SUMMARY
Brain abnormalities acquired early in life may cause schizophrenia, characterized by adulthood onset of psychosis, affective flattening, and cognitive impairments. Cognitive symptoms like impaired cognitive control are now recognized to be important treatment targets but cognition-promoting treatments are ineffective. We hypothesized that cognitive training during the adolescent period of neuroplastic development can tune compromised neural circuits to develop in the service of adult cognition and attenuate schizophrenia-related cognitive impairments that manifest in adulthood. We report, using neonatal ventral hippocampus lesion rats (NVHL), an established neurodevelopmental model of schizophrenia, that adolescent cognitive training prevented the adult cognitive control impairment in NVHL rats. The early intervention also normalized brain function, enhancing cognition-associated synchrony of neural oscillations between the hippocampi, a measure of brain function that indexed cognitive ability. Adolescence appears to be a critical window during which prophylactic cognitive therapy may benefit people at risk of schizophrenia.
INTRODUCTION
Cognitive impairment is a core feature of schizophrenia (Elvevag and Goldberg, 2000) and the best predictor of functional outcome (Green, 1996), but effective procognitive treatments are unknown (Weinberger and Gallhofer, 1997). Antipsychotic medications minimally improve cognition, if at all (Hill et al., 2010), and although cognitive remediation therapy may hold promise (Demily and Franck, 2008; McGurk et al., 2007; Penades et al., 2006; Wykes et al., 2007; Wykes et al., 1999), the gains of targeted remediation are variable and do not generalize substantially beyond the training tasks (Dickinson et al., 2010; Medalia et al., 2000; van der Gaag et al., 2002). The limited success of cognitive remediation therapy in schizophrenia may be due to the timing of the therapy, as it is given to adults with schizophrenia after the onset of psychotic symptoms, which may be too late. In fact, treatments of any kind are more likely to be effective at the disease prodrome than they are after onset (Lieberman et al., 2001; Perkins et al., 2005), which has generated considerable optimism that initiating treatments at the earliest indications of the disease may be optimal. Indeed, the benefits of cognitive remediation therapy are greater in younger patients (Wykes et al., 2009).
Premorbid motor and cognitive impairments in schizophrenia have been reported in children (Fish, 1957; Jones et al., 1994; Walker et al., 1994) and young adults (Reichenberg et al., 2005) who later developed schizophrenia (Fuller et al., 2002; MacCabe et al., 2008), and in children who are genetically at high-risk for schizophrenia (Gunnell et al., 2002; Maccabe, 2008; Ozan et al., 2010) (Koenen et al., 2009; Woodberry et al., 2008) supporting the idea that schizophrenia is a neurodevelopmental disorder that involves alterations in brain circuits (Insel, 2010; Lewis and Levitt, 2002; Weinberger, 1996). We examined whether adolescence, characterized by substantial neuroplastic maturation (Keshavan and Hogarty, 1999; Shen et al., 2010; Uhlhaas et al., 2009; Yurgelun-Todd, 2007), is an opportune window for prophylactic cognitive therapy. We found that cognitive training in adolescence prevents the onset of adult cognitive deficits in neonatal ventral hippocampal lesion (NVHL) rats, an established neurodevelopmental animal model of schizophrenia (Lipska, 2004; Lipska and Weinberger, 2002; McDannald et al., 2011; Tseng et al., 2009). Despite the persistence of the brain lesion into adulthood, the early intervention 1) prevented cognitive control deficits when NVHL rats are adult, 2) extended the procognitive effects beyond the training task, and 3) improved brain function assessed by inter-hippocampal synchrony of cognition-related neural oscillations. These findings suggest that prophylactic cognitive therapy at an early age can offer tremendous promise for improving functional outcomes of people at risk for schizophrenia.
RESULTS AND DISCUSSION
Impaired cognitive control in NVHL rats
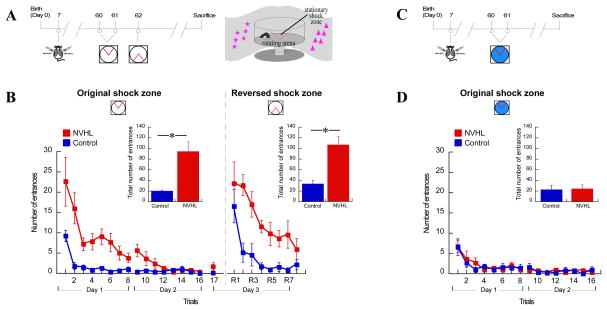
We first established that there is a cognitive control impairment in NVHL rats because this feature closely resembles the core cognitive deficit that can be measured in schizophrenia (Barch et al., 2009; Wobrock et al., 2009). We operationally define cognitive control as the ability to use relevant information and ignore irrelevant information. We measured this ability in adult (P60) NVHL and control rats using the active place avoidance task (Fig. 1A). The task requires a rat on a slowly rotating disk-shaped arena to avoid entering a stationary shock zone. In the two-frame variant of the task, the rat must dissociate locations of shock in the spatial frames of the stationary room and rotating arena by using only the relevant room cues and ignoring the irrelevant arena cues to locate the shock zone (Cimadevilla et al., 2001; Kelemen and Fenton, 2010; Wesierska et al., 2005). Adult NVHL rats tested on the two-frame task were impaired compared to sham control rats as assessed both by the learning curve (Fig. 1B left) and the total entrances across all training trials shown as a performance summary (Fig. 1B, p=0.004). Control rats quickly reduced entering the shock zone, whereas NVHL rats required prolonged training to reach the same level of avoidance. Retention of the avoidance after a 24-hour delay was tested by comparing performance on trial 16 to trial 17. Retention was not impaired in NVHL rats (t8 = 1.83; p = 0.10), suggesting that long-term memory approached normal in the NVHL rats, once they had reached the performance asymptote.
Figure 1. Impaired cognitive control in adult NVHL rats.
A. Timeline of the lesion and two-frame active place avoidance training (left). Schematic of the behavioral apparatus (right). B. Two-frame avoidance learning deficit in adult NVHL rats. Learning curve and inset: total number of entrances into the original shock zone location (left: t8 = 4.02, p = 0.004) and the reversed location in the conflict session (right: t8 = 4.35, p = 0.002). Error bars indicate SEM. C. Timeline of lesion and training in the one-frame task variant. D. Adult NVHL and sham control rats were indistinguishable in learning the one-frame task variant as shown by the learning curve. Inset: total number of entrances (t8 = 0.19, p = 0.85, 5 rats/group). *p < 0.05.
We then investigated whether the NVHL improvement in place avoidance to the level of controls was a sign of remediation that can be transferred to another task. The shock zone location was changed 180°, creating a conflict task variant that normal rats solve by inhibiting avoidance of the original shock location and learning the reversed location of shock. Control rats quickly avoided the reversed shock zone, whereas avoidance in the NVHL rats was severely impaired (Fig. 1B, p = 0.002). Although the place avoidance deficit appeared to attenuate with training in constant conditions, the impairment reappeared with changes in what information should be used and ignored.
We verified that the two-frame active place avoidance deficit is a cognitive control impairment rather than an impairment of motivation, spatial perception, memory, or navigation, which are essential components of the avoidance behavior (Fig. 1C). We used a one-frame control task in which the arena continues to rotate, just as in the two-frame task, but is covered with shallow water to remove the olfactory cues that were present but irrelevant for avoiding shock in the two-frame task (Wesierska et al., 2005). This essentially allows the rat to use the relevant room cues to locate the shock zone without interference from the hidden arena cues. Naïve adult NVHL and sham rats rapidly learned the one-frame avoidance as well as control animals (Fig. 1D). Rats in both groups rapidly decreased entering the shock zone, demonstrating intact motivation to avoid shock, spatial perception, place learning, and place avoidance in adult NVHL rats. These data show that adult NVHL rats have intact motivation, spatial perception, place learning, and place avoidance, which are abilities that cannot account for the impairment in the two-frame task variant that requires cognitive control.
It is unlikely that the impaired two-frame avoidance was due to low motivation to avoid the shock, or an inability or unwillingness to move during the two-frame task (and not during the one-frame task). That possibility was excluded by analysis of how fast the rats were actively moving (i.e. speed in the arena frame) during the place avoidance trials (Fig. S1). Instead of unwillingness to move and thus avoid the shock zone, NVHL rats moved more than the controls, which is opposite to the expectations of reduced motivation in NVHL rats. Furthermore, whether or not NVHL rats appeared hyperactive had no obvious relationship to place avoidance performance. NVHL rats were hyperactive on the initial one- and two-frame trials despite being no different than control rats in the one-frame task and being severely impaired in the two-frame task. We stress that because the only difference between the one-and two-frame task variants is the presence of water to attenuate irrelevant stimuli in the one-frame variant. Spared one-frame avoidance and impaired two-frame avoidance demonstrates a frank cognitive control deficit in adult NVHL rats as was also shown in prior work (Kelemen and Fenton, 2010; Wesierska et al., 2005).
Early cognitive training prevented adult deficits
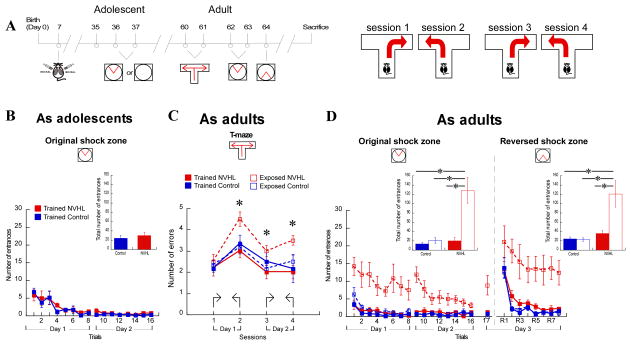
We then tested whether adolescent cognitive training could prevent the cognitive control deficit. NVHL and control rats were trained in the two-frame task as adolescents (P35) and tested in a T-maze alternation task as adults (Fig. 2A). In addition, to control for the non-cognitive components of the two-frame experience, separate groups of adolescent NVHL and control rats were exposed to the two-frame conditions but were never shocked. The trained NVHL and control groups were indistinguishable as adolescents (Fig. 2B: p = 0.52). On the T-maze, each adult rat was required to make a left or a right turn to escape shock in the other arm during a 15-trial session. Cognitive control of memory for the location of the safe arm was tested in subsequent sessions by reversing the safe and the shock arms. This required the rats to ignore the previously correct arm and use the new locations of shock for the avoidance. Performance in the first session was similar amongst all the groups, (Fig. 2C) indicating normal prerequisite abilities for good performance in the absence of a demand for cognitive control.
Figure 2. Cognitive training during adolescence prevents the cognitive control impairment in adult NVHL rats and the benefits generalize beyond the training task.
A. Schematics and timeline of experimental procedures. B. Two-frame avoidance learning during adolescence is similar in NVHL and sham control rats. Inset: total number of entrances (t9 = 0.67, p = 0.52). C. Relative to control performance in session 1 (lesion: F1,19 = 0.12, p = 0.72; treatment: F1,19 = 0.69, p = 0.41; interaction: F1,19 = 0.05, p = 0.81), alternation learning in subsequent sessions was impaired in NVHL rats that were only exposed to the rotating arena during adolescence. Adult NVHL rats that received adolescent training were better that those that were exposed during adolescence: session 2 (t9 = 3.53, p = 0.006), session 3 (t9 = 3.15, p = 0.01), and session 4 (t9 = 4.26, p = 0.002). Comparison of NVHL and control rats that were trained during adolescence: session 2 (t9 = 0.52, p = 0.52), session 3 (t9 = 0.19, p = 0.85), session 4 (t9 = 0.73, p = 0.49). D. Two-frame avoidance of both the original (lesion: F1,19 = 13.01, p = 0.002; treatment: F1,19 = 14.12, p = 0.001; interaction: F1,19 = 10.11, p = 0.005) and reversed shock zone locations (lesion: F1,19 = 12.16, p = 0.003; treatment: F1,19 = 7.21, p = 0.01; interaction: F1,19 = 7.62, p = 0.01) was impaired in the NVHL rats that were only exposed to the rotating arena during adolescence (Tukey HSD post-hoc p’s<0.05). Inset: total number of entrances. 24-h retention of the place avoidance on trial 17 relative to performance on trial 16 was not worse in the exposed NVHL rats (t8 = 1.97; p = 0.08). *p < 0.05
When cognitive control was required in subsequent sessions, the trained NVHL rats learned to alternate the safe and shock arms like the control rats, but the exposed NVHL rats were impaired (Fig. 2C). The trained NVHL rats were significantly better than the exposed NVHL rats in sessions 2–4, indicating adolescent training promoted adult cognition. We then compared the trained NVHL and the trained control rats to assess whether adolescent cognitive training was normalizing. The two groups did not differ (Fig. 2C) suggesting that adolescent cognitive training improved cognitive control to normal.
We verified that the improved cognitive performance of NVHL rats in the T-maze was due to adolescent training by retesting all the rats on the two-frame place avoidance task (Fig. 2D). The NVHL rats that were trained as adolescents were not impaired, but the NVHL rats that were only exposed to the rotating arena as adolescents were consistently impaired in avoiding both the original shock zone (Fig. 2D left) as well as the reversed shock zone in the conflict avoidance test (Fig. 2D right). Only the exposed NVHL rats were significantly impaired compared to the other groups in both the original and reversed shock zones (p’s<0.05). We conclude that adolescent cognitive training has adult procognitive effects that include preventing cognitive control deficits following a neonatal lesion, and that this benefit can generalize to other tests of cognition.
Early cognitive training changed adult neural function
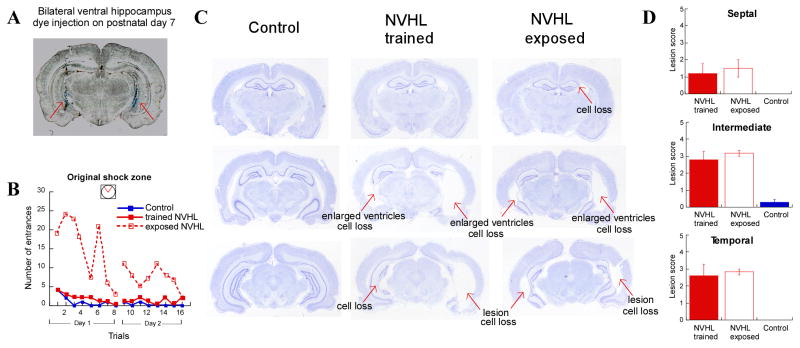
Physical changes in the degree of the adult hippocampal lesion could not account for the cognitive benefits of adolescent training because there was no correspondence between lesion extent and cognitive performance (Fig. 3). Although the adolescent trained and exposed NVHL rats show similar degree of lesion of septal, intermediate, and temporal hippocampus, cognitive performance was markedly different.
Figure 3. Cognitive training during adolescence prevented the adult cognitive impairment despite the persistence of the brain lesion.
A. Histological verification of the neonatal (P7) infusion site using 0.1% trypan blue (indicated by red arrows). B. Learning curves of the three rats for which histological characterization of brain lesions are shown in (C). C. Nissl-stained sections from the septal, intermediate, and temporal regions of the hippocampus in three representative rats. D. Lesion scores of trained NVHL and exposed NVHL rats did not differ at different septo-temporal levels of hippocampus (septal: t9 = 0.39, p = 0.70; intermediate: t9 = 0.76, p = 0.46; temporal: t9 = 0.36, p = 0.72).
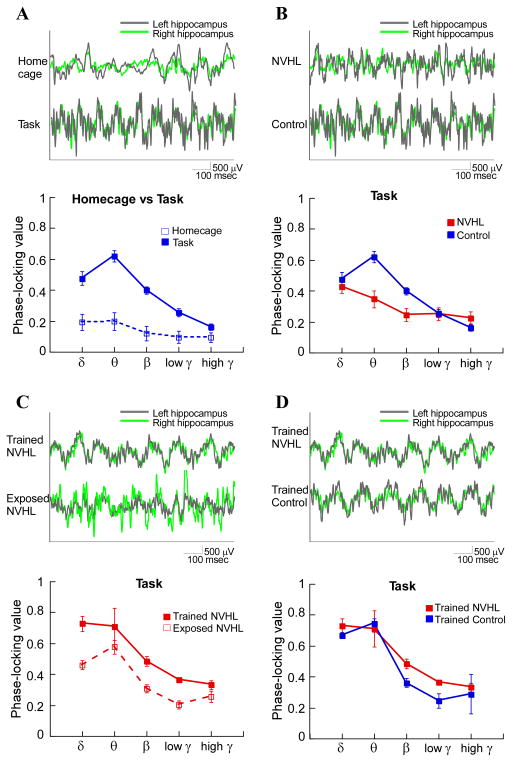
We then tested whether early cognitive training caused functional changes, focusing on neural synchrony, which may be disturbed in patients with schizophrenia (Gandal et al., 2011; Moran and Hong, 2011; Uhlhaas and Singer, 2010). Local field potentials (LFPs) in hippocampus and the medial prefrontal cortex (mPFC) of adult control rats were compared from recordings during home cage behaviors and during the two-frame task to first identify changes that were related to cognitive performance. Neural synchrony between two electrode locations was measured as the frequency-specific phase locking value (Fig. S2). In sham control rats, compared to being in the home cage, performing the task produced a robust increase of inter-hippocampus phase synchrony across delta, theta, and beta frequencies but not gamma (Fig. 4A). These changes were specific to hippocampus because no such differences were found in either inter-mPFC or inter hippocampus-mPFC synchrony (Fig. S3). Because inter-hippocampus synchrony was related to two-frame performance but synchrony involving the mPFC was not, we focused on hippocampal synchrony in further analyses. We then compared inter-hippocampal synchrony of adult control and NVHL rats while they were performing the two-frame task. The adult NVHL rats that showed impaired cognitive control (Fig. 1B) also had lower inter-hippocampal synchrony compared to control rats (Fig. 4B).
Figure 4. Cognitive training during adolescence increased cognition-associated inter-hippocampal phase synchrony in adult NVHL rats.
Raw EEG traces (top); inter-hippocampal phase synchrony (bottom) A. In sham control rats, phase synchrony across delta, theta, and beta frequencies but not gamma was significantly increased during two-frame avoidance compared to home cage behaviors (group: F1,35 = 95.77, p = 10−11; frequency: F4,19 = 17.01, p = 10−6; interaction: F4,35 = 5.96, p = 0.0009; Tukey HSD post-hoc p’s < 0.05). B. Adult NVHL rats had significantly lower phase synchrony than control rats during the two-frame task (group: F1,40 = 10.79, p = 0.002; frequency: F4,40 = 19.69, p = 10−9; interaction: F4,40 = 5.56, p = 0.001; Tukey HSD post-hoc p’s < 0.05). C. While performing the two-frame task, adult NVHL rats that were trained during adolescence had significantly greater phase synchrony than adult NVHL rats that were only exposed to the rotating arena during adolescence (group: F1,20 = 35.32, p = 10−6; frequency: F4,20 = 29.96, p = 10−8; interaction: F4,20 = 1.28, p = 0.31). D. Phase synchrony did not significantly differ between adult NVHL and control rats that were given the two-frame training during adolescence (group: F1,10 = 2.51, p = 0.14; frequency: F4,10 = 22.06, p = 10−5; interaction: F4,10 = 0.55, p = 0.71). *p < 0.05.
Since adolescent cognitive training improved adult cognition in NVHL rats, we asked whether the early experience also increased inter-hippocampal synchrony. Adult NVHL rats that received cognitive training as adolescents had higher inter-hippocampal synchrony compared to the adult NVHL rats that were just exposed to the rotating arena as adolescents (Fig. 4C). In fact, inter-hippocampal synchrony in the trained NVHL rats could not be distinguished from that of the trained control rats (Fig. 4D), suggesting that adolescent cognitive training normalized inter-hippocampal synchrony in NVHL rats.
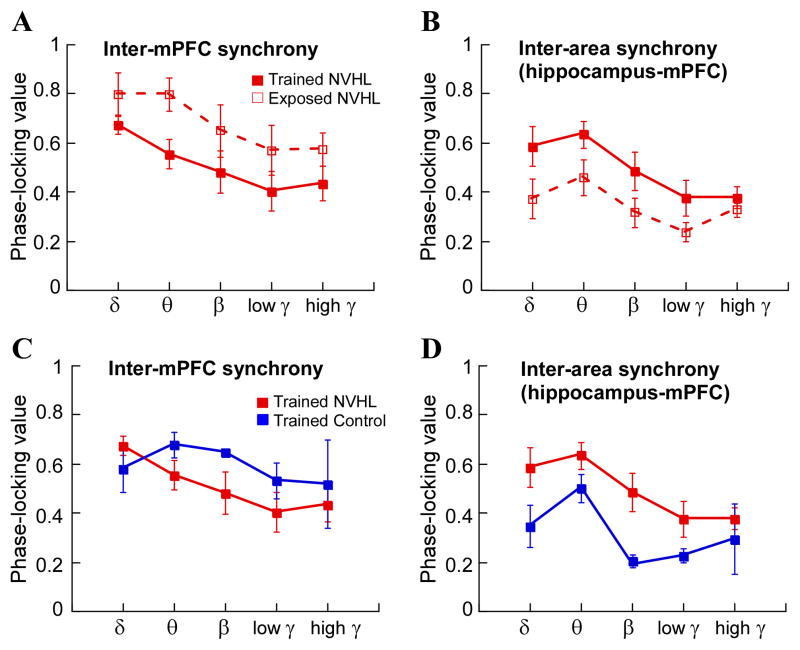
Beyond normalizing the synchrony of LFP oscillations between the two dorsal hippocampi of adult NVHL rats, the juvenile cognitive experience caused additional changes in neural synchrony during the two-frame task. Compared to the NVHL rats that were just exposed to the rotating arena as juveniles, the phase synchrony between the left and right mPFC tended to be lower at all the frequency bands, from delta to fast gamma, in the NVHL rats that had juvenile cognitive training (Fig. 5A). An essentially opposite pattern of differences in synchrony between the mPFC and hippocampus was observed between the adult NVHL rats that had been trained or exposed as juveniles. Phase synchrony between the hippocampus and mPFC tended to be higher at all the frequency bands in the NVHL rats that had juvenile cognitive training compared to the NVHL rats that had only been exposed to the rotating arena (Fig. 5B). The same variables were compared between the NVHL and sham control animals that were trained in adolescence. No significant differences were identified in left-right mPFC phase synchrony (Fig. 5C) but phase synchrony between the hippocampus and mPFC sites was reliably greater in the NVHL animals (Fig. 5D). Because synchrony between the left and right mPFC and synchrony between the mPFC and hippocampus was not different during home cage behavior and during the two frame task (Fig. S3), it is unclear whether these differences are relevant for cognitive function in the two-frame task. Nonetheless, these findings provide additional unambiguous evidence that the adolescent cognitive experience had potentially widespread functional consequences in brain networks known to be involved in a variety of cognitive operations, including cognitive control (Kelemen and Fenton, 2010; Miller and Cohen, 2001).
Figure 5. Cognitive training during adolescence altered phase synchrony between mPFC sites and between mPFC and hippocampus in adult NVHL rats.
Significant group differences between adolescent trained NVHL and adolescent exposed NVHL rats during the two-frame task. A. Inter-mPFC synchrony: group (F1,30 = 11.27, p = 0.002), frequency (F4,30 = 3.80, p = 0.01), interaction (F4,30 = 0.17, p = 0.95) and B. inter hippocampus-phase synchrony: group (F1,30 = 8.61, p = 0.006), frequency (F4,25 = 2.83, p = 0.04), interaction (F4,25 = 0.29, p = 0.88). C. No significant group difference between adolescent trained NVHL and adolescent trained control rats in inter-mPFC synchrony: group (F1,20 = 2.21, p = 0.15), frequency (F4,20 = 1.55, p = 0.23), interaction (F4,20 = 0.71, p = 0.60) but D. a significant group difference in inter hippocampus-mPFC synchrony: group (F1,10 = 14.48, p = 0.004), frequency (F4,10 = 4.41, p = 0.03), interaction (F4,10 = 0.65, p = 0.64).
Early cognitive training changed adult parvalbumin labeling in the mPFC
We sought additional evidence that cognitive training in adolescence could alter brain structure or function in adulthood. Four groups were examined: NVHL animals that had training (n=4) or were exposed (n=5), and saline-treated animals that had training (n=3) or were exposed (n=5) in adolescence (P35). We began by investigating whether training in adolescence increased mPFC thickness as an account for the cognitive improvements because prefrontal cortical thickness is related to a variety of learned and innate behaviors in the rat (Kolb and Whishaw, 1981) and frontal cortical thickness may be decreased in schizophrenia, even in first-episode patients (Wiegand et al., 2004). Measurements of mPFC thickness did not reveal any effect of training (Two-way ANOVA, F1,13 = 0. 56, p = 0.5), lesion (F1,13 = 0.42, p = 0.5), or the training x lesion interaction (F1,13 = 0.04, p = 0.84), causing us to examine a marker that is related to oscillatory function.
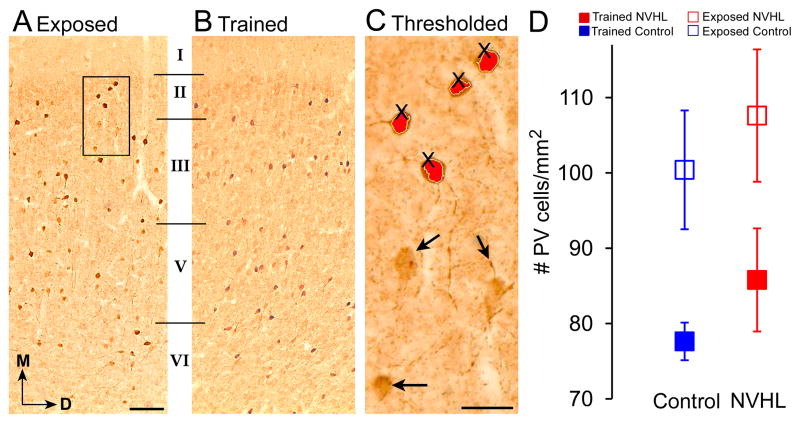
We investigated whether training in adolescence altered the expression of the calcium binding protein parvalbumin (PV) in the adult mPFC. We studied PV because GABAergic neurotransmission is a major contributor to long-range hippocampal synchrony in the theta and beta frequency ranges (Bibbig et al., 2002; Brazhnik and Fox, 1997, 1999; Stewart and Fox, 1990) as well as control of the theta phase at which principal cells discharge (Royer et al., 2012), and dysfunction in PV-positive GABAergic interneurons has been hypothesized for schizophrenia (Lewis and Moghaddam, 2006). Figures 6A,B show a representative comparison of PV-labeled cells in the prelimbic division of mPFC. No qualitative differences were detected in the morphology of the cells that were labeled, which were all very similar to mPFC GABAergic neurons that have been previously described (Gabbott et al., 1997b). Quantification of PV-labeled cells did not show differences in the NVHL and control groups but it showed that training decreased PV-labeling. Two-way ANOVA confirmed a significant effect of training (F1,13 = 7.77, p = 0.02) but no effects of lesion (F1,13 = 0.92, p = 0.4) or the lesion X training interaction (F1,13 = 0.00, p = 0.95).
Figure 6. Quantification of parvalbumin (PV)-labeled cells in the medial prefrontal cortex.
A. A representative coronal section from an exposed animal shows the laminar distribution of PV in the mPFC. The layers of the PL (I–VI) are marked as defined by Gabbott et. al (1997a). D = dorsal, M = medial. B. A representative coronal section from a trained animal shows the PL area in the mPFC labeled with PV. Scale bar for A and B = 100 μm shown in B. C. A high power image of the area marked by the rectangle in part A illustrates the method that was used to count PV-labeled neurons. A threshold was set so that only cells with strong PV expression were counted. These cells are indicated in green with a pink “x”. The threshold was set conservatively so that cells with weak PV expression were not counted (arrows). However, we also calculated the number of PV-expressing cells with weak or strong expression and the results were not different (data not shown). Scale bar for C = 10 μm. D. A scatter plot showing the number of PV-labeled cells per mm2 in exposed (white) and trained (black) control and NVHL adult animals.
Summary
The main finding is that early cognitive training prevents the adult cognitive control deficit in NVHL rats and this apparent prophylaxis is associated with improved cognition-related brain function, measured as normalized inter-hippocampal synchrony of field potential oscillations. To our knowledge, this is the first demonstration of prophylactic cognitive treatment in an animal model of schizophrenia. Although cognitive control is a core untreated deficit in schizophrenia, this deficit had not been definitely demonstrated in the NVHL model, or any other schizophrenia-related animal model for that matter. We wish to stress that synchrony between LFPs in the two hippocampi was identified as a correlate of two-frame place avoidance (Fig. 4a) in control rats whereas we did not identify any such relationship in two-frame avoidance and the synchrony between LFPs in the mPFC sites or between LFPs in the mPFC and dorsal hippocampus (Fig. S3). Consequently, we focused on inter-hippocampal synchrony as a measure of brain function that is relevant to the cognitive task we used. Synchrony involving mPFC LFPs was clearly altered (Figs. 6; S3) and exaggerated hippocampus-mPFC synchrony may have even been a compensatory alteration (Fig. 5D). It is likely that mPFC oscillations may reflect a more general functional anomaly in the NVHL rat, whereas unambiguous cognition-related electrophysiological measures of mPFC function may only emerge at the level of single unit discharge or in tasks with different cognitive challenges (Gruber et al., 2010). Indeed, direct electrophysiological evidence of cognitive control was provided by decoding the spatial information in the neural ensemble discharge of hippocampus during a two-frame task variant with both a stationary and a rotating shock zone (Kelemen and Fenton, 2010). As the rat moved through the space, positional information in hippocampus discharge switched between the two spatial frames, reflecting the frame of the nearby shock zone. The neurons within a single hippocampus formed transient, functionally-defined neural groups by discharging together at the timescales of gamma and theta oscillations. Here, we observed inter-hippocampal task- and experience-dependent synchrony changes in the theta range (Fig. 4) but not in the gamma range. These data add to the evidence that gamma oscillations only organize neural activity locally and that lower frequency oscillations, including theta, are more likely to provide for long-range temporal organization between brain regions (Kopell et al., 2000; Siapas et al., 2005), including the theta-gamma coupling that may channel information from different sources into the hippocampus (Colgin et al., 2009; Fries, 2009; Tort et al., 2009).
Support for the neurodevelopmental and discoordination hypotheses
Adolescent cognitive training prevented both the cognitive and neural synchrony abnormalities in adult NVHL rats, providing strong support for the neurodevelopmental hypothesis. The hypothesis, which focuses on etiology, asserts that schizophrenia is caused by a defect in early brain development (Weinberger, 1995, 1996). The hypothesis emphasizes the vulnerabilities due to continuing development of the brain into early adulthood (Insel, 2010). This perspective also makes the optimistic prediction that treatments could be prophylactic if administered sufficiently early before abnormalities manifest, a prediction that is confirmed by the present study.
A unifying idea we call the discoordination hypothesis has been proposed to account for the syndrome, whatever the etiology (Fenton, 2008; Gordon, 2001; Lee et al., 2003; Phillips and Silverstein, 2003; Tononi and Edelman, 2000; Uhlhaas and Singer, 2006; Wright and Kydd, 1986). This view acknowledges that schizophrenia may turn out to be heterogeneous and that multiple factors contribute, which include genetic alterations, infectious, toxic, and stressful events. The idea is rooted in the concept of cognitive coordination, the brain’s ability to selectively and dynamically activate and suppress information in order to organize knowledge and perception into useable representations.
The physiological basis of this mental coordination is hypothesized to involve the organized timing of spike discharge across ensembles of neurons (Buzsaki, 2010; Hebb, 1949; Kelemen and Fenton, 2010; Kelemen and Fenton, in press; Phillips and Silverstein, 2003; Phillips and Singer, 1997). To minimize confusion between what is mental and what is physiological, we use the term “neural coordination” to refer specifically to the physiological coordination of neural activity, and the term “cognitive coordination” to refer to interpretations of behavioral observations. The experiments presented here were also formulated within the framework of the discoordination hypothesis, which asserts that disruption of neural coordination produces abnormalities in cognitive coordination, resulting in the core cognitive dysfunctions of schizophrenia. This physiological hypothesis is agnostic to etiology of the disease and to whether there are abnormalities in dopaminergic, glutamatergic, GABAergic or other neurotransmitter systems. It is thus remarkable that abnormal synchrony between the two hippocampi was associated with cognitive control difficulties in the adult NVHL rats and that the adolescent cognitive training corrected both the neural and cognitive abnormalities, which confirmed basic predictions of the discoordination hypothesis. We conclude that the present work offers an experimental platform for evaluating both the neurodevelopmental and discoordination hypotheses. An important next step is to use the platform to evaluate other neurodevelopmental schizophrenia models with distinct etiologies such as the drug-induced methylazoxymethanol (MAM) model (Chen and Hillman, 1986; Featherstone et al., 2007), the polyriboinosinic–polyribocytidilic acid (PolyI:C) immune challenge model (Meyer et al., 2005; Pearce, 2001) and genetic models such as the DISC1 (Kim et al., 2012) and other mutant mouse models (Belforte et al., 2010; Sigurdsson et al., 2010).
A procognitive treatment opportunity?
The benefits of early cognitive training demonstrated in the NVHL model indicate the possibility of a critical window for procognitive intervention in schizophrenia and related disorders. This offers preclinical support for the idea that it is more effective to treat schizophrenia patients in the prodrome of the disease, at the very earliest signs of a disorder (Bird et al., 2010; Lieberman et al., 2001; Perkins et al., 2005). In fact, the present findings suggest there may be substantial merit to explore the effectiveness of behavioral interventions even earlier in a preemptive effort. While further work in animal models will be needed to define and possibly expand (Maya Vetencourt et al., 2008) the boundaries of the opportunity, adolescence may be a natural target since it is characterized by substantial brain maturation (Shen et al., 2010; Uhlhaas et al., 2009; Yurgelun-Todd, 2007). The mechanism for the increase of inter-hippocampal synchrony we observed in the adolescent trained NVHL rats is unknown, and may be multifaceted. We can only speculate that experience-dependent changes in synaptic plasticity and functional connectivity could help to guide maturation of neural circuits that subserve cognition in a manner that is tailored by use in the event that the normal developmental program has been disturbed. Nonetheless, precedent for this idea has been observed in children following intensive remedial reading (Keller and Just, 2009) and even in adult, post-onset schizophrenia patients. A recent study of chronic schizophrenia patients showed that computerized cognitive training improved the ability to distinguish between self- and externally-generated material in a test of reality monitoring and that the cognitive improvement was associated with increased mPFC activity and improved social functioning that persisted for at least 6 months (Subramaniam et al., 2012). In fact, preemptive cognitive therapy as a general strategy may hold substantial merit in overcoming the poor motivation of schizophrenia patients to participate in cognitive therapy. The present findings suggest substantial benefits if cognitive therapy is preemptive, as early as in adolescence when symptoms are at best mild, before the full onset of the debilitating positive, negative and cognitive symptoms of the disease. The present data compel us to suggest that adolescence may be a critical window of opportunity for cognitive treatment, and that prophylactic cognitive therapy during this period may offer tremendous promise for improving intellectual competence in people at risk for schizophrenia, and perhaps other neurodevelopmental disorders with a significant impact on cognitive function.
EXPERIMENTAL PROCEDURES
Neonatal ventral hippocampal lesion
The neonatal lesions procedure followed the manual provided Barbara Lipska and Daniel Weinberger (Lipska et al., 1993). Briefly, time-pregnant (13 or 14 days in gestation) female Long-Evans rats were obtained from Charles River Laboratories (Wilmington, MA). Pups were born at the Downstate animal facility. On postnatal day 7 (P7), male pups were anaesthetized by hypothermia. Bilateral puncture holes (relative to bregma AP: −3.0mm, ML: ±3.5mm) were made in the skull with a 30-ga injection needle. Bilateral infusions (0.3 μl/side) of saline or ibotenic acid solution (10μg/μl) was delivered to each ventral hippocampus (relative to skull surface DV: −5.0mm).
Two-frame place avoidance task
Adolescent and adult rats were placed one at a time on an 82-cm diameter circular arena that rotated at 1 rpm to test active place avoidance. A mild constant current (< 0.4 mA) foot shock was delivered for 500 ms whenever the rat entered a computer defined 60° shock zone that was fixed in the room. The arena rotated in both the one-frame and two-frame task variants. In the one-frame task variant, the arena surface was covered by shallow water and in the two-frame task variant the arena was dry. Each place avoidance trial was 10 minutes long and the interval between trials was at least 10 minutes. Each trial was repeated 8 times per day for two days. Memory retention of the initial shock zone location was tested during a single trial on day 3. For the subsequent session of 8 conflict avoidance trials, the shock zone location was changed 180°. Place avoidance was measured as the number of times the rat entered the shock zone.
T-maze task
Rats were given two 15-trial sessions per day for 2 days. Rats were placed in the start arm and a 0.5 mA constant current foot shock was activated after 5 s and thereafter every 10 s until the rat escaped to the correct arm. Once in the correct arm, the rat was placed there for 30 s before the start of the next trial. The correct arm was constant within a session but alternated between sessions.
Histological characterization of lesions
Rats were deeply anesthetized and perfused with 4% paraformaldehyde (pH 7.4). Coronal sections (40 μm) were collected from the septal, intermediate, and ventral regions of the hippocampus identified according to the stereotaxic atlas (Paxinos and Watson, 2007). Lesions were evaluated by light microscope examination of cresyl violet-stained sections and appeared similar to those that have been published for NVHL Long-Evans rats (McDannald et al., 2011). A lesion score for each region was computed as the sum of the scores from three categories: cell layer damage (0 = none, 1 = disorganized, 2 = gross cell loss); tissue damage (0 = none, 1 = unilateral, 2 = bilateral), ventricular enlargement (0 = none, 1 = enlarged). With maximal damage, the highest score for a region is 5.
Parvalbumin immunohistochemistry
A mouse monoclonal antibody against parvalbumin (PV; clone PARV-19, MAB 1575, Millipore, Billerica, MA) was used to evaluate GABAergic interneurons in mPFC. The characterization of the antibody by the manufacturer using Western blot shows that the antibody labels a single 12 kD band, corresponding to the molecular weight of PV. Immunocytochemistry using this antibody suggests that it labels a similar population of GABAergic interneurons as other antibodies to PV, with qualitatively similar patterns of expression (Blurton-Jones and Tuszynski, 2006). Coronal sections from control and NVHL rats were prepared as described in the histology section above. The sections were labeled with the PV antibody (1:10,000 dilution) using previously described immunohistochemical procedures (Duffy et al., 2011; Scharfman et al., 2002). Sections were mounted and coverslipped and the slides were analyzed using a brightfield microscope (BX61, Olympus, Center Valley, PA, U.S.A.), and photographed using a digital camera (RET 2000R-F-CLR-12, Q Imaging, Surrey, BC, Canada).
Quantification of PV-labeled cells was performed using Bioquant software (Bioquant Image Analysis Corporation, Nashville, TN, U.S.A.). Briefly, both hemispheres were analyzed from at least 2 sections from saline treated exposed control (n = 5), saline treated trained (n= 3), NVHL exposed control (n = 5) and NVHL trained (n = 4) animals. The experimenter was blind to the origin of the tissue. The sections that were analyzed were located approximately +2.5 and +3.2 anterior to Bregma according to a standard mouse stereotaxic atlas (Franklin and Paxinos, 2007; Gabbott et al., 1997a). An area of at least 2.4 mm2 within the prelimbic cortex (PL) of the mPFC, containing all layers from pial surface to the layer VI/white matter border, was analyzed in each animal. The nomenclature used for the PL has been described previously (Gabbott et al., 1997a). Immunolabeled cells were defined by computerized thresholding and counted using Bioquant software. The threshold was set so that PV-expressing cells with robust PV expression were always above the threshold, and cells that the software determined were not different from background were not counted.
The cortical thickness of PL mPFC was calculated from sections that were stained with cresyl violet. These sections were adjacent to those that were used to quantify PV expression. Cortical thickness was defined as the distance from the pial surface to the border of layer VI with the underlying white matter, and measured using Bioquant software.
Local field potential recordings
Local field potentials in the dorsal hippocampus (AP: −4 mm; ML: ±2.5 mm; DV: −3 mm) and in the mPFC (AP: +3 mm; ML: ±1 mm; DV: −4 mm) were recorded by implanting rats with 75-μm Nichrome wire (Fig. S4). All electrodes were referred to an electrode implanted in the cerebellar white matter (AP: −10 mm; ML: +2 mm; DV: −3 mm). Recordings were made with a wireless digital telemetry system (Bio-Signal Group, Brooklyn, NY) (Fenton et al., 2010). The signals at the electrode connector were amplified (300 times), low-pass filtered (6 kHz) and digitized (24-bits, 12 kHz using delta-sigma analog-digital convertors). The digital signals were transmitted wirelessly to a recording system (dacqUSB, Axona Ltd., St. Albans, U.K.) for bandpass filtering (1–500 Hz), digital amplification and down-sampling (16-bits, 2000 Hz) using digital signal processors. The digital EEG data were stored on computer hard drives for off-line analysis.
Phase locking value (PLV)
The pairs of EEG channels that were selected for PLV analysis were: left-hippocampus and right-hippocampus; left-mPFC and right-mPFC; left-hippocampus and left-mPFC; right-hippocampus and right-mPFC. Using custom software written in Matlab, all signals were first low-pass filtered (250Hz) and then down-sampled from 2 kHz to 1 kHz. The phase of a signal at time t, sample n φ(t, n) was obtained by filtering the signal with a narrow-band Finite Input Response (FIR) filter using a zero phase-shift filtering algorithm followed by a Hilbert Transform. The filters were designed using the Matlab filter design toolbox. Given a pair of EEG signals of N samples, the PLV was defined as follows (Lachaux et al., 1999):
where θ(t, n) is the phase difference φ1(t, n) − φ2(t, n) between the two signals at time t sample n; N is the total number of samples; and i is the imaginary unit.
Statistics
Statistical comparisons were done by t tests or two-way ANOVA with Tukey HSD post-hoc tests when appropriate.
Supplementary Material
HIGHLIGHTS.
Impaired cognitive control in adult neonatal ventral hippocampus lesion (NVHL) rats.
Inter-hippocampal field potential theta/beta phase synchrony indexes cognition.
Cognitive training in adolescence prevents the adult cognitive control deficit.
Cognitive training in adolescence also normalizes cognition-related phase synchrony.
Acknowledgments
Supported by NIMH grant R01MH084038-01 and a Young Investigator’s Award from NARSAD to AAF. We are grateful to Drs. Steven Silverstein and Daniel Weinberger for advice.
Footnotes
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
HL collected and analysed data and wrote the manuscript. DD analysed data, H-YK performed preliminary experiments, AD and HS performed and analysed histological studies, AAF designed and supervised research, and wrote the manuscript. All authors discussed the results and manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barch DM, Braver TS, Carter CS, Poldrack RA, Robbins TW. CNTRICS final task selection: executive control. Schizophr Bull. 2009;35:115–135. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbig A, Traub RD, Whittington MA. Long-range synchronization of gamma and beta oscillations and the plasticity of excitatory and inhibitory synapses: a network model. J Neurophysiol. 2002;88:1634–1654. doi: 10.1152/jn.2002.88.4.1634. [DOI] [PubMed] [Google Scholar]
- Bird V, Premkumar P, Kendall T, Whittington C, Mitchell J, Kuipers E. Early intervention services, cognitive-behavioural therapy and family intervention in early psychosis: systematic review. Br J Psychiatry. 2010;197:350–356. doi: 10.1192/bjp.bp.109.074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estradiol-induced modulation of estrogen receptor-β and GABA within the adult neocortex: A potential transsynaptic mechanism for estrogen modulation of BDNF. J Comp Neurol. 2006;499:603–612. doi: 10.1002/cne.21122. [DOI] [PubMed] [Google Scholar]
- Brazhnik ES, Fox SE. Intracellular recordings from medial septal neurons during hippocampal theta rhythm. Exp Brain Res. 1997;114:442–453. doi: 10.1007/pl00005653. [DOI] [PubMed] [Google Scholar]
- Brazhnik ES, Fox SE. Action potentials and relations to the theta rhythm of medial septal neurons in vivo. Exp Brain Res. 1999;127:244–258. doi: 10.1007/s002210050794. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Hillman DE. Selective ablation of neurons by methylazoxymethanol during pre- and postnatal brain development. Exp Neurol. 1986;94:103–119. doi: 10.1016/0014-4886(86)90275-x. [DOI] [PubMed] [Google Scholar]
- Cimadevilla JM, Wesierska M, Fenton AA, Bures J. Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociation of arena cues from room cues by rotation of the arena. Proc Natl Acad Sci U S A. 2001;98:3531–3536. doi: 10.1073/pnas.051628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Demily C, Franck N. Cognitive remediation: a promising tool for the treatment of schizophrenia. Expert Rev Neurother. 2008;8:1029–1036. doi: 10.1586/14737175.8.7.1029. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Tenhula W, Morris S, Brown C, Peer J, Spencer K, Li L, Gold JM, Bellack AS. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Am J Psychiatry. 2010;167:170–180. doi: 10.1176/appi.ajp.2009.09020264. [DOI] [PubMed] [Google Scholar]
- Duffy AM, Schaner MJ, Wu SH, Staniszewski A, Kumar A, Arevalo JC, Arancio O, Chao MV, Scharfman HE. A selective role for ARMS/Kidins220 scaffold protein in spatial memory and trophic support of entorhinal and frontal cortical neurons. Experimental Neurology. 2011;229:409–420. doi: 10.1016/j.expneurol.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Nobrega JN, Kapur S, Fletcher PJ. Gestational methylazoxymethanol acetate treatment impairs select cognitive functions: parallels to schizophrenia. Neuropsychopharmacology. 2007;32:483–492. doi: 10.1038/sj.npp.1301223. [DOI] [PubMed] [Google Scholar]
- Fenton AA. Neural coordination and psychotic disorganization. In: Holscher C, Munk MH, editors. Information Processing by Neuronal Populations. London: Cambridge University Press; 2008. pp. 387–408. [Google Scholar]
- Fenton AA, Jeffery KJ, Donnett JG. Neural Recording Using Digital Telemetry In Electrophysiological Recording Techniques. In: Vertes RP, Stackman RWJ, editors. NeuroMethods Series. Totowa, NJ: Humana Press; 2010. [Google Scholar]
- Fish B. The detection of schizophrenia in infancy; a preliminary report. J Nerv Ment Dis. 1957;125:1–24. doi: 10.1097/00005053-195701000-00001. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3. San Diego: Academic Press; 2007. [Google Scholar]
- Fries P. The model- and the data-gamma. Neuron. 2009;64:601–602. doi: 10.1016/j.neuron.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159:1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Dickie BGM, Vaid RR, Headlam AJN, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: Morphology and quantitative distribution. J Comp Neurol. 1997a;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Dickie BGM, Vaid RR, Headlam AJN, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: Morphology and quantitative distribution. J Comp Neurol. 1997b;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: Towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. Integrative psychophysiology. Int J Psychophysiol. 2001;42:95–108. doi: 10.1016/s0167-8760(01)00160-x. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell D, Harrison G, Rasmussen F, Fouskakis D, Tynelius P. Associations between premorbid intellectual performance, early-life exposures and early-onset schizophrenia. Cohort study. Br J Psychiatry. 2002;181:298–305. doi: 10.1192/bjp.181.4.298. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior, a neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- Hill SK, Bishop JR, Palumbo D, Sweeney JA. Effect of second-generation antipsychotics on cognition: current issues and future challenges. Expert Rev Neurother. 2010;10:43–57. doi: 10.1586/ern.09.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Kelemen E, Fenton AA. Dynamic grouping of hippocampal neural activity during cognitive control of two spatial frames. PLoS Biol. 2010;8:e1000403. doi: 10.1371/journal.pbio.1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen E, Fenton AA. The organization of neuronal discharge on timescales of milliseconds and seconds is related to the spatial response properties of hippocampal neurons. In: Sato N, Omori T, editors. Advances in Cognitive Neurodynamics (III) Proceedings of the Third International Conference on Cognitive Neurodynamics. Heidelberg: Springer; 2011. (in press) [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Dev Psychopathol. 1999;11:525–543. doi: 10.1017/s0954579499002199. [DOI] [PubMed] [Google Scholar]
- Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, Feighery E, Lu B, Rujescu D, St Clair D, et al. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell. 2012;148:1051–1064. doi: 10.1016/j.cell.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, Poulton R, Caspi A. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry. 2009;166:50–57. doi: 10.1176/appi.ajp.2008.08030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Neonatal Frontal Lesions in the rat: sparing of learned but not species-typical behavior in the presence of reduced brain weight and cortical thickness. J Comp Physiol Psychol. 1981;95:863–879. doi: 10.1037/h0077849. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Perkins D, Belger A, Chakos M, Jarskog F, Boteva K, Gilmore J. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- Lipska BK. Using animal models to test a neurodevelopmental hypothesis of schizophrenia. J Psychiatry Neurosci. 2004;29:282–286. [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. A neurodevelopmental model of schizophrenia: neonatal disconnection of the hippocampus. Neurotox Res. 2002;4:469–475. doi: 10.1080/1029842021000022089. [DOI] [PubMed] [Google Scholar]
- Maccabe JH. Population-based cohort studies on premorbid cognitive function in schizophrenia. Epidemiol Rev. 2008;30:77–83. doi: 10.1093/epirev/mxn007. [DOI] [PubMed] [Google Scholar]
- MacCabe JH, Lambe MP, Cnattingius S, Torrang A, Bjork C, Sham PC, David AS, Murray RM, Hultman CM. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychol Med. 2008;38:1133–1140. doi: 10.1017/S0033291707002048. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- McDannald MA, Whitt JP, Calhoon GG, Piantadosi PT, Karlsson RM, O’Donnell P, Schoenbaum G. Impaired reality testing in an animal model of schizophrenia. Biol Psychiatry. 2011;70:1122–1126. doi: 10.1016/j.biopsych.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Revheim N, Casey M. Remediation of memory disorders in schizophrenia. Psychol Med. 2000;30:1451–1459. doi: 10.1017/s0033291799002913. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moran LV, Hong LE. High vs low frequency neural oscillations in schizophrenia. Schizophr Bull. 2011;37:659–663. doi: 10.1093/schbul/sbr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozan E, Deveci E, Oral M, Karahan U, Oral E, Aydin N, Kirpinar I. Neurocognitive functioning in a group of offspring genetically at high-risk for schizophrenia in Eastern Turkey. Brain Res Bull. 2010;82:218–223. doi: 10.1016/j.brainresbull.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson M. The rat brain in stereotaxic coordinates. 6. San Diego: Academic Press; 2007. [Google Scholar]
- Pearce BD. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Mol Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- Penades R, Catalan R, Salamero M, Boget T, Puig O, Guarch J, Gasto C. Cognitive remediation therapy for outpatients with chronic schizophrenia: a controlled and randomized study. Schizophr Res. 2006;87:323–331. doi: 10.1016/j.schres.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Gu H, Boteva K, Lieberman JA. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162:1785–1804. doi: 10.1176/appi.ajp.162.10.1785. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 2003;26:65–82. doi: 10.1017/s0140525x03000025. discussion 82–137. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Singer W. In search of common foundations for cortical computation. Behav Brain Sci. 1997;20:657–683. doi: 10.1017/s0140525x9700160x. discussion 683–722. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rapp MA, Rabinowitz J, Caspi A, Schmeidler J, Knobler HY, Lubin G, Nahon D, Harvey PD, Davidson M. Elaboration on premorbid intellectual performance in schizophrenia: premorbid intellectual decline and risk for schizophrenia. Arch Gen Psychiatry. 2005;62:1297–1304. doi: 10.1001/archpsyc.62.12.1297. [DOI] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsaki G. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience. 2002;111:71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A Critical Role for {alpha}4{beta}{delta} GABAA Receptors in Shaping Learning Deficits at Puberty in Mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990;13:163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73:842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Edelman GM. Schizophrenia and the mechanisms of conscious integration. Brain Res Brain Res Rev. 2000;31:391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci U S A. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- van der Gaag M, Kern RS, van den Bosch RJ, Liberman RP. A controlled trial of cognitive remediation in schizophrenia. Schizophr Bull. 2002;28:167–176. doi: 10.1093/oxfordjournals.schbul.a006919. [DOI] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. From neuropathology to neurodevelopment. Lancet. 1995;346:552–557. doi: 10.1016/s0140-6736(95)91386-6. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology. 1996;14:1S–11S. doi: 10.1016/0893-133X(95)00199-N. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Gallhofer B. Cognitive function in schizophrenia. Int Clin Psychopharmacol. 1997;12(Suppl 4):S29–36. doi: 10.1097/00004850-199709004-00006. [DOI] [PubMed] [Google Scholar]
- Wesierska M, Dockery C, Fenton AA. Beyond memory, navigation, and inhibition: behavioral evidence for hippocampus-dependent cognitive coordination in the rat. J Neurosci. 2005;25:2413–2419. doi: 10.1523/JNEUROSCI.3962-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand LC, Warfield SK, Levitt JJ, Hirayasu Y, Salisbury DF, Heckers S, Dickey CC, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Prefrontal cortical thickness in first-episode psychosis: a magnetic resonance imaging study. Biol Psychiatry. 2004;55:131–140. doi: 10.1016/j.biopsych.2003.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobrock T, Ecker UK, Scherk H, Schneider-Axmann T, Falkai P, Gruber O. Cognitive impairment of executive function as a core symptom of schizophrenia. World J Biol Psychiatry. 2009;10:442–451. doi: 10.1080/15622970701849986. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Wright JJ, Kydd RR. Schizophrenia as a disorder of cerebral state transition. Aust N Z J Psychiatry. 1986;20:167–178. doi: 10.3109/00048678609161329. [DOI] [PubMed] [Google Scholar]
- Wykes T, Newton E, Landau S, Rice C, Thompson N, Frangou S. Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophr Res. 2007;94:221–230. doi: 10.1016/j.schres.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Corner J, Williams C, Everitt B. The effects of neurocognitive remediation on executive processing in patients with schizophrenia. Schizophr Bull. 1999;25:291–307. doi: 10.1093/oxfordjournals.schbul.a033379. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Landau S, Matthiasson P, Haworth E, Hutchinson C. Does age matter? Effects of cognitive rehabilitation across the age span. Schizophr Res. 2009;113:252–258. doi: 10.1016/j.schres.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.