Abstract
How renal epithelial cells respond to increased pressure and the link with kidney disease states remain poorly understood. Pkd1 knock out or expression of a PC2 pathogenic mutant, mimicking the autosomal dominant polycystic kidney disease, dramatically enhances mechanical stress-induced tubular apoptotic cell death. We show the presence of a stretch-activated K+ channel dependent on the TREK-2 K2P subunit in proximal convoluted tubule epithelial cells. Our findings further demonstrate that polycystins protect renal epithelial cells against apoptosis in response to mechanical stress and this function is mediated through the opening of stretch-activated K2P channels. Thus, we establish for the first time, both in vitro and in vivo, a functional relationship between mechanotransduction and mechanoprotection. We propose that this mechanism is at play in other important pathologies associated with apoptosis and in which pressure or flow stimulation is altered, including heart failure or atherosclerosis.
Keywords: apoptosis, cysts, filamin A, mechanotransduction, polycystins, TREK/TRAAK K2P channels, TRP channels
Mechanical forces play a central role in early development, as well as in various important physiological functions, including hearing, touch or the regulation of heart rate (Chalfie, 2009; Garcia-Anoveros and Corey, 1997; Lumpkin and Caterina, 2007; Pedersen and Nilius, 2007; Wozniak and Chen, 2009). Various organs have the ability to adapt and cope with high mechanical forces. For instance, resistance arteries reduce their diameter in response to hypertension so that, according to the law of Laplace, they maintain their wall tension constant (Mulvany, 2002). Failure to adapt to high mechanical forces (flow or pressure) results in cell death and contributes to pathological states, including atherosclerosis and cardiac hypertrophy (Hahn and Schwartz, 2009; Jaalouk and Lammerding, 2009). How cells sense mechanical forces and how they adapt to mechanical stress is not yet fully understood.
Stretch-activated ion channels (SACs) show an increase in open probability in response to pressure (Kung, 2005; Sachs and Morris, 1998). The TREK-1, TREK-2 and TRAAK two-pore potassium channel (K2P) subunits underlie the stretch-activated K+-selective channels (SAKs) (for review (Honoré, 2007)). TREK-1, the most thoroughly studied SAK, is thought to be directly activated by tension in the lipid bilayer (Honoré et al., 2006; Patel et al., 1998). Moreover, channel opening can also be reversibly induced, in the absence of mechanical stimulation, by a variety of anionic amphipathic molecules, including the long chain polyunsaturated fatty acid docosahexaenoic acid (DOHA), as well as by intracellular acidosis (Honoré, 2007). TREK-2 shares all the functional properties of TREK-1, besides its sensitivity to external pH (Bang et al., 2000; Lesage et al., 2000; Sandoz et al., 2009). Transcellular Na+/glucose or Na+/amino-acids co-transports in amphibian renal proximal convoluted tubules (PCT) have been associated with water influx and an increase in cellular volume resulting in the opening of basolateral SAKs sharing the functional properties of the cloned TREK/TRAAK K2P channels (Beck and Potts, 1990; Cemerikic and Sackin, 1993; Sackin, 1989).
Autosomal dominant polycystic kidney disease (ADPKD) is caused by mutations in either PKD1 (85% of the patients) or PKD2 (15% of the patients) genes, encoding the polycystins PC1 and PC2 (Delmas, 2004; Harris and Torres, 2009; Patel and Honore, 2010; Wilson, 2004; Zhou, 2009). PC1 includes a prominent extracellular amino terminal domain, 12 transmembrane segments and a short intracellular carboxy terminal domain. PC2 is a member of the TRP family of calcium channels containing a pore sequence between transmembrane segments 5 and 6. Both proteins interact through their cytosolic carboxy terminal coiled-coil domains. The polycystin complex has been previously shown to act as a flow sensor in the primary cilium of both renal epithelial and endothelial cells (Nauli et al., 2003; Nauli et al., 2008). Moreover, polycystin dosage was recently demonstrated to regulate arterial pressure sensing (Sharif-Naeini et al., 2009). In arterial myocytes, we have shown that polycystins regulate the activity of the stretch-activated ion channels responsible for the myogenic tone, but the molecular identity of these channels was not defined (Sharif-Naeini et al., 2009).
Although less than 1% of the tubules become cystic in ADPKD, a gradual decrease in glomerular filtration rate (GFR) ultimately leads to kidney failure (Grantham et al., 2011). Why so few cysts impair the function of so many nephrons (about 1 million) in the kidney is still an open question. Although cystogenesis results from an increase in cell proliferation, apoptosis of both cystic and non-cystic tubular cells is also documented in ADPKD (Boca et al., 2006; Boletta et al., 2000; Edelstein, 2005; Goilav, 2011; Tao et al., 2005; Woo, 1995). In an experimental model of ADPKD, up to 50% of the glomeruli become a tubular, with loss of the glomerulotubular junction cells (Tanner et al., 2002). Compression/obstruction of non-cystic “healthy” tubules by growing cysts and/or fibrosis was proposed to result in an upstream tubular dilation (Grantham et al., 2011; Power et al., 2004). Moreover, abnormal fluid accumulation causes the cyst wall to stretch (Derezic and Cecuk, 1982). Thus, an increase in intra-renal mechanical stress leading to apoptosis is also proposed to be associated with kidney failure in ADPKD (Grantham et al., 2011).
In the present report, we demonstrate that polycystins play a key role in protecting renal epithelial cells against apoptosis in response to mechanical stress and this function is mediated through the opening of stretch-activated K2P channels.
Results
Mechanical stress-induced PCT cell death is influenced by polycystins
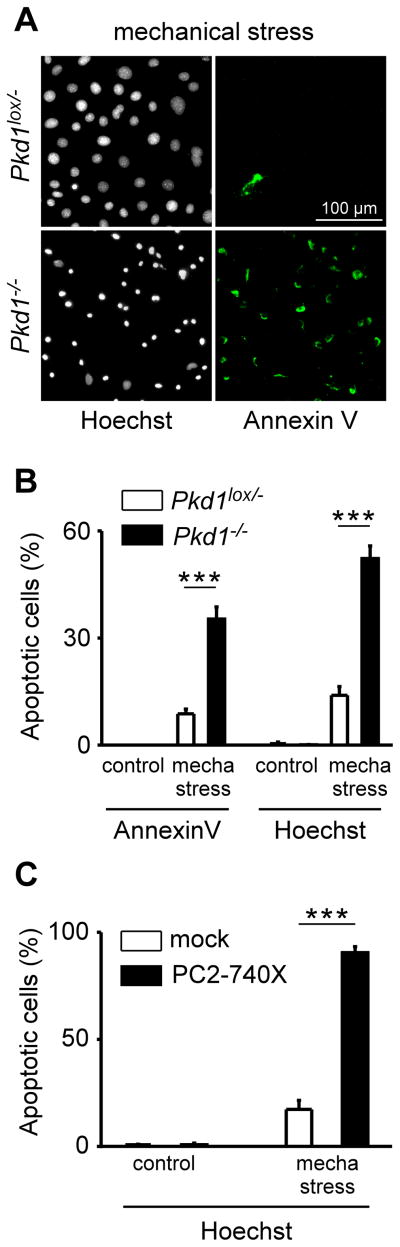
In order to study the effect of mechanical stress on cultured PCT cells, we developed an in vitro assay based on centrifugal force. Mouse PCT cells plated on glass coverslips were spun for 4 hours at 2800 g and after a recovery period of 3 hours, early apoptosis was quantified by detecting the externalization of phosphatidylserine (annexin V assay) and a later event of cell death by visualizing DNA condensation (Hoechst staining) (Fig. 1A). To examine the role of PC1, we used an immortalized mouse PCT Pkd1−/− cell line derived from a parental Pkd1lox/− clone following transfection with Cre recombinase (Wei et al., 2008) (Fig. 1A–B). Homozygote inactivation of Pkd1 significantly increased PCT cell death induced by mechanical stress, which was absent in the control condition (Fig. 1A–B). In subsequent experiments, we studied the effect of the pathogenic mutant PC2-740X expressed in wild-type mouse PCT cells (Fig. 1C). Similarly, PC2-740X expression dramatically increased the level of PCT cell death induced by mechanical stress (Fig. 1C).
Figure 1. Polycystins and mechanical stress-induced PCT cell death.
A) Early apoptosis detected by Annexin V labeling (visualized in green as shown on the right panels) induced by mechanical stress (4 hours at ~2800 g) in both Pkd1lox/− (top) and Pkd1−/− (bottom) cultured plated PCT cells. Total number of nuclei and late cell death are detected by a Hoechst staining (shown in black and white on the left panels). B) Histogram showing the amount of early (Annexin V) and late (Hoechst) apoptosis induced by mechanical stress in both Pkd1lox/− (n = 18 plates with 19 000 cells analyzed) and Pkd1−/− PCT cells (n = 18 plates with 36 000 cells analyzed). C) Histogram showing the amount of late apoptosis (Hoechst staining) induced by mechanical stress in both mock- (n = 12 plates with 1300 transfected cells analyzed) and PC2-740X (n = 11 plates with 250 transfected cells analyzed) transiently transfected cultured PCT cells. Transfected cells were visualized by EGFP fluorescence (green) and nuclear fragmentation evaluated by Hoechst staining. Cell death was determined 3 hours after mechanical stress. Data represent mean ± standard error of the mean.
These findings indicate that polycystins greatly influence the sensitivity of PCT cells to mechanical stress and associated cell death.
The stretch sensitivity of SAKs/K2P channels is conditioned by polycystins
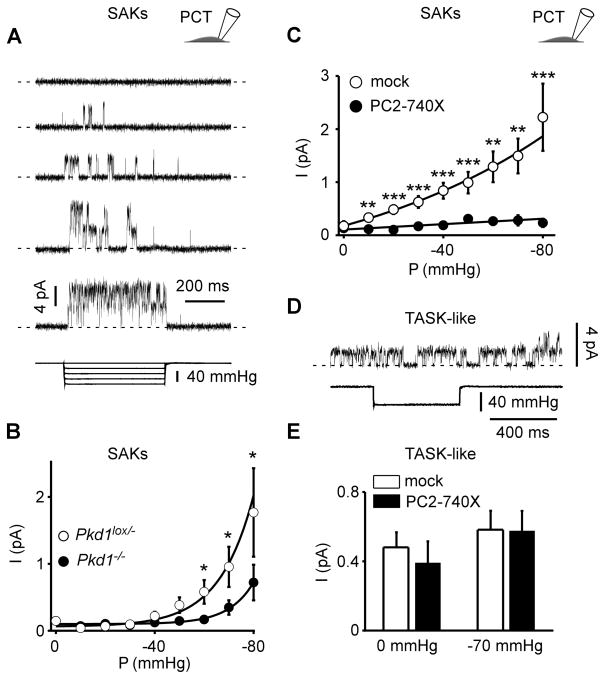
We next examined whether stretch-activated ion channels (SACs) might be involved in the response of renal cells to mechanical stimulation. Using the cell-attached patch clamp configuration coupled to a fast pressure-clamp system, we identified SAKs in mouse PCT epithelial cells (Fig. 2A). These channels were recorded at a holding potential of 0 mV in the presence of TEA (10 mM), 4-aminopyridine (3 mM) and glibenclamide (10 μM) in the pipette medium in order to minimize possible contamination by BK, Kv or KATP channels. The single channel conductance of SAKs recorded in the presence of 5 mM extracellular K+ was 49.7 ± 0.2 pS (n = 5) and a reversal potential was extrapolated to be about −80 mV, indicating K+ selectivity (Fig. S1). SAKs were recorded in tubular epithelial cells either maintained in primary culture or following immortalization (Fig. 2A and see later Fig. Supp3C). Channel openings gradually and reversibly increased with applied negative pressure and eventually reached saturation (Fig. 2A).
Figure 2. Polycystins regulate SAK activity in PCT cells.
A) Stretch-activated channels were recorded in the cell-attached patch configuration at a holding potential of 0 mV in immortalized mouse PCT cells. The pipette solution contained 5 mM K+ and the reversal potential of these channels was estimated to be about −80 mV (see Supp Fig. 1). Channel activity is illustrated at increasing negative pressure applied at the back of the patch pipette. Pressure pulses are shown at the bottom. Two channels were active in this patch. The zero current is indicated by a dashed line. B) Pressure-effect curves for mean pressure induced currents recorded in the cell-attached patch configuration in Pkd1lox/− (empty circles; n = 142) and in Pkd1−/− (filled circles; n = 146) PCT cells. Same conditions as in A. C) Mean SAK currents in PCT cells transiently transfected with either a mock empty pIRES2-DsRed plasmid (empty circles; n = 97) or together with PC2-740X (filled circles; n = 82). Currents were recorded in the cell-attached patch configuration at a holding potential of 0 mV. D) TASK-like channel activity recorded in the same conditions as in A. These channels are not responsive to an increase in pressure as shown in the lower trace. E) The histogram shows the effect of PC2-740X (n = 31) as compared to the mock empty expression vector (n = 33) on TASK-like channel activity in PCT cells. Data represent mean ± standard error of the mean.
Interestingly, SAK activity was significantly reduced in the Pkd1−/− cells, as compared to the heterozygote parental Pkd1lox/− cells (Fig. 2B). Next we studied the effect of the pathogenic mutant PC2-740X transiently expressed in WT PCT cells (Fig. 2C). PC2-740X expression similarly induced a dramatic inhibition of SAK activity at all pressures studied (Fig. 2C). The single channel current amplitude (i) of SAKs measured at 0 mV was not altered by PC2-740X (4.1 ± 0.1 pA, n = 35 and 4.1 ± 0.1 pA, n = 14 for mock and PC2-740X, respectively), indicating that either the number of active channels (n) or the open probability (Po) is decreased by PC2-740X (as I = n × Po × i; with I being the mean current). PCT cells also express a constitutively active K+ channel resistant to TEA, 4AP and glibenclamide and which is not modulated by membrane stretch (Fig. 2D–E). This channel has previously been documented to be responsible for volume regulation of PCT cells and shown to be encoded by the alkaline activated K2P channel subunit TASK-2 (Barriere et al., 2003; L’Hoste et al., 2007). Importantly, the native TASK-like channels in PCT cells were not affected by expression of PC2-740X, although SAKs were inhibited in the same cells (Fig. 2C–E).
These findings indicate that polycystins specifically modulate native SAK activity in PCT cells.
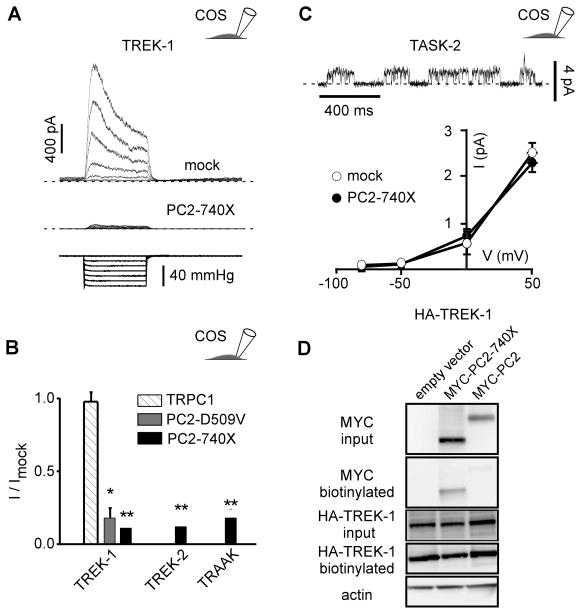
Since SAKs in PCT cells share the functional properties of the cloned TREK/TRAAK K2P channels (Honoré, 2007), we investigated whether these recombinant channels might similarly be regulated by polycystins. The TREK-1, TREK-2 and TRAAK K2P channel subunits were expressed transiently in COS cells and stretch-induced activity was recorded in the cell-attached patch configuration as previously described (Fig. 3A–B). No endogenous SAKs were present in this mock-transfected cell line (Patel et al., 1998). Co-expression with the mutant PC2-740X significantly reduced the stretch-induced TREK-1, TREK-2 or TRAAK currents (Fig. 3A–B). Stretch activation of TREK-1 was also significantly impaired by PC2-D509V co-expression, another pathogenic mutant reported to exert a dominant negative effect (Bai et al., 2008; Ma et al., 2005; Sammels et al., 2010) (Fig. 3B). In contrast, TREK-1 channel activity was not altered by co-expression with TRPC1, a PC2 interacting TRP subunit (Tsiokas et al., 1999) (Fig. 3B). Notably, PC2-740X failed to affect exogenous TASK-2 channels co-expressed in COS cells (Fig. 3C).
Figure 3. Stretch activation of TREK/TRAAK K2P channels expressed in COS cells is inhibited by PC2-740X.
A) TREK-1 currents (top trace) recorded in the cell-attached patch configuration at a holding potential of 0 mV in transiently transfected COS cells (co-transfected with a mock empty pIRES2-DsRed plasmid) in response to increasing negative pipette pressure from 0 to −60 mm Hg in steps of −10 mm Hg (bottom trace). When co-expressed with PC2-740 X, the amplitude of the stretch-activated current is dramatically reduced (middle trace). B) Mean current ratios (I/Imock) for TREK-1, TREK-2 or TRAAK at a holding potential of 0 mV in COS cells co-transfected with empty expression vector (n = 112), TRPC1 (n = 20), PC2-740X (n = 27, 27 and 22) or PC2-D509V (n = 28) in the cell attached patch configuration at a pressure of −60 mm Hg. C) TASK-2 currents in a transfected COS cell (together with a mock empty pIRES2-DsRed plasmid) recorded in the cell-attached patch configuration at a holding potential of 0 mV. I-V curves for TASK-2 in the absence (together with the empty expression vector; n = 9) or in the presence of PC2-740X (n = 6). D) Biotinylation experiments of TREK-1 in transfected COS cells. Neither PC2-740X nor WT PC2 affects expression of TREK-1 at the plasma membrane in COS cells. The three conditions tested are: HA-TREK 1 + empty vector; HA-TREK 1 + MYC-PC2-740X; HA-TREK 1 + MYC-PC2. Input blot and biotinylation blot were either probed with anti-MYC to detect PC2 or anti-HA to detect TREK-1. Blots were then stripped and probed with anti-actin. Only the input blot showed an actin signal which was equivalent for all 3 lanes. No detectable difference in TREK-1 expression at the cell surface was seen between any of these conditions. Data represent mean ± standard error of the mean.
A possible mechanism for TREK/TRAAK inhibition by PC2 mutation may involve an effect on the biosynthesis and/or trafficking of the TREK/TRAAK channels. However, biotinylation experiments performed in transiently transfected COS cells demonstrate that the plasma membrane expression of the TREK-1 subunits is not altered by overexepression of PC2-740X, suggesting that instead channel gating might be altered (Fig. 3D). Biotinylation experiments also confirm that WT PC2 is mostly retained in the ER, while the PC2-740X mutant is targeted to the plasma membrane (Chen et al., 2001) (Fig. 3D).
These results indicate that stretch sensitivity of the cloned TREK/TRAAK K2P channels is impaired by expression of PC2 pathogenic mutants.
Are the general gating properties of SAKs modulated by polycystins?
Since TREK/TRAAK channels are polymodal, activated by both physical and chemical stimuli, we next investigated whether the general gating properties of SAKs/TREK/TRAAK channels might be affected by PC2-740X.
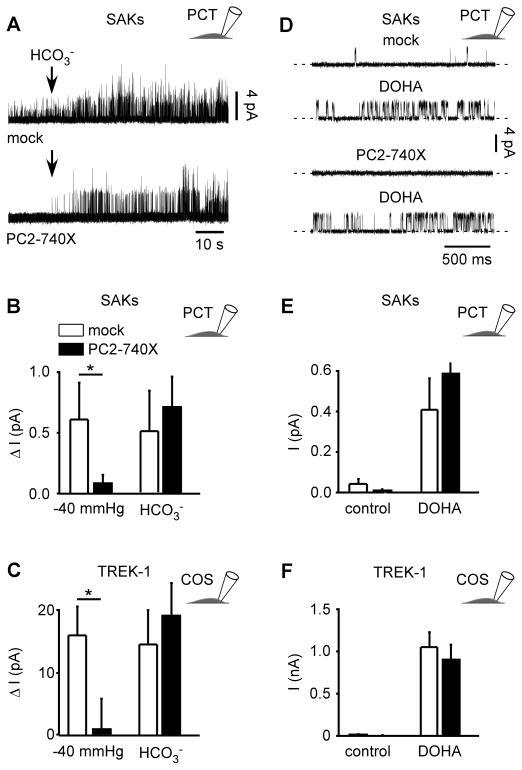
Besides activation by membrane stretch, native SAKs are also opened by intracellular acidosis, either induced by addition of 90 mM extracellular HCO3− in the cell attached patch configuration or directly by lowering intracellular pH in the inside out configuration (Maingret et al., 1999) (Fig. 4A; Fig. S2A–B). Remarkably, the activation by HCO3− was not affected by PC2-740X (Fig. 4A–B). However, in the same patches and in the absence of HCO3−, pressure activation of native SAKs in PCT cells was strongly inhibited by PC2-740X (Fig. 4B). In TREK-1 transfected COS cells, PC2-740X expression similarly failed to affect HCO3− current stimulation in the cell-attached patch configuration (Fig. 4C). Intracellular acidosis has been previously shown to protonate the residue E306 in the cytosolic carboxy terminal domain of TREK-1 resulting in constitutive channel opening (Honoré et al., 2002). Substitution of E306 by an alanine mimics protonation and locks the channel in the open conformation. The E306A mutant expressed in COS cells, which shows a background activity resistant to membrane stretch, was again not altered by co-expression with PC2-740X (Fig. S2C).
Figure 4. Stimulation of SAKs/TREK-1 activity by intracellular acidosis or DOHA is not altered by PC2-740X.
A) Addition of 90 mM HCO3− in the bath solution induced SAK activity in the cell-attached patch configuration at a holding potential of 0 mV in mock transfected PCT cells (top) or in PC2-740X transfected PCT cells (bottom). B) Changes in mean SAK current amplitude in mock (empty bars; n = 5) or PC2-740X (filled bars; n = 5) expressing PCT cells induced in the same patches by stretch (−40 mm Hg) or HCO3− addition (0 mm Hg). C) Changes in TREK-1 mean current amplitude in mock (empty bars; n = 28) or PC2-740X (filled bars; n = 21) co-expressing COS cells induced by HCO3− addition (0 mm Hg) or stretch (−40 mm Hg). D) Cell-attached patch recording of SAKs in a mock transfected PCT cell at a holding potential of 0 mV in control condition (0 mm Hg) including 0.01 % ethanol (vehicle; top trace) or after extracellular addition of 10 μM DOHA (second trace). Same with expression of PC2-740X (bottom two traces). E) Changes in mean SAK current amplitude (0 mm Hg) in the absence or in the presence of DOHA (10 μM) in PCT cells transfected either with a mock empty plasmid (empty bars; n = 8) or together with PC2-740X (black bars; n = 8). F) Changes in mean TREK-1 current amplitude (0 mm Hg) in the absence or in the presence of DOHA (10 μM) in co-expressing COS cells transfected either with a mock empty plasmid (empty bars; n = 12) or together with PC2-740X (black bars; n = 13). Data represent mean ± standard error of the mean.
Native SAKs in PCT cells, as well as TREK-1 expressed in transfected COS cells, were also reversibly stimulated by external addition of the long chain polyunsaturated fatty acid DOHA (Fig. 4D–F). Again, the DOHA-induced activity was not significantly altered by expression of PC2-740X (Fig. 4D–F).
These results show that although the stretch sensitivity of the SAKs/TREK/TRAAK channels is strongly inhibited by PC2-740X, activation by intracellular acidosis or polyunsaturated fatty acids is resistant. Thus, these findings indicate that polycystins specifically regulate SAKs/TREK/TRAAK mechano-gating.
Inhibition of SAKs mechano-gating by PC2-740X involves the actin cytoskeletal network
Our previous findings have established that TREK-1 channel activity induced by stretch is repressed by F-actin (Lauritzen et al., 2005). We explored the possibility that polycystins may affect pressure-dependent TREK/TRAAK channels activity through the F-actin cytoskeleton network.
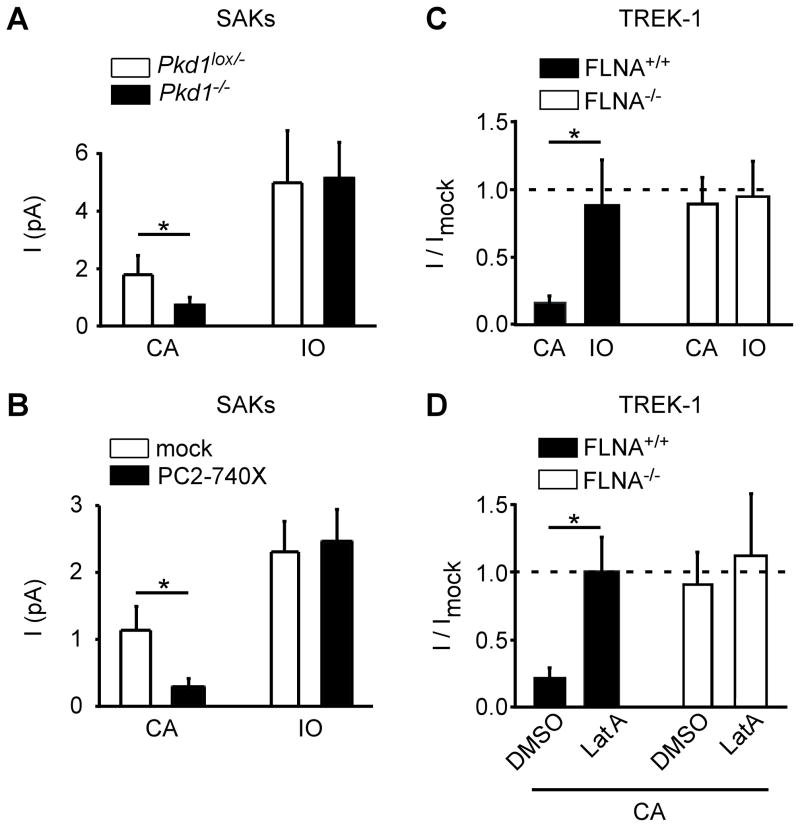
The actin cytoskeleton can be mechanically disrupted by excision of the patches in the inside-out configuration (Lauritzen et al., 2005). When Pkd1 is inactivated or when PC2-740X is expressed in PCT cells, SAK inhibition, which is observed in the cell-attached patch configuration, disappeared upon excision of the patches in the inside-out configuration (Fig. 5A–B). Similarly, when transfected in FLNA+/+ cells (A7 cells), which express the PC2 interactor filamin A, an actin cross-linking protein, again TREK-1 inhibition is reversed by patch excision (Fig. 5C). By contrast, in the FLNA−/− cells (M2 cells), inhibition was absent in both cell-attached and inside out patch configurations (Fig. 5C). Rescue of channel activity upon patch excision suggests that the cytoskeleton is involved in the down-regulation of SAKs/TREK/TRAAK by polycystins. Indeed, treating FLNA+/+ cells with latrunculin A, which disrupts the F-actin cytoskeleton, also reversed TREK-1 inhibition in the cell-attached patch configuration, although it failed to affect channel activity in the FLNA−/− cells lacking filamin A (Fig. 5D).
Figure 5. Role of the actin cystoskeleton in the regulation of SAKs/TREK-1 mechano-gating by polycystins.
A) SAK activity elicited by membrane stretch (−80 mm Hg) in the cell-attached (CA) patch configuration is significantly reduced in the Pkd1−/− (n = 20) as compared to the Pkd1lox/− PCT cells (n = 21). SAK activity induced by stretch in the Pkd1−/− cells is rescued following patch excision in the inside-out (IO) patch configuration. B) SAK activity elicited by membrane stretch (−60 mm Hg) in the cell-attached patch configuration is significantly reduced by PC2-740X expression (black bars; n= 12),as compared to mock-transfectedPCT cells (white bars; n = 15). SAK activity induced by stretch in the PC2-740X expressing cellsis rescued following patch excision in the inside-out patch configuration. C) SAK activity in A7 cells (FLNA+/+) is strongly reduced by PC2-740X expression (black bars; n = 21), as compared to the mock condition (n = 21). Patch excision in the inside out configuration rescues channel activity (black bar; n = 17). When transfected in M2 cells (FLNA−/−), PC2-740X fails to affect TREK-1 channel activity elicited by stretch in both the cell attached (white bar; n = 44) and the inside out (white bar; n = 15) patch configurations. Pressure stimulation was −60 mm Hg. D) Latrunculin A treatment (3 μM) reverses TREK-1 inhibition by PC2-740X expression in FLNA+/+ cells in the cell-attached patch configuration (black bars; n = 33), as compared to mock condition (n= 17). In FLNA−/− cells, no inhibition by PC2-740X is seen and latrunculin A fails to affect channel activity (white bars; n = 21 and n = 13). Pressure stimulation was −60 mm Hg. Data represent mean ± standard error of the mean.
These findings indicate that F-actin and filamin A are critically required for the regulation of SAKs/TREK/TRAAK mechano-gating by polycystins.
SAKs knock-out enhances tubular cell death induced by mechanical stress
Native SAKs recorded in PCT cells share the functional properties of both TREK-1 and TREK-2 subunits which are similarly activated by stretch, DOHA and intracellular acidosis, unlike TRAAK which is activated by intracellular alkalosis. In the subsequent experiments, we aimed to identify which TREK subunit encodes for the native SAKs in PCT cells. The TREK-2 subunit has previously been shown to be expressed in the kidney (Bang et al., 2000; Lesage et al., 2000). Indeed, we detected expression of TREK-2 in whole tubules and in cultured immortalized PCT cells by QPCR (Fig. S3A–B). While TRAAK was also found in whole tubules (including distal tubules), it was very low in PCT cells (Fig. S3A–B). SAK activity was lost in TREK-2−/− PCT cells either maintained in primary culture or after immortalization, recorded both in the cell-attached and excised inside-out patch configurations (Fig. S3C). The TASK-like channel activity was, however, still present in the TREK-2−/− cells (Fig. S3D).
We examined the renal structure of constitutive TREK-2 knock-out adult mice maintained in basal conditions. These kidneys were not different from those of control WT mice (Fig. S4). Furthermore, in the Pkd1+/− background, TREK-2 homozygote knock-out did not show any obvious structural defect (Fig. S4). To avoid any possible confounding genetic compensation mechanisms and to be able to inactivate the three stretch-activated K2P channels at the same time, in subsequent experiments we used a mouse model in which the genes encoding TREK-1, TREK-2 and TRAAK have been knocked-out altogether (SAK KO) (Guyon et al., 2009). Again, no kidney structural defect was visible in adult SAK KO mice (Fig. S4).
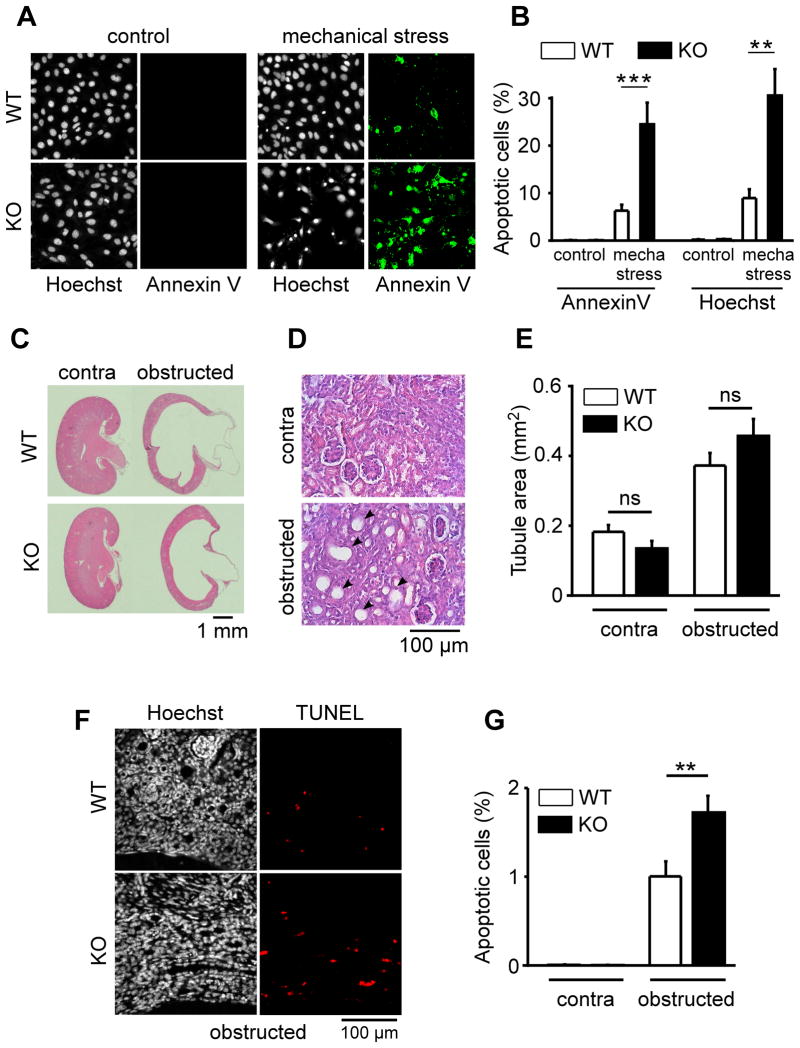
We next hypothesized that a loss of SAK activation may influence PCT cell death induced by mechanical stress. We compared the sensitivity to centrifugal force (as described earlier) of WT PCT cells with those of SAK KO PCT cells in which the stretch-activated TREK/TRAAK K2P channels have been deleted altogether. SAK KO PCT cells were consistently more sensitive to mechanical stress-induced cell death, as compared to WT PCT cells (Fig. 6 A–B).
Figure 6. SAKs knock-out increases mechanical stress-induced PCT cell death both in vitro and in vivo.
A) Early apoptosis visualized by Annexin V labeling (panels in green on the right) induced by mechanical stress (centrifugal force) in both WT (top) and SAK KO (TREK-1−/−/TREK-2−/−/TRAAK−/−) (bottom) cultured PCT cells. Total number of nuclei and late apoptosis are detected by a Hoechst staining (panels in black and white on the left). B) Histogram showing the amount of early (Annexin V) and late (Hoechst) apoptosis induced by mechanical stress in both WT (n = 11) and SAK KO PCT cells (n = 12). Cell death was determined 3 hours after mechanical stress (centrifugation of plated cells at 2800 g for 4 hours). C) Sections of WT and SAK KO (TREK-1−/−/TREK-2−/−/TRAAK−/−) mouse contralateral (contra; left panel) or obstructed (right panel) kidneys stained with eosin hematoxylin. D) Effect of ureteral ligation on the tubular diameter of a WT kidney from a 10-day-old mouse ligated for 3 days (bottom), as compared to the contralateral kidney (top). Kidney sections were stained with eosin hematoxylin. E) Tubular area cross section of contralateral (contra) and obstructed kidneys from WT (white bars; n = 7) and SAK KO (black bars; n = 6) mice. F) Ureteral ligation increases the number of apoptotic cells as detected by a double Hoechst (left panels) and TUNEL staining (right panels in red). G) Effect of ureteral ligation on apoptotic cell death (TUNEL staining) in kidneys from WT (white bars; n = 14) and SAK KO (black bars; n = 13) mice. Data represent mean ± standard error of the mean.
We reasoned that a loss of SAK stretch sensitivity may influence tubular cell death in a high intra-renal pressure condition. We used a mouse model of ureteral obstruction which is associated with increased intra-renal pressure and wall stress (Power et al., 2004; Quinlan et al., 2008; Rohatgi and Flores, 2010; Wyker et al., 1981). We performed unilateral ureteral ligation for 3 days in 10-day-old mice (Fig. 6 C–G). The dimension of the obstructed kidneys after three days of obstruction significantly increased to a similar extent in both WT and SAK KO mice (Fig. 6C; Fig. S5). A close up view of the kidney structure demonstrates that tubules were significantly dilated upon ureteral obstruction, irrespective of the genotype (Fig. 6D–E). Obstructive uropathy is associated with apoptosis of epithelial cells and tubular atrophy (Power et al., 2004; Quinlan et al., 2008; Rohatgi and Flores, 2010). We measured apoptosis by a double Hoechst and TUNEL staining in the obstructed, in the unligated contralateral, as well as in the sham-operated kidneys from both WT and SAK KO mice. No apoptosis was detected in sham-operated kidneys (n = 9; not shown) or in the contralateral kidneys (Fig. 6G; Fig. S6). By contrast, about 1 % of the tubular cells were found apoptotic in the WT obstructed kidneys, in agreement with previous reports (Power et al., 2004; Quinlan et al., 2008; Rohatgi and Flores, 2010; Wyker et al., 1981) (Fig. 6F–G). Remarkably, the number of apoptotic cells almost doubled in the obstructed kidneys from SAK KO mice lacking the stretch-activated K2P channels (Fig. 6G).
These findings demonstrate that TREK/TRAAK K2P channels are protective against tubular epithelial cell death induced by mechanical stress both in vitro and in vivo.
Discussion
The present study shows that when SAKs are inactivated, similarly to Pkd1 knock out or PC2 pathogenic mutant expression mimicking ADPKD, PCT cells in vitro become highly sensitive to mechanical stress and undergo apoptosis. Moreover, our in vivo findings further indicate that the opening of the TREK/TRAAK channels is protective against apoptosis associated with high intra-renal pressure. We identify, and demonstrate at the molecular level, the regulation of SAKs (K2P channels) by polycystins in the kidney. All together these findings show that mechanoprotection by polycystins against apoptosis is mediated through the opening of stretch-activated K2P channels. These molecular findings are significant to better understand how polycystins regulate pressure sensing in the kidney.
Is the regulation of SAKs by polycystins specific? Native or exogenous TASK-2 channels (another K2P channel) are not altered by PC2-740X expression, whereas in the same PCT cells, SAKs are inhibited. PC2-740X also fails to affect other types of ion channels such as voltage-gated K+ channels or ASICS (Sharif-Naeini et al., 2009). In addition, PC2-740X does not influence the E306A TREK-1 gain of function mutant. SAKs inhibition is not seen with TRPC1, another TRP channel subunit. Importantly, we provide evidence using biotinylation experiments, that the plasma membrane expression of TREK-1 is not altered by PC2-740X. Remarkably, when we excise patches in the inside out configuration, SAKs activity is fully rescued, demonstrating that channels are still present at the plasma membrane, but their stretch sensitivity is specifically repressed by PC2-740X expression in the cell-attached configuration. Moreover, low pHi or DOHA activation of SAKs in the cell-attached configuration is not altered, again demonstrating that channels are functional at the plasma membrane when PC2-740X is expressed, although their stretch activation is strongly repressed. These findings indicate that the pathogenic mutant PC2-740X selectively inhibits the stretch sensitivity of K2P channels. Our previous study demonstrated that polycystins also regulate the stretch sensitivity of non-selective SACs in arterial myocytes (Sharif-Naeini et al., 2009). Since the loss of function PC2 D509V mutant similarly inhibits SAKs mechano-gating, PC2 permeation is unlikely to be involved in this effect.
Potassium channel activity has been shown to be pro-apoptotic in both neuronal and non-neuronal cells (for review (Patel and Lazdunski, 2004)). For instance, the TASK-2 K2P channels play a key role in PCT cells apoptotic volume decrease (AVD) (L’Hoste et al., 2007). By contrast, in the present study we demonstrate a protective role for SAKs (i.e. TREK/TRAAK K2P channels) against mechanical stress-induced cell death. Failure to repolarize could be an important factor in the initiation of stretch-induced cell death (Kainulainen et al., 2002). Opening of SAKs in PCT cells during mechanical stimulation is anticipated to protect cells from excessive depolarization. Another possible explanation for mechanoprotection by SAKs might be related to cell swelling associated with Na+/solute co-transport, previously shown to be coupled to SAK activity at the basolateral membrane of PCT cells (Beck and Potts, 1990; Cemerikic and Sackin, 1993; Sackin, 1989). Although the present findings suggest that stretch activation of SAKs during mechanical stress protects PCT cells from apoptosis, constitutive TREK/TRAAK channel activity is anticipated to have an opposite effect. Indeed, our previous work indicates that constitutive (or leak) K2P channel activity such as TREK-1 E306A is pro-apoptotic, unlike WT TREK-1 (Lauritzen et al., 2003). These results indicate that SAKs mechano-gating is probably central to its protective effect. Cells will hyperpolarize during mechanical stress because of the opening of SAKs. Is the hyperpolarization, or the change in intracellular K+ resulting from the K+ efflux, or both, linked with cellular mechanoprotection? We have performed in vitro mechanical stimulation of PCT cells for four hours (centrifugal force) and subsequently measured intracellular K+ concentration. No significant difference is seen between control and SAKs knock-out cells before or after mechanical stress (not shown). Thus, it is unlikely that K+ itself is involved in the protective effect of SAKs. We propose that cell hyperpolarization is a key parameter in mechanoprotection by SAKs.
Urinary tract obstruction, which is the leading cause of pediatric end stage renal failure, notably provokes tubular cell apoptosis (Chevalier, 2008). In line with this clinical observation, previous experimental findings indicate that there is a close association between tubular distension and apoptosis in the kidney (Grantham et al., 2011; Power et al., 2004; Quinlan et al., 2008; Wyker et al., 1981). In the present study, inactivation of SAKs K2P channel subunits significantly enhances tubular cells apoptosis in an experimental model of ureteral obstruction in the newborn mouse. These results show that opening of SAKs exerts a protective effect on tubular epithelial cells subjected to chronic stretch. It would be interesting to stimulate SAK opening during ADPKD, with an expected protection of renal cells. Unfortunately, since neither a specific opener nor a SAK gain of function mouse model is yet available, this experiment cannot be performed at the present time.
Thus, resistance to apoptosis induced by high intra-renal pressure involves mechanotransduction (i.e. opening of mechano-gated potassium channels). To our knowledge, this is the first time that a functional link is established between mechanotransduction and mechanoprotection. TREK and TRAAK channels are broadly expressed, including in cardiac and arterial myocytes, as well as in endothelial cells (Blondeau et al., 2007; Garry et al., 2007; Terrenoire et al., 2001). Thus, our findings may be extended to other pathologies associated with apoptosis and in which pressure or flow stimulation is altered, including cardiac hypertrophy/heart failure or atherosclerosis (Hahn and Schwartz, 2009; Jaalouk and Lammerding, 2009).
Here, we demonstrate that upon Pkd1 inactivation or expression of a PC2 pathogenic mutant, mimicking ADPKD, inhibition of the stretch sensitivity of SAKs is deleterious and contributes to increase tubular apoptosis. In ADPKD, a ≪ two hit ≫ mechanism was put forward to explain focal cystogenesis, slow progression of the disease and the interfamily phenotypic variability (Qian et al., 1996; Wu et al., 1998). However, several observations also suggest that an additional dosage mechanism may be at play in the disease (Lantinga-van Leeuwen et al., 2004; Pei, 2001). If polycystin dosage is indeed involved, it is anticipated that SAK activity will be decreased in both cystic and non-cystic tubules of ADPKD kidneys where apoptosis is detected (Woo, 1995).
The present results also demonstrate the critical role of the F-actin/filamin A network in the regulation of SAKs by polycystins in kidney epithelial cells. We previously introduced the “Upholstery Model” to explain how polycystins may affect the conversion of intraluminal pressure to local bilayer tension (Sharif Naeini et al., 2009). We proposed that PC2 through interaction with filamin A and cross-linking of F actin may influence the radius of membrane curvature in microdomains and thus according to Laplace’s Law control membrane tension (Sharif Naeini et al., 2009). The PC2/filamin A interaction is predicted to occur whether PC2 is in the endoplasmic reticulum or at the plasma membrane because in both cases the C terminal domain of PC2 will be facing the cytosol (Sharif Naeini et al., 2009). The deletion of the carboxy terminal domain of PC2 at position 690, unlike at position 740, impairs the interaction with filamin A (our proteomic data (Sharif-Naeini et al., 2009)) and moreover, PC2-690X fails to influence stretch-activated channels (not shown). In the present study, we identify the molecular identity of the mechano-gated ion channels regulated by polycystins through the actin cytoskeletal network in renal tubular epithelial cells, with TREK-2 playing a key role in PCT cells. Since stimulation of SAKs by low pHi or polyunsaturated fatty acids is not altered by polycystins, these modes of K2P channels activation are likely to be independent of membrane tension or of the actin cytoskeletal network.
In conclusion, we put forward a mechanism whereby a loss of mechanoprotection by SAKs (i.e. TREK/TRAAK K2P channels) enhances tubular cell death and contributes to kidney failure in ADPKD. All together, these results allow a better understanding of the molecular basis of renal mechanotransduction, mechanoprotection and involvement in disease states.
Methods
Electrophysiology
Electrophysiological procedure has been previously described elsewhere (Sharif Naeini et al., 2009). Briefly, single channel cell-attached patch clamp recordings were performed on primary cultures or immortalized PCT cells, as well as on transiently transfected COS-7, M2 or A7 cells. The pipette medium contained (in mM): NaCl 150, KCl 5, CaCl2 1, and HEPES 10 (pH 7.4 with NaOH). The pipette solution also contained 10 mM TEA, 5 mM 4AP and 10 μM glibenclamide to inhibit eventual contaminating potassium channels. The bath medium contained (in mM): KCl 155, EGTA 5, MgCl2 3, and HEPES 10 (pH 7.2 with KOH). The osmolality of all solutions was adjusted to 310 mOsm. For HCO3− stimulation, 90 mM KCl was substituted with 90 mM KHCO3. Membrane patches were stimulated with brief negative pressure pulses of −10 mm Hg increments, through the recording electrode using a pressure-clamp device (ALA High Speed Pressure Clamp-1 system, ALA-scientific). The holding voltage for all experiments was 0 mV for SAK recordings. Detailed information about materials and methods is available in the supplementary information.
Supplementary Material
Acknowledgments
We are grateful to the ANR 2008 du gène à la physiopathologie, to the Fondation de la recherche mèdicale, to EEC Marie-Curie fellowship 039328 (JHAF), to the Fondation de recherche sur l’hypertension artérielle, to the Fédération de recherche sur le cerveau, to Human Frontier Science Program long term fellowship LT-00555 (RSN), to the Fondation de France, to the Association Française contre les Myopathies (RP), to the Association pour l’information et la recherche sur les maladies rénales génétiques France, to the Région Provence Alpes Côte d’Azur, to the Société Française d’hypertension artérielle (CE), to the Université de Nice Sophia Antipolis and to CNRS for financial support. We are grateful to Dr. Boris Martinac and Dr. Ardem Patapoutian for helpful suggestions. We thank Dr. L. Tsokias and to Dr. T.P. Stossel for providing the mouse Pkd2 clones and the M2 and A7 cell lines, respectively.
References
- Bai CX, Kim S, Li WP, Streets AJ, Ong AC, Tsiokas L. Activation of TRPP2 through mDia1-dependent voltage gating. EMBO J. 2008;27:1345–1356. doi: 10.1038/emboj.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem pore K+ channel family. J Biol Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- Barriere H, Belfodil R, Rubera I, Tauc M, Lesage F, Poujeol C, Guy N, Barhanin J, Poujeol P. Role of TASK2 potassium channels regarding volume regulation in primary cultures of mouse proximal tubules. J Gen Physiol. 2003;122:177–190. doi: 10.1085/jgp.200308820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JS, Potts DJ. Cell swelling, co-transport activation and potassium conductance in isolated perfused rabbit kidney proximal tubules. J Physiol. 1990;425:369–378. doi: 10.1113/jphysiol.1990.sp018108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau N, Petrault O, Manta S, Giordanengo V, Gounon P, Bordet R, Lazdunski M, Heurteaux C. Polyunsaturated Fatty Acids Are Cerebral Vasodilators via the TREK-1 Potassium Channel. Circ Res. 2007;101:176–184. doi: 10.1161/CIRCRESAHA.107.154443. [DOI] [PubMed] [Google Scholar]
- Boca M, Distefano G, Qian F, Bhunia AK, Germino GG, Boletta A. Polycystin-1 induces resistance to apoptosis through the phosphatidylinositol 3-kinase/Akt signaling pathway. J Am Soc Nephrol. 2006;17:637–647. doi: 10.1681/ASN.2005050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boletta A, Qian F, Onuchic LF, Bhunia AK, Phakdeekitcharoen B, Hanaoka K, Guggino W, Monaco L, Germino GG. Polycystin-1, the gene product of PKD1, induces resistance to apoptosis and spontaneous tubulogenesis in MDCK cells. Mol Cell. 2000;6:1267–1273. doi: 10.1016/s1097-2765(00)00123-4. [DOI] [PubMed] [Google Scholar]
- Cemerikic D, Sackin H. Substrate activation of mechanosensitive, whole cell currents in renal proximal tubule. Am J Physiol. 1993;264:F697–714. doi: 10.1152/ajprenal.1993.264.4.F697. [DOI] [PubMed] [Google Scholar]
- Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol. 2009;10:44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- Chen XZ, Segal Y, Basora N, Guo L, Peng JB, Babakhanlou H, Vassilev PM, Brown EM, Hediger MA, Zhou J. Transport function of the naturally occurring pathogenic polycystin-2 mutant, R742X. Biochem Biophys Res Commun. 2001;282:1251–1256. doi: 10.1006/bbrc.2001.4720. [DOI] [PubMed] [Google Scholar]
- Chevalier RL. Chronic partial ureteral obstruction and the developing kidney. Pediatr Radiol. 2008;38(Suppl 1):S35–40. doi: 10.1007/s00247-007-0585-z. [DOI] [PubMed] [Google Scholar]
- Delmas P. Polycystins: from mechanosensation to gene regulation. Cell. 2004;118:145–148. doi: 10.1016/j.cell.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Derezic D, Cecuk L. Hydrostatic pressure within renal cysts. Br J Urol. 1982;54:93–94. doi: 10.1111/j.1464-410x.1982.tb13525.x. [DOI] [PubMed] [Google Scholar]
- Edelstein CL. What is the role of tubular epithelial cell apoptosis in polycystic kidney disease (PKD)? Cell Cycle. 2005;4:1550–1554. doi: 10.4161/cc.4.11.2185. [DOI] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Corey DP. The molecules of mechanosensation. Annu Rev Neurosci. 1997;20:567–594. doi: 10.1146/annurev.neuro.20.1.567. [DOI] [PubMed] [Google Scholar]
- Garry A, Fromy B, Blondeau N, Henrion D, Brau F, Gounon P, Guy N, Heurteaux C, Lazdunski M, Saumet JL. Altered acetylcholine, bradykinin and cutaneous pressure-induced vasodilation in mice lacking the TREK1 potassium channel: the endothelial link. EMBO Rep. 2007;8:354–359. doi: 10.1038/sj.embor.7400916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goilav B. Apoptosis in polycystic kidney disease. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbadis.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7:556–566. doi: 10.1038/nrneph.2011.109. [DOI] [PubMed] [Google Scholar]
- Guyon A, Tardy MP, Rovere C, Nahon JL, Barhanin J, Lesage F. Glucose inhibition persists in hypothalamic neurons lacking tandem-pore K+ channels. J Neurosci. 2009;29:2528–2533. doi: 10.1523/JNEUROSCI.5764-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PC, Torres VE. Polycystic Kidney Disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E. The neuronal background K2P channels: focus on TREK-1. Nature reviews neuroscience. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Honoré E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K2P channels. Proc Natl Acad Sci U S A. 2006;103:6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainulainen T, Pender A, D’Addario M, Feng Y, Lekic P, McCulloch CA. Cell death and mechanoprotection by filamin a in connective tissues after challenge by applied tensile forces. J Biol Chem. 2002;277:21998–22009. doi: 10.1074/jbc.M200715200. [DOI] [PubMed] [Google Scholar]
- Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- L’Hoste S, Poet M, Duranton C, Belfodil R, e Barriere H, Rubera I, Tauc M, Poujeol C, Barhanin J, Poujeol P. Role of TASK2 in the control of apoptotic volume decrease in proximal kidney cells. J Biol Chem. 2007;282:36692–36703. doi: 10.1074/jbc.M703933200. [DOI] [PubMed] [Google Scholar]
- Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, Verbeek S, Deruiter MC, Breuning MH, de Heer E, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13:3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Chemin J, Honoré E, Jodar M, Guy N, Lazdunski M, Jane Patel A. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6:642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen I, Zanzouri M, Honoré E, Duprat F, Ehrengruber MU, Lazdunski M, Patel AJ. K+-dependent cerebellar granule neuron apoptosis: role of TASK leak K+ channels. J Biol Chem. 2003;278:32068–32076. doi: 10.1074/jbc.M302631200. [DOI] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol. 2005;25:8285–8298. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci. 2002;17:105–109. doi: 10.1152/nips.01366.2001. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Honore E. Polycystins and renovascular mechanosensory transduction. Nat Rev Nephrol. 2010;6:530–538. doi: 10.1038/nrneph.2010.97. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M. The 2P-domain K+ channels: role in apoptosis and tumorigenesis. Pflugers Arch. 2004;448:261–273. doi: 10.1007/s00424-004-1255-8. [DOI] [PubMed] [Google Scholar]
- Pedersen SA, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymology. 2007;428:183–207. doi: 10.1016/S0076-6879(07)28010-3. [DOI] [PubMed] [Google Scholar]
- Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med. 2001;7:151–156. doi: 10.1016/s1471-4914(01)01953-0. [DOI] [PubMed] [Google Scholar]
- Power RE, Doyle BT, Higgins D, Brady HR, Fitzpatrick JM, Watson RW. Mechanical deformation induced apoptosis in human proximal renal tubular epithelial cells is caspase dependent. J Urol. 2004;171:457–461. doi: 10.1097/01.ju.0000091106.61065.e3. [DOI] [PubMed] [Google Scholar]
- Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996;87:979–987. doi: 10.1016/s0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- Quinlan MR, Docherty NG, Watson RW, Fitzpatrick JM. Exploring mechanisms involved in renal tubular sensing of mechanical stretch following ureteric obstruction. Am J Physiol Renal Physiol. 2008;295:F1–F11. doi: 10.1152/ajprenal.00576.2007. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Flores D. Intratubular hydrodynamic forces influence tubulointerstitial fibrosis in the kidney. Curr Opin Nephrol Hypertens. 2010;19:65–71. doi: 10.1097/MNH.0b013e32833327f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F, Morris CE. Mechanosensitive ion channels in nonspecialized cells. Rev Physiol Biochem Pharmacol. 1998;132:1–77. doi: 10.1007/BFb0004985. [DOI] [PubMed] [Google Scholar]
- Sackin H. A stretch-activated K+ channel sensitive to cell volume. Proc Natl Acad Sci U S A. 1989;86:1731–1735. doi: 10.1073/pnas.86.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammels E, Devogelaere B, Mekahli D, Bultynck G, Missiaen L, Parys JB, Cai Y, Somlo S, De Smedt H. Polycystin-2 activation by inositol 1,4,5-trisphosphate-induced Ca2+ release requires its direct association with the inositol 1,4,5-trisphosphate receptor in a signaling microdomain. J Biol Chem. 2010;285:19794–18805. doi: 10.1074/jbc.M109.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc Natl Acad Sci U S A. 2009;106:14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- Tanner GA, Tielker MA, Connors BA, Phillips CL, Tanner JA, Evan AP. Atubular glomeruli in a rat model of polycystic kidney disease. Kidney Int. 2002;62:1947–1957. doi: 10.1046/j.1523-1755.2002.00689.x. [DOI] [PubMed] [Google Scholar]
- Tao Y, Kim J, Faubel S, Wu JC, Falk SA, Schrier RW, Edelstein CL. Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease. Proc Natl Acad Sci U S A. 2005;102:6954–6959. doi: 10.1073/pnas.0408518102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrenoire C, Lauritzen I, Lesage F, Romey G, Lazdunski M. A TREK-1-like potassium channel in atrial cells inhibited by b-adrenergic stimulation and activated by volatile anesthetics. Circ Res. 2001;89:336–342. doi: 10.1161/hh1601.094979. [DOI] [PubMed] [Google Scholar]
- Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci U S A. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Karihaloo A, Yu Z, Marlier A, Seth P, Shibazaki S, Wang T, Sukhatme VP, Somlo S, Cantley LG. Neutrophil gelatinase-associated lipocalin suppresses cyst growth by Pkd1 null cells in vitro and in vivo. Kidney Int. 2008;74:1310–1318. doi: 10.1038/ki.2008.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- Woo D. Apoptosis and loss of renal tissue in polycystic kidney diseases. N Engl J Med. 1995;333:18–25. doi: 10.1056/NEJM199507063330104. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, D’Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- Wyker AT, Ritter RC, Marion D, Gillenwater JY. Mechanical factors and tissue stresses in chronic hydronephrosis. Invest Urol. 1981;18:430–436. [PubMed] [Google Scholar]
- Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.