Abstract
Several acyclic hydroxy-methylthio-amines with 3 to 5 carbon atoms were prepared and coupled via a methylene link to 9-deazaadenine. The products were tested for inhibition against human MTAP and E. coli and N. meningitidis MTANs and gave Ki values as low as 0.23 nM. These results were compared to those obtained with 1st and 2nd generation inhibitors (1S)-1-(9-deazaadenin-9-yl)-1,4-dideoxy-1,4-imino-5-methylthio-d-ribitol (MT-Immucillin-A, 3) and (3R,4S)-1-[9-deazaadenin-9-yl)methyl]3-hydroxy-4-methylthiomethylpyrrolidine (MT-DADMe-Immucillin-A, 4). The best inhibitors were found to exhibit binding affinities of approximately 2- to 4-fold those of 3 but were significantly weaker than 4. Cleavage of the 2,3 carbon–carbon bond in MT-Immucillin-A (3) gave an acyclic product (79) with a 21,500 fold loss of activity against E. coli MTAN. In another case, N-methylation of a side chain secondary amine resulted in a 250-fold loss of activity against the same enzyme [(±)-65 vs (±)-68]. The inhibition results were also contrasted with those acyclic derivatives previously prepared as inhibitors for a related enzyme, purine nucleoside phosphorylase (PNP), where some inhibitors in the latter case were found to be more potent than their cyclic counterparts.
Keywords: Human MTAP, Bacterial MTANs, Ribooxacarbenium ion mimics, Inhibitors, Acyclic hydroxy-methylthio-amines
1. Introduction
The human methylthioadenosine phosphorylase (MTAP)1,2 and bacterial methylthioadenosine/S-adenosylhomocysteine nucleosidase (MTAN)3,4 enzymes are targets for drug design. MTAP is a target for cancer therapy through its importance in polyamine biosynthesis and S-adenosylmethionine (SAM) metabolism.5–10 Efficient inhibitors of MTAP are expected to lead to accumulation of methylthioadenosine (MTA) and consequent inhibition of spermidine and spermine synthases while MTA itself has antiproliferative properties.11–17 In addition, the metabolism of SAM is likely to be prevented along with the disruption of SAM-dependent methylation. We have developed potent inhibitors of MTAP that show strong anti-cancer effects18,19 thereby providing validation for MTAP as a cancer target.
MTAN is involved in bacterial polyamine biosynthesis and quorum sensing.20–22 Certain gram-negative organisms utilize species-specific acyl-homoserine lactones as quorum sensing molecules. These are synthesized from N-acylated S-adenosylmethionine generating MTA as a by-product of the reaction.23 Efficient inhibitors of MTAN will lead to elevated concentrations of MTA which may induce feedback inhibition of acyl-homoserine lactone synthesis. S-Ribosylhomocysteine, the product of MTAN-catalysed hydrolysis of S-adenosylhomocysteine, is catabolized by S-ribosylhomocysteinase (LuxS) into l-homocysteine and (4S)-4,5-dihydroxy-2,3-pentanedione.24 The latter forms the basis of the bacterial quorum sensing signal AI-2.21,25,26 The LuxS enzyme has been identified in more than 55 gram-negative and gram-positive bacteria and the evidence suggests that the autoinducing signal AI-2 is common to many bacterial species. Thus, without MTAN activity there can be no synthesis of AI-2. In consequence, inhibition of MTAN would be expected to inhibit polyamine biosynthesis, block salvage pathways for methionine and adenine as well as blocking the synthesis of two types of quorum sensing autoinducer molecules.
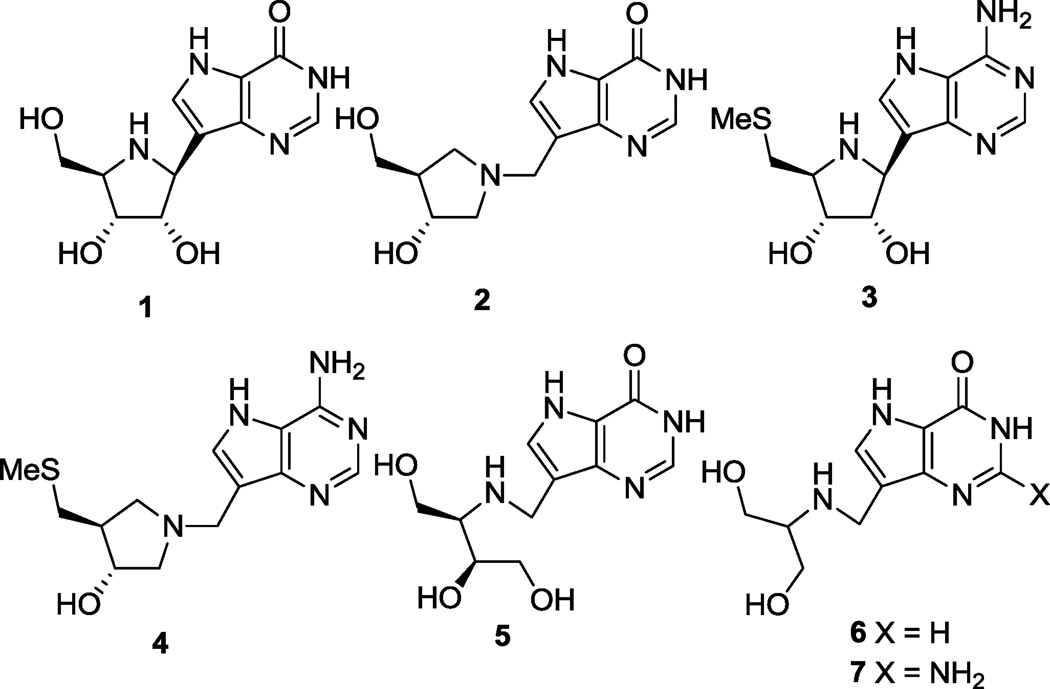
We have been engaged for a number of years in the design and synthesis of transition state analogue inhibitors of a number of N-ribosyltransferase enzymes, including those mentioned above. The use of measured kinetic isotope effects has enabled the identification of the nature of the transition states of many of these enzymes.27–29 With this knowledge, inhibitor design and synthesis has provided exceedingly potent inhibitors of human and Plasmodium falciparum purine nucleoside phosphorylases (PNPs),30–33 human MTAP,34–37 protozoan nucleoside hydrolases38,39 and bacterial MTANs.36,40–47 Immucillin-H (1, Forodesine) and DADMe-Immucillin-H (2, BCX4208) are first and second generation inhibitors of the PNPs, and are in clinical development for the treatment of T-cell proliferative disorders and gout (Fig. 1). MT-Immucillin-A (3) and MT-DADMe-Immucillin-A (4) are corresponding first and second generation inhibitors of MTAP and bacterial MTANs and the second generation inhibitor 4 is in preclinical development for oncology indications.18,19 Recently, we reported on third generation inhibitors of PNP with acyclic aza-sugar mimics, some of which showed surprising activity. For example, DATMe-Immucillin-H 5 and SerMe-Immucillin-H 6 had exceptional activity with the achiral serinol derivative 7 being the most potent PNP inhibitor yet discovered (Ki* = 2.1 pM).48
Figure 1.
Structures of known transition state inhibitors of various N-ribosyltransferases.
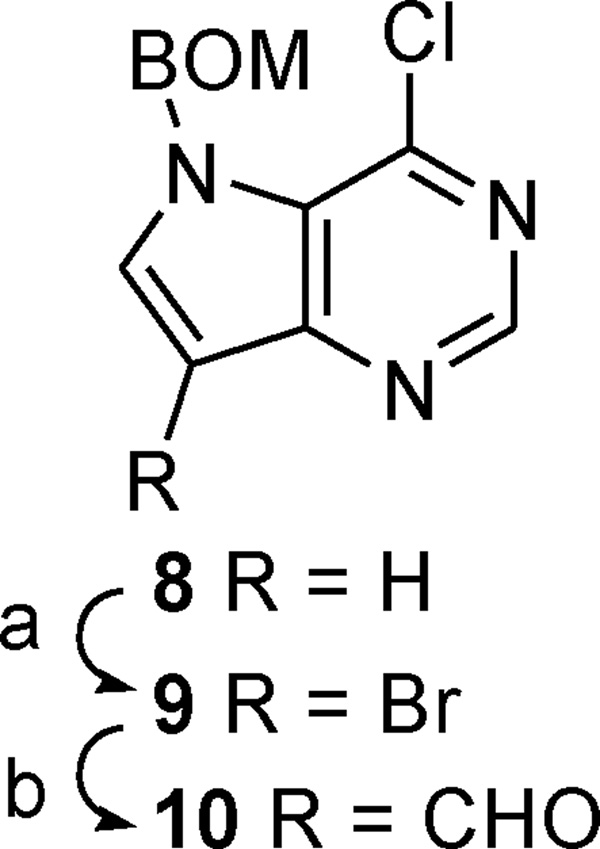
Therefore we wished to investigate whether replacement of the hydroxymethyl and 9-deazahypoxanthine or 9-deazaguanine units, found in acyclic PNP inhibitors, with methylthio and 9-deazaadenine ones, respectively, would lead to potent inhibitors of the related MTAP and MTAN enzymes. In our previous work on the preparation of acyclic PNP inhibitors, including compounds 5 to 7, we found reductive amination/alkylation and Mannich reactions useful in their construction.48 In both cases several primary and secondary amino alcohols were prepared and coupled through their amino functions, via a methylene link, to the 9-position of either deazahypoxanthine or deazaguanine. In this paper we describe the synthesis of several hydroxymethylthio-substituted primary and secondary amines and their couplings to aldehyde 10 or 9-deazaadenine,49 substrates for the reductive amination/alkylation (Schemes 1 to 8) and Mannich reactions (Schemes 9 to 11), respectively. In addition, the direct convertion of MT-Immucillin-A (3) into an acyclic derivative is also described (Scheme 12).
Scheme 1.
(a) NBS, 0 °C → rt, 1 h, 71%; (b) (i) n-BuLi, anisole-Et2O (1:3), −78 °C, 3 min, (ii) DMF,−78 → −40 °C, (iii) H2O, −40 °C → rt, 57%.
Scheme 8.
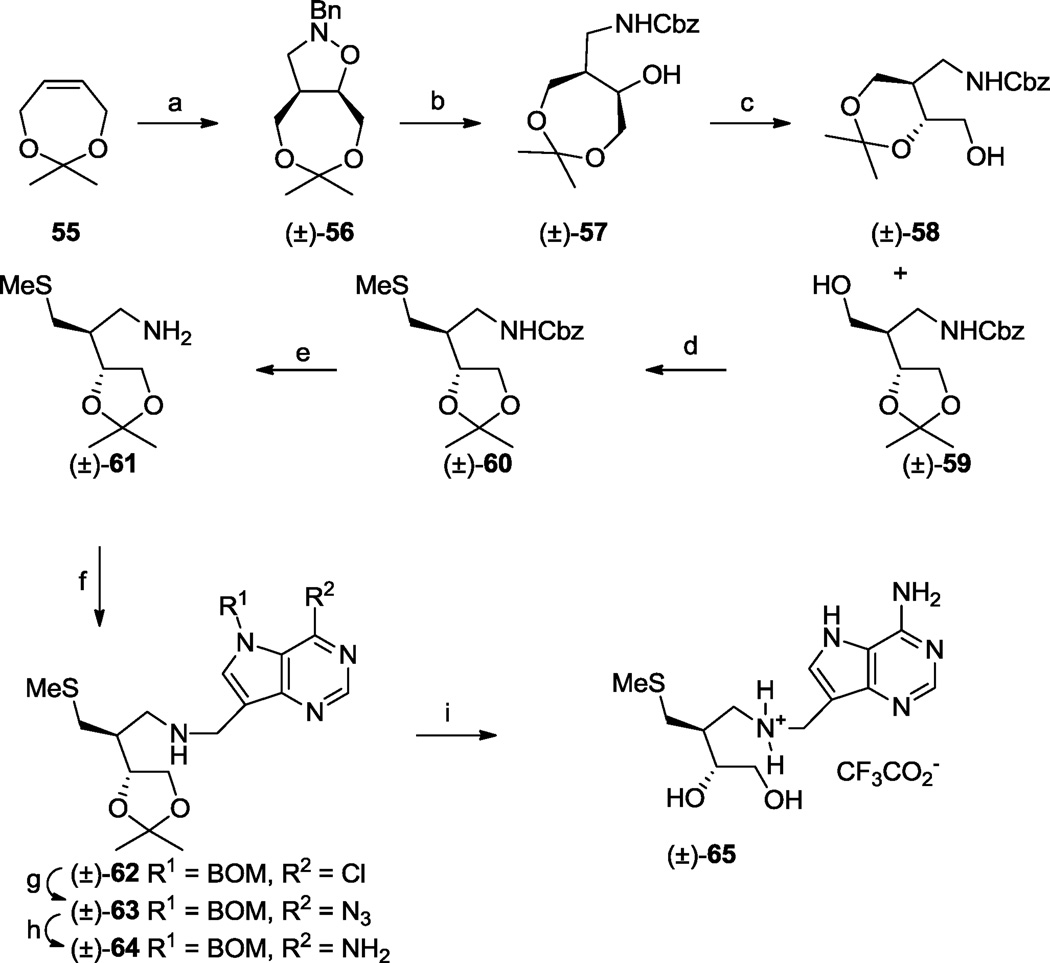
One enantiomer series present in the racemic mixtures is drawn to illustrate relative stereochemistry. (a) BnNHOH•HCl, aq. CH2O, NaOAc, EtOH, reflux, 6 h, 43%; (b) (i) H2, Pd/C. EtOH, rt, 2 days, (ii) BnOCOCl, Et3N, 0 °C → rt, 30 min, 63%; (c) camphor sulfonic acid, Me2CO, rt, 40 min, (±)-58 (52%), (±)-59 (44%); (d) (i) MsCl, i-Pr2NEt, CH2Cl2, rt 15 min, (ii) NaSMe, DMF, rt, 1 h, 83%; (e) KOH, i-PrOH, reflux, 2 h, 83%; (f) 10, Na(OAc)3BH, 1,2-dichloroethane, rt, 40 min, 64%; (g) NaN3, DMF, 90 °C, 1 h, 100%; (h) PMe3, THF, aq. NH3, rt, 1.5 h, 91%; (i) TFA-H2O, rt, 10 min, then NH2NH2•H2O, Pd black, 7M NH3-MeOH, rt 1 h, 100%.
Scheme 9.
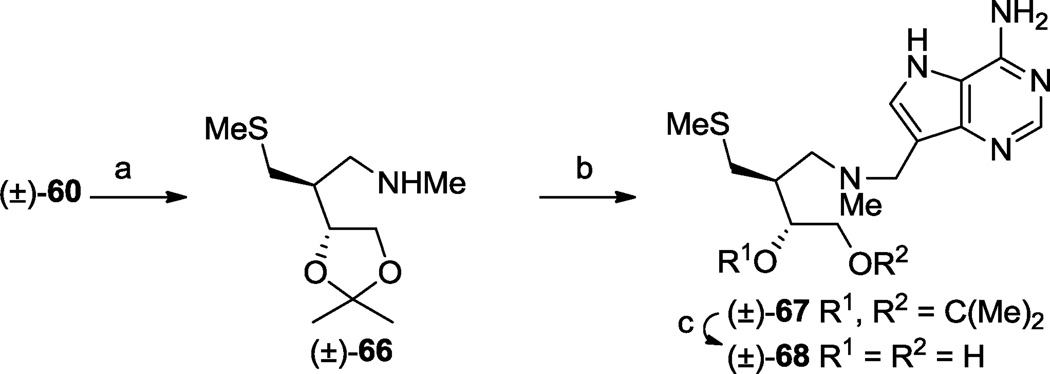
One enantiomer series present in the racemic mixtures is drawn to illustrate relative stereochemistry. (a) (i) NaH, THF, MeI, rt, 30 min, (ii) KOH, i-PrOH, reflux, 5 h, 77%; (b) 9-deazaadenine, CH2O, 1,4-dioxane, H2O, 90 °C, 1 h, (c) aq. HCl (37%)- MeOH, rt, 61% over 2 steps.
Scheme 11.
One enantiomer series present in the racemic mixtures is drawn to illustrate relative stereochemistry. (a) (Boc)2O, MeOH, rt, 1 h, 85%; (b) (i) MsCl, Et3N, CH2Cl2, rt, 30 min, (ii) NaSMe, DMF, rt 2 h, 60%; (c) LAH, THF, reflux, 1 h, 74%; (d) 9-deazaadenine, CH2O, 1,4-dioxane, H2O, 90 °C, 1 h; (e) aq. HCl (37%), MeOH, rt, 63% over 2 steps.
Scheme 12.
(a) (i) NaIO4, H2O, rt, 1 h, (ii) NaBH4, rt, 15 min, 47%.
2. Chemistry
2.1. Synthesis using the reductive amination/alkylation reaction
The key aldehyde 10 was synthesized by brominating chlorodeazapurine 850 followed by trapping of the lithium-bromine-exchanged derivative with N,N-dimethylformamide (DMF) (Scheme 1). The structure of bromide 9 was characterized spectroscopically and further confirmed by X-ray crystallography.51
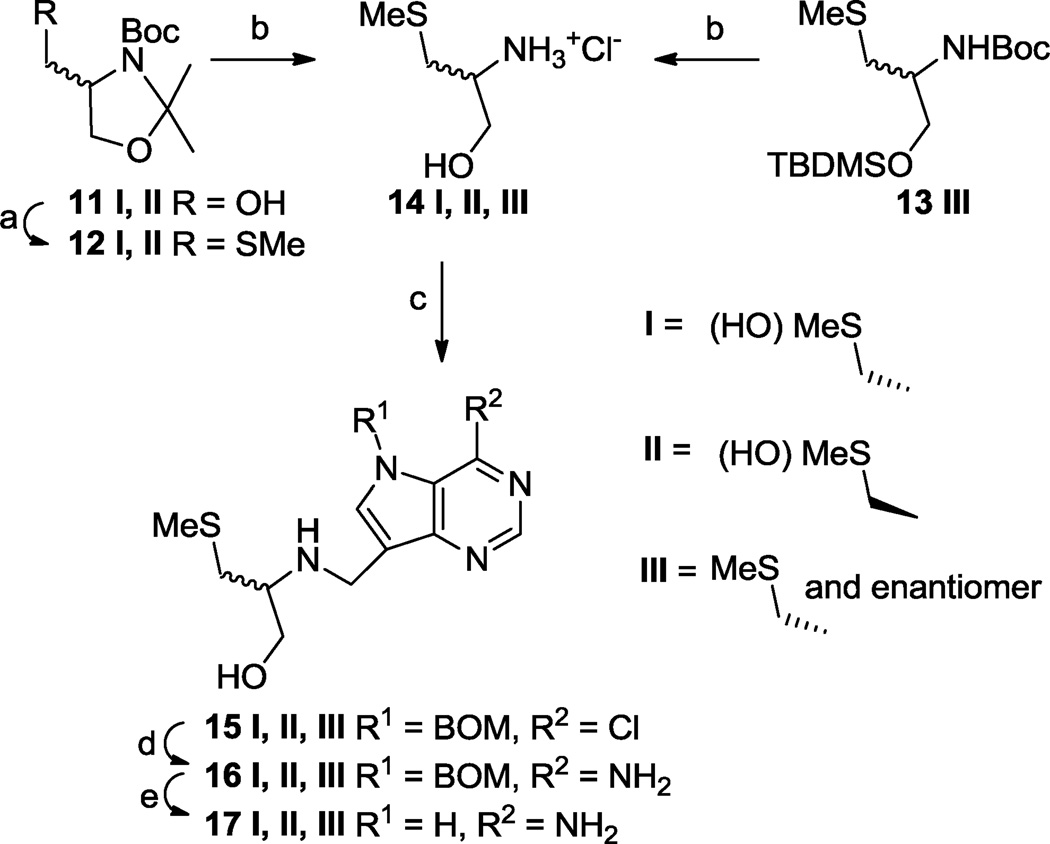
Serinol-linked 9-deazahypoxanthine or 9-deazaguanine derivatives 6 and 7 (Fig. 1) have been shown to be low pM inhibitors of human PNP48 and so we prepared the analogous methylthio-serinol-based 9-deazaadenine derivative 17 III to see if this would also be the case with human MTAP and bacterial MTANs. Treatment of racemic methylthio-serinol derivative 13 III52 with hydrochloric acid gave amine salt 14 III. This was reductively alkylated with aldehyde 10 using sodium cyanoborohydride53, after adjusting the pH of the mixture to between 6 and 7 with sodium hydrogen carbonate, to give the benzyloxymethyl (BOM) protected chloride 15 III (Scheme 2). Displacement of the chloride in 15 III with ammonia was effected in a sealed tube at 135 °C to give 16 III. Finally, the BOM protecting group was efficiently removed hydrogenolytically using hydrazine hydrate and palladium black in methanolic ammonia solution to produce 17 III.
Scheme 2.
(a) (i) MsCl, Et3N, CH2Cl2, 0 °C → rt, 30 min, (ii) NaSMe, DMF, rt, 1 h, 75% (I), 91% (II); (b) aq. HCl (37%), MeOH, 0 °C → rt, 1 h, 100% (I, II and III); (c) 10, 2-picoline-borane complex (for I and II) or NaCNBH3 (for III), MeOH, Et3N (for I and II) or NaHCO3 (for III), rt, 16 h, 67% (I), 78% (II), 71% (III); (d) 7M NH3-MeOH, 135 °C, sealed tube, 24–30 h, 74% (I), 86% (II), 59% (III); (e) NH2NH2•H2O, Pd black, 7M NH3-MeOH, rt 1 h, 84% (I), 79% (II), 76% (III).
Encouraged by the enzyme inhibition results (Table 1) obtained for 17 III, we next prepared the individual enantiomers 17 I and 17 II. Readily obtainable (S)-alcohol 11 I, prepared as for its enantiomer,54 was converted through its mesylate into methylthio compound 12 I and then into (R)-amine salt 14 I on treatment with hydrochloric acid (Scheme 2). Similarly, (R)-alcohol 11 II54 was transformed into (S)-amino hydrochloride 14 II.
Table 1.
Inhibitory constants (nM) for acyclic compounds vs human MTAP and bacterial MTANs
| Compound |
Ki
human MTAPa nM |
Ki
E. coli MTANa nM |
Ki
N. men. MTANa nM |
|---|---|---|---|
| 3 | 1.0 ± 0.5b,c | 0.08 ± 0.02d | 0.36e |
| 4 | 0.09 ± 0.01c | 0.002 ± .0002d | 0.14e |
| 17 III | 12 ± 1 | 1.7 ± 0.2 | 0.80 ± .06 |
| 17 I | 4.4 ± 0.2 | 0.23 ± 0.2 | NDf |
| 17 II | 34 ± 3 | 1.1 ± 0.4 | NDf |
| (±)-25 | 10 ± 1 | 0.36 ± 0.04 | 1.2 ± 0.1 |
| 33 | 5.2 ± 0.4 | 0.8 ± 0.1 | 0.9 ± 0.1 |
| ent-33 | 87 ± 11 | 3.9 ± 0.5 | 6 ± 1 |
| 40 | 87 ± 8 | 10 ± 2 | 10 ± 1 |
| ent-40 | 34 ± 18 | 2.1 ± 0.5 | 1.8 ± 0.4 |
| 47 | 34 ± 24 | 5.8 ± 0.8 | 4 ± 2 |
| 54 | 602 ± 83 | 40 ± 5 | 36 ± 4 |
| (±)-65 | 34 ± 6 | 9 ± 2 | 5 ± 1 |
| (±)-68 | 12 ± 2 | 2300 ± 600 | 61 ± 5 |
| (±)-72 | 105 ± 30 | 401 ± 37 | 132 ± 9 |
| (±)-78 | 368 ± 153 | 202 ± 35 | 85 ± 6 |
| 79 | 130 ± 20 | 1720 ± 850 | 47 ± 4 |
Conversions of 14 I and 14 II into enantiomeric products 17 I and 17 II, respectively, were achieved as for the racemate above except 2-picoline-borane complex55 was used as a less toxic alternative to sodium cyanoborohydride, and triethylamine was used as a more convenient base for adjusting the pH. Treatment of amines 14 I and 14 II with (S)-α-methoxy-α-trifluoromethylphenylacetyl chloride [(S)-MTPACl]56 gave Mosher amides with d.e.’s of ~94%.
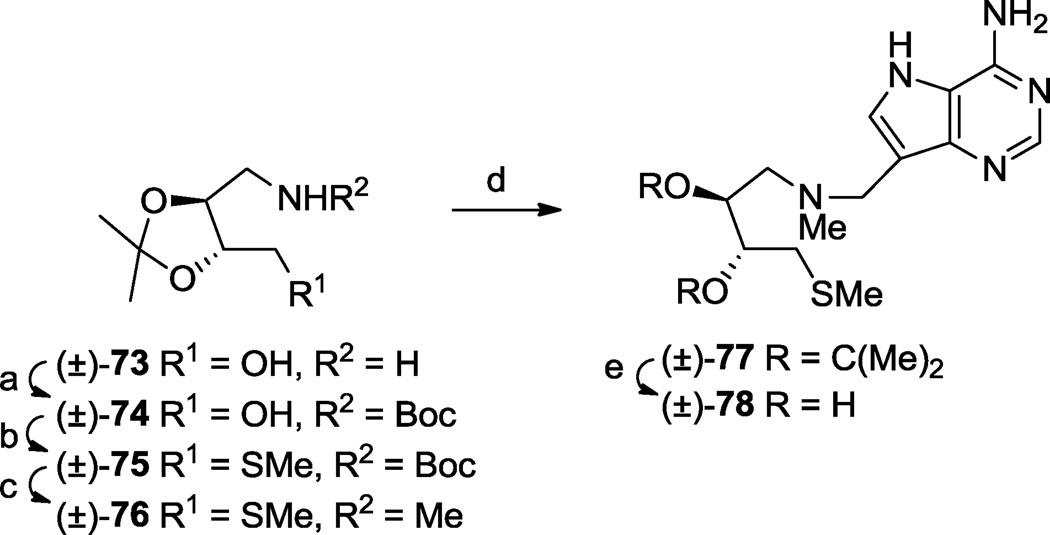
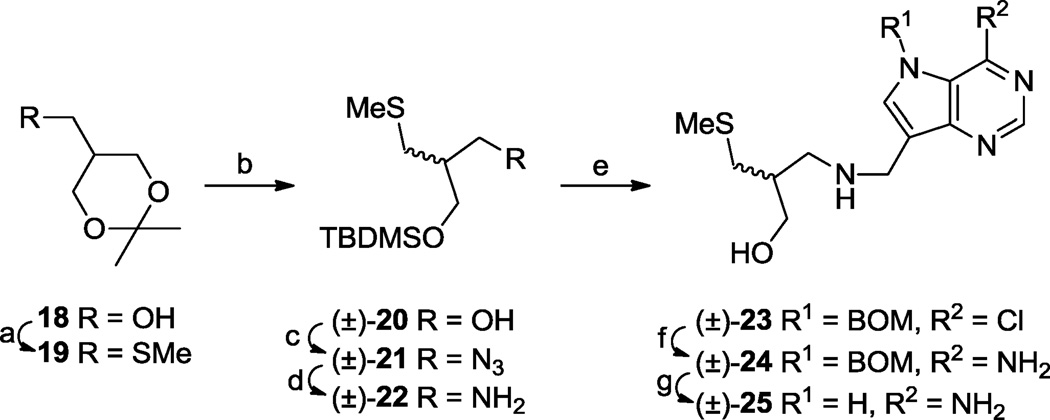
The (S)-enantiomer 17 II structurally resembles 3 with a CHOH group removed from the latter’s pyrrolidine ring. Similarly, the (S)-enantiomer present in (±)-25 resembles the structure resulting from the formal removal of a methylene at the 2-position of the pyrrolidine ring in 4. We therefore synthesized (±)-25 as shown (Scheme 3). Acetonide alcohol 1857 was methylthiolated via its mesylate, then the isopropylidene protecting group was removed by acid-catalysed transacetalization, and the resulting diol selectively mono-silylated58 to give alcohol (±)-20. Displacement of the mesylate derivative of (±)-20 with azide followed by hydrogenation furnished amine (±)-22 which was de-silylated then reductively alkylated with aldehyde 10 to afford (±)-23. Transformations (±)-23 → (±)-24 → (±)-25 were carried out as described for the conversions of 15 I to III → 17 I to III above.
Scheme 3.
(a) (i) MsCl, Et3N, 0 °C → rt, 30 min, (ii) NaSMe, DMF, rt, 16 h, 76%; (b) (i) AcCl, MeOH, rt, 1 h, (ii), NaH, TBDMSCl, rt, 2 h, 75%; (c) (i) MsCl, Et3N, 0 °C → rt, 30 min, (ii) NaN3, DMF, 80 °C, 3 h, 80%; (d) NH2NH2•H2O, Pd black, MeOH, rt 1 h, 82%; (e) (i) aq. HCl (37%), MeOH, rt, 1 h, (ii) 10, NaCNBH3, NaHCO3, MeOH, (iii) 7M NH3-MeOH, 135 °C, sealed tube, 24 h, 20%; (f) NH2NH2•H2O, Pd black, 7M NH3-MeOH, rt 1 h, 54%.
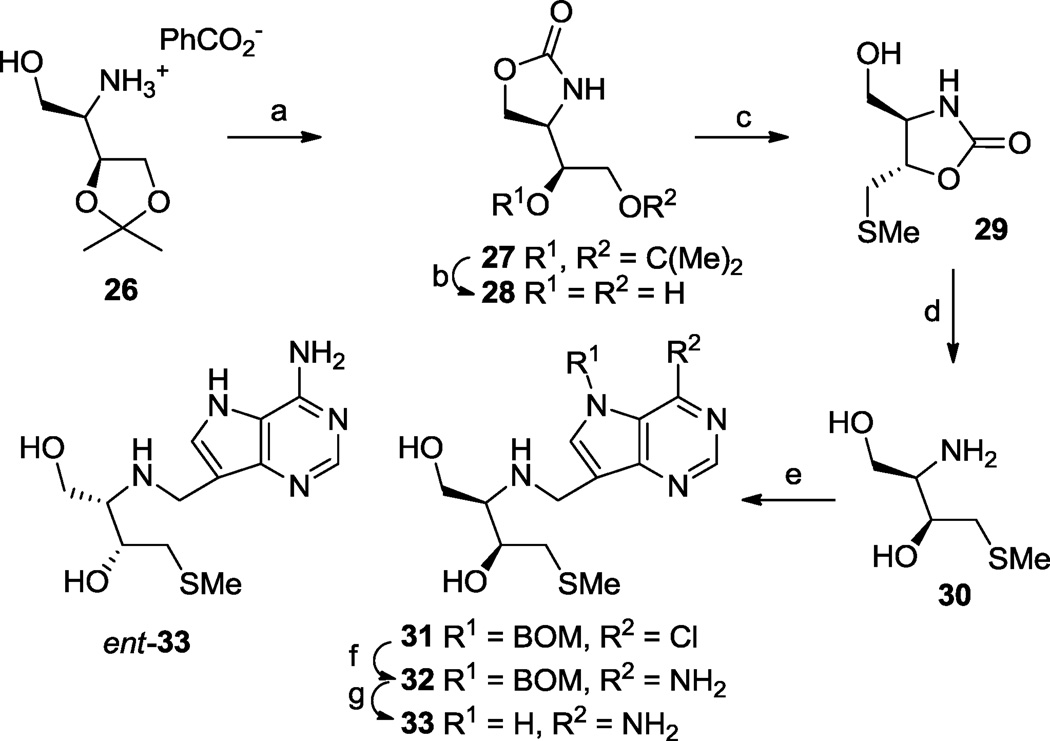
DATMe-Immucillin-H (5) has been identified, amongst its enantiomer and diastereomers, as a powerful PNP inhibitor (Fig. 1)48. Human MTAP and PNP share similar active sites and overall structural homology59 and this, together with a crystal structure of 5 in the active site of human PNP60, suggested that the methylthio 9-deazadenine analogue 33 was preferred as a target for MTAP/ MTANs inhibition, rather than the structure in which the alternative hydroxymethyl was substituted by methylthio (Scheme 4). The free amine present in salt 26, prepared as for its enantiomer61 and liberated from the benzoic acid with basic ion exchange resin, was converted to the oxazolidinone 27 with triphosgene, then deacetalized under acid-catalysed conditions to give diol 28. Tosylation of the primary hydroxyl then displacement with sodium thiomethoxide in DMF led unexpectedly to the rearranged oxazolidinone 29. X-ray crystallography62 of 29 with molybdenum Kα radiation confirmed both the relative and absolute stereochemistry. Base-catalysed hydrolysis of the oxazolidinone ring in 29 led to amine 30 which was reductively alkylated with aldehyde 10 to give 31. The standard conversion of 31 → 32 → 33 proceeded uneventfully as shown in the Scheme. The enantiomer (ent-33) of 33 was also prepared from ent-2661 in the same way as described for 33 (Scheme 4).
Scheme 4.
(a) (i) Amberlyst A26 (OH−) resin, MeOH; (ii) triphosgene, CH2Cl2, Et3N, rt, 90 min, 92%; (b) AcCl, MeOH, rt, 5 h, 93%; (c) (i) TsCl, pyridine, 0 °C → rt, 16 h; (ii) NaSMe, DMF, rt, 3 h, 44%; (d) KOH, i-PrOH, 80 °C, 4 h, 94%; (e) 10, AcCl, MeOH, NaCNBH3, rt, 3 h, 52%; (f) 7M NH3-MeOH, 135 °C, sealed tube, 24 h, 60%; (g) NH2NH2•H2O, Pd black, 7M NH3-MeOH, rt 1 h, 74%.
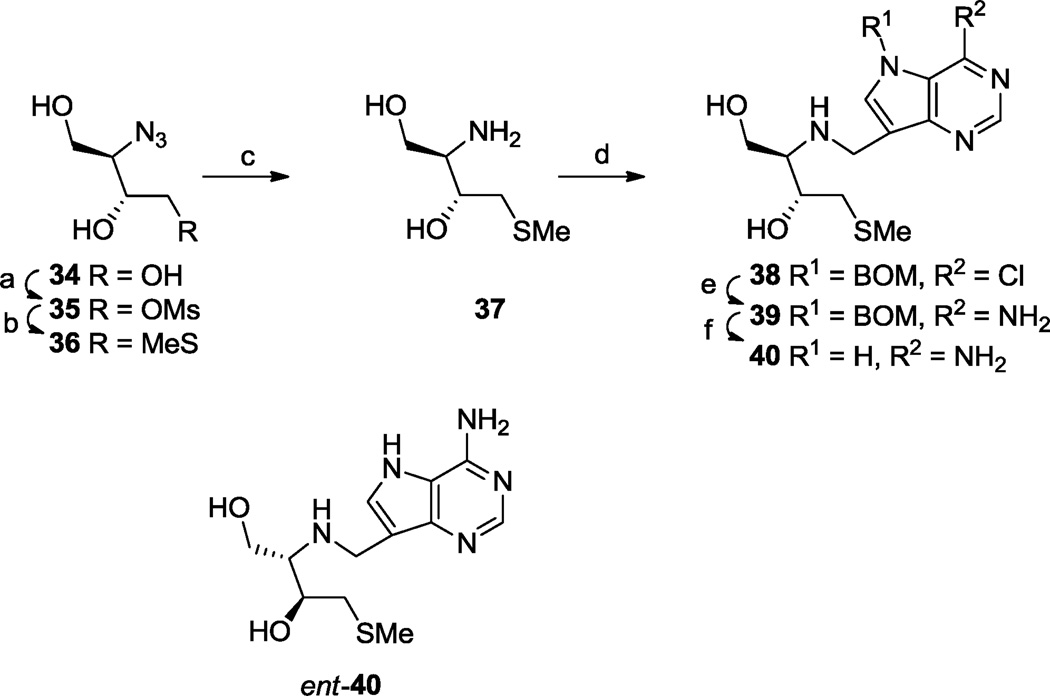
Two further diastereomers (40 and its enantiomer ent-40) of 33 were also prepared (Scheme 5). Azido triol 3463 was converted into methylthio derivative 36 through regioselective formation of an intermediate 5-membered ring stannylene acetal. The structure of 36 was confirmed by 1H-1H DQF-COSY NMR. After reduction of the azido group in 36, the resulting amine 37 was reductively alkylated with aldehyde 10 to give 38 then further transformed into product by the standard end sequence 38 → 39 → 40. The enantiomer (ent-40) of 40 was made in the same way starting from ent-3463
Scheme 5.
(a) (i) Bu2SnO, toluene, reflux, 30 min, (ii) MsCl, rt, 16 h; (b) NaSMe, DMF, rt, 2 h, 37% over 2 steps; (c) LAH, THF, 0 °C → rt, 1 h, 65%; (d) 10, NaCNBH3, HCl, MeOH, 61%; (e) 6M NH3-EtOH, 130 °C, sealed tube, 48 h, 73%; (g) NH2NH2•H2O, Pd black, 7M NH3-MeOH, rt 1 h, 61%.
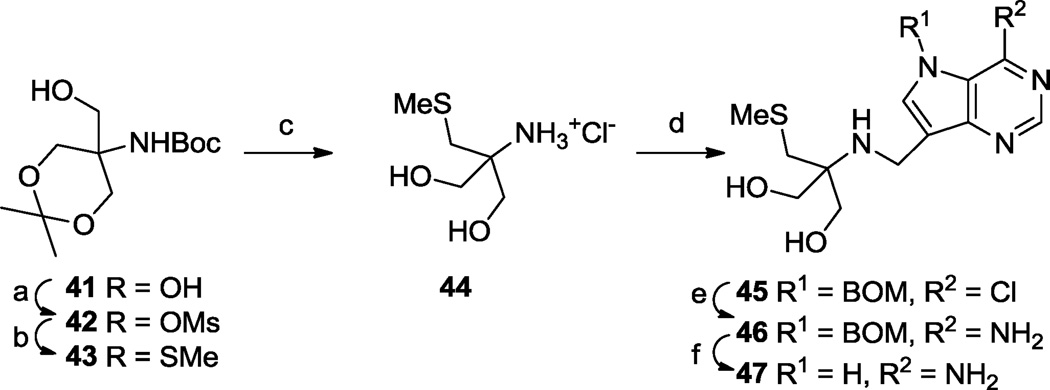
In order to explore steric congestion effects on the side chain adjacent to the amino group, the site that when protonated mimics the ribosyl cation in the transition state, we prepared compound 47 - formally equivalent to adding a hydroxymethyl group to compounds 17 I to III. In similar fashion to the methods presented above, the tris(hydroxymethyl)aminomethane acetonide 4164 was converted into the N-donor 44 and coupled to aldehyde 10 to provide the chloro-deazapurine derivative 45 (Scheme 6). The usual transformation of the deazapurine Cl → NH2 (45 → 46) then BOM removal furnished 47.
Scheme 6.
(a) MsCl, Et3N, CH2Cl2, 0 °C → rt, 90 min; (b) NaSMe, DMF, rt, 15 h; (c) aq. HCl (37%), MeOH, rt, 65% over 3 steps; (d) 10, NaCNBH3, MeOH, rt, 15 h; (e) 7M NH3-MeOH, 135 °C, sealed tube, 20 h, 78% over 2 steps; (f) NH2NH2•H2O, Pd black, 7M NH3-MeOH, rt 1 h, 56%.
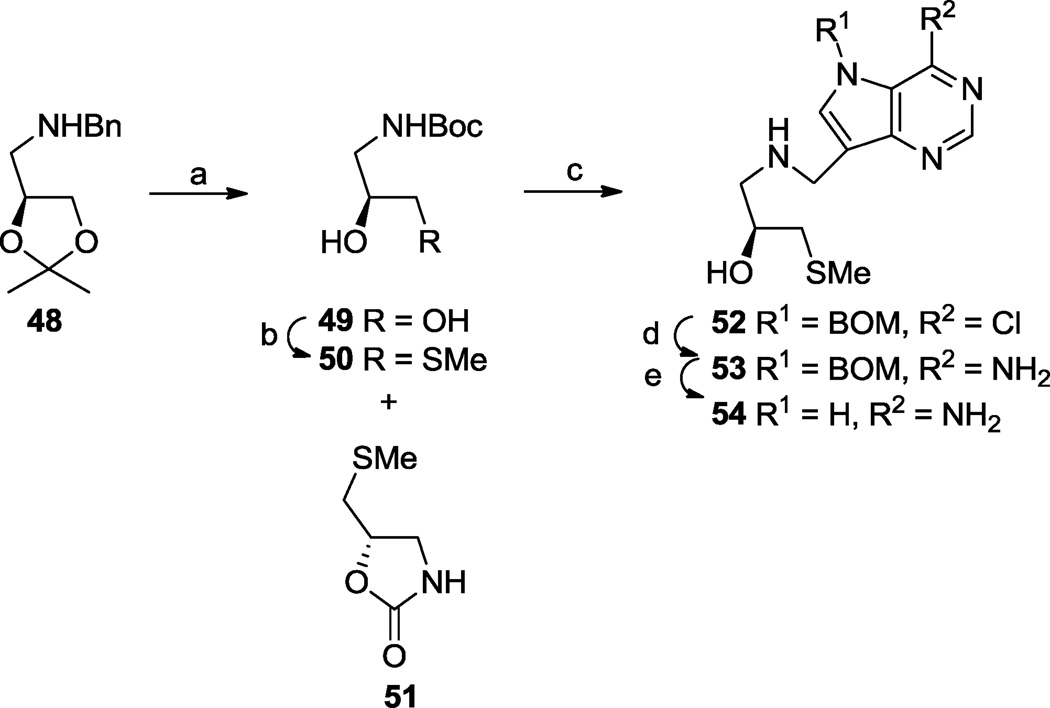
On the other hand, removal of a hydroxymethyl group from 33 would lead to compound 54 containing just one hydroxyl on a 3-carbon acyclic framework. The synthesis of 54 was accomplished through conversion of N-benzyl amine 4848 to the known tert-butyl carbamate (Boc) protected amino alcohol 4965 followed by successive treatment with tosyl chloride then sodium thiomethoxide to give oxazolidinone 51 in addition to the expected product 50 (Scheme 7). After removal of the N-Boc group in 50 with hydrochloric acid, the resulting amine salt was reductively alkylated with aldehyde 10 and the deazapurine ring modified as shown in the Scheme to give 54.
Scheme 7.
(a) (i) H2, Pd/C, EtOH, rt, 16 h, (ii) 6M HCl, 100 °C, 30 min, (iii) (Boc)2O, Et3N, MeOH, rt, 2 h, 66%; (b) (i) TsCl, pyridine, 0 °C → rt, 42 h, (ii) NaSMe, DMF, rt, 2 h, 22% (50), 16% (51); (c) (i) aq. HCl (37%), MeOH, rt, 5 min, (ii) 10, NaCNBH3, MeOH, rt, 60 h, 44%; (d) 7M NH3-MeOH, 135 °C, sealed tube, 24 h, 72%; (e) NH2NH2•H2O, Pd black, 7M NH3-MeOH, rt, 40 min, 76%.
The compounds described so far were constructed with either 3- or 4-carbon atoms in their acyclic side chains. We expanded the scope of the study by preparing products with 5-carbon side chains (Scheme 8). The 1,3-dioxepine acetal 5566 underwent a 1,3-dipolar cycloaddition with N-benzyl hydroxylamine and formaldehyde to afford (±)-56 which after reductive cleavage of the 1,2-isoxazolidine ring, then amino protection as a benzyl carbamate (Cbz), gave (±)-57. Acid-catalysed rearrangement of the acetonide moiety in (±)-57 was accomplished in acetone and gave a separable mixture of the 1,3-dioxane (±)-58 and 1,3-dioxolane (±)-59. Standard transformation of the hydroxyl in (±)-59 into a methylthio substituent followed by base-catalysed hydrolysis of the carbamate moiety gave amine (±)-61. Reductive amination of aldehyde 10 with (±)-61, using sodium triacetoxyborohydride67 as a less toxic alternative to sodium cyanoborohydride, gave the expected chloro-deazapurine (±)-62. At this stage we tried a different end sequence that avoided the use of sealed pressure vessels. Treatment of chloride (±)-62 with sodium azide gave (±)-63 which underwent a Staudinger reaction to produce protected amine (±)-64. The acetonide and BOM protecting groups were then removed by acid-catalysed hydrolysis and hydrogenolysis, respectively, to furnish (±)-65.
2.2. Synthesis using the Mannich reaction
N-Methylation of the secondary amine products above would provide derivatives with an altered affinity towards protonation whilst at the same time removing an N-H bond which may be important for enzyme inhibitor interaction. We therefore prepared a small subset of such compounds. Carbamate (±)-60 was N-methylated under anhydrous, basic conditions then the Cbz group was removed by basic solvolysis to give N-methyl amine (±)-66 (Scheme 9). This latter compound underwent a Mannich reaction with formaldehyde and 9-deazaadenine49 to afford (±)-67, then the acetal function was hydrolysed with hydrochloric acid to give, after neutralization, (±)-68, the N-methyl analogue of (±)-65 as the free base form.
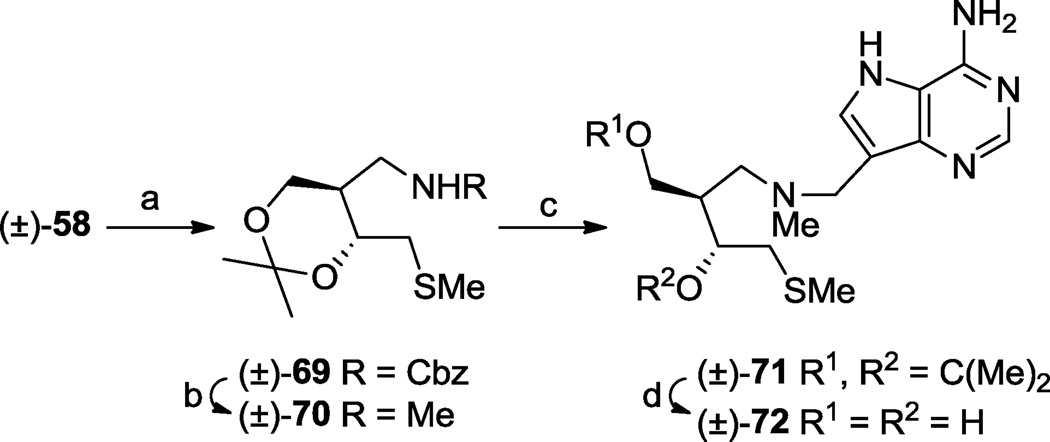
An isomer [(±)-72] of (±)-68 was also prepared by Mannich reaction except in this case the amine precursor (±)-70 was obtained by direct reduction of the N-Cbz group in (±)-69 with lithium aluminium hydride (Scheme 10).
Scheme 10.
One enantiomer series present in the racemic mixtures is drawn to illustrate relative stereochemistry. (a) (i) MsCl, i-Pr2NEt, CH2Cl2, rt 15 min, (ii) NaSMe, DMF, rt, 1 h, 86%; (b) LAH, THF, rt, 18 h, 57%; (c) 9-deazaadenine, CH2O, 1,4-dioxane, H2O, 85 °C, 15 min, 68%; (d) aq. HCl (37%), MeOH, rt, 79%.
By way of further example we also prepared (±)-78, equivalent to the formal removal of a methylene from the hydroxymethyl side chain found in (±)-72 (Scheme 11). It was conveniently prepared from racemic diethyl tartrate-derived amino alcohol (±)-7348 using similar chemistry to that used in the previous two schemes. In this case N-methylation was accomplished by reduction of the N-Boc function in (±)-75 with lithium aluminium hydride.
2.3. Synthesis from a cyclic precursor
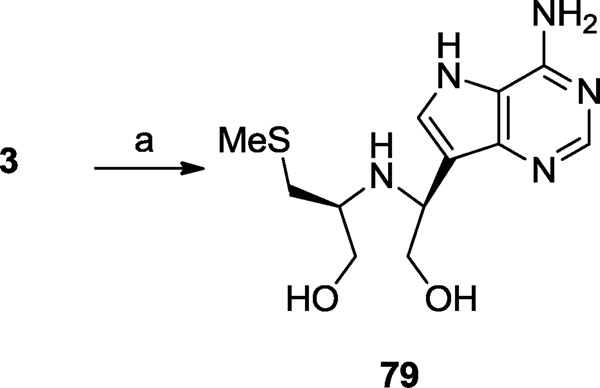
MT-Immucillin-A (3), a first generation MTAP/MTAN inhibitor, is ideally set up for conversion to an acyclic system through oxidative cleavage of the vicinal diol. In a one-pot reaction 3 was selectively oxidized with periodate and the resulting dialdehyde reduced with sodium borohydride to afford 79 (Scheme 12).
3. Biological evaluation
The inhibition of the phosphorolysis or hydrolysis of MTA catalysed by human MTAP or bacterial MTANs, respectively, was evaluated with 3rd generation acyclic inhibitors and the results compared with 1st and 2nd generation inhibitors, 3 and 4 (Table 1). Compounds 17 I – III, (±)-25 and 33 were the most potent inhibitors with the (R)-enantiomer (17 I) having Ki’s of 4.4 (Km/Ki = 1200) and 0.23 (Km/Ki = 1870) nM against human MTAP and E. coli MTAN, respectively. Although 17 I was not tested against N. meningitidis MTAN it is likely that this compound would also be a strong inhibitor of this enzyme given that the racemate (17 III) was the strongest inhibitor tested with a Ki of 0.80 nM (Km/Ki = 1750). Racemic compound (±)-25 was found to be just 1.5 times weaker an inhibitor than 17 I against E. coli MTAN suggesting one of the enantiomers present in (±)-25 could be of similar potency to 17 I.
As shown in the schemes, compounds 17 I and (±)-25 can be drawn such that they resemble cyclic compounds 3 and 4, respectively, with one carbon atom removed. Compound 17 I had binding affinities in the order of 3- and 4-fold that of 3 aginst E. coli MTAN and human MTAP, respectively. The racemate 17 III was about half as potent against N. meningitidis MTAN indicating 17 I could have a similar potency to 3 against the latter enzyme. Thus it appeared that compounds 17, despite the larger number of degrees of freedom, were able to adopt conformations similar to that taken by 3 in the enzymes’ active sites, resulting in comparable enzyme inhibition. Compared with 4 however, 17 I and (±)-25 showed approximately 110- and 180-fold weaker binding affinities, respectively, against E. coli MTAN and approximately 50- and 110-fold weaker binding affinities, respectively, against human MTAP. In contrast 17 III and (±)-25, had smaller 6- and 9-fold differences, respectively, with N. meningitidis MTAN. The large differences seen with the first two enzymes were attributed to compound 4 being a better match for the late dissociative transition state found with human MTAP37 and E. coli MTAN44 than for the early transition state of N. meningitidis MTAN40.
Compound 54 contains a side chain one carbon atom shorter than one of the enantiomers found in (±)-25 and had significantly reduced inhibitory activity, particularly against E. coli MTAN, experiencing a drop in binding affinity in the region of 110-fold. Alternatively, compound 54 can be viewed as the product produced by the removal of a hydroxymethyl group from 33. In contrast, compound 47 which had an extra hydroxymethyl group compared to compounds 17 I to III still retained moderate activity.
Compounds 33, 40 and their respective enantiomers ent-33 and ent-40 are the structures that would be formed from the formal cleavage of the 1,2-bond in the pyrrolidine ring of 3 with subsequent transposition of the terminal CH2SMe and CH2OH groups. Compound 33 was the strongest inhibitor of this group having approximately 7- to 17-fold greater affinity over the other compounds for human MTAP and approximately 3- to 13-fold increased affinity for the bacterial MTANs. Again compound 33 showed comparable efficacy to compound 3 being within about 2 to 10 fold of its activity against all three enzymes. Compound 33 was also of a similar potency as 17 I was against human MTAP and as 17 III was against N. meningitides MTAN, respectively, but was about 4-fold less active than 17 I was against E. coli MTAN. By comparison, oxidative cleavage of the vicinal diol bond in 3 without transposition of the CH2SMe and CH2OH units gave 79, and resulted in a severe loss of inhibitory activity against E. coli MTAN together with more moderate losses suffered against the other two enzymes.
A dramatic 250-fold loss of binding affinity occured in E. coli MTAN when (±)-65, a moderate inhibitor against all three enzymes, was N-methylated to (±)-68, making it the weakest inhibitor with a Ki of 2300 nM (Km/Ki = 19). In contrast, inhibition of N. meningitidis MTAN suffered only an approximate 12-fold loss in activity by this change but inhibition improved about 3-fold against human MTAP. This loss of activity against E. coli MTAN may be due to removal of a critical N-H bond. A similar case was also observed in the related enzyme PNP where N-methylation of DATMe-Immucillin-H (5) and SerMe-Immucillin-H (6) also resulted in a significant loss of activity48.
The isomeric N-methyl compounds (±)-68 and (±)-72 differ only by having their CH2SMe and CH2OH groups transposed. The latter compound showed a gain in binding affinity in the order of 6-fold with E. coli MTAN but a reduction of approximately 2- and 9-fold for N. meningitidis MTAN and human MTAP, respectively. On the other hand compound (±)-78 which differs from (±)-72 by one carbon atom, resulting in the transformation of a primary alcohol to a secondary one, produced around a 2-fold increases in potency for the bacterial MTANs but an approximate 4-fold loss in activity with human MTAP.
4. Conclusions
Cyclic compounds 3 and 4 are known powerful transition state analogue inhibitors of human MTAP and bacterial MTANs and therefore we have prepared a series of acyclic compounds to see if improved activity and simpler structures could be found as was the case with the related enzyme PNP48. Compounds 17 I to III, (±)-25 and 33 were identified as the best inhibitors with 17 I being around 3- and 4-fold as active as compound 3 against E. Coli MTAN human MTAP, respectively, but significantly weaker than 4 against the same enzymes. (S)-enantiomer 17 II, the product of the formal removal of a CHOH group from the pyrrolidine ring of 3, was found to be a weaker inhibitor than its (R)-enantiomer 17 I. Racemate (±)-25 containing the enantiomer present in the product of formal removal of the 2-methylene from the pyrrolidine in 4 was the second most potent inhibitor found against E. coli MTAN but it was not determined which enantiomer had the best activity. It was also found that simple bond cleavage of a cyclic inhibitor (3 → 79) or N-methylation of a secondary amine to a tertiary one [(±)-65 → (±)-68] led to dramatic losses in activity. In our previous work on the related PNP enzyme, acyclic compounds were found that were comparable or better inhibitors than the best cyclic forms and it was further shown that unfavourable binding entropies present in these acyclic derivatives were offset by favourable enthalpies of binding.68 In the present study such thermodynamic properties remain to be determined but it is clear that with MTAP/MTANs the acyclic inhibitors described here have not achieved the same level of potency as seen with our previous PNP study, particularly when compared to 4. The best compounds were nevertheless novel and potent inhibitors of human MTAP and E. coli and N. meningitidis MTANs.
Supplementary Material
Acknowledgements
This work was supported by research grant C08X0701 from the New Zealand Foundation for Research, Science & Technology and by research grants GM41916 and CA135405 from the National Institutes for Health. We are grateful to Drs Herbert Wong and Yinrong Lu for NMR and MS measurements, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting information
The full experimental details and the 1H and 13C NMR spectra of 17 I (17 II, 17 III), (±)-25, 33, (ent-33), 40, (ent-40), 47, 54, (±)-65, (±)-68, (±)-72, (±)-78 and 79 and of the Mosher amide derivatives of 14 I and 14 II can be found, in the online version, at
References
- 1.Della Ragione F, Carteni-Farina M, Gragnaniello V, Schettino MI, Zappia V. J. Biol. Chem. 1986;261:12324. [PubMed] [Google Scholar]
- 2.Toorchen D, Miller RL. Biochem. Pharmacol. 1991;41:2023. doi: 10.1016/0006-2952(91)90145-u. [DOI] [PubMed] [Google Scholar]
- 3.Miller CH, Duerre JA. J. Biol. Chem. 1968;243:92. [PubMed] [Google Scholar]
- 4.Ragione D, Porcelli FM, Carteni-Farina M, Zappia V, Pegg AE. Biochem. J. 1985;232:335. doi: 10.1042/bj2320335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marton LJ, Pegg AE. Ann. Rev. Pharmacol. Toxicol. 1995;35:55. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 6.Seiler N, Atanassov CL, Raul F. Int. J. Oncol. 1998;13:993. doi: 10.3892/ijo.13.5.993. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Ahmad N, Marengo SR, MacLennan GT, Greenberg NM, Mukhtar H. Cancer Res. 2000;60:5125. [PubMed] [Google Scholar]
- 8.Belting M, Borsig L, Fuster MM, Brown JR, Persson L, Fransson LA, Esko JD. Proc. Natl. Acad. Sci. U SA. 2002;99:371. doi: 10.1073/pnas.012346499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace HM, Fraser AV. Biochem. Soc. Trans. 2003;31:393. doi: 10.1042/bst0310393. [DOI] [PubMed] [Google Scholar]
- 10.Meyskens FL, Jr, Gerner EW. Clin. Cancer Res. 1999;5:945. [PubMed] [Google Scholar]
- 11.Wolford RW, MacDonald MR, Zehfus B, Rogers TJ, Ferro A. J. Cancer Res. 1981;41:3035. [PubMed] [Google Scholar]
- 12.Williams-Ashman HG, Seidenfeld J, Galletti P. Biochem. Pharmacol. 1982;31:277. doi: 10.1016/0006-2952(82)90171-x. [DOI] [PubMed] [Google Scholar]
- 13.Pegg AE, Borchardt RT, Coward JK. Biochem. J. 1981;194:79. doi: 10.1042/bj1940079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pajula RL, Raina A. FEBS Letters. 1979;99:343. doi: 10.1016/0014-5793(79)80988-6. [DOI] [PubMed] [Google Scholar]
- 15.Carteni-Farina M, Cacciapuoti G, Porcelli M, Ragione FD, Lancieri M, Geraci G, Zappia V. Biochim. Biophys. Acta (BBA) – Mol. Cell Res. 1984;805:158. doi: 10.1016/0167-4889(84)90163-0. [DOI] [PubMed] [Google Scholar]
- 16.Avila MA, Garcia-Trevijano ER, Lu SC, Corrales FJ, Mato JM. Int. J. Biochem. Cell Biol. 2004;36:2125. doi: 10.1016/j.biocel.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Andreu-Pérez P, Hernandez-Losa J, Moliné T, Gil R, Grueso J, Pujol A, Cortés J, Avila MA, Recio JA. BMC Cancer. 2010;10:265. doi: 10.1186/1471-2407-10-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basu I, Cordovano G, Das I, Belbin TJ, Guha C, Schramm VL. J. Biol. Chem. 2007;282:21477. doi: 10.1074/jbc.M702287200. [DOI] [PubMed] [Google Scholar]
- 19.Basu I, Locker J, Cassera MB, Belbin TJ, Merino EF, Dong X, Hemeon I, Evans GB, Guha C, Schramm VL. J. Biol. Chem. 2011;286:4902. doi: 10.1074/jbc.M110.198374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xavier KB, Bassler BL. Curr. Opin. Microbiol. Rev. 2003;6:191. doi: 10.1016/s1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Schauder S, Potier N, Dorsselaer VA, Pelczer I, Bassler BL, Hughson FM. Nature. 2002;415:545. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 22.Miller MB, Bassler BL. Ann. Rev. Microbiol. 2001;55:165. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 23.Fuqua C, Parsek MR, Greenberg EP. Annu. Rev. Genet. 2001;35:439. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Dizin E, Hu X, Wavreille AS, Park J, Pei D. Biochemistry. 2003;42:4717. doi: 10.1021/bi034289j. [DOI] [PubMed] [Google Scholar]
- 25.Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM. Mol. Cell. 2004;15:677. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Meijler MM, Horn LG, Kaufmann GF, McKenzie KM, Sun C, Moss JA, Matsushita M, Janda KD. Angew. Chem. Int. Ed. 2004;43:2106. doi: 10.1002/anie.200353150. [DOI] [PubMed] [Google Scholar]
- 27.Schramm VL. Acc. Chem. Res. 2003;36:588. doi: 10.1021/ar0200495. [DOI] [PubMed] [Google Scholar]
- 28.Schramm VL. Arch. Biochem. Biophys. 2005;433:13. doi: 10.1016/j.abb.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Schramm VL, Tyler PC. In: In iminosugars from synthesis to therapeutic applications. Compain P, Martin OR, editors. England: John Wiley & Sons Ltd; pp. 177–208. [Google Scholar]
- 30.Kline PC, Schramm VL. Biochemistry. 1993;32:13212. doi: 10.1021/bi00211a033. [DOI] [PubMed] [Google Scholar]
- 31.Lewandowicz A, Tyler PC, Evans GB, Furneaux RH, Schramm VL. J. Biol. Chem. 2003;278:31465. doi: 10.1074/jbc.C300259200. [DOI] [PubMed] [Google Scholar]
- 32.Lewandowicz A, Schramm VL. Biochemistry. 2004;43:1458. doi: 10.1021/bi0359123. [DOI] [PubMed] [Google Scholar]
- 33.Miles RW, Tyler PC, Furneaux RH, Bagdassarian CK, Schramm VL. Biochemistry. 1998;37:8615. doi: 10.1021/bi980658d. [DOI] [PubMed] [Google Scholar]
- 34.Evans GB, Furneaux RH, Schramm VL, Singh V, Tyler PC. J. Med. Chem. 2004;47:3275. doi: 10.1021/jm0306475. [DOI] [PubMed] [Google Scholar]
- 35.Evans GB, Furneaux RH, Lenz DH, Painter GF, Schramm VL, Singh V, Tyler PC. J. Med. Chem. 2005;48:4679. doi: 10.1021/jm050269z. [DOI] [PubMed] [Google Scholar]
- 36.Singh V, Shi W, Evans GB, Tyler PC, Furneaux RH, Almo SC, Schramm VL. Biochemistry. 2004;43:9. doi: 10.1021/bi0358420. [DOI] [PubMed] [Google Scholar]
- 37.Singh V, Schramm VL. J. Am. Chem. Soc. 2006;128:14691. doi: 10.1021/ja065419p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horenstein BA, Parkin DW, Estupinan B, Schramm VL. Biochemistry. 1991;30:10788. doi: 10.1021/bi00108a026. [DOI] [PubMed] [Google Scholar]
- 39.Parkin DW, Schramm VL. Biochemistry. 1995;34:13961. doi: 10.1021/bi00042a030. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez JA, Luo M, Singh V, Li L, Brown RL, Norris GE, Evans GB, Furneaux RH, Tyler PC, Painter GF, Lenz DH, Schramm VL. ACS Chemical Biology. 2007;2:725. doi: 10.1021/cb700166z. [DOI] [PubMed] [Google Scholar]
- 41.Lee JE, Singh V, Evans GB, Tyler PC, Furneaux RH, Cornell KA, Riscoe MK, Schramm VL, Howell PL. J. Biol. Chem. 2005;280:18274. doi: 10.1074/jbc.M414471200. [DOI] [PubMed] [Google Scholar]
- 42.Luo M, Schramm VL. J. Am. Chem. Soc. 2008;130:11617. doi: 10.1021/ja804578m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh V, Lee JE, Nunez S, Howell PL, Schramm VL. Biochemistry. 2005;44:11647. doi: 10.1021/bi050863a. [DOI] [PubMed] [Google Scholar]
- 44.Singh V, Evans GB, Lenz DH, Mason JM, Clinch K, Mee S, Painter GF, Tyler PC, Furneaux RH, Lee JE, Howell PL, Schramm VL. J. Biol. Chem. 2005;280:18265. doi: 10.1074/jbc.M414472200. [DOI] [PubMed] [Google Scholar]
- 45.Singh V, Shi W, Almo SC, Evans GB, Furneaux RH, Tyler PC, Painter GF, Lenz DH, Mee S, Zheng R, Schramm VL. Biochemistry. 2006;45:12929. doi: 10.1021/bi061184i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh V, Luo M, Brown RL, Norris GE, Schramm VL. J. Am. Chem. Soc. 2007;129:13831. doi: 10.1021/ja0754204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh V, Schramm VL. J. Am. Chem. Soc. 2007;129:2783. doi: 10.1021/ja065082r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinch K, Evans GB, Fröhlich RFG, Furneaux RH, Kelly PM, Legentil L, Murkin AS, Li L, Schramm VL, Tyler PC, Woolhouse AD. J. Med. Chem. 2009;52:1126. doi: 10.1021/jm801421q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evans GB, Kelly PM, Luxenburger A, Guan R, Suarez J, Thomas K, Schramm VL, Tyler PC. Manuscript in preparation. [Google Scholar]
- 50.Evans GB, Furneaux RH, Hutchison TL, Kezar HS, Morris PE, Schramm VL, Tyler PC. J. Org. Chem. 2001;66:5723. doi: 10.1021/jo0155613. [DOI] [PubMed] [Google Scholar]
- 51.Gainsford GJ, Mason JM, Gulab SA. Acta Crystallogr. Sect. E. 2010;66:o138. doi: 10.1107/S1600536809050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murkin AS, Clinch K, Mason JM, Tyler PC, Schramm VL. Bioorg. Med. Chem. Lett. 2008;18:5900. doi: 10.1016/j.bmcl.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borch RF, Bernstein MD, Durst HD. J. Am Chem. Soc. 1971;93:2897. [Google Scholar]
- 54.Dondoni A, Perrone D. Org. Synth. 2004;Coll. Vol. 10:320. Org. Synth.2000, 77, 64. [Google Scholar]
- 55. Sato S, Sakamoto T, Miyazawa E, Kikugawa Y. Tetrahedron. 2004;60:78–99. 2-Picoline-borane complex is now the reagent of choice for conducting reductive amination/alkylation reactions in our laboratories.
- 56.Ward DE, Rhee CK. Tetrahedron Lett. 1991;32:7165. [Google Scholar]
- 57.Harnden MR, Wyatt PG, Boyd MR, Sutton D. J. Med. Chem. 1990;33:187. doi: 10.1021/jm00163a031. [DOI] [PubMed] [Google Scholar]
- 58.McDougal PG, Rico JG, Oh YI, Condon BD. J. Org. Chem. 1986;51:3388. [Google Scholar]
- 59.Appleby TC, Erion MC, Ealick SE. Structure. 1999;7:629. doi: 10.1016/s0969-2126(99)80084-7. [DOI] [PubMed] [Google Scholar]
- 60.Ho M-C, Shi W, Rinaldo-Matthis A, Tyler PC, Evans GB, Clinch K, Almo SC, Schramm VL. Proc. Nat. Acad. Sci. 2010;107:4805. doi: 10.1073/pnas.0913439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inaba T, Yamada Y, Abe H, Sagawa S, Cho H. J. Org. Chem. 2000;65:1623. doi: 10.1021/jo991793e. [DOI] [PubMed] [Google Scholar]
- 62.Gainsford GJ, Clinch K. Acta Crystallogr. Sect. E. 2012;68:o2082. doi: 10.1107/S1600536812025809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calderón F, Doyagüez EG, Fernández-Mayoralas A. J. Org. Chem. 2006;71:6258. doi: 10.1021/jo060568b. [DOI] [PubMed] [Google Scholar]
- 64.Ooi H, Ishibashi N, Iwabuchi Y, Ishihara J, Hatakeyama S. J. Org. Chem. 2004;69:7765. doi: 10.1021/jo048817o. [DOI] [PubMed] [Google Scholar]
- 65.Kokotos G, Verger R, Chiou A. Chem. Eur. J. 2000;6:4211. doi: 10.1002/1521-3765(20001117)6:22<4211::aid-chem4211>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 66.Elliott WJ, Fried J. J. Org. Chem. 1976;41:2469. doi: 10.1021/jo00876a027. [DOI] [PubMed] [Google Scholar]
- 67.Abdel-Magid AF, Maryanoff CA, Carson KG. Tetrahedron Lett. 1990;31:5595. [Google Scholar]
- 68.Edwards AA, Mason JM, Clinch K, Tyler PC, Evans GB, Schramm VL. Biochemistry. 2009;48:5226. doi: 10.1021/bi9005896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.