Abstract
Objective: To review the efficacy and safety of rituximab therapy for systemic lupus erythematosus (SLE).Methods: We searched for randomized controlled trails and observational studies that evaluated the effect of rituximab based on the systemic lupus erythematosus disease activity index (SLEDAI), British Isles lupus assessment group index (BILAG), urine protein levels, and the prednisolone dose, and had adequate data to calculate the mean, standard deviation (SD), and 95% confidence intervals, and to systematically review and meta-analyze observational studies with fixed effects model or random effects model. Results: We included 2 randomized controlled studies and 19 observational clinical studies. We summarized the data from the 19 observational studies, analyzed the heterogeneity of the literature, and then used fixed effect model or random effect model for statistical analysis. The SLEDAI, BILAG, and urine protein levels and the prednisolone dosage were decreased after rituximab treatment, and the decreases in the BILAG, urine protein levels, and the prednisolone dose were found to be significant (P<0.05), when compared with baseline level. Rituximab’s adverse effects generally could be controlled with an effective dosing regimen. Conclusions: Although there are still controversies about rituximab’s treatment on SLE, but our study had showed that rituximab had favorable effects on refractory lupus. The long-term efficacy and safety of rituximab require further study.
Keywords: Systemic lupus erythematosus, Rituximab, Meta-analysis
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease caused by cellular and humoral immune dysfunction. Renal involvement occurs in up to 60% of SLE patients, and lupus nephritis (LN) remains a predominant cause of morbidity and mortality (Waldman and Appel, 2006). At present, the main drug treatments for SLE include corticosteroids and immunosuppressive drugs, such as cyclophosphamide (CYC), azathioprine (AZA), mycophenolate mofetil (MMF), and tacrolimus (Houssiau et al., 2002). Unfortunately, many patients experience the adverse drug reactions of the currently available immunosuppressants (which are used due to the increased risk of infection), which contribute to increased mortality. Therefore, there is an urgent need to identify new, more effective therapeutic methods with more favorable safety profiles.
Rituximab is a chimeric monoclonal antibody against the protein CD20 and is used in the treatment of lymphoma, leukemia, transplant rejection, and some autoimmune disorders (Scott, 1998). As a chimeric antibody directed against CD20 on B lymphocytes, rituximab has become a hopeful therapy on SLE (Thatayatikom and White, 2006), and there are a number of observational studies with evidence that rituximab is effective in reducing the levels of certain auto-antibodies, resulting in clinical improvement (Levine and Pestronk, 1999). However, these results are in contrast with two recently conducted controlled trials: the ‘Explorer’ (Merrill et al., 2011) and ‘Lunar’ (Furie et al., 2009) trials. These trials were randomized, double blind placebo-controlled studies. The ‘Explorer’ trial accessed the efficacy of rituximab added to standard immunosuppressive therapy in moderate or severe SLE. The ‘Lunar’ trial investigated the efficacy and safety of rituximab in active proliferative LN. Both studies failed to show clinically significant differences between rituximab and placebo. In this study, we systematically assessed the efficacy and safety of rituximab in SLE patients. In accordance with the guidelines of the meta-analysis of observational studies in epidemiology (MOOSE GROUP), we designed this study as a systematic review and meta-analysis of observational studies.
2. Materials and methods
2.1. Identification of eligible studies and data extraction
We performed a search to identify observational studies and randomized controlled trial (RCT) that examined rituximab therapy for SLE patients. Literature searches were performed using the PubMed database (between Jan. 1,2002 and Dec. 31,2011). We also searched the American College of Rheumatology (ACR) and the Europe League against Rheumatology (EULAR), the following key words and Medical Subject Headings (MeSH) terms were used: ‘Lupus’, ‘Systemic lupus erythematosus’, ‘Rituximab’, and ‘Anti-CD20’. We reviewed all references in the studies included to determine extra works not included in the electronic databases. No language restrictions were considered.
2.2. Criteria for considering articles for review
We reviewed RCTs and further included cohort studies, case control studies, and case series (>5 cases). Studies were included if they met the following criteria: (1) the study that examined rituximab as an induction therapy for SLE; (2) the study that recorded the necessary data about therapy efficacy and safety; and (3) patients with a diagnosis of SLE based on the ACR criteria. We excluded studies that included pediatric patients.
In the studies included in our review, the complete remission criteria of LN were defined as a normal value for serum creatinine, normal serum albumin, inactive urinary sediment, and a 24-h urinary albumin level <0.5 g. Partial remission criterion of LN was defined as a ≥50% improvement in all renal parameters that were abnormal at baseline without deterioration in any parameter. The studies with imputed standard deviations (SDs) were defined as low quality studies.
One reviewer (Dr. Lan LAN) extracted the data and another reviewer (Dr. Fei HAN) verified that the data had been accurately recorded.
2.3. Statistical analysis
Data were extracted and summarized using the medians or means and the SDs as provided by the authors. Missing data were requested from the study authors by e-mail. Analysis of the indicators of heterogeneity between studies was performed to determine whether these indicators could be combined, heterogeneity was analyzed using χ 2 test with N−1 degrees of freedom. A P value of 0.05 was regarded as the critical value for homogeneity. Continuous outcome data from individual trials were meta-analyzed using the weighted mean difference (WMD) as combined effect. If the studies included were homogeneous, they were meta-analyzed with using the fixed effects model to estimate the combined effect. If the studies included were heterogeneous, they were analyzed using random effects model to estimate the combined effect. All statistical analyses were performed using Review Manager 4.2 statistical software.
3. Results
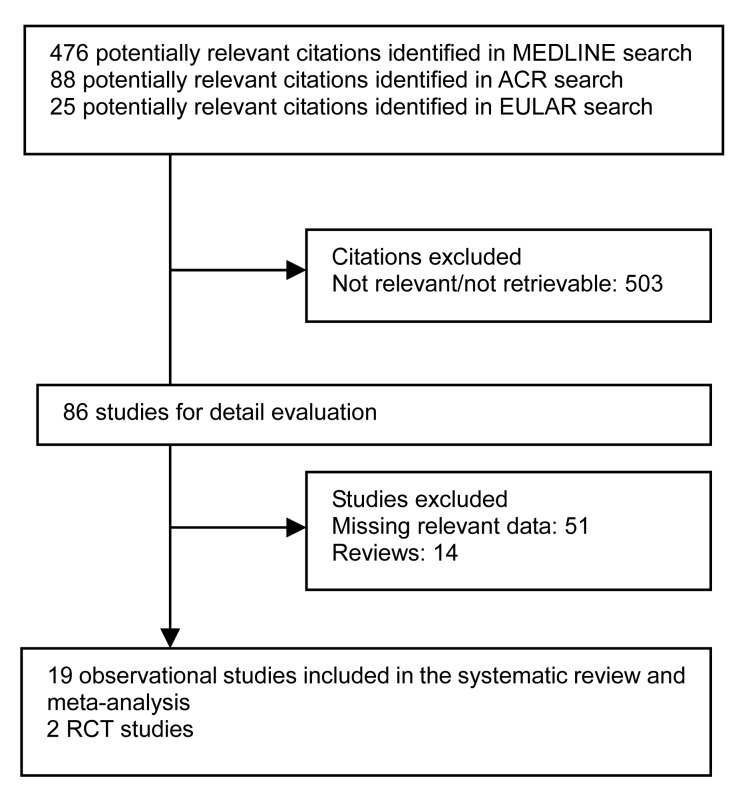
Our search returned 589 publications and abstracts, of which 503 were clearly not relevant to the study and excluded. Fifty-one studies were excluded due to the absence of required data, and 14 reviews were excluded. We included 19 observational studies and 2 RCT studies for systematic review (Fig. 1).
Fig. 1.
MEDLINE, the American College of Rheumatology (ACR), and the Europe League against Rheumatology (EULAR) search: process selection
3.1. Characteristics of the included studies and their quality
Tables 1 and 2 show a summary of included studies. We found 2 RCTs (Furie et al., 2009; Merrill et al., 2011) and 19 observational studies (Leandro et al., 2002; Leandro et al., 2005; Vigna-Perez et al., 2006; Gunnarsson et al., 2007; Tokunaga et al., 2007; Sutter et al., 2008; Tamimoto et al., 2008; Melander et al., 2009; Pepper et al., 2009; Catapano et al., 2010; Lateef et al., 2010; Ramos-Casals et al., 2010; Terrier et al., 2010; Pinto et al., 2011; Roccatello, 2011; Tony et al., 2011; Turner-Stokes et al., 2011; Vital et al., 2011; Arce-Salinas et al., 2012).
Table 1.
Characters of 19 observational studies
| No. | Study | Patient characters | n e | Dose | Other immunosuppressant agents | t m | Remission rate | SLEDAI change | BILAG change | Achieving BCD | Relapse rate | Relapse date | Adverse effect |
| 1 | Leandro et al., 2002 | Patient with active SLE and resistant to standard immunosuppressive therapy | 6 | 500 mg 2 infusions 1 week apart | 750 mg cyclophosphamide 2 infusions, prednisolone 30/60 mg for 5 d, continue hydroxychloroquine, and prednisolone | 12 | Not mentioned | 5 (83%) | 6 (100%) | Not registered | Not registered | Acute respiratory infection (3), acute gastroenteritis (3), shingles (1), folliculitis (1), oral candidiasis (1) | |
| 2 | Leandro et al., 2005 | Active SLE failed to conventional immunosuppressive therapy | 24 | 6 patients 2 infusions of 500 mg, 18 patients 2 infusions of 1000 mg, all 2 weeks apart | Infusion with cyclophosphamide/prednisolone, continue prednisolone, and hydroxychloroquine | 6 | Not mentioned | 19 (80%) | 23 (95.8%) | 7 (29.2%) | Not registered | Thrombocytopenia (1), infusion reaction (1) | |
| 3 | Vigna-Perez et al., 2006 | Active SLE and renal involvement refractory to conventional therapy | 22 | 0.5 to 1.0 g on Days 1 and 15 | Continue previous immunosuppressant including GC, CYC, MMF, AZA | 3 | PR: 7 (32%); CR: 5 (23%) | 20 (90%) | 20 (90%) | Not registered | Not registered | 1 patient died for invasive histoplasmosis | |
| 4 | Gunnarsson et al., 2007 | Active SLE and renal involvement refractory to conventional therapy | 7 | 375 mg/m2 of body surface area on Days 2, 9, 16, 23 | Infusion with methylprednisolone 250 mg and CYC 0.5 g/m2, continue with prednisolone 0.5–1.0 kg/d | 6 | PR: 1 (14%); CR: 3 (43%) | 7 (100%) | 7 (100%) | Not registered | Not registered | Photosensitive eruption (1), herpes zoster (limited) (1), neutrogena fever (1), urinary tract infection (1) | |
| 5 | Tokunaga, 2007 | Active SLE, with CNS | 10 | 375 mg/m2 2 infusions for 6, 500 mg 4 infusions for 1, 1 week apart; 1000 mg 4 infusions for 2, 2 weeks apart; 375 mg/m2 single for 1 | Infusion and continue with (15–40 mg of prednisolone, 1–3 mg betamethasone | 24 | Not mentioned | 9 (90%) | 10 (100%) | 6 (60%) | 4–23 months | Pneumonia (2), herpes zoster (1), chickenpox (1), intractable infection of decubitus ulceration (1) | |
| 6 | Sutter et al., 2008 | Active SLE failed to previous immunosuppressive therapy | 12 | 375 mg/m2 intravenously 4 infusions, 1 week apart | Pretreated with 50 mg diphenhydramine, 650 mg of acetaminophen, and 100 mg of intravenous methylprednisolone | 12 | Not mentioned | 10 (83.3%) | 11 (91.7%) | Not registered | Not registered | Not mentioned | |
| 7 | Tamimoto et al., 2008 | Active SLE failed to previous immunosuppressive therapy | 8 | 100 mg/m2 for 3, 250 mg/m2 for 2, 375 mg/m2 for 3, on Days 1, 8, 15 and 22 | Prednisolone 12.5–50.0 mg, CSA 75–175 mg | 12 | PR: 3 (38%); CR: 2 (25%) | 7 (87.5%) | 7 (87.5%) | 4 (50%) | 3–9 months | Nasopharyngitis (1), bacterial bronchitis (1), bacterial pneumonia (1), cutaneous candidiasis (1) | |
| 8 | Melander et al., 2009 | Lupus nephritis refractory to previous therapy (12) and relapse (8) | 20 | 375 mg/m2 of body surface area 4 infusions, 1 week apart | 3 patients receive CYC infusion with rituximab, 13 companied with high dose GC | 22 | PR: 7 (35%); CR: 5 (25%) | Not mentioned | Not mentioned | 12 (70.6%) | 1 | 9 months | RTX injection (2), infection (5), cutaneous herpes zoster (2) |
| 9 | Lateef et al., 2010 | Severe,refractory SLE patients | 10 | 375 mg/m2 infusions, 1 week apart | Infusion with CYC 500 mg | 12 | PR: 4 (57%); CR: 3 (43%) | 10 (100%) | 10 (100%) | 9 | 6–12 months | No obvious adverse effect observed | |
| 10 | Vital et al., 2011 | Active SLE refractory to previous therapy | 39 | 1000 mg rituximab on Days 1 and 14 | Infusion with methylprednisolone 100 mg and continue prednisolone 30–60 mg and background immunosuppressants | 12 | PR: 12 (31%); CR: 20 (51%) | 27 (69.2%) | 18 (46.2%) | 14, 14 | 12 months, 33 months | 1 patient died at 92 weeks non opportunisitic infections (4) | |
| 11 | Turner-Stokes et al., 2011 | Patients received at least two cycles of BCD with rituximab refractory to treatment with other immunosuppressive agents | 18 | 1000 mg rituximab given in two infusions 2 weeks apart | Infusion with 750 mg CYC and 100–250 mg methylprednisolone, continue with oral prednisolone, but the dose gradually tapered | 12 | PR: 7 (41%); CR: 4 (24%) | 15 (83%) | 15 (83%) | 8 (45%) | 12 months | Severe allergic reactions (2), 1 patient died for varicella pneumonia, another died for septicaemia | |
| 12 | Roccatello et al., 2011 | Severe SLE refractory to previous immunosuppressive agents | 8 | 375 mg/m2 on Days 2, 8, 15 and 22, 2 more dose in Days 30, 60 | Infusion with methyl prednisolone 1.5 mg/kg, continue with oral prednisone, 50 mg for 2 weeks rapidly tapered until 5 mg in 2 months | 36 | Not mentioned | 8 (100%) | 8 (100%) | 8 (100%) | 2 | 41 months | Show significant mild-to-moderate infusion reactions nor clinically relevant infection sequelas |
| 13 | Arce-Salinas et al., 2012 | Refractory lupus nephritis | 8 | 375 mg/m2 4 infusions 1 week apart | Infusion with steroids (1.5 mg/kg), tapered after 6 weeks, continue with no modification of steroids, AZA, or MM doses | 24 | PR: 2 (25%); CR: 2 (250%) | 4 (50%) | Not mentioned | 3 | 12 months | Minor acute adverse reactions (blood pressure variations, chills, and some mild rash), no major reactions or infections follow-up period | |
| 14 | Catapano et al., 2010 | Refractory or relapsing SLE | 31 | 15: a dose of 375 mg/m2 4 infusions; 1 week apart; 16: 1 000 mg with a 2-week interval 2 infusions | Infusion with cyclophosphamide (500 mg) and IV methyl prednisolone (500–1 000 mg) | 30 | PR: 10 (32.2%); CR: 17 (54.8%) | 27/31 (87%) | 30 (97%) | 18/27 (67%) | 11 months | Sever adverse effect (pericarditis, serum sickness reaction, throat swelling, et al.), patient have 11 severe infection | |
| 15 | Pepper et al., 2009 | Patients with class III/IV/V lupus nephritis | 18 | 2 doses of rituximab, 1 g, given at Days 1 and 15 | Infusion with 500 mg methyl prednisolone IV maintenance with MMF 1 g/d | 12 | PR: 6 (333.3%); CR: 6 (33.3%) | Not registered | Not registered | 10 (55.5%) | 2 (11.1%) | 4.5 months | Infection-related admissions (3), cannula site cellulites (1) |
| 16 | Pinto et al., 2011 | Colombian patients with severe and refractory SLE | 42 | 1 g of RTX every 2 weeks 2 infusions | Infusion with paracetamol 1 g, diphenhy 50 mg, methyl prednisolone 200 mg, continue with prednisolone 1 mg/(kg∙d) | 24 | 80% PR+CR | 25 (60%) | Not mentioned | 9 (21.4%) | 0–44 months | Urinary infection (13),bacteremia (3), delayed infusion reaction (2), respiratory infection (1) | |
| 17 | Terrier et al., 2010 | 136 active SLE patient from 44 centers, refractory to previous treat-ments | 136 | 82: 1 g (2 infusions); 48:375 mg/m2 (4 infusions) | 125 associate prednisone wit dosage of 29.9 mg/d, 72 patients (53%) receive HCQ and 52% associate other concomitant immunosuppressive agents | 18 | In LN, PR: 6 (29%), CR: 14 (45%) | 80 (71%) | Not mentioned | 31/76 (41%) | 16.6 months | Sever infection (12 (9%)), serum sick-ness (5), acute infusion reactions 12 (9%) | |
| 18 | Ramos-Casals et al., 2010 | 196 SAD patients refractory to standard therapy | 107* | 375 mg/m2 of rituximab weekly for 4 weeks (85%), 1000 mg 2 infusions, 2 weeks apart (15%) | All patients continue with corticosteroid 60% patients continue with previous immunosuppressive agents | 27 | CR: 45%; PR: 32% | Not mentioned | Not mentioned | Not mentioned | 20/81 (25%) | 0–26 months | Infections in 12 patients, including respiratory infection, urinary tract infection, cutaneous infection |
| 19 | Tony et al., 2011 | 370 patients from 42 centers with a diagnosis of an autoimmune, condition other than RA or NHL | 85# | Mean dose of rituximab was 2 440 mg each patient over a median period of 194 d | 7 SLE patients receive CYC with infusion of rituximb, 67 patients continue immunosuppressive agents | 12 | CR: 30%; PR: 20% | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | 13.2% patients experience infection, serious infections was 5.3 per 100 patient-years in the total 370 patients |
SLEDAI: systemic lupus erythematosus disease activity index; BILAG: British isles lupus assessment group index; BCD: B-cell depletion; PR: partial remission; CR: complete remission; SLE: systemic lupus erythematosus; LN: lupus nephritis; SAD: systemic autoimmune diseases, GC: glucocorticoid; CYC: cyclophosphamide; MMF: mycophenolate mofetil; AZA: azathioprine; HCQ: hydroxychloroquine. n e:number enrolled; t m:median follow month
including 107 SLE patients
23.0% (85 patients) were SLE
Table 2.
Characters of 2 RCT studies
| Study | n e | n c | Subject | Mean age (year) | Follow-up period (week) | Treatment | Disease activity | Flare | Prednisone dose | Immunologic parameters | Adverse effect | Conclusion |
| Explorer | 169 | 88 | Moderately to severely active extra renal SLE | 40.2 | 56 | Rituximab 1 000 mg 2 infusions at entry, again at 6 months, continue with prednisone dose | Low disease activity was achieved prior to Week 52 in 58 (66.0%) patients in the placebo group and in 127 (75.1%) patients in the rituximab group | No difference of median time to first moderate or severe flare and flare rate between two groups | The rate of prednisone rescue for A flare was similar | The decrease in rituximab was greater than that in the placebo group | Infusion related adverse effect similar in two groups. Four serum sickness adverse events in rituximab group | Explorer study did not meet the primary and secondary points, but suggested that rituximab may lessen severe flares as defined by BILAG A criteria |
| Lunar | 72 | 72 | Class III/IV and active lupus nephritis | 30 | 52 | Rituximab on Days 1, 15, 168, and 182 | There were no statistically significant differences in the primary or clinical secondary end points | Not registered | Rituximab had a greater effect on levels of anti-dsDNA and complement at Week 52 | Serious adverse events were similar in two groups | Lunar did not show a statistically significant difference in primary or clinical secondary end points |
n e: number enrolled; n c: control number
3.1.1. Characteristics of the patients in 19 observational studies
We enrolled 19 observational studies included a total of 611 patients (520 female (85.1%), 91 male (14.9%)) with an average age of 33.6 years (SD=4.37 years). Of these patients, 222 (36.3%) were diagnosed with LN. The median follow-up time was 18.2 months.
The LN cases consisted of 139 (62.6%) class IV, 31 (14%) class V, 8 (3.6%) class IV+V, 21 (9.5%) class III, 13 (5.8%) other type, and 10 (4.5%) cases that were not classified. All patients fulfilled the ACR criteria for SLE and were measured using the 2003 International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification of LN. Additionally, 576 (94.3%) of the SLE patients enrolled in these observational studies had active disease that was refractory to standard immunosuppressive therapy or had relapsed. Previously applied immunosuppressive agents included glucocorticoids (GC), CYC, MMF, and AZA.
1. Dose of rituximab
The dosing of rituximab in SLE patients varied between studies; some followed the rheumatoid arthritis guidelines ((0.5–1.0 g)×2 infusions) (Leandro et al., 2002; 2005; Vigna-Perez et al., 2006; Pepper et al., 2009; Terrier et al., 2010; Pinto et al., 2011), and some followed a lymphoma schedule (375 mg/m2×4 weeks) (Gunnarsson et al., 2007; Tokunaga et al., 2007; Sutter et al., 2008; Tamimoto et al., 2008; Melander et al., 2009; Catapano et al., 2010; Lateef et al., 2010; Ramos-Casals et al., 2010; Terrier et al., 2010; Roccatello et al., 2011; Vital et al., 2011; Arce-Salinas et al., 2012). In our review, except for the German Registry of Autoimmune Diseases (GRAID) studies in Germany, 268 (50.9%) cases received a rituximab (0.5–1.0 g)×2 infusions, 211 (40.1%) received a rituximab infusion of 375 mg/m2×4 weeks, and 46 (8.7%) cases received a dose of rituximab ≤375 mg/m2×2 weeks. Rituximab was commonly co-administered with corticosteroids, with 428 (70.0%) patients receiving methylprednisolone/prednisolone (100–250 mg) or a full dose of prednisolone as induction therapy and 99 (16.2%) patients receiving CYC with the first infusion of rituximab. Additionally, 329 (53.8%) patients received other immunosuppressive agents. We analyzed the response of 569 patients to receiving methylprednisolone and nonmethylprednisolone induction therapy, and the remission rates were 74.9% and 64.2%, respectively (Table 3).
Table 3.
Role of methylprednisolone as an induction therapy
| Infusion | n e | n a | n f |
| Methylprednisolone as induction therapy | 223 | 167 (74.9%) | 56 (25.1%) |
| Low dose prednisolone | 346 | 222 (64.2%) | 124 (35.8%) |
n e:number enrolled; n a:number achieving remission; n f:number failing to achieve remission
2. B-cell depletion (BCD)
Five studies did not mention the number of patients with BCD. In the remaining 232 cases, 187 (80.6%) patients achieved satisfactory BCD. Most of these patients achieved BCD in 12 weeks, and this depletion lasted for 12–48 months. We extracted the available data from 173 cases and analyzed the dose effect on BCD (Table 4).
Table 4.
Different doses of rituximab in BCD
| Dose | n e | n a |
| 1 000 mg×2 infusions | 122 | 92 (75.4%) |
| 500 mg×2 infusions | 12 | 12 (100%) |
| 375 mg/m2×4 infusions | 27 | 27 (100%) |
| 375 mg/m2×2 infusions | 9 | 9 (100%) |
| 100 mg/m2×4 infusions | 3 | 2 (66.6%) |
n e:number enrolled; n a:number achieving BCD
3. Overall clinical response
The remission rate was registered in 460 cases, where 116 (25.2%) patients achieved partial remission and 153 (33.3%) achieved complete remission. In the study of Pinto et al. (2011), 34 complete and partial remissions were reported, accounting for 80.9% of patients. Based on this information, 65.9% of patients achieved partial/complete remission. There were 253 patients in our review that reported a change of SLEDAI, with 80.2% of patients achieving reduction of SLEDAI. Furthermore, 160 patients reported change of BILAG, with 75.6% of patients achieving decreases in BILAG score.
4. Outcome of LN
There were 220 LN patients included in our review, and 66 patients recorded the change of proteinuria, amounting to 54 (81.8%) patients achieving significant decreases in 24-h proteinuria. Of the 220 lupus patients, 8 studies enrolling 116 patients reported on remission rates. Among these, 44 (37.9%) cases achieved complete remission and 40 (34.5%) cases reached partial remission. We also investigated the response of patients with different pathology types to rituximab (Table 5). Patients with LN class IV seemed to have the highest sensitivity to rituximab.
Table 5.
LN behavior with rituximab therapy
| Pathology | n e | n c | n p | n f |
| Class IV | 49 | 19 (39.6%) | 18 (37.6%) | 12 (22.8%) |
| Class V | 14 | 4 (28.6%) | 5 (35.7%) | 5 (35.7%) |
| Class III | 4 | 1 (20.0%) | 1 (20.0%) | 2 (40.0%) |
| Class IV+V | 3 | 0 | 2 (66.6%) | 1 (33.3%) |
n e: number enrolled; n c: number of complete remission; n p: number of partial remission; n f: number failing to achieve remission
5. Corticosteroid sparing effect
One hundred percent of patients in our review received corticosteroid or prednisone therapy after rituximab, and the dose was tapered during follow-up. There were 5 studies that detailed the dose change in prednisone, and 137 cases indicated a significant decrease in prednisone dose, in a mean observational period of 18.8 months, with a mean reduction of 12.13 mg/year.
6. Serology and complement levels
It was noteworthy that the serology and complement levels changed in the studies we included. For example, Leandro et al. (2002) indicated that after rituximab therapy, the IgA, IgM, and IgG levels all decreased significantly (P<0.05). Gunnarsson et al. (2007) enrolled seven patients, and all were found to have a significant reduction in anti-dsDNA, and five patients achieved significant increases in C3 levels and GFR. Melander et al. (2009), Lateef et al. (2010), and Vital et al. (2011) all found decreased anti-dsDNA levels after rituximab therapy (P<0.05). Terrier et al. (2010) also found a median C3 level increase from 0.68 to 0.80 ng/ml and a median anti-dsDNA level decrease from 119 to 31 IU/ml (P<0.05) in 18 cases.
7. Adverse effects
Adverse events (AE) were recorded in 111 (16.8%) patients, including infection (70 (63.1%) cases), acute infusion reaction (21 (18.9%) cases), severe allergic reaction (11 (9.9%) cases), serum sickness (7 (6.3%) cases), and delayed infusion reaction (2 (1.8%) cases). In the 70 patients who experienced an infection, there were 15 cases of urinary tract infections, 9 cases of respiratory infection, 2 cases of candidiasis infection, 3 cases of bacteremia, 1 case of chickenpox, and 1 case of septicemia. The other 39 infection cases were no clearly described (Table 6).
Table 6.
Adverse effects in 111 SLE patients
| Adverse effect | Number of patients |
| Severe allergic reaction | 11 (9.9%) |
| Acute infusion reaction | 21 (18.9%) |
| Delayed infusion reaction | 2 (1.8%) |
| Severe sickness | 7 (6.2%) |
| Infection | 70 (63.1%) |
| Urinary tract infection | 15 (21.5%) |
| Respiratory infection | 9 (12.3%) |
| Candidiasis infection | 2 (2.9%) |
| Chickenpox | 1 (1.5%) |
| Bacteremia | 3 (4.4%) |
| Septicemia | 1 (1.5%) |
| Not clear | 39 (55.8%) |
8. Relapse rate
Fourteen studies (478 cases) reported that 148 (31.0%) patients experienced relapse during follow-up, with the date of relapse varying from 3 to 44 months. Of these, 61 (41.2%) patients were treated with rituximab (treated with the same dose as the previous therapy) during the relapse. Eight (5.4%) patients reached remission after the dose of steroid was increased. Furthermore, 11 patients with flare-ups were successfully treated with immunosuppressants (e.g., MMF, AZA, CYC). In the 61 cases who were treated with rituximab during relapse, 46 (75.4%) patients achieved complete remission, 9 (14.8%) patients achieved partial remission, 3 (4.9%) patients were lost to follow-up, and 3 (4.9%) patients had no response.
3.1.2. Efficacy and safety of rituximab in randomized controlled studies
We included the RCTs ‘Explorer’ (Merrill et al., 2011) and ‘Lunar’ (Furie et al., 2009) and summarized the characteristics of the two RCTs (Table 2).
1. ‘Explorer’ study
The ‘Explorer’ trial was a 52-week, multicenter, randomized, double blind placebo-controlled trial of rituximab in 257 patients with moderately to severely active extra renal SLE. In this trial, 257 SLE patients were randomized in a 2:1 ratio to receive intravenous rituximab (2 times 1 000 mg dose given every 14 d) or placebo on Days 1, 15, 168, and 182. At entry, patients were assigned to receive a new or increased prednisone dose (0.50, 0.75, or 1.00 mg/kg) based on their baseline steroid use and disease severity. The prednisone dose was tapered over the following 56 weeks. The previous immunosuppressive regimen was continued throughout follow-up. The primary endpoint was the effect of placebo versus rituximab in achieving and maintaining a major clinical response. Secondary endpoints were (1) the time-adjusted area under the curve minus baseline (AUCMB) of the BILAG, (2) the proportion of patients who achieved major clinical response, (3) the proportion of patients who got better in all organs or with a BILAG C at Week 24, (4) the time from remission to the first moderate or severe flare-up, (5) quality of life (QoL) measured by the Lupus QoL questionnaire, (6) the proportion of patients who achieved a major clinical response with a prednisone dose of 10 mg/d from Weeks 24–52.
In conclusion, the ‘Explorer’ trial did not meet its primary or secondary efficacy outcomes, and none of these outcomes were significantly different between the two treatment groups.
In the ‘Explorer’ study, there were no statistically significant differences in the numbers of AEs and serious adverse events (SAEs) between the two treatment groups, although the frequency of treatment-emergent infectious SAEs was higher in the placebo group than in the group of patients receiving rituximab. There was no difference in musculoskeletal and connective tissue disorders between the two groups.
2. ‘Lunar’ study
The objective of the ‘Lunar’ study was to assess the efficacy and safety of rituximab in active, proliferative LN. This study was a randomized, double-blind phase III study of 144 enrolled class III/IV LN participants with a urine protein to creatinine ratio (UPCR) of >1. Participants were randomized to receive either 1 000 mg of rituximab or placebo on Days 1, 15, 168, and 182. The primary endpoint was the proportion of patients who achieved either complete or partial remission. The secondary outcomes included: (1) decrease of C3 and C4 complement levels from baseline; and (2) proportion of patients who achieve a complete renal response. There were no statistically significant differences in the primary or secondary outcomes between the two treatment groups. AEs and SAEs were similar between the two groups.
3.2. Meta-analysis
We combined the data at baseline and after rituximab therapy from the 19 observational studies. The data after rituximab therapy was defined as the intervention group and the data at baseline was defined as the comparison group. We did not include the data from the 2 RCTs, because we did not get enough data of randomized trials.
3.2.1. Effect on BILAG
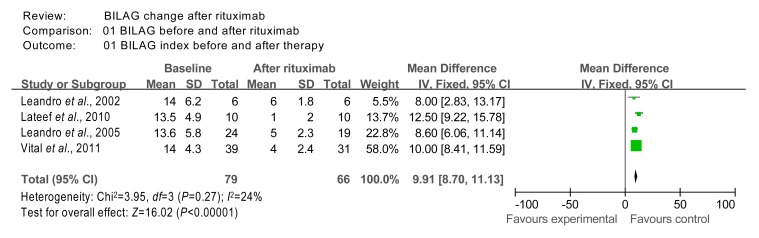
The BILAG data from studies 1 (Leandro et al., 2002), 2 (Leandro et al., 2005), 9 (Lateef et al., 2010), and 10 (Vital et al., 2011) were included in the meta-analysis. Although studies 11 (Turner-Stokes et al., 2011), 12 (Roccatello et al., 2011), and 14 (Catapano et al., 2010) also reported on BILAG, they were excluded due to missing data. The homogeneity χ 2 test value of BILAG data from studies 1, 2, 9, and 10 was 3.95 (P=0.27 (>0.05)); therefore, the 4 studies were considered homogeneous. The overall effect was measured to be Z=16.02 (P<0.000 01), indicating that treatment with rituximab had significant effects on BILAG when compared to baseline (Fig. 2).
Fig. 2.
Comparison of BILAG index at baseline and after rituximab therapy
3.2.2. Effect on SLEDAI
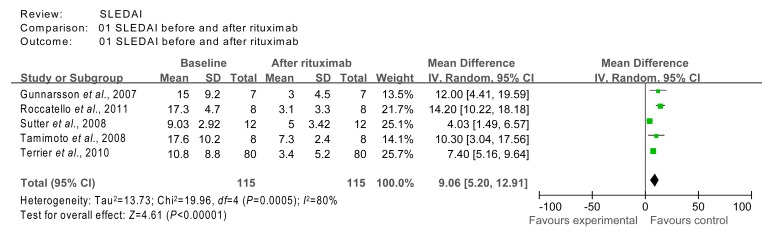
The SLEDAI data from studies 4 (Gunnarsson et al., 2007), 6 (Sutter et al., 2008), 7 (Tamimoto et al., 2008), 12 (Roccatello et al., 2011), and 17 (Terrier et al., 2010) were included in the meta-analysis. Although studies 3 (Vigna-Perez et al., 2006), 5 (Tokunaga, 2007), 13 (Arce-Salinas et al., 2012), and 16 (Pinto et al., 2011) also reported on SLEDAI, they were excluded due to missing data. The homogeneity test χ 2 value of SLEDAI data from studies 4, 6, 7, 12, and 17 was 19.96 (P=0.000 5 (<0.01)) and these studies were therefore, not considered homogeneous. The combined effect was calculated using the random effects model, and was found to be Z=4.61 (P<0.000 01), indicating that treatment with rituximab had significant effects on SLEDAI when compared to baseline (Fig. 3).
Fig. 3.
Comparison of SLEDAI index at baseline and after rituximab therapy
3.2.3. Effect on prednisone dose
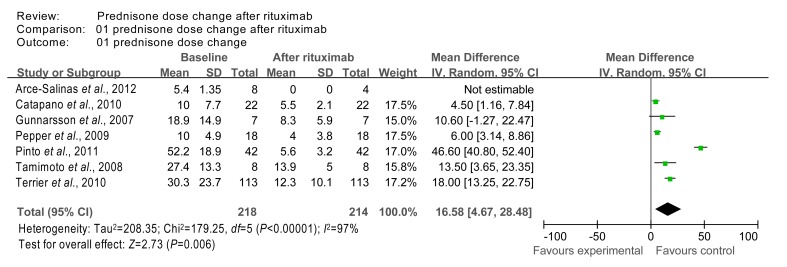
The homogeneity test χ 2 value of prednisone dose data from studies 4 (Gunnarsson et al., 2007), 7 (Tamimoto et al., 2008), 13 (Arce-Salinas et al., 2012), 14 (Catapano et al., 2010), 15 (Pepper et al., 2009), 16 (Pinto et al., 2011), and 17 (Terrier et al., 2010) was 179.25 (P=0.00001 (<0.05)) and these studies were therefore, not considered homogeneous. The combined effect was calculated using the random effects model, and was found to be Z=2.73 (P=0.006 (<0.05)), indicating that treatment with rituximab had significant effects on prednisone dose when compared to baseline (Fig. 4).
Fig. 4.
Comparison of prednisone dose index at baseline and after rituximab therapy
3.2.4. Effect on 24-h urine proteinuria
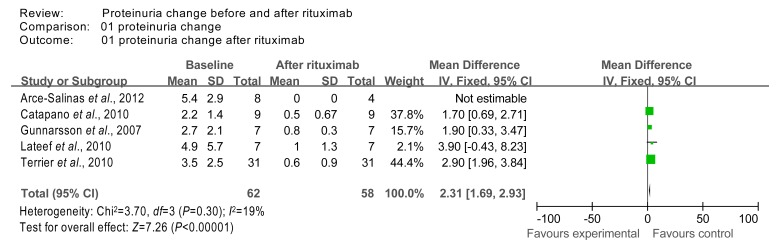
The 24-h urine proteinuria data from studies 4 (Gunnarsson et al., 2007), 9 (Lateef et al., 2010), 13 (Arce-Salinas et al., 2012), 14 (Catapano et al., 2010), and 17 (Terrier et al., 2010) were combined in a meta-analysis. We did not include the studies that had missing data. The homogeneity test χ 2 value of the 24-h urine proteinuria data from studies 4, 9, 13, 14, and 17 was 3.70 (P=0.30 (>0.1)); therefore the 5 studies were considered homogeneous. Test for overall effect was calculated using the fixed effects model and found to be Z=7.26 (P<0.000 01), indicating that after rituximab treatment the 24-h urine proteinuria decreased significantly compared to baseline (Fig. 5).
Fig. 5.
Comparison of 24-h urine proteinuria index at baseline and after rituximab therapy
3.2.5. Sensitivity analysis
Given the heterogeneity across studies that reported on SLEDAI and prednisone dose, we conducted sensitivity analysis for these outcomes.
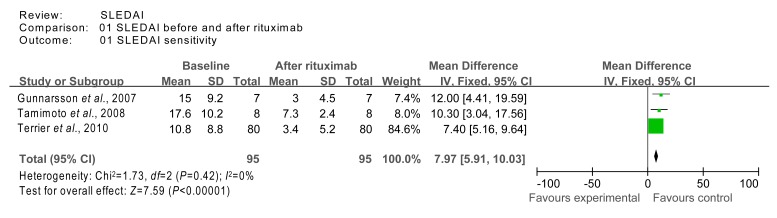
For the SLEDAI outcome, we excluded the low quality studies (with imputed SDs), and re-calculated the WMD using the fixed effects model. The homogeneity test χ 2 value for studies 4 (Gunnarsson et al., 2007), 7 (Tamimoto et al., 2008), and 17 (Terrier et al., 2010) was 1.73 (P=0.42 (>0.1)), indicating homogeneity. The test for overall effect was calculated to be Z=7.59 (P<0.000 01), which indicated that the SLEDAI significantly decreased after rituximab therapy (Fig. 6).
Fig. 6.
Sensitivity analysis of SLEDAI at baseline and after rituximab therapy
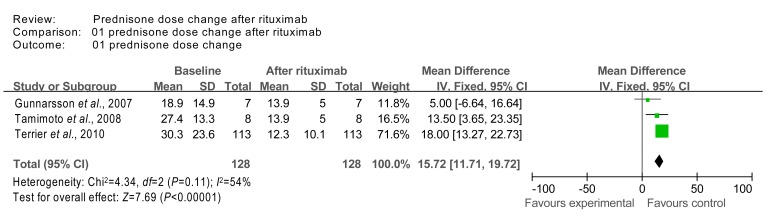
For the prednisone dose data, we also excluded the low quality studies (with imputed SDs), and re-calculated the WMD using the fixed effects model. As a result, the data from studies 4 (Gunnarsson et al., 2007), 7 (Tamimoto et al., 2008), and 17 (Terrier et al., 2010), had a homogeneity test χ 2 value of 4.34 (P=0.11 (>0.05)). The test for overall effect was calculated to be Z=7.69 (P<0.00001), which indicates that prednisone dose significantly decreased after rituximab therapy (Fig. 7).
Fig. 7.
Sensitivity analysis of prednisone dose at baseline and after rituximab therapy
4. Discussion
B-cells have been demonstrated to play a key role in the pathogenesis of lupus beyond their polyclonal activation and the production of auto-antibodies against self-antigens (Odendahl et al., 2000). Targeting the B-cell compartment is therefore an attractive alternative to current available therapies.
Rituximab, a chimeric anti-CD20 antibody, leads to the depletion of most peripheral B-cells. The mechanism of rituximab inducing B-cell depletion is unclear; however, in vitro studies have found that rituximab induces the lysis of CD20-positive lymphoma cells by three mechanisms: antibody-dependent cell-mediated cytotoxicity (ADCC) (Reff et al., 1994), complement-dependent cytotoxicity and direct signaling leading to apoptosis (Shan et al., 2000).
There have been a few published RCTs that have shown good efficacy and safety profiles for this drug in patients with rheumatoid arthritis (Bingham et al., 2010; Emery et al., 2010; Rigby et al., 2011); however, there is a paucity of evidence assessing the use of rituximab therapy in SLE.
Because there is no standardized dose for rituximab in clinical practice, the included studies followed different schedules. From our review, 40.4% of patients received a dose of 375 mg/m2, 51.6% of patients received 1 000 mg×2 infusions, and very few patients took a low dose of 100–250 mg/m2 or a single dose of 500/1 000 mg. As shown in Table 4, we found no significant differences for dose on the effect of BCD. However, the RCT, which used a dose of 1 000 mg×2 infusions, had a lower BCD (75.4%). BCD may be related to genotype, and one study has indicated a unique relationship between BCD and FcgRIIIa genotype (Pepper et al., 2009).
In our review, 65.9% of patients achieved complete/partial remission, which was lower than that found in Murray and Perry (2010)’s study. In addition, 80.2% of patients had a reduction in SLEDAI and 75.6% of patients showed a reduction in BILAG. These data indicate overall improvement in the reduction of disease activity after therapy. There were 418 (68.5%) patients receiving methylprednisolone (100–150 mg) as induction therapy, but similar to the Pepper et al. (2009)’s study, our analysis indicated that additional methylprednisolone did not significantly alter disease outcomes (Table 5).
Three hundred and eleven (50.9%) patients continued with previously administered immunosuppressive agents. Through analysis of the groups either continuing with immunosuppressives or not receiving immunosuppressive agents, Catapano et al. (2010) found no significant difference in the remission rate between the two groups.
Our review also indicated a decrease in serum levels of anti-dsDNA, IgA, IgG, and IgM, a rise in serum C3 levels, and a decrease in the prednisone dose. These results were consistent with the ‘Explorer’ and ‘Lunar’ RCTs.
The main adverse effects of rituximab included infusion reactions such as headache, nausea, and chest discomfort, and were mostly well-tolerated. In our review, we summarized the adverse effects from 111 patients, and found that acute infusion reactions accounted for 18.9% of the 111 cases. Most of these acute infusion reactions could be reversed with cortisone. Infection accounted for 63.1% of the adverse effects, and almost could be controlled. These results were similar to those of previous studies. From our review, we can conclude that rituximab was safe for the treatment of SLE. The ‘Explorer’ and ‘Lunar’ studies also indicated that there were no significant differences between the experimental and control groups.
Progressive multifocal leukoencephalopathy PML) is a rare, usually fatal disease caused by opportunistic infection caused by the JC virus (JCV). Although there were no participants with PML included in our review, PML has attracted the attention of rheumatologists for reports about its association with the use of rituximab (Calabrese et al., 2007). In December 2006, the US Food and Drug Administration (FDA) issued an alert about the death of two PML cases with SLE, both of whom had been treated with rituximab (Molloy and Calabrese, 2010).
The potential pathogenic mechanism of rituximab-related PML remains unknown. The loss of other B-cell functions, such as those of antigen-presenting cells or cytokine production, may lead to a defect in cell-mediated immunity. PML is a rare AE. It occurs in fewer than 1 in 10 000 rituximab-treated patients (Molloy, 2011). A better understanding of PML’s mechanism is necessary for risk prediction and guidance of therapy.
In our study, 148 (31.0%) patients experienced relapse during follow-up, while 46 (75.4%) patients achieved complete remission after retreatment with rituximab. It is believed that relapse time is associated with the repopulation of B-cells. Vital et al. (2011) confirmed that the repopulation of B-cells predicted relapse of the disease, as incomplete B-cell depletion at 6 weeks was associated with lower clinical response rates. In the study by Catapano et al. (2010), however, 6 patients relapsed before the recovery of B-cells.
In our systematic review and meta-analysis, we only included adult SLE patients (≥18 years), but there have been studies, which have focused on childhood SLE or juvenile-onset lupus patients. Compared with adult patients, the pediatric patients always took a lower dose of rituximab. Marks et al. (2005) and Podolskaya et al. (2008) reported that 7 patients with median age of 14.8 years received a dose of 750 mg/m2 (about up to 100 mg, the maximum dose was 1 g) on Days 1 and 15. The participants in Nwobi et al. (2008)’s study took a dose of 188 and 375 mg/m2. Similar to the adult patients, most of the pediatric patients were administered methylprednisolone prior to the injection of rituximab; oral prednisolone was decreased gradually after rituximab therapy. Similar to the studies of adult patients, the rituximab’s therapy on childhood SLE patients observed positive results. Five patient experienced improvement in BILAG, renal function, and proteinuria in Marks et al. (2005)’s study. In Podolskaya et al. (2008)’s study, 11 (58.8%) patients achieved remission, and 7 (36.8%) patients had improved outcomes. The two studies reported five cases of herpes zoster. In Nwobi et al. (2008)’s study, 14 of the 15 patients achieved complete/partial remission and decreases in SLEDAI, proteinuria, Scr, and prednisone dose. Rituximab was well tolerated in most pediatric patients.
Although observational and retrospective studies of rituximab showed improved outcomes in SLE patients, they were not consistent with the results from the RCTs. Although many observational studies showed a satisfactory safety profile and clinical efficacy of rituximab in SLE patients, the two RCT studies did not achieve their primary and secondary endpoints. No significant difference was found between the rituximab and placebo in preventing or delaying moderate to severe flares. Possible reasons for this discrepancy are explained below.
First, the patients included in both the rituximab and placebo groups in the ‘Explorer’ and ‘Lunar’ studies had active SLE and had previously received moderate to high doses of corticosteroids and immunosuppressive agents. These studies excluded patients who had previously used CYC. Including patients with active SLE in both groups is necessary to establish efficacy unless a satisfactory control group is difficult to recruit (Pinto et al., 2011). The patients included in the RCTs were different from patients included in the observational studies, as 76% of the LN patients enrolled in the AIR registry are refractory to MMF and/or CYC compared with 0% in the ‘Lunar’ trial. The difference in ethnic factors should also be considered.
Second, the background therapy should be emphasized. Ramos-Casals et al. (2009) viewed a possible synergistic effect for rituximab in combination with CYC and associated CYC with significant advantages in the treatment of complicated, refractory SLE. Other factors, such as degree of resistance to other therapies and the ability of instruments to capture disease activity, should also be considered.
The present systematic review has several limitations. First, only two RCT studies were included. Also, given the heterogeneity between the RCTs and observational studies, the RCTs were not included in the meta-analysis. Second, several of the included studies had small sample sizes. Third, the follow-up period varied from 3 to 24 months. Finally, clinical heterogeneity was an issue in study type, subject, LN class, rituximab dosage, and follow-up duration.
The observational studies indicated that rituximab was effective in severe and refractory SLE patients, and they reported decreased SLEDAI, BILAG, urine protein levels, and prednisolone dosage. In addition, some of the LN patients achieved complete or partial remission. In contrast, both of the RCTs did not achieve primary and second endpoints, which makes us question the true effect attributed to rituximab.
References
- 1.Arce-Salinas CA, Rodríguez-García F, Gómez-Vargas JI. Long-term efficacy of anti-CD20 antibodies in refractory lupus nephritis. Rheumatol Int. 2012;32(5):1245–1249. doi: 10.1007/s00296-010-1755-0. [DOI] [PubMed] [Google Scholar]
- 2.Bingham CO, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, Trzaskoma B, Martin F, Agarwal S, Kelman A. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62(1):64–74. doi: 10.1002/art.25034. [DOI] [PubMed] [Google Scholar]
- 3.Calabrese LH, Molloy ES, Huang D, Ransohoff RM. Progressive multifocal leukoencephalopathy in rheumatic diseases: evolving clinical and pathologic patterns of disease. Arthritis Rheum. 2007;56(7):2116–2128. doi: 10.1002/art.22657. [DOI] [PubMed] [Google Scholar]
- 4.Catapano F, Chaudhry AN, Jones RB, Smith KG, Jayne DW. Long-term efficacy and safety of rituximab in refractory and relapsing systemic lupus erythematosus. Nephrol Dial Transplant. 2010;25(11):3586–3592. doi: 10.1093/ndt/gfq256. [DOI] [PubMed] [Google Scholar]
- 5.Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, Latinis K, Abud-Mendoza C, Szczepanski LJ, Roschmann RA, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)) Ann Rheum Dis. 2010;69(9):1629–1635. doi: 10.1136/ard.2009.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furie R, Looney RJ, Rovin B, Latinis Kevin M, Appel G, Sanchez-Guerrero J. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR study [abstract] Arthritis Rheum. 2009;60(s10):1149. doi: 10.1002/art.26223. [DOI] [Google Scholar]
- 7.Gunnarsson I, Sundelin B, Jónsdóttir T, Jacobson SH, Henriksson EW, van Vollenhoven RF. Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum. 2007;56(4):1263–1272. doi: 10.1002/art.22505. [DOI] [PubMed] [Google Scholar]
- 8.Houssiau FA, Vasconcelos C, D′Cruz D. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46(8):2121–2131. doi: 10.1002/art.10461. [DOI] [PubMed] [Google Scholar]
- 9.Lateef A, Lahiri M, Teng GG, Vasoo S. Use of rituximab in the treatment of refractory systemic lupus erythematosus: Singapore experience. Lupus. 2010;19(6):765–770. doi: 10.1177/0961203309358599. [DOI] [PubMed] [Google Scholar]
- 10.Leandro MJ, Edwards JC, Cambridge G, Ehrenstein MR, Isenberg DA. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 2002;46(10):2673–2677. doi: 10.1002/art.10541. [DOI] [PubMed] [Google Scholar]
- 11.Leandro MJ, Cambridge G, Edwards JC, Ehrenstein MR, Isenberg DA. Isenberg, B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology. 2005;44(12):1542–1545. doi: 10.1093/rheumatology/kei080. [DOI] [PubMed] [Google Scholar]
- 12.Levine TD, Pestronk A. IgM antibody-related polyneuropathies: B-cell depletion chemotherapy using rituximab. Neurology. 1999;52(8):1701–1704. doi: 10.1212/WNL.52.8.1701. [DOI] [PubMed] [Google Scholar]
- 13.Marks SD, Patey S, Brogan PA, Hasson N, Pilkington C, Woo P, Tullus K. B lymphocyte depletion therapy in children with refractory systemic lupus erythematosus. Arthritis Rheum. 2005;52(10):3168–3174. doi: 10.1002/art.21351. [DOI] [PubMed] [Google Scholar]
- 14.Melander C, Sallée M, Trolliet P, Candon S, Belenfant X, Daugas E, Rémy P, Zarrouk V, Pillebout E, Jacquot C, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol. 2009;4(3):579–587. doi: 10.2215/CJN.04030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrill JT, Buyon JP, Furie RA, Latinis LM, Gordon C, Hsieh HJ, Brunetta P. Assessment of flares in lupus patients enrolled in a phase II/III study of rituximab (EXPLORER) Lupus. 2011;20(7):709–716. doi: 10.1177/0961203310395802. [DOI] [PubMed] [Google Scholar]
- 16.Molloy ES. PML and rheumatology: the contribution of disease and drugs. Cleve Clin J Med. 2011;78(s2):S28–S32. doi: 10.3949/ccjm.78.s2.07. [DOI] [PubMed] [Google Scholar]
- 17.Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun Rev. 2010;8(2):144–146. doi: 10.1016/j.autrev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Murray E, Perry M. Off-label use of rituximab in systemic lupus erythematosus: a systematic review. Clin Rheumatol. 2010;29(7):707–716. doi: 10.1007/s10067-010-1387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nwobi O, Abitbol CL, Chandar J, Seeherunvong W, Zilleruelo G. Rituximab therapy for juvenile-onset systemic lupus erythematosus. Pediatr Nephrol. 2008;23(3):413–419. doi: 10.1007/s00467-007-0694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165(10):5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 21.Pepper R, Griffith M, Kirwan C, Levy J, Taube D, Pusey C, Lightstone L, Cairns T. Rituximab is an effective treatment for lupus nephritis and allows a reduction in maintenance steroids. Nephrol Dial Transplant. 2009;24(12):3717–3723. doi: 10.1093/ndt/gfp336. [DOI] [PubMed] [Google Scholar]
- 22.Pinto LF, Velásquez CJ, Prieto C, Mestra L, Forero E, Márquez JD. Rituximab induces a rapid and sustained remission in Colombian patients with severe and refractory systemic lupus erythematosus. Lupus. 2011;20(11):1219–1226. doi: 10.1177/0961203311409273. [DOI] [PubMed] [Google Scholar]
- 23.Podolskaya A, Stadermann M, Pilkington C, Marks SD, Tullus K. B cell depletion therapy for 19 patients with refractory systemic lupus erythematosus. Arch Dis Child. 2008;93(5):401–406. doi: 10.1136/adc.2007.126276. [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Casals M, Díaz-Lagares C, Khamashta MA. Rituximab and lupus: good in real life, bad in controlled trials. Comment on the article by Lu et al. Arthritis Rheum. 2009;61(9):1281–1282. doi: 10.1002/art.24726. [DOI] [PubMed] [Google Scholar]
- 25.Ramos-Casals M, García-Hernández FJ, de Ramón E, Callejas JL, Martínez-Berriotxoa A, Pallarés L, Caminal-Montero L, Selva-O′Callaghan A, Oristrell J, Hidalgo C, et al. Off-label use of rituximab in 196 patients with severe, refractory systemic autoimmune diseases. Clin Exp Rheumatol. 2010;28(4):468–476. [PubMed] [Google Scholar]
- 26.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–445. [PubMed] [Google Scholar]
- 27.Rigby W, Ferraccioli G, Greenwald M, Zazueta-Montiel B, Fleischmann R, Wassenberg S, Ogale S, Armstrong G, Jahreis A, Burke L, et al. Effect of rituximab on physical function and quality of life in patients with rheumatoid arthritis previously untreated with methotrexate. Arthritis Care Res. 2011;63(5):711–720. doi: 10.1002/acr.20419. [DOI] [PubMed] [Google Scholar]
- 28.Roccatello D, Sciascia S, Rossi D, Alpa M, Naretto C, Baldovino S, Menegatti E, La Grotta R, Modena V. Intensive short-term treatment with rituximab, cyclophosphamide and methylprednisolone pulses induces remission in severe cases of SLE with nephritis and avoids further immunosuppressive maintenance therapy. Nephrol Dial Transplant. 2011;26(12):3987–3992. doi: 10.1093/ndt/gfr109. [DOI] [PubMed] [Google Scholar]
- 29.Scott SD. Rituximab: a new therapeutic monoclonal antibody for non-Hodgkin’s lymphoma. Cancer Pract. 1998;6(3):195–197. doi: 10.1046/j.1523-5394.1998.006003195.x. [DOI] [PubMed] [Google Scholar]
- 30.Shan D, Ledbetter JA, Press OW. Signaling events involved in anti-CD20-induced apoptosis of malignant human B cells. Cancer Immunol Immunother. 2000;48(12):673–683. doi: 10.1007/s002620050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutter JA, Kwan-Morley J, Dunham J, Du YZ, Kamoun M, Albert D, Eisenberg RA, Luning Prak ET. A longitudinal analysis of SLE patients treated with rituximab (anti-CD20): factors associated with B lymphocyte recovery. Clin Immunol. 2008;126(3):282–290. doi: 10.1016/j.clim.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Tamimoto Y, Horiuchi T, Tsukamoto H, Otsuka J, Mitoma H, Kimoto Y, Nakashima H, Muta K, Abe Y, Kiyohara C, et al. A dose-escalation study of rituximab for treatment of systemic lupus erythematosus and Evans’ syndrome: immunological analysis of B cells, T cells and cytokines. Rheumatology. 2008;47(6):821–827. doi: 10.1093/rheumatology/ken071. [DOI] [PubMed] [Google Scholar]
- 33.Terrier B, Amoura Z, Ravaud P, Hachulla E, Jouenne R, Combe B, Bonnet C, Cacoub P, Cantagrel A, de Bandt M, et al. Safety and efficacy of rituximab in systemic lupus erythematosus. Arthritis Rheum. 2010;62(8):2458–2466. doi: 10.1002/art.27541. [DOI] [PubMed] [Google Scholar]
- 34.Thatayatikom A, White AJ. Rituximab: a promising therapy in systemic lupus erythematosus. Autoimmun Rev. 2006;5(1):18–24. doi: 10.1016/j.autrev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Tokunaga M, Saito K, Kawabata D, Imura Y, Fujii T, Nakayamada S, Tsujimura S, Nawata M, Iwata S, Azuma T, et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007;66(4):470–475. doi: 10.1136/ard.2006.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tony HP, Burmester G, Schulze-Koops H, Grunke M, Henes J, Kötter I, Haas J, Unger L, Lovric S, Haubitz M, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID) Arthritis Res Ther. 2011;13(3):R75. doi: 10.1186/ar3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner-Stokes T, Lu TY, Ehrenstein MR, Giles I, Rahman A, Isenberg DA. The efficacy of repeated treatment with B-cell depletion therapy in systemic lupus erythematosus: an evaluation. Rheumatology. 2011;50(8):1401–1408. doi: 10.1093/rheumatology/ker018. [DOI] [PubMed] [Google Scholar]
- 38.Vigna-Perez M, Hernández-Castro B, Paredes-Saharopulos O, Portales-Pérez D, Baranda L, Abud-Mendoza C, González-Amaro R. Clinical and immunological effects of rituximab in patients with lupus nephritis refractory to conventional therapy: a pilot study. Arthritis Res Ther. 2006;8(3):R83. doi: 10.1186/ar1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, Ponchel F, Rawstron AC, Emery P. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 2011;63(10):3038–3047. doi: 10.1002/art.30466. [DOI] [PubMed] [Google Scholar]
- 40.Waldman M, Appel GB. Update on the treatment of lupus nephritis. Kidney Int. 2006;70(8):1403–1412. doi: 10.1038/sj.ki.5001777. [DOI] [PubMed] [Google Scholar]