Abstract
Rod and cone photoreceptors possess ribbon synapses that assist in the transmission of graded light responses to second-order bipolar and horizontal cells of the vertebrate retina. Proper functioning of the synapse requires the juxtaposition of presynaptic release sites immediately adjacent to postsynaptic receptors. In this review, we focus on the synaptic, cytoskeletal, and extracellular matrix proteins that help to organize photoreceptor ribbon synapses in the outer plexiform layer. We examine the proteins that foster the clustering of release proteins, calcium channels, and synaptic vesicles in the presynaptic terminals of photoreceptors adjacent to their postsynaptic contacts. Although many proteins interact with one another in the presynaptic terminal and synaptic cleft, these protein–protein interactions do not create a static and immutable structure. Instead, photoreceptor ribbon synapses are remarkably dynamic, exhibiting structural changes on both rapid and slow time scales.
Keywords: Cone, Rod, Outer retina, Neurotransmission, Cytoskeleton
Introduction
Synapses are highly specialized contacts between neurons that have evolved to transmit signals effficiently and reliably throughout the nervous system. At chemical synapses, changes in the electrical potential of a cell are converted into a chemical signal. The classical mechanism involves a depolarization-induced Ca2+ influx causing SNARE proteins to intertwine and pull vesicles to the plasma membrane (Pang & Südhof, 2010). The subsequent fusion event results in vesicles emptying their neurotransmitter contents into the synaptic cleft to signal through receptors on postsynaptic neurons. Synapses require a highly organized placement of pre-synaptic exocytotic proteins, endocytotic proteins, calcium channels, postsynaptic receptors, and other associated proteins. In photoreceptor terminals, synapses are organized around a large electron-dense structure known as the ribbon. In this review, we examine the molecular architecture of ribbon synapses in photore-ceptor terminals with a focus on interactions with the cytoskeleton and extracellular matrix (ECM). We also summarize studies showing that photoreceptor ribbon synapses are not static structures but can be altered dynamically on both fast and slow time scales.
CaV channels cluster at the active zone
Physiological experiments have established that the voltage-gated calcium (CaV) channels in rod and cone photoreceptors are high voltage-activated L-type channels (Bader et al., 1982; Corey et al., 1984; Barnes & Hille, 1989; Lasater & Witkovsky, 1991; Wilkinson & Barnes, 1996). There is no evidence for non-L-type CaV channels in photoreceptors. Freeze-fracture electron micrographs of photoreceptor terminals show a random array of hexagonal-shaped particles at the apex of the synaptic ridge that are thought to be CaV channels (Raviola & Gilula, 1975; Schaeffer et al., 1982). Terminals of rods and cones in the outer plexiform layer (OPL) label with antibodies to poreforming CaV1.2 (α1C), CaV1.3 (α1D), and CaV1.4 (α1F) subunit isoforms (Nachman-Clewner et al., 1999; Firth et al., 2001; Henderson et al., 2001; Morgans, 2001; Morgans et al., 2001, 2005; Wu et al., 2003; Mansergh et al., 2005; tom Dieck et al., 2005; Xiao et al., 2007; Specht et al., 2009; Kersten et al., 2010; Mercer et al., 2011a,b). High levels of CaV1.3 and CaV1.4 messenger RNA (mRNA) are present in retina (Bech-Hansen et al., 1998; Strom et al., 1998; Xiao et al., 2007) but only low levels of CaV1.2 mRNA (Kamphuis & Hendriksen, 1998), suggesting that the labeling of photoreceptors by CaV1.2 antibodies may be nonspecific. Mutations in CaV1.4 greatly diminish the electroretinogram (ERG) b-wave in knockout mice, produce malformed ribbons, and cause congenital stationary night blindness (CSNB) in humans. Combined with immunohistochemical evidence, these findings suggest that CaV1.4 is the principle CaV channel subtype in rods (Bech-Hansen et al., 1998; Strom et al., 1998; Mansergh et al., 2005; Chang et al., 2006; Striessnig et al., 2010).
The contribution of CaV1.3 at photoreceptor synapses is less clear. Immunoelectron micrographs show labeling for antibodies to CaV1.3 at the base of ribbons in mouse retina (Kersten et al., 2010). However, CaV1.3 mutations in mouse retina do not significantly diminish the b-wave (Wu et al., 2007), and b-waves of CaV1.3/CaV1.4 double mutants do not appear to differ from those of CaV1.4 single mutants (McCall & Gregg, 2008). It may be that CaV1.3 channels are present only in a subpopulation of cones. For example, CaV1.3 antibodies label M cones but not S cones in the tree shrew retina (Morgans, 1999). Subtype differences among cones also occur in salamander retina where protein kinase A (PKA) inhibits Ca2+ currents in rods and short wavelength-sensitive small single cones but enhances Ca2+ currents of long wavelength-sensitive large single cones (Stella & Thoreson, 2000). PKA activation also enhances currents from heterologously expressed CaV1.3 channels (Qu et al., 2005; Liang & Tavalin, 2007). The pharmacological properties of Ca2+ currents, including partial block by omega-conotoxin, are consistent with the presence of CaV1.3 channels in salamander cones (Wilkinson & Barnes, 1996). Antibodies to α2δ4 subunits label photoreceptor terminals (Mercer et al., 2011a), and mutations in β2 or α2δ4 subunits cause diminished ERG b-waves and CSNB, suggesting that these are the principle CaV channel accessory subunits in photoreceptors (Ball et al., 2002; Wycisk et al., 2006a,b).
Excision of the synaptic terminal diminishes rod calcium currents by 95%, indicating that most of the CaV channels are located in the terminal (Xu & Slaughter, 2005). As illustrated in Fig. 1, sites of intraterminal Ca2+ influx also colocalize with synaptic ribbons in rod photoreceptor terminals. In this example, changes in intracellular Ca2+ levels were visualized in a salamander rod using the Ca2+-sensitive dye, Oregon Green BAPTA-6F, (Invitrogen, Carlsbad, CA) (KD = 3 μM Ca2+), and the ribbon was labeled with a rhodamine-conjugated consensus peptide that binds selectively to the ribbon protein, ribeye (Zenisek et al., 2004). Both dyes were introduced into the rod through a patch-clamp pipette. Sites of Ca2 + influx and ribbons are also colocalized in cones (Choi et al., 2008). Consistent with close proximity between CaV channels and the ribbon, immunohistochemical studies provide evidence for clusters of CaV channels at the base of the ribbon (Nachman-Clewner et al., 1999; Morgans, 2001; Morgans et al., 2005; Specht et al., 2009; Mercer et al., 2011b). Furthermore, individual CaV channels labeled with quantum dots conjugated to α2δ4 antibodies colocalize with fluorescently labeled ribeye consensus peptides (Mercer et al., 2011a), and immunoelectron micrographs show CaV channels along the synaptic ridge at the base of the ribbon (tom Dieck et al., 2005; Kersten et al., 2010).
Fig. 1.
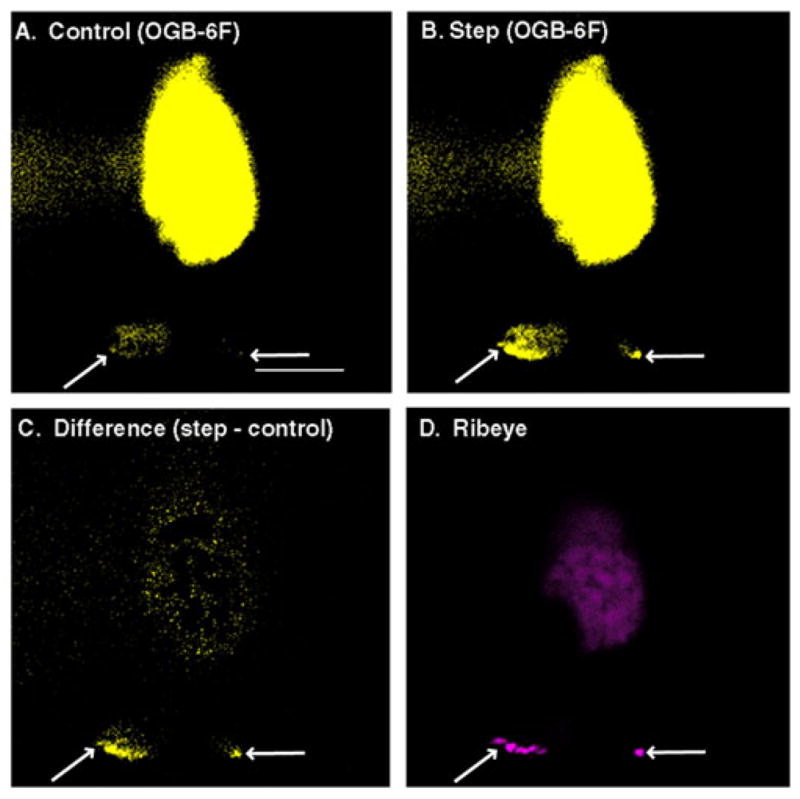
Colocalization of Ca2+ channels to ribbon release sites. A voltage-clamped rod from a salamander retinal slice preparation was loaded with Oregon Green BAPTA-6F (OGB-6F) (200 μM) to visualize intracellular Ca2+ changes and a rhodamine-conjugated ribeye-binding peptide (50 μM; Zenisek et al., 2004) to visualize the ribbon. Both were introduced into the rod through a patch pipette. To activate L-type Ca2+ channels, the voltage-clamped rod was depolarized from −70 to −10 mV for 100 ms. Panel (A) shows a single confocal section (55 ms exposure) illustrating OGB-6F fluorescence in the rod prior to the test step. Panel (B) shows OGB-6F fluorescence in the same confocal section during the depolarizing test step. The difference image (test step minus control) in panel (C) shows localized hot spots of Ca2+ increase at the base of the synaptic terminal (arrows). Panel (D) shows synaptic ribbons labeled with rhodamine-conjugated ribeye-binding peptide in the same rod. Note that locations of the ribbons match the sites of Ca2+ influx in the two terminals of the rod. OGB-6F was imaged with 488 nm excitation and 525 nm emission filters, and rhodamine was visualized using 568 nm excitation and 607 nm emission filters. Scale bar = 10 μm.
Ca2+-dependent vesicle release and replenishment at photoreceptor synapses
At most synapses, Ca2+ needs to attain high micromolar levels to stimulate release, and therefore, exocytosis is typically restricted to release sites within nanodomains of high Ca2+ very close to CaV channels. A Ca2+ nanodomain is defined as the 10–100 nm range in the immediate vicinity of a Ca2+ channel where Ca2+ is not in equilibrium with fast buffers (Naraghi & Neher, 1997). Examples of synapses where release involves Ca2+ nanodomains include the squid giant synapse (Adler et al., 1991), mammalian rod bipolar cell (Jarsky et al., 2010), GABAergic basket cells in the hippocampus (Bucurenciu et al., 2008), and mature calyx of Held (Fedchyshyn & Wang, 2005; Weber et al., 2010). The release mechanism in rods and cones is much more sensitive to Ca2+ than at other synapses, and exocytosis can thus be triggered by submicromolar Ca2+ levels (Rieke & Schwartz, 1996; Thoreson et al., 2004; Sheng et al., 2007; Duncan et al., 2010). The high Ca2+ afffinity of the release mechanism at photoreceptor synapses suggests that release might involve spatially averaged Ca2+ levels in the terminal and not the high Ca2+ levels found in nanodomains. However, experiments comparing effects of the diffusible synthetic Ca2+ chelators ethylene glycol tetraacetic acid (EGTA) and 1,2-bis (o-aminophenoxy) ethane, N,N,N′,N′-tetraacetic acid (BAPTA) on release indicate that release sites are within 50–100 nm of Ca2+ channels in amphibian cones (Mercer et al., 2011b). This was shown by the finding that when 0.5 mM EGTA, 5 mM EGTA, or 5 mM BAPTA were introduced into cones, only 5 mM BAPTA reduced the amplitude of glutamatergic post-synaptic currents in second-order neurons (Mercer et al., 2011b). Comparisons of the calcium current to the size of the readily releasable pool of synaptic vesicles suggest that only a few Ca2+ channel openings accompany fusion of each vesicle during the first few milliseconds of release from cones (Bartoletti et al., 2010). Thus, when cones are depolarized by a light decrement, the release of vesicles is triggered by the emergence of nanodomains of [Ca2+]i beneath open CaV channels close to ribbon release sites, providing a tight coupling between channel opening and vesicle fusion (Mercer et al., 2011b).
In darkness, cones appear to switch from a tight coupling between CaV channel opening and vesicle fusion to a mode of release whereby the rate of sustained release is governed by the rate of replenishment. At the dark potential, cones are depolarized to a membrane potential of approximately −40 mV, allowing the continued influx of Ca2+, which in turn causes a depletion of the readily releasable pool of vesicles at the base of the ribbon (Jackman et al., 2009; Bartoletti et al., 2010). Under these conditions, the rate of synaptic release is limited not by the rate of individual channel openings but by the rate at which release-ready vesicles can be replenished (Jackman et al., 2009). The replenishment process is Ca2+ dependent (Babai et al., 2010) and so, even when the releasable pool is depleted in darkness, release is nonetheless regulated by Ca2+ influx through CaV channels. However, the Ca2+-dependent sites involved in replenishment are >200 nm from CaV channels, further than the distance between release sites and channels (Babai et al., 2010). Because these Ca2+-dependent sites of replenishment are not located within Ca2+ nanodomains beneath individual channels, replenishment is regulated by the spatially averaged levels of Ca2+ entering through multiple channels. In bright light, random channel openings are rare, and individual channel openings are closely synchronized with decrements in light intensity. Under these conditions, close coupling between channel openings and release events can provide an accurate read out of stimulus-dependent changes in membrane potential. When cones are depolarized in darkness, many Ca2+ channel openings occur at random intervals. Controlling release under these conditions by regulating the rate of replenishment offers the benefit of reducing noise that can be introduced by stochastic channel openings.
Evidence that the Ca2+-dependent replenishment mechanism involves sites >200 nm from CaV channel suggests that proteins along the ribbon may participate in this process. At the calyx of Held, calmodulin plays a key role in the Ca2+-dependent acceleration of replenishment (Sakaba & Neher, 2001). One target of calmodulin is the GTP-binding protein, Rab3a (Park et al., 2002), and Rab3a has been shown to play a role in replenishment (Leenders et al., 2001). Rab3a interacts with RIM2 proteins along the ribbon and has been proposed to tether vesicles to the ribbon (Fukuda, 2003; Dulubova et al., 2005; tom Dieck et al., 2005; Deguchi-Tawarada et al., 2006; Uthaiah & Hudspeth, 2010). Along with calmodulin, Rab3a is an appealing candidate for regulating replenishment at the cone ribbon.
Synaptic Ca2+ buffering and extrusion
Ca2+ influx can induce vesicle release and regulate replenishment, but rising [Ca2+]i levels are balanced by Ca2+ extrusion and buffering mechanisms. One of the first studies of synaptic Ca2+ extrusion in photoreceptors was performed by Morgans et al. (1998) to determine whether the Na+/Ca2+ exchanger or the plasma membrane Ca2+ ATPase (PMCA) tempered increases in [Ca2+]i following a depolarizing stimulus. PMCA is considered critical for Ca2+ handling at other synapses (Juhaszova et al., 2000; Wanaver-becq et al., 2003; Gover et al., 2007), but the Na+/Ca2+ exchanger regulates Ca2+ levels in the outer segments of rod photoreceptors (Thoreson et al., 1997; Lagnado et al., 1998; Krizaj & Copenhagen, 2002). Morgans et al. (1998) demonstrated that the PMCA handled Ca2+ extrusion in terminals of cone photoreceptors from the tree shrew, and that photoreceptors in the tree shrew, mouse, goldfish, and rat express a PMCA protein using a pan-PMCA channel antibody. Labeling with PMCA antibodies is found just above antibodies to CaV1.3 channels, colocalized with presynaptic post-synaptic density-95 (PSD-95) complexes and the Crumbs complex protein membrane palmitoylated protein 4 (MPP4) (Yang et al., 2007; Aartsen et al., 2009). Therefore, it seems reasonable to suggest that PMCA transporters lie some distance from synaptic release sites to avoid quenching brief Ca2+ signals in the active zone during dim light conditions but capable of limiting global increases in [Ca2+]i throughout the terminal. Similar PMCA expression patterns are found in the retinas of mice (Krizaj et al., 2002; Cia et al., 2005; Johnson et al., 2007), tiger salamanders (Krizaj et al., 2003, 2004), and chickens (Tolosa de Talamoni et al., 2002). However, using a C57Bl/6J mouse model, Johnson et al. (2007) found that PMCA is critical for Ca2+ extrusion in rod terminals, whereas the Na+/Ca2+ exchanger is more important for maintaining Ca2+ homeostasis in cone terminals. This corroborates the finding that PMCA2 knockout mice exhibit deflcits only in rod-mediated ERG responses (Duncan et al., 2006) and calls into question the idea that PMCAs are solely responsible for Ca2+ homeostasis in the synaptic terminals of all photoreceptor cells.
Although Ca2+ extrusion can modulate Ca2+ homeostasis at photororeceptor synapses, endogenous buffering systems are also important (Thoreson, 2007). Extrusion of Ca2+ through PMCA or the Na+/Ca2+ exchanger is slow compared to the kinetics of Ca2+ buffering (Klingauf & Neher, 1997; Thoreson et al., 1997; Krizaj et al., 2004; Mercer et al., 2011b). Local calcium buffering shapes diffusion of Ca2+ influx at the active zone (Klingauf & Neher, 1997) and thereby shapes the kinetics of neurotransmission. Endogenous neuronal buffers include calbindin, calretinin, and parvalbumin (Bennis et al., 2005; Rusakov, 2006). Although there is significant species-to-species variability, cone photoreceptors in most species contain calbindin, a fast low mobility buffer, while calretinin and parvalbumin are only observed in the photoreceptors of a handful of species.
The calcium-binding proteins CaBP4 (Haeseleer et al., 2004; Lee et al., 2007) and calmodulin (Miller, 1991; Griessmeier et al., 2009) are also present at photoreceptor synapses and can buffer relatively high (>1 μM) increases in [Ca2+]i. CaBP4 shifts the voltage activation range of CaV1.4 towards more negative potentials, allowing channels to be effectively activated by physiological voltage levels below −40 mV (Haeseleer et al., 2004). CaBP4 weakly inhibits calcium-dependent inactivation of CaV1.3 (Cui et al., 2007) and is a phosphorylation target of intracellular kinase pathways (Lee et al., 2007).
The synaptic ribbon
A striking feature of many sensory synapses is the presence of a large electron-dense structure known as the ribbon. Although its function in vesicle release is not yet fully understood, it is believed that the ribbon assists sensory neurons in maintaining graded tonic neurotransmission. Ribbons were initially proposed to function as conveyor belts that help transport vesicles to the active zone for exocytosis (Bunt, 1971). However, cytosolic ATP is not needed to release vesicles that have already been primed and attached to the ribbon, indicating that vesicle movement along the ribbon does not involve a molecular motor requiring ATP hydrolysis (Heidelberger, 1998). In fact, rather than accelerating vesicle replenishment at the base of the ribbon, the observation that vesicles are depleted from the base of the ribbon in darkness suggests that ribbons actually slow vesicle delivery (Jackman et al., 2009). Other proposed functions include the possibility that ribbons may prime vesicles for release, act like a flytrap to capture and deliver vesicles to the active zone, assist with endocytosis of previously released vesicles, or coordinate multivesicular release (Prescott & Zenisek, 2005).
Ribbons are found in bipolar and photoreceptor cells of the retina, pineal photoreceptors, hair cells, and electroreceptors. Ultrastructural studies show differences in the shapes of ribbons in different cell types. For example, ribbons of rod and cone photoreceptors and bipolar cells are typically more plate-like, whereas ribbons in hair cells are often spherical in shape (tom Dieck & Brandstätter, 2006). Synaptic ribbons in vertebrate photoreceptors are first expressed as similar precursor spheres (Regus-Leidig et al., 2009). Spherical ribbons are also found in photoreceptors from the hagfish retina suggesting that this may represent the primordial shape of the ribbon (Holmberg & Ohman, 1976). The main protein in the ribbon, ribeye, does not appear to be present in invertebrates, but photoreceptors from Drosophila melanogaster have T-bar structures that are sometimes compared to ribbons (Fouquet et al., 2009; Hamanaka & Meinertzhagen, 2010). Conventional vertebrate synapses also sometimes exhibit structures that resemble ribbons, such as spherical structures at the frog neuromuscular junction and punctate electron-dense projections at synapses between human hippocampal neurons (Zhai & Bellen, 2004).
Ribbons at synapses of vertebrate rod and cone photoreceptors are typically 30–40 nm wide and project 200–400 nm vertically into the cytoplasm (reviewed by Heidelberger et al., 2005; Sterling & Matthews, 2005; Schmitz, 2009). Cone ribbons have a base extending 200–700 nm along the membrane, whereas rod ribbons are 800–1500 nm long. Bipolar cell ribbons are normally smaller than photoreceptor ribbons (Sterling & Matthews, 2005). Terminals of mammalian rods typically contain one ribbon, whereas amphibian rods have multiple ribbons (Carter-Dawson & LaVail, 1979; Townes-Anderson et al., 1985; Migdale et al., 2003; Zampighi et al., 2011), but both mammalian and nonmammalian cones have larger terminals with a dozen or more ribbons. Vesicles tethered along the bottom one or two rows of the ribbon contact the plasma membrane and appear to form a readily releasable pool of vesicles that can fuse rapidly following an increase in [Ca2+]i (Sterling & Matthews, 2005; Bartoletti et al., 2010). A single ribbon at a rod synapse tethers ~25 vesicles to the plasma membrane at the base of the ribbon with a total tethered pool of ~700 vesicles (Thoreson et al., 2004; Heidelberger et al., 2005), whereas cone ribbons have a readily releasable pool of ~20 vesicles and a total tethered pool of ~110 vesicles (Innocenti & Heidelberger, 2008; Bartoletti et al., 2010).
Ribeye
Technological advances over the past 20 years have allowed scientists to characterize many of the key structural and functional domains of photoreceptor ribbons. The primary protein constituent of the ribbon is ribeye, which was first identified, characterized, and cloned by Schmitz et al. (2000). Ribeye is composed of a novel proline-rich A domain and a carboxyterminal B domain that is almost identical in sequence to the nuclear repressor, CtBP2. Both protein products are encoded by a single gene, but ribeye is expressed only at ribbon synapses (Wan et al., 2005; Magupalli et al., 2008). Synaptic ribbons have been visualized by fluorescent and electron microscopy using antibodies to ribeye (Schmitz et al., 2000), antibodies to CtBP2 (tom Dieck et al., 2005; Uthaiah & Hudspeth, 2010), and fluorescently labeled ribeye consensus peptides (Zenisek et al., 2004; Choi et al., 2008; LoGiudice et al., 2008; Zenisek, 2008).
The A domain of ribeye constitutes most of the internal scaffold of the ribbon structure, while the B domain faces into the cytoplasm where it can interact with other ribbon-related proteins (Schmitz et al., 2000; Alpadi et al., 2008; Magupalli et al., 2008). The scaffold of the ribbon is constructed from homotypic and heterotypic interactions between A and B domains of adjacent ribeye molecules. Heterotypic interactions between A and B domains are inhibited by nicotinamide adenine dinucleotide [NAD(H)] (Magupalli et al., 2008).
Ribbon-associated proteins
Although the backbone of a ribbon is constructed from interlinking ribeye peptides, a number of accessory cytomatrix proteins at the synapse appear to help organize vesicle trafficking near the ribbon (Zanazzi & Matthews, 2009). Directly beneath the ribbon in photoreceptor cells, but not most other ribbon synapses, is a trough-like electron-dense structure called the arciform density (Dowling & Boycott, 1966; Gray & Pease, 1971; Lasansky, 1973; Raviola & Gilula, 1975; Raviola & Raviola, 1982). The arciform density may help to anchor the ribbon to the plasma membrane and perhaps shape the diffusion of Ca2+ entering through Ca2+ channels beneath it, but the molecular constituents of the arciform density have not been identified.
The protein bassoon has received a great deal of attention as a ribbon coorganizer (Dick et al., 2003; tom Dieck et al., 2005; Frank et al., 2010; Regus-Leidig et al., 2010), although it is not localized exclusively to ribbon synapses (tom Dieck et al., 1998; Richter et al., 1999; Altrock et al., 2003; Hallermann et al., 2010). Bassoon is a large 420 KD protein that comigrates with ribeye in precursor spheres during development (Regus-Leidig et al., 2009) and has been localized to the base of the synaptic ribbon (Brand-stätter et al., 1999; tom Dieck et al., 2005). Bassoon may function as a ribbon-anchoring protein, but it is not clear if it is directly associated with the arciform density (Zanazzi & Matthews, 2009). Bassoon knockout animals have detached ribbons, deformed Ca2+ channel clusters, and impaired vesicle attachment to the membrane in both the retina (Dick et al., 2003; tom Dieck et al., 2005) and auditory hair cells (Khimich et al., 2005; Frank et al., 2010). In the retina, bassoon knockout mice exhibit diminished ERG b-waves. In hair cells of bassoon knockout mice, fast synchronous release is inhibited more strongly than slower sustained release (Khimich et al., 2005), suggesting a role for nonribbon sites in maintaining tonic neurotransmission in these cells. At ribbon and nonribbon synapses, bassoon mutants also show defects in vesicle replenishment, particularly for fast refilling (Frank et al., 2010; Hallermann et al., 2010). Bassoon and ribeye often colocalize with the protein piccolo (Cases-Langhoff et al., 1996), in both initial precursor spheres (Dresbach et al., 2006; Regus-Leidig et al., 2010) and fully functional ribbon-associated active zones (Dick et al., 2001; tom Dieck et al., 2005). Bassoon and piccolo share a similar structure and may share similar functions (Takao-Rikitsu et al., 2004; Mukherjee et al., 2010).
Cytomatrix at the active zone-associated structural protein (CAST) helps to organize the active zone at many synapses by interacting with bassoon, piccolo, RIM1, and RIM2, as well as indirectly with Munc13 (Hida & Ohtsuka, 2010). CAST is concentrated at the base of the ribbon along with RIM2, piccolo, and bassoon (Takao-Rikitsu et al., 2004; Deguchi-Tawarada et al., 2006). RIM proteins interact with many of the key proteins in release including synaptotagmin 1, SNAP-25, Rab3A, Munc13, and CaV channels (Matteoli et al., 1991; Geppert et al., 1997; Wang et al., 1997; von Kriegstein et al., 1999; Coppola et al., 2001; Schoch et al., 2002; Deguchi-Tawarada et al., 2006; Gracheva et al., 2008; Kaeser et al., 2011).
RIM1 and RIM2 are differentially located with RIM1 found along the face of the ribbon and RIM2 clustered at the base of the ribbon (tom Dieck et al., 2005). ELKS has a high degree of homology to CAST and shares similar binding partners including bassoon and RIM1 (Inoue et al., 2006), but it is not concentrated as tightly at the ribbon base (Deguchi-Tawarada et al., 2006). This suggests that RIM2 may interact with CAST near the base of the ribbon, whereas interactions between RIM1 and ELKS may predominate further away from the base. CAST, ELKS, bassoon, piccolo, and RIM all appear to interact with an N-terminal region of Munc13. Interactions among these proteins may form an important node for organizing the synaptic active zone (Wang et al., 2009; Hida & Ohtsuka, 2010).
Interactions between RIM proteins and the C-terminal region of CaV channel β subunits promote the clustering CaV channels close to synaptic vesicles (Coppola et al., 2001; Kiyonaka et al., 2007; Gebhart et al., 2010; Han et al., 2011; Kaeser et al., 2011). Interactions between CaV channels and RIM proteins can also be promoted by intermediary RIM-binding proteins (Hibino et al., 2002).
CaV β subunits possess SH3-HOOK-GK domains that can potentially mediate multiple binding interactions with cytoskeletal elements, classifying them as membrane-associated guanylate kinase (MAGUK) proteins (Chen et al., 2004; Takahashi et al., 2004; Buraei & Yang, 2010). Although CaV channel β subunits are MAGUKs with homology to other scaffolding proteins like PSD-95, their interactions with other cytoskeletal proteins are limited because the guanylate kinase domain is normally shielded by interactions with the α1 pore-forming subunit and the SH3 domain is shielded by other domains of the β subunit (Buraei & Yang, 2010).
In addition to potential cytoskeletal interactions with CaV β subunits, the pore-forming α1 subunit of CaV1.3 channels has a carboxyterminal sequence that recognizes class I PDZ domains (Zhang et al., 2005). CaV1.3 channels in photoreceptors can interact through this sequence with PDZ domains on the Usher proteins whirlin (Kersten et al., 2010) and harmonin (Gregory et al., 2011). This recognition sequence may also facilitate interactions between Ca2+ channels and other PDZ domain proteins (Nourry et al., 2003; Feng & Zhang, 2009).
Unc119 is located near the base of the ribbon where it plays a role in endocytosis (Karim et al., 2010) and interacts with both ribeye and the calcium-binding protein CaBP4 (Higashide et al., 1998; Alpadi et al., 2008; Haeseleer, 2008). CaBP4 can in turn interact with CaV1.4 or CaV1.3 channels (Haeseleer et al., 2004; Maeda et al., 2005; Yang et al., 2006), and these interactions may further help to localize CaV channels near the base of the ribbon.
Endocytic proteins like dynamin, amphiphysin, and clathrin are also expressed at ribbon synapses (Ullrich & Südhof, 1994: Sherry & Heidelberger, 2005). An amphiphysin splice variant has been identified in retinal ribbon synapses (Hosoya et al., 2004). Synaptojanin is a polyphosphoinositide phosphatase that plays a role in clathrin-mediated endocytosis (Song & Zinsmaier, 2003; Chang-Ileto et al., 2011). Synaptojanin antibodies label cone terminals more prominently than rod terminals (Holzhausen et al., 2009), and loss of synaptojanin produces floating ribbons in cone terminals (Van Epps et al., 2004). Unlike cone synapses, rod synapses develop normally without synaptojanin suggesting different roles for this protein and thus perhaps different endocytic pathways in the two cell types (Holzhausen et al., 2009).
Exocytotic proteins
There is evidence that release from ribbon synapses of inner hair cells does not involve neuronal SNARE proteins (Nouvian et al., 2011). However, photoreceptor ribbon synapses, like those of most other neurons, appear to employ SNARE proteins during vesicle fusion: synaptobrevin (VAMP1 and 2), SNAP-25, and syntaxin (Ullrich & Südhof, 1994; Brandstätter et al., 1996a,b; Greenlee et al., 1996, 2001; von Kriegstein et al., 1999; Bergmann et al., 2000; Morgans, 2000; Sherry et al., 2001, 2006; Heidelberger et al., 2003). The v-SNARE, synaptobrevin, is located exclusively on synaptic vesicles. Immunoelectron micrographs suggest that the t-SNARE, SNAP-25, may be present not only on the plasma membrane but also on vesicles along the ribbon (Brändstatter et al., 1996b; Morgans et al., 1996; Morgans & Brändstatter, 2000). It is possible that vesicular SNAP-25 may promote vesicle priming or compound fusion prior to synaptic release. Ribbon synapses also possess Munc18 proteins, which assist in assembling the SNARE complex and priming vesicles (Ullrich & Südhof, 1994). One difference between photoreceptors and most other neurons is the reliance on syntaxin 3 rather than the syntaxin 1 isoform used at many synapses (Morgans et al., 1996; Curtis et al., 2008). Additionally, retinal ribbon synapses use complexins 3 and 4 rather than complexins 1 and 2 used at most other terminals (Reim et al., 2005; Zanazzi & Matthews, 2010). In complexin 3/4 knockout mice, ~25% of the synaptic terminals exhibit free floating spherical ribbons perhaps as a result of altered synaptic activity (Reim et al., 2005).
The identity of the calcium sensor molecules that regulate exocytosis from photoreceptors is unclear. Experiments on non-mammalian rods and cones show that the sensor exhibits an unusually high affinity for Ca2+ with a threshold of 400 nM and low cooperativity of approximately two Ca2+ ions (Rieke & Schwartz, 1996; Thoreson et al., 2004; Sheng et al., 2007; Duncan et al., 2010). This is quite different from release at synapses employing synaptotagmin 1, which show a cooperativity of five Ca2+ ions and a requirement for much higher Ca2+ levels (Heidelberger et al., 1994; Bollmann et al., 2000; Schneggenburger & Neher, 2000; Beutner et al., 2001). Kreft et al. (2003) found that salamander rod terminals could be labeled with an antibody to synaptotagmin 1, but other synaptotagmin 1 antibodies do not label terminals of photoreceptors in salamander or goldfish (Berntson & Morgans, 2003; Heidelberger et al., 2003; Fox & Sanes, 2007). By contrast with these results in lower vertebrates, antibodies to synaptotagmin 1 consistently label photoreceptor ribbon synapses in the OPL of mammalian and chick retina (Greenlee et al., 1996; Bergmann et al., 2000; Berntson & Morgans, 2003; Heidelberger et al., 2003; von Kriegstein & Schmitz, 2003; Lazzell et al., 2004; Fox & Sanes, 2007; Wahlin et al., 2008). Antibodies for synaptotagmin 2 do not label photoreceptor terminals in chick, mouse, salamander, or goldfish, although there is evidence for labeling of terminals in ray retina (Fox & Sanes, 2007). In hair cells, it has been proposed that otoferlin or synaptotagmin 4 might be the calcium sensor (Roux et al., 2006; Johnson et al., 2010). Otoferlin is absent from photoreceptor ribbon synapses (Uthaiah & Hudspeth, 2010); the distribution of synaptotagmin 4 in the retina has not been examined.
Cytoskeletal components
Ribeye and ribbon-associated proteins interact extensively with the intracellular cytoskeleton. Much of the early work elucidating cytoskeletal networks in photoreceptors was performed using species whose rods and cones show photosensitive contractile motion (Burnside, 1986; Nagle et al., 1986) or regenerative properties that require cytoskeletal-dependent trafficking (Mandell et al., 1993; Vecino & Avila, 2001; Hasegawa et al., 2007). These studies focused more on changes in cellular morphology than on specific molecular interactions, but they offered insight into the subcellular organization of the cytoskeleton and generally agreed that there are moderate levels of actin within the synaptic terminals of both rod and cone photoreceptors (Nagle et al., 1986; Schmitz & Drenckhahn, 1993; Schmitz et al., 1993; Katsumata et al., 2009). Actin and microtubule networks in photoreceptors help to stabilize active zone components and to aid in cargo trafficking to and from the synapse (Schmitz & Drenckhahn, 1997; Zanazzi & Matthews, 2009).
Interactions between actin and various binding partners help to sculpt pre- and postsynaptic structure (Doussau & Augustine, 2000; Hotulainen & Hoogenraad, 2010). For example, actin interacts with dystrophin and closely related spectrin proteins, all of which are found in photoreceptor terminals (Lazarides et al., 1984; Isayama et al., 1991; Spencer et al., 1991; Schmitz et al., 1993; Ueda et al., 1995, 1998; Drenckhahn et al., 1996; Blank et al., 1997, 1999; Morgans, 2000; Jastrow et al., 2006; Uthaiah and Hudspeth, 2010). One of the more common binding partners for actin in most presynaptic terminals is synapsin, but synapsin is absent from photoreceptor terminals (Mandell et al., 1990; Koontz & Hendrickson, 1993). Its absence at ribbon synapses is thought to explain the high mobility of synaptic vesicles at ribbon synapses (Holt et al., 2004; Rea et al., 2004). The network of actin fibers can exhibit tremendous plasticity as shown in hair cells where the entire actin cytoskeleton turns over every 48 h (Schneider et al., 2002). The regulation of actin varies among different retinal cell types but often involves Ca2+-dependent and CaV-associated processes (Fukuda et al., 1981; Johnson & Byerly, 1993; Job & Lagnado, 1998; Schubert & Akopian, 2004, 2006; Oertner & Matus, 2005; Cristofanilli et al., 2007; Mizuno et al., 2010). At photoreceptor synapses, single particle tracking studies show that individual CaV channels diffuse more freely after the disruption of actin with cytochalasin, raising the possibility that an actin network helps to maintain the spatial position of photoreceptor synaptic proteins (Mercer et al., 2011a).
In addition to actin, the other two primary cytoskeletal proteins, intermediate filaments and microtubules, are present in photoreceptors (Gray, 1976; Nagle et al., 1986). Intermediate filaments are present in the axon and perinuclear region, whereas microtubules are found throughout the cell (Nagle et al., 1986). At many synapses, microtubules play an important role in vesicle delivery (Elluru et al., 1995; Lin-Jones et al., 2003; Gonzalez-Bellido et al., 2009) and, consistent with such a possibility, they can sometimes be observed close to the ribbon and ribbon-associated vesicles (Gray, 1976; Nagle et al., 1986; Zanazzi & Matthews, 2009).
Deficits in cytoskeletal and cytomatrix proteins can cause neurological defects including Usher syndrome (Williams, 2008), which is the most common form of deaf-blindness. Clinically, there are three subtypes of Usher syndrome, caused by at least 12 identified gene loci (Kremer et al., 2006; Maerker et al., 2008; Friedman et al., 2011; Richardson et al., 2011). Seven USH1 loci have been identified to date and are responsible for the most severe form of deaf-blindness syndrome (Ahmed et al., 2003; Yan & Liu, 2010). These gene regions encode for various cytomatrix molecules including the scaffold proteins harmonin (USH1C) and “scaffold protein containing ankyrin repeats/SAM domain” (USH1G), the motor protein myosin VIIa (USH1B), and the cell–cell adhesion molecules cadherin 23 (USH1D) and protocadherin 15 (USH1F) (Reiners et al., 2003, 2005a, 2006; Adato et al., 2005; El-Amraoui & Petit, 2005). Three USH2 loci encode for the scaffold proteins usherin (USH2A) and whirlin (USH2D) as well as very large G-protein-coupled receptor 1b (USH2C) (Reiners et al., 2005b; Yang et al., 2010). The downstream product of the lone USH3 gene is the synaptic protein clarin-1 (Adato et al., 2002; Isosomppi et al., 2009). Many of the Usher proteins interact with one another, as well as with the actin cytoskeleton (Boëda et al., 2002; Inoue et al., 2006; Rzadzinska et al., 2004) and cell–cell adhesion molecules (Küssel-Andermann et al., 2000; Reiners et al., 2006). In hair cell stereocilia and the connecting cilium in photoreceptors, these protein networks appear to be important for intracellular protein trafficking and structural organization. In addition to being found in cilia, whirlin, myosin VIIa, harmonin, cadherin 23, protocadherin 15, and clarin-1 are also present in photoreceptor terminals where they likely help organize the structure of the ribbon synapse (El-Amraoui et al., 1996; Reiners et al., 2003, 2005a; Williams et al., 2009; Zallocchi et al., 2009).
Lipid rafts
Along with cytoskeletal interactions, the distribution of lipids within the plasma membrane is a major mechanism for segregating and compartmentalizing membrane proteins (Lenne et al., 2006). Using electron microscopy and a cholesterol-binding antibiotic, filipin, Cooper and McLaughlin (1984) found that photoreceptors display a high concentration of cholesterol in lipid rafts at margins of the ribbon-style active zone. Consistent with the presence of lipid rafts in photoreceptor terminals, Mercer et al. (2011a) found that labeling of lipid raft ganglioside GM1 glycoproteins with fluorescein isothiocyanate-conjugated toxin B stained the OPL. Cholesterol-containing lipid rafts were absent from nonribbon locations in presynaptic terminals and were not observed in the post-synaptic dendrites of horizontal and bipolar cells (Cooper & McLaughlin, 1984). P-/Q-type CaV channels and α2δ CaV channel accessory subunits have been shown to localize preferentially to lipid rafts (Taverna et al., 2004; Davies et al., 2006), suggesting that membrane cholesterol may assist in clustering ion channels close to vesicle release machinery (Lang et al., 2001; Gil et al., 2004; Salaün et al., 2005; Taverna et al., 2007; Linetti et al., 2010). Consistent with such a possibility at photoreceptor synapses, depleting membrane cholesterol from photoreceptors expanded the synaptic confinement area for CaV channels (Mercer et al., 2011a). These results also support the hypothesis that lipid rafts act as diffusion barriers to limit the lateral mobility of membrane proteins (Renner et al., 2009).
Summary of protein interactions within the photoreceptor ribbon synapse
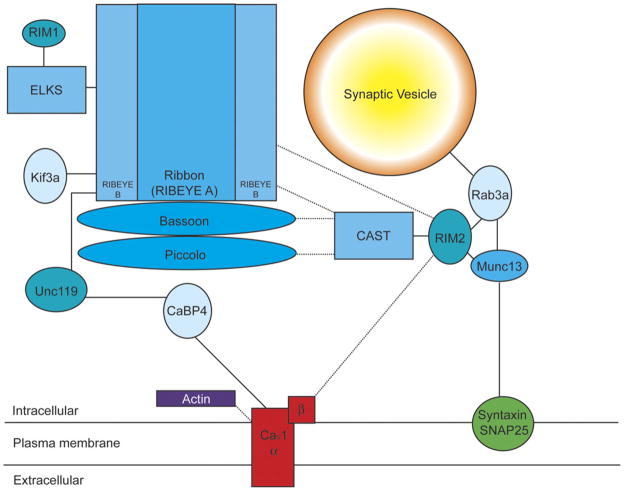
Fig. 2 illustrates the coassembly of proteins at the photoreceptor ribbon synapse. Interactions among ribeye A domains form the backbone of the ribbon (Schmitz et al., 2000; Magupalli et al., 2008), whereas ribeye B domains on the outer face of the ribbon appear to link it to scaffold proteins at the base such as bassoon, piccolo, and Unc119 (Alpadi et al., 2008). Bassoon and piccolo also interact with CAST and the homologous protein ELKS (Takao-Rikitsu et al., 2004; Tokoro et al., 2007). The protein “hub” formed by CAST, bassoon, piccolo, RIM, and Munc13 proteins helps to cluster a number of proteins near the ribbon (Ohara-Imaizumi et al., 2005; Inoue et al., 2006; Wang et al., 2009; Hida & Ohtsuka, 2010). Contacts between SNARE proteins and Munc13 may help to localize SNARE complexes beneath the ribbon (Betz et al., 1997; Guan et al., 2008; Deng et al., 2011), while interactions between RIM and Munc13 with Raba may link this ribbon complex directly to vesicles (Dulubova et al., 2005). CaV channels can associate with the ribbon by virtue of interactions with RIM proteins, CaBP4, and Unc119 (Hibino et al., 2002; Kiyonaka et al., 2007; Han et al., 2011; Kaeser et al., 2011). Many other proteins are found at photoreceptor ribbon synapses (Uthaiah and Hudspeth, 2010), and thus, interactions with other synaptic, scaffolding, and motor proteins (Muresan et al., 1999; tom Dieck et al., 2005; Katsumata et al., 2009) are also likely to help organize CaV channels, synaptic vesicles, and fusion machinery at the ribbon synapse.
Fig. 2.
Protein–protein interactions at the photoreceptor ribbon synapse. The synaptic ribbon anchors to bassoon and piccolo at the terminals of rods and cones. The vertical ribbon is complexed at the base through CAST to a hub including RIM2, Rab3a, and Munc13. This synaptic node may help to position primed vesicles close to CaV channels and the fusion machinery (e.g., syntaxin, SNAP-25). CaV channels also appear to interact with the actin cytoskeleton and, by virtue of interactions with CaBP4, RIM, and Unc119, may couple directly to the ribbon itself. Kif3a and a complex of ELKS and RIM1 appear to be located at more distant sites up the vertical face of the ribbon. In Figs. 2 and 3, solid lines show direct interactions and dashed lines show putative interactions, for which direct interactions have not yet been definitively established.
The ECM
In addition to interactions with intracellular proteins and intra-membrane proteins, active zone-associated proteins can interact with the ECM in the synaptic cleft. A number of different synaptic cleft proteins are important for positioning presynaptic release sites across from postsynaptic receptors (Specht & Triller, 2008; Feng & Zhang, 2009; Lajoie et al., 2009). We review ECM proteins, both retina specific (e.g., pikachurin, retinoschisin, and nyctalopin) and otherwise, that have been shown to help organize photoreceptor synapses and their postsynaptic contacts in the OPL.
Alterations in the proteins dystrophin and dystroglycan cause muscular dystrophy and can produce visual deficits (Straub & Campbell, 1997; Waite et al., 2009). Both dystrophin and dystroglycan are expressed at the synapses of rod and cone photoreceptors (Schmitz et al., 1993; Ueda et al., 1995; Drenckhahn et al., 1996; Blank et al., 1997, 1999; Ueda et al., 1998; Morgans, 2000; Jastrow et al., 2006). Futhermore, mutations in dystrophin and dystroglycan can cause reductions in the ERG b-wave (Cibis et al., 1993; Fitzgerald et al., 1994; Pillers et al., 1999). A retina-specific form of dystrophin, Dp260, is localized to photoreceptor synapses (D’Souza et al., 1995), and reductions in this protein cause a selective loss of dystroglycan from the OPL (Kameya et al., 1997). Dystroglycan precursor proteins are cleaved into two subunits: β-dystroglycan and α-dystroglycan. α-dystroglycan is a heavily glycosylated extracellular protein that interacts with multiple ECM partners including β-dystroglycan, pikachurin, fibulin, and laminin (Ibraghimov-Beskrovnaya et al., 1992; Talts et al., 1999; Sato et al., 2008). In addition to binding to α-dystroglycan outside the cell, β-dystroglycan spans the plasma membrane and binds to dystrophin inside the cell. Dystrophin in turn interacts with the actin cytoskeleton. Disruption of β-dystroglycan causes changes in K+ channel clustering in Müller cells but does not appear to alter dystrophin localization in the outer retina or cause changes in retinal lamination patterns (Satz et al., 2009). By contrast, as discussed below, impaired interactions between α-dystroglycan and pikachurin produce significant outer retinal defects.
The ECM protein pikachurin interacts with glycosylated α-dystroglycan at photoreceptor terminals (Sato et al., 2008; Kanagawa et al., 2010; Hu et al., 2011). The presence of pikachurin is critical for establishing contacts between rods and bipolar cells (Sato et al., 2008). Pikachurin knockout mice yield viable offspring but show morphological defects in OPL organization (Kanagawa et al., 2010) and blunted ERG responses (Sato et al., 2008). Hypoglycosylation of α-dystroglycan can impede dystroglycan–pikachurin interactions, causing defects in the OPL similar to those observed in pikachurin knockout mice (Kanagawa et al., 2010; Hu et al., 2011). Although a postsynaptic protein partner for pikachurin has not been identified, these data suggest that pikachurin provides an intermediary tether between the terminal of a photoreceptor and dendrite of a bipolar cell.
Laminin isoforms have been implicated in the functional organization of the OPL and photoreceptor outer segment layer (Libby et al., 1999; Biehlmaier et al., 2007). Laminin interacts with multiple ECM proteins including α-dystroglycan and retinoschisin (Claudepierre et al., 2005; Steiner-Champliaud et al., 2006). Seven laminin subtypes have been described in the retina (Libby et al., 2000), and mutations of at least two laminin subtypes result in floating ribbons (Biehlmaier et al., 2007).
Retinoschisin is a secreted retina-specific OPL matrix protein, and mutations cause the retinopathy X-linked retinoschisis (Grayson et al., 2000; Tantri et al., 2004; Vijayasarathy et al., 2008). This protein is a large disulfide-linked ECM organizing protein, with a critical adhesion site called the discoidin domain. Defects in the discoidin domain of retinoschisin disrupt trafficking and secretion (Wu & Molday, 2003) and are thought to contribute to developmentally dependent changes in retina morphology and subsequent retinal dysfunction (Weber et al., 2002; Takada et al., 2004, 2008). The discoidin domain of retinoschisin can interact with a number of binding partners: anionic phospholipids (Vijayasarathy et al., 2007; Kotova et al., 2010), galactose (Dyka et al., 2008), Na+/K+-ATPase (Molday et al., 2007; Shi et al., 2009; Friedrich et al., 2011), photoreceptor CaV channels (Shi et al., 2009), laminin, alpha crystallin, and the binding partner for peanut agglutinin (Steiner-Champliaud et al., 2006). Like pikachurin, a postsynaptic binding partner for retinoschisin has not been identified, but retinoschisin is nevertheless essential for proper development and function of the retina (Park et al., 2009; Sergeev et al., 2010).
The retina-specific ECM protein nyctalopin, derived from the NYX gene, has been implicated in a form of CSNB (Bech-Hansen et al., 2000; Pusch et al., 2000) and the mouse “no b-wave” (NYXnob) mutant (Gregg et al., 2003, 2007). Analysis of ERGs in CSNB patients and NYXnob mutant mice indicates a failure of transmission from photoreceptors to ON bipolar cells (Khan et al., 2005; Bahadori et al., 2006; Leroy et al., 2009). This is similar to effects of postsynaptic mutations in the glutamate receptor, mGluR6, or the transduction channel, TRPM1, at the ON bipolar synapse (McCall & Gregg, 2008; Shen et al., 2009; van Genderen et al., 2009; Koike et al., 2010; Morgans et al., 2010). Nyctalopin appears to target TRPM1 to the tips of ON bipolar cell dendrites, which may explain the similarity in ERG phenotypes when nyctalopin or TRPM1 are mutated (Cao et al., 2011; Pearring et al., 2011). Like mGluR6 and TRPM1 knockout animals, nyctalopin knockout animals show normal morphology in the OPL (Ball et al., 2003). This contrasts with mutations of presynaptic proteins (e.g., CaV channels, bassoon, or ribeye) that lead to disorganization of the OPL, including the sprouting of ectopic synapses (McCall & Gregg, 2008). The relatively normal OPL structure of NYX mutants is consistent with the hypothesis that nyctalopin is more important for maintaining the dendritic organization of ON bipolar cells, whereas the disorganization of the OPL that accompanies mutations in pikachurin or retinoschisin suggests that these proteins are more important for presynaptic organization.
Crumbs (Crb), a transmembrane ECM protein, acts as a scaffold and is a critical element in the apical–basal development of photoreceptors (reviewed in Gosens et al., 2008). Three CRB proteins have been identified in humans and mice. Deletion or missense mutations of Crumbs genes can lead to Leber’s congenital amaurosis and retinitis pigmentosa type 12 (den Hollander et al., 1999; Lotery et al., 2001; Meuleman et al., 2004; van den Hurk et al., 2005) indicating a pivotal role in the normal assembly and function of the retina. Gain-of-function mutations also suggest that proper expression of CRB during development is required to produce the normal laminar structure of the outer retina (Fan et al., 2003). The CRB protein assembly links photoreceptors to surrounding Müller glia at the outer limiting membrane, just beyond the outer nuclear layer (Mehalow et al., 2003; Van de Pavert et al., 2004). CRB1, CRB2, and CRB3 bind directly to protein 4.1 (EBP41)-L5 and MPP5 family to maintain neural–glial connectivity at adherens junctions in the outer limiting membrane (Knust & Bossinger, 2002; Meuleman et al., 2004; van de Pavert et al., 2004). At photoreceptor synapses, there is evidence for CRB2, CRB3, MPP4, MPP5, and Veli3 expression but not CRB1 expression (van de Pavert et al., 2004; Kantardzhieva et al., 2005, 2006; Stöhr et al., 2005; Aartsen et al., 2006). Among other functions, these proteins are thought to regulate the synaptic localization of PMCA proteins in rods (Yang et al., 2007).
Despite its name, PSD-95 is not limited to postsynaptic locations but can also be found presynaptically in terminals of photoreceptors (Koulen et al., 1998). PSD-95 contains PDZ domains that can interact with interact with a number of proteins (Nourry et al., 2003; Feng and Zhang, 2009). In photoreceptor terminals, PSD-95 has been shown to interact with Crumbs-related proteins (Aartsen et al., 2006), PMCA (Aartsen et al., 2009), and the putative calcium-activated chloride channel, TMEM16B (Stöhr et al., 2009). PMCA and calcium-activated chloride channels are not clustered tightly near the synaptic ribbon but distributed more diffusely throughout photoreceptor terminals (Morgans et al., 1998; Mercer et al., 2011b). PSD-95 may thus be more important for organizing proteins in surrounding parts of the synaptic terminal, whereas other proteins discussed earlier (e.g., RIM and Munc13) may be more important for organizing proteins near the ribbon.
Yamagata and Sanes (2010) showed evidence for a transsynaptic scaffold in photoreceptors involving MAGI proteins. Like PSD-95, MAGI proteins contain multiple PDZ domains (Nourry et al., 2003). MAGI proteins in the presynaptic terminals of photoreceptors appear to be linked across the synaptic cleft by extracellular Sidekick-2 proteins and cadherins to MAGI proteins in postsynaptic dendrites (Yamagata et al., 2002; Yamagata & Sanes, 2010). Sidekick-2 may be retina specific since homologous transsynaptic scaffold proteins have not yet been described at other synapses.
Summary of protein interactions with the ECM at the photoreceptor ribbon synapse
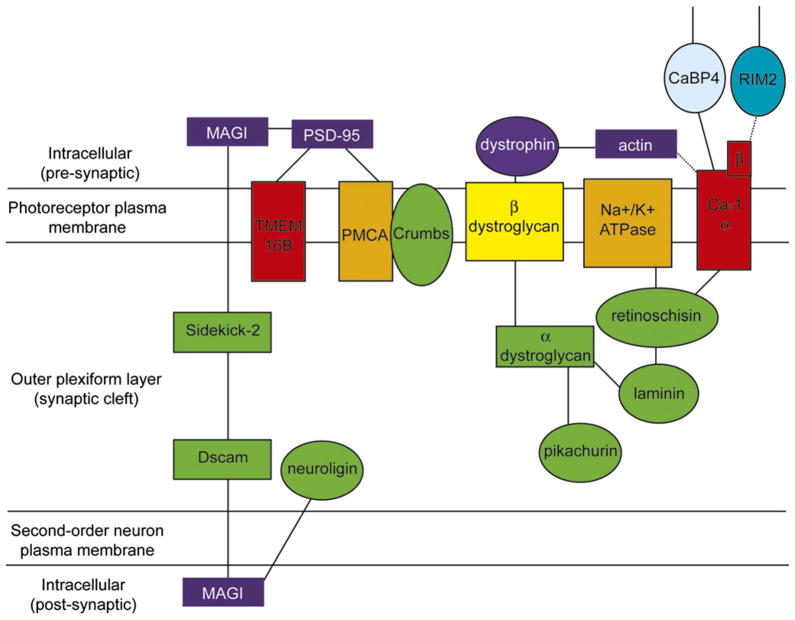
Fig. 3 summarizes various extracellular protein–protein interactions across the OPL. As described above, PSD-95 in photoreceptor terminals can link to PMCA (Aartsen et al., 2009), TMEM16B calcium-activated chloride channels (Stöhr et al., 2009), and the Crumbs complex (Yang et al., 2007). Whereas RIM and Munc13 proteins appear to be particularly important for organizing active zone proteins close to the ribbon (Fig. 2), PSD-95 may be more important for organizing proteins in neighboring nonribbon regions of the terminal. By virtue of its interactions with laminin, CaV channels, and Na+/K+ ATPases, retinoschisin may be an important node for organizing ECM proteins (Molday et al., 2007; Shi et al., 2009; Friedrich et al., 2011). Laminin and pikachurin can also interact with the dystroglycan/dystrophin complex (Claudepierre et al., 2005; Kanagawa et al., 2010). Other links among ECM proteins remain to be discovered, such as binding partners for nyctalopin and Crumbs complex proteins.
Fig. 3.
Structural organization and protein interactions across the OPL. There appear to be at least two major nodes of protein–protein interactions in the ECM within the OPL. The Sidekick-2 transsynaptic scaffold attaches MAGI proteins in apposed cells, with putative lateral presynaptic interactions to PSD-95. PSD-95 also appears to anchor the Cl(Ca) channel TMEM16B and PMCA extrusion pump at nonribbon sites in the terminal. The second hub of scaffolding interactions occurs between a transmembrane dystroglycan complex and the retina-specific ECM proteins pikachurin and retinoschisin. These complexes are linked through synaptic laminin and cytoplasmic actin, while also attaching to the extracellular face of transmembrane CaV channels and the Na+/K+-ATPase.
Dynamic changes in ribbon proteins
The need to maintain efficient and reliable neuron-to-neuron communication would appear to dictate a relatively fixed arrangement of calcium channels, vesicle release sites, and postsynaptic receptors at many synapses, including photoreceptor synapses. The many interactions among cytoskeletal, intramembrane, ribbon-associated, and ECM proteins described above also suggest a static and heavily reinforced structure. However, despite these expectations, there is evidence for a surprising degree of structural plasticity at photoreceptor ribbon synapses, some of which operates on a very rapid time scale.
As mentioned above, mutations in a number of different presynaptic proteins can cause neurodegenerative changes that are accompanied by changes in ribbon structure. For example, mutations in CaV1.4, bassoon, complexin, synaptojanin, and laminin can produce floating ribbons (Dick et al., 2003; Reim et al., 2005; tom Dieck et al., 2005; Chang et al., 2006; Biehlmaier et al., 2007). Mutations in tubby-like protein 1 (TULP1), which underlies a form of autosomal recessive retinitis pigmentosa, also causes changes in the structure of photoreceptor ribbons (Grossman et al., 2009). TULP1 appears to play a role in intracellular membrane trafficking but its role in synaptic function is unclear. Additionally, mutations in cysteine string protein alpha, a chaperone protein that prevents SNAP-25 degradation and SNARE complex formation (Sharma et al., 2011), cause photoreceptor degeneration accompanied by floating ribbons and the appearance of electron-dense aggregates (Schmitz et al., 2006). In addition to changes in ribbon structure, mutations in presynaptic CaV channel subunits (α1F, β2α, α2δ4), and the calcium channel-associated protein CaBP4 can also promote the extension of dendritic processes from bipolar and horizontal cells into the outer nuclear layer and formation of ectopic synapses (Haeseleer et al., 2004; McCall & Gregg, 2008).
More surprising than the ability of mutations and neurodegenerative changes to induce long-term structural changes, ribbons in rods and cones can alter their structure reversibly on a diurnal basis (Abe & Yamamoto, 1984; Vollrath & Spiwoks-Becker, 1996; Adly et al., 1999; Balkema et al., 2001; Spiwoks-Becker et al., 2004). At night, rod ribbons form plate-like structures, but in daytime, ribbons become much shorter and are associated with adjacent electron-dense spherical bodies. In the transition between these two forms during crepuscular conditions, the ribbons often assume a club-like appearance (Abe & Yamamoto, 1984; Vollrath & Spiwoks-Becker, 1996; Adly et al., 1999). It has recently been reported that these differences are much more pronounced in C57Bl/6J mice than Balb/cJ mice (Fuchs et al., 2011). Diurnal changes in ribbon shape appear to be driven by light, not circadian rhythms (Spiwoks-Becker et al., 2004). Diurnal structural changes have also been observed in ribbons from pineal photoreceptors (Kurumado & Mori, 1977; Kikuchi et al., 2000) and retinal bipolar cells (Hull et al., 2006). The finding that heterotypic interactions between A and B domains of ribeye are inhibited by NAD(H) has led to the proposal that metabolically driven changes in NAD(H) levels might regulate diurnal changes in ribbon structure (Magupalli et al., 2008).
Ribbons are also capable of dynamic movements on very short time scales. Bipolar cell ribbons labeled with fluorescently conjugated ribeye-binding peptide can exhibit both small (Zenisek et al., 2004; Mercer et al., 2011a) and occasional large rapid excursions through the cell (Zenisek et al., 2004).
Diurnal changes in postsynaptic processes have been described. However, these appear to occur only in fish retina where exposure to light stimulates the tips of horizontal cell dendrites (known as spinules) to extend into the cone pedicle and darkness causes them to retract (reviewed by Wagner & Djamgoz, 1993).
The possibility of rapid changes in the arrangement of pre-synaptic proteins has been investigated by visualizing the movements of individual CaV channels labeled with brightly fluorescent quantum dots (Mercer et al., 2011a). Streptavidin-coated quantum dots, bound through a biotinylated secondary antibody, were attached to the extracellular domain of the α2δ4 accessory subunit of L-type CaV channels in photoreceptors. Even though CaV channels interact with multiple proteins, single particle tracking experiments show that individual CaV channels are continuously in motion within the plasma membrane of photoreceptors (Mercer et al., 2011a). Movements of CaV channels are confined to a small region near the ribbon that roughly matches the size of the active zone, consistent with evidence that CaV channels cluster beneath the ribbon in photoreceptors and hair cells (Raviola & Gilula, 1975; tom Dieck et al., 2005; Frank et al., 2010). Some of the CaV channel movement may reflect movement of the overlying ribbon but ribbon movements are much smaller than CaV channel movements (Mercer et al., 2011a).
Depolarization-evoked fusion of vesicles at the base of the ribbon can abruptly increase CaV channel movements, propelling them away from the center of the confinement domain (Mercer et al., 2011a). The lipid constituents of vesicle membranes do not appear to differ from plasma membrane lipids (Takamori et al., 2006), suggesting that this increased channel movement is probably not due to changes in local lipid composition but more likely due to a brief expansion of the active zone membrane. Channels rebound towards the center of the confinement domain soon after the termination of exocytosis with a time course similar to that of endocytotic membrane retrieval. Thus, changes in the rate of vesicle fusion may cause the synaptic membrane to inflate and deflate like a balloon. These results are consistent with the suggestion that the fusion of synaptic vesicles may disorganize the arrangement of nearby exocytotic proteins and thereby contribute to postsynaptic depression (Neher & Sakaba, 2008; Hosoi et al., 2009; Kim & von Gersdorff, 2009). Disorganization of the synapse by altered membrane fusion and retrieval may also explain the presence of malformed ribbons produced by mutations of proteins involved in exocytosis and endocytosis.
Disruption of the actin cytoskeleton or depletion of cholesterol from cholesterol-rich lipid rafts at the base of the ribbon expand the size of the CaV channel confinement domain, allowing channels to move further away from the ribbon (Mercer et al., 2011a). Ca2+ channels are normally confined to a region within 50–100 nm of release sites (Mercer et al., 2011b). Increasing that distance would be expected to reduce the probability that an individual channel opening could induce vesicle fusion and thereby diminish the coupling efficiency between channel opening and vesicle fusion. Changes in postsynaptic receptor mobility can produce changes in synaptic strength (Heine et al., 2008; Petrini et al., 2009; Rust et al., 2010). For example, increasing the lateral mobility of AMPA receptors in the hippocampus promotes recovery from desensitization by speeding the return of undesensitized receptors to the synapse (Heine et al., 2008). Turnover of actin promoted by n-cofilin enhances AMPA receptor mobility and impairs both long-term potentiation and long-term depression (Rust et al., 2010). By analogy, we hypothesize that changes in CaV channel mobility caused by changes in endocytotic activity, actin, cholesterol-rich lipid rafts, or other partner proteins might also be a mechanism for regulating release presynaptically.
Conclusion
This review summarizes many of the different cytoskeletal, cytosolic, membrane, and ECM proteins that participate in assembling the synapse during development and maintaining the position of proteins in locations needed for efficient and reliable transmission. Photoreceptors share many intracellular and ECM proteins with other central nervous system synapses, but there are also a number of unique architectural elements (e.g., synaptic ribbon). A handful of proteins appear to form particularly important nodes for organizing proteins both in and around the active zone (e.g., RIM, Munc13, and PSD-95). Despite the many interactions among many different proteins, the synaptic terminals of photoreceptors are surprisingly dynamic. For example, ribbons can reversibly assemble and disassemble on a diurnal basis. On shorter time scales, CaV channels move continuously within the membrane and the ability of vesicle fusion to perturb these movements suggests that fusion can alter the arrangements of these and perhaps other presynaptic proteins.
In recent years, investigators have made great progress in identifying many of the key synaptic, cytoskeletal, and ECM proteins that regulate the anatomy and physiology of conventional and ribbon synapses. The more difficult task of understanding the complex functional and structural interrelationships linking these many proteins has only begun, and future studies are likely to reveal an even more complex and dynamic web of interactions.
Acknowledgments
This work was supported by Research to Prevent Blindness, NIH grant EY10542 (W.B.T.), and a University of Nebraska Medical Center Graduate Student Fellowship (A.J.M.).
References
- Aartsen WM, Arsanto JP, Chauvin JP, Vos RM, Versteeg I, Cardozo BN, Bivic AL, Wijnholds J. PSD95beta regulates plasma membrane Ca2+ pump localization at the photoreceptor synapse. Molecular and Cellular Neurosciences. 2009;41:156–165. doi: 10.1016/j.mcn.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Aartsen WM, Kantardzhieva A, Klooster J, van Rossum AG, van de Pavert SA, Versteeg I, Cardozo BN, Tonagel F, Beck SC, Tanimoto N, Seeliger MW, Wijnholds J. Mpp4 recruits Psd95 and Veli3 towards the photoreceptor synapse. Human Molecular Genetics. 2006;15:1291–1302. doi: 10.1093/hmg/ddl047. [DOI] [PubMed] [Google Scholar]
- Abe H, Yamamoto TY. Diurnal changes in synaptic ribbons of rod cells of the turtle. Journal of Ultrastructure Research. 1984;86:246–251. doi: 10.1016/s0022-5320(84)90104-7. [DOI] [PubMed] [Google Scholar]
- Adato A, Michel V, Kikkawa Y, Reiners J, Alagramam KN, Weil D, Yonekawa H, Wolfrum U, El-Amraoui A, Petit C. Interactions in the network of Usher syndrome type 1 proteins. Human Molecular Genetics. 2005;14:347–356. doi: 10.1093/hmg/ddi031. [DOI] [PubMed] [Google Scholar]
- Adato A, Vreugde S, Joensuu T, Avidan N, Hamalainen R, Belenkiy O, Olender T, Bonne-Tamir B, Ben-Asher E, Espinos C, Millán JM, Lehesjoki AE, Flannery JG, Avraham KB, Pietrokovski S, Sankila EM, Beckmann JS, Lancet D. USH3A transcripts encode clarin-1, a four-transmembrane-domain protein with a possible role in sensory synapses. European Journal of Human Genetics. 2002;10:339–350. doi: 10.1038/sj.ejhg.5200831. [DOI] [PubMed] [Google Scholar]
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. The Journal of Neuroscience. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adly MA, Spiwoks-Becker I, Vollrath L. Ultrastructural changes of photoreceptor synaptic ribbons in relation to time of day and illumination. Investigative Ophthalmology & Visual Science. 1999;40:2165–2172. [PubMed] [Google Scholar]
- Ahmed ZM, Riazuddin S, Riazuddin S, Wilcox ER. The molecular genetics of Usher syndrome. Clinical Genetics. 2003;63:431–444. doi: 10.1034/j.1399-0004.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- Alpadi K, Magupalli VG, Käppel S, Köblitz L, Schwarz K, Seigel GM, Sung CH, Schmitz F. RIBEYE recruits Mnc119, a mammalian ortholog of the Caenorhabditis elegans protein unc 119, to synaptic ribbons of photoreceptor synapses. The Journal of Biological Chemistry. 2008;283:26461–26467. doi: 10.1074/jbc.M801625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altrock WD, tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C, Fässler R, Richter K, Boeckers TM, Potschka H, Brandt C, Löscher W, Grimberg D, Dresbach T, Hempelmann A, Hassan H, Balschun D, Frey JU, Brand-stätter JH, Garner CC, Rosenmund C, Gundelfinger ED. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37:787–800. doi: 10.1016/s0896-6273(03)00088-6. [DOI] [PubMed] [Google Scholar]
- Babai N, Bartoletti TM, Thoreson WB. Calcium regulates vesicle replenishment at the cone ribbon synapse. The Journal of Neuroscience. 2010;30:15866–15877. doi: 10.1523/JNEUROSCI.2891-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader CR, Bertrand D, Schwartz EA. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. The Journal of Physiology. 1982;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadori R, Biehlmaier O, Zeitz C, Labhart T, Makhankov YV, Forster U, Gesemann M, Berger W, Neuhauss SC. Nyctalopin is essential for synaptic transmission in the cone dominated zebrafish retina. The European Journal of Neuroscience. 2006;24:1664–1674. doi: 10.1111/j.1460-9568.2006.05053.x. [DOI] [PubMed] [Google Scholar]
- Balkema GW, Cusick K, Nguyen TH. Diurnal variation in synaptic ribbon length and visual threshold. Visual Neuroscience. 2001;18:789–797. doi: 10.1017/s0952523801185123. [DOI] [PubMed] [Google Scholar]
- Ball SL, Pardue MT, McCall MA, Gregg RG, Peachey NS. Immunohistochemical analysis of the outer plexiform layer in the nob mouse shows no abnormalities. Visual Neuroscience. 2003;20:267–272. doi: 10.1017/s0952523803203059. [DOI] [PubMed] [Google Scholar]
- Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG. Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Investigative Ophthalmology & Visual Science. 2002;43:1595–1603. [PubMed] [Google Scholar]
- Barnes S, Hille B. Ionic channels of the inner segment of tiger salamander cone photoreceptors. The Journal of General Physiology. 1989;94:719–743. doi: 10.1085/jgp.94.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoletti TM, Babai N, Thoreson WB. Vesicle pool size at the salamander cone ribbon synapse. Journal of Neurophysiology. 2010;103:419–423. doi: 10.1152/jn.00718.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nature Genetics. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, Birch DG, Bergen AA, Prinsen CF, Polomeno RC, Gal A, Drack AV, Musarella MA, Jacobson SG, Young RS, Weleber RG. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nature Genetics. 2000;26:319–333. doi: 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- Bennis M, Versaux-Botteri C, Repérant J, Armengol JA. Calbindin, calretinin and parvalbumin immunoreactivity in the retina of the chameleon (Chamaeleo chamaeleon) Brain, Behavior and Evolution. 2005;65:177–187. doi: 10.1159/000083683. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Grabs D, Rager G. Expression of presynaptic proteins is closely correlated with the chronotopic pattern of axons in the retinotectal system of the chick. The Journal of Comparative Neurology. 2000;418:361–372. [PubMed] [Google Scholar]
- Berntson AK, Morgans CW. Distribution of the presynaptic calcium sensors, synaptotagmin I/II and synaptotagmin III, in the goldfish and rodent retinas. Journal of Vision. 2003;3:274–280. doi: 10.1167/3.4.3. [DOI] [PubMed] [Google Scholar]
- Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. The Journal of Biological Chemistry. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Biehlmaier O, Makhankov Y, Neuhauss SC. Impaired retinal differentiation and maintenance in zebrafish laminin mutants. Investigative Ophthalmology & Visual Science. 2007;48:2887–2894. doi: 10.1167/iovs.06-1212. [DOI] [PubMed] [Google Scholar]
- Blank M, Koulen P, Blake DJ, Kröger S. Dystrophin and beta-dystroglycan in photoreceptor terminals from normal and mdx3Cv mouse retinae. The European Journal of Neuroscience. 1999;11:2121–2133. doi: 10.1046/j.1460-9568.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- Blank M, Koulen P, Kröger S. Subcellular concentration of beta-dystroglycan in photoreceptors and glial cells of the chick retina. The Journal of Comparative Neurology. 1997;389:668–678. [PubMed] [Google Scholar]
- Boëda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J, Houdusse A, Legrain P, Wolfrum U, Richardson G, Petit C. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. The EMBO Journal. 2002;21:6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Löhrke S, Morgans CW, Wässle H. Distributions of two homologous synaptic vesicle proteins, synaptoporin and synaptophysin, in the mammalian retina. The Journal of Comparative Neurology. 1996a;370:1–10. doi: 10.1002/(SICI)1096-9861(19960617)370:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wässle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. The European Journal of Neuroscience. 1999;11:3683–3693. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Wässle H, Betz H, Morgans CW. The plasma membrane protein SNAP-25, but not syntaxin, is present at photoreceptor and bipolar cell synapses in the rat retina. The European Journal of Neuroscience. 1996b;8:823–828. doi: 10.1111/j.1460-9568.1996.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Bunt AH. Enzymatic digestion of synaptic ribbons in amphibian retinal photoreceptors. Brain Research. 1971;25:571–577. doi: 10.1016/0006-8993(71)90461-6. [DOI] [PubMed] [Google Scholar]
- Buraei Z, Yang J. The b subunit of voltage-gated Ca2+ channels. Physiological Reviews. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside B. Microtubules and actin filaments in teleost visual cone elongation and contraction. Journal of Supramolecular Structure. 1986;5:257–275. doi: 10.1002/jss.400050302. [DOI] [PubMed] [Google Scholar]
- Cao Y, Posokhova E, Martemyanov KA. TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. The Journal of Neuroscience. 2011;31:11521–11526. doi: 10.1523/JNEUROSCI.1682-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. The Journal of Comparative Neurology. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Cases-Langhoff C, Voss B, Garner AM, Appeltauer U, Takei K, Kindler S, Veh RW, De Camilli P, Gundelfinger ED, Garner CC. Piccolo, a novel 420 kDa protein associated with the presynaptic cytomatrix. European Journal of Cell Biology. 1996;69:214–223. [PubMed] [Google Scholar]
- Chang B, Heckenlively JR, Bayley PR, Brecha NC, Davisson MT, Hawes NL, Hirano AA, Hurd RE, Ikeda A, Johnson BA, McCall MA, Morgans CW, Nusinowitz S, Peachey NS, Rice DS, Vessey KA, Gregg RG. The nob2 mouse, a null mutation in Cacna1f: Anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Visual Neuroscience. 2006;23:11–24. doi: 10.1017/S095252380623102X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Ileto B, Frere SG, Chan RB, Voronov SV, Roux A, Di Paolo G. Synaptojanin 1-mediated PI(4,5)P2 hydrolysis is modulated by membrane curvature and facilitates membrane fission. Developmental Cell. 2011;20:206–218. doi: 10.1016/j.devcel.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- Choi SY, Jackman S, Thoreson WB, Kramer RH. Light regulation of Ca2+ in the cone photoreceptor synaptic terminal. Visual Neuroscience. 2008;25:693–700. doi: 10.1017/s0952523808080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cia D, Bordais A, Varela C, Forster V, Sahel JA, Rendon A, Picaud S. Voltage-gated channels and calcium homeostasis in mammalian rod photoreceptors. Journal of Neurophysiology. 2005;93:1468–1475. doi: 10.1152/jn.00874.2004. [DOI] [PubMed] [Google Scholar]
- Cibis GW, Fitzgerald KM, Harris DJ, Rothberg PG, Rupani M. The effects of dystrophin gene mutations on the ERG in mice and humans. Investigative Ophthalmology & Visual Science. 1993;34:3646–3652. [PubMed] [Google Scholar]
- Claudepierre T, Manglapus MK, Marengi N, Radner S, Champliaud MF, Tasanen K, Bruckner-Tuderman L, Hunter DD, Brunken WJ. Collagen XVII and BPAG1 expression in the retina: Evidence for an anchoring complex in the central nervous system. The Journal of Comparative Neurology. 2005;487:190–203. doi: 10.1002/cne.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NG, McLaughlin BJ. The distribution of filipin-sterol complexes in photoreceptor synaptic membranes. The Journal of Comparative Neurology. 1984;230:437–443. doi: 10.1002/cne.902300311. [DOI] [PubMed] [Google Scholar]
- Coppola T, Magnin-Luthi S, Perret-Menoud V, Gattesco S, Schiavo G, Regazzi R. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. The Journal of Biological Chemistry. 2001;276:32756–32762. doi: 10.1074/jbc.M100929200. [DOI] [PubMed] [Google Scholar]
- Corey DP, Dubinsky JM, Schwartz EA. The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. The Journal of Physiology. 1984;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Akopian A. Calcium channel and glutamate receptor activities regulate actin organization in salamander retinal neurons. The Journal of Physiology. 2006;575:543–554. doi: 10.1113/jphysiol.2006.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Mizuno F, Akopian A. Disruption of actin cytoskeleton causes internalization of Ca(v)1.3 (alpha 1D) L-type calcium channels in salamander retinal neurons. Molecular Vision. 2007;13:1496–1507. [PubMed] [Google Scholar]
- Cui G, Meyer AC, Calin-Jageman I, Neef J, Haeseleer F, Moser T, Lee A. Ca2+-binding proteins tune Ca2+-feedback to Cav1.3 channels in mouse auditory hair cells. The Journal of Physiology. 2007;585:791–803. doi: 10.1113/jphysiol.2007.142307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis LB, Doneske B, Liu X, Thaller C, McNew JA, Janz R. Syntaxin 3b is a t-SNARE specific for ribbon synapses of the retina. The Journal of Comparative Neurology. 2008;510:550–559. doi: 10.1002/cne.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Douglas L, Hendrich J, Wratten J, Tran Van Minh A, Foucault I, Koch D, Pratt WS, Saibil HR, Dolphin AC. The calcium channel alpha2delta-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: Implications for localization and function. The Journal of Neuroscience. 2006;26:8748–8757. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi-Tawarada M, Inoue E, Takao-Rikitsu E, Inoue M, Kitajima I, Ohtsuka T, Takai Y. Active zone protein CAST is a component of conventional and ribbon synapses in mouse retina. The Journal of Comparative Neurology. 2006;495:480–496. doi: 10.1002/cne.20893. [DOI] [PubMed] [Google Scholar]
- Deng L, Kaeser PS, Xu W, Südhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, ten Brink JB, de Kok YJ, van Soest S, van den Born LI, van Driel MA, van de Pol DJ, Payne AM, Bhattacharya SS, Kellner U, Hoyng CB, Westerveld A, Brunner HG, Bleeker-Wagemakers EM, Deutman AF, Heckenlively JR, Cremers FP, Bergen AA. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12) Nature Genetics. 1999;23:217–221. doi: 10.1038/13848. [DOI] [PubMed] [Google Scholar]
- Dick O, Hack I, Altrock WD, Garner CC, Gundelfinger ED, Brandstätter JH. Localization of the presynaptic cytomatrix protein Piccolo at ribbon and conventional synapses in the rat retina: Comparison with Bassoon. The Journal of Comparative Neurology. 2001;439:224–234. doi: 10.1002/cne.1344. [DOI] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermüller J, Weiler R, Garner CC, Gundelfinger ED, Brandstätter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Doussau F, Augustine GJ. The actin cytoskeleton and neurotransmitter release: An overview. Biochimie. 2000;82:353–363. doi: 10.1016/s0300-9084(00)00217-0. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: Electron microscopy. Proceedings of the Royal Society of London Series B, Biological Sciences. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D, Holbach M, Ness W, Schmitz F, Anderson LV. Dystrophin and the dystrophin-associated glycoprotein, beta-dystroglycan, co-localize in photoreceptor synaptic complexes of the human retina. Neuroscience. 1996;73:605–612. doi: 10.1016/0306-4522(96)00069-3. [DOI] [PubMed] [Google Scholar]
- Dresbach T, Torres V, Wittenmayer N, Altrock WD, Zamorano P, Zuschratter W, Nawrotzki R, Ziv NE, Garner CC, Gundelfinger ED. Assembly of active zone precursor vesicles: Obligatory trafficking of presynaptic cytomatrix proteins Bassoon and Piccolo via a trans-Golgi compartment. The Journal of Biological Chemistry. 2006;281:6038–6047. doi: 10.1074/jbc.M508784200. [DOI] [PubMed] [Google Scholar]
- D’Souza VN, Nguyen TM, Morris GE, Karges W, Pillers DA, Ray PN. A novel dystrophin isoform is required for normal retinal electrophysiology. Human Molecular Genetics. 1995;4:837–842. doi: 10.1093/hmg/4.5.837. [DOI] [PubMed] [Google Scholar]
- Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggen-burger R, Südhof TC, Rizo J. A Munc13/RIM/Rab3 tripartite complex: From priming to plasticity? The EMBO Journal. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G, Rabl K, Gemp I, Heidelberger R, Thoreson WB. Quantitative analysis of synaptic release at the photoreceptor synapse. Biophysical Journal. 2010;98:2102–2110. doi: 10.1016/j.bpj.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JL, Yang H, Doan T, Silverstein RS, Murphy GJ, Nune G, Liu X, Copenhagen D, Tempel BL, Rieke F, Krizaj D. Scotopic visual signaling in the mouse retina is modulated by high-affinity plasma membrane calcium extrusion. The Journal of Neuroscience. 2006;26:7201–7211. doi: 10.1523/JNEUROSCI.5230-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyka FM, Wu WW, Pfeifer TA, Molday LL, Grigliatti TA, Molday RS. Characterization and purification of the discoidin domain-containing protein retinoschisin and its interaction with galactose. Biochemistry. 2008;47:9098–9106. doi: 10.1021/bi800938g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amraoui A, Petit C. Usher I syndrome: Unravelling the mechanisms that underlie the cohesion of the growing hair bundle in inner ear sensory cells. Journal of Cell Science. 2005;118:4593–4603. doi: 10.1242/jcs.02636. [DOI] [PubMed] [Google Scholar]
- El-Amraoui A, Sahly I, Picaud S, Sahel J, Abitbol M, Petit C. Human Usher 1B/mouse shaker-1: The retinal phenotype discrepancy explained by the presence/absence of myosin VIIA in the photoreceptor cells. Human Molecular Genetics. 1996;5:1171–1178. doi: 10.1093/hmg/5.8.1171. [DOI] [PubMed] [Google Scholar]
- Elluru RG, Bloom GS, Brady ST. Fast axonal transport of kinesin in the rat visual system: Functionality of kinesin heavy chain isoforms. Molecular Biology of the Cell. 1995;6:21–40. doi: 10.1091/mbc.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SS, Chen MS, Lin JF, Chao WT, Yang VC. Use of gain-of-function study to delineate the roles of crumbs in Drosophila eye development. Journal of Biomedical Science. 2003;10:766–773. doi: 10.1159/000073964. [DOI] [PubMed] [Google Scholar]
- Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of Held synapse. The Journal of Neuroscience. 2005;25:4131–4140. doi: 10.1523/JNEUROSCI.0350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nature Reviews. Neuroscience. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Firth SI, Morgan IG, Boelen MK, Morgans CW. Localization of voltage-sensitive L-type calcium channels in the chicken retina. Clinical & Experimental Ophthalmology. 2001;29:183–187. doi: 10.1046/j.1442-9071.2001.00401.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KM, Cibis GW, Giambrone SA, Harris DJ. Retinal signal transmission in Duchenne muscular dystrophy: Evidence for dysfunction in the photoreceptor/depolarizing bipolar cell pathway. The Journal of Clinical Investigation. 1994;93:2425–2430. doi: 10.1172/JCI117250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, Sigrist SJ. Maturation of active zone assembly by Drosophila Bruchpilot. The Journal of Cell Biology. 2009;186:129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Sanes JR. Synaptotagmin I and II are present in distinct subsets of central synapses. The Journal of Comparative Neurology. 2007;503:280–296. doi: 10.1002/cne.21381. [DOI] [PubMed] [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangršic T, Khimich D, Fetjova A, Gundelfinger ED, Liberman MC, Harke B, Bryan KE, Lee A, Egner A, Riedel D, Moser T. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman TB, Schultz JM, Ahmed ZM, Tsilou ET, Brewer CC. Usher syndrome: Hearing loss with vision loss. Advances in Otorhino-laryngology. 2011;70:56–65. doi: 10.1159/000322473. [DOI] [PubMed] [Google Scholar]
- Friedrich U, Stöhr H, Hilfinger D, Loenhardt T, Schachner M, Langmann T, Weber BH. The Na/K-ATPase is obligatory for membrane anchorage of retinoschisin, the protein involved in the pathogenesis of X-linked juvenile retinoschisis. Human Molecular Genetics. 2011;20:1132–1142. doi: 10.1093/hmg/ddq557. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Sendelbeck A, Brandstätter JH. Examining the molecular basis of light adaptation at the photoreceptor ribbon synapse. Investigative Ophthalmology & Visual Science. 2011;52:ARVO E-Abstract 1177. [Google Scholar]
- Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2. Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. The Journal of Biological Chemistry. 2003;278:15373–15380. doi: 10.1074/jbc.M212341200. [DOI] [PubMed] [Google Scholar]
- Fukuda J, Kameyama M, Yamaguchi K. Breakdown of cytoskeletal filaments selectively reduces Na and Ca spikes in cultured mammal neurons. Nature. 1981;294:82–85. doi: 10.1038/294082a0. [DOI] [PubMed] [Google Scholar]
- Gebhart M, Juhasz-Vedres G, Zuccotti A, Brandt N, Engel J, Trockenbacher A, Kaur G, Obermair GJ, Knipper M, Koschak A, Striessnig J. Modulation of Cav1.3 Ca2+ channel gating by Rab3 interacting molecule. Molecular and Cellular Neurosciences. 2010;44:246–259. doi: 10.1016/j.mcn.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Stevens CF, Südhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- Gil C, Soler-Jover A, Blasi J, Aguilera J. Synaptic proteins and SNARE complexes are localized in lipid rafts from rat brain synaptosomes. Biochemical and Biophysical Research Communications. 2004;329:117–124. doi: 10.1016/j.bbrc.2005.01.111. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bellido PT, Wardill TJ, Kostyleva R, Meinertzhagen IA, Juusola M. Overexpressing temperature-sensitive dynamin decelerates phototransduction and bundles microtubules in Drosophila photoreceptors. The Journal of Neuroscience. 2009;29:14199–14210. doi: 10.1523/JNEUROSCI.2873-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosens I, den Hollander AI, Cremers FP, Roepman R. Composition and function of the Crumbs protein complex in the mammalian retina. Experimental Eye Research. 2008;86:713–726. doi: 10.1016/j.exer.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Gover TD, Moreira TH, Kao JP, Weinreich D. Calcium regulation in individual peripheral sensory nerve terminals of the rat. The Journal of Physiology. 2007;578:481–490. doi: 10.1113/jphysiol.2006.119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Hadwiger G, Nonet ML, Richmond JE. Direct interactions between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density. Neuroscience Letters. 2008;444:137–142. doi: 10.1016/j.neulet.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. Microtubules in synapses of the retina. Journal of Neurocytology. 1976;5:361–370. doi: 10.1007/BF01175121. [DOI] [PubMed] [Google Scholar]
- Gray EG, Pease HL. On understanding the organisation of the retinal receptor synapses. Brain Research. 1971;35:1–15. doi: 10.1016/0006-8993(71)90591-9. [DOI] [PubMed] [Google Scholar]
- Grayson C, Reid SN, Ellis JA, Rutherford A, Sowden JC, Yates JR, Farber DB, Trump D. Retinoschisin, the X-linked retinoschisis protein, is a secreted photoreceptor protein, and is expressed and released by Weri-Rb1 cells. Human Molecular Genetics. 2000;9:1873–1879. doi: 10.1093/hmg/9.12.1873. [DOI] [PubMed] [Google Scholar]
- Greenlee MH, Roosevelt CB, Sakaguchi DS. Differential localization of SNARE complex proteins SNAP-25, syntaxin, and VAMP during development of the mammalian retina. The Journal of Comparative Neurology. 2001;430:306–320. doi: 10.1002/1096-9861(20010212)430:3<306::aid-cne1032>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Greenlee MH, Swanson JJ, Simon JJ, Elmquist JK, Jacobson CD, Sakaguchi DS. Postnatal development and the differential expression of presynaptic terminal-associated proteins in the developing retina of the Brazilian opossum, Monodelphis domestica. Brain Research. Developmental Brain Research. 1996;96:159–172. [PubMed] [Google Scholar]
- Gregg RG, Kamermans M, Klooster J, Lukasiewicz PD, Peachey NS, Vessey KA, McCall MA. Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. Journal of Neurophysiology. 2007;98:3023–3033. doi: 10.1152/jn.00608.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RG, Mukhopadhyay S, Candille SI, Ball SL, Pardue MT, McCall MA, Peachey NS. Identification of the gene and the mutation responsible for the mouse nob phenotype. Investigative Ophthalmology & Visual Science. 2003;44:378–384. doi: 10.1167/iovs.02-0501. [DOI] [PubMed] [Google Scholar]
- Gregory FD, Bryan KE, Pangršic T, Calin-Jageman IE, Moser T, Lee A. Harmonin inhibits presynaptic Ca(v)1.3 Ca(2+) channels in mouse inner hair cells. Nature Neuroscience. 2011;14:1109–1111. doi: 10.1038/nn.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griessmeier K, Cuny H, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. Calmodulin is a functional regulator of Cav1.4 L-type Ca2+ channels. The Journal of Biological Chemistry. 2009;284:29809–29816. doi: 10.1074/jbc.M109.048082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman GH, Pauer GJ, Narendra U, Peachey NS, Hagstrom SA. Early synaptic defects in tulp1−/− mice. Investigative Ophthalmology & Visual Science. 2009;50:3074–3083. doi: 10.1167/iovs.08-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Dai H, Rizo J. Binding of the Munc13-1 MUN domain to membrane-anchored SNARE complexes. Biochemistry. 2008;47:1474–1481. doi: 10.1021/bi702345m. [DOI] [PubMed] [Google Scholar]
- Haeseleer F. Interaction and colocalization of CaBP4 and Unc119 (MRG4) in photoreceptors. Investigative Ophthalmology & Visual Science. 2008;49:2366–2375. doi: 10.1167/iovs.07-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Rieke F, Palczewski K. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nature Neuroscience. 2004;7:1079–1087. doi: 10.1038/nn1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallermann S, Fejtova A, Schmidt H, Weyhersmüller A, Silver RA, Gundelfinger ED, Eilers J. Bassoon speeds vesicle reloading at a central excitatory synapse. Neuron. 2010;68:710–723. doi: 10.1016/j.neuron.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka Y, Meinertzhagen IA. Immunocytochemical localization of synaptic proteins to photoreceptor synapses of Drosophila melanogaster. The Journal of Comparative Neurology. 2010;518:1133–1155. doi: 10.1002/cne.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Südhof TC, Schneggenburger R. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa A, Hisatomi O, Yamamoto S, Ono E, Tokunaga F. Stathmin expression during newt retina regeneration. Experimental Eye Research. 2007;85:518–527. doi: 10.1016/j.exer.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Heidelberger R. Adenosine triphosphate and the late steps in calcium-dependent exocytosis at a ribbon synapse. The Journal of General Physiology. 1998;111:225–241. doi: 10.1085/jgp.111.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Thoreson WB, Witkovsky P. Synaptic transmission at retinal ribbon synapses. Progress in Retinal and Eye Research. 2005;24:682–720. doi: 10.1016/j.preteyeres.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Wang MM, Sherry DM. Differential distribution of synaptotagmin immunoreactivity among synapses in the goldfish, salamander, and mouse retina. Visual Neuroscience. 2003;20:37–49. doi: 10.1017/s095252380320105x. [DOI] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Béïque JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D, Doerr TA, Gottesman J, Miller RF. Calcium channel immunoreactivity in the salamander retina. Neuro-report. 2001;12:1493–1499. doi: 10.1097/00001756-200105250-00039. [DOI] [PubMed] [Google Scholar]
- Hibino H, Pironkova R, Onwumere O, Vologodskaia M, Hudspeth AJ, Lesage F. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca(2+) channels. Neuron. 2002;34:411–423. doi: 10.1016/s0896-6273(02)00667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida Y, Ohtsuka T. CAST and ELKS proteins: Structural and functional determinants of the presynaptic active zone. Journal of Biochemistry. 2010;148:131–137. doi: 10.1093/jb/mvq065. [DOI] [PubMed] [Google Scholar]
- Higashide T, McLaren MJ, Inana G. Localization of HRG4, a photoreceptor protein homologous to Unc-119, in ribbon synapse. Investigative Ophthalmology & Visual Science. 1998;39:690–698. [PubMed] [Google Scholar]
- Holmberg K, Ohman P. Fine structure of retinal synaptic organelles in lamprey and hagfish photoreceptors. Vision Research. 1976;16:237–239. doi: 10.1016/0042-6989(76)90105-x. [DOI] [PubMed] [Google Scholar]
- Holt M, Cooke A, Neef A, Lagnado L. High mobility of vesicles supports continuous exocytosis at a ribbon synapse. Current Biology. 2004;14:173–183. doi: 10.1016/j.cub.2003.12.053. [DOI] [PubMed] [Google Scholar]
- Holzhausen LC, Lewis AA, Cheong KK, Brockerhoff SE. Differential role for synaptojanin 1 in rod and cone photoreceptors. The Journal of Comparative Neurology. 2009;517:633–644. doi: 10.1002/cne.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi N, Holt M, Sakaba T. Calcium dependence of exo-and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Hosoya O, Tsutsui K, Tsutsui K. Localized expression of amphiphysin Ir, a retina-specific variant of amphiphysin I, in the ribbon synapse and its functional implication. The European Journal of Neuroscience. 2004;19:2179–2187. doi: 10.1111/j.0953-816X.2004.03340.x. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC. Actin in dendritic spines: Connecting dynamics to function. The Journal of Cell Biology. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Li J, Zhang Z, Yu M. Pikachurin interaction with dystroglycan is diminished by defective O-mannosyl glycosylation in congenital muscular dystrophy models and rescued by LARGE over-expression. Neuroscience Letters. 2011;489:10–15. doi: 10.1016/j.neulet.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Studholme K, Yazulla S, von Gersdorff H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. Journal of Neurophysiology. 2006;96:2025–2033. doi: 10.1152/jn.00364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Innocenti B, Heidelberger R. Mechanisms contributing to tonic release at the cone photoreceptor ribbon synapse. Journal of Neurophysiology. 2008;99:25–36. doi: 10.1152/jn.00737.2007. [DOI] [PubMed] [Google Scholar]
- Inoue E, Deguchi-Tawarada M, Takao-Rikitsu E, Inoue M, Kitajima I, Ohtsuka T, Takai Y. ELKS, a protein structurally related to the active zone protein CAST, is involved in Ca2+-dependent exocytosis from PC12 cells. Genes to Cells. 2006;11:659–672. doi: 10.1111/j.1365-2443.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- Isayama T, Goodman SR, Zagon IS. Spectrin isoforms in the mammalian retina. The Journal of Neuroscience. 1991;11:3531–3538. doi: 10.1523/JNEUROSCI.11-11-03531.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isosomppi J, Västinsalo H, Geller SF, Heon E, Flannery JG, Sankila EM. Disease-causing mutations in the CLRN1 gene alter normal CLRN1 protein trafficking to the plasma membrane. Molecular Vision. 2009;15:1806–1818. [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Choi SY, Thoreson WB, Rabl K, Bartoletti TM, Kramer RH. Role of the synaptic ribbon in transmitting the cone light reponse. Nature Neuroscience. 2009;12:303–310. doi: 10.1038/nn.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. The Journal of Neuroscience. 2010;30:11885–11895. doi: 10.1523/JNEUROSCI.1415-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrow H, Koulen P, Altrock WD, Kröger S. Identification of a beta-dystroglycan immunoreactive subcompartment in photoreceptor terminals. Investigative Ophthalmology & Visual Science. 2006;47:17–24. doi: 10.1167/iovs.05-0597. [DOI] [PubMed] [Google Scholar]
- Job C, Lagnado L. Calcium and protein kinase C regulate the actin cytoskeleton in the synaptic terminal of retinal bipolar cells. The Journal of Cell Biology. 1998;143:1661–1672. doi: 10.1083/jcb.143.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Byerly L. A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+ Neuron. 1993;10:797–804. doi: 10.1016/0896-6273(93)90196-x. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Franz C, Kuhn S, Furness DN, Rüttiger L, Münkner S, Rivolta MN, Seward EP, Herschman HR, Engel J, Knipper M, Marcotti W. Synaptotagmin IV determines the linear Ca2+ dependence of vesicle fusion at auditory ribbon synapses. Nature Neuroscience. 2010;13:45–52. doi: 10.1038/nn.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Jr, Perkins GA, Giddabasappa A, Chaney S, Xiao W, White AD, Brown JM, Waggoner J, Ellisman MH, Fox DA. Spatiotemporal regulation of ATP and Ca2+ dynamics in vertebrate rod and cone ribbon synapses. Molecular Vision. 2007;13:887–919. [PMC free article] [PubMed] [Google Scholar]
- Juhaszova M, Church P, Blaustein MP, Stanley EF. Location of calcium transporters at presynaptic terminals. The European Journal of Neuroscience. 2000;12:839–846. doi: 10.1046/j.1460-9568.2000.00974.x. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Südhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameya S, Araki E, Katsuki M, Mizota A, Adachi E, Nakahara K, Nonaka I, Sakuragi S, Takeda S, Nabeshima Y. Dp260 disrupted mice revealed prolonged implicit time of the b-wave in ERG and loss of accumulation of beta-dystroglycan in the outer plexiform layer of the retina. Human Molecular Genetics. 1997;6:2195–2203. doi: 10.1093/hmg/6.13.2195. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Hendriksen H. Expression patterns of voltage-dependent calcium channel alpha 1 subunits (alpha 1A-alpha 1E) mRNA in rat retina. Brain Research. Molecular Brain Research. 1998;55:209–220. doi: 10.1016/s0169-328x(97)00363-x. [DOI] [PubMed] [Google Scholar]
- Kanagawa M, Omori Y, Sato S, Kobayashi K, Miyagoe-Suzuki Y, Takeda S, Endo T, Furukawa T, Toda T. Post-translational maturation of dystroglycan is necessary for pikachurin binding and ribbon synaptic localization. The Journal of Biological Chemistry. 2010;285:31208–31216. doi: 10.1074/jbc.M110.116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantardzhieva A, Alexeeva S, Versteeg I, Wijnholds J. MPP3 is recruited to the MPP5 protein scaffold at the retinal outer limiting membrane. The FEBS Journal. 2006;273:1152–1165. doi: 10.1111/j.1742-4658.2006.05140.x. [DOI] [PubMed] [Google Scholar]
- Kantardzhieva A, Gosens I, Alexeeva S, Punte IM, Versteeg I, Krieger E, Neefjes-Mol CA, den Hollander AI, Letteboer SJ, Klooster J, Cremers FP, Roepman R, Wijnholds J. MPP5 recruits MPP4 to the CRB1 complex in photoreceptors. Investigative Ophthalmology & Visual Science. 2005;46:2192–2201. doi: 10.1167/iovs.04-1417. [DOI] [PubMed] [Google Scholar]
- Karim Z, Vepachedu R, Gorska M, Alam R. UNC119 inhibits dynamin and dynamin-dependent endocytic processes. Cellular Signalling. 2010;22:128–137. doi: 10.1016/j.cellsig.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Katsumata O, Ohara N, Tamaki H, Niimura T, Naganuma H, Watanabe M, Sakagami H. IQ-ArfGEF/BRAG1 is associated with synaptic ribbons in the mouse retina. The European Journal of Neuroscience. 2009;30:1509–1516. doi: 10.1111/j.1460-9568.2009.06943.x. [DOI] [PubMed] [Google Scholar]
- Kersten FF, van Wijk E, van Reeuwijk J, van der Zwaag B, Märker T, Peters TA, Katsanis N, Wolfrum U, Keunen JE, Roepman R, Kremer H. Association of whirlin with Cav1.3 (alpha1D) channels in photoreceptors, defining a novel member of the usher protein network. Investigative Ophthalmology & Visual Science. 2010;51:2338–2346. doi: 10.1167/iovs.09-4650. [DOI] [PubMed] [Google Scholar]
- Khan NW, Kondo M, Hiriyanna KT, Jamison JA, Bush RA, Sieving PA. Primate retinal signaling pathways: Suppressing ON-pathway activity in monkey with glutamate analogues mimics human CSNB1-NYX genetic night blindness. Journal of Neurophysiology. 2005;93:481–492. doi: 10.1152/jn.00365.2004. [DOI] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Chiba A, Aoki K. Daily melatonin injections entrain the circadian change of synaptic ribbon number in the pineal organ of the Japanese newt. Neuroscience Letters. 2000;285:181–184. doi: 10.1016/s0304-3940(00)01058-2. [DOI] [PubMed] [Google Scholar]
- Kim JH, von Gersdorff H. Traffic jams during vesicle cycling lead to synaptic depression. Neuron. 2009;63:143–145. doi: 10.1016/j.neuron.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Kiyonaka S, Wakamori M, Miki T, Uriu Y, Nonaka M, Bito H, Beedle AM, Mori E, Hara Y, De Waard M, Kanagawa M, Itakura M, Takahashi M, Campbell KP, Mori Y. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nature Neuroscience. 2007;10:691–701. doi: 10.1038/nn1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Neher E. Modeling buffered Ca2+ diffusion near the membrane: Implications for secretion in neuroendocrine cells. Biophysical Journal. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz MA, Hendrickson AE. Comparison of immunolocalization patterns for the synaptic vesicle proteins p65 and synapsin I in macaque monkey retina. Synapse. 1993;14:268–282. doi: 10.1002/syn.890140405. [DOI] [PubMed] [Google Scholar]
- Kotova S, Vijayasarathy C, Dimitriadis EK, Ikonomou L, Jaffe H, Sieving PA. Retinoschisin (RS1) interacts with negatively charged lipid bilayers in the presence of Ca2+: An atomic force microscopy study. Biochemistry. 2010;2049:7023–7032. doi: 10.1021/bi1007029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Fletcher EL, Craven SE, Bredt DS, Wässle H. Immunocytochemical localization of the postsynaptic density protein PSD-95 in the mammalian retina. The Journal of Neuroscience. 1998;18:10136–10149. doi: 10.1523/JNEUROSCI.18-23-10136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- Kreft M, Krizaj D, Grilc S, Zorec R. Properties of exocytotic response in vertebrate photoreceptors. Journal of Neurophysiology. 2003;90:218–225. doi: 10.1152/jn.01025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer H, van Wijk E, Märker T, Wolfrum U, Roepman R. Usher syndrome: Molecular links of pathogenesis, proteins and pathways. Human Molecular Genetics. 2006;15(Suppl 2):R262–R270. doi: 10.1093/hmg/ddl205. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Copenhagen DR. Calcium regulation in photoreceptors. Frontiers in Bioscience. 2002;7:d2023–d2044. doi: 10.2741/a896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Demarco SJ, Johnson J, Strehler EE, Copenhagen DR. Cell-specific expression of plasma membrane calcium ATPase isoforms in retinal neurons. The Journal of Comparative Neurology. 2002;451:1–21. doi: 10.1002/cne.10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Lai FA, Copenhagen DR. Ryanodine stores and calcium regulation in the inner segments of salamander rods and cones. The Journal of Physiology. 2003;547:761–774. doi: 10.1113/jphysiol.2002.035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Liu X, Copenhagen DR. Expression of calcium transporters in the retina of the tiger salamander (Ambystoma tigrinum) The Journal of Comparative Neurology. 2004;475:463–480. doi: 10.1002/cne.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurumado K, Mori W. A morphological study of the circadian cycle of the pineal gland of the rat. Cell and Tissue Research. 1977;182:565–568. doi: 10.1007/BF00219839. [DOI] [PubMed] [Google Scholar]
- Küssel-Andermann P, El-Amraoui A, Safieddine S, Nouaille S, Perfettini I, Lecuit M, Cossart P, Wolfrum U, Petit C. Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin-catenins complex. The EMBO Journal. 2000;19:6020–6029. doi: 10.1093/emboj/19.22.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L, Cervetto L, McNaughton PA. Ion transport by the Na-Ca exchange in isolated rod outer segments. Proceedings of the National Academy of Sciences of the United States of America. 1998;85:4548–4552. doi: 10.1073/pnas.85.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: Domain regulation of receptor signaling at the plasma membrane. The Journal of Cell Biology. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. The EMBO Journal. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1973;265:471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Lasater EM, Witkovsky P. The calcium current of turtle cone photoreceptor axon terminals. Neuroscience Research Supplement. 1991;15:S165–S173. [PubMed] [Google Scholar]
- Lazarides E, Nelson WJ, Kasamatsu T. Segregation of two spectrin forms in the chicken optic system: A mechanism for establishing restricted membrane-cytoskeletal domains in neurons. Cell. 1984;36:269–278. doi: 10.1016/0092-8674(84)90220-4. [DOI] [PubMed] [Google Scholar]
- Lazzell DR, Belizaire R, Thakur P, Sherry DM, Janz R. SV2B regulates synaptotagmin 1 by direct interaction. The Journal of Biological Chemistry. 2004;279:52124–52131. doi: 10.1074/jbc.M407502200. [DOI] [PubMed] [Google Scholar]
- Lee A, Jimenez A, Cui G, Haeseleer F. Phosphorylation of the Ca2+-binding protein CaBP4 by protein kinase C zeta in photoreceptors. The Journal of Neuroscience. 2007;27:12743–12754. doi: 10.1523/JNEUROSCI.4264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders AG, Lopes da Silva FH, Ghijsen WE, Verhage M. Rab3a is involved in transport of synaptic vesicles to the active zone in mouse brain nerve terminals. Molecular Biology of the Cell. 2001;12:3095–3102. doi: 10.1091/mbc.12.10.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenne PF, Wawrezinieck L, Conchonaud F, Wurtz O, Boned A, Guo XJ, Rigneault H, He HT, Marguet D. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. The EMBO Journal. 2006;25:3245–3256. doi: 10.1038/sj.emboj.7601214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy BP, Budde BS, Wittmer M, De Baere E, Berger W, Zeitz C. A common NYX mutation in Flemish patients with X linked CSNB. The British Journal of Ophthalmology. 2009;93:692–696. doi: 10.1136/bjo.2008.143727. [DOI] [PubMed] [Google Scholar]
- Liang Y, Tavalin SJ. Auxiliary beta subunits differentially determine pka utilization of distinct regulatory sites on Cav1.3 L type Ca2+ channels. Channels (Austin) 2007;1:102–112. doi: 10.4161/chan.4284. [DOI] [PubMed] [Google Scholar]
- Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ. Laminin expression in adult and developing retinae: Evidence of two novel CNS laminins. The Journal of Neuroscience. 2000;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD. Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. The Journal of Neuroscience. 1999;19:9399–9411. doi: 10.1523/JNEUROSCI.19-21-09399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetti A, Fratangeli A, Taverna E, Valnegri P, Francolini M, Cappello V, Matteoli M, Passafaro M, Rosa P. Cholesterol reduction impairs exocytosis of synaptic vesicles. Journal of Cell Science. 2010;123:595–605. doi: 10.1242/jcs.060681. [DOI] [PubMed] [Google Scholar]
- Lin-Jones J, Parker E, Wu M, Knox BE, Burnside B. Disruption of kinesin II function using a dominant negative-acting transgene in Xenopus laevis rods results in photoreceptor degeneration. Investigative Ophthalmology & Visual Science. 2003;44:3614–3621. doi: 10.1167/iovs.03-0164. [DOI] [PubMed] [Google Scholar]
- LoGiudice L, Sterling P, Matthews G. Mobility and turnover of vesicles at the synaptic ribbon. The Journal of Neuroscience. 2008;28:3150–3158. doi: 10.1523/JNEUROSCI.5753-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotery AJ, Jacobson SG, Fishman GA, Weleber RG, Fulton AB, Namperumalsamy P, Héon E, Levin AV, Grover S, Rosenow JR, Kopp KK, Sheffield VC, Stone EM. Mutations in the CRB1 gene cause Leber congenital amaurosis. Archives of Ophthalmology. 2001;119:415–420. doi: 10.1001/archopht.119.3.415. [DOI] [PubMed] [Google Scholar]
- Maeda T, Lem J, Palczewski K, Haeseleer F. A critical role of CaBP4 in the cone synapse. Investigative Ophthalmology & Visual Science. 2005;46:4320–4327. doi: 10.1167/iovs.05-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerker T, van Wijk E, Overlack N, Kersten FF, McGee J, Goldmann T, Sehn E, Roepman R, Walsh EJ, Kremer H, Wolfrum U. A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Human Molecular Genetics. 2008;17:71–86. doi: 10.1093/hmg/ddm285. [DOI] [PubMed] [Google Scholar]
- Magupalli VG, Schwarz K, Alpadi K, Natarajan S, Seigel GM, Schmitz F. Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons. The Journal of Neuroscience. 2008;28:7954–7967. doi: 10.1523/JNEUROSCI.1964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JW, MacLeish PR, Townes-Anderson E. Process outgrowth and synaptic varicosity formation by adult photoreceptors in vitro. The Journal of Neuroscience. 1993;13:3533–3548. doi: 10.1523/JNEUROSCI.13-08-03533.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JW, Townes-Anderson E, Czernik AJ, Cameron R, Greengard P, De Camilli P. Synapsins in the vertebrate retina: Absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron. 1990;5:19–33. doi: 10.1016/0896-6273(90)90030-j. [DOI] [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Human Molecular Genetics. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Takei K, Cameron R, Hurlbut P, Johnston PA, Südhof TC, Jahn R, De Camilli P. Association of Rab3A with synaptic vesicles at late stages of the secretory pathway. The Journal of Cell Biology. 1991;115:625–633. doi: 10.1083/jcb.115.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall MA, Gregg RG. Comparisons of structural and functional abnormalities in mouse B-wave mutants. The Journal of Physiology. 2008;586:4385–4392. doi: 10.1113/jphysiol.2008.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehalow AK, Kameya S, Smith RS, Hawes NL, Denegre JM, Young JA, Bechtold L, Haider NB, Tepass U, Heckenlively JR, Chang B, Naggert JK, Nishina PM. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Human Molecular Genetics. 2003;12:2179–2189. doi: 10.1093/hmg/ddg232. [DOI] [PubMed] [Google Scholar]
- Mercer AJ, Chen M, Thoreson WB. Lateral mobility of presynaptic L-type calcium channels at photoreceptor ribbon synapses. The Journal of Neuroscience. 2011a;31:4397–4406. doi: 10.1523/JNEUROSCI.5921-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer AJ, Rabl K, Riccardi GE, Brecha NC, Stella SL, Jr, Thoreson WB. Location of release sites and calcium-activated chloride channels relative to calcium channels at the photoreceptor ribbon synapse. Journal of Neurophysiology. 2011b;105:321–335. doi: 10.1152/jn.00332.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuleman J, van de Pavert SA, Wijnholds J. Crumbs homologue 1 in polarity and blindness. Biochemical Society Transactions. 2004;32:828–830. doi: 10.1042/BST0320828. [DOI] [PubMed] [Google Scholar]
- Migdale K, Herr S, Klug K, Ahmad K, Linberg K, Sterling P, Schein S. Two ribbon synaptic units in rod photoreceptors of macaque, human, and cat. The Journal of Comparative Neurology. 2003;455:100–112. doi: 10.1002/cne.10501. [DOI] [PubMed] [Google Scholar]
- Miller RJ. The control of neuronal Ca2+ homeostasis. Progress in Neurobiology. 1991;37:255–285. doi: 10.1016/0301-0082(91)90028-y. [DOI] [PubMed] [Google Scholar]
- Mizuno F, Barabas P, Krizaj D, Akopian A. Glutamate-induced internalization of Ca(v)1.3 L-type Ca(2+) channels protects retinal neurons against excitotoxicity. The Journal of Physiology. 2010;588:953–966. doi: 10.1113/jphysiol.2009.181305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday LL, Wu WW, Molday RS. Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex. The Journal of Biological Chemistry. 2007;282:32792–32801. doi: 10.1074/jbc.M706321200. [DOI] [PubMed] [Google Scholar]
- Morgans C, Brandstätter JH. SNAP-25 is present on the Golgi apparatus of retinal neurons. Neuroreport. 2000;11:85–88. doi: 10.1097/00001756-200001170-00017. [DOI] [PubMed] [Google Scholar]
- Morgans CW. Calcium channel heterogeneity among cone photoreceptors in the tree shrew retina. The Europena Journal of Neuroscience. 1999;11:2989–2993. doi: 10.1046/j.1460-9568.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- Morgans CW. Presynaptic proteins of ribbon synapses in the retina. Microscopy Research and Technique. 2000;50:141–150. doi: 10.1002/1097-0029(20000715)50:2<141::AID-JEMT6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Morgans CW. Localization of the alpha(1F) calcium channel subunit in the rat retina. Investigative Ophthalmology & Visual Science. 2001;42:2414–2418. [PubMed] [Google Scholar]
- Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L, Taylor WR. Photoreceptor calcium channels: Insight from night blindness. Visual Neuroscience. 2005;22:561–568. doi: 10.1017/S0952523805225038. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Brandstätter JH, Kellerman J, Betz H, Wässle H. A SNARE complex containing syntaxin 3 is present in ribbon synapses of the retina. The Journal of Neuroscience. 1996;16:6713–6721. doi: 10.1523/JNEUROSCI.16-21-06713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Brown RL, Duvoisin RM. TRPM1: The endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. BioEssays. 2010;32:609–614. doi: 10.1002/bies.200900198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, El Far O, Berntson A, Wässle H, Taylor WR. Calcium extrusion from mammalian photoreceptor terminals. The Journal of Neuroscience. 1998;18:2467–2474. doi: 10.1523/JNEUROSCI.18-07-02467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Gaughwin P, Maleszka R. Expression of the alpha1F calcium channel subunit by photoreceptors in the rat retina. Molecular Vision. 2001;7:202–209. [PubMed] [Google Scholar]
- Mukherjee K, Yang X, Gerber SH, Kwon HB, Ho A, Castillo PE, Liu X, Südhof TC. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6504–6509. doi: 10.1073/pnas.1002307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan V, Lyass A, Schnapp BJ. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. The Journal of Neuroscience. 1999;19:1027–1037. doi: 10.1523/JNEUROSCI.19-03-01027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman-Clewner M, St Jules R, Townes-Anderson E. L-type calcium channels in the photoreceptor ribbon synapse: Localization and role in plasticity. The Journal of Comparative Neurology. 1999;415:1–16. [PubMed] [Google Scholar]
- Nagle BW, Okamoto C, Taggart B, Burnside B. The teleost cone cytoskeleton. Localization of actin, microtubules, and intermediate filaments. Investigative Ophthalmology & Visual Science. 1986;27:689–701. [PubMed] [Google Scholar]
- Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. The Journal of Neuroscience. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Nourry C, Grant SG, Borg JP. PDZ domain proteins: Plug and play! Science’s STKE. 2003:RE7. doi: 10.1126/stke.2003.179.re7. [DOI] [PubMed] [Google Scholar]
- Nouvian R, Neef J, Bulankina AV, Reisinger E, Pangrši T, Frank T, Sikorra S, Brose N, Binz T, Moser T. Exocytosis at the hair cell ribbon synapse apparently operates without neuronal SNARE proteins. Nature Neuroscience. 2011;14:411–413. doi: 10.1038/nn.2774. [DOI] [PubMed] [Google Scholar]
- Oertner TG, Matus A. Calcium regulation of actin dynamics in dendritic spines. Cell Calcium. 2005;37:477–482. doi: 10.1016/j.ceca.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Ohtsuka T, Matsushima S, Akimoto Y, Nishiwaki C, Nakamichi Y, Kikuta T, Nagai S, Kawakami H, Watanabe T, Nagamatsu S. ELKS, a protein structurally related to the active zone-associated protein CAST, is expressed in pancreatic beta cells and functions in insulin exocytosis: Interaction of ELKS with exocytotic machinery analyzed by total internal reflection fluorescence microscopy. Molecular Biology of the Cell. 2005;16:3289–3300. doi: 10.1091/mbc.E04-09-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Südhof TC. Cell biology of Ca2+-triggered exocytosis. Current Opinion in Cell Biology. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Kim JS, Lee JY, Kim J, Seo JY, Kim AR. GTP binds to Rab3A in a complex with Ca2+/calmodulin. The Biochemical Journal. 2002;362:651–657. doi: 10.1042/0264-6021:3620651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TK, Wu Z, Kjellstrom S, Zeng Y, Bush RA, Sieving PA, Colosi P. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Therapy. 2009;16:916–926. doi: 10.1038/gt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearring JN, Bojang P, Jr, Shen Y, Koike C, Furukawa T, Nawy S, Gregg RG. A role for nyctalopin, a small leucine-rich repeat protein, in localizing the TRP melastatin 1 channel to retinal depolarizing bipolar cell dendrites. The Journal of Neuroscience. 2011;31:10060–10066. doi: 10.1523/JNEUROSCI.1014-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillers DA, Fitzgerald KM, Duncan NM, Rash SM, White RA, Dwinnell SJ, Powell BR, Schnur RE, Ray PN, Cibis GW, Weleber RG. Duchenne/Becker muscular dystrophy: Correlation of phenotype by electroretinography with sites of dystrophin mutations. Human Genetics. 1999;105:2–9. doi: 10.1007/s004399900111. [DOI] [PubMed] [Google Scholar]
- Prescott ED, Zenisek D. Recent progress towards understanding the synaptic ribbon. Current Opinion in Neurobiology. 2005;15:431–436. doi: 10.1016/j.conb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Pusch CM, Zeitz C, Brandau O, Pesch K, Achatz H, Feil S, Scharfe C, Maurer J, Jacobi FK, Pinckers A, Andreasson S, Hardcastle A, Wissinger B, Berger W, Meindl A. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nature Genetics. 2000;26:324–327. doi: 10.1038/81627. [DOI] [PubMed] [Google Scholar]
- Qu Y, Baroudi G, Yue Y, El-Sherif N, Boutjdir M. Localization and modulation of {alpha}1D (Cav1.3) L-type Ca channel by protein kinase A. American Journal of Physiology. Heart and Circulatory Physiology. 2005;288:H2123–H2130. doi: 10.1152/ajpheart.01023.2004. [DOI] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. The Journal of Cell Biology. 1975;65:192–222. doi: 10.1083/jcb.65.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, Raviola G. Structure of the synaptic membranes in the inner plexiform layer of the retina: A freeze-fracture study in monkeys and rabbits. The Journal of Comparative Neurology. 1982;209:233–248. doi: 10.1002/cne.902090303. [DOI] [PubMed] [Google Scholar]
- Rea R, Li J, Dharia A, Levitan ES, Sterling P, Kramer RH. Streamlined synaptic vesicle cycle in cone photoreceptor terminals. Neuron. 2004;41:755–766. doi: 10.1016/s0896-6273(04)00088-1. [DOI] [PubMed] [Google Scholar]
- Regus-Leidig H, tom Dieck S, Brandstätter JH. Absence of functional active zone protein Bassoon affects assembly and transport of ribbon precursors during early steps of photoreceptor synaptogenesis. European Journal of Cell Biology. 2010;89:468–475. doi: 10.1016/j.ejcb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Regus-Leidig H, tom Dieck S, Specht D, Meyer L, Brandstätter JH. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: The involvement of precursor spheres. The Journal of Comparative Neurology. 2009;512:814–824. doi: 10.1002/cne.21915. [DOI] [PubMed] [Google Scholar]
- Reim K, Wegmeyer H, Brandstätter JH, Xue M, Rosenmund C, Dresbach T, Hofmann K, Brose N. Structurally and functionally unique complexins at retinal ribbon synapses. The Journal of Cell Biology. 2005;169:669–680. doi: 10.1083/jcb.200502115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiners J, Märker T, Jürgens K, Reidel B, Wolfrum U. Photoreceptor expression of the Usher syndrome type 1 protein protocadherin 15 (USH1F) and its interaction with the scaffold protein harmonin (USH1C) Molecular Vision. 2005a;11:347–355. [PubMed] [Google Scholar]
- Reiners J, Nagel-Wolfrum K, Jürgens K, Märker T, Wolfrum U. Molecular basis of human Usher syndrome: Deciphering the meshes of the Usher protein network provides insights into the pathome-chanisms of the Usher disease. Experimental Eye Research. 2006;83:97–119. doi: 10.1016/j.exer.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Reiners J, Reidel B, El-Amraoui A, Boëda B, Huber I, Petit C, Wolfrum U. Differential distribution of harmonin isoforms and their possible role in Usher-1 protein complexes in mammalian photoreceptor cells. Investigative Ophthalmology & Visual Science. 2003;44:5006–5015. doi: 10.1167/iovs.03-0483. [DOI] [PubMed] [Google Scholar]
- Reiners J, van Wijk E, Märker T, Zimmermann U, Jürgens K, te Brinke H, Overlack N, Roepman R, Knipper M, Kremer H, Wolfrum U. Scaffold protein harmonin (USH1C) provides molecular links between Usher syndrome type 1 and type 2. Human Molecular Genetics. 2005b;14:3933–3943. doi: 10.1093/hmg/ddi417. [DOI] [PubMed] [Google Scholar]
- Renner M, Choquet D, Triller A. Control of the postsynaptic membrane viscosity. The Journal of Neuroscience. 2009;29:2926–2937. doi: 10.1523/JNEUROSCI.4445-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, de Monvel JB, Petit C. How the genetics of deafness illuminates auditory physiology. Annual Review of Physiology. 2011;73:311–334. doi: 10.1146/annurev-physiol-012110-142228. [DOI] [PubMed] [Google Scholar]
- Richter K, Langnaese K, Kreutz MR, Olias G, Zhai R, Scheich H, Garner CC, Gundelfinger ED. Presynaptic cytomatrix protein bassoon is localized at both excitatory and inhibitory synapses of rat brain. The Journal of Comparative Neurology. 1999;408:437–448. doi: 10.1002/(sici)1096-9861(19990607)408:3<437::aid-cne9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rieke F, Schwartz EA. Asynchronous transmitter release: Control of exocytosis and endocytosis at the salamander rod synapse. The Journal of Physiology. 1996;493:1–8. doi: 10.1113/jphysiol.1996.sp021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Rusakov DA. Ca2+-dependent mechanisms of presynaptic control at central synapses. The Neuroscientist. 2006;12:317–326. doi: 10.1177/1073858405284672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust MB, Gurniak CB, Renner M, Vara H, Morando L, Görlich A, Sassoè-Pognetto M, Banchaabouchi MA, Giustetto M, Triller A, Choquet D, Witke W. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. The EMBO Journal. 2010;29:1889–1902. doi: 10.1038/emboj.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. The Journal of Cell Biology. 2004;164:887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- Salaün C, Gould GW, Chamberlain LH. The SNARE proteins SNAP-25 and SNAP-23 display different affinities for lipid rafts in PC12 cells. Regulation by distinct cysteine-rich domains. The Journal of Biological Chemistry. 2005;280:1236–1240. doi: 10.1074/jbc.M410674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado T, Furukawa T. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nature Neuroscience. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- Satz JS, Philp AR, Nguyen H, Kusano H, Lee J, Turk R, Riker MJ, Hernández J, Weiss RM, Anderson MG, Mullins RF, Moore SA, Stone EM, Campbell KP. Visual impairment in the absence of dystroglycan. The Journal of Neuroscience. 2009;29:13136–13146. doi: 10.1523/JNEUROSCI.0474-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer SF, Raviola E, Heuser JE. Membrane specializations in the outer plexiform layer of the turtle retina. The Journal of Comparative Neurology. 1982;204:253–267. doi: 10.1002/cne.902040305. [DOI] [PubMed] [Google Scholar]
- Schmitz F. The making of synaptic ribbons: How they are built and what they do. The Neuroscientist. 2009;15:611–624. doi: 10.1177/1073858409340253. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Drenckhahn D. Distribution of actin in cone photoreceptor synapses. Histochemistry. 1993;100:35–40. doi: 10.1007/BF00268876. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Drenckhahn D. Localization of dystrophin and beta-dystroglycan in bovine retinal photoreceptor processes extending into the postsynaptic dendritic complex. Histochemistry and Cell Biology. 1997;108:249–255. doi: 10.1007/s004180050165. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Holbach M, Drenckhahn D. Colocalization of retinal dystrophin and actin in postsynaptic dendrites of rod and cone photoreceptor synapses. Histochemistry. 1993;100:473–479. doi: 10.1007/BF00267828. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Königstorfer A, Südhof TC. RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Tabares L, Khimich D, Strenzke N, de la Villa-Polo P, Castellano-Muñoz M, Bulankina A, Moser T, Fernández-Chacón R, Südhof TC. CSPalpha-deficiency causes massive and rapid photoreceptor degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2926–2931. doi: 10.1073/pnas.0510060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Schneider ME, Belyantseva IA, Azevedo RB, Kachar B. Rapid renewal of auditory hair bundles. Nature. 2002;418:837–838. doi: 10.1038/418837a. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Südhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Schubert T, Akopian A. Actin filaments regulate voltage-gated ion channels in salamander retinal ganglion cells. Neuroscience. 2004;125:583–590. doi: 10.1016/j.neuroscience.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Sergeev YV, Caruso RC, Meltzer MR, Smaoui N, MacDonald IM, Sieving PA. Molecular modeling of retinoschisin with functional analysis of pathogenic mutations from human X-linked retinoschisis. Human Molecular Genetics. 2010;19:1302–1313. doi: 10.1093/hmg/ddq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Burré J, Südhof TC. CSPα promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nature Cell Biology. 2011;13:30–39. doi: 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. The Journal of Neuroscience. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z, Choi SY, Dharia A, Li J, Sterling P, Kramer RH. Synaptic Ca2+ in darkness is lower in rods than cones, causing slower tonic release of vesicles. The Journal of Neuroscience. 2007;27:5033–5042. doi: 10.1523/JNEUROSCI.5386-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DM, Heidelberger R. Distribution of proteins associated with synaptic vesicle endocytosis in the mouse and goldfish retina. The Journal of Comparative Neurology. 2005;484:440–457. doi: 10.1002/cne.20504. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Mitchell R, Standifer KM, du Plessis B. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neuroscience. 2006;7:54. doi: 10.1186/1471-2202-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DM, Yang H, Standifer KM. Vesicle-associated membrane protein isoforms in the tiger salamander retina. The Journal of Comparative Neurology. 2001;431:424–436. doi: 10.1002/1096-9861(20010319)431:4<424::aid-cne1080>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Shi L, Jian K, Ko ML, Trump D, Ko GY. Retinoschisin, a new binding partner for L-type voltage-gated calcium channels in the retina. The Journal of Biological Chemistry. 2009;284:3966–3975. doi: 10.1074/jbc.M806333200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Zinsmaier KE. Endophilin and synaptojanin hook up to promote synaptic vesicle endocytosis. Neuron. 2003;40:665–667. doi: 10.1016/s0896-6273(03)00726-8. [DOI] [PubMed] [Google Scholar]
- Specht CG, Triller A. The dynamics of synaptic scaffolds. BioEssays. 2008;30:1062–1074. doi: 10.1002/bies.20831. [DOI] [PubMed] [Google Scholar]
- Specht D, Wu SB, Turner P, Dearden P, Koentgen F, Wolfrum U, Maw M, Brandstätter JH, tom Dieck S. Effects of presynaptic mutations on a postsynaptic Cacna1s calcium channel colocalized with mGluR6 at mouse photoreceptor ribbon synapses. Investigative Ophthalmology & Visual Science. 2009;50:505–515. doi: 10.1167/iovs.08-2758. [DOI] [PubMed] [Google Scholar]
- Spencer M, Moon RT, Milam AH. Membrane skeleton protein 4.1 in inner segments of retinal cones. Investigative Ophthalmology & Visual Science. 1991;32:1–7. [PubMed] [Google Scholar]
- Spiwoks-Becker I, Glas M, Lasarzik I, Vollrath L. Mouse photoreceptor synaptic ribbons lose and regain material in response to illumination changes. The European Journal of Neuroscience. 2004;19:1559–1571. doi: 10.1111/j.1460-9568.2004.03198.x. [DOI] [PubMed] [Google Scholar]
- Steiner-Champliaud MF, Sahel J, Hicks D. Retinoschisin forms a multi-molecular complex with extracellular matrix and cytoplasmic proteins: Interactions with beta2 laminin and alphaB-crystallin. Molecular Vision. 2006;12:892–901. [PubMed] [Google Scholar]
- Stella SL, Jr, Thoreson WB. Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. The European Journal of Neuroscience. 2000;12:3537–3548. doi: 10.1046/j.1460-9568.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends in Neurosciences. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Stöhr H, Heisig JB, Benz PM, Schöberl S, Milenkovic VM, Strauss O, Aartsen WM, Wijnholds J, Weber BH, Schulz HL. TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. The Journal of Neuroscience. 2009;29:6809–6818. doi: 10.1523/JNEUROSCI.5546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr H, Molday LL, Molday RS, Weber BH, Biedermann B, Reichenbach A, Krämer F. Membrane-associated guany-late kinase proteins MPP4 and MPP5 associate with Veli3 at distinct intercellular junctions of the neurosensory retina. The Journal of Comparative Neurology. 2005;481:31–41. doi: 10.1002/cne.20367. [DOI] [PubMed] [Google Scholar]
- Straub V, Campbell KP. Muscular dystrophies and the dystrophin-glycoprotein complex. Current Opinion in Neurology. 1997;10:168–175. doi: 10.1097/00019052-199704000-00016. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Bolz HJ, Koschak A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflügers Archive. 2010;460:361–374. doi: 10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Rüther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nature Genetics. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- Takada Y, Fariss RN, Tanikawa A, Zeng Y, Carper D, Bush R, Sieving PA. A retinal neuronal developmental wave of retinoschisin expression begins in ganglion cells during layer formation. Investigative Ophthalmology & Visual Science. 2004;45:3302–3312. doi: 10.1167/iovs.04-0156. [DOI] [PubMed] [Google Scholar]
- Takada Y, Vijayasarathy C, Zeng Y, Kjellstrom S, Bush RA, Sieving PA. Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery. Investigative Ophthalmology & Visual Science. 2008;49:3677–3686. doi: 10.1167/iovs.07-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi SX, Miriyala J, Colecraft HM. Membrane-associated guanylate kinase-like properties of beta-subunits required for modulation of voltage-dependent Ca2+ channels. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7193–7198. doi: 10.1073/pnas.0306665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Takao-Rikitsu E, Mochida S, Inoue E, Deguchi-Tawarada M, Inoue M, Ohtsuka T, Takai Y. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. The Journal of Cell Biology. 2004;164:301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talts JF, Andac Z, Göhring W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix proteins. The EMBO Journal. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantri A, Vrabec TR, Cu-Unjieng A, Frost A, Annesley WH, Jr, Donoso LA. X-linked retinoschisis: A clinical and molecular genetic review. Survey of Ophthalmology. 2004;49:214–230. doi: 10.1016/j.survophthal.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Taverna E, Saba E, Linetti A, Longhi R, Jeromin A, Righi M, Clementi F, Rosa P. Localization of synaptic proteins involved in neurosecretion in different membrane microdomains. Journal of Neurochemistry. 2007;100:664–677. doi: 10.1111/j.1471-4159.2006.04225.x. [DOI] [PubMed] [Google Scholar]
- Taverna E, Saba E, Rowe J, Francolini M, Clementi F, Rosa P. Role of lipid microdomains in P/Q-type calcium channel (Cav2.1) clustering and function in presynaptic membranes. The Journal of Biological Chemistry. 2004;279:5127–5134. doi: 10.1074/jbc.M308798200. [DOI] [PubMed] [Google Scholar]
- Thoreson WB. Kinetics of synaptic transmission at ribbon synapses of rods and cones. Molecular Neurobiology. 2007;36:205–223. doi: 10.1007/s12035-007-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, Miller RF. Reducing extracellular Cl− suppresses dihydropyridine-sensitive Ca2+ currents and synaptic transmission in amphibian photoreceptors. Journal of Neurophysiology. 1997;77:2175–2190. doi: 10.1152/jn.1997.77.4.2175. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Rabl K, Townes-Anderson E, Heidelberger R. A highly Ca2+-sensitive pool of vesicles contributes to linearity at the rod photoreceptor ribbon synapse. Neuron. 2004;42:595–605. doi: 10.1016/s0896-6273(04)00254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoro T, Higa S, Deguchi-Tawarada M, Inoue E, Kitajima I, Ohtsuka T. Localization of the active zone proteins CAST, ELKS, and Piccolo at neuromuscular junctions. Neuroreport. 2007;18:313–316. doi: 10.1097/WNR.0b013e3280287abe. [DOI] [PubMed] [Google Scholar]
- Tolosa de Talamoni N, Pérez A, Riis R, Smith C, Norman ML, Wasserman RH. Comparative immunolocalization of the plasma membrane calcium pump and calbindin D28K in chicken retina during embryonic development. European Journal of Histochemistry. 2002;46:333–340. doi: 10.4081/1745. [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtová A, Bracko O, Gundelfinger ED, Brandstätter JH. Molecular dissection of the photoreceptor ribbon synapse: Physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. The Journal of Cell Biology. 2005;168:825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Brandstätter JH. Ribbon synapses of the retina. Cell and Tissue Research. 2006;326:339–346. doi: 10.1007/s00441-006-0234-0. [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Sanmartí-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla KH, Kämpf U, Fränzer JT, Stumm M, Garner CC, Gundelfinger ED. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. The Journal of Cell Biology. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes-Anderson E, MacLeish PR, Raviola E. Rod cells dissociated from mature salamander retina: Ultrastructure and uptake of horseradish peroxidase. The Journal of Cell Biology. 1985;100:175–188. doi: 10.1083/jcb.100.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Gohdo T, Ohno S. Beta-dystroglycan localization in the photoreceptor and Müller cells in the rat retina revealed by immunoelectron microscopy. The Journal of Histochemistry and Cytochemistry. 1998;46:185–191. doi: 10.1177/002215549804600207. [DOI] [PubMed] [Google Scholar]
- Ueda H, Kobayashi T, Mitsui K, Tsurugi K, Tsukahara S, Ohno S. Dystrophin localization at presynapse in rat retina revealed by immunoelectron microscopy. Investigative Ophthalmology & Visual Science. 1995;36:2318–2322. [PubMed] [Google Scholar]
- Ullrich B, Südhof TC. Distribution of synaptic markers in the retina: Implications for synaptic vesicle traffic in ribbon synapses. Journal of Physiology, Paris. 1994;88:249–257. doi: 10.1016/0928-4257(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Uthaiah RC, Hudspeth AJ. Molecular anatomy of the hair cell’s ribbon synapse. The Journal of Neuroscience. 2010;30:12387–12399. doi: 10.1523/JNEUROSCI.1014-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk JA, Rashbass P, Roepman R, Davis J, Voesenek KE, Arends ML, Zonneveld MN, van Roekel MH, Cameron K, Rohrschneider K, Heckenlively JR, Koenekoop RK, Hoyng CB, Cremers FP, den Hollander AI. Characterization of the Crumbs homolog 2 (CRB2) gene and analysis of its role in retinitis pigmentosa and Leber congenital amaurosis. Molecular Vision. 2005;11:263–273. [PubMed] [Google Scholar]
- van de Pavert SA, Kantardzhieva A, Malysheva A, Meuleman J, Versteeg I, Levelt C, Klooster J, Geiger S, Seeliger MW, Rashbass P, Le Bivic A, Wijnholds J. Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. Journal of Cell Science. 2004;117:4169–4177. doi: 10.1242/jcs.01301. [DOI] [PubMed] [Google Scholar]
- Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, Brockerhoff SE. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. The Journal of Neuroscience. 2004;24:8641–8650. doi: 10.1523/JNEUROSCI.2892-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen MM, Bijveld MM, Claassen YB, Florijn RJ, Pearring JN, Meire FM, McCall MA, Riemslag FC, Gregg RG, Bergen AA, Kamermans M. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. American Journal of Human Genetics. 2009;85:730–736. doi: 10.1016/j.ajhg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecino E, Avila J. Distribution of the phosphorylated form of microtubule associated protein 1B in the flsh visual system during optic nerve regeneration. Brain Research Bulletin. 2001;56:131–137. doi: 10.1016/s0361-9230(01)00618-9. [DOI] [PubMed] [Google Scholar]
- Vijayasarathy C, Takada Y, Zeng Y, Bush RA, Sieving PA. Retinoschisin is a peripheral membrane protein with affinity for anionic phospholipids and affected by divalent cations. Investigative Ophthalmology & Visual Science. 2007;48:991–1000. doi: 10.1167/iovs.06-0915. [DOI] [PubMed] [Google Scholar]
- Vijayasarathy C, Takada Y, Zeng Y, Bush RA, Sieving PA. Organization and molecular interactions of retinoschisin in photoreceptors. Advances in Experimental Medicine and Biology. 2008;613:291–297. doi: 10.1007/978-0-387-74904-4_34. [DOI] [PubMed] [Google Scholar]
- Vollrath L, Spiwoks-Becker I. Plasticity of retinal ribbon synapses. Microscopy Research and Technique. 1996;35:472–487. doi: 10.1002/(SICI)1097-0029(19961215)35:6<472::AID-JEMT6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Schmitz F. The expression pattern and assembly profile of synaptic membrane proteins in ribbon synapses of the developing mouse retina. Cell and Tissue Research. 2003;311:159–173. doi: 10.1007/s00441-002-0674-0. [DOI] [PubMed] [Google Scholar]
- Von Kriegstein K, Schmitz F, Link E, Südhof TC. Distribution of synaptic vesicle proteins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. The European Journal of Neuroscience. 1999;11:1335–1348. doi: 10.1046/j.1460-9568.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Djamgoz MB. Spinules: A case for retinal synaptic plasticity. Trends in Neurosciences. 1993;16:201–206. doi: 10.1016/0166-2236(93)90155-f. [DOI] [PubMed] [Google Scholar]
- Wahlin KJ, Moreira EF, Huang H, Yu N, Adler R. Molecular dynamics of photoreceptor synapse formation in the developing chick retina. The Journal of Comparative Neurology. 2008;506:822–837. doi: 10.1002/cne.21582. [DOI] [PubMed] [Google Scholar]
- Waite A, Tinsley CL, Locke M, Blake DJ. The neurobiology of the dystrophin-associated glycoprotein complex. Annals of Medicine. 2009;41:344–359. doi: 10.1080/07853890802668522. [DOI] [PubMed] [Google Scholar]
- Wan L, Almers W, Chen W. Two ribeye genes in teleosts: The role of Ribeye in ribbon formation and bipolar cell development. The Journal of Neuroscience. 2005;25:941–949. doi: 10.1523/JNEUROSCI.4657-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanaverbecq N, Marsh SJ, Al-Qatari M, Brown DA. The plasma membrane calcium-ATPase as a major mechanism for intracellular calcium regulation in neurones from the rat superior cervical ganglion. The Journal of Physiology. 2003;550:83–101. doi: 10.1113/jphysiol.2002.035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu B, Zieba A, Neumann NG, Kasper-Sonnenberg M, Honsbein A, Hultqvist G, Conze T, Witt W, Limbach C, Geitmann M, Danielson H, Kolarow R, Niemann G, Lessmann V, Kilimann MW. A protein interaction node at the neurotransmitter release site: Domains of Aczonin/Piccolo, Bassoon, CAST, and rim converge on the N-terminal domain of Munc13-1. The Journal of Neuroscience. 2009;29:12584–12596. doi: 10.1523/JNEUROSCI.1255-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Südhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- Weber BH, Schrewe H, Molday LL, Gehrig A, White KL, Seeliger MW, Jaissle GB, Friedburg C, Tamm E, Molday RS. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6222–6227. doi: 10.1073/pnas.092528599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AM, Wong FK, Tufford AR, Schlichter LC, Matveev V, Stanley EF. N-type Ca2+ channels carry the largest current: Implications for nanodomains and transmitter release. Nature Neuroscience. 2010;13:1348–1350. doi: 10.1038/nn.2657. [DOI] [PubMed] [Google Scholar]
- Wilkinson MF, Barnes S. The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. The Journal of General Physiology. 1996;107:621–630. doi: 10.1085/jgp.107.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS. Usher syndrome: Animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Research. 2008;48:433–441. doi: 10.1016/j.visres.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Aleman TS, Lillo C, Lopes VS, Hughes LC, Stone EM, Jacobson SG. Harmonin in the murine retina and the retinal phenotypes of Ush1c-mutant mice and human USH1C. Investigative Ophthalmology & Visual Science. 2009;50:3881–3889. doi: 10.1167/iovs.08-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Marmorstein AD, Striessnig J, Peachey NS. Voltage-dependent calcium channel CaV1.3 subunits regulate the light peak of the electroretinogram. Journal of Neurophysiology. 2003;97:3731–3735. doi: 10.1152/jn.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Marmorstein AD, Striessnig J, Peachey NS. Voltage-dependent calcium channel CaV1.3 subunits regulate the light peak of the electroretinogram. Journal of Neurophysiology. 2007;97:3731–3735. doi: 10.1152/jn.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WW, Molday RS. Defective discoidin domain structure, subunit assembly, and endoplasmic reticulum processing of retinoschisin are primary mechanisms responsible for X-linked retinoschisis. The Journal of Biological Chemistry. 2003;278:28139–28146. doi: 10.1074/jbc.M302464200. [DOI] [PubMed] [Google Scholar]
- Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nürnberg P, Ruether K, Berger W. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Investigative Ophthalmology & Visual Science. 2006a;47:3523–3530. doi: 10.1167/iovs.06-0271. [DOI] [PubMed] [Google Scholar]
- Wycisk KA, Zeitz C, Feil S, Wittmer M, Forster U, Neidhardt J, Wissinger B, Zrenner E, Wilke R, Kohl S, Berger W. Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. American Journal of Human Genetics. 2006b;79:973–977. doi: 10.1086/508944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Chen X, Steele EC., Jr Abundant L-type calcium channel Ca(v)1.3 (alpha1D) subunit mRNA is detected in rod photoreceptors of the mouse retina via in situ hybridization. Molecular Vision. 2007;13:764–771. [PMC free article] [PubMed] [Google Scholar]
- Xu JW, Slaughter MM. Large-conductance calcium-activated potassium channels facilitate transmitter release in salamander rod synapse. The Journal of Neuroscience. 2005;25:7660–7668. doi: 10.1523/JNEUROSCI.1572-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Synaptic localization and function of Sidekick recognition molecules require MAGI scaffolding proteins. The Journal of Neuroscience. 2010;30:3579–3588. doi: 10.1523/JNEUROSCI.6319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: Synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Yan D, Liu XZ. Genetics and pathological mechanisms of Usher syndrome. Journal of Human Genetics. 2010;55:327–335. doi: 10.1038/jhg.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PS, Alseikhan BA, Hiel H, Grant L, Mori MX, Yang W, Fuchs PA, Yue DT. Switching of Ca2+-dependent inactivation of Ca(v)1.3 channels by calcium binding proteins of auditory hair cells. The Journal of Neuroscience. 2006;26:10677–10689. doi: 10.1523/JNEUROSCI.3236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu X, Zhao Y, Adamian M, Pawlyk B, Sun X, McMillan DR, Liberman MC, Li T. Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss. PLoS Genetics. 2010;6:e1000955. doi: 10.1371/journal.pgen.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Pawlyk B, Wen XH, Adamian M, Soloviev M, Michaud N, Zhao Y, Sandberg MA, Makino CL, Li T. Mpp4 is required for proper localization of plasma membrane calcium ATPases and maintenance of calcium homeostasis at the rod photoreceptor synaptic terminals. Human Molecular Genetics. 2007;16:1017–1029. doi: 10.1093/hmg/ddm047. [DOI] [PubMed] [Google Scholar]
- Zallocchi M, Meehan DT, Delimont D, Askew C, Garige S, Gratton MA, Rothermund-Franklin CA, Cosgrove D. Localization and expression of clarin-1, the Clrn1 gene product, in auditory hair cells and photoreceptors. Hearing Research. 2009;255:109–120. doi: 10.1016/j.heares.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA, Schietroma C, Zampighi LM, Woodruff M, Wright EM, Brecha NC. Conical tomography of a ribbon synapse: Structural evidence for vesicle fusion. PLoS One. 2011;6:e16944. doi: 10.1371/journal.pone.0016944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanazzi G, Matthews G. The molecular architecture of ribbon presynaptic terminals. Molecular Neurobiology. 2009;39:130–148. doi: 10.1007/s12035-009-8058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanazzi G, Matthews G. Enrichment and differential targeting of complexins 3 and 4 in ribbon-containing sensory neurons during zebrafish development. Neural Development. 2010;5:24. doi: 10.1186/1749-8104-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D. Vesicle association and exocytosis at ribbon and extraribbon sites in retinal bipolar cell presynaptic terminals. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4922–4927. doi: 10.1073/pnas.0709067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Horst NK, Merrifield C, Sterling P, Matthews G. Visualizing synaptic ribbons in the living cell. The Journal of Neuroscience. 2004;24:9752–9759. doi: 10.1523/JNEUROSCI.2886-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 2004;19:262–270. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]
- Zhang H, Maximov A, Fu Y, Xu F, Tang TS, Tkatch T, Surmeier DJ, Bezprozvanny I. Association of CaV1.3 L-type calcium channels with Shank. The Journal of Neuroscience. 2005;25:1037–1049. doi: 10.1523/JNEUROSCI.4554-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]