Abstract
Telomeres are synthesized by telomerase, a specialized reverse transcriptase, which contains a template in its intrinsic RNA component. In Kluyveromyces lactis, the repeats synthesized by the wild-type telomerase are 25 nucleotides (nt) in length and uniform in sequence. To determine the role of the 5-nt repeats defining the ends of the K. lactis telomerase RNA template in telomerase translocation, we have made mutations in and around them and observed their effects on telomere length and the sequence of newly made telomeric repeats. These template mutations typically result in telomeres that are shorter than those of wild-type cells. The mismatches between the telomerase template and the telomeric tip that occur after telomerase-mediated incorporation of the mutations are normally not removed. Instead, the mutations lead to the synthesis of aberrant repeats that range in size from 31 to 13 bp. Therefore, the specificity with which the telomeric tip aligns with the telomere is critical for the production of the uniform repeats seen in K. lactis. In addition, the region immediately 3′ of the template may play an important role in translocation of the enzyme.
Telomeres, the DNA-protein complexes found at the ends of linear eukaryotic chromosomes, contain DNA composed of short, repetitive sequences. The sequences are maintained by telomerase, a specialized reverse transcriptase. The enzyme is a ribonucleoprotein, with the template for telomerase synthesis contained within the RNA moiety (1, 2, 3, 14b). The RNA is bound by the reverse transcriptase component, called Est2p in Saccharomyces cerevisiae (14b) and TERT (telomerase reverse transcriptase) in humans (21a), which contains conserved features found in reverse transcriptases (1).
At the telomere, there is a duplex region that is involved in regulating the length of the telomere and also a single-stranded region (reviewed in reference 37). In yeast, the single-stranded overhang forms in a cell cycle-specific manner and is not dependent on telomerase for its formation (3a, 34a). It is at this part of the telomere that the access of telomerase to the telomere occurs. Cdc13p binds the single-stranded region (14a), where it plays a critical role in mediating access to the telomere through its interaction with Est1p, a protein that interacts with the telomerase complex (1a, 4, 23a). Stn1p, a negative regulator of telomere length, interacts with Cdc13p, and both are critical for capping the telomere (12). Competition between the negative activity of Stn1p and telomerase-mediated telomere addition is thought to be a component of telomere length regulation. Telomerase is conveyed to the telomere by a number of proteins, including Est1p (4) and the Ku complex (11, 24), both of which interact with the RNA component of telomerase. Est1p also interacts with the single-stranded region of the telomere, both through direct DNA binding and via interactions with Cdc13p (4, 28). Ku is a generalized DNA end-binding protein that is involved in nonhomologous end joining in addition to its role at telomeres (23).
The template of the telomerase RNA is defined by terminal repeats (TRs) of 3 to 5 nucleotides (nt) at both ends (5, 17, 36). These repeats are thought to be involved in aligning the template with the telomeric end prior to the synthesis of a new telomeric repeat. The sequence copied from the 5′ TR of the template would perfectly base pair with the sequence at the 3′ end of the template, providing 3 to 5 nt of complementarity between the telomere and the template. This could account for much of the uniformity in telomeric repeat sequence within most species. One notable exception to the trend toward uniform telomeric repeats is found in S. cerevisiae, which synthesizes highly heterogeneous telomeric repeats (26, 30, 32, 33). Work explaining the mechanisms by which a single template gives rise to heterogeneous telomeric repeats has led to two important conclusions (6, 7). First, S. cerevisiae telomerase does not always copy the entire length of the template, which would thus preclude it from always using the TR to align properly. Second, there are multiple binding sites within the template that have very similar sequences, such that alignment could occur at any of them and lead to the synthesis of variant repeats. The telomerase template has also been shown to play a role in the active-site function of telomerase. In Tetrahymena thermophila, mutations in the telomerase RNA template lead to a decrease in enzyme fidelity and premature synthesis termination (10).
The translocation step, in which telomerase dissociates from the newly synthesized repeat and realigns to synthesize a new repeat, offers the potential for variations in telomere synthesis. Despite this, telomeres in the budding yeast Kluyveromyces lactis are composed of uniform copies of a 25-bp repeat. The repeat is synthesized from a 30-nt template, which contains 5-nt TRs at its ends (8, 17). A critical determinant of the telomerase stop site involves an 8-nt sequence just 5′ of the template. This sequence, only 3 nt from the end of the template, is a base-paired structure involved in a long-range interaction with a region more than 300 nt away. Elimination of the base pairing in this region in vivo causes telomerase to read through the template and incorporate sequences of the nontemplate region adjacent to the template into the telomere (34). The base-paired structure therefore defines a stop signal beyond the end of the template, but previous studies have shown that, in vitro, K. lactis telomerase normally stops copying before reaching the end of the template (8). The precise stop sites in vivo, however, were not determined. The factors influencing the start site are also unknown, although it is presumed that base pairing between the telomere and the 3′ end of the template plays a role.
In order to examine the determinants affecting normal alignment between the telomerase template with the telomeric tip in vivo, a collection of K. lactis strains containing point mutations in the telomerase template was examined. By cloning telomeres templated by various telomerase RNA (TER1) mutants, it was possible to determine whether an aberrant translocation event had occurred. These mutants were also used to examine the minimum base-pairing interactions required for translocation in vivo.
MATERIALS AND METHODS
Mutagenesis and strain construction.
Plasmid pTER-BX-UA (17) was mutated via one of two methods. Some mutations were constructed by using a single-stranded template and a mutagenic oligonucleotide (14). The majority of the mutations were made with the Quik-change mutagenesis kit as described by the manufacturer (Stratagene, La Jolla, Calif.). Oligonucleotides of approximately 25 nt were used.
Strains used in this study are derivatives of haploid K. lactis 7B520 (ura3 his3 trp1) (35). A His+ revertant was used to construct many of the ter1 template mutant strains. The wild type is 7B520, while the control strain used in the experiments is strain dhBcl+His1, a His+ revertant strain containing the phenotypically silent TER1-Bcl allele. Previously characterized ter1 template mutants (16-20) were constructed in the 7B520 strain. Mutants were constructed primarily in the dhBcl+His1 strain, although ter1-3C, ter1-28C, ter1-28A, and ter1-28A/30G were constructed in the 7B520 strain, with the plasmid loop-in-loop-out method as described previously (17). When two telomerase alleles were present in the same cell, they existed as heteroalleles and were the product of a plasmid loop in.
Passaging of the mutants was carried out by serial restreaking on rich medium (yeast extract-peptone-dextrose [YPD] plates). They were streaked to single colonies every 3 to 4 days. Each streak is estimated to represent 20 to 25 cell divisions. Transformation was carried out with a modified procedure identical to that used for S. cerevisiae. The procedure was scaled down proportionately so that for each transformation a 1-ml overnight culture was grown to saturation. On the next day, 0.5 ml of YPD liquid was added to the culture to return the cells to the growth phase. The cells were grown for 1 to 2 h, and then 1.5 ml of cells was pelleted at 3,000 × g for 5 min. The supernatant was removed, and the cells were resuspended in 500 μl of cold H2O and repelleted. The cells were resuspended in 80 μl of cold H2O. Ten microliters each of 10× Tris-EDTA and 1 M Li acetate was added, and the mixture was incubated at 30°C for 45 min. The cells were incubated for an additional 15 min after the addition of 2.5 μl of 1 M dithiothreitol. Four hundred microliters of cold H2O was added, and the cells were repelleted. The cells were serially resuspended and pelleted in 250 μl of cold H2O, 200 μl of cold 1 M sorbitol, and 30 μl of cold 1 M sorbitol. The cells were incubated on ice for 2 min after the addition of DNA. The cells were then electroporated at 1,500 or 1,700 V in an Eppendorf electroporator (model 2510), resuspended in 1 M sorbitol, and plated on appropriate selective plates.
Southern blots and hybridizations.
Restriction enzyme-digested yeast genomic DNA was run on 0.8 or 1% agarose gels, stained with ethidium bromide, and blotted onto Hybond N+ membranes (Amersham Biosciences, Piscataway, N.J.). Hybridizations were carried out in Na2HPO4 and sodium dodecyl sulfate at 45 to 55°C (1b). The telomeric oligonucleotide used was the G-strand oligonucleotide Klac1-25 (ACGGATTTGATTAGGTATGTGGTGT).
Telomere cloning.
The types of telomeric repeats synthesized in vivo by the mutated TER1 alleles were determined by direct cloning of telomeres from yeast cells. Telomeres were cloned from the longest-streaked strains available at the time of the experiment. In some cases, telomeres were cloned after as few as 3 serial restreaks, while in other cases as many as 40 serial restreaks had occurred. The two methods for telomere cloning used in this study are described below.
(i) Use of a linear plasmid.
Plasmid pHISLIN1 (20) contains yeast CEN and ARS sequences and has two telomeric tracts in opposite orientations, separated by the URA3 gene. The plasmid was cut with XhoI and BamHI to excise the URA3 gene and yield a linear plasmid with K. lactis telomeres at both ends. The linear DNA was introduced into ter1 template mutants, and transformants were selected for the presence of the plasmid by growth on plates lacking histidine. The DNA prepared from the transformed yeast cells was digested with SmaI, which cleaved one telomere from the plasmid. The remaining plasmid was then ligated into a circle and transformed into Escherichia coli. The telomeres contained in the resulting plasmids were then sequenced.
(ii) Use of an integrative plasmid.
The majority of the telomeres cloned in this study were recovered with one of two integrative plasmids used to clone telomeres from yeast cells. The first, pSTH (subtelomeric HIS3), was made by inserting an ∼600-bp EcoRI-SalI fragment of subtelomeric DNA from pAK25 (18) into pRS423 cut with EcoRI and SalI (31). The plasmid was transformed into his3 ter1 mutants, and transformants were selected by growth on medium lacking histidine. The plasmid was integrated at telomeres by subtelomeric homology. In some instances, transformants were pooled in the cultures used to prepare the genomic DNA. Telomeres were recovered by digesting the genomic DNA with either SnaBI or XhoI, removing the overhangs with T4 DNA polymerase, and ligating the blunt ends into a circle. After transformation into E. coli, the plasmids were prepared and the telomeres were sequenced.
The second plasmid, pMya, is a derivative of the pAK25 plasmid (18) in which the telomeric repeats were deleted by releasing a SacI fragment and then ligating the cohesive ends together. Transformants were selected on medium lacking uracil; the plasmid was integrated into the subtelomeric sequences via homology with the plasmid. Once genomic DNA was prepared from the transformed cells and treated with T4 DNA polymerase and deoxynucleoside triphosphates to make the ends blunt, a linker oligonucleotide containing a SacI restriction site was ligated onto the DNA ends. The DNA was then cut with SacI and ligated to form circles. After transformation into E. coli, plasmids containing a telomere were identified by hybridization of Southern blots with a telomeric probe. Sequencing was carried out either with the Sequenase kit (Amersham Biosciences) or by automated florescent dideoxy sequencing.
RESULTS
Mutations in the TRs of the TER1 template compromise telomere function.
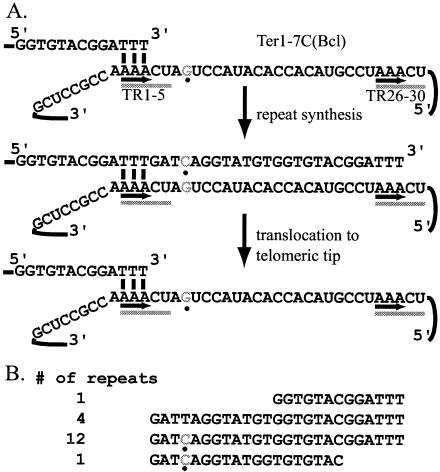
In all known telomerase RNAs, the template region is flanked by short TRs. These TRs are thought to be important for telomerase translocation. The model for the synthesis of new telomeric repeats suggests that alignment occurs between the 3′ end of the telomere and the 3′ TR of the telomerase template. The telomeric repeat would then be reverse transcribed from the template, and the telomeric sequence copied from the 5′ TR would subsequently be used to realign the 3′ TR of the RNA with the telomeric tip in order to initiate the synthesis of another telomeric repeat. Figure 1A shows K. lactis telomerase translocation as described by this model, which will be examined in vivo in this work. In K. lactis, the telomerase RNA (Ter1) has a 30-nt template region that contains 5-nt TRs, referred to as TR1-5 and TR26-30. The terms TR1-5 and TR26-30 will be used to refer to both the 5-nt TRs in the Ter1 template and the sequences in the gene that encodes them but not the telomeric nucleotides that they template. Telomeres in K. lactis are composed of perfect 25-bp repeats, consistent with the idea that K. lactis normally aligns with the telomere with TR1-5.
FIG. 1.
Wild-type telomerase translocation. (A) A diagram of telomerase translocation in K. lactis is shown; the labeling in this diagram will be continued in all subsequent diagrams. The allele TER1-7C(Bcl) is shown, with the point mutation shown in gray and marked with a black dot. The mutation name refers to the point mutation incorporated into the telomeric strand. A short region of the telomeric repeat is shown base pairing with TR1-5 of the template. TR1-5 and TR26-30 are shown underlined with a gray bar. The region between the underlined segments is the 20-nt core of the template. The nucleotides involved in base pairing are underlined with a black arrow; in this model, telomerase synthesis is shown to stop at position 28, so there are 3 nt available to align with the template. Alignment begins at position 1 of the template. Once synthesis is complete, telomerase can dissociate and realign for the next round of telomeric repeat synthesis. (B) The sequence of a telomere cloned from a strain containing TER1-7C(Bcl) is shown. This telomere contains 16 complete telomeric repeats, in addition to the partial repeats at each end. The sequence is shown from the subtelomere-adjacent sequence (top) to the tip of the telomere (bottom). The point mutation is copied into each newly synthesized telomeric repeat. In all figures, the first full line of sequence is the complete 25-bp repeat; all variations can be compared to this line. The point mutations are shown in gray type and marked with a black dot. The number of each type of repeat is shown at the right. The repeat at the tip varies from the penultimate repeat only because it is not a complete repeat.
To examine the role of the Ter1 TRs, mutations were made such that either TR1-5 or TR26-30 was deleted. RNAs containing each deletion also contained different phenotypically silent point mutations, allowing the activity of the mutant telomerase molecules to be examined in vivo. For mutants containing either deletion allele as the sole source of telomerase RNA, the colonies were rough in appearance and exhibited somewhat slower growth than wild-type K. lactis. Although the growth phenotype was similar to that of an early-stage ter1 null mutant (16), neither deletion mutant displayed the growth senescence and eventual cell death that are characteristic of ter1 null mutants. The cells maintained the ability to grow indefinitely, albeit at the somewhat slower than normal rate. Examination of the telomeres by Southern blotting indicated that they were very short and became slightly shorter after cleavage with BclI or ApaLI (the enzyme sites specified by the mutant templates) (data not shown). The lack of senescence and the detectable shortening after cleavage with BclI or ApaLI indicate that these telomerases were at least partially functional. Consistent with this, a single cloned telomere recovered from ter1-Δ1-5 showed a single ApaLI site, present in the most TR of the telomere (data not shown). When the ter1-Δ1-5 and ter1-Δ26-30 alleles were present in the same cell, telomeres were, at most, slightly longer than in either single mutant (data not shown). Cloning of the telomeres, however, revealed that both of the phenotypically silent point mutations could be incorporated into a single telomeric repeat (Fig. 2, shown with gray nucleotides), indicating that telomerase molecules containing either TR deletion could function. Incorporation of both base changes was seen in one of two cloned telomeres. In the second cloned telomere, only one mutation was incorporated. Both of the cloned telomeres were extremely short (less than 125 bp), consistent with the short telomeres seen on the Southern blot. To minimize the possibility of artifacts, all of the telomeres described in this report were cloned with plasmid rescue techniques instead of PCR-based protocols (see Materials and Methods). Thus, when ter1-ΔTR1-5 and ter1-ΔTR26-30 were both present in the same cell, it was possible for chromosome ends to acquire more than one newly synthesized telomeric repeat. These results show that, although the TRs of the telomerase template were important for proper telomerase function, their deletion did not abolish telomerase activity.
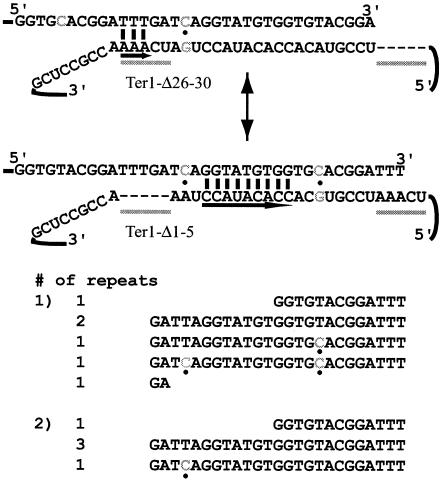
FIG. 2.
Two telomerase molecules can work cooperatively to synthesize a telomeric repeat. A model for telomerase translocation in cells containing ter1-Δ26-30 and ter1-Δ26-30 is shown. The positions of the deleted nucleotides are marked with dashes, and the regions of the TRs are underlined with gray bars. The telomerase shown at the top, from the ter1-Δ26-30 mutant, also contains a 7C point mutation (shown in gray and marked with a dot). This molecule can base pair by using TR1-5 but lacks TR26-30. The second molecule, from the ter1-Δ1-5 mutant, could base pair by using nucleotides in the template core (underlined with a long black arrow) and is marked with the 20C (shown in gray and marked with a dot) mutation. Note that, in order for the 20C mutation to be incorporated, loss of some telomeric sequence is necessary after synthesis by the ter1-Δ26-30 telomerase. Telomeres cloned from the heteroallelic strain are shown below the model. In one telomere, incorporation of both point mutations can be seen (gray nucleotides, marked with dots); in the other, only one has been incorporated.
Base substitutions in the 20-nt core of the Ter1 template found between TR1-5 and TR26-30 appear to typically be incorporated into the 25-bp telomeric repeat as the expected base changes. First, telomeres from a strain containing the phenotypically silent allele TER1-7C(Bcl) (previously published as TER1-Bcl) were cloned and contained only the expected single-base change in each newly synthesized telomeric repeat (20, 34) (Fig. 1B). Telomeres were also recovered from three mutants that contained telomerase alleles that led to the formation of very long telomeres, and the telomeres from all three strains contained only the expected change (D. H. Underwood et al., unpublished data) (17). These results suggest that mutations made in the core of the template do not routinely affect the process of translocation.
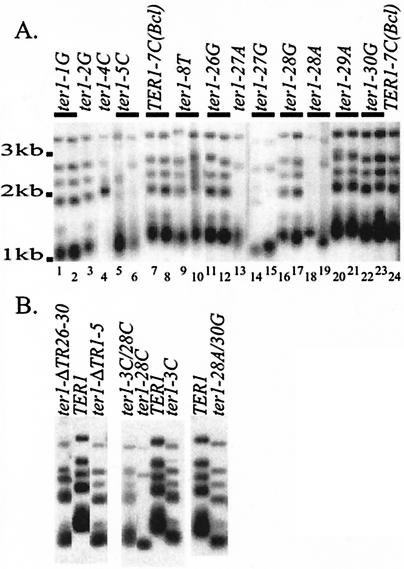
In order to examine the role of the K. lactis telomerase TRs in telomerase translocation, a series of point mutations were made in TR1-5 and TR26-30. The mutations are named in terms of the position that was mutated, such that ter1-2G indicates that position 2 of the template was altered and the sequence that would be copied into the telomere from that position is a G. K. lactis strains containing TER1 alleles with template point mutations as their only source of the telomerase RNA were constructed. The mutants described in this work were part of a larger project involving mutagenesis of each position in the template (Underwood et al., unpublished). The telomere length in the TR mutants was typically found to be shorter than that of wild-type cells. A Southern blot assay of many of the TR mutants after 40 serial restreaks is shown in Fig. 3. Mutations in the first three positions of TR1-5 led to significant telomere shortening, while mutations in positions 4 and 5 of TR1-5 initially exhibited mild telomere shortening that persisted over an extended growth period (Fig. 3). After prolonged passaging, the telomeres in ter1-4T became nearly wild type in length, while ter1-5T telomeres sometimes became elongated by hundreds of bases (Underwood et al., unpublished; data not shown). The alleles ter1-27A, ter1-27G, ter1-28A, and ter1-28C led to telomere shortening. However, ter1-26G, ter1-30G, and ter1-28G had no apparent effect on telomere length and ter1-29A led to very mild telomere elongation (Fig. 3A and B).
FIG. 3.
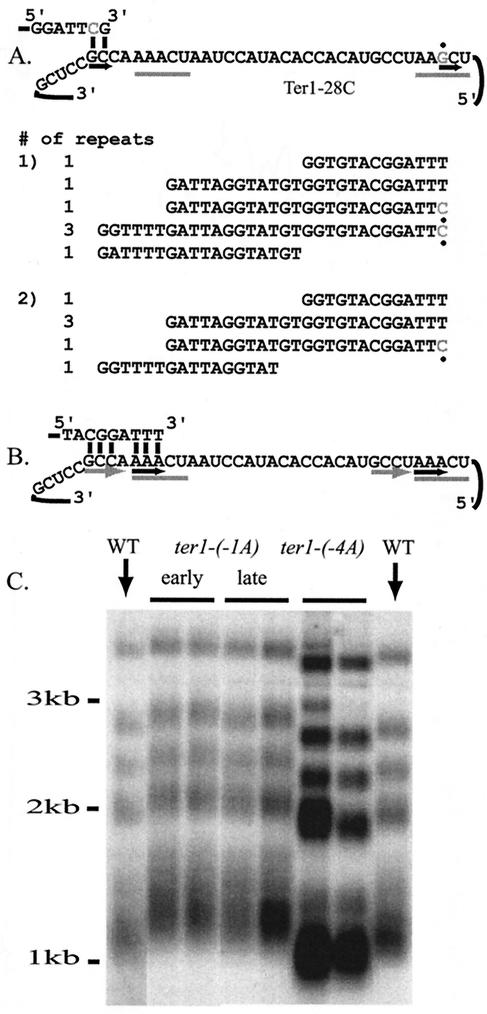
Point mutations in TR1-5 and TR26-30 often lead to telomere shortening. (A) Southern blots of EcoRI-digested genomic DNA hybridized with a telomeric probe are shown. The names of the TER1 alleles are shown above the lanes. Lines above the lanes indicate that two clones containing the mutation are shown. The 40th streak was used. Lanes labeled TER1-7C(Bcl) show telomeres that were wild type in length. (B) Southern blots of telomeres from strains containing the TR deletion mutations and double mutations are shown as in panel A. The mutants are shown at the 1st (ter1-ΔTR1-5, ter1-ΔTR26-30, and ter1-28A/30G), 5th (ter1-3C and ter1-3C/28C), or 10th (ter1-28C) streak. In each mutant, telomere lengths did not change after streak 1.
In addition to changes in telomere length, long-term passaging of the TER1 template mutants revealed that many had elevated levels of subtelomeric recombination. This was indicated by loss of EcoRI restriction fragments in Southern blot assays (Fig. 3A, compare lanes 2, 4, 5, 6, 9, 13, 15, 18, and 19 with lanes 7 and 8, which showed a banding pattern identical to that of the wild type, and B, second lane of the second part). Such band loss has been shown to be due to gene conversion events that homogenize subtelomeric polymorphisms caused by a compromised telomere-capping function (18).
Three point mutations in TR1-5 and TR26-30 lead to minor shifts in alignment.
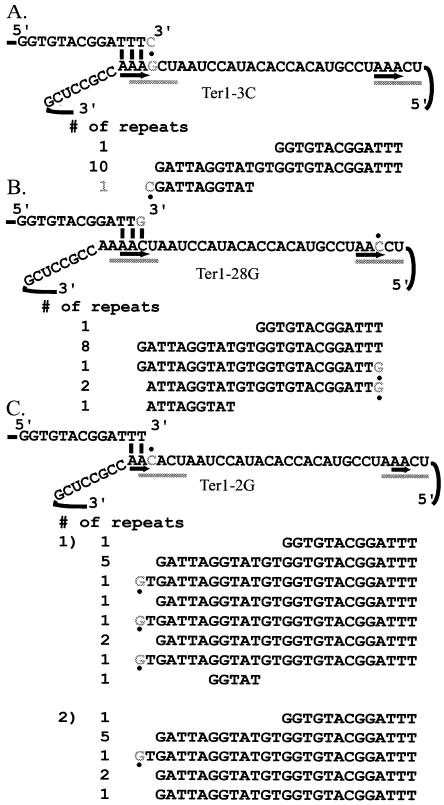
In order to study the effects of the TER1 template mutations on telomerase translocation, telomeres were cloned from the mutants. The sequencing of telomeres allowed models to be made that explained the translocation events that would have occurred in order for those particular sequences to be copied by telomerase. Telomeres were cloned from 10 TR mutants; three mutations, ter1-3C, ter1-28G, and ter1-2G, led to 1- or 2-nt shifts in the normal alignment between telomerase and the telomere. When telomeres were cloned from the ter1-3C strain, a 26-bp repeat was seen near the terminus of two of the three telomeres cloned, instead of the 25-bp length of wild-type repeats. The small number of mutant repeats recovered may be because the mutants had only been grown for 90 cell divisions prior to cloning of the telomeres and therefore contained very few newly synthesized telomeric repeats. Synthesis of the 26-bp repeat appeared to be due to a shift of the template, such that instead of the alignment being between the telomeric tip and positions 1 to 3 of the template it instead involved the position immediately 3′ of the template (position −1) and positions 1 and 2 (Fig. 4A). This assumes that the telomeric tip terminates in TTT, copied from positions 26 to 28. Previous work had indicated that position 28 was the predominant stop site for K. lactis telomerase in vitro (8). The mutation at position 3 would disrupt base pairing of the 3-nt complementary region normally used for alignment. Therefore, the region of telomerase used for alignment was shifted by 1 nt in order to restore perfect base pairing between the 3′ end of the telomere and the template.
FIG. 4.
Some point mutations in TR1-5 and TR26-30 lead to minor shifts in alignment with telomerase. (A) The telomerase allele ter1-3C causes the synthesis of a 26-bp repeat. The presence of the mutation at position 3 of the template causes telomerase alignment to shift by 1 nt, such that alignment begins at a nucleotide 3′ of the normal start site. (B) A 24-bp repeat is synthesized in the strain containing ter1-28G. When a G is copied from position 28, the alignment shifts by 1 nt toward the 5′ end of the template. Base pairing then occurs with positions 2 to 4 of the template. (C) ter1-2G can cause a 27-bp repeat to be synthesized. When a C is present at position 2 of the template, telomerase alignment is sometimes shifted by 2 nt. In this case, only 2 nt of base pairing is apparent between the tip of the telomere and the template.
Similarly, the ter1-28G mutation caused a 1-nt shift into the template where the base-pairing interaction apparently occurred with positions 2 to 4 of the template (Fig. 4B). This led to the synthesis of a 24-bp repeat that was observed three times in a single telomere. Finally, the allele ter1-2G sometimes caused a 27-bp repeat to be synthesized. This long repeat was observed a total of four times in the two telomeres examined (Fig. 4C). For this to occur, the TT sequence copied from positions 27 and 28 was predicted to base pair with positions −1 and 1 at the start of the template. When this happened, the mutation at position 2 could be copied into the telomere. The shifts in the region of telomerase that was aligned with the telomere in the ter1-3C, ter1-28G, and ter1-2C mutants indicate that mismatches between telomerase and the telomeric tip are not typically removed by a proofreading activity; instead, they lead to the synthesis of aberrantly sized telomeric repeats.
Translocation to sites within the core of the template can occur.
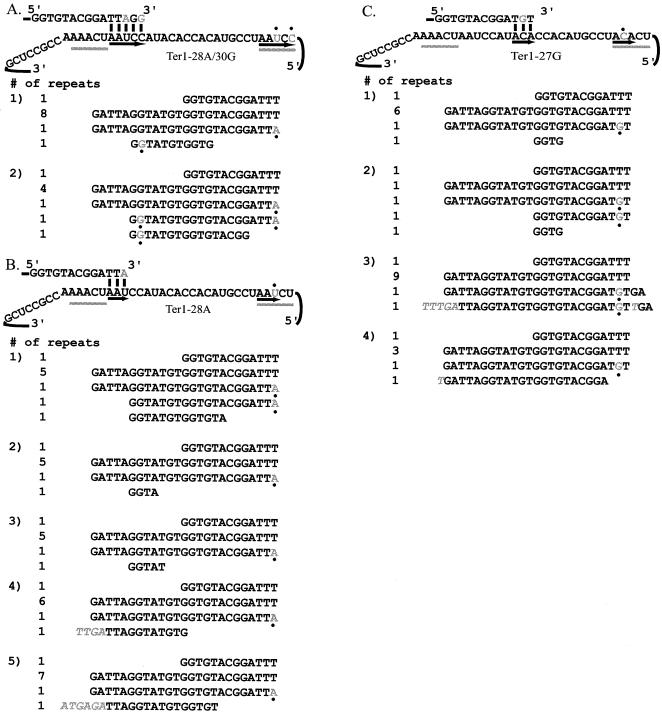
In order to see if translocation to sites within the core of the template can occur, double point mutations were made at positions 28 and 30 of the template. The ter1-28A/30G mutation caused the sequence of TR26-30 to no longer be identical to TR1-5; instead, it was identical to the 5 nt at template positions 6 to 10 (Fig. 5A). This allele was thus predicted to lead to the synthesis of 20-bp repeats that lacked the sequence from TR1-5. When telomeres were cloned from a strain containing ter1-28A/30G as its only telomerase RNA allele, 20-bp repeats of the predicted sequence were found at the tips of the telomeres (Fig. 5A). This indicated that the sequence added at the tip of the telomere by the mutated TER1 allele could base pair with a sequence within the core of the template for the next round of telomere synthesis.
FIG. 5.
The telomere can align with nucleotides within the core of the template. (A) ter1-28A/30G copies a 20-bp repeat. The telomeric repeats made in the ter1-28A/30G strain are 20 bp long. It is unclear whether position 30 is typically copied in vivo; however, the mutations cause the sequence in TR26-30 to be identical to the sequence at positions 6 to 10 (underlined with arrows). The change potentially templated by position 30 is shown in gray, as is the mutation introduced by position 28 (marked with dots). (B) ter1-28A copies a 20-nt repeat or stutters. Telomeres cloned from a strain containing ter1-28A often contain 20-bp repeats identical to those made in the ter1-28A/30G mutant strain. Sometimes, however, a sequence that is not easily explained was found (shown in gray italics). (C) ter1-27G leads to the synthesis of 13-nt repeats or unusually long repeats. When a G is copied from position 27, the telomeric tip can base pair with positions 13 to 15 of the template, leading to the synthesis of a 13-nt repeat. In other cases, aberrant synthesis occurs (extra sequence shown in gray italics).
If template position 30 is not normally copied by telomerase, as suggested by in vitro data (8), it would be expected that the single mutant ter1-28A might lead to the synthesis of the same 20-bp repeats synthesized by ter1-28A/30G. To examine this, the allele ter1-28A was made. When ter1-28A mutant telomeres were cloned, it was found that the same 20-bp repeat found in the double point mutant was often copied (Fig. 5B). This could occur whether telomerase typically stopped copying at either template position 28, where the single mutation could direct the synthesis of the 20-bp repeat, or template position 29, which already matched the sequence needed to base pair with position 9 and would thus be expected to have no effect. In two of the five clones, however, aberrant translocation events occurred. In these instances, 4 or 6 bp of additional sequence was found between the incorporation of the mutation at the end of the repeat and the start of the next newly synthesized repeat. In the example in which four extra bases were seen (Fig. 5B, tip repeat of telomere 4), a single imperfectly base-paired alignment event with the region near TR1-5 could have led to their incorporation. In the second case (Fig. 5B, tip repeat of telomere 5), however, no single misalignment could account for the extra 4 nt. One possible explanation is that the additional sequence was incorporated when telomerase stuttered as it tried different alignments in the TR1-5 region before finding an appropriate one and synthesizing a full repeat. The fact that the repeats made by ter1-28A were not all 20 bp in length may indicate that position 30 is copied at low frequency. The nucleotide copied from position 30 might, when incorporated, also be involved in base pairing during translocation.
The most dramatic example of aberrant translocation leading to an altered telomeric repeat size was observed in a strain with the ter1-27G allele (Fig. 3A). In this case, the sequence copied from positions 26 to 28 would be TGT. As with the previously described allele, cloned telomeres indicate that more than one type of repeat was synthesized by ter1-27G. The most easily explained was a 13-bp repeat that apparently occurred when the telomeric tip base paired with positions 13 to 15 of the template (Fig. 5C). As the TGT sequence is also found at positions 17 to 19, it is possible that copying position 29 required additional base pairing that forced alignment to occur with positions 13 to 16. The 13-bp repeat was observed a combined total of three times in two of the four telomeres cloned (Fig. 5C, telomeres 1 and 2). No repeat shorter than 13 bp has been found to be synthesized by any K. lactis TER1 allele to date. In three other repeats, additional sequences were found (Fig. 5C, telomeres 3 and 4, shown in gray italics) between the incorporation of the mutation at the end of one repeat and the start of the next newly synthesized repeat. Although the 5 nt at the beginning of the TR of telomere 3 matched TR1-5, it was unclear what base-pairing interactions led to their incorporation into the telomere. For the small additions found at the end of the TR in telomere 3 and the start of the tip repeat in telomere 4, it was again unclear what base-pairing interactions caused their incorporation, although they could have been copied from TR1-5 or mutated TR26-30.
Mutations within the core of the template can cause aberrant translocation.
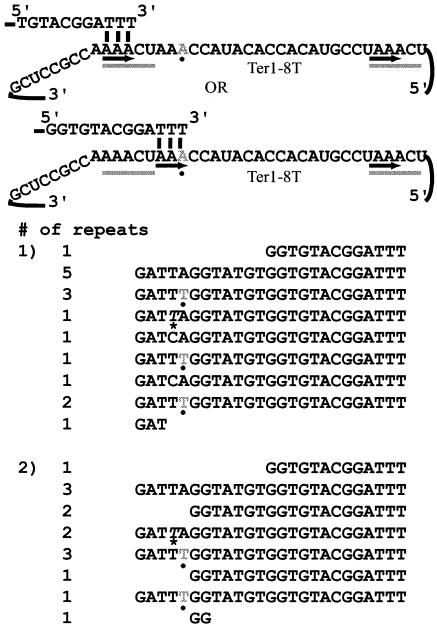
The mutations described thus far have all been within either TR1-5 or TR26-30. In order to test whether mutations within the core of the template could also cause aberrant translocation events, the mutation ter1-8T was created. This mutation could potentially function in a manner similar to that of ter1-28A in that the sequence copied from position 26 to position 29 would be a perfect complement to template positions 6 to 9. The unusual characteristic of this mutant, however, was that template positions 1 to 4 and 6 to 9 are identical. Therefore, it was expected that telomerase might be able to base pair at either location.
It was found that two types of repeat were copied by ter1-8T (Fig. 6). One type was 25 bp long and contained a single-base change at position 8, consistent with incorporation of the mutant nucleotide into the telomere following normal alignment between the telomere and TR1-5. The other repeats were 20 bp in length, consistent with the telomeric tip base pairing with telomerase positions 6 to 9. In the six cloned telomeres examined, there was a significant bias toward the 25-bp repeat containing the point mutation; 44 such repeats were found, compared with 8 of the 20-bp repeats.
FIG. 6.
Mutations in the template core can alter translocation. The mutation ter1-8T (shown in gray, marked with a dot) can lead to the synthesis of two types of repeat. In the first type, the telomere base pairs with TR1-5 (shown in the upper alignment) and incorporates the mutation as a single-base change (shown in gray in the upper sequence). In the second type (lower alignment), base pairing occurs within the template and leads to the synthesis of a 20-bp repeat. Sequences from two telomeres cloned from the ter1-8T mutant are shown. Wild-type repeats (identified by the italicized nucleotide at position 7 and an asterisk) are interspersed with the ter1-8T and TER1-7C(Bcl) repeats (which have a C at position 7) made by telomerase alleles in the cell.
Unexpectedly, there was evidence of telomeric recombination in four of the six telomeres cloned from the ter1-8T mutant. This ter1 mutant, like most of those in this study (see Materials and Methods), was made in a background that initially contained phenotypically silent TER1-7C(Bcl). Therefore, its telomeres contained wild-type repeats basally and Bcl repeats [templated by the TER1-7C(Bcl) telomerase] distally prior to introduction of the ter1-8T mutant allele. For a brief time, TER1-7C(Bcl) and the ter1-8T allele were in the cell simultaneously and thus the telomere might contain both types of mutant repeats intermingled. In this strain, however, wild-type, TER1-7C(Bcl)-templated, and ter1-8T-templated repeats were interspersed at the telomere (Fig. 6, wild-type sequences marked with asterisks). The presence of wild-type repeats nearer to the telomeric tip than repeats templated by ter1-8T is not an arrangement that could have been generated by the action of telomerase, as the wild-type TER1 allele was deleted in the strain prior to the introduction of ter1-8T. A likely explanation is the occurrence of recombination between the arrays of telomeric repeats.
Template positions 29 and 30 are sometimes copied.
Analysis of telomeres cloned from the ter1-28A mutant supports the hypothesis that, in vivo, K. lactis telomerase normally copies position 28 of the template. Telomeres cloned from strains with TER1 mutations at positions 4, 5, 29, and 30 provided a way to examine whether telomerase also copied positions 29 and 30 in vivo. If a position was copied in vivo, then the presence of a TER1 allele mutated at that position would lead to incorporation of a mutated nucleotide into the newly synthesized telomeric repeats and could potentially cause aberrant translocation and thus abnormally sized telomeric repeats. Telomeres cloned from a strain containing ter1-5C indicated that position 5 was usually copied by telomerase. If the repeats that had incorporated the mutation at position 5 were counted, starting from the first repeat that was known to be synthesized by ter1-5C, position 5 was incorporated 18 out of 24 times in the two telomeres examined. When the same calculation was made with telomeres cloned from the ter1-4C mutant, position 4 was found to be incorporated 17 out of 25 times in the four telomeres examined. In both mutants, the mutation was incorporated into 25-bp repeats as the single expected base change.
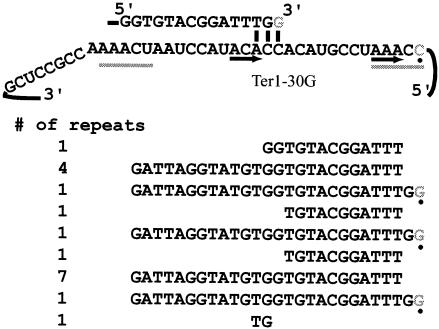
The results obtained from strains containing ter1-4C and ter1-5C were expected to have an inverse relationship with the numbers obtained when strains mutated at position 29 or 30 were used. The model shown in Fig. 1 predicted that, for each repeat synthesized, the telomeric sequence was copied from either position 4 or 29 in a mutually exclusive manner. The same would be true for positions 5 and 30. When six telomeres from ter1-29A were cloned, the repeats were observed to be completely wild type. Possible reasons for the complete lack of mutant repeats will be described in the Discussion. The telomeres cloned from this strain were slightly longer than average for K. lactis (data not shown), consistent with the slightly long appearance of the telomeres on a Southern blot (Fig. 2). Only one telomere was recovered from the strain containing ter1-30G, but the telomere contained three repeats that showed incorporation of the mutation at position 30. Interestingly, this mutation led to an aberrant translocation event that produced a 13-bp repeat; this apparently occurred through base pairing between the telomere and positions 15 to 17 of the template (Fig. 7). Although additional base-pairing interactions are not shown in Fig. 7, several nucleotides within the telomeric repeat could potentially be involved in base pairing with the template. These include the nucleotides at positions 7, 8, 10, 12, and 13. This repeat is the same 13 bp copied by ter1-27G, although they differ by 2 nt owing to the sites of the mutations. The results presented here indicate that, although telomerase can copy the 5′ end of the template, up to and including position 30, copying of positions 4 and 5 is more common.
FIG. 7.
ter1-30G can synthesize a 13-bp repeat. A telomere cloned from a strain containing ter1-30G contained repeats in which position 30 was copied. In each case, the subsequent repeat synthesized was 13 bp long.
The region immediately 3′ of the template is available for base pairing and is important in vivo.
The third TER1 allele made with a mutation at position 28 was ter1-28C. Like ter1-28A, ter1-28C led to shortened telomeres (18) and translocation defects. When telomeres were cloned from a strain containing ter1-28C, telomeric repeats that were 31 bp long were found (Fig. 8A). In order for these repeats to be made, the telomere must have base paired with nucleotides 3′ of template position 1 (referred to as −3 and −4, respectively). This indicates that this region of the telomerase RNA was accessible for base pairing with the telomere. Interestingly, the sequence found at template positions −4, −3, and −2 was identical to that found at template positions 22, 23, and 24 (Fig. 8B). This sequence identity, coupled with the fact that the region 3′ of the template was available for base pairing, led to the idea that these positions might be involved in stabilizing the initial alignment between telomerase and the telomere in wild-type cells. As an initial test of this, two point mutations were made in the sequence immediately 3′ of the telomere. The first, ter1-(−4A), disrupted the potential for base pairing between that position and the telomere and led to moderate telomere shortening (Fig. 8C). The second was ter1-(−1A). This position, which was normally a mismatch for any potential interaction with the telomeric nucleotide copied from position 25, was altered so that base pairing could occur. This mutation led to mild telomere elongation. Two cloned telomeres recovered from ter1-(−1A) showed no evidence of an abnormal telomeric sequence (data not shown). Our results clearly indicate that the sequence immediately 3′ of the template plays an important role in telomerase function.
FIG. 8.
The region 3′ of the template is available for base pairing. (A) ter1-28C leads to the synthesis of 31-bp repeats. When position 28, and possibly position 29, is copied, the telomeric tip base pairs with the nucleotides 3′ of template position 1. The positions, referred to as −3 and −4, are underlined with a black arrow at the left. Multiple repeats were found in one cloned telomere, while a single long repeat was found in the other. (B) The region 3′ of the template may stabilize alignment during wild-type translocation. Alignment of a wild-type telomeric repeat with the telomerase template reveals that additional base pairing might occur outside TR1-5. The region 3′ of the template, at positions −2, −3, and −4, is identical to that at template positions 22, 23, and 24. The telomere copied from these positions might base pair with the region 3′ of the template. Both regions are underlined with gray arrows. (C) Mutations 3′ of the template can cause telomere length phenotypes. Two mutations were made in the region 3′ of the template. The first, ter1-(−1A), changes the nucleotide at the −1 position so that it could base pair with the nucleotide templated by position 25 in the template. This mutant exhibits a mild but stable elongation of the telomeres. The second mutation, at nt −4 [ter1-(−4A)], disrupts its base-pairing potential. This mutation leads to telomere shortening. WT, wild type.
DISCUSSION
Proper telomerase translocation requires that each of several steps in the process occur correctly. Therefore, in order to examine telomerase translocation in vivo, it is necessary to address a number of different questions. First, it is necessary to determine what nucleotides in the telomerase template are reverse transcribed into the telomeric repeat. It is also necessary to determine what positions within both the telomere and the telomerase template contribute to base pairing during translocation. Another issue is whether the K. lactis telomerase has a nuclease activity that could remove mismatched bases from the telomere upon alignment with the Ter1 RNA. A related question involves determining whether mismatches between the tip nucleotides of the telomere and the telomerase RNA inhibit the ability of telomerase to extend the telomeric tip. It is also possible that the structure of the telomerase complex imposes steric constraints on the alignment region. All of the factors above combine to determine what telomeric repeats are synthesized by telomerase.
In order to test the base-pairing requirements for telomerase translocation, mutations in TR1-5 and TR26-30 were made and telomeres from the resulting mutants were cloned and sequenced. Models were then created to explain the aberrant translocation events observed in the mutants. Several factors complicated the construction of the models. First, it was not possible to determine how all of the factors described above were affecting a given mutant; although incorporation of the mutation could be easily discerned from the telomere sequences, the exact stop site of telomeric repeat synthesis, for example, might not be obvious. It was therefore necessary to view the totality of the data before many conclusions could be made. Also, in some cases only a very few mutated repeats were sequenced. Because many of the mutations lead to telomere shortening, it is often impossible to recover long tracts of mutated repeats. However, the plasmid rescue method used is unlikely to introduce errors into the sequence, making the sequences recovered likely to be reliable. The aberrant telomeres recovered contrast sharply with wild-type telomeres, in which the only aberrant translocation events found are rare 26-bp repeats that contain an extra T before the three T's of positions 1 to 3 (Y. Tzfati, personal communication). Therefore, because each mutation examined caused different translocation events, specific models were created for each mutant. Although the models may describe only a subset of the events that actually occur, they provide valuable insight into how telomerase functions.
Mismatches between the telomeric tip and the template are not typically removed.
A nuclease activity has been reported to be associated with telomerase in Euplotes crassus, Tetrahymena thermophila, and Saccharomyces cerevisiae (2, 3, 13, 15, 21, 22, 26). In E. crassus, the nuclease has been shown to cleave by an endonucleolytic mechanism that preferentially removes mismatches by cleaving single-stranded DNA at the junctions of mismatches between the telomeric primer and the telomerase template (13, 21). In S. cerevisiae, mismatches between the telomeric sequence and the telomerase template have been shown to stimulate cleavage of the single-stranded telomeric primer by the telomerase-associated nuclease, which would also lead to removal of the mismatch (25). However, there was also no evidence that a nuclease activity acted on mutated telomeric repeats in Tetrahymena in vivo, where mutations in the telomerase template led to the synthesis of aberrantly sized telomeric repeats (36). In K. lactis, mismatches between telomerase and the template introduced by telomerase template mutations do not typically appear to be resolved via the action of a nuclease. In the majority of the mutants described here, the mismatch between telomerase and the telomere could have been eliminated by the removal of 1 or 2 nt from the tip of the telomere. Instead, however, aberrant translocation events were the mechanism by which telomerase dealt with the mismatch. When mutations outside the template caused telomerase to copy the Ter1 sequence beyond TR26-30, the additional sequence also did not appear to be cleaved (34; Tzfati, personal communication). Instead, when multiple mutant telomeric repeats were present, aberrant translocation events involving perfect base pairing between the sequence at the telomeric tip and the telomerase template had occurred. Although there is no evidence that a proofreading activity acts during translocation, the presence of a proofreading activity that acts during the synthesis step of telomere addition cannot be ruled out.
Telomerase can copy the entire 30-nt template in vivo.
An important step in examining telomerase translocation in vivo is determining where in the template telomerase synthesis stops. In vitro evidence indicates that K. lactis telomerase normally stops copying at position 28 of the template (8), and in creating models of aberrant translocation events it is useful to know if this is also the case in vivo. In all 10 of the telomeres cloned from strains in which position 28 of the template was mutated, the mutated nucleotide was incorporated into the telomere. No repeats failing to incorporate the mutated nucleotide were found distal to the most basal mutated repeats. This indicates that position 28 is normally copied into the telomere. Telomeres were cloned from all four template mutants that could be informative in answering the question of whether the last 2 nt of the template, positions 29 and 30, are copied. On the basis of the results obtained with the cloned telomeres, it appears that positions 4, 5, 29, and 30 all are copied sometimes. The mutation predicted by each specific allele was sometimes incorporated, and in the case of ter1-30G, it led to the synthesis of aberrantly sized repeats that were 13 bp in length. Although no clone containing a mutated telomere was recovered from the strain containing ter1-29A, it is clear that if position 30 is copied, then position 29 must also be copied at some frequency. There are several possible reasons why the ter1-29A mutation was not observed in the telomeres examined. First, it is clear that position 30, and possibly position 29, is not copied during every translocation event. When the mutation templated by ter1-29A is copied, however, it is not clear what translocation event(s) would allow another repeat to be synthesized. It is possible that telomeric termini containing the mutation templated by ter1-29A are not readily extended because they cannot base pair efficiently with any part of the template. The nucleotides at the tip, and thus the mutated nucleotides, would therefore be lost during the normal turnover at the telomere. It is also possible that the mutation is incorporated and then the resulting mismatch is cleaved by a telomerase-associated nuclease; this seems unlikely in view of the results obtained with other mutations. Finally, it is possible that it is by chance that no mutated repeats were recovered from this strain.
If positions 29 and/or 30 are not always copied, then positions 4 and/or 5 must sometimes be copied. Telomeres cloned from the ter1-4C and ter1-5C mutants showed that positions 4 and 5 are often copied. However, wild-type repeats were intermingled with the mutant repeats in a manner similar to the telomeres cloned from the ter1-8T mutant (see below). One possible explanation for this is that these repeats were incorporated by telomeric recombination. It is also possible that these repeats were incorporated when telomerase copied the wild-type sequence at position 29 or positions 29 and 30 and subsequently extended a mismatched base pair. Mismatch extension did not typically occur in the other mutants examined in this study (see below); in the strain containing ter1-30G, the mismatch would also be between template position 5 and the telomere, and yet aberrant translocation rather than mismatch extension was observed. Therefore, it seems unlikely that a mismatch at the same position would be extended in the strain containing ter1-5C. The results obtained with strains with mutations at template positions 4, 5, 29, and 30 therefore suggest that, although telomerase can copy the entire template, the stop site for telomere synthesis is usually position 28 of the template.
Mismatches at the 3′-terminal nucleotides are not typically extended.
More than 250 telomeric repeats, synthesized by different telomerase alleles, have been examined, and of these, only 6 contain sequence that cannot be ascribed to a single translocation event that involves extending a telomere that base pairs perfectly with the telomerase RNA. This emphasizes the importance of base-pairing interactions in telomerase function. However, extension of mismatched primers by the reverse transcriptase from spleen necrosis virus has been reported to occur ∼20% of the time in vivo (27), so it was of interest to determine whether telomerase also extends such mismatches. Both mismatches at the terminal nucleotide of the telomere-Ter1 alignment region and those within the alignment region were examined. Mismatches at the terminal nucleotide of the telomere, which would cause a mismatch to have to be directly extended, will be addressed first. Telomeric repeat synthesis often stops at position 28, and any mutation at that position would be expected to cause a mismatch to be present at the tip of the alignment region. If base pairing with the wild-type positions of TR1-5 occurred, telomerase would be forced to extend mismatched nucleotides. In the cases of all three mutations at position 28 of the template, aberrant translocation occurs rather than the extension of a mismatch. The aberrant alignments ranged from 1-nt shifts to more dramatic changes causing 20- or 31-nt repeats to be synthesized. One mutation, ter1-28C, causes the telomere to align with a region outside of the template. This alignment, which, at the most, involves only 2 contiguous complementary nt between the telomere and telomerase, maintains perfect base pairing at the tip of the alignment region. A final example of the preference for perfect base pairing at the tip of the alignment region is found when telomeres synthesized by ter1-30G are examined. When the mutated nucleotide incorporated at position 30 is observed, an aberrant translocation event, which leads to the synthesis of a 13-bp repeat, always occurs despite the potential for base pairing between TR1-5 and the wild-type sequence copied from position 26 to position 29. This base-pairing interaction also involves a region that is only 2 nt long. In the last two examples, aberrant translocation occurs despite the fact that there are a minimum of 2 or 4 nt, respectively, that could be involved in base pairing with TR1-5; the potential for additional base pairing that could stabilize alignment with TR1-5 will be discussed below. In all of the examples, alignment with TR1-5, the site of alignment in wild-type cells, would have required only that a single mismatch at the end of the alignment region be tolerated.
The second class of mismatches involves those that are not at the tip of the region used for base pairing and would thus not be directly extended by telomerase. The mutations described in this work offer some evidence that mismatches at the penultimate telomeric nucleotide can also affect alignment during translocation. A mutation at position 2 of the template, as found in the ter1-2G allele, might be predicted to have no effect, since base pairing at the tip of the alignment region would be maintained. The telomeres recovered from a strain containing ter1-2G, however, show that an alternative base pairing occurred in a significant percentage of the repeats. This indicates that a mismatch between telomerase and the telomere that is not predicted to be at the tip of the alignment region can still affect translocation. The alternative alignment did not occur in every repeat, which could be due to the specific site where telomere synthesis stopped. Copying position 29 would still allow 2 nt of perfect base pairing with TR1-5 beyond the mismatch at position 2, while stopping at position 28 would only allow 1 nt of base pairing after the mismatch. Thus, a shift might sometimes occur to avoid alignments with single base pairs. The telomeres cloned from the ter1-27G mutant indicate that a similar situation occurs in this strain. Telomeric repeats synthesized by ter1-27G are predicted to most often terminate in the sequence TGT; if position 29 is the terminal position copied, then the sequence at the telomeric tip would be TGTG. If a telomeric repeat containing the mutation at position 27 base paired with TR1-5, the mismatch would be with position 2 of the template. Despite the fact that the alignment region would likely terminate with 1 or 2 nt of perfect base pairing, no 25-bp repeats containing the G mutation templated by position 27 were recovered. Instead, alignment occurred at an alternate site within the core of the template, which allowed the template to maintain perfect base pairing with the 3 nt at the telomeric tip.
The results described in this section emphasize two important points. First, mismatches between the telomeric tip and the telomerase RNA are not typically extended. Second, the minimum preferred amount of base pairing at the telomeric tip appears to be 2 nt. The great majority of the telomeric repeats examined were synthesized following translocation events that maintained perfect base pairing between the terminal 2 or 3 nt at the telomeric tip and the telomerase RNA. In all of the strains, with one possible exception, at least 2 nt were involved in the alignment between the telomere and telomerase. The one possible exception is found with the allele ter1-28C. With this allele, only 1 bp is used unless position 29 is also copied. Since the entire template can be copied, it is not unreasonable to think that position 29 might have been copied in this instance.
A possible new determinant of telomerase translocation in K. lactis.
The data presented in this work suggest that telomere-template alignment preferentially occurs in the area of the template near TR1-5. The few repeats seen in this study that could not be explained by a single translocation event contained sequences that appeared to be copied from this region. In addition, the wild-type alignment is always with TR1-5 of the template, despite the potential for a few nucleotides of base pairing elsewhere in the template, and single-base changes in TR1-5 caused only minor shifts in the region of Ter1 involved in base pairing. Together, these results suggest that TR1-5 is not the sole determinant of telomerase translocation. It is possible that telomere-associated proteins play a role in positioning telomerase correctly. Another possibility is that 3 nt, located 3′ of the template, may be involved in base pairing with nt 22 to 24 of the telomeric repeat (Fig. 8). This extra base pairing could stabilize the interaction between the telomeric repeat and the telomerase template. A mutation in the region 3′ of the template that disrupted base-pairing potential led to telomere shortening, while a mutation that added additional base-pairing potential caused mild telomere elongation, indicating that this region may play a role in telomerase-mediated telomere maintenance. However, it is difficult to test the base-pairing model directly. Compensatory changes in the region 3′ of the template and in the template itself—for example, changing position −4 and also position 22—could be difficult to interpret. Mutations in positions 22 to 24 lead to short telomeres, indicating that the positions are involved in a positive telomeric function (34; Underwood et al., unpublished). These telomeric positions are part of the Rap1p binding site and have been proposed to be part of the binding site for a single-strand telomere binding protein, such as Cdc13p or Est1p. Although it is possible that the telomere shortening is due to defects in translocation, the length phenotype is more severe than that of the ter-(−4)A mutant strain. This is consistent with the idea that the region may have another function and complicates analysis of the base-pairing requirement.
Our data show that many mutations in TR1-5 and TR26-30 cause aberrant translocation events and lead to telomere shortening. Although the disruption in normal telomerase translocation could account for the shortened overall telomere length, it is important to keep in mind that abnormal telomere function might have other contributing causes. It is possible that some TER1 template mutations could also disrupt the active site of the telomerase complex. Mutations outside the Ter1 template in K. lactis (29) and within the S. cerevisiae and Tetrahymena telomerase RNA templates (9, 10) have previously been shown to affect telomerase function. Alternatively, template mutations might act through the telomere, perhaps affecting the binding of telomeric proteins and disrupting telomere length regulation or telomere capping. Considerable work is needed to resolve these issues.
Acknowledgments
This work was supported by the American Cancer Society, grants RPG-00-082-01 GMC and RPG-02-253-01 GMC, and NIH GM61645. D.H.U. was supported by an NIH training grant and University of Georgia fellowships.
We thank Jennifer Caffarella, Will McRae, Shobhana Natarajan, Myia Thomas, and Amin Hajiani for help in constructing and using the telomere rescue plasmids.
REFERENCES
- 1.Cech, T. R., T. M. Nakamura, and J. Lingner. 1997. Telomerase is a true reverse transcriptase. A review. Biochemistry (Moscow) 62:1202-1205. [PubMed]
- 1a.Chandra, A., T. R. Hughes, C. I. Nugent, and V. Lundblad. 2001. Cdc13 both positively and negatively regulates telomere replication. Genes Dev. 15:404-414. [DOI] [PMC free article] [PubMed]
- 1b.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn, M., and E. H. Blackburn. 1995. Telomerase in yeast. Science 269:396-400. [DOI] [PubMed] [Google Scholar]
- 3.Collins, K., and C. W. Greider. 1993. Tetrahymena telomerase catalyzes nucleolytic cleavage and nonprocessive elongation. Genes Dev. 7:1364-1376. [DOI] [PubMed] [Google Scholar]
- 3a.Dionne, I., and R. J. Wellinger. 1996.. Cell cycle-regulated generation of single-stranded G-rich DNA in the absence of telomerase. Proc. Natl. Acad. Sci. USA 93:13902-13907. [DOI] [PMC free article] [PubMed]
- 4.Evans, S. K., and V. Lundblad. 1999. Est1 and Cdc13 as comediators of telomerase access. Science 286:117-120. [DOI] [PubMed] [Google Scholar]
- 5.Feng, J., W. D. Funk, S. S. Wang, S. L. Weinrich, A. A. Avilion, C. P. Chiu, R. R. Adams, E. Chang, R. C. Allsopp, and J. Yu. 1995. The RNA component of human telomerase. Science 269:1236-1241. [DOI] [PubMed] [Google Scholar]
- 6.Forstemann, K., M. Hoss, and J. Lingner. 2000. Telomerase-dependent repeat divergence at the 3′ ends of yeast telomeres. Nucleic Acids Res. 28:2690-2694. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forstemann, K., and J. Lingner. 2001. Molecular basis for telomere repeat divergence in budding yeast. Mol. Cell. Biol. 21:7277-7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulton, T. B., and E. H. Blackburn. 1998. Identification of Kluyveromyces lactis telomerase: discontinuous synthesis along the 30-nucleotide-long templating domain. Mol. Cell. Biol. 18:4961-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilley, D., and E. H. Blackburn. 1996. Specific RNA residue interactions required for enzymatic functions of Tetrahymena telomerase. Mol. Cell. Biol. 16:66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilley, D., M. S. Lee, and E. H. Blackburn. 1995. Altering specific telomerase RNA template residues affects active site function. Genes Dev. 9:2214-2226. [DOI] [PubMed] [Google Scholar]
- 11.Grandin, N., C. Damon, and M. Charbonneau. 2000. Cdc13 cooperates with the yeast Ku proteins and Stn1 to regulate telomerase recruitment. Mol. Cell. Biol. 20:8397-8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandin, N., S. I. Reed, and M. Charbonneau. 1997. Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev. 11:512-527. [DOI] [PubMed] [Google Scholar]
- 13.Greene, E. C., J. Bednenko, and D. E. Shippen. 1998. Flexible positioning of the telomerase-associated nuclease leads to preferential elimination of nontelomeric DNA. Mol. Cell. Biol. 18:1544-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunkel, T. A., J. D. Roberts, and R. A. Zakour. 1987. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 154:367-382. [DOI] [PubMed] [Google Scholar]
- 14a.Lin, J. J., and V. A. Zakian. 1996. The Saccharomyces CDC13 protein is a single-strand TG1-3 telomeric DNA-binding protein in vitro that affects telomere behavior in vivo. Proc. Nat. Acad. Sci. USA 93:13760-13765. [DOI] [PMC free article] [PubMed]
- 14b.Lingner, J., T. R. Cech, T. R. Hughes, and V. Lundblad. 1997. Three ever shorter telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc. Natl. Acad. Sci. USA 94:11190-11195. [DOI] [PMC free article] [PubMed]
- 15.Lue, N. F., and Y. Peng. 1997. Identification and characterization of a telomerase activity from Schizosaccharomyces pombe. Nucleic Acids Res. 25:4331-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEachern, M. J., and E. H. Blackburn. 1996. Cap-prevented recombination between terminal telomeric repeat arrays (telomere CPR) maintains telomeres in Kluyveromyces lactis lacking telomerase. Genes Dev. 10:1822-1834. [DOI] [PubMed] [Google Scholar]
- 17.McEachern, M. J., and E. H. Blackburn. 1995. Runaway telomere elongation caused by telomerase RNA gene mutations. Nature 376:403-409. [DOI] [PubMed] [Google Scholar]
- 18.McEachern, M. J., and S. Iyer. 2001. Short telomeres in yeast are highly recombinogenic. Mol. Cell 7:695-704. [DOI] [PubMed] [Google Scholar]
- 19.McEachern, M. J., S. Iyer, T. B. Fulton, and E. H. Blackburn. 2000. Telomere fusions caused by mutating the terminal region of telomeric DNA. Proc. Natl. Acad. Sci. USA 97:11409-11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEachern, M. J., D. H. Underwood, and E. H. Blackburn. 2002. Dynamics of telomeric DNA turnover in yeast. Genetics 160:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melek, M., E. C. Greene, and D. E. Shippen. 1996. Processing of nontelomeric 3′ ends by telomerase: default template alignment and endonucleolytic cleavage. Mol. Cell. Biol. 16:3437-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Nakamura, T. M., G. B. Morin, K. B. Chapman, S. L. Weinrich, W. H. Andrews, J. Lingner, C. B. Harley, and T. R. Cech. 1997. Telomerase catalytic subunit homologs from fission yeast and human. Science 277:955-959. [DOI] [PubMed]
- 22.Niu, H., J. Xia, and N. F. Lue. 2000. Characterization of the interaction between the nuclease and reverse transcriptase activity of the yeast telomerase complex. Mol. Cell. Biol. 20:6806-6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nugent, C. I., G. Bosco, L. O. Ross, S. K. Evans, A. P. Salinger, J. K. Moore, J. E. Haber, and V. Lundblad. 1998. Telomere maintenance is dependent on activities required for end repair of double-strand breaks. Curr. Biol. 8:657-660. [DOI] [PubMed] [Google Scholar]
- 23a.Pennock, E., K. Buckley, and V. Lundblad. 2001. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104:387-396. [DOI] [PubMed]
- 24.Peterson, S. E., A. E. Stellwagen, S. J. Diede, M. S. Singer, Z. W. Haimberger, C. O. Johnson, M. Tzoneva, and D. E. Gottschling. 2001. The function of a stem-loop in telomerase RNA is linked to the DNA repair protein Ku. Nat. Genet. 27:64-67. [DOI] [PubMed] [Google Scholar]
- 25.Prescott, J., and E. H. Blackburn. 1997. Functionally interacting telomerase RNAs in the yeast telomerase complex. Genes Dev. 11:2790-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prescott, J., and E. H. Blackburn. 1997. Telomerase RNA mutations in Saccharomyces cerevisiae alter telomerase action and reveal nonprocessivity in vivo and in vitro. Genes Dev. 11:528-540. [DOI] [PubMed] [Google Scholar]
- 27.Pulsinelli, G. A., and H. M. Temin. 1994. High rate of mismatch extension during reverse transcription in a single round of retrovirus replication. Proc. Natl. Acad. Sci. USA 91:9490-9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi, H., and V. A. Zakian. 2000. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated est1 protein. Genes Dev. 14:1777-1788. [PMC free article] [PubMed] [Google Scholar]
- 29.Roy, J., T. B. Fulton, and E. H. Blackburn. 1998. Specific telomerase RNA residues distant from the template are essential for telomerase function. Genes Dev. 12:3286-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shampay, J., J. W. Szostak, and E. H. Blackburn. 1984. DNA sequences of telomeres maintained in yeast. Nature 310:154-157. [DOI] [PubMed] [Google Scholar]
- 31.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singer, M. S., and D. E. Gottschling. 1994. TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science 266:404-409. [DOI] [PubMed] [Google Scholar]
- 33.Szostak, J. W., and E. H. Blackburn. 1982. Cloning yeast telomeres on linear plasmid vectors. Cell 29:245-255. [DOI] [PubMed] [Google Scholar]
- 34.Tzfati, Y., T. B. Fulton, J. Roy, and E. H. Blackburn. 2000. Template boundary in a yeast telomerase specified by RNA structure. Science 288:863-867. [DOI] [PubMed] [Google Scholar]
- 34a.Wellinger, R. J., A. J. Wolf, and V. A. Zakian. 1993. Saccharomyces telomeres acquire single-strand TG1-3 tails late in S phase. Cell 72:51-60. [DOI] [PubMed]
- 35.Wray, L. V., M. M. Witte, R. C. Dickson, and M. I. Riley. 1987. Characterization of a positive regulatory gene, LAC9, that controls induction of the lactose-galactose regulon of Kluyveromyces lactis: structural and functional relationships to GAL4 of Saccharomyces cerevisiae. Mol. Cell. Biol. 7:1111-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu, G. L., J. D. Bradley, L. D. Attardi, and E. H. Blackburn. 1990. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature 344:126-132. [DOI] [PubMed] [Google Scholar]
- 37.Zakian, V. A. 1996. Structure, function, and replication of Saccharomyces cerevisiae telomeres. Annu. Rev. Genet. 30:141-172. [DOI] [PubMed]