Abstract
U small nuclear RNAs (snRNAs) and mRNAs are both transcribed by RNA polymerase II (Pol II), but the snRNAs have unusual TATA-less promoters and are neither spliced nor polyadenylated; instead, 3′ processing is directed by a highly conserved 3′ end formation signal that requires initiation from an snRNA promoter. Here we show that the C-terminal domain (CTD) of Pol II is required for efficient U2 snRNA transcription, as it is for mRNA transcription. However, CTD kinase inhibitors, such as 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) and 1-(5-isoquinolinesulfonyl)-2-methylpiperazine (H7), that block mRNA elongation do not affect U2 transcription, although 3′ processing of the U2 primary transcript is impaired. We show further that U2 transcription is preferentially inhibited by low doses of UV irradiation or actinomycin D, which induce CTD kinase activity, and that UV inhibition can be rescued by treatment with DRB or H7. We propose that Pol II complexes transcribing snRNAs and mRNAs have distinct CTD phosphorylation patterns. mRNA promoters recruit factors including kinases that hyperphosphorylate the CTD, and the CTD in turn recruits proteins needed for mRNA splicing and polyadenylation. We predict that snRNA promoters recruit factors including a CTD kinase(s) whose snRNA-specific phosphorylation pattern recruits factors required for promoter-coupled 3′ end formation.
RNAs that encode proteins are transcribed by RNA polymerase II (Pol II) in almost all eukaryotes. In contrast, untranslated RNAs are transcribed by all three RNA polymerases: 5.8, 18, and 28S rRNA by Pol I; 5S rRNA, tRNA, and U6 small nuclear RNA (snRNA) by Pol III (56); and the other U snRNAs, which function in mRNA splicing and various RNA processing events, by Pol II (27). Kinetoplastid protozoa, a class of early diverging eukaryotes, are exceptions to these rules. Kinetoplastid snRNAs are transcribed not by Pol II but by Pol III (65), and certain mRNAs, including the immunologically important variant surface glycoprotein message, are hybrids of a U snRNA-like spliced leader transcribed by Pol II and a protein-coding mRNA body transcribed by Pol I (19).
Although U snRNAs and mRNAs are both transcribed by Pol II in mammals, the genes are very different. U snRNA promoters have no TATA box and rely instead upon a dedicated U snRNA-specific promoter consisting of a highly conserved proximal sequence element (PSE) and an enhancer-like distal sequence element spaced one nucleosome apart (27). In addition, U snRNA genes are short (typically only a few hundred base pairs) and lack introns, whereas genes encoding mRNAs can span megabases and usually contain many introns. Also, U snRNA genes are typically present in multiple copies in higher eukaryotes—the human U1 and U2 genes are tandemly repeated (6, 40, 66, 68)—whereas most protein-coding genes are present in only one or a few copies per haploid genome.
U snRNA processing also differs from mRNA processing. U snRNAs are neither spliced nor polyadenylated; instead, formation of the first U snRNA intermediate (U2+10 in the case of U2 snRNA) is directed by a highly conserved 3′-end formation signal (3′ box) located just downstream of the mature 3′ end of the snRNA (26, 72). Intriguingly, a U snRNA promoter is required for efficient 3′ end formation directed by the 3′ box (18, 28). Moreover, 3′ end formation appears to be an RNA processing event, because U2 (but apparently not U1) transcription continues for up to 800 nucleotides (nt) beyond the 3′ box (15, 47). Over the next 90 min (12, 51), the processed intermediate is exported to the cytoplasm, where the mature 3′ end is generated by 3′ trimming (21, 29), the 5′ monomethyl cap is trimethylated, Sm proteins are assembled onto the snRNA (44, 45), and the nearly mature small nuclear ribonucleoprotein particle is imported back into the nucleus (31) where it undergoes further base modifications (17, 71) before it can function in RNA processing. Little is known about either the nuclear or cytoplasmic 3′ processing events, but the dependence of efficient 3′-box-directed processing on a PSE-bearing snRNA promoter links an early step at the promoter to later events at the 3′ end of the gene, perhaps through specific modification of the polymerase and/or recruitment of factors that travel along with it.
In the case of mRNAs, early events at the promoter are known to be coupled to subsequent events in splicing, cleavage and polyadenylation, and transcription termination through the C-terminal domain (CTD) of the large subunit of Pol II. The CTD consists of tandem heptapeptide motifs ranging from 26 repeats in Saccharomyces cerevisiae (2) to 42 repeats in Drosophila melanogaster (73) and 52 repeats in humans (14). Although a CTD-less polymerase is catalytically active (23), almost no mRNA transcription is observed in vivo in the absence of the CTD (48). The heptapeptide repeat unit, YSPTSPS, provides a complex platform for modification by phosphorylation. Serines 2 and 5 are phosphorylated during transcription, and CTD phosphorylation by kinases cdk7, cdk8, and cdk9 has been linked not only to initiation and mRNA elongation (13, 57) but to capping, splicing, and cleavage and polyadenylation as well (11, 46). In addition to phosphorylation on Ser2, Ser5, and also Tyr1 (3, 4), CTD dephosphorylation may be regulated by Pin1 isomerization of Pro3 and/or Pro6 (34).
Here we examine the role of the CTD and CTD phosphorylation in U2 snRNA transcription and processing by modifying the CTD in several ways. First, using a Pol II construct that lacks the CTD entirely (22), we show that the CTD is required for efficient U2 snRNA transcription and 3′ processing in vivo. Next, using the CTD kinase inhibitors 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB), 1-(5-isoquinolinesulfonyl)-2-methylpiperazine (H7), and N-[2-(methylamino)ethyl]-5-isoquinolinesulfonamide (H8), we show that U2 transcription is unaffected by inhibition of CTD phosphorylation but 3′ processing is impaired. These results are in agreement with recent work of Medlin et al. (47). We then show that both U2 transcription and processing are impaired by low doses of actinomycin D (ActD) or UV irradiation, both of which promote CTD hyperphosphorylation (8, 41, 43, 60). Finally, underscoring the link between CTD hyperphosphorylation and transcription inhibition, we show that UV-mediated inhibition of transcription (but not 3′ processing) can be rescued by CTD kinase inhibitors.
Our results suggest that CTD phosphorylation plays different roles in the transcription and processing of mRNAs and U snRNAs. CTD hypophosphorylation inhibits mRNA elongation (32, 43) yet has no effect on U2 snRNA transcription; however, CTD hypophosphorylation impairs U2 snRNA 3′ processing. In contrast, CTD hyperphosphorylation preferentially inhibits U2 snRNA transcription and also impairs 3′ processing. Given the dependence of U2 snRNA 3′ processing on a PSE-bearing snRNA promoter (18, 28), we suggest that key differences between mRNA and U snRNA transcription units may be established as early as the promoter and communicated to subsequent steps, such as elongation and 3′ processing, through differential phosphorylation of the Pol II CTD.
MATERIALS AND METHODS
Chemicals.
α-Amanitin (Sigma) was dissolved in water at a concentration of 1 mg/ml. Actinomycin D (95%, high-performance liquid chromatography purified; Sigma) was dissolved in ethanol at a concentration of 1 or 0.1 mg/ml. 5,6-Dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) (Calbiochem) was dissolved in dimethyl sulfoxide at a concentration of 50 mM. H7 [1-(5-isoquinolinesulfonyl)-2-methylpiperazine, 2HCl; Calbiochem] and H8 {N-[2-(methylamino)ethyl]-5-isoquinolinesulfonamide, 2HCl; Calbiochem} were dissolved in phosphate-buffered saline (PBS) at 10 mM. All were stored at −20°C and protected from light.
Transfection.
For the Pol II large subunit (LS) transfections, HT1080 human fibrosarcoma cells were transfected at 50% confluence with Fugene 6 reagent (Roche) as recommended by the manufacturer. After 24 h, the cells were split 1:2, α-amanitin was added to a final concentration of 2 μg/ml where appropriate, and RNA and proteins were harvested 24 h later. Transfection efficiency was determined by staining for β-galactosidase (38). For the U2 maxigene transient transfections, HT1080 cells at 90% confluence were transfected with Fugene 6 reagent (Roche) as recommended by the manufacturer. After 4 h, medium was replaced with fresh media containing the appropriate drugs, and 5 h later, RNA was harvested from the cells as described below.
UV irradiation.
Cells at 60 to 80% confluence were washed with PBS and irradiated at 10 J/m2 under a biosafety cabinet UV light (General Electric; 254-nm peak) calibrated with a UV voltimeter (Ultra Violet Products). The medium was replaced (including drugs where appropriate), and the plates were incubated at 37°C under a 5% CO2 atmosphere.
RNA isolation and Western blotting.
RNA was isolated using TRIzol reagent (Gibco-BRL) as recommended by the manufacturer. Total RNA was used for RNase protection assays. RNA preparations were quantified by UV spectrometry and examined for integrity by agarose gel electrophoresis.
Cells extracts were prepared for Western blots from 80-to-90%-confluent cells. Cells on 100-mm-diameter plates were washed once with cold PBS, scraped in cold PBS plus 1× protease inhibitor cocktail (Roche), and collected by centrifugation. The supernatant was removed by aspiration, the was pellet resuspended in 50 μl of cold PBS with protease inhibitors, and cells were lysed in an equal-volume 2× Laemmli gel loading buffer before sonication. Western blotting and ECL chemiluminescence detection (Pierce) were performed using the following antibodies: a rabbit polyclonal antibody against the N terminus of Pol II (Santa Cruz) at a 1:100 dilution, a mouse monoclonal antibody against the B10 E-tag (an epitope from the B region of the human estrogen receptor) (Chemicon) at a 1:1,000 dilution, and a hypoxanthine phosphoribosyltransferase-conjugated goat antirabbit or antimouse antibody (Santa Cruz) as a secondary antibody at 1:5,000 dilution.
Northern blotting.
Twenty-five micrograms of total RNA per lane was ethanol precipitated, resuspended in 10 μl of formamide loading buffer, incubated at 90°C for 2 min, and resolved by electrophoresis through a 7 M urea-15% polyacrylamide gel until the xylene cyanol dye front was halfway down the 42-cm gel. Gels were transferred to a Zeta-probe membrane (Bio-Rad) overnight in a Trans-blot apparatus (Bio-Rad) at 4°C in 1× Tris-borate-EDTA. Transfer was verified by ethidium bromide staining of the gel. The membrane was UV irradiated and prehybridized for 1 h at 45°C in 5× SSPE (0.75 M NaCl, 50 mM NaH2PO4, 5 mM EDTA [pH 7.4]) containing 5× Denhardt's reagent, 1% sodium dodecyl sulfate (SDS), and 100 μg of sheared denatured salmon sperm DNA/ml. The membrane was hybridized overnight with 106 cpm of a deoxyoligonucleotide/ml, labeled at the 5′ end with [γ-32P]ATP by polynucleotide kinase (NEB). Sequential washes were for 5 min at room temperature in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-1% SDS, 1 h at 50°C in 2× SSC-1% SDS, and 1 h at 50°C in 1× SSC-1% SDS. Washed membranes were phosphorimaged (Molecular Dynamics) overnight and quantified (IQ Mac v1.2).
Nuclear run-on assays.
Nuclear run-on transcription was performed as described previously (35) with the following modifications: slot blots (see below) were prehybridized overnight at 42°C in 50% formamide containing 6× SSC, 5 mM sodium pyrophosphate, 2× Denhardt's reagent, 0.5% SDS, and 100 μg of sheared denatured salmon sperm DNA/ml. Labeled RNA transcribed in isolated nuclei (with 2 μg of α-amanitin/ml where appropriate) was then added, and the slot blots were incubated for 24 h. Blots were then rinsed once with 2× SSC containing 0.1% SDS, washed for 3 h at 42°C in the same buffer, incubated with RNase A at 42°C for 1 h in 2× SSC without SDS to reduce nonspecific signals, and finally washed for 1 h at 42°C in 1× SSC containing 0.1% SDS. Washed slot blots were phosphorimaged (Molecular Dynamics) and the image quantified (IQ Mac v1.2).
Primer extension.
Oligonucleotide primers were labeled at the 5′ end with [γ-32P]ATP by polynucleotide kinase (NEB) in 15 μl of 1× avian myeloblastosis virus (AMV) reverse transcriptase (RT) buffer (Roche). Ten micrograms of total RNA was denatured and annealed by slow cooling from 65 to 42°C with primers (U2+10 and U6-60, 5 × 104 cpm each). Ten microliters of RT cocktail (1× AMV RT buffer, 10 mM dithiothreitol, 0.1-μg/ml ActD, 5 μM deoxynucleoside triphosphates) and 5 U of AMV RT (Roche) were added, and the reactions incubated for 15 min at 42°C. After addition of 0.5 μg of RNase A (Roche)/ml, the reactions were incubated for another 15 min at 42°C and then ethanol precipitated, resuspended in 10 μl of formamide loading buffer, incubated at 90°C for 2 min, and resolved by electrophoresis through a 7 M urea-15% polyacrylamide gel until the xylene cyanol dye front was halfway down the 42-cm gel. Gels were transferred to filter paper, dried, phosphorimaged (Molecular Dynamics) overnight (for U6) and then for 2 days (for U2+10), and quantified (IQ Mac v1.2).
RNase protection assays.
The RNase protection probes were transcribed by T7 polymerase (Ambion) in vitro in the presence of [α-32P]UTP, gel purified, and eluted overnight at 37°C. The template, pNE BS SK+, was linearized with ApaLI, which cuts close to the 3′ end of the U2 coding region; the undigested probe consists of 225 nt, containing 6 nt of 3′ U2 coding sequence, 152 nt downstream of U2 including the 3′ box, and 67 nt of vector-derived sequence. The β-actin template was cut with DraI; the undigested probe is 390 nt, including 67 nt of vector-derived sequence and 323 nt of β-actin mRNA sequence. RNase protection was performed using RNase T1 (RPA III; Ambion) with 50 μg of total RNA. Protected products were resuspended in 10 μl of formamide loading buffer and resolved by 6% polyacrylamide-7 M urea gel electrophoresis until the bromophenol blue dye front was at the bottom of the 42-cm gel. Gels were transferred to Whatman paper, dried, phosphorimaged (Molecular Dynamics) overnight, and quantified (IQ Mac v. 1.2).
Constructs and primers.
The U2+10 processing intermediate was detected by primer extension using the reverse primer U2+10: 5′-TTGTATCCCGGAGGGGGTGCA-3′. Mature U6 snRNA was detected by primer extension with the reverse primer U6-60, complementary to nt 37 to 60 of U6: 5′-GCCATGCTAATCTTCTCTGTATCG-3′. U2 maxigene products were detected using the oligonucleotide 5′-GATACTACACTTGATCCTCTAG-3′, which cross-reacts weakly with endogenous U2. Sequences complementary to both endogenous U2 snRNA and U2 maxigene snRNA are underlined. The U2 maxigene plasmid, pNE (42), contains a complete U2 gene with 152 bp of 3′ flanking sequence and a 10-nt Xba linker inserted into the Sau3A site of the U2 coding region. pNE was used for slot blotting in the nuclear run-on assay. The human β-actin probe for the slot blotting assay was a TA clone generated by PCR using 5′-AGCCATGTACGTTGCTATCCA-3′ and 5′-GGCACGAAGGCTCATCAT-3′ as forward and reverse primers, respectively. The human 5S probe was a TA clone also generated by PCR using 5′-CCGGGCTGGCGGTGTCGGCTGC-3′ and 5′-AGCACCCGGTATTCCCAGGCGG-3′ forward and reverse primers, respectively. Plasmids were linearized with HindIII and denatured for 3 min at 90°C in 0.1 M NaOH, and 5 μg of plasmid DNA/slot was transferred to a Zeta-probe membrane (Bio-Rad) with a vacuum slot blotting apparatus (Schleicher & Schuell). The template for in vitro transcription of the U2 antisense probe was plasmid pNE BS SK+, generated by cloning the EcoRI-HindIII fragment of pNE (containing all the U2 sequences) between the EcoRI and HindIII sites of pBlueScript SK+. The template for T7 in vitro transcription of the β-actin antisense probe was the β-actin TA clone described above.
RESULTS
Efficient U2 snRNA transcription requires the CTD.
Mature U2 snRNA is abundant (∼5 × 105 molecules/cell) and stable, with a half-life greater than a cell generation (67, 75). As a result, levels of mature U2 snRNA do not reflect rates of transcription. Instead, we used three different assays to detect nascent or newly transcribed U2 snRNA intermediates and thus to compare instantaneous rates of U2 snRNA transcription under different conditions (Fig. 1). First, we used a nuclear run-on assay to measure Pol II density on U2 snRNA genes; in this assay, U2 snRNA transcribed in isolated nuclei is labeled with [α-32P]UTP, and nascent snRNA is detected by hybridization to membrane-immobilized U2 DNA fragments (slot blotting). Second, we used a primer extension assay that detects U2+10 but not mature U2 snRNA, by relying on a primer that spans the 3′ end of U2+10. Although this assay can also detect precursors longer than U2+10, under normal steady-state conditions U2+10 is much more abundant than the primary transcript. The primer extension assay also detects any accumulation of unprocessed U2 primary transcripts when 3′ processing is blocked. Third, we used RNase protection to assay scarce, short-lived U2 primary transcripts that extend beyond U2+10, the most stable 3′ processing intermediate.
FIG. 1.
U2 snRNA constructs, primers, and probes used in the analysis. DSE, distal sequence element; PSE, proximal sequence element; 3′ box, 3′ end formation signal. Sequence elements upstream of the mature U2 snRNA coding region are indicated by negative numbers, and those downstream are indicated by positive numbers. The slanting line indicates 67 nt of vector sequence at the 5′ end of the RNase protection probe.
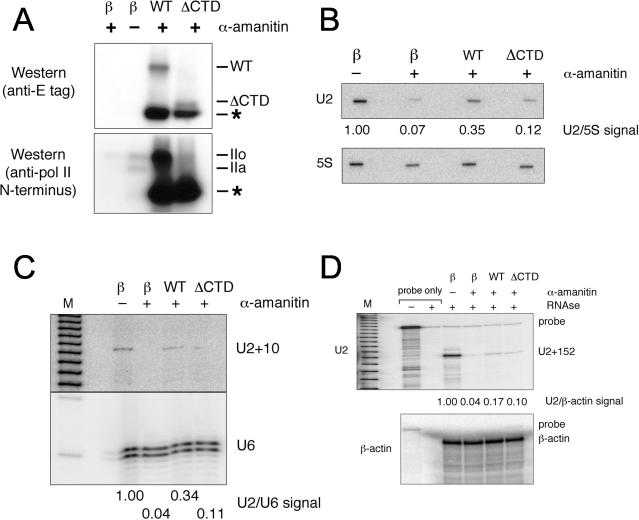
To determine whether the Pol II CTD is required for U2 snRNA transcription, as it is for mRNA transcription in vivo (48), we transiently transfected the human HT1080 fibrosarcoma line with constructs that encode an α-amanitin-resistant Pol II LS with (WT) or without (ΔCTD) an intact CTD (22) (Fig. 2); transfection with a β-galactosidase expression construct provided a negative control. Only the α-amanitin-resistant LS constructs are seen by Western blotting against the E-tag (Fig. 2A, upper panel); however, blotting against the N terminus of Pol II reveals that the α-amanitin-resistant LS is vastly overexpressed compared to endogenous Pol II (Fig. 2A, lower panel, compare right two lanes with left two) and that much of the overexpressed LS is degraded to a stable C-terminally truncated species (indicated by an asterisk), as also seen by others (14, 24, 32). Following degradation of endogenous Pol II LS (52) by exposure to 2 μg of α-amanitin/ml for 24 h (Fig. 2A, lower panel, compare left two lanes), we assayed U2 snRNA transcription by nuclear run-on using 5S rRNA transcription as an internal control (Fig. 2B) and by primer extension using U6 snRNA as an internal control (Fig. 2C). In the presence of α-amanitin, the WT construct transcribes U2 more efficiently than the ΔCTD construct (Fig. 2B, compare WT and ΔCTD lanes) and also generates more U2 transcripts detected by the U2+10 primer (Fig. 2C, compare WT and ΔCTD lanes). Although the ΔCTD polymerase may drive some residual U2 transcription, transcription is not significantly higher with ΔCTD than with no exogenous Pol II LS (Fig. 2B). U2 transcription driven by the WT construct is lower in the presence of α-amanitin than U2 transcription without the drug (Fig. 2B and C, compare β− and WT lanes) because only 30% of the cells are typically transfected as judged by staining for β-galactosidase activity (data not shown).
FIG. 2.
U2 snRNA transcription and 3′ processing require the CTD of Pol II. (A) Transient expression in HT1080 cells of E-tagged, α-amanitin-resistant Pol II LS constructs with (WT) or without (ΔCTD) an intact CTD. As assayed by Western blotting against the E-tag (upper panel), the WT construct generates an intact, phosphorylated LS (IIo); the ΔCTD construct generates comparable levels of a truncated LS; both the WT and ΔCTD constructs generate large amounts of an N-terminal LS fragment (asterisk), as seen previously (14, 24, 32); and no tagged LS is seen upon transfection with a control β-galactosidase expression construct. Assayed by Western blotting against the N terminus of Pol II (lower panel), the α-amanitin-resistant LS constructs are vastly overexpressed compared to endogenous Pol II (compare right two lanes with left two), and endogenous Pol II is degraded after exposure to 2 μg of α-amanitin/ml for 24 h (compare left two lanes). (B) Nascent U2 snRNA assayed by run-on transcription, using 5S rRNA as an internal control. Cells were harvested 24 h after transfection, and nuclear run-on transcription was conducted in the presence or absence of α-amanitin (2 μg/ml). (C) U2+10 precursor assayed by primer extension. α-Amanitin was added 24 h after transfection, and cells were incubated for an additional 24 h to degrade endogenous Pol II LS before harvesting RNA. (D) U2 snRNA primary transcript assayed by RNase protection. RNA was harvested as for panel C and assayed using the probe shown in panel A. The U2+152 signal reflects transcripts extending beyond position +152 downstream of the U2 coding region. The small amount of full-length probe seen reflects incomplete RNase digestion. RNase protection conducted with RNase A as well as T1 results in the absence of undigested probe and sharper U2+152 bands but also reveals a spurious band at U2+110 (data not shown), perhaps due to an AT-rich region in this area. Minor degradation of the high-specific-activity probe reflects autoradiolysis and does not affect our results because the probe cannot protect itself (see lane with probe only plus RNase).
U2 snRNA 3′ processing requires the CTD.
To determine whether the Pol II CTD is required for 3′ processing of the U2 primary transcript, we performed RNase protection assays using total cellular RNA and a uniformly labeled probe extending from the 3′ end of U2 through the 3′ box to +152 downstream of the U2 coding sequence; the 5′ end of this probe, corresponding to 67 nt of vector sequence, is not complementary to U2 RNA (Fig. 1). The RNase protection product seen, U2+152, corresponds to full protection of the RNase probe and represents the U2 primary transcript. As expected, little 3′-extended U2 precursor is seen when cells are transfected with the β-galactosidase construct and exposed to α-amanitin (Fig. 2D, β+ lane). Deletion of the CTD reduced U2 transcription about threefold (Fig. 2B), but U2+152 levels were only slightly decreased (Fig. 2D, compare WT and ΔCTD lanes). We conclude that CTD deletion not only reduces U2 transcription but blocks 3′ processing and causes accumulation of unprocessed U2 primary transcripts. As a control, RNase protection was performed in parallel with a probe corresponding to the stable and abundant β-actin mRNA. As previously shown (48), β-actin mRNA steady-state levels do not change significantly after a 24-h exposure to α-amanitin.
U2 snRNA transcription is unaffected by CTD hypophosphorylation, but 3′ processing is impaired.
Having established that the CTD is required for efficient U2 transcription and 3′ processing, we next asked if the phosphorylation state of the CTD is important for transcription or 3′ processing. As monitored by Western blotting against the N terminus of the Pol II LS (Fig. 3A), the CTD kinase inhibitors DRB, H7, and H8 shift the distribution of the Pol II CTD from the hyper- to the hypophosphorylated state (IIo and IIa, respectively) within 2 h as expected (33, 49, 64). However, DRB and H7 have little effect on U2 transcription as assayed by nuclear run-on (Fig. 3B). Similarly, as judged by a primer extension assay for the 3′ processing intermediate U2+10, these same CTD kinase inhibitors do not affect U2 transcription even after 6 h (Fig. 3C). Importantly, no evidence is seen in the primer extension assay for aberrant initiation in the presence of CTD kinase inhibitors (Fig. 3C). Although DRB and H7 do not inhibit U2 transcription, the RNase protection assay revealed that all three CTD kinase inhibitors (DRB, H7, and H8) cause a dramatic increase in the U2+152 signal corresponding to 3′ unprocessed U2 primary transcripts (Fig. 3D). The stable β-actin mRNA served as a positive control for the RNase protection assay. As a negative control to abolish transcription, we chose ActD instead of α-amanitin because it takes effect in vivo within minutes instead of in >12 h (52). It is formally possible that the 3′ unprocessed U2 transcripts accumulating in the presence of kinase inhibitors might not be authentic U2 snRNA precursors; however, transient transfection of a U2 maxigene shows that mature maxigene levels are indeed reduced in the presence of CTD kinase inhibitors relative to levels with mature endogenous U2 as an internal control (Fig. 4). Taken together, these results indicate that CTD phosphorylation is not required for U2 snRNA transcription, but it may be required for 3′ processing of the U2 primary transcript.
FIG. 3.
Effect of CTD kinase inhibitors on U2 snRNA transcription and 3′ processing. (A) Western blots against the N terminus of the Pol II LS. CTD kinase inhibitors DRB, H7, and H8 cause a rapid shift of the Pol II LS from the hyperphosphorylated form (IIo) to the hypophosphorylated form (IIa). (B) U2 transcription assayed by nuclear run-on. U2+10 levels are not significantly affected by DRB or H7. (C) U2+10 precursor detected by primer extension, using U6 as an internal control. The U2+10 and U6 primer extensions were performed in a single reaction and resolved on a single gel; the upper panel of this gel (U2+10) was exposed for longer than the lower panel (U6 internal control) to reveal the weaker U2+10 signal. U2 snRNA is unaffected by exposure to DRB or H7 for 4 h. (D) RNase protection assay for U2 primary transcripts as performed for Fig. 1E. Significant accumulation of 3′-extended U2 species is seen upon treatment with DRB (50 μM), H7 (40 μM), or H8 (40 μM) for 4 h. As a negative control, cells were treated with a high level of ActD (2 μg/ml, 2 h) to block U2 transcription (also see Fig. 4 and 6).
FIG. 4.
Northern blot against a transiently transfected U2 maxigene. Mature U2 maxigene accumulation is reduced in the presence of the CTD kinase inhibitors DRB (50 μM) and H7 (40 μM) for 5 h. The maxigene has a 10-nt insertion within the U2 coding region; the construct has previously been shown to produce normally processed U2 (42). The oligonucleotide probe used to detect the maxigene cross-reacts weakly with endogenous mature U2, providing an internal control. As a negative control, cells were treated with a high level of ActD (2 μg/ml, 5 h) to block U2 transcription.
U2 snRNA transcription and 3′ processing are inhibited by CTD hyperphosphorylation.
UV (41, 60) and low concentrations of ActD (8) promote CTD phosphorylation, at least in part by relieving 7SK RNA repression of the cdk9 kinase component of elongation factor P-TEFb (53, 70). Indeed, as monitored by Western blotting against the N terminus of the Pol II LS (Fig. 5A), both UV and a low concentration of ActD shift the distribution of the Pol II LS within 2 h from a balance between the IIo and IIa forms to the hyperphosphorylated IIo form exclusively. Interestingly, UV- or ActD-induced CTD hyperphosphorylation inhibited U2 transcription about 10-fold as judged by the nuclear run-on assay (Fig. 5B) and by primer extension for U2+10 (Fig. 5C), whereas β-actin mRNA synthesis was inhibited less than twofold under these same conditions (Fig. 5B). Moreover, UV- and ActD-induced CTD hyperphosphorylation inhibited U2 transcription (Fig. 5B) more severely than it did levels of unprocessed U2 species, as judged by the RNase protection assay for 3′-extended U2 primary transcripts (Fig. 5D). These data suggest that CTD hyperphosphorylation impairs both transcription and 3′ processing, and as a result the large reduction in U2 transcription (as judged by primer extension and nuclear run-on) is accompanied by only a modest reduction in the level of unprocessed U2 primary transcripts. As in Fig. 3, stable β-actin mRNA served as a positive control for the RNase protection assay, and a high concentration of ActD served as a negative control to abolish all transcription.
FIG. 5.
Effect of CTD hyperphosphorylation on U2 snRNA transcription and 3′ processing. (A) Western blot against the N terminus of the Pol II LS. A low dose of UV irradiation or ActD shifts the Pol II LS from the hypophosphorylated (IIa) form to the hyperphosphorylated (IIo) form. (B) U2 transcription, assayed by nuclear run-on, is reduced by UV irradiation (6 h) or a low dose of ActD (50 ng/ml, 2 h), whereas β-actin transcription is less severely affected. (C) U2+10 precursor, detected by primer extension, is reduced by UV or ActD treatment. U6 was used as a control as for Fig. 2C. (D) RNase protection assay for U2 primary transcripts, as in Fig. 2D. A moderate reduction in 3′-extended U2 species is seen after treatment with UV (6 h) or a low level of ActD (50 ng/ml, 2 h), whereas a high level of ActD (2 μg/ml, 2 h) completely blocks U2 transcription.
CTD kinase inhibitors can rescue inhibition of transcription by CTD hyperphosphorylation but not 3′ processing.
Although U2 transcription correlates well with CTD phosphorylation status, this does not necessarily imply that UV and ActD inhibit U2 transcription through CTD phosphorylation. For example, UV-induced DNA damage could shut down transcription while damage was repaired (5), or UV could block U2 transcription by preferentially damaging active U2 transcription units; alternatively, UV could inhibit U2 transcription indirectly by inducing stress response mRNAs. Similarly, ActD, which binds duplex DNA tenaciously, could physically block progression of RNA polymerase (63).
To provide a more direct link between UV-induced CTD hyperphosphorylation and inhibition of U2 snRNA transcription, we asked whether the CTD kinase inhibitors DRB and H7 could rescue U2 transcription in UV-irradiated cells. UV irradiation shifts Pol II from the IIa form to the IIo form (Fig. 5A) and inhibits U2 transcription (Fig. 5B); treatment of UV-irradiated cells with DRB or H7 shifts Pol II from the IIo form to the IIa form as judged by Western blots (Fig. 6A) and rescues U2 transcription as assayed by nuclear run-on transcription (Fig. 6B) or by primer extension (Fig. 6C). Interestingly, U2+152 precursor still accumulates to high levels in the presence of UV and either DRB or H7 (Fig. 6D), indicating that while UV transcription inhibition is ameliorated by the drugs, there remains a profound defect in 3′ end formation. We conclude that UV irradiation preferentially inhibits U2 snRNA transcription through CTD hyperphosphorylation.
FIG. 6.
Effects of CTD kinase inhibitors on UV inhibition of U2 snRNA transcription and 3′ processing. (A) As shown by Western blotting with an antibody against the N terminus of Pol II LS, DRB (50 μM) and H7 (40 μM) shift the distribution of Pol II LS to the hypophosphorylated (IIa) form and prevent UV-mediated redistribution of Pol II LS to the hyperphosphorylated (IIo) form. (B) U2+10 precursor as detected by primer extension is drastically reduced after UV irradiation but restored in the presence of DRB (50 μM) or H7 (40 μM). (C) As shown by nuclear run-on, UV inhibition of U2 transcription 6 h postirradiation is rescued by DRB (50 μM) or H7 (40 μM). (D) RNase protection with the U2+152 probe complementary to the 3′ region of the primary transcript indicates accumulation of 3′-extended U2 species 6 h after UV irradiation in the presence of DRB or H7 compared to results with either untreated cells or UV-irradiated cells alone. A high dose of ActD (2 μg/ml, 2 h) served as a negative control to block all transcription.
DISCUSSION
In this study, we have shown that efficient U2 snRNA transcription requires the Pol II CTD, as is the case for mRNAs (Fig. 2). Our other results, however, highlight differences between snRNA and mRNA transcription. U2 snRNA transcription, unlike mRNA transcription, is not sensitive to the CTD kinase inhibitors DRB, H7, and H8 (Fig. 3). In fact, exposure to these drugs causes accumulation of 3′-extended primary transcripts, probably through inhibition of 3′ box-directed processing. This is a seemingly paradoxical result: CTD kinase inhibitors, which prevent elongation of mRNA transcripts, promote accumulation of longer U2 snRNA transcripts. The resistance of U2 snRNA transcription to kinase inhibitors cannot be attributed to the small size of mature U2 snRNA (188 nt), since U2 primary transcripts can be as long as 1 kb (15), exceeding the length of mRNA transcripts observed in the presence of CTD kinase inhibitors (47).
We also show that snRNA transcription is preferentially sensitive to treatments that promote CTD hyperphosphorylation (Fig. 5) and that UV-induced inhibition of transcription (but not 3′ processing) can be rescued by CTD kinase inhibitors (Fig. 6). This is strong evidence that the UV-induced defect in U2 transcription is caused by CTD hyperphosphorylation. However, it is important to note that H7 (but not DRB) has also been shown to inhibit a kinase involved in the UV response (10), and thus, we cannot exclude the possibility that these kinase inhibitors block UV damage detection or UV signal transduction at some step prior to CTD hyperphosphorylation.
More than 22 years ago, DRB was shown to prevent accumulation of mature U snRNA (25); however, we (Fig. 3) and others (47) have now shown that transcription of U2 snRNA is not affected by DRB. These results can be reconciled: Hellung-Larsen et al. (25) used polyacrylamide gel electrophoresis to monitor accumulation of mature snRNAs labeled in vivo with [32P]orthophosphate. U2 transcripts made in the presence of DRB would have escaped detection not only because little mature snRNA is made when 3′ processing is inhibited (30) but also because the unprocessed transcripts are heterogeneous (15) and unlikely to be stable over the period of in vivo labeling (data not shown).
More than 24 years ago, U snRNA transcription was found to be inhibited by low doses of UV irradiation (20). An even earlier report that accumulation of mature snRNAs and rRNA are equally sensitive to ActD had led to speculation that snRNAs might be Pol I transcripts (74), although snRNAs and mRNAs were also equally sensitive to α-amanitin (9, 59). We show that U2 snRNA transcription is more sensitive to UV and ActD than is transcription of β-actin mRNA (Fig. 5), and we provide evidence that inhibition is caused by CTD hyperphosphorylation (Fig. 6). As detailed below, this mechanism suggests the existence of a specialized snRNA-specific transcription and processing complex.
The CTD plays an important role in transcription and processing of both mRNAs and U snRNAs, but there are intriguing similarities and differences that are likely have functional consequences. For mRNAs, capping, splicing, and polyadenylation factors can be recruited to the initiating polymerase through binding to the CTD (11, 46). For snRNAs, events at the promoter are also tightly coupled to downstream processing. Although both snRNAs and mRNAs are capped, promoter swap experiments have shown that transcripts originating from an mRNA promoter are not efficiently processed at the U snRNA 3′ end formation signal (3′ box) (18, 28), whereas transcripts originating from U snRNA promoters can be spliced (69) but not efficiently polyadenylated (16). We show that some level of CTD phosphorylation is required for efficient 3′ processing of the U2 primary transcript (Fig. 3 and 4), but U2 transcription itself does not require CTD phosphorylation and is in fact inhibited by CTD hyperphosphorylation (Fig. 3 and 5). This may indicate that the CTD of Pol II transcribing mRNAs and snRNAs is differentially phosphorylated and that these differences may be established as early as assembly of the preinitiation complex on the promoter.
Recent reports have implicated small ribonucleoprotein complexes in the regulation of mRNA transcription. The small cytoplasmic 7SK RNA has been found to sequester and inhibit cdk9, an essential kinase component of the Pol II elongation factor P-TEFb (53, 70). UV irradiation or treatment with ActD disrupts the small ribonucleoprotein complex, freeing P-TEFb to function in elongation. Indeed, recruitment of P-TEFb by Tat protein bound to the TAR element is an important survival strategy for the human immunodeficiency type 1 provirus, which is regulated at the level of transcription elongation (7). Similarly, the basal transcription factor TFIIH, which contains the kinase cdk7 (61, 62), exhibits increased transcription initiation activity in vitro when complexed with a form of U1 snRNA (37).
We speculate that CTD hyperphosphorylation may have multiple roles—promoting transcription of stress promoters, such as heat shock protein 70, where polymerases are poised early in elongation (54), facilitating DNA damage repair (39, 55), and transiently shutting down transcription of vulnerable snRNAs until danger has passed. Consistent with this hypothesis, artificial recruitment of P-TEFb to an snRNA promoter impairs transcription (58).
TFIIH, like the P-TEFb complex, also responds to UV. After UV irradiation, TFIIH relocates to sites of DNA damage, where it functions in repair (50). Conceivably, U1 snRNA could modulate the activity of TFIIH in response to noxious agents like UV and ActD, just as 7SK modulates P-TEFb activity. TFIIH is the only basal transcription factor not thought to be required for snRNA transcription (36), perhaps playing a role in negative regulation instead. However, unlike the cdk9-mediated CTD kinase activity of P-TEFb, the cdk7-mediated CTD kinase activity of TFIIH seems to be reduced rather than stimulated by UV irradiation (1).
Many important questions remain. We do not know the identity of the CTD kinase(s) required for U2 snRNA 3′ processing or for inhibition of U2 transcription. Nor do we know the positions within the CTD heptad repeat that must be phosphorylated for 3′ processing or that have the potential to inhibit U2 transcription. A more difficult problem will be to understand the topography of the CTD itself, i.e., whether phosphorylation is a bulk property of all repeats or must be properly distributed over particular heptad repeats (or regions of repeats) in order to create the binding surfaces required to recruit distinct processing factors as previously proposed (47). Finally, we do not know the identity of the U snRNA 3′ processing factors, how these factors recognize the 3′ end formation signal (3′ box) to direct 3′ cleavage of the U snRNA primary transcript, or whether the specialized U snRNA promoter plays a role in loading these factors onto the CTD of initiating Pol II.
Acknowledgments
We thank Anton Krumm and Mark Groudine for advice on nuclear run-on transcription and Tom Pavelitz for assistance cloning pNE BS SK+. We also thank Arnold Bailey and John Newman for critical reading of the manuscript. The α-amanitin-resistant Pol II LS constructs were kind gifts of David Bentley (University of Colorado Health Sciences Center, Denver).
This work was supported by NIH GM41624 and NRSA T32 GM07270.
REFERENCES
- 1.Adamczewski, J. P., M. Rossignol, J. P. Tassan, E. A. Nigg, V. Moncollin, and J. M. Egly. 1996. MAT1, cdk7 and cyclin H form a kinase complex which is UV light-sensitive upon association with TFIIH. EMBO J. 15:1877-1884. [PMC free article] [PubMed] [Google Scholar]
- 2.Allison, L. A., M. Moyle, M. Shales, and C. J. Ingles. 1985. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell 42:599-610. [DOI] [PubMed] [Google Scholar]
- 3.Baskaran, R., G. G. Chiang, T. Mysliwiec, G. D. Kruh, and J. Y. Wang. 1997. Tyrosine phosphorylation of RNA polymerase II carboxyl-terminal domain by the Abl-related gene product. J. Biol. Chem. 272:18905-18909. [DOI] [PubMed] [Google Scholar]
- 4.Baskaran, R., M. E. Dahmus, and J. Y. Wang. 1993. Tyrosine phosphorylation of mammalian RNA polymerase II carboxyl-terminal domain. Proc. Natl. Acad. Sci. USA 90:11167-11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender, K., C. Blattner, A. Knebel, M. Iordanov, P. Herrlich, and H. J. Rahmsdorf. 1997. UV-induced signal transduction. J. Photochem. Photobiol. B 37:1-17. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein, L. B., T. Manser, and A. M. Weiner. 1985. Human U1 small nuclear RNA genes: extensive conservation of flanking sequences suggests cycles of gene amplification and transposition. Mol. Cell. Biol. 5:2159-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieniasz, P. D., T. A. Grdina, H. P. Bogerd, and B. R. Cullen. 1999. Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter proximal RNA target is both necessary and sufficient for full activation of transcription. Proc. Natl. Acad. Sci. USA 96:7791-7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casse, C., F. Giannoni, V. T. Nguyen, M. F. Dubois, and O. Bensaude. 1999. The transcriptional inhibitors, actinomycin D and alpha-amanitin, activate the HIV-1 promoter and favor phosphorylation of the RNA polymerase II C-terminal domain. J. Biol. Chem. 274:16097-16106. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekharappa, S. C., J. H. Smith, and G. L. Eliceiri. 1983. Biosynthesis of small nuclear RNAs in human cells. J. Cell Physiol. 117:169-174. [DOI] [PubMed] [Google Scholar]
- 10.Chernov, M. V., L. J. Bean, N. Lerner, and G. R. Stark. 2001. Regulation of ubiquitination and degradation of p53 in unstressed cells through C-terminal phosphorylation. J. Biol. Chem. 276:31819-31824. [DOI] [PubMed] [Google Scholar]
- 11.Cho, E. J., T. Takagi, C. R. Moore, and S. Buratowski. 1997. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 11:3319-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhury, K., I. Choudhury, and G. L. Eliceiri. 1989. Metabolism of small RNAs in cultured human cells. J. Cell Physiol. 138:433-438. [DOI] [PubMed] [Google Scholar]
- 13.Conaway, J. W., A. Shilatifard, A. Dvir, and R. C. Conaway. 2000. Control of elongation by RNA polymerase II. Trends Biochem. Sci. 25:375-380. [DOI] [PubMed] [Google Scholar]
- 14.Corden, J. L., D. L. Cadena, J. M. Ahearn, Jr., and M. E. Dahmus. 1985. A unique structure at the carboxyl terminus of the largest subunit of eukaryotic RNA polymerase II. Proc. Natl. Acad. Sci. USA 82:7934-7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuello, P., D. C. Boyd, M. J. Dye, N. J. Proudfoot, and S. Murphy. 1999. Transcription of the human U2 snRNA genes continues beyond the 3′ box in vivo. EMBO J. 18:2867-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlberg, J. E., and E. T. Schenborn. 1988. The human U1 snRNA promoter and enhancer do not direct synthesis of messenger RNA. Nucleic Acids Res. 16:5827-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darzacq, X., B. E. Jady, C. Verheggen, A. M. Kiss, E. Bertrand, and T. Kiss. 2002. Cajal body-specific small nuclear RNAs: a novel class of 2′-O-methylation and pseudouridylation guide RNAs. EMBO J. 21:2746-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vegvar, H. E., E. Lund, and J. E. Dahlberg. 1986. 3′ end formation of U1 snRNA precursors is coupled to transcription from snRNA promoters. Cell 47:259-266. [DOI] [PubMed] [Google Scholar]
- 19.Donelson, J. E. 2003. Antigenic variation and the African trypanosome genome. Acta Trop. 85:391-404. [DOI] [PubMed] [Google Scholar]
- 20.Eliceiri, G. L. 1979. Sensitivity of low molecular weight RNA synthesis to UV radiation. Nature 279:80-81. [DOI] [PubMed] [Google Scholar]
- 21.Eliceiri, G. L., and M. S. Sayavedra. 1976. Small RNAs in the nucleus and cytoplasm of HeLa cells. Biochem. Biophys. Res. Commun. 72:507-512. [DOI] [PubMed] [Google Scholar]
- 22.Fong, N., and D. L. Bentley. 2001. Capping, splicing, and 3′ processing are independently stimulated by RNA polymerase II: different functions for different segments of the CTD. Genes Dev. 15:1783-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber, H. P., M. Hagmann, K. Seipel, O. Georgiev, M. A. West, Y. Litingtung, W. Schaffner, and J. L. Corden. 1995. RNA polymerase II C-terminal domain required for enhancer-driven transcription. Nature 374:660-662. [DOI] [PubMed] [Google Scholar]
- 24.Guilfoyle, T. J., G. Hagen, and S. Malcolm. 1984. Size heterogeneity of the largest subunit of nuclear RNA polymerase II. An immunological analysis. J. Biol. Chem. 259:649-653. [PubMed] [Google Scholar]
- 25.Hellung-Larsen, P., E. G. Jensen, and S. Frederiksen. 1981. Effect of 5,6-dichloro-1-beta-d-ribofuranosylbenzimidazole on the synthesis of low molecular weight, RNA components. Biochem. Biophys. Res. Commun. 99:1303-1310. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez, N. 1985. Formation of the 3′ end of U1 snRNA is directed by a conserved sequence located downstream of the coding region. EMBO J. 4:1827-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez, N. 2001. Small nuclear RNA genes: a model system to study fundamental mechanisms of transcription. J. Biol. Chem. 276:26733-26736. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez, N., and A. M. Weiner. 1986. Formation of the 3′ end of U1 snRNA requires compatible snRNA promoter elements. Cell 47:249-258. [DOI] [PubMed] [Google Scholar]
- 29.Huang, Q., M. R. Jacobson, and T. Pederson. 1997. 3′ processing of human pre-U2 small nuclear RNA: a base-pairing interaction between the 3′ extension of the precursor and an internal region. Mol. Cell. Biol. 17:7178-7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang, Q., and T. Pederson. 1999. A human U2 RNA mutant stalled in 3′ end processing is impaired in nuclear import. Nucleic Acids Res. 27:1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber, J., U. Cronshagen, M. Kadokura, C. Marshallsay, T. Wada, M. Sekine, and R. Luhrmann. 1998. Snurportin1, an m3G-cap-specific nuclear import receptor with a novel domain structure. EMBO J. 17:4114-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, W. Y., and M. E. Dahmus. 1989. The major late promoter of adenovirus-2 is accurately transcribed by RNA polymerases IIO, IIA, and IIB. J. Biol. Chem. 264:3169-3176. [PubMed] [Google Scholar]
- 33.Kim, Y. K., C. F. Bourgeois, C. Isel, M. J. Churcher, and J. Karn. 2002. Phosphorylation of the RNA polymerase II carboxyl-terminal domain by CDK9 is directly responsible for human immunodeficiency virus type 1 Tat-activated transcriptional elongation. Mol. Cell. Biol. 22:4622-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kops, O., X. Z. Zhou, and K. P. Lu. 2002. Pin1 modulates the dephosphorylation of the RNA polymerase II C-terminal domain by yeast Fcp1. FEBS Lett. 513:305-311. [DOI] [PubMed] [Google Scholar]
- 35.Krumm, A., L. B. Hickey, and M. Groudine. 1995. Promoter-proximal pausing of RNA polymerase II defines a general rate-limiting step after transcription initiation. Genes Dev. 9:559-572. [DOI] [PubMed] [Google Scholar]
- 36.Kuhlman, T. C., H. Cho, D. Reinberg, and N. Hernandez. 1999. The general transcription factors IIA, IIB, IIF, and IIE are required for RNA polymerase II transcription from the human U1 small nuclear RNA promoter. Mol. Cell. Biol. 19:2130-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwek, K. Y., S. Murphy, A. Furger, B. Thomas, W. O'Gorman, H. Kimura, N. J. Proudfoot, and A. Akoulitchev. 2002. U1 snRNA associates with TFIIH and regulates transcriptional initiation. Nat. Struct. Biol. 9:800-805. [DOI] [PubMed] [Google Scholar]
- 38.Lim, K., and C. B. Chae. 1989. A simple assay for DNA transfection by incubation of the cells in culture dishes with substrates for beta-galactosidase. BioTechniques 7:576-579. [PubMed] [Google Scholar]
- 39.Ljungman, M., H. M. O'Hagan, and M. T. Paulsen. 2001. Induction of ser15 and lys382 modifications of p53 by blockage of transcription elongation. Oncogene 20:5964-5971. [DOI] [PubMed] [Google Scholar]
- 40.Lund, E., and J. E. Dahlberg. 1984. True genes for human U1 small nuclear RNA. Copy number, polymorphism, and methylation. J. Biol. Chem. 259:2013-2021. [PubMed] [Google Scholar]
- 41.Luo, Z., J. Zheng, Y. Lu, and D. B. Bregman. 2001. Ultraviolet radiation alters the phosphorylation of RNA polymerase II large subunit and accelerates its proteasome-dependent degradation. Mutat. Res. 486:259-274. [DOI] [PubMed] [Google Scholar]
- 42.Mangin, M., M. Ares, Jr., and A. M. Weiner. 1986. Human U2 small nuclear RNA genes contain an upstream enhancer. EMBO J. 5:987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall, N. F., J. Peng, Z. Xie, and D. H. Price. 1996. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J. Biol. Chem. 271:27176-27183. [DOI] [PubMed] [Google Scholar]
- 44.Mattaj, I. W. 1986. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell 46:905-911. [DOI] [PubMed] [Google Scholar]
- 45.Mattaj, I. W., and E. M. De Robertis. 1985. Nuclear segregation of U2 snRNA requires binding of specific snRNP proteins. Cell 40:111-118. [DOI] [PubMed] [Google Scholar]
- 46.McCracken, S., N. Fong, K. Yankulov, S. Ballantyne, G. Pan, J. Greenblatt, S. D. Patterson, M. Wickens, and D. L. Bentley. 1997. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature 385:357-361. [DOI] [PubMed] [Google Scholar]
- 47.Medlin, J. E., P. Uguen, A. Taylor, D. L. Bentley, and S. Murphy. 2003. The C-terminal domain of pol II and a DRB-sensitive kinase are required for 3′ processing of U2 snRNA. EMBO J. 22:925-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meininghaus, M., R. D. Chapman, M. Horndasch, and D. Eick. 2000. Conditional expression of RNA polymerase II in mammalian cells. Deletion of the carboxyl-terminal domain of the large subunit affects early steps in transcription. J. Biol. Chem. 275:24375-24382. [DOI] [PubMed] [Google Scholar]
- 49.Mitsui, A., and P. A. Sharp. 1999. Ubiquitination of RNA polymerase II large subunit signaled by phosphorylation of carboxyl-terminal domain. Proc. Natl. Acad. Sci. USA 96:6054-6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mone, M. J., M. Volker, O. Nikaido, L. H. Mullenders, A. A. van Zeeland, P. J. Verschure, E. M. Manders, and R. van Driel. 2001. Local UV-induced DNA damage in cell nuclei results in local transcription inhibition. EMBO Rep. 2:1013-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuman de Vegvar, H. E., and J. E. Dahlberg. 1990. Nucleocytoplasmic transport and processing of small nuclear RNA precursors. Mol. Cell. Biol. 10:3365-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen, V. T., F. Giannoni, M. F. Dubois, S. J. Seo, M. Vigneron, C. Kedinger, and O. Bensaude. 1996. In vivo degradation of RNA polymerase II largest subunit triggered by alpha-amanitin. Nucleic Acids Res. 24:2924-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen, V. T., T. Kiss, A. A. Michels, and O. Bensaude. 2001. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414:322-325. [DOI] [PubMed] [Google Scholar]
- 54.O'Brien, T., S. Hardin, A. Greenleaf, and J. T. Lis. 1994. Phosphorylation of RNA polymerase II C-terminal domain and transcriptional elongation. Nature 370:75-77. [DOI] [PubMed] [Google Scholar]
- 55.Ostapenko, D., and M. J. Solomon. 2003. Budding yeast CTDK-I is required for DNA damage-induced transcription. Eukaryot. Cell 2:274-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paule, M. R., and R. J. White. 2000. Survey and summary: transcription by RNA polymerases I and III. Nucleic Acids Res. 28:1283-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Payne, J. M., P. J. Laybourn, and M. E. Dahmus. 1989. The transition of RNA polymerase II from initiation to elongation is associated with phosphorylation of the carboxyl-terminal domain of subunit IIa. J. Biol. Chem. 264:19621-19629. [PubMed] [Google Scholar]
- 58.Ratnasabapathy, R., M. Sheldon, L. Johal, and N. Hernandez. 1990. The HIV-1 long terminal repeat contains an unusual element that induces the synthesis of short RNAs from various mRNA and snRNA promoters. Genes Dev. 4:2061-2074. [DOI] [PubMed] [Google Scholar]
- 59.Ro-Choi, T. S., N. B. Raj, L. M. Pike, and H. Busch. 1976. Effects of alpha-amanitin, cycloheximide, and thioacetamide on low molecular weight nuclear RNA. Biochemistry 15:3823-3828. [DOI] [PubMed] [Google Scholar]
- 60.Rockx, D. A., R. Mason, A. van Hoffen, M. C. Barton, E. Citterio, D. B. Bregman, A. A. van Zeeland, H. Vrieling, and L. H. Mullenders. 2000. UV-induced inhibition of transcription involves repression of transcription initiation and phosphorylation of RNA polymerase II. Proc. Natl. Acad. Sci. USA 97:10503-10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serizawa, H., T. P. Makela, J. W. Conaway, R. C. Conaway, R. A. Weinberg, and R. A. Young. 1995. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature 374:280-282. [DOI] [PubMed] [Google Scholar]
- 62.Shiekhattar, R., F. Mermelstein, R. P. Fisher, R. Drapkin, B. Dynlacht, H. C. Wessling, D. O. Morgan, and D. Reinberg. 1995. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature 374:283-287. [DOI] [PubMed] [Google Scholar]
- 63.Sobell, H. M. 1985. Actinomycin and DNA transcription. Proc. Natl. Acad. Sci. USA 82:5328-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song, C. Z. 1996. Requirement for phosphorylation of RNA polymerase II C-terminal domain in transcription is both transcript length and promoter dependent. Biochem. Biophys. Res. Commun. 229:810-816. [DOI] [PubMed] [Google Scholar]
- 65.Tschudi, C., and E. Ullu. 2002. Unconventional rules of small nuclear RNA transcription and cap modification in trypanosomatids. Gene Expr. 10:3-16. [PMC free article] [PubMed] [Google Scholar]
- 66.Van Arsdell, S. W., and A. M. Weiner. 1984. Human genes for U2 small nuclear RNA are tandemly repeated. Mol. Cell. Biol. 4:492-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weinberg, R. A., and S. Penman. 1968. Small molecular weight monodisperse nuclear RNA. J. Mol. Biol. 38:289-304. [DOI] [PubMed] [Google Scholar]
- 68.Westin, G., J. Zabielski, K. Hammarstrom, H. J. Monstein, C. Bark, and U. Pettersson. 1984. Clustered genes for human U2 RNA. Proc. Natl. Acad. Sci. USA 81:3811-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White, R. A., and G. R. Kunkel. 1993. Pre-messenger RNA splicing of transcripts synthesized from human small nuclear RNA gene promoters. Biochem. Biophys. Res. Commun. 195:1394-1400. [DOI] [PubMed] [Google Scholar]
- 70.Yang, Z., Q. Zhu, K. Luo, and Q. Zhou. 2001. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414:317-322. [DOI] [PubMed] [Google Scholar]
- 71.Yu, Y. T., M. D. Shu, A. Narayanan, R. M. Terns, M. P. Terns, and J. A. Steitz. 2001. Internal modification of U2 small nuclear (sn)RNA occurs in nucleoli of Xenopus oocytes. J. Cell Biol. 152:1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuo, C. Y., M. Ares, Jr., and A. M. Weiner. 1985. Sequences required for 3′ end formation of human U2 small nuclear RNA. Cell 42:193-202. [DOI] [PubMed] [Google Scholar]
- 73.Zehring, W. A., J. M. Lee, J. R. Weeks, R. S. Jokerst, and A. L. Greenleaf. 1988. The C-terminal repeat domain of RNA polymerase II largest subunit is essential in vivo but is not required for accurate transcription initiation in vitro. Proc. Natl. Acad. Sci. USA 85:3698-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zieve, G., B. J. Benecke, and S. Penman. 1977. Synthesis of two classes of small RNA species in vivo and in vitro. Biochemistry 16:4520-4525. [DOI] [PubMed] [Google Scholar]
- 75.Zieve, G., and S. Penman. 1976. Small RNA species of the HeLa cell: metabolism and subcellular localization. Cell 8:19-31. [DOI] [PubMed] [Google Scholar]