(+) B Cells Are Restrained by an Intrinsic Cell Fate Predisposition. Immunity. 2012;36(5):857–872. doi: 10.1016/j.immuni.2012.02.009. S1074-7613(12)00083-0 [pii] [DOI] [PubMed] [Google Scholar]
- 81.Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, Lee WP, Egen JG, Austin CD, Xu M, Wu LC. IgE(+) memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol. 2012;13(4):396–404. doi: 10.1038/ni.2256. ni.2256 [pii] [DOI] [PubMed] [Google Scholar]
- 82.Kehry MR, Yamashita LC. Low-affinity IgE receptor (CD23) function on mouse B cells: role in IgE-dependent antigen focusing. Proc Natl Acad Sci U S A. 1989;86 (19):7556–7560. doi: 10.1073/pnas.86.19.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pirron U, Schlunck T, Prinz JC, Rieber EP. IgE-dependent antigen focusing by human B lymphocytes is mediated by the low-affinity receptor for IgE. Eur J Immunol. 1990;20(7):1547–1551. doi: 10.1002/eji.1830200721. [DOI] [PubMed] [Google Scholar]
- 84.Montagnac G, Molla-Herman A, Bouchet J, Yu LC, Conrad DH, Perdue MH, Benmerah A. Intracellular trafficking of CD23: differential regulation in humans and mice by both extracellular and intracellular exons. J Immunol. 2005;174 (9):5562–5572. doi: 10.4049/jimmunol.174.9.5562. [DOI] [PubMed] [Google Scholar]
- 85.Tu Y, Salim S, Bourgeois J, Di Leo V, Irvine EJ, Marshall JK, Perdue MH. CD23-mediated IgE transport across human intestinal epithelium: inhibition by blocking sites of translation or binding. Gastroenterology. 2005;129 (3):928–940. doi: 10.1053/j.gastro.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 86.Palaniyandi S, Tomei E, Li Z, Conrad DH, Zhu X. CD23-dependent transcytosis of IgE and immune complex across the polarized human respiratory epithelial cells. J Immunol. 2011;186(6):3484–3496. doi: 10.4049/jimmunol.1002146. jimmunol.1002146 [pii] [DOI] [PubMed] [Google Scholar]
- 87.Li H, Chehade M, Liu W, Xiong H, Mayer L, Berin MC. Allergen-IgE complexes trigger CD23-dependent CCL20 release from human intestinal epithelial cells. Gastroenterology. 2007;133 (6):1905–1915. doi: 10.1053/j.gastro.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blazquez AB, Knight AK, Getachew H, Bromberg JS, Lira SA, Mayer L, Berin MC. A functional role for CCR6 on proallergic T cells in the gastrointestinal tract. Gastroenterology. 2010;138(1):275–284. e271–274. doi: 10.1053/j.gastro.2009.09.016. S0016-5085(09)01657-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Henningsson F, Ding Z, Dahlin JS, Linkevicius M, Carlsson F, Gronvik KO, Hallgren J, Heyman B. IgE-mediated enhancement of CD4+ T cell responses in mice requires antigen presentation by CD11c+ cells and not by B cells. PLoS One. 2011;6(7):e21760. doi: 10.1371/journal.pone.0021760. PONE-D-11-03750 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Getahun A, Heyman B. How antibodies act as natural adjuvants. Immunol Lett. 2006;104(1–2):38–45. doi: 10.1016/j.imlet.2005.11.005. S0165-2478(05)00351-2 [pii] [DOI] [PubMed] [Google Scholar]
- 91.Getahun A, Hjelm F, Heyman B. IgE enhances antibody and T cell responses in vivo via CD23+ B cells. J Immunol. 2005;175 (3):1473–1482. doi: 10.4049/jimmunol.175.3.1473. [DOI] [PubMed] [Google Scholar]
- 92.Gustavsson S, Hjulstrom S, Liu T, Heyman B. CD23/IgE-mediated regulation of the specific antibody response in vivo. J Immunol. 1994;152 (10):4793–4800. [PubMed] [Google Scholar]