Abstract
Heterodimers of the retinoid X receptor (RXR) with the thyroid hormone receptor (TR) are considered to be nonpermissive. It is believed that within these complexes RXR acts as a “silent partner.” We demonstrate here that a permissive heterodimer mediates stimulation of prolactin expression by the thyroid hormone T3 and by 9-cis retinoic acid (9-cis-RA). A response element located in the prolactin distal enhancer mediates transactivation by both ligands in pituitary cells, and RXR recruits coactivators when bound to this element as a heterodimer with TR. Furthermore, transcription by the RXR agonist can be obtained in CV-1 cells only after overexpression of coactivators, and overexpression of corepressors inhibits the response in pituitary cells. Thus, cell type-specific differences in coregulator recruitment can determine the cellular response to both ligands. Coactivator recruitment by 9-cis-RA requires the ligand-dependent transactivation domains (AF-2) of both heterodimeric partners. Interestingly, the presence of the RXR ligand can overcome the deleterious effect of the AF-2 mutation E401Q on association with coactivators and transactivation. These results demonstrate an unexpected role for RXR in TR signaling and show that in particular cellular environments this receptor can act as a “nonsilent” partner of TR, allowing stimulation by RXR agonists.
The actions of the thyroid hormone triiodothyronine (T3) are mediated by binding to nuclear thyroid hormone receptors (TRs). TRs are ligand-dependent transcription factors which regulate transcription by binding to T3 response elements (TREs) in target genes (53). TREs are composed of at least two copies of the consensus motif PuG/TGTCA, configured as a palindrome, an inverted palindrome, or a direct repeat normally separated by four intervening nucleotides (DR4). TRs, as well as many other nuclear receptors, bind DNA preferentially as heterodimers with retinoid X receptors (RXRs). Heterodimerization strongly increases binding to the TRE and transcriptional activity. Therefore, RXR plays a dual role in nuclear receptor signaling. On one hand, it can bind to its own response element, a DR1, as a homodimer and activate transcription in response to its ligand 9-cis-retinoic acid (9-cis-RA) (21, 27), and on the other hand it serves as a partner for other nuclear receptors (31).
The existence of two types of nuclear receptor heterodimers, nonpermissive and permissive, has been described. Permissive heterodimers can be indistinctly activated by ligands of either RXR or its partner receptor and are synergistically activated in the presence of both ligands (1). However, in nonpermissive heterodimers the ligand-induced transcriptional activities of RXR are suppressed, and it is believed that formation of the heterodimer actually precludes the binding of ligand to RXR (15). Thus, in these complexes, RXR is said to be a “silent partner.” TRs as well as the receptors for vitamin D or for retinoic acid (RARs) were thought to be nonpermissive.
The effects of TRs, as well as other nuclear receptors, on transcription are mediated through recruitment of coregulators. TRs bind corepressor factors and actively repress target gene expression in the absence of ligand. Corepressors are found within multicomponent complexes, which contain histone deacetylase activity (22). Upon ligand binding the receptors undergo a conformational change, which allows the recruitment of multiple coactivator complexes through a ligand-dependent transcriptional activation function (AF-2) located in helix 12 at the C terminus in the ligand binding domain (2). Some of these proteins are chromatin-remodeling factors, others (such as CBP/p300 and the p160 coactivators) possess histone acetylase activity, and others (such as the TRAP/DRIP complex) may interact directly with the basic transcriptional machinery. These coactivators cause chromatin decompaction, RNA polymerase II recruitment, and transcriptional activation (16, 24, 32, 39, 40).
Results arguing against the current silent-partner model for RXR in the RXR/TR heterodimer have been recently obtained with a derepression assay system (28). According to this model RXR would bind ligand and this binding would lead to dissociation of corepressors from TR, thus modulating heterodimer activity. Since RXR was believed not to bind ligand, it was assumed that coactivators could not be recruited to a nonpermissive heterodimer in response to 9-cis-RA. However, recent data indicate that RXR can recruit coactivators as a heterodimer with RAR. Lack of autonomous transcription on binding of the RXR agonist would be due to the fact that in the usual cellular environment corepressors do not dissociate from RAR and they prohibit coactivator access because corepressor binding and coactivator binding are mutually exclusive (18). This model predicts that transcription by RXR agonists (rexinoids) could be obtained under some conditions, for instance, in cells expressing high coactivator levels, although evidence for natural genes regulated in this manner had not been obtained.
The rat prolactin (PRL) gene provides an excellent model for the study of cell-specific and multihormonal regulation. Multiple hormones, growth factors, and oncogenes act in conjunction with the pituitary-specific transcription factor GHF-1/Pit-1 to regulate PRL gene expression in the lactotroph cells of the anterior pituitary. Transcription of the PRL gene is governed by two domains, a proximal promoter and a distal enhancer (located between bp −1500 and −1800), both containing binding sites for GHF-1/Pit-1. PRL-producing rat pituitary cell lines expressing high levels of different members of the nuclear receptor superfamily are available. Several nuclear receptors, among them estrogen receptors (ERs) (12), peroxisome proliferator-activated receptors (46), vitamin D receptors (5), and glucocorticoid receptors (41), are known to play an important role in PRL gene transcription in these cells. In addition, it has been reported that the thyroid hormone regulates both positively and negatively PRL gene transcription in rat pituitary cell lines. Thus, it was reported that T3 inhibits and stimulates PRL transcription in GH1 cells and GH4C1 cells, respectively, through sequences located in the proximal promoter (44). In contrast, it has been found that in GH3 cells a region close to the ER response element (ERE) in the distal enhancer mediates stimulation, whereas sequences contained in the proximal promoter mediate inhibition by T3 (11). However, a detailed analysis of these sequences has not been performed, and the role of RXR in this regulation has not been analyzed.
In this work we have examined the role of RXR/TR heterodimers in PRL gene expression. We find that not only T3 but also 9-cis-RA increase PRL transcripts in GH4C1 cells. We demonstrate binding of RXR/TR heterodimers to a positive TRE, configured as a DR4, in the distal enhancer between nucleotides −1551 and −1566. This TRE mediates stimulation of the PRL gene by ligands of both heterodimeric partners in transient-transfection assays. Furthermore, expression of TR in pituitary 235-1 cells lacking this receptor confers responsiveness not only to T3 but also to 9-cis-RA. The TRE does not mediate regulation by 9-cis-RA in CV-1 cells, but overexpression of coactivators allows stimulation by this ligand, fulfilling the prediction that transcription by RXR agonists can be obtained in cells expressing high coactivator levels. These results strongly suggest that RXR does not act as a silent partner in the RXR/TR heterodimer to stimulate PRL gene transcription. This hypothesis is further proved by the finding that the TRE-bound heterodimer can recruit p160 coactivators or the TRAP205 subunit of the DRIP/TRAP complex in response to either agonist. Interestingly, deletion of the TR AF-2 domain inhibits coactivator recruitment, as well as activation of the TRE-containing construct, by the RXR ligand. Furthermore, the presence of the RXR ligand overcomes the deleterious effect of a point mutation (E401Q) in the TR AF-2 domain on association with coactivators and transcriptional stimulation. These results show that different conformations of the heterodimer can be induced by both agonists and that binding to either agonist results in a linked conformational change in the other receptor subunit. Therefore, our results prove for the first time that RXR/TR heterodimers, previously regarded as nonpermissive, can mediate stimulation of transcription of a natural gene by the RXR agonist 9-cis-RA.
MATERIALS AND METHODS
RNA extraction and hybridization.
For the experiments the cells were incubated for 24 h in a medium containing a hormone-stripped serum and treated for 48 h with different ligands. Total RNA was used for Northern blot analysis with a cDNA probe for rat PRL as described previously (5). The RNA was stained with 0.02% methylene blue to detect rRNA as a control for loading.
Plasmids.
Reporter plasmids containing different fragments of the rat PRL 5′-flanking region have been previously described (5, 29). The mutated constructs were obtained with the Pfu Turbo DNA polymerase (Stratagene), by using the oligonucleotide 5′-TGCTTTGGTCTCAGAAGATTCAG-3′ (boldface indicates mutated bases). The mutations were confirmed by sequencing. Oligonucleotides containing sequences −1551 to −1573 and −1551 to −1593 of the rat PRL distal enhancer were cloned upstream of the thymidine kinase promoter into pBL-CAT2, from which an AP-1-like sequence, which could mask some promoter responses, had been deleted by digestion with AatII and NarI. Expression vectors for wild-type and mutant RXRα and chick TRα have been previously described and were cloned in pSG5 (2, 3). Expression vectors for TIF-2, SRC-1, ACTR, DRIP205, SMRT, and NcoR (6, 7, 20, 39, 47) were cloned in the same vector. The glutathione S-transferase (GST)-ACTR, GST-TIF-2, GST-SRC-1, GST-DRIP205, and GST-SMRT vector constructs code for protein fragments containing the nuclear receptor-interacting domains of these proteins. The His-tagged nuclear receptor-interacting domain of TIF-2, as well as this domain with mutations in box II (M2) and box III (M3) have also been described (18).
Transfections.
HeLa and CV-1 cells were transfected by calcium phosphate coprecipitation as described previously (46), typically with 5 μg of reporter. GH4C1 and 235-1 cells were transfected by electroporation with 15 μg of reporter plasmids as previously described (17, 42). After transfection cells were plated in medium containing hormone-stripped serum; after an overnight incubation, cells were shifted to serum-free medium and treatments were started. When appropriate, the reporter plasmid was cotransfected with the amounts of expression vectors for the receptors or coregulators indicated in the figure legends, and in this case equivalent amounts of empty vectors were used. Experiments were performed with triplicate cultures, and each experiment was repeated at least three times. Data are represented as means ± standard deviations.
Gel retardation assays.
Oligonucleotides corresponding to the PRL TRE (5′-TGCTTTGGGGTCAGAAGAGGCAG-3′) and to a consensus DR4 element (5′-AGCTCAGGTCACAGGAGGTCAG-3′) were used in the assays. Wild-type and mutant TR and RXR coding sequences cloned in pSG5 were used for in vitro transcription and translation with TNT Quick (Promega). Assays were performed as previously described (5, 35, 46), with 1 μl of each receptor subunit in the presence and absence of 400 to 600 ng of the GST-fused coactivators, 450 ng of His-tagged TIF-2, or 1.5 μg of GST-SMRT.
RESULTS
T3 and 9-cis-RA stimulate PRL gene transcription.
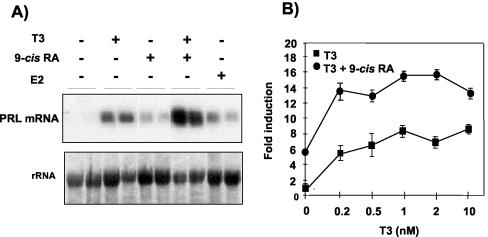
Figure 1A shows that 9-cis-RA increases PRL mRNA levels in pituitary GH4C1 cells and strongly potentiates the stimulatory effect of T3. In addition, 9-cis-RA and estrogen were similarly potent in inducing the levels of PRL transcripts in these cells. The combined effects of T3 and 9-cis-RA in transient-transfection assays with a reporter plasmid containing 5′-flanking sequences of the rat PRL gene are shown in Fig. 1B. Physiological concentrations of T3 caused a dose-dependent stimulation of the activity of this construct, and, in parallel with the cooperation shown in Fig. 1A, this response was further increased in the presence of 9-cis-RA, which by itself also increased reporter activity.
FIG. 1.
T3 and 9-cis-RA stimulate PRL gene expression in rat pituitary GH4C1 cells. (A) Northern blot analyses were carried out with RNA from control cells and cells incubated for 48 h with 5 nM T3, 1 μM 9-cis-RA, or 100 nM estradiol (E2), as indicated. The blot was hybridized with a labeled cDNA probe for rat PRL. (Bottom) 18S rRNA. (B) GH4C1 cells were transfected with a PRL promoter construct containing the distal enhancer fused to the sequence from −422 to +34, and luciferase activity was determined after 48 h in untreated cells and in cells treated with increasing concentrations of T3 in the presence and absence of 1 μM 9-cis-RA. The data are expressed relative to those found in the untreated cells.
PRL sequences involved in regulation by T3 and 9-cis-RA.
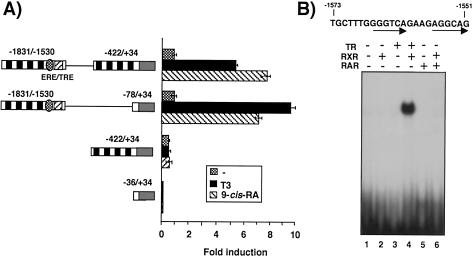
The influence of T3 and 9-cis-RA on PRL reporter plasmids containing only the proximal promoter, only the distal enhancer, or both was also analyzed. As shown in Fig. 2A, T3 caused a strong induction of the activity of a plasmid containing the distal enhancer (−1831 to −1530) fused to sequence −422 to +34. This construct was stimulated by 9-cis-RA to a similar extent. Both ligands also increased the activity of a plasmid in which the region between nucleotides −422 and −78, which contains the proximal GHF-1/Pit-1 binding sites, has been deleted. However, neither T3 nor 9-cis-RA stimulated the activity of plasmids lacking the distal enhancer, showing that this region contains the sequences responsible for induction by both ligands. Inspection of the PRL distal enhancer suggested the existence of a putative TRE next to the ERE, with the configuration of a direct repeat separated by 4 nucleotides (DR4). Figure 3B shows that an oligonucleotide containing sequences between −1573 and −1551 does not bind the α isoform of TR, RAR, or RXR alone but binds strongly RXR/TR heterodimers. In contrast, with RXR/TR heterodimers, neither RXR homodimers nor RXR/RAR heterodimers bind this DNA motif with high affinity.
FIG. 2.
The PRL distal enhancer contains an RXR/TR binding site. (A) PRL luciferase reporter constructs containing the indicated combinations of the proximal promoter (−422/+34), the distal enhancer (−1530/−1831), or basal sequences (−36/+34) were transfected into GH4C1 cells, and reporter activity was determined after 48 h in control cells and in cells treated with 5 nM T3 or 1 μM 9-cis-RA. Results are expressed relative to the values obtained in the untreated cells transfected with the longest construct. The TRE is depicted as a shaded rectangle adjacent to the ERE. Black boxes, binding sites for GHF-1/Pit-1. (B) Mobility shift assays with in vitro-translated TRα, RXR, and RAR (1 μl) and an oligonucleotide encompassing the sequence from −1551 to −1573 of the PRL distal enhancer.
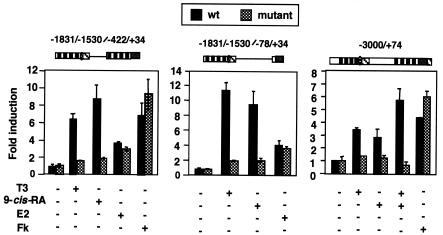
FIG. 3.
The PRL TRE mediates regulation by T3 and 9-cis-RA. The TRE was mutated in the PRL constructs indicated at the top. The wild-type (wt) and mutant plasmids were transfected into GH4C1 cells, and reporter activity was determined after 48 h of incubation with 5 nM T3, 1 μM 9-cis-RA, 10 μM forskolin (Fk), or 100 nM estradiol (E2). Results are expressed as factors by which induction exceeded that obtained in the corresponding control untreated cells.
A common hormone response element mediates regulation of PRL gene transcription by T3 and 9-cis-RA.
To test the functionality of the RXR/TR binding site in the distal enhancer of the PRL gene, this element was mutated in the context of the reporter constructs shown in Fig. 3. The inserted mutation affected both hemisites of the response element and abolished binding of RXR/TR in gel retardation assays (data not shown). In all cases, mutation of this motif abolished the response to T3 and, interestingly, also blocked stimulation by 9-cis-RA. The effect of this mutation was specific for these ligands, since the response to estradiol was not affected. Stimulation by forskolin, which is mediated by proximal promoter sequences, was not affected either.
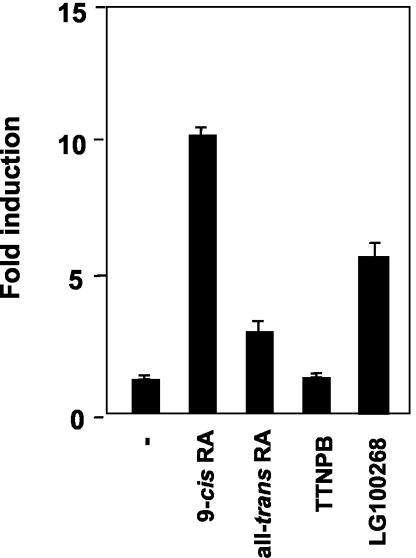
The finding that the same element mediates regulation by T3 and 9-cis-RA could be explained if RXR could act as a nonsilent partner of TR to mediate PRL gene transcription in pituitary cells. This is suggested by the finding that the TRE binds neither RXR homodimers nor RXR/RAR heterodimers. To further dismiss participation of these receptor complexes in PRL stimulation by 9-cis-RA, the influence of different retinoids on transient-transfection studies with the PRL promoter was determined. For this purpose, besides 9-cis-RA, a natural ligand that binds with similarly high affinity to both RXR and RAR, we used all-trans-RA, which shows a higher affinity for RAR; the RAR-selective agonist TTNPB; and the rexinoid LG100268, a specific agonist for RXR homodimers. Figure 4 shows that, compared with 9-cis-RA, all-trans-RA displays a markedly reduced ability to stimulate the PRL construct. In addition, TTNPB was unable to induce a response by itself or to cooperate with T3, demonstrating that RAR is not involved in regulation of the PRL gene by 9-cis-RA. TTNPB is active in pituitary cells, since this RAR-selective retinoid stimulated the activity of the RARβ2 promoter, which is mediated by RXR/RAR heterodimers (25), with the same potency as all-trans-RA and 9-cis-RA (not illustrated). Furthermore, the RXR-selective ligand increased reporter activity, although it was less effective than 9-cis-RA Taken together these results strongly suggest that a permissive RXR/TR heterodimer could mediate stimulation of the PRL gene by T3, 9-cis-RA, or both. However, our results are also compatible with the existence of a different permissive heterodimer of RXR with another still-unidentified receptor.
FIG. 4.
The effect of 9-cis-RA is mediated by RXR. The PRL construct containing the distal enhancer was transfected into GH4C1 cells, and luciferase activity was measured after 48 h of incubation in the presence of 1 μM concentrations of the compounds indicated.
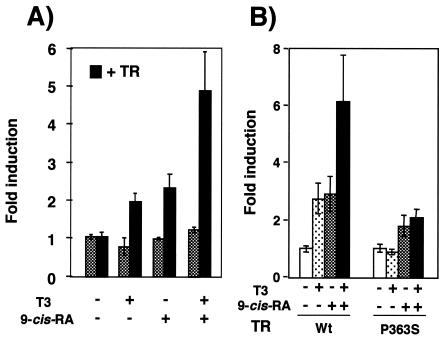
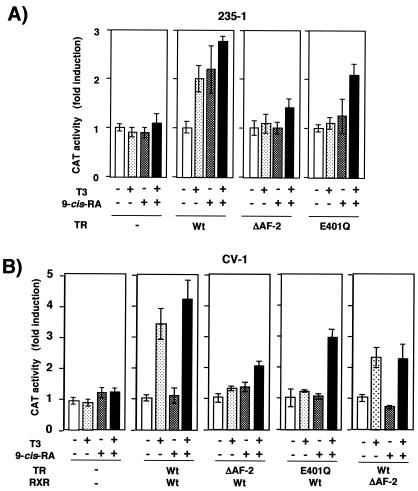
To directly test whether TR is required for the response to 9-cis-RA, we used the lactotroph 235-1 cell line, which expresses very low TR levels but which shows retinoid responses (14, 42). As shown in Fig. 5A, incubation with T3, 9-cis-RA, or the combination of both did not increase PRL transcription in 235-1 cells. In contrast, when the PRL construct was cotransfected with an expression vector for TR, a response to T3, although weaker than that observed in GH4C1 cells, was observed. Strikingly, 9-cis-RA caused a similar stimulation, and the two ligands were able to cooperate to stimulate reporter activity. These data were obtained with TRα, but similar results were found with the TRβ isoform (data not shown).
FIG. 5.
Expression of TR confers responsiveness to both T3 and 9-cis-RA in 235-1 cells. (A) The PRL construct was transfected into pituitary 235-1 cells together with 35 μg of an expression vector for TR or with the same amount of a noncoding vector. (B) The cells were cotransfected with the PRL promoter and either a wild-type receptor or the P363S mutant TR (20 μg). Reporter activity was measured after 48 h in the presence of 50 nM T3 and/or 1 μM 9-cis-RA, as indicated.
To analyze further whether the response to 9-cis-RA is mediated by a permissive RXR/TR heterodimer, a heterodimerization-defective mutant TR with P636S, a mutation present in the dimerization interface of the TR viral counterpart v-erbA (3), was also used. This mutant TR has been reported to interact with RXR in mammalian two-hybrid experiments (54), but we have observed that in gel retardation assays this mutant TR did not bind the TRE with high affinity in the presence of RXR, although some binding was observed when 9-cis-RA was present in the assay (not illustrated). As shown in Fig. 5B, this mutation reduced the response to the retinoid and totally abolished the response to T3 and the synergistic effect of both ligands.
The PRL TRE confers responsiveness to T3 and 9-cis-RA to a heterologous promoter.
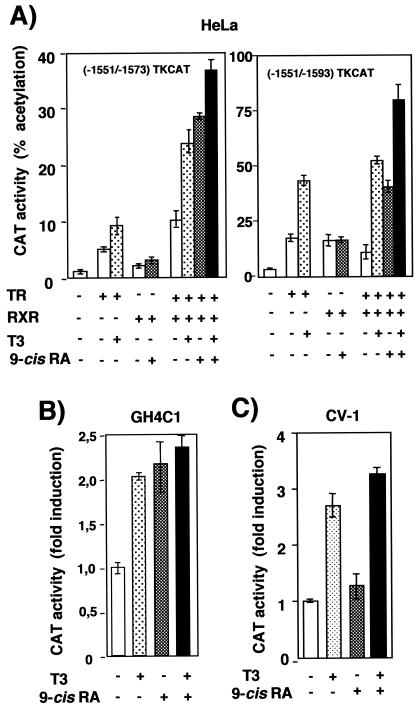
To further prove that the RXR/TR binding site can mediate regulation by ligands of both receptors, fragments of the PRL enhancer containing the TRE alone or the TRE plus the adjacent ERE were cloned in front of the thymidine kinase promoter and transfected into HeLa cells, which express low receptor levels, together with expression vectors for TRα, RXR, or both. Figure 6A shows that, in these cells, expression of TR and/or RXR did not repress transcription but rather caused a ligand-independent stimulation of both reporter plasmids. In addition, incubation with T3 caused a weak increase of promoter activity in TR-expressing cells, whereas 9-cis-RA was unable to stimulate this promoter in cells transfected with RXR. However, in cells expressing TR and RXR, 9-cis-RA increased reporter activity and was able to cooperate with T3, a finding similar to that observed with the natural promoter in pituitary cells. However, none of the constructs containing fragments of the PRL promoter were stimulated by T3 or the retinoid in nonpituitary HeLa cells (data not shown). As shown in Fig. 6B, the heterologous promoter containing the response element was stimulated by T3 and 9-cis-RA in GH4C1 cells, although stimulation was much weaker than that observed with the constructs containing the element in the context of its natural environment in the PRL distal enhancer. In contrast with the results obtained with GH4C1 or HeLa cells, the TRE-containing reporter plasmid was stimulated by T3 but not by 9-cis-RA in CV-1 cells, which also express very low receptor levels, after expression of TR and RXR (Fig. 6C). Therefore, in some cell types but not in others, RXR/TR appears to function as a permissive heterodimer, allowing stimulation by 9-cis-RA of constructs containing the TRE present in the PRL distal enhancer.
FIG. 6.
RXR acts as a silent partner for TR in CV-1 cells but not in GH4C1 or HeLa cells. (A) HeLa cells were transfected with plasmids in which PRL sequences from −1551 to −1573 (which contain the TRE) or −1551 to −1593 (which contain both the TRE and the ERE) were fused to a thymidine kinase (TK)-chloramphenicol acetyltransferase (CAT) reporter gene. The reporter gene was cotransfected with the expression vector for TR (2.5 μg) and/or that for RXR (0.5 μg) as indicated, and luciferase activity was determined after 48 h of incubation with T3 (200 nM) and 9-cis-RA (1 μM). (B) Stimulation of the −1551 to −1593 TK-CAT plasmid by the endogenous receptors in GH4C1 cells treated for 48 h with 5 nM T3 and 9-cis-RA (1 μM). (C) Reporter activity was determined in CV-1 cells transfected with the −1551 to −1593 TK-CAT plasmid and vectors for TR (200 ng) and RXR (50 ng). Cells were treated as for panel A.
Both RXR and TR can bind ligand and recruit coactivators when bound to the PRL TRE.
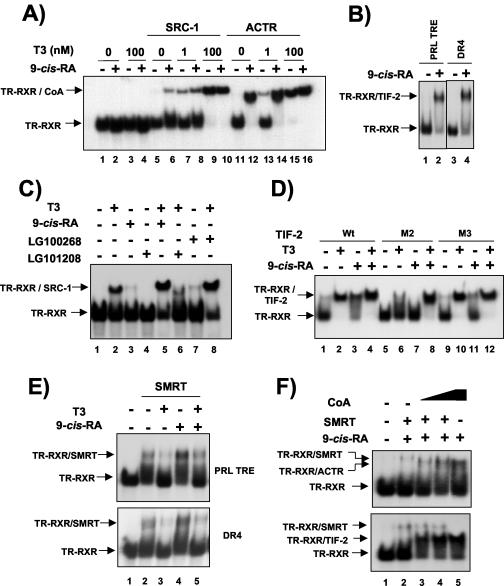
As stated above, the RXR/TR heterodimer has been considered to be nonpermissive. It is believed that in nonpermissive heterodimers RXR is incapable of ligand binding and therefore acts as a silent partner (15). Although this hypothesis has been recently challenged in a study using a derepression assay system (28), the possibility that 9-cis-RA could stimulate PRL gene transcription through a permissive RXR/TR heterodimer requires demonstration that the binding of RXR ligand can cause coactivator recruitment by the DNA-bound receptors. Gel retardation assays illustrated in Fig. 7A show association of the receptor-interacting domains of the coactivators SRC-1 and ACTR fused to GST upon the binding of T3 and 9-cis-RA to the RXR/TR heterodimer bound to the PRL response element. It can be seen that both heterodimeric partners form complexes with the coactivators in the presence of either T3 or 9-cis-RA, implying that RXR can bind its ligand even in the context of the DNA-bound heterodimer. Interestingly, there is some coactivator specificity for this response, since 9-cis-RA was more efficient in recruiting ACTR than SRC-1 (compare lanes 6 and 12). Furthermore, depending on the ligand used, a slight but consistent change in the mobility of the retarded band containing the ternary complex of the coactivators with RXR/TR was detected. This change was better observed when a longer probe was used (data not shown). Figure 7B shows that recruitment of the coactivators by 9-cis-RA is not a particular characteristic of the PRL TRE, since this ligand also caused binding of other p160 coactivator, TIF-2, when the heterodimer was bound to a consensus DR4 element. As illustrated in Fig. 7C, the RXR agonist LG100268 was also able to induce binding of a p160 coactivator to the heterodimer (lane 7), whereas LG101208, a RXR-selective antagonist, did not cause coactivator interaction (lane 4). These results again demonstrate that both TR and RXR autonomously bind their ligands and that upon agonist binding each of them can recruit coactivators. Cooperation of T3 and 9-cis-RA in activation of PRL gene expression could result from cooperative coactivator binding. Under conditions in which the retarded heterodimer is not totally supershifted in the assay (Fig. 7C), it could be indeed observed that the combination of 9-cis-RA and T3 was more efficient in causing heterodimer-coactivator association than either ligand alone. Furthermore, cooperation of T3 and the RXR agonist was found, whereas the RXR antagonist did not increase, but rather decreased, coactivator recruitment in the presence of T3. Again, slight changes in complex mobility with the different ligands were observed.
FIG. 7.
RXR agonists cause coactivator recruitment by the RXR/TR heterodimer. (A) Gel retardation assays with the PRL TRE oligonucleotide and in vitro-translated TR and RXR. Assays were performed in the presence of the receptor-interacting domains of the p160 coactivators SRC-1 and ACTR fused to GST. In lanes without coactivators (lanes 1 to 4), the same amount of GST alone was added. As indicated, 9-cis-RA (1 μM) and T3 (1 and 100 nM) were present in the binding assays. Arrows, mobilities of the heterodimer and the complexes containing the heterodimer and the coactivator (CoA). (B) Gel retardation assays were performed with oligonucleotides conforming to either the PRL TRE or a consensus DR4. The binding of the p160 coactivator TIF-2 to RXR/TR was analyzed in the presence and absence of 1 μM 9-cis-RA. (C) Similar assays were performed with SRC-1 in the presence of 100 nM T3 or 1 μM 9-cis-RA, the rexinoid LG100268, or the RXR-selective antagonist LG101208. (D) Association of the RXR/TR heterodimer with wild-type His-tagged TIF-2 or with TIF-2 with mutations in the second (M2) or third (M3) LXXLL motif (450 ng). Assays were performed with the PRL TRE and in the presence of 20 nM T3 and 1 μM 9-cis-RA as indicated. (E) Gel retardation assays with the receptor-interacting domain of the corepressor SMRT fused to GST, the receptor heterodimer, and either the PRL TRE (top) or DR4 (bottom). Assays were performed with 9-cis-RA (1 μM) and/or T3 (20 nM). (F) PRL TRE was incubated with the corepressor in the presence of increasing amounts of GST-ACTR (200 to 600 ng) (top) or His-tagged TIF-2 (300 to 900 ng) (bottom). When indicated, 1 μM 9-cis-RA was present in the assays.
The receptor-interacting domain of p160 coactivators is composed of three boxes, each containing the LXXLL motif (19). As shown in Fig. 7D, mutation of box III (M3) had little effect on recruitment of the His-tagged TIF-2 receptor-interacting domain by T3 or 9-cis-RA, whereas mutation of box II (M2) strongly decreased the response to T3 and inhibited the response to 9-cis-RA. However, the M2 mutant interacted strongly with the heterodimer in the presence of both ligands. Therefore, boxes I and III, which are inefficient, can act synergistically and promote interaction with the receptors when both ligands of the heterodimer are present.
To test the possibility that 9-cis-RA could alter corepressor binding to the RXR/TR heterodimer, gel retardation assays were performed with the C-terminal fragment of the corepressor SMRT fused to GST. The amount of corepressor used in the assays to detect binding was high, since heterodimers do not recruit corepressors with the same potency as receptor homodimers (8, 51). As shown in Fig. 7E, a retarded complex was detected in the absence of T3 and, as expected, the hormone caused the disappearance of the retarded band. In contrast, 9-cis-RA produced a detectable increase in the intensity of the retarded complex and was able to partially antagonize the effect of T3 on corepressor release. An increase of SMRT binding to the heterodimer in the presence of 9-cis-RA was also found with a consensus DR4 (Fig. 7D). To analyze whether under these conditions the RXR/TR heterodimer can still recruit coactivators, assays were performed in the presence of 9-cis-RA and SMRT. Figure 7F shows that 9-cis-RA caused recruitment of ACTR in the presence of SMRT and that the coactivator inhibited the complex of the heterodimer with the corepressor, which has a slightly lower mobility (top). To better resolve these complexes, additional assays were carried out with SMRT fused to GST and the His-tagged TIF-2 protein. As shown in Fig. 7F (bottom), strong biding of the coactivator to the heterodimer in response to 9-cis-RA was found, even in the presence of SMRT.
Role of helix 12 of TR and RXR on coactivator recruitment by T3 and 9-cis-RA.
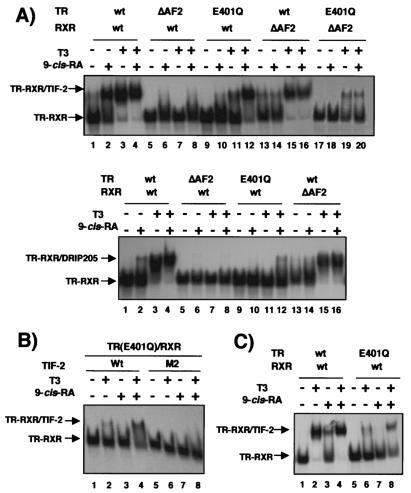
To analyze the contribution of the AF-2 domains of each heterodimeric partner to coactivator recruitment by the agonists, gel retardation assays with mutant receptors in which the core AF-2 domain contained in helix 12 was either mutated or deleted were performed. Figure 8A shows the results obtained with the p160 coactivator TIF-2 (top) and with the DRIP205 subunit of the DRIP/TRAP complex (bottom). It was observed that 9-cis-RA was also able to induce association of these coactivators with the receptors, more efficiently in the case of TIF-2 (lane 2). Deletion of helix 12 in TR not only abolished the expected recruitment by T3 but also blocked the association of TIF-2 with the heterodimer in response to 9-cis-RA (lanes 5 to 8). Therefore, the TR AF-2 plays a key role in coactivator recruitment by both ligands. The influence of mutation of a conserved glutamic acid residue (E401Q) in helix 12 of TR on the effect of both ligands was also explored. This mutation reduced very significantly association of TIF-2 with the receptors upon incubation with T3 or with 9-cis-RA. However, a synergistic effect of both agonists could be observed, and with the combination of 9-cis-RA and T3 a strong recruitment of the coactivator to the AF-2-defective heterodimer was found (lanes 9 to 12). This synergistic effect also required the RXR AF-2 domain, since 9-cis-RA did not cause coactivator recruitment and was unable to cooperate with T3 in the heterodimer composed of TR with the E401Q mutation [TR(E401Q)] and RXR with AF-2-deleted (lanes 17 to 20). On the other hand, deletion of the RXR AF-2 domain abolished the response to 9-cis-RA but allowed TIF-2 recruitment in response to T3 (lanes 13 to 16). Similar qualitative results were obtained with DRIP205, although the effect of 9-cis-RA was less marked and TR(E401Q) only weakly recruited the coactivator in the presence of the combination of both agonist ligands. As shown in Fig. 8B, the synergistic effect of T3 and 9-cis-RA on TIF-2 recruitment by the AF-2 mutant TR required the integrity of the receptor-interacting domain of the coactivator, as mutation of the second LXXLL motif abolished ligand-dependent association of TIF-2 with the RXR/TR(E401Q) heterodimer. The influence of the helix 12 of TR(E401Q) on coactivator recruitment by both agonists on the consensus DR4 was also tested As shown in Fig. 8C, mutation of the E401 residue also abolished interaction with the coactivator in response to 9-cis-RA, and a synergistic effect of both ligands was observed again (lane 8).
FIG. 8.
The TR AF-2 domain is required for coactivator recruitment by the RXR ligand. (A) Gel retardation assays with the receptor-interacting domains of TIF-2 (top) and DRIP205 (bottom) fused to GST and the PRL TRE oligonucleotide. As indicated, wild-type (wt) TR and RXR, receptors lacking helix 12 (ΔAF2), and the point mutant TR TR(E401Q) were used. (B) Assays were performed with TR(E401Q), native RXR, and wild-type (Wt) His-tagged TIF-2 or TIF-2 with a mutation in the second LXXLL box (M2). (C) The consensus DR4 oligonucleotide was incubated with GST-TIF-2 and either wild-type receptors or TR(E401Q). In all panels experiments were performed in the presence and absence of T3 (20 nM) and 9-cis-RA (1 μM) as indicated.
Transcriptional regulation by AF-2 mutant receptors.
To analyze the effect of the AF-2 mutant receptors on transcriptional regulation by T3 and 9-cis-RA, the PRL construct was transfected into 235-1 cells together with expression vectors for wild-type and AF-2 mutant receptors (Fig. 9A). Whereas, as already observed in Fig. 5, both T3 and 9-cis-RA increased reporter activity upon expression of the native receptor, deletion of helix 12 of TR abolished the response to both ligands. This is in agreement with the lack of coactivator recruitment by the RXR/TR(ΔAF-2) heterodimer shown in Fig. 8. In addition, mutation of the E401 residue inhibited, as expected, the response to T3, but a significant activation was observed when the hormone was combined with 9-cis-RA. This result is also compatible with the in vitro coactivator association with this AF-2-defective heterodimer observed in the presence of both agonists.
FIG. 9.
Transactivation by AF-2 mutant receptors. (A) Pituitary 235-1 cells were transfected with the PRL reporter, which contains the distal enhancer fused to proximal promoter sequences, and expression vectors (15 μg) for wild-type TR, TR(ΔAF-2), TR(E401Q). Luciferase activity was measured after treatment with 50 nM T3 and/or 1 μM 9-cis-RA for 48 h and is expressed as the factor by which induction exceeded the value for the untreated control within each group. (B) CV-1 cells were transfected with the PRL TRE fused to the heterologous thymidine kinase (TK) promoter and vectors for native TR (200 ng) and RXR (50 ng) or with the same amount of the specified AF-2 mutant receptors. Chloramphenicol acetyltransferase (CAT) activity was determined after treatment with T3 (5 nM) and/or 9-cis-RA (1 μM).
The effect of the mutant receptors was also examined in nonpituitary CV-1 cells transfected with the construct containing the PRL TRE fused to the heterologous thymidine kinase promoter. Figure 9B shows again that in this cell type expression of native TR conferred regulation by T3, but not by 9-cis-RA. Additionally, no response to T3 was observed upon expression of the AF-2-defective mutant TRs. However, confirming that the lack of transcriptional activity of the E401 mutant TR can be reversed when both heterodimeric partners are occupied, a significant activation in the presence of T3 plus 9-cis-RA was observed in cells transfected with this mutant TR. In agreement with the finding that the RXR AF-2 domain does not play a major role in in vitro recruitment with coactivators in response to T3, expression of RXR lacking helix 12 allowed stimulation by T3, although stimulation was somewhat weaker than that found upon expression of native RXR.
Coactivator and corepressor availability modulates the response to 9-cis-RA.
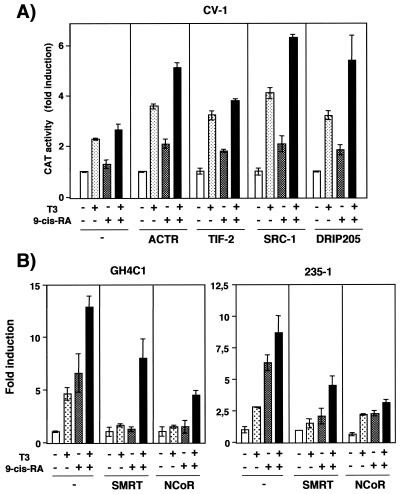
The different transcriptional responses of pituitary and CV-1 cells to 9-cis-RA could be due to different availabilities of coregulators. To test this possibility, the construct containing the PRL TRE was cotransfected into CV-1 cells with expression vectors for the p160 coactivators ACTR, TIF-2, and SRC-1, as well as for DRIP205. As shown in Fig. 10A, expression of the coactivators increased the response to T3 and, more importantly, allowed stimulation by 9-cis-RA. In contrast, expression of coactivators was unable to increase the response of the PRL promoter to either T3 or 9-cis-RA in pituitary GH4C1 or 235-1 cells (not illustrated), showing that in these cells the amount of endogenous coactivators is sufficient to elicit a maximal transcriptional response.
FIG. 10.
Expression of coregulators modulates responsiveness to 9-cis-RA. (A) CV-1 cell were transfected with the thymidine kinase (TK)-chloramphenicol acetyltransferase (CAT) construct containing the PRL TRE and expression vectors for TR (200 ng) and RXR (50 ng). Vectors (100 ng) for the coactivators ACTR, TIF-2, SRC-1, and DRIP205 or a noncoding vector (100 ng) was cotransfected with the reporter and the receptors, and CAT activity was determined after treatment with T3 (5 nM) and/or 9-cis-RA (1 μM) for 48 h. Data are expressed as the factors by which induction exceeded the value obtained for the corresponding control untreated cells. (B) Pituitary GH4C1 cells were cotransfected with the PRL promoter construct and 200 ng of expression vectors for SMRT or NcoR. Luciferase activity was determined after incubation with 5 nM T3 and/or 1 μM 9-cis-RA. In 235-1 cells the PRL reporter was cotransfected with 15 μg of TR and 500 ng of SMRT or NcoR.
To analyze whether the availability of corepressors could also modulate the response to 9-cis-RA in cells in which this ligand promotes transactivation, the PRL construct was cotransfected into GH4C1 and 235-1 cells together with vectors for the corepressors SMRT and NcoR. Figure 10B shows that expression of both corepressors reduced the response to T3 and 9-cis-RA in the pituitary cells. However, when both ligands were combined, a synergistic effect was still observed and significant reporter stimulation was found. Interestingly, transfection of amounts of corepressors higher than those used in Fig. 9B did not further reduce these responses but rather allowed a stronger ligand-dependent stimulation (not illustrated).
DISCUSSION
It has been previously found that lactotroph tumor cells respond to T3 with either an increase or a decrease in PRL gene expression (11, 44). In this work we have shown that in the rat GH4C1 somatolactotroph cell line T3 increases PRL transcripts as well as PRL promoter activity in transient-transfection studies. Most likely this represents a physiological action of the hormone, since we have also observed a profound decrease of PRL mRNA levels in pituitaries of thyroidectomized rats that is reversed upon thyroid hormone treatment (G. Bedó and A. Aranda, unpublished observations). This regulation contrasts with the observation that hypothyroidism in human patients is often associated with an increase in serum PRL levels (48). Although this is possibly due to central effects, it has been shown that T3 represses the activity of the human PRL promoter. The human promoter appears to contain both a positive and a negative TRE, and the negative effect is stronger and could involve cross talk between the thyroid hormone receptor and AP-1 (38). This demonstrates the existence of species specificity in regulation of PRL gene expression.
We also show in this work that 9-cis-RA increases the levels of PRL transcripts and stimulates the activity of reporter plasmids containing the PRL enhancer in GH4C1 cells. A common element in the enhancer region, the rat PRL TRE, mediates regulation by both T3 and the RXR agonist. As in many TREs, the hemisites of the PRL TRE are arranged as DRs separated by 4 nucleotides (1, 53). As expected from this configuration, the PRL TRE binds RXR/TR heterodimers with high affinity but shows little affinity for TR or RXR homodimers or monomers. The PRL TRE is adjacent to the ERE, but it functions as a separate element, as mutation of the TRE abolishes stimulation by both T3 and the 9-cis-RA but does not affect activation by estradiol.
The TRE functions as a bona fide response element, since it can confer ligand responsiveness to a heterologous promoter both in pituitary and nonpituitary cells. The finding that the element fused to the heterologous promoter is weakly stimulated by T3 and 9-cis-RA in pituitary cells, in comparison with the strong stimulation of the natural PRL sequences, suggests that the promoter context is important in determining the functional strength of the response element. On the other hand, the PRL constructs which contain the TRE were not activated by these ligands in nonpituitary cells. The lack of functionality of the PRL TRE in these cells could be related to the absence of the pituitary-specific factor GHF-1/Pit-1, which binds to the PRL gene and which is needed for ligand-dependent transcription of the PRL and growth hormone (GH) genes by other nuclear receptors (5, 12, 34, 36, 45, 46). The requirement of GHF-1/Pit-1 for ligand-dependent stimulation of PRL gene transcription is also deduced from the finding that a construct extending to nucleotide −1597 that contains the PRL TRE but that lacks the distal enhancer binding sites for the pituitary factor is not activated by T3 or 9-cis-RA in GH4C1 cells (A. I. Castillo and A. Aranda, unpublished observations).
It was normally assumed that in TR signaling the only function of RXR was to facilitate the binding of TR to the TRE. This was based on evidence obtained by Forman et al. (15) showing that ligand binding by RXR was abolished when this receptor heterodimerizes with TR. However, our results do not support this model, in which RXR ligand binding is possible only when TR is occupied by the hormone, and rather show that RXR does not act as a silent partner for TR in stimulation of PRL gene expression. In fact, a main finding of this work is the demonstration that RXR/TR can act as a permissive heterodimer, allowing stimulation of transcription by the ligands of both subunits of the heterodimer. The use of a RAR-selective ligand, together with the finding that RXR/RAR heterodimers do not associate with this element, dismisses the possibility that 9-cis-RA could stimulate PRL transcription through binding to RAR. Furthermore, the RXR-selective ligand LG100268 was able to stimulate PRL transactivation, although with less potency than the natural RXR agonist. The rexinoid, which acts as an agonist for RXR homodimers (26), presents some dissimilarities from the natural agonist 9-cis-RA which could explain this difference. The crystal structure of RXR bound to this retinoid has shown that the C-terminal helix 12, which contains the receptor AF-2 domain, is trapped in a novel position not seen in other liganded nuclear receptors (30). Furthermore, LG100268 is unable to release corepressors from RXR unless coactivators are present (30).
Stimulation by 9-cis-RA was found in pituitary 235-1 cells transfected with the PRL distal enhancer upon expression of TR. Furthermore, the effect of the retinoid was decreased in cells expressing a heterodimerization-defective mutant TR. Also, in HeLa cells transfected with the PRL TRE fused to a heterologous promoter, expression of the RXR/TR heterodimer conferred responsiveness to both T3 and 9-cis-RA. These results clearly prove that a permissive RXR/TR heterodimer can support transcription by agonists of both receptors under appropriate circumstances. However, whereas a response to T3 was found in pituitary and HeLa cells, stimulation of the TRE-containing promoter by 9-cis-RA was not observed in CV-1 cells. This shows that the cellular context can be crucial in determining whether a given element can confer regulation by one or both ligands of the receptor heterodimer.
A common element in the 5′-flanking region of the rat GH gene mediates regulation by both T3 and retinoids (4, 17), and an RXR-selective ligand has been used to demonstrate that RXR activates the rat GH promoter in pituitary cells through the TRE (10). In the light of our present results, and since RXR does not bind as a heterodimer to this element of the GH promoter (35), it is likely that the liganded RXR could also function as a partner for TR and that a permissive heterodimer could be responsible for stimulation of the pituitary GH gene by the rexinoid. Permissiveness of RXR/TR heterodimers for PRL and GH gene expression could be due to the promoter context or to selective interaction with other factors that bind these promoters. However, this does not appear to be the case, because the PRL TRE confers T3 and 9-cis-RA responsiveness to a heterologous promoter. Moreover, we have found cooperation of T3 and all-trans-RA in stimulation of reporter plasmids containing consensus TREs in pituitary cells (17). Therefore, somatolactotrophs could present a cellular environment particularly advantageous to overcome RXR subordination within heterodimers with TR.
We have been able to demonstrate that, in the RXR/TR heterodimer bound to the PRL TRE, each partner can independently bind ligand and recruit coactivators. Similar results have been recently shown for the RXR/RAR heterodimer (18), previously considered to be unable to bind RXR ligand when RAR was unoccupied. The RXR ligand could elicit interaction of coactivators with this receptor or could induce a conformational change in TR, which then would recruit the coactivator. Our observation that deletion of the RXR AF-2 domain abolishes association of coactivators in response to 9-cis-RA suggests that a coactivator molecule binds directly to the RXR moiety. Furthermore, we have observed that the conformation of the coactivator-heterodimer complexes formed is different depending of the bound ligand, as their mobilities in gel retardation assays differ. Therefore, our data are compatible with a model in which each subunit of the heterodimer creates a surface for coactivator interaction. This is in agreement with recent data indicating that a single coactivator molecule binds to receptor heterodimers. The receptor-interacting domains of the coactivators contain three copies of the signature motif LXXLL (19), and each partner of the heterodimer appears to recognize a different LXXLL box of the coactivator (13, 49). In the presence of both ligands synergy would originate from the cooperative binding of the two receptor-interacting motifs (18). Our results have shown that the second LXXLL box is required for the binding of the coactivator TIF-2 to the heterodimer in response to T3 and 9-cis-RA. However, in the absence of box II, both ligands act synergistically and the weak I and III motifs are sufficient for efficient binding to the receptors.
Although each receptor in the heterodimer can autonomously bind coactivators, our results also demonstrate that there is an important cross talk between the receptor partners. For instance, deletion of the TR AF-2 core domain abolishes coactivator recruitment not only in response to T3 but also in response to 9-cis-RA, and this is translated into loss of the transcriptional response to both compounds in cells expressing the truncated receptor. This is reminiscent of the ligand phantom effect observed with other heterodimers such as LXR/RXR, in which the activation potential of LXR is enabled by ligand binding to its partner (50) or by the binding of a synthetic ligand to RXR that mimics the effects observed when the hormone is bound to RAR (43).
An interesting novel observation was obtained with a TR with a mutated conserved residue that is involved in association with the LXXLL box (13) and that is required for ligand-dependent transcriptional activation (2). We have shown that the heterodimer of RXR with TR(E401Q) displays a strongly reduced ability to bind coactivators in response to T3, but binding is significantly restored when RXR is occupied. Remarkably, the mutated receptor is transcriptionally inactive in cells incubated with T3 alone but can stimulate transcription when the hormone is combined with 9-cis-RA. Since point mutations of the TRβ isoform in helix 12 are present in some patients with thyroid hormone resistance syndrome (9), our results open the interesting possibility that some transcriptional activity of AF-2-deficient receptors could be found under conditions in which the RXR ligand is present. From our data it can be also inferred that the effects of 9-cis-RA on TRE-dependent transactivation should depend on the cellular pattern of coactivator expression. We have shown that there is some specificity in coactivator binding and that some coactivators are more efficiently recruited than others in response to 9-cis-RA. Therefore, it can be expected that rexinoid responses through an RXR/TR heterodimer should be selectively found in those cell types expressing a favorable combination of coactivators and that RXR could be silent in other cellular contexts.
The cellular levels of corepressors could also play an important role in the transcriptional response mediated by the agonists of the RXR/TR heterodimer. For RXR/RAR, rexinoids induce coactivator recruitment to RXR but cannot dissociate corepressors (18). Since coactivators and corepressors have similar interaction surfaces in the receptor (23, 33, 37), binding of both is mutually exclusive. It has been proposed that RXR ligands can transactivate only when the heterodimeric partner interacts weakly with corepressors or when coactivator expression in a particular cell dominates corepressor content (18). In agreement with this hypothesis we have observed that overexpression of corepressors in pituitary cells inhibits PRL promoter transactivation not only by 9-cis-RA but also by T3. Interestingly, a synergistic effect of both ligands is still observed even in the presence of high corepressor levels. These data, as well as our results showing that expression of coactivators allows the response to 9-cis-RA in CV-1 cells, underline the importance of the levels of these coregulators for RXR to act as a nonsilent partner of TR.
For RXR/TR it has been recently proposed that the binding of the RXR ligand could induce dissociation of corepressors from TR and thus may serve to modulate TR activity (28). However, our data do not support this model, since incubation with 9-cis-RA increases the binding of the corepressor SMRT to the heterodimer in gel retardation assays using both the PRL TRE and a consensus DR4. Since unliganded RXR does not bind corepressors because its helix 12 masks the corepressor binding surface (52), these results again suggest that the binding of ligand to RXR results in a linked conformational change in TR. However, it is also possible that RXR could bind corepressors upon ligand binding. The increase in corepressor recruitment by 9-cis-RA is in apparent contradiction with the stimulation of gene expression seen in the functional assays. However, we have observed that the retinoid causes strong recruitment of coactivators even in the presence of corepressors, and under these conditions stimulation would be favored. In any case, the enhancement in corepressor recruitment by 9-cis-RA could also contribute to the silencing of the transcriptional response mediated by this ligand, particularly in cells with high corepressor content or with a high corepressor-to-coactivator ratio.
In summary, our findings indicate that RXR ligands can elicit PRL gene transcription through a permissive heterodimer with TR. This demonstrates an unexpected role for RXR in TR signaling and argues against a silent-partner model for RXR. Therefore, future studies are needed to analyze the function of RXR/TR heterodimers and to reevaluate the actions of both receptors and agonist and antagonist ligands in different genes and cell types.
Acknowledgments
We thank D. Barettino, R. Evans, L. Freedman, M. Parker, and H. Gronemeyer for plasmids used in this study, and M. D. Leibowitz from Ligand Pharmaceuticals for LG100268 and LG101208.
This work was supported by grant BMC2001-2275 from the Ministerio de Ciencia and Tecnología (Spain).
REFERENCES
- 1.Aranda, A., and A. Pascual. 2001. Nuclear hormone receptors and gene expression. Physiol. Rev. 81:1269-1304. [DOI] [PubMed] [Google Scholar]
- 2.Barettino, D., M. M. Vivanco Ruiz, and H. G. Stunnenberg. 1994. Characterization of the ligand-dependent transactivation domain of thyroid hormone receptor. EMBO J. 13:3039-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barettino, D., T. H. Bugge, P. Bartunek, M. M. Vivanco Ruiz, V. Sontag-Buck, H. Beug, M. Zenke, and H. G. Stunnenberg. 1993. Unliganded T3R, but not its oncogenic variant, v-erbA, suppresses RAR-dependent transactivation by titrating out RXR. EMBO J. 12:1343-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedó, G., P. Santisteban, and A. Aranda. 1989. Retinoic acid regulates growth hormone gene expression. Nature 339:231-234. [DOI] [PubMed] [Google Scholar]
- 5.Castillo, A. I., A. M. Jimenez-Lara, R. M. Tolon, and A. Aranda. 1999. Synergistic activation of the prolactin promoter by vitamin D receptor and GHF-1: role of the coactivators CREB-binding protein and steroid hormone receptor coactivator 1. Mol. Endocrinol. 13:1141-1154. [DOI] [PubMed] [Google Scholar]
- 6.Chen, H., R. J. Lin, R. L. Schiltz, D. Chakravarti, A. Nash, L. Nagy, M. L. Privalsky, Y. Nakatani, and R. M. Evans. 1997. Nuclear receptor coactivator ACTR is a histone acetyltransferase and forms a multimeric activation complex with p/CAF and CBP/p300. Cell 90:569-580. [DOI] [PubMed] [Google Scholar]
- 7.Chen, J. D., and R. M. Evans. 1995. A transcriptional corepressor that interacts with nuclear hormone receptors. Nature 377:455-457. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, R. N., A. Putney, F. E. Wondisford., and A. N. Hollenberg. 2000. The nuclear corepressors recognize distinct nuclear receptor complexes. Mol. Endocrinol. 14:900-914. [DOI] [PubMed] [Google Scholar]
- 9.Collingwood, T. N., O. Rajanayagam, M. Adams, R. Wagner, V. Cavaillès, E. Kalkhoven, C. Matthews, E. Nystrom, K. Stenlof, G. Lindstedt, L. Tisell, R. J. Fletterick, M. G. Parker, and V. K. K. Chatterjee. 1997. A natural transactivation mutation in the thyroid hormone β receptor: impaired interaction with putative transcriptional mediators. Proc. Natl. Acad. Sci. USA 94:248-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, K. D., T. J. Berrodin, J. E. Stelmach, J. D. Winkler, and M. A. Lazar. 1994. Endogenous retinoid X receptors can function as hormone receptors in pituitary cells. Mol. Cell. Biol. 14:7105-7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day, R. N., and R. Maurer. 1989. Thyroid hormone-responsive elements of the prolactin gene: evidence for both positive and negative regulation. Mol. Endocrinol. 3:931-938. [DOI] [PubMed] [Google Scholar]
- 12.Day, R. N., S. Koike, M. Sakai, M. Muramatsu, and R. Maurer. 1990. Both Pit-1 and the estrogen receptor are required for estrogen responsiveness of the rat prolactin gene. Mol. Endocrinol. 4:1964-1971. [DOI] [PubMed] [Google Scholar]
- 13.Feng, W., R. C. Ribeiro, R. L. Wagner, H. Nguyen, J. W. Apriletti, R. J. Fletterick, D. J. Baxter, P. J. Kushner, and B. L. West. 1998. Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 280:1747-1749. [DOI] [PubMed] [Google Scholar]
- 14.Forman, B. M., C.-R. Yang, F. Stanley, J. Casanova, and H. H. Samuels. 1988. c-erbA protooncogenes mediate thyroid hormone-dependent and -independent regulation of the rat growth hormone and prolactin genes. Mol. Endocrinol. 2:902-911. [DOI] [PubMed] [Google Scholar]
- 15.Forman, B. M., K. Umesono, J. Chen, and R. M. Evans. 1995. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell 81:541-550. [DOI] [PubMed] [Google Scholar]
- 16.Freedman, L. P. 1999. Increasing the complexity of coactivation in nuclear receptor signaling. Cell 97:5-8. [DOI] [PubMed] [Google Scholar]
- 17.García-Villalba, P., A. M. Jiménez-Lara, and A. Aranda. 1996. Vitamin D interferes the transactivation of the growth hormone gene by thyroid hormone and retinoic acid. Mol. Cell. Biol. 16:318-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germain, P., J. Iyer, C. Zechel, and H. Gronemeyer. 2002. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 415:187-192. [DOI] [PubMed] [Google Scholar]
- 19.Heery, D. M., E. Kalkhoven, S. Hoare, and M. G. Parker. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733-736. [DOI] [PubMed] [Google Scholar]
- 20.Henttu, P. M., E. Kalkhoven, and M. G. Parker. 1997. AF-2 activity and recruitment of steroid receptor coactivator 1 to the estrogen receptor depend on a lysine residue conserved in nuclear receptors. Mol. Cell. Biol. 17:1832-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyman, R. A., D. J. Mangelsdorf, J. A. Dyck, R. B. Stein, R. M. Evans, and C. Thaller. 1992. 9-cis-Retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68:397-406. [DOI] [PubMed] [Google Scholar]
- 22.Hu, X., and M. A. Lazar. 2000. Transcriptional repression by nuclear hormone receptors. Trends Endocrinol. Metab. 11:6-10. [DOI] [PubMed] [Google Scholar]
- 23.Hu, X., Y. Li, and M. A. Lazar. 2001. Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol. Cell. Biol. 21:1747-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito, M., and R. G. Roeder. 2001. The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol. Metab. 12:127-134. [DOI] [PubMed] [Google Scholar]
- 25.Jiménez-Lara, A. M., and A. Aranda. 1999. Vitamin D represses retinoic acid-dependent transactivation of the retinoic acid-b2 promoter: the AF-2 domain of the vitamin D receptor is involved in transrepression. Endocrinology 140:2898-2907. [DOI] [PubMed] [Google Scholar]
- 26.Lala, D. S., R. Mukherjee, I. G. Schulman, S. S. C. Koch, L. J. Dardashti, A. M. Nadzan, G. E. Croston, R. M. Evans, and R. A. Heyman. 1996. Activation of specific RXR heterodimers by an antagonist of RXR homodimers. Nature 383:450-453. [DOI] [PubMed] [Google Scholar]
- 27.Levin, A. A., L. J. Sturzenbecker, S. Kazmer, A. T. Bosakowski, C. Huselton, C. Allenby, J. Speck, C. Kratzeisen, M. Rosenberg, Lovey, and J. F. Grippo. 1992. 9-cis-Retinoic acid steroisomer binds and activates the nuclear RXRα. Nature 355:359-361. [DOI] [PubMed] [Google Scholar]
- 28.Li, D., T. Li, F. Wang, H. Tian, and H. H. Samuels. 2002. Functional evidence for retinoid X receptor (RXR) as a nonsilent partner in the thyroid hormone receptor/RXR heterodimer. Mol. Cell. Biol. 22:5782-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López-Fernández, J., D. Palacios, A. I. Castillo, R. M. Tolón, A. Aranda, and M. Karin. 2000. Differentiation of lactotrope precursor GHFT cells in response to fibroblast growth factor-2. J. Biol. Chem. 275:21653-21660. [DOI] [PubMed] [Google Scholar]
- 30.Love, J. D., J. T. Gooch, S. Benko, C. Li, L. Nagy, K. K. Chatterjee, R. M. Evans, and W. R. Schwabe. 2002. The structural basis for the specificity of retinoid-X receptor-selective agonists: new insights into the role of helix 12. J. Biol. Chem. 277:11385-11391. [DOI] [PubMed] [Google Scholar]
- 31.Mangelsdorf, D. J., and R. M. Evans. 1995. The RXR heterodimers and orphan receptors. Cell 83:841-850. [DOI] [PubMed] [Google Scholar]
- 32.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465-474. [DOI] [PubMed] [Google Scholar]
- 33.Nagy, L., H. Y. Kao, J. D. Love, C. Li, E. Banayo, J. T. Gooch, K. V. Chatterjee, R. M. Evans, and J. W. Schwabe. 1999. Mechanism of corepressor binding and release from nuclear receptors. Genes Dev. 15:3209-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowakowski, B. E., and R. Maurer. 1994. Multiple Pit-1-binding sites facilitate estrogen responsiveness of the prolactin gene. Mol. Endocrinol. 8:1742-1749. [DOI] [PubMed] [Google Scholar]
- 35.Palomino, T., A. Sánchez-Pacheco, P. Peña, and A. Aranda. 1998. A direct protein to protein interaction is involved in the cooperation between thyroid hormone and retinoic acid receptors and the transcription factor GHF-1. FASEB J. 12:1201-1209. [DOI] [PubMed] [Google Scholar]
- 36.Palomino, T., D. Barettino, and A. Aranda. 1998. Role of GHF-1 in the regulation of the rat growth hormone gene promoter by thyroid hormone and retinoic acid receptors. J. Biol. Chem. 273:27541-27547. [DOI] [PubMed] [Google Scholar]
- 37.Perissi, V., L. M. Staszewski, E. M. McInerney, R. Kurokawa, A. Krones, D. W. Rose, M. H. Lambert, M. V. Milburn, C. K. Glass, and M. G. Rosenfeld. 1999. Molecular determinants of nuclear receptor-corepressor interactions. Genes Dev. 13:3198-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pernasseti, F., L. Caccavelli, C. Van de Weerdt, J. A. Martial, and M. Muller. 1997. Thyroid hormone inhibits the human prolactin gene promoter by interfering with activating protein-1 and estrogen stimulations. Mol. Endocrinol. 11:986-996. [DOI] [PubMed] [Google Scholar]
- 39.Rachez, C., B. D. Lemon, Z. Suldan, V. Bromleigh, M. Gamble, A. M. Naar, H. Erdjument-Bromage, P. Tempst, and L. P. Freedman. 1999. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature 398:824-828. [DOI] [PubMed] [Google Scholar]
- 40.Rosenfeld, M. G., and C. K. Glass. 2001. Coregulator codes of transcriptional regulation by nuclear receptors. J. Biol. Chem. 276:36865-36868. [DOI] [PubMed] [Google Scholar]
- 41.Sakai, D. D., S. Helms, J. Carlstedt-Duke, J.-A. Gustafsson, F. M. Rottman, and K. R. Yamamoto. 1988. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 2:1144-1154. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Pacheco, A., T. Palomino, and A. Aranda. 1995. Retinoic acid induces expression of the transcription factor GHF-1/Pit-1 in pituitary growth hormone and prolactin-producing cell lines. Endocrinology 136:5391-5398. [DOI] [PubMed] [Google Scholar]
- 43.Schulman, L. G., C. Li, J. W. Schwabe, and R. M. Evans. 1997. The phantom ligand effect: allosteric control of transcription by the retinoid X receptor. Genes Dev. 11:299-308. [DOI] [PubMed] [Google Scholar]
- 44.Stanley, F. 1989. Transcriptional regulation of prolactin gene expression by thyroid hormone—alternate suppression and stimulation in different GH cell lines. Mol. Endocrinol. 3:1627-1633. [DOI] [PubMed] [Google Scholar]
- 45.Tolon, R. M., A. I. Castillo, and A. Aranda. 1998. Activation of the prolactin gene by peroxisome proliferator activated receptor-α appears to be DNA binding-independent. J. Biol. Chem. 273:26652-26661. [DOI] [PubMed] [Google Scholar]
- 46.Tolón, R. M., A. I. Castillo, A. M. Jimenez-Lara, and A. Aranda. 2000. Association with Ets-1 causes ligand- and AF2-independent activation of nuclear receptors. Mol. Cell. Biol. 20:8793-8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voegel, J. J., M. J. S. Heine, M. Tini, V. Vivat, P. Chambon, and H. Gronemeyer. 1998. The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J. 17:507-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe, H., and S. Sasaki. 1995. Effect of thyroid status on the prolactin-releasing action of vasoactive intestinal peptide in humans: comparison with the action of thyrotropin-releasing hormone. Neuroendocrinology 61:207-212. [DOI] [PubMed] [Google Scholar]
- 49.Westin, S., R. Kurokawa, R. T. Nolte, G. B. Wisely, E. M. McInerney, D. W. Rose, M. V. Milburn, M. G. Rosenfeld, and C. K. Glass. 1998. Interactions controlling the assembly of nuclear-receptor heterodimers and co-activators. Nature 395:199-202. [DOI] [PubMed] [Google Scholar]
- 50.Willy, P. J., and D. J. Mangelsdorf. 1997. Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR. Genes Dev. 11:289-298. [DOI] [PubMed] [Google Scholar]
- 51.Yoh, S. M., and M. L. Privalsky. 2001. Transcriptional repression by thyroid hormone receptors. A role for receptor homodimers in the recruitment of SMRT corepressor. J. Biol. Chem. 276:16847-16867. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, J., X. Hu, and M. A. Lazar. 1999. A novel role for helix 12 of retinoid X receptor in regulating repression. Mol. Cell. Biol. 19:6448-6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, J., and M. A. Lazar. 2000. The mechanism of action of thyroid hormones. Annu. Rev. Physiol. 62:439-466. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, J., I. Zamir, and M. A. Lazar. 1997. Differential recognition of liganded and unliganded thyroid hormone receptor by retinoid X receptor regulates transcriptional repression. Mol. Cell. Biol. 17:6887-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]