Abstract
Conditioning lesion of the peripheral branch of dorsal column axons is a well-known paradigm enabling the central branch to regenerate after injury to the spinal cord. However, only a small number of regenerating axons enter grafted substrates, and they do not grow beyond the lesion. We found that conditioning lesion induces, in addition to growth-stimulating genes, related to receptor tyrosine kinase (Ryk), a potent repulsive receptor for Wnts. Wnts are expressed around the site of spinal cord injury, and we found that grafted bone marrow stromal cells secreting the Wnt inhibitors secreted frizzled-related protein 2 or Wnt inhibitory factor 1 enhanced regeneration of the central branch after peripheral conditioning lesion. Furthermore, we found that Wnt4-expressing grafts caused dramatic long-range retraction of the injured central branch of conditioned dorsal root ganglion neurons. Macrophages accumulate along the path of receding axons but not around Wnt4-expressing cells, suggesting that the retraction of dorsal column axons is not a secondary effect of increased macrophages attracted by Wnt4. Therefore, Wnt-Ryk signaling is an inhibitory force co-induced with growth-stimulating factors after conditioning lesion. Overcoming Wnt inhibition may further enhance therapies being designed on the basis of the conditioning-lesion paradigm.
Keywords: axon regeneration, axon retraction, Wnt signaling
Repair of the central nervous system after axon injury remains a major challenge. Long-projecting sensory and motor axons in the spinal cord are resistant to manipulations designed to promote regeneration and functional reconnection. Peripheral conditioning lesion has provided a promising tool for understanding how regeneration can be promoted. The work of many laboratories has demonstrated that peripheral injury conditions large-diameter dorsal root ganglia (DRG) sensory neurons for regeneration by increasing the intrinsic growth capacity through gene regulation (1, 2). Peripheral conditioning, therefore, primes sensory neurons to regenerate through grafted, growth-permissive substrates such as peripheral nerve and dissociated cell grafts (1). However, conditioning lesion can promote only moderate central regeneration into grafts, and the growth of regenerated axons is often restricted to the grafted substrates, with axons failing to grow beyond the lesion into the rostral regions of the injured spinal cord (1).
In this study, we found that peripheral conditioning injury induces the expression of the repulsive Wnt receptor, related to receptor tyrosine kinase (Ryk), within the axons of large-diameter sensory neurons of the DRG. We found that grafting syngeneic bone marrow stromal cells (BMSCs) secreting Wnt inhibitors, secreted frizzled-related protein 2 (SFRP2), or Wnt inhibitory factor 1 (WIF1) blocked endogenous Wnt repulsion and enhanced the regeneration of peripherally conditioned dorsal column axons by 60%. In stark contrast, grafting BMSCs overexpressing Wnt4 resulted in long-range retraction of conditioned dorsal column axons for an average of 3.1 mm and, in some cases, up to 6–7 mm. Macrophages were found to be concentrated along the path of retracting axons rather than at the Wnt4-expressing cell grafts, suggesting that the increased axon retraction was not caused secondarily by increased macrophages attracted by Wnt4 cell grafts, but rather directly by the repulsive function of Wnt-Ryk signaling. Therefore, we propose that the induced Wnt-Ryk signaling system limits the regeneration capacity of the conditioning lesion and that the combination of Wnt signaling manipulation and conditioning-lesion–mimicking growth enhancement may produce greater regeneration.
Results
Induction of Wnt Receptors in DRG Neurons by Conditioning Lesion.
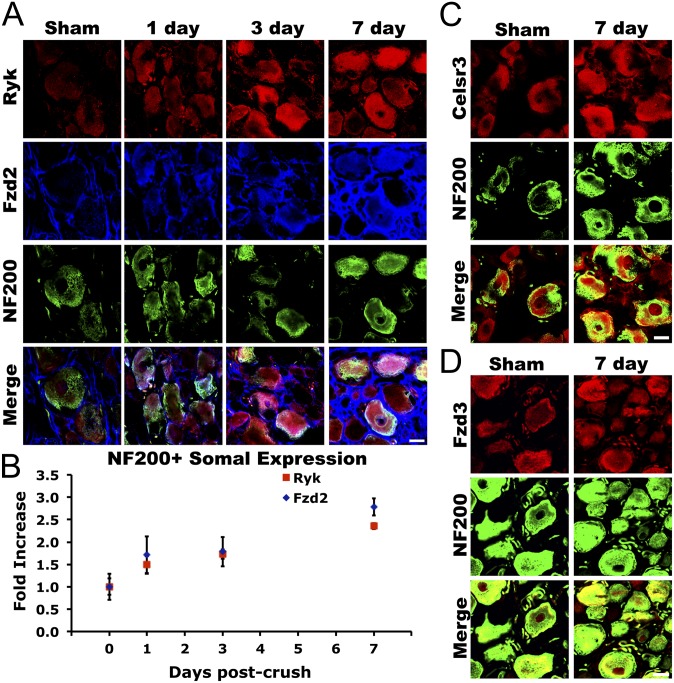
To characterize the role of Wnt signaling in conditioning lesion, we first sought to determine if Wnt receptors were expressed in DRG neurons after peripheral conditioning injury by sciatic nerve crush. We were particularly interested in the large-diameter neurofilament 200 (NF200)-immunoreactive sensory neurons that respond to peripheral conditioning injury with increased regenerative capacity. Adult female Fischer 344 rats underwent unilateral sciatic nerve crush with fine forceps, except those in the intact sham group. Animals were transcardially perfused with 4% (wt/vol) paraformaldehyde (PFA) at 1, 3, or 7 d post nerve crush and were examined for Wnt-receptor expression by immunohistochemistry. We observed a 2.5-fold increase in immunoreactivity of the Wnt receptors Ryk and Frizzled2 in DRG neuronal somatae over the course of 1 wk following peripheral conditioning injury (Fig. 1; ANOVA P < 0.0001 Ryk, P < 0.01 Fzd2; n = 6). Over 80% of induced Ryk expression overlapped with NF200 immunoreactivity in the neuronal somatae (Fig. 1 A and B). Remarkably, Ryk expression in proximal DRG axons increased over 12-fold 1 wk after peripheral conditioning injury (Fig. 2 A and D; ANOVA P < 0.001; n = 6). Two other components of Wnt signaling critical for axon guidance, Frizzled3 and Celsr3, were found to be expressed in normal adult NF200-immunoreactive DRG neurons, but were not regulated by peripheral injury (Fig. 1 C and D).
Fig. 1.
Induction of Wnt signaling components in large-diameter sensory neurons after conditioning lesion. (A) Over the course of 1 wk after peripheral conditioning injury, expression of the Wnt receptors Ryk and Frizzled2 increased in NF200-immunoreactive neurons in L4 and L5 DRGs. (B) Quantification of Ryk and Frizzled2 staining in NF200-immunoreactive neurons revealed an approximately 2.5-fold increase in expression in large-diameter sensory neurons (ANOVA P < 0.0001 Ryk, P < 0.01 Fzd2; n = 6). (C and D) Expression of the Wnt planar cell polarity signaling components Celsr3 and Frizzled3 in NF200-immunoreactive neurons was not affected by peripheral conditioning lesion. (Scale bars, 20 μm.)
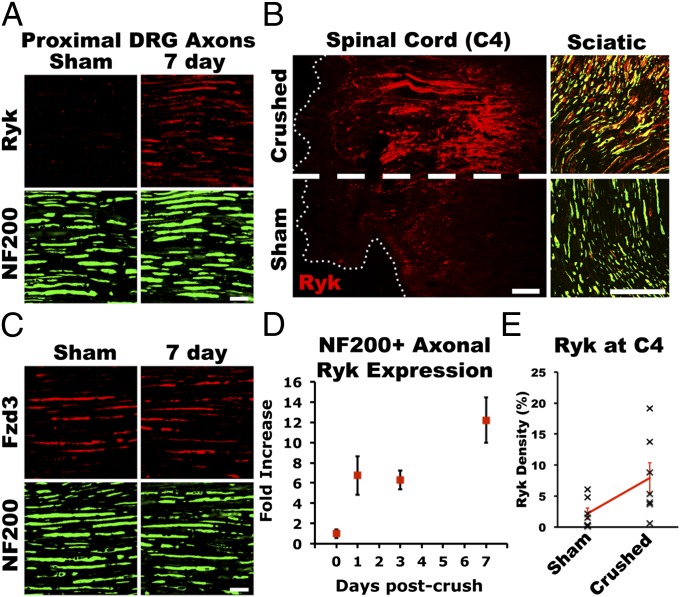
Fig. 2.
Induced Ryk was present in peripheral and central axons of large-diameter sensory neurons. (A) Ryk induced by peripheral conditioning was present in large-diameter sensory axons proximal to the DRG at 1 wk after injury. (B) At 2 wk after unilateral peripheral conditioning injury and 1 wk after a central lesion at cervical level 4, Ryk was detected in both the central and the peripheral axons of large-diameter sensory neurons. Horizontal spinal cord section demonstrates that Ryk expression was not induced in ascending sensory axons by central injury alone. (C) Axonal expression of the Wnt receptor Frizzled3 was not regulated by peripheral conditioning lesion. (D) Quantification of Ryk expression demonstrated a greater than 12-fold increase in Ryk staining in NF200-immunoreactive axons proximal to the DRG (ANOVA P < 0.001; n = 6). (E) Quantification of Ryk immunoreactivity in dorsal columns of horizontal sections 2 wk after peripheral conditioning and 1 wk after C4 dorsal column lesion demonstrates significant elevation of Ryk ipsilateral to peripheral conditioning lesion (paired t test P < 0.05; n = 7). [Scale bars, 20 μm (A and C); 100 μm (B).]
Conditioning-Lesion–Induced Ryk Protein Is Present on both the Peripheral Branch and the Injured Central Branch of Proprioceptive Axons.
To determine whether induced Ryk is present on the injured central branches after peripheral conditioning injury, animals were given a dorsal column wire-knife lesion at cervical level 4 (C4) 1 wk after unilateral sciatic nerve crush. One week after spinal cord injury (SCI) and 2 wk after unilateral conditioning injury, the induced Ryk receptor was robustly detected on both the central and the peripheral branches of large-diameter DRG axons (Fig. 2B). Lesioned ascending dorsal column sensory axons expressed significant levels of Ryk ipsilateral to the peripheral nerve crush. We quantified the fold of induction in the dorsal column caudal to the C4 lesion in horizontal spinal cord sections with ImageJ and observed an 8.2- ± 2.7-fold increase in the density of Ryk immunostaining ipsilateral to the sciatic nerve crush compared with the contralateral side (Fig. 2E; paired t test P < 0.05; n = 7). Only sparse Ryk immunolabeling was observed on the contralateral central axon branches of DRGs that had not been conditioned.
Bone Marrow Stromal Cells Expressing Wnt Inhibitors Grafted to Dorsal Column Lesion Promoted Regenerative Growth of Conditioning-Lesioned Sensory Axons.
Blocking Wnt-Ryk signaling after injury attenuates retraction of corticospinal axons after injury; therefore, we sought to determine if blocking Wnt signaling would enhance the regenerative capacity of the central branch of ascending sensory axons after peripheral conditioning. Additionally, we tested the effects of two Wnt inhibitors, WIF1 and SFRP2, on regeneration. Wnt4 is up-regulated at the central injury site after SCI (3). Therefore, we examined the response of preconditioned ascending dorsal column axons to Wnt4. Syngeneic BMSCs were isolated from adult female Fischer 344 rats and transduced ex vivo to secrete Wnt4, WIF1 (which shares sequence homology with the Wnt-binding domain of Ryk), or SFRP2, an inhibitor of Wnt-Frizzled binding (4). Animals received bilateral peripheral conditioning injury, and 1 wk later underwent a C4 dorsal column lesion of the ascending sensory fibers followed by the immediate grafting of ∼200,000 BMSCs: naive, Wnt4-, WIF1-, or SFRP2-secreting. Animals were injected bilaterally into the sciatic nerve with a 1% (wt/vol) solution of the transganglionic tracer cholera toxin B (CTB) to label ascending sensory neurons 3 d before sacrifice. One month after C4 dorsal column lesion, animals were transcardially perfused with 4% (wt/vol) PFA, and sagittal spinal cord sections were examined by immunohistochemistry. The host–graft interface was determined with DAPI nuclear stain and immunoreactivity of the reactive astrocyte marker glial fibrillary acidic protein (GFAP).
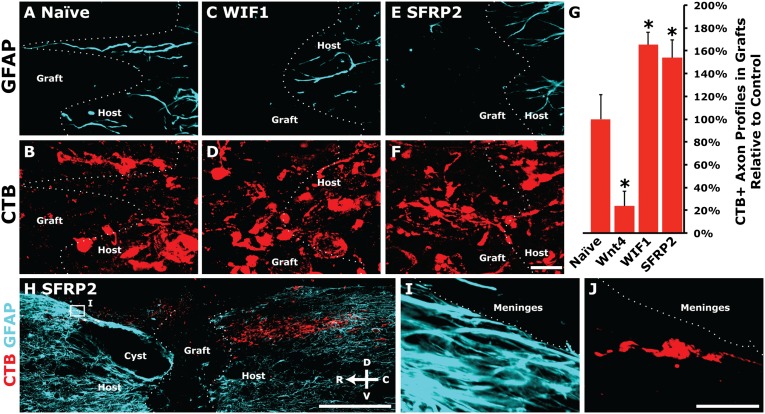
To assess regeneration, continuous segments of CTB-labeled regenerated axons, or axon profiles, that were beyond the caudal host–graft interface and within BMSC grafts were counted in every seventh sagittal section. In animals grafted with WIF1- or SFRP2-secreting BMSCs, a significantly higher proportion of all CTB-labeled axons were able to regenerate than in animals with naive BMSC grafts [Fig. 3 A–G; ANOVA P < 0.0001, *post hoc t tests with Bonferroni correction P < 0.05; n = 5 (naive, Wnt4), n = 6 (WIF1, SFRP2)]. Additionally, in three of six animals grafted with SFRP2-secreting BMSCs, CTB-labeled axons were observed bridging BMSC grafts and re-entering host spinal cord (Fig. 3 H–J). No animals grafted with other BMSCs exhibited bridging axons, and no animals in any of the groups exhibited CTB labeling in serial sections through the brainstem target gracile nucleus, indicating complete transection of sciatic-projecting sensory axons at C4 in all animals. Few CTB-labeled axons were able to penetrate Wnt4-secreting BMSC grafts (Fig. 3G).
Fig. 3.
Wnt inhibitors blocked endogenous Wnt-mediated repulsion and increased regeneration of peripherally conditioned axons. (A–F) Peripherally conditioned axons showed increased axon regeneration into BMSC grafts secreting the Wnt inhibitors WIF1 or SFRP2 compared with regenerative growth into naive BMSC grafts. (G) Quantification of the total number of CTB+ axon profiles in Wnt inhibitor-secreting grafts relative to controls shows a 50–65% increase in axon regeneration with inhibition of endogenous Wnt signaling [ANOVA P < 0.0001, *post hoc t tests with Bonferroni correction P < 0.05; n = 5 (naive, Wnt4), n = 6 (WIF1, SFRP2)]. (H–J) Three of six animals grafted with SFRP2-secreting BMSCs exhibited bridging of axons beyond the rostral host–graft interface. No other animals exhibited bridging axons, and no CTB-labeled axons were detected in serial sections of the nucleus gracilis. [Scale bars, 20 μm (A–F); 500 μm (H); 25 μm (I and J).]
Wnt4-Secreting Bone Marrow Stromal Cells Grafted in Dorsal Column Lesion Caused Long-Range Retraction of the Central Branch of Conditioning-Lesioned Sensory Axons.
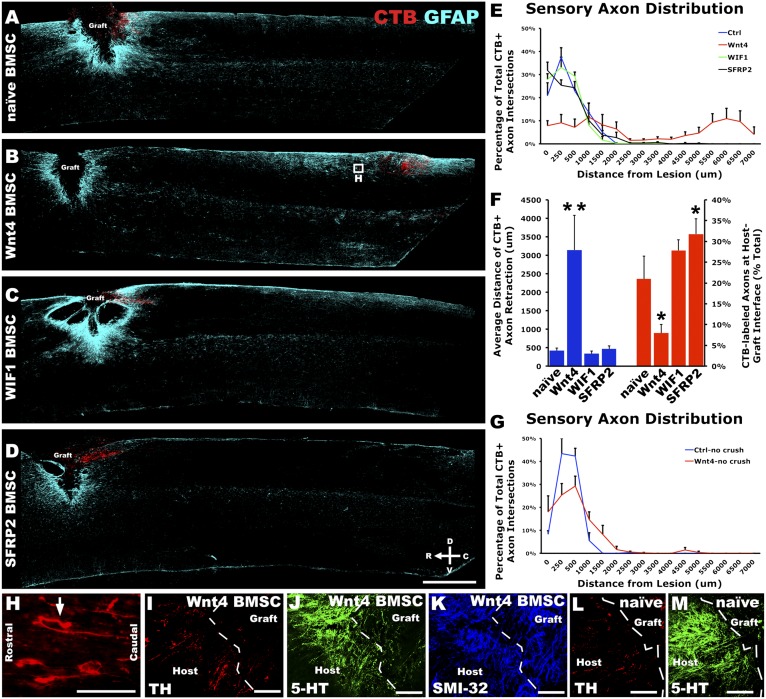
In animals grafted with Wnt4-secreting BMSCs, we not only found a drastically reduced number of regenerated sensory axons, but also observed repulsion of conditioned sensory axons from Wnt4-secreting BMSC grafts. To assess axon retraction, the caudal host–graft interface was traced and CTB-labeled axons were counted as they transected the tracing at set distances caudal to the lesion. BMSC grafts secreting Wnt4, but not WIF1 or SFRP2, had a dramatic effect on preconditioned sensory axons by inducing retraction of peripherally conditioned sensory axons an average of 3,143 ± 939 μm from the caudal lesion edge compared with 423 ± 71 μm in animals grafted with naive BMSCs with axons in some Wnt4-BMSC grafted animals repelled up to 6–7 mm [Fig. 4 B and F; repeated measures ANOVA P < 0.005, n = 5 (naive, Wnt4), n = 6 (WIF1, SFRP2)]. Moreover, Wnt4-secreting BMSCs induced repulsive turning of axons away from the high expression of Wnt4 by BMSCs (arrow, Fig. 4H). Additionally, SFRP2 reduced basal levels of retraction from endogenous Wnt expression as SFRP2 BMSC grafts exhibited a significantly higher proportion of CTB-labeled axons at the caudal host–graft interface compared with naive BMSC grafts (Fig. 4F; post hoc t test P < 0.05).
Fig. 4.
Peripheral conditioning lesion sensitized large-diameter sensory axons to Wnt signaling. (A) Adult rats 4 wk after C4 dorsal column lesion exhibit minimal axon retraction of CTB-labeled, peripherally conditioned dorsal column sensory neurons. Animals were grafted at C4 with naive syngeneic BMSCs 1 wk after bilateral peripheral conditioning injury. (B) Wnt4-secreting BMSCs induced massive retraction of peripherally conditioned dorsal column sensory axons. (C and D) Neither of the Wnt signaling inhibitors tested, WIF1 nor SFRP2, significantly affected axon retraction after C4 dorsal hemisection. (E) Quantification of CTB-labeled axons caudal to the host–graft interface demonstrated a robust retraction of peripherally conditioned sensory axons from Wnt4-secreting BMSC grafts [repeated measures ANOVA P < 0.005; n = 5 (naive, Wnt4), n = 6 (WIF1, SFRP2)]. (F) The average distance of conditioned axon retraction was significantly higher with Wnt4 BMSC grafts (**post hoc t test with Bonferroni correction P < 0.0005, ANOVA P < 0.0005). Additionally, at the host–graft interface, SFRP2-reduced axon retraction compared with naive as a higher proportion of CTB-labeled axons remained at the host–graft interface (*post hoc t test with Bonferroni correction P < 0.05, ANOVA P < 0.002). (G) In the absence of peripheral conditioning, Wnt4 did not significantly affect axon retraction compared with control naive BMSC grafts [n = 3 (naive), n = 7 (Wnt4)]. (H) Inset from B illustrates individual peripherally conditioned axons that have retracted from Wnt4 grafts; arrow indicates an axon that attempted to turn away from a Wnt4-secreting graft. (I–K) Descending serotonergic (5-HT) raphaespinal axons, dopaminergic (TH-immunoreactive) coerulospinal axons, and locally projecting, heavy-chain, nonphosphorylated neurofilament (SMI-32)-labeled axons do not show any induced repulsion from Wnt4-secreting BMSC grafts. (L and M) Examples of TH and 5-HT–immunoreactive axons in the presence of naive BMSC grafts. [Scale bars, 1 mm (A–D); 100 μm (H–M).]
An additional contingent of SCI animals was grafted with either naive or Wnt4-secreting BMSCs in the absence of peripheral conditioning injury. Animals grafted with Wnt4-secreting BMSCs did not exhibit significant retraction in these animals [Fig. 4G; repeated measures ANOVA P = 0.55, n = 3 (naive), n = 7 (Wnt4)]. Populations of descending supraspinal axons immunoreactive for serotonin (raphaespinal) and tyrosine hydroxylase (coerulospinal), as well as locally projecting axons, labeled with the neurofilament marker SMI-32, exhibited no increased retraction from Wnt4-secreting BMSC grafts compared with other BMSC grafts (Fig. 4 I–M).
Macrophages Accumulate Along the Receding Path of Retracting Axons but Not Around Wnt4-Secreting Bone Marrow Stromal Cells.
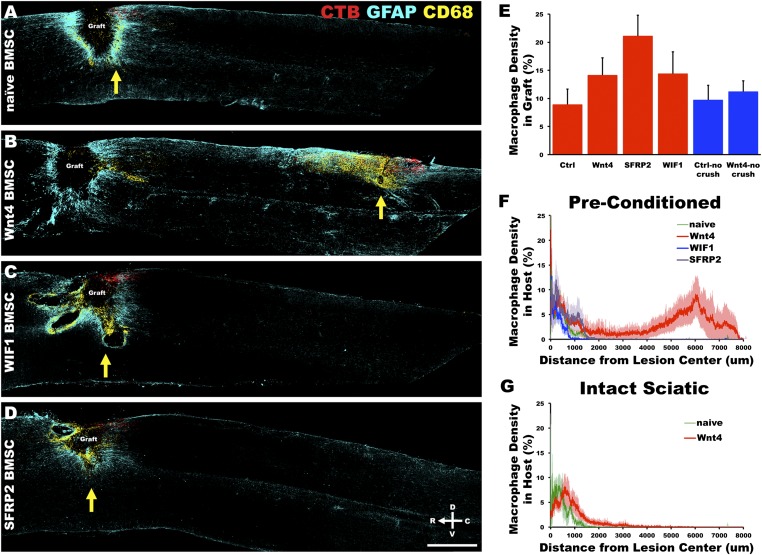
To test whether the increased retraction by Wnt4-expressing bone marrow stromal cells was caused by Wnt4-mediated repulsion or by increased macrophages potentially attracted by high levels of Wnt4, we characterized the distribution of macrophages. At the injury site, CD68- and CD86-immunoreactive macrophages were present in the graft and around the injury site as well as in the dorsal columns rostral to the lesion along the distal sensory axon fragments, where Wallerian degeneration occurs. However, the peak of the macrophage distribution did not follow the introduced Wnt4 gradient, but rather concentrated immediately rostral to the retracting tips of the injured axons (Fig. 5 A–D).
Fig. 5.
Macrophage clearance of axons followed Wnt4-mediated repulsion. (A) Four weeks after C4 dorsal column lesion, CD68-immunoreactive macrophages infiltrate the BMSC graft, surround the injury site, and associate with the lesioned ends of dorsal column sensory axons. (B) With Wnt4-secreting BMSC grafts, macrophages infiltrate the host spinal cord and are associated with the retracting peripherally conditioned axons. (C and D) Wnt inhibitors do not alter patterns of macrophage activity after C4 dorsal column lesion. Yellow arrows indicate peak areas of CD68 density caudal to the lesion center in A–D. (E) Density of CD68-immunoreactive macrophage staining in BMSC grafts is not significantly altered by modulation of Wnt signaling [ANOVA P = 0.11; n = 5 (naive, Wnt4), n = 6 (WIF1, SFRP2), n = 3 (nonconditioned naive), n = 7 (nonconditioned Wnt4)]. (F) CD68 density caudal to the lesion center demonstrated that macrophage infiltration of spinal cord tissue immediately surrounding the graft was similar in all groups. However, beyond 3 mm caudal to the lesion center only Wnt4-secreting grafts resulted in significant infiltration of macrophages within the spinal cord [repeated measures ANOVA P = 0.05; n = 5 (naive, Wnt4), n = 6 (WIF1, SFRP2)]. (G) In the absence of peripheral conditioning, Wnt4 did not significantly affect macrophage infiltration compared with control naive BMSC grafts [n = 3 (naive), n = 7 (Wnt4)]. (Scale bar, 1 mm.)
There are four main pieces of evidence to suggest that Wnt4 acts in a direct manner upon the peripherally conditioned axons and not through a secondary action dependent upon macrophage activation. First, Wnt4 did not affect macrophage penetration of BMSC grafts, where Wnt4 is expressed at the highest level, although there was a statistically insignificant trend toward increased macrophage infiltration with Wnt inhibition by SFRP2 [Fig. 5E; ANOVA P = 0.11; n = 5 (naive, Wnt4), n = 6 (WIF1, SFRP2), n = 3 (nonconditioned naive), n = 7 (nonconditioned Wnt4)]. Second, macrophage density immediately caudal to the lesion center was similar in all groups. Third, a second (and greater) peak of macrophage distribution is immediately rostral to the tips of the central branches of the conditioning-lesioned sensory axons retracting from Wnt4-secreting BMSC grafts (Figs. 5F and 6A) corresponding to the freshly degenerating axons [Fig. 6A; repeated measures ANOVA P = 0.05; n = 5 (naive, Wnt4), n = 6 (WIF1, SFRP2)]. Fourth, in the absence of peripheral conditioning, Wnt4 did not significantly affect macrophage infiltration compared with control naive BMSC grafts [Fig. 5G; n = 3 (naive), n = 7 (Wnt4)].
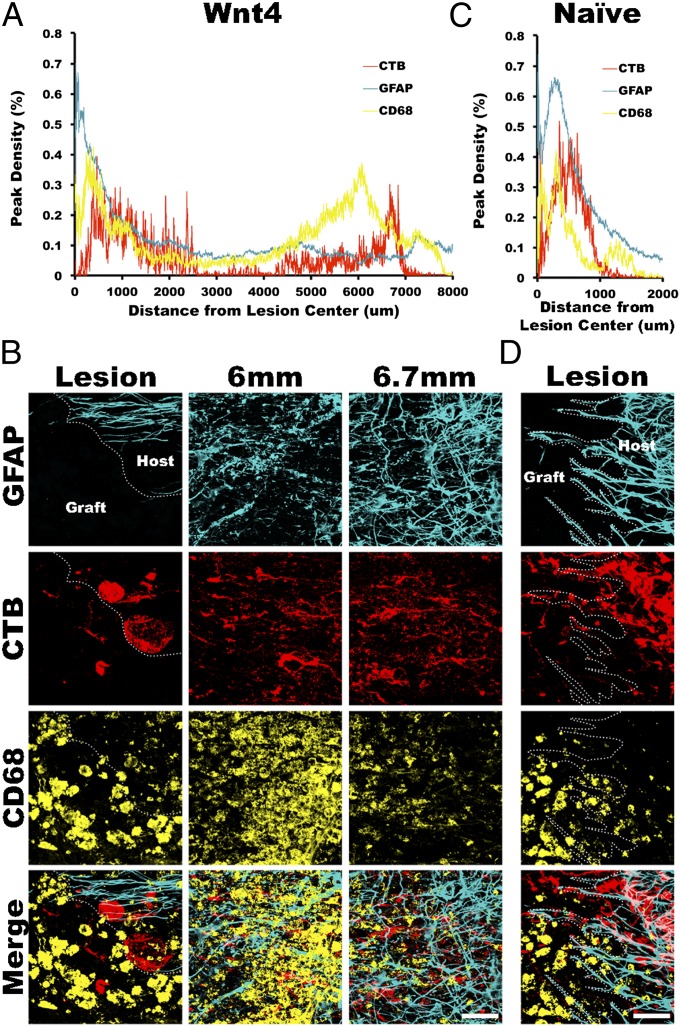
Fig. 6.
Infiltrating macrophages associated with the lesioned ends of ascending sensory axons. (A) CD68+ macrophage density in host spinal cord peaked rostral to the lesioned ends of preconditioned sensory axons that had retracted from Wnt4-secreting BMSC grafts. (B) High magnification images of CTB-labeled sensory axons at the lesion edge as well as at the peak density of CD68 immunostaining and at the peak density of CTB within the spinal cord demonstrate the association of infiltrating macrophages with retracted sensory axons. (C) Macrophage density in naive BMSC grafted animals also peaked just rostral to the lesioned ends of ascending sensory axons as shown in D. n = 5 (naive, Wnt4). (Scale bars, 50 μm.)
As alternatively activated M2 macrophages are able to promote neurite outgrowth of DRG neurons in vitro (5), we sought to determine if the increased regeneration observed in WIF1- and SFRP2-secreting grafts corresponded to a shift in macrophage subtypes. We examined expression of the M2 macrophage marker Arginase 1 and found no deviation from expression levels in naive BMSC control grafts at 1 mo after SCI in any group (not shown).
The peak distribution of macrophages within the injured spinal cord was located immediately rostral to the lesioned tips of conditioned sensory axons, whether they were retracting a few hundred micrometers from a naive BMSC graft or over several millimeters from a Wnt4-secreting BMSC graft (Fig. 6 A and C). Therefore, the long-range retraction caused by Wnt4-expressing cells grafted in the lesion was not caused by increased macrophage migration toward the Wnt4 or by increased infiltration of macrophages in the lesioned spinal cord, suggesting a direct Wnt4-Ryk–mediated axon repulsion.
Discussion
In recent years, it has become apparent that many developmental guidance cues—Semaphorins, ephrins, netrin, Wnts, and Shh—are reinduced after SCI (3, 6–10). In addition, other molecular guidance systems are still expressed in the adult central nervous system even in uninjured animals. However, it remains unclear whether these guidance molecules in injured spinal cord have similar functions as in development. So far, only Wnt-Ryk signaling has been demonstrated to cause similar repulsive axon response to corticospinal tract axons and dorsal column sensory axons after injury in vivo, and EphA4 is involved in a very different manner (regulation of gliosis) (3, 6, 7).
Wnts are indispensable in establishing the anterior–posterior, or rostro-caudal, organization of axons within the brainstem and spinal cord, driving the formation of circuits that govern both the transmission of sensory information to the brain as well as the formation of those controlling supraspinal motor input to the spinal cord (11–18). Gradients of Wnt expression have been demonstrated to direct the growth of ascending commissural sensory axons, dopaminergic and serotonergic axons, and corticospinal motor axons (13, 15–18). Within the developing spinal cord, expression gradients of multiple Wnts (Wnt4, Wnt5a, Wnt7a, and Wnt7b) direct anterior turning of postcrossing commissural axons through the Frizzled3 receptor (16) and descending growth of corticospinal motor axons through the Ryk receptor, following a decreasing anterior-to-posterior gradient of Wnts (Wnt1 and Wnt5a) in the developing spinal cord (15, 18). After spinal cord injury, Ryk expression is reinduced on the very same corticospinal axons whereas Wnts1, -4, and -5a are re-expressed in the spinal cord surrounding the injury site (3). We previously found that Wnt-Ryk signaling after SCI induces corticospinal axon retraction and limits corticospinal axon plasticity (3).
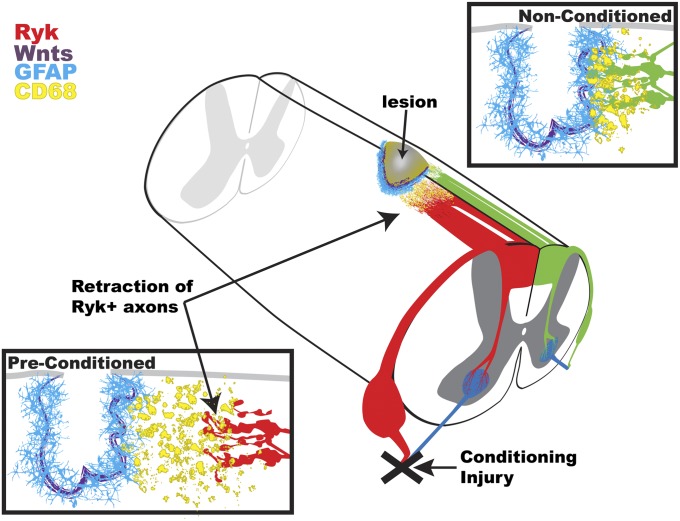
Here we show that the regeneration potential of the central branch of the conditioning-lesioned adult sensory neurons is limited in the injured spinal cord by repulsive Wnt signaling co-induced with a growth-stimulating program during conditioning lesion. In the absence of peripheral conditioning, Ryk was not expressed in ascending sensory axons, and Wnt4 was unable to cause axon repulsion. We observed a slight, nonsignificant amount of retraction from Wnt4 grafts without a preconditioning lesion, which was likely due to mild peripheral conditioning induced by CTB tracer injection 3 d before sacrifice. Three days after a peripheral conditioning injury, we observed a significant induction of Ryk in large-diameter sensory neurons (Fig. 1A). By blocking Wnt signaling through cellular delivery of the Wnt signaling inhibitors WIF1 and SFRP2, we were able to augment regeneration of conditioned ascending sensory axons. A proposed component of axon retraction after injury is macrophage-mediated die-back (19). Here we found that macrophages were associated with the retracting tips of sensory axons but not accumulated at the peak of Wnt4 expression. Therefore, we propose that the massive retraction of preconditioned DRG axons was likely caused by direct axon repulsion by Wnt4-secreting BMSC grafts, rather than by increased macrophages potentially attracted by Wnt4-secreting cells (Fig. 7). In fact, we did not find any evidence that Wnt4 attracts macrophages.
Fig. 7.
Peripheral conditioning sensitizes sensory axons to Wnt-mediated axon repulsion after spinal cord injury. Exongenously applied and endogenous Wnts up-regulated after spinal cord injury limit the enhanced regeneration of peripherally conditioned sensory neurons. Furthermore, an exogenous source of Wnt protein is able to mediate drastic macrophage-mediated die-back of sensory axons through repulsive Wnt signaling.
In addition to our previous finding of a repulsive function of Wnt-Ryk signaling in corticospinal axons after injury, we observed strong repulsive effects of Wnts on conditioned sensory axons via Ryk. This suggests that Wnt-Ryk signaling may be responsible for retraction of multiple classes of axons following traumatic CNS injury and that Wnt signaling should be a major consideration in therapeutic design. It should be noted that in this study only a handful of axons were found to bridge beyond the lesion site with Wnt inhibition. Although the increase of regeneration is 60%, this is an improvement from the relatively modest baseline regeneration induced by peripheral conditioning lesion. Therefore, additional inhibition, such as that mediated by myelin-associated inhibitors or chondroitin sulfate proteoglycans, may need to be removed, and the intrinsic growth potential will need to be further enhanced via combinatorial approaches to achieve greater regeneration and functional recovery. Interestingly, reinduced Ryk is also expressed in the peripheral branches, suggestive of a role for Ryk in regulating peripheral regeneration.
Wnts are powerful morphogens capable of mediating many processes in the developing nervous system—from neuronal migration and polarization, dendritic arbor growth, and axon guidance to regulation of synapse formation (20). Here we report their role in regulating axon growth after adult CNS injury. On the basis of our observation, it is possible that Wnts have additional roles, such as regulating the inflammatory or glial responses to injury. For example, some animals grafted with BMSCs secreting Wnt inhibitors exhibited an exacerbation of cystic cavitation (Fig. 3C). Although axon regeneration is a critical to the final goal of neural circuit repair, understanding the potential roles of Wnts in adult CNS injury responses other than regulation of axonal growth will be important for effective therapeutic design. The increased complexity of the adult CNS, compared with the developing CNS, presents unique challenges as treatment effects on axons and the surrounding milieu of the injured spinal cord cannot be dealt with in isolation.
Materials and Methods
Sciatic Nerve Crush.
All animal work in this research was approved by the University of California at San Diego Institutional Animal Care and Use Committee. Unilateral or bilateral sciatic nerve crush was performed on adult female Fischer 344 rats (150–165 g). An area over the hind limb was shaved and cleaned with povidone-iodine before incision caudal and parallel to the femur. The sciatic nerve was exposed and crushed for 10 s with a pair of fine (#55) forceps. After crush, the skin was closed with surgical staples.
C4 Dorsal Column Lesion.
Animals were deeply anesthetized with 2 mL/kg of ketamine mixture (25 mg/mL ketamine, 1.3 mg/mL xylazine, and 0.25 mg/mL acepromazine). Spinal level C4 was exposed by laminectomy, and the dorsal columns were lesioned with two passes of a Scouten wire-knife (David Kopf Instruments) at 1.2 mm lateral to the midline and at a depth of 1 mm. The intact dura was pressed against the wire-knife to ensure complete transection of ascending dorsal column sensory axons. The lesion cavity was filled with 200,000 BMSCs (in PBS) secreting myc-tagged Wnt4, HA-tagged WIF1, or HA-tagged SFRP2 via a glass micropipette connected to a picospritzer (General Valve) (Fig. S1).
Transganglionic Labeling of Sciatic-Projecting Sensory Axons.
Three days before sacrifice, sciatic nerves were injected bilaterally with a 1% (wt/vol) solution of CTB (List Biological Laboratories). The sciatic nerve was exposed as in nerve crush experiments, and 1 μL was injected into each tibial and common peroneal branch bilaterally (4 μL total).
Sacrifice and Tissue Processing.
Animals were transcardially perfused with ice-cold PBS followed by 4% (wt/vol) PFA in PBS. Spinal columns, sciatic nerves, and DRGs were postfixed overnight at 4 °C in 4% wt/vol PFA. Tissue was cryoprotected with 30% wt/vol sucrose and sectioned sagittally on a cryostat at 40 μm thick (Leica). See SI Appendix for immunohistochemistry and antibody information.
Image Acquisition and Analysis.
Images were acquired on an inverted Zeiss LSM510 confocal microscope with LSM acquisition software (Carl Zeiss Microscopy). An Axiovert 40 CFL with an AxioCam MRm and AxioVision software (Carl Zeiss Microscopy) was used to assess 3′,3′-diaminobenzidine (DAB) chromogenic staining of CTB in measurements of labeled axon profiles. Image density quantification was done on thresholded fluorescent images using ImageJ (National Institutes of Health). Statistical tests were performed using JMP 9 software (SAS Institute). An investigator blinded to the experimental group performed all analyses.
Supplementary Material
Acknowledgments
The authors thanks Kristine Tolentino for assistance with sample processing and Anna Tury, Keisuke Onishi, and Virginia Hazen for critical reading of the manuscript. This work was supported by the Roman Reed Foundation, the Wings for Life Foundation, and National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) Grant R01 NS 047484 (to Y.Z.), NIH/NINDS Neurobiology Training Grant T32 NS007220-27, and Craig H. Neilsen Foundation Fellowships (to E.R.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.J. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206218109/-/DCSupplemental.
References
- 1.Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- 2.Stam FJ, et al. Identification of candidate transcriptional modulators involved in successful regeneration after nerve injury. Eur J Neurosci. 2007;25:3629–3637. doi: 10.1111/j.1460-9568.2007.05597.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, et al. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 5.Kigerl KA, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabes J, Anderson P, Brennan C, Bolsover S. Regeneration-enhancing effects of EphA4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur J Neurosci. 2007;26:2496–2505. doi: 10.1111/j.1460-9568.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24:10064–10073. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Löw K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasterkamp RJ, et al. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13(2):143–166. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- 10.Pasterkamp RJ, Verhaagen J. Semaphorins in axon regeneration: Developmental guidance molecules gone wrong? Philos Trans R Soc Lond B Biol Sci. 2006;361:1499–1511. doi: 10.1098/rstb.2006.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holstege JC, Kuypers HG. Brainstem projections to spinal motoneurons: An update. Neuroscience. 1987;23:809–821. doi: 10.1016/0306-4522(87)90160-6. [DOI] [PubMed] [Google Scholar]
- 12.Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Brain Res Rev. 2008;57(1):183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Fenstermaker AG, et al. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010;30:16053–16064. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollis ER, II, Zou Y. 2012. Expression of the Wnt signaling system in central nervous system axon guidance and regeneration. Front Mol Neurosci 5, 10.3389/fnmol.2012.00005.
- 15.Liu Y, et al. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- 16.Lyuksyutova AI, et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 17.Blakely BD, et al. Wnt5a regulates midbrain dopaminergic axon growth and guidance. PLoS ONE. 2011;6:e18373. doi: 10.1371/journal.pone.0018373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Hutchins BI, Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J Neurosci. 2009;29:5873–5883. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: Activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28:9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.