Abstract
Isopentenyl diphosphate isomerase (IPPI) is an enzyme involved in the synthesis of juvenile hormone (JH) in the corpora allata (CA) of insects. IPPI catalyzes the conversion of isopentenyl pyrophosphate (IPP) to dimethylallyl pyrophosphate (DMAPP); afterwards IPP and DMAPP condense in a head-to-tail manner to produce geranyl diphosphate (GPP), this head-to-tail condensation can be repeated, by the further reaction of GPP with IPP, yielding the JH precursor farnesyl diphosphate. An IPPI expressed sequence tag (EST) was obtained from an Aedes aegypti corpora-allata + corpora cardiaca library. Its full-length cDNA encodes a 244-aa protein that shows a high degree of similarity with type I IPPIs from other organisms, particularly for those residues that have important roles in catalysis, metal coordination and interaction with the diphosphate moiety of the IPP. Heterologous expression produced a recombinant protein that metabolized IPP into DMAPP; treatment of DMAPP with phosphoric acid produced isoprene, a volatile compound that was measured with an assay based on a solid-phase micro extraction protocol and direct analysis by gas chromatography. A. aegypti IPPI (AaIPPI) required Mg2+ or Mn2+ but not Zn2+ for full activity and it was entirely inhibited by iodoacetamide. Real time PCR experiments showed that AaIPPI is highly expressed in the CA. Changes in AaIPPI mRNA levels in the CA in the pupal and adult female mosquito corresponded well with changes in JH synthesis (Li et al., 2003). This is the first molecular and functional characterization of an isopentenyl diphosphate isomerase involved in the production of juvenile hormone in the CA of an insect.
Keywords: Mosquito, IPP isomerase, juvenile hormone, corpora allata, Aedes
INTRODUCTION
Isoprenoids are a large family of organic compounds derived from C5 isoprene units. They are present in Archaea, Bacteria and Eukarya and have essential roles in signal transduction, electron transport, photosynthesis and regulation of development and reproductive cycles (Sacchettini and Poulter, 1997; Ramos-Valdivia et al., 1997; Satterwhite, 1985; Spurgeon and Porter, 1981; Bonano et al., 2001). An important building block of all isoprenoids is the compound isopentenyl pyrophosphate (IPP) (Ramos-Valdivia et al., 1997). Almost all organisms synthesize IPP by the condensation of three units of acetyl-CoA through the mevalonate pathway (MVA) (Ramos-Valdivia et al., 1997). In contrast, in some prokaryotes and plant plastids, IPP production is accomplished via the 2-C-methyl-D-erythritol-4-phosphate pathway (MEP) (Rohmer, 1999).
The synthesis of isoprenoids includes the conversion of IPP to the electrophile dimethylallyl pyrophosphate (DMAPP), catalyzed by isopentenyl diphosphate isomerase (IPPI). IPPI generates DMAPP by the isomerization of the carbon-carbon double bond of IPP via the stereoselective antarafacial transposition of hydrogen (Durbecq et al., 2001). There are two types of IPPIs, type I is found in almost all eukaryotes while type II has only been observed in prokaryotes (Ramos-Valdivia et al., 1997). The optimal functioning of IPPI type I requires a divalent metal cation (Mg2+ or Mn2+) (Durbecq et al., 2001). Zinc has also been identified as an essential cofactor for the activity of recombinant IPPI type I from Escherichia coli (Carrigan and Poulter, 2003). Type II IPPI requires a reduced flavin coenzyme in addition to a divalent metal cation (de Ruyck et al., 2008).
In the corpora allata (CA) of insects, IPPI is involved in the synthesis of juvenile hormone (JH) (Belles, et al., 2005; Goodman and Granger, 2005); IPP and DMAPP can condense in a head-to-tail manner to produce geranyl diphosphate (GPP). This type of head-to-tail condensation can be repeated by the further reaction of GPP with IPP, yielding the JH precursor farnesyl diphosphate (Goodman and Granger, 2005). In insect species, IPPIs have been only partially characterized from extracts of the silkworm Bombyx mori larvae (Koyama et al., 1985). We cloned an IPPI expressed in the CA of the mosquito Aedes aegypti (Noriega et al., 2006). Heterologous expression produced a recombinant protein that metabolizes IPP into DMAPP. The protein requires Mg2+ or Mn2+ but not Zn2+ for full activity and is entirely inhibited by alkylation of the catalytic cysteine residue by iodoacetamide. Real time PCR experiments showed that IPPI mRNA levels in the CA match the changes in JH synthesis.
MATERIAL AND METHODS
2.1. Insects
Aedes aegypti of the Rockefeller strain were reared at 28 °C and 80% relative humidity under a photoperiod of 16 h light: 8 h dark. Mated adults were offered a cotton pad soaked in 3% sucrose solution. The cotton pad sucrose-fed adults are referred to as sugar fed. Four-day-old female mosquitoes were fed porcine blood equilibrated to 37 °C. Adenosine triphosphate was added to the blood meal to a final concentration of 1 mM immediately before use (Noriega et al., 1999).
2.2. Chemicals
Isoprene was purchased from Sigma-Aldrich (St Louis, MO). IPP and DMAPP were obtained from Echelon Biosciences (Salt Lake City, UT).
2.3 Identification of the A. aegypti IPP isomerase cDNA
An IPPI expressed sequence tag (EST) was obtained from an A. aegypti corpora-allata + corpora cardiaca library, constructed and sequenced as previously described (Noriega et al., 2006). The IPPI EST sequence was queried against the A. aegypti database at VectorBase (Lawson et al., 2009); it revealed a single identical sequence (Accession number: AAEL006144). The A. aegypti IPPI (AaIPPI) cDNA was PCR-amplified from cDNA obtained from the thorax of mosquitoes (containing the corpora allata) using the following two primers:
Forward: AaIPPI-F, 5′-CATATGTCTCTTCTGGCTCGCTT-3′.
Reverse: AaIPPI-R, 5′- GCGGCCGCTCAAAATCGTTCGATTTCGTTG-3′.
2.4. Expression and purification of recombinant AaIPPI
The coding region of the AaIPPI cDNA was ligated into the pET-28a(+) expression vector (Novagen, Gibbstown, NJ). E. coli strain BL21 (DE3) were transformed with the construct and expressed as previously described (Mayoral et al., 2009). Recombinant protein containing a C-terminal His-tag was purified with a cobalt column (Pierce, Rockford, IL) and desalted using a PD-10 column (Amersham, Pharmacia Piscataway, NJ) (Mayoral et al., 2009). The purified protein was concentrated (225 ng/μL) using Centricon YM-10 centrifugal filters (Millipore, Billerica, MA), aliquoted and stored at −20 °C until use.
2.5. IPPI enzymatic assay
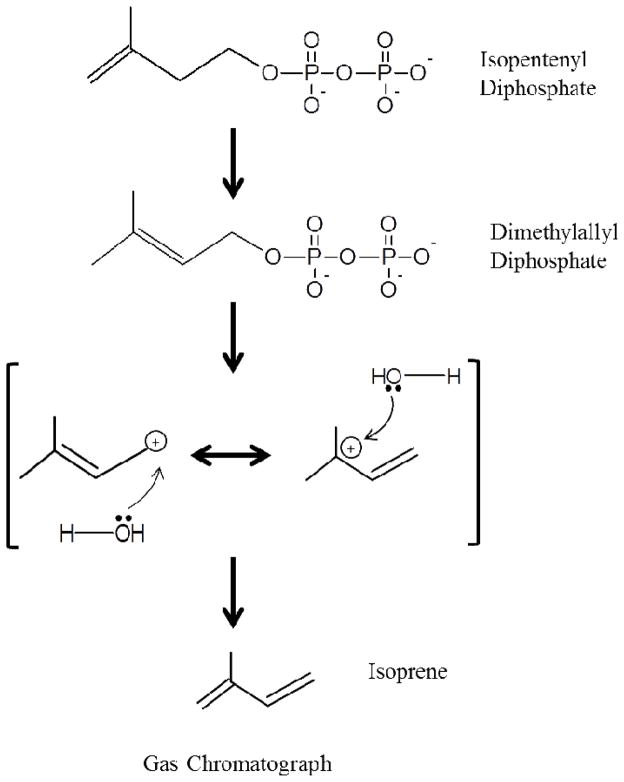
IPPI activity was determined by conversion of DMAPP to isoprene by treatment with phosphoric acid, followed by gas chromatographic (GC) analysis of isoprene (Fisher et al., 2001; Bruggemann and Schnitzler, 2002) (Fig. 1). We modified previously described methods by using a solid-phase micro extraction protocol (SPME) to adsorb the volatile isoprene for direct GC analysis. A sample containing 93 μL of assay buffer (0.4 M Tris, 1 mM DTT, 10mM MgCl2, pH 8.0), 5 μL of IPP (1 μg/μL) and 2 μL of the recombinant AaIPPI (200 ng/μL) was incubated at 35 °C for 1 h and the reaction was terminated by the addition of 30 μL of phosphoric acid (14.8 M). After incubation the solution was transferred to a 2-mL glass vial sealed with a silicone septum vial (Agilent) and the needle of the holder carrying the SPME fiber was introduced through the septum. The SPME holder and the 30 μm divinylbenzene-carboxen-polydimethylsiloxane (DVD-CAR-PDMS) fibers used to absorb isoprene were supplied by Supelco (Bellefonte, PA). The vial was placed on a heating block at 50 °C and the fiber was exposed to the atmosphere in the vial (Supplementary Fig. 1). After 1 hour of incubation at 50 °C, the fiber was retracted into the needle. The holder was removed and transferred to the GC equipment. The needle was then introduced into the injection port of the GC equipment preheated at 200 °C; isoprene was immediately desorbed from the fiber and the GC run started.
Figure 1. IPPI assay outline.
Isomerization of IPP to DMAPP is catalyzed by IPPI. During the reaction an electrophilic attack by a proton from water occurs on the IPP double bond to yield the carbocation intermediate, afterwards the C-2 pro-R hydrogen of the intermediate is stereospecifically eliminated. DMAPP is converted to isoprene by acidic hydrolysis and dehydration using phosphoric acid. Volatile isoprene is adsorbed using a SPME fiber and detected by gas chromatography.
GC determinations of isoprene were performed on a Trace Gas Chromatography Ultra apparatus (Thermo Electron Corporation, Waltham, MA) equipped with a non-polar capillary column DB-5MS (30 m × 0.25 mm i.d.; film thickness 0.25 μm) (Agilent, Santa Clara, CA) and a flame ionization detector. The output was recorded using a ChromQuest version 4.1 data system. Helium was employed as the gas carrier at a flow rate of 1 mL/min. The injector and detector temperatures were 200 °C and 230 °C, respectively. The column oven temperature was isothermally held at 30 °C. The amounts of isoprene detected were expressed as GC peak areas without using corrections factors. Prior to re-use, the fiber was conditioned for 10 min in the GC injection port at 200 °C.
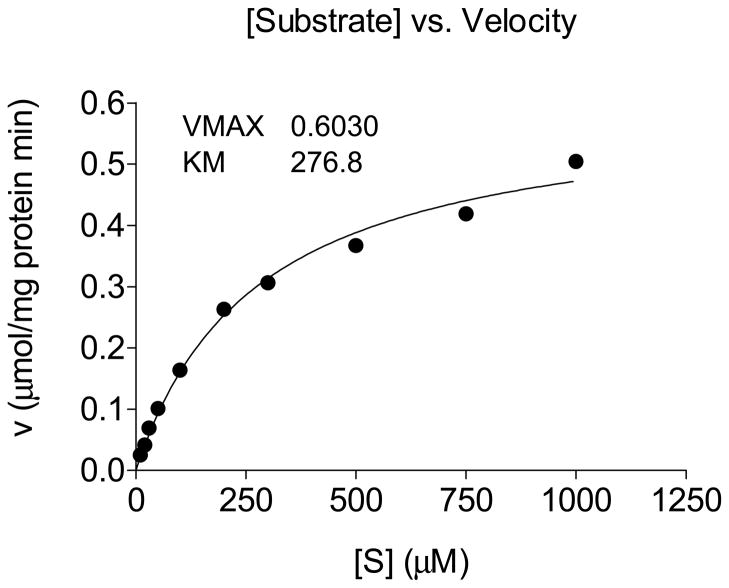
For the kinetic studies, substrate concentrations ranged from 10 to 100 μM in a reaction mixture of 100 μL, containing 400 ng of recombinant enzyme. Reactions were incubated for 1 h at 35 °C. The kinetic values were calculated by nonlinear regression analysis of the Michaelis-Menten equation using GraphPad Prism software 3.03. A Lineweaver-Burk plot was derived from transformation based upon nonlinear regression analysis.
2.6. RNA extraction and Real Time-PCR
Corpora allata-corpora cardiac complexes (CA-CC) (20 pairs per replicate) were dissected in a drop of sterile DNA-RNAse free phosphate buffered saline (PBS). Total RNA was isolated using RNA-binding glass powder as previously described (Noriega and Wells, 1993). RNA samples were treated with rDNAse I using DNA-free™ kit (Ambion, Austin, TX, US) according to the manufacturer’s recommendations. Reverse transcription was carried out using the SuperScript® III first strand synthesis kit. Real-time PCR was performed in a 7300 Real Time PCR System (Applied Biosystems, Foster City, CA, US) using TaqMan® Gene Expression Assays together with TaqMan® Universal PCR Master Mix (Applied Biosystems). The primers and probes for the house keeping gene 60S ribosomal protein rpL32 (Accession number: AAEL003396) and the AaIPPI gene are included in Supplementary table 1. Primer/probes were synthesized by Applied Biosystems and reactions were carried out in 20 μL volume according to the manufacturer’s recommendations for Custom TaqMan® Gene Expression Assays. Reactions were run in triplicate using 1 μL of cDNA per reaction. Standard curves to quantify relative gene copy number were made from ten-fold serial dilutions of plasmids containing rpL32 or AaIPPI (from 300,000 to 30 copies of a plasmid per reaction). Real-time data were collected by 7300 System SDS Software and analyzed in Microsoft Excel. Transcript levels were normalized with rpL32 mRNA levels in the same sample. Relative transcript levels are expressed as a number of copies of transcript per 10,000 copies of rpL32.
2.7. Phylogenetic analysis
IPPI sequences were obtained from GenBank and VectorBase (Lawson et al., 2009) databases and used for the alignments and phylogenetic analysis. We aligned the IPPI sequences using ClustalW (Higgins et al., 1994). A Maximum-Likelihood tree was built using MEGA software version 5.1 (Tamura et al., 2011) with a bootstrapping of 1000 (Felsenstein, 1985). Partial deletion method was selected for the gap/missing data in the software.
2.8. Modeling A. aegypti IPPI
The tertiary structure model of the AaIPPI was created using the protein structure homology-modeling server Swiss-Model v.8.05 (Arnold et al., 2006; Schwede et al., 2003) using as template the Human IPPI (2i6kA) that has 46.6% identity with AaIPPI. The QMEAN6 (Benkert et al., 2011) score obtained for the model obtained was 0.720, the secondary structure agreement 80.0% and the solvent accessibility agreement 82.3%.
3. RESULTS
3.1. Molecular characterization of A. aegypti IPPI
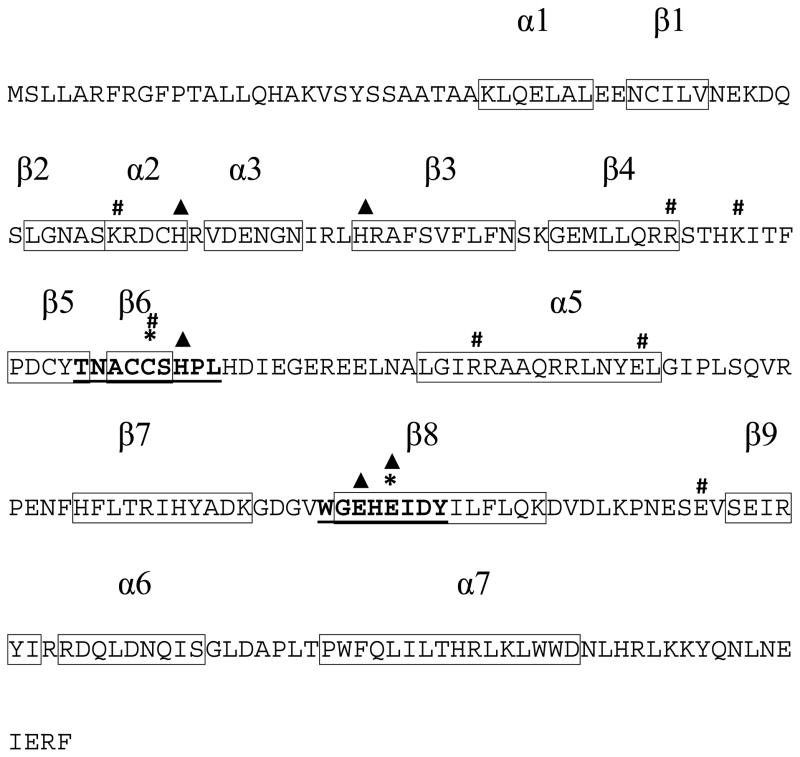
A single orthologue gene to the AaIPPI EST was found in the genome of A. aegypti (VectorBase) (Lawson et al., 2009). The IPPI gene is located on supercontig 1.191, and it is composed of three exons interrupted by two introns with lengths of 4,158 and 9,351 bp. It encodes a 244 amino acid protein with an estimated molecular weight of 28.5 kDa. Analysis of the AaIPPI sequence revealed the presence of a typical Nudix hydrolase superfamily domain (Nudix: NUcleoside DIphosphate linked to some other moiety X) (Fig. 2). Residues previously described as important for metal coordination and interaction with the diphosphate moiety of the IPP are well conserved in AaIPPI; including two important motifs that have been associated with IPPI type I functionality: a TNACCSHPL motif containing a conserved cysteine residue and a WGEHEIDY motif that contains a conserved glutamate residue (Fig. 2).
Figure 2. AaIPPI amino acid sequence.
Predicted α-helices and β-sheets are labeled on top and shown inside a box. IPPI conserved sequence motifs are highlighted in bold and underlined. (▲): Residues important for metal coordination. (*): Residues important for catalysis. (#): Residues important for interaction with the diphosphate moiety of the substrate. The putative Nudix motif is highlighted by a grey box.
IPPI orthologues were searched in other species of insects. An alignment of the AaIPPI with related insect proteins is shown in the Supplementary Fig. 2. A cladogram of the phylogenetic relationship of the insect’s IPPI sequences was generated (Supplementary Fig. 3).
3.2. Establishment of an improved protocol to measure IPPI activity
We developed a convenient and simple IPPI activity assay based on the non-enzymatic acidic hydrolysis of DMAPP, which generates the volatile compound isoprene (Fisher et al., 2001). Our modification was to introduce the use of solid-phase micro extraction (SPME) to adsorb the volatile isoprene that can be later directly quantified by GC (Fig. 1). Optimal conditions for hydrolysis were explored; a temperature of 50 °C was chosen because at this temperature isoprene was efficiently adsorbed by the SPME fiber. Higher temperatures could also be used, but heating the sample over 50 °C caused spontaneous hydrolysis of IPP and unwanted production of isoprene. At 50 °C formation and adsorption of isoprene derived from DMAPP was very effective and generation of isoprene from IPP was minimalized (Supplementary Fig. 4). Analysis of different reaction times showed that most of the isoprene formed from DMAPP was recovered within 1 hour. Using these conditions three standard curves were constructed by non-enzymatic hydrolysis of mixtures containing 1) increasing amounts of DMAPP, 2) increasing amounts of IPP and 3) increasing amounts of DMAPP and decreasing amounts of IPP (Supplementary Fig. 4). We used a mix of IPP and DMAPP to mimic the situation of enzymatic reactions in which increasing amounts of IPP were transformed into DMAPP, but still there was a mix of both compounds in the solution that was hydrolyzed by phosphoric acid. A linear increase in non-enzymatic generation of isoprene formation was observed as the amount of DMAPP was increased and IPP decreased (Supplementary Fig. 4). The DMAPP standard curve was adjusted for IPP interference and developed into a corrected DMAPP standard curve that was used for product quantification (Supplementary Fig. 4). Under these conditions, the SPME method was sensitive and reproducible.
3.3. AaIPPI activity in the presence of cofactors and inhibitors
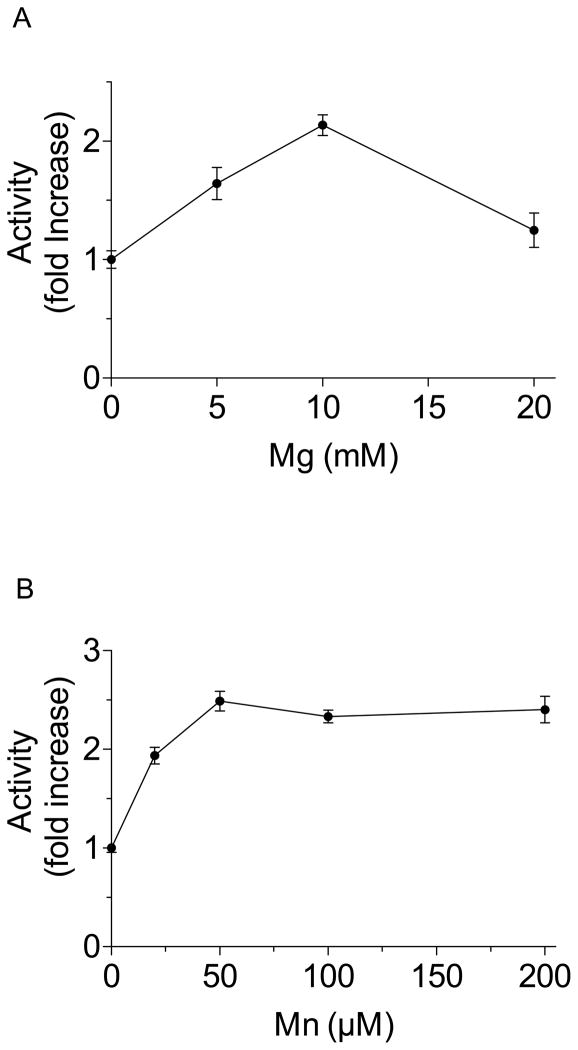
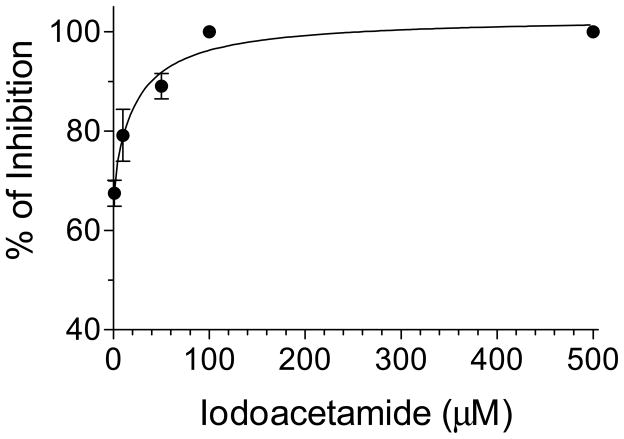
To analyze the effect of metal cofactors, Mg2+ in the form of MgCl2 was added to samples to obtain final concentrations of 5, 10 and 20 mM. There was a 2-fold increase in activity when 10 mM was added; increasing the concentration to 20 mM also stimulated AaIPPI activity but it was less pronounced (Fig. 3). Buffer without metal cofactor was used as control (0.4 M Tris, 1 mM DTT, pH 8.0). The same process was repeated with Mn2+ in the form of MnCl2 to produce concentrations of 0.02, 0.05, 0.1 and 0.2 mM. There was a 2.5-fold increase in activity when 0.1 mM was added; increasing the concentration to 0.2 mM also stimulated AaIPPI activity but it was less marked (Fig. 3). The effect of Zn2+ as a cofactor was analyzed with the addition of ZnCl2 to produce concentrations ranging from 50 μM to 10 mM (50 μM, 100 μM, 200 μM, 1 mM, 5 mM and 10mM). Addition of Zn2+ had no effect on AaIPPI activity. The 0.1 mM Mn2+ samples yielded the highest rate of IPP isomerization, thus this concentration was chosen to examine the effect of the irreversible inhibitor iodoacetamide. The inhibitor was added to the sample reactions at concentrations between 1 μM and 5 mM. The inhibitor was very effective; 1 μM iodoacetamide caused a relative inhibition of ~ 70%, with complete inhibition at 100 μM (Fig. 4).
Figure 3. Effect of metal cofactors.
Different concentrations of Mg2+ (A) or Mn2+ (B) were added to samples and IPPI activity was analyzed. Activities are expressed as increases relative to the activity of the control without metal cofactor (fold increase). Each data point is the mean ± S.E.M of 2–4 independent replicates.
Figure 4. Iodoacetamide inhibition of AaIPPI.
Percentages of inhibition increased as increasing concentrations of iodoacetamide were added to the sample up to 500 μM. Controls had no inhibitors. Each data point is the mean ± S.E.M of 2 independent replicates.
3.4. Kinetic properties of recombinant AaIPPI
Kinetic constants were measured for purified recombinant AaIPPI. A plot of the initial velocity of DMAPP production versus IPP concentration was hyperbolic (Fig. 5). The values for Km and Vmax were calculated by non-linear regression analysis. A Lineweaver-Burk double reciprocal plot was derived from transformation based upon nonlinear regression analysis (Supplementary Fig. 5). The Km was 276.8 μM and Vmax was 0.6 μmol of substrate (IPP) converted to product (DMAPP) per minute per mg of enzyme. The equilibrium constant, Keq = [IPP]/[DMAPP] = 0.6 was uniform over the logarithmic phase of the enzymatic reaction (Fig. 5).
Figure 5. Saturation kinetics of AaIPPI.
Plot of initial velocity of AaIPPI activity versus IPP concentration. Each data point is the mean ± S.E.M of 3 independent replicates.
3.5. Tissue distribution and developmental expression of AaIPPI mRNA
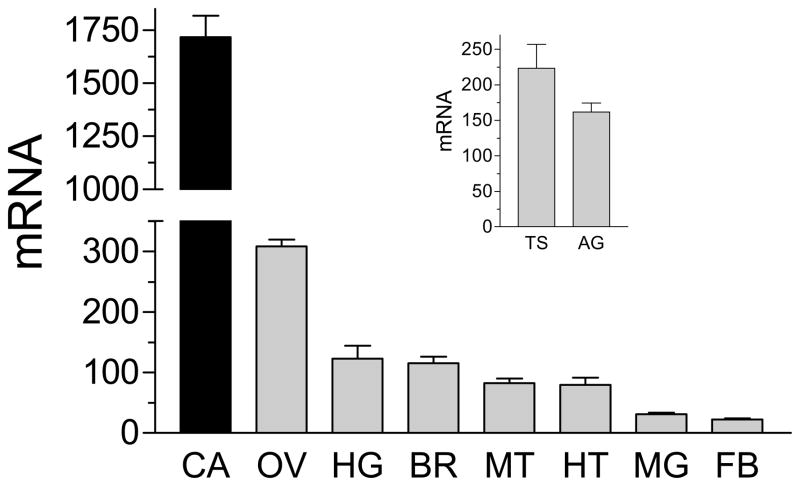
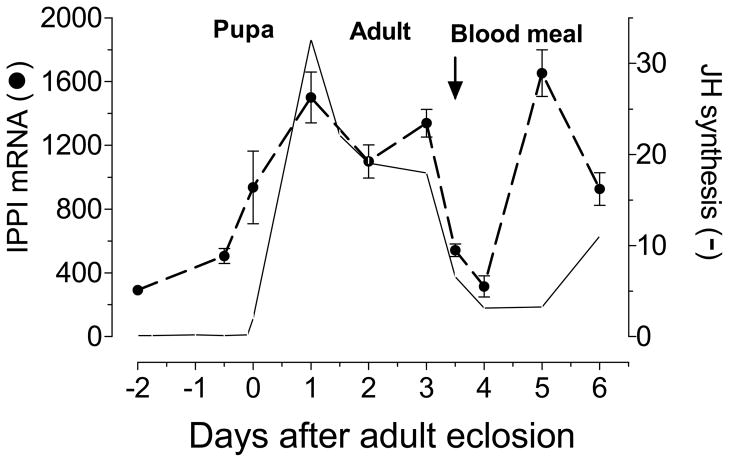
Real time PCR was used to analyze the expression of AaIPPI mRNA in adult female and male tissues. AaIPPI mRNA expression was higher in the female CA-CC. Lower levels of mRNA transcripts were detected in the ovary, hindgut and brain of the female, as well as the testis and accessory glands of the male (Fig. 6). AaIPPI mRNA was also detected in the female heart, midgut, fat body and Malpighian tubules. In addition, the levels of the AaIPPI transcripts were studied in the CA-CC during female pupal and adult development. In the female CA-CC, the AaIPPI mRNA levels were low in the early pupae, started increasing 6 hours before adult eclosion and reached a maximum 24 hours after female emergence. Blood feeding resulted in a decrease in transcript levels. The pattern of changes of AaIPPI mRNA resembled the changes in JH biosynthesis (Fig. 7).
Figure 6. Tissue specific expression of AaIPPI mRNA.
All female tissues were dissected from three-day old sugar-fed females. CA: corpora allata-corpora cardiaca; OV: ovary; HG: hindgut; BR: brain; MT: Malpighian tubules; HT: heart; MG: midgut and FB: fat body. The insert shows the male testis (TS) and accessory glands (AG). AaIPPI mRNAs are expressed as copy number/10,000 copies of rpL32 mRNA. Each RT-PCR data point is mean ± S.E.M of two independent biological replicates of 10 tissue samples. These studies are an expansion of studies published by Nouzova et al. (2011).
Figure 7. Developmental expression of AaIPPI mRNA in the CA.
Expression of AaIPPI mRNA in CA-CC of pupa, sugar-fed and blood-fed adult female. AaIPPI mRNA is expressed as copy number of AaIPPI mRNA/10,000 copies of rpL32 mRNA. Each RT-PCR data point is mean ± S.E.M of two independent biological replicates of 20 CA-CC. JH biosynthesis values are based on Li et al. (2003) and are expressed as fmol/CA/h. These studies are an expansion of studies published by Nouzova et al. (2011).
4. DISCUSSION
4.1. Molecular and functional characterization of an insect IPPI
We have molecularly and functionally characterized an A. aegypti IPPI involved in the synthesis of JH in the CA. An IPPI expressed sequence tag (EST) was obtained from an A. aegypti corpora-allata + corpora cardiaca library, constructed and sequenced as previously described (Noriega et al., 2006). The AaIPPI 244 amino acid sequence is slightly longer than the 182 amino acid long IPPI from E. coli, considered to represent the basic IPPI core necessary for IPP isomerization (Durbecq et al., 2001). The extra 62 amino acid present in A. aegypti IPPI are mostly an N-terminus extension. Analysis of the AaIPPI sequence revealed the presence of a typical Nudix hydrolase superfamily domain. Members of the Nudix family are found in a wide variety of organisms and they are capable of hydrolyzing organic pyrophosphates. Nudix family proteins are characterized by the conserved motif, G-X5-E-X7-R-E-U-X-E-E-X2-U, where X is any residue and U denotes a large hydrophobic side chain (Midvan et al., 2005). The corresponding sequence within the IPPIs contains a similar but changed conserved motif, G-X3-A-X2-R-R/K-U-X2-E-L-G-U (Fig. 2) (Bonano et al., 2001). The Nudix motif forms a loop–á helix–loop structure which functions as a versatile Mg2+-binding and catalytic site (Lin et al., 2008). This motif is part of a shared á/â/á sandwich or Nudix fold containing additional regions which differ, depending on the detailed mechanism and specificity of the enzyme (Midvan et al., 2005; Lin et al., 2008). AaIPPI exhibits a compact globular α/β structure very similar to that described for human IPPI (Zheng et al., 2007) (Supplementary Fig. 6). AaIPPI shows a high degree of similarity with IPPIs from other organisms, especially in two important motifs that have been associated with IPPI type I functionality: a TNACCSHPL motif containing a conserved cysteine residue and a WGEHEIDY motif that contains a conserved glutamate residue (Fig. 2) (Zheng et al., 2007). The antarafacial stereochemistry of the isomerization is consistent with an active site containing two residues located on opposite faces of the allyl moiety in IPP, both residues are indispensable to accomplish the protonation/deprotonation steps during IPP isomerization; in the sequence of AaIPPI these two residues have been identified as C105 and E156, located face-to-face close to the bottom of the active site pocket. W213 and Y168 are two additional active site residues with important roles in catalysis that are located at the bottom of the deeply buried active site cavity. These four residues are highly conserved among the insect IPPIs (Supplementary Fig. 2). E. coli type I IPPI requires two divalent metal atoms for activity. In its native resting form, E. coli type I IPPI seems to be a zinc metalloprotein, but this zinc can be replaced by a Mn2+ (Carrigan and Poulter, 2003). The divalent metal is located in a hexacoordinate His3Glu2 pocket, stabilizes the N-terminus of the enzyme, and is a part of the catalytic machinery for protonating the carbon-carbon double bond in IPP. In addition to zinc and manganese, the enzyme-substrate complex contains an atom of magnesium that facilitates substrate binding through the diphosphate moiety (Lee and Poulter, 2006). Residues previously described as important for metal coordination and interaction with the diphosphate moiety of the IPP are well conserved in AaIPPI (Fig. 2), as well as in other insect IPPIs (Supplementary Fig. 2).
Measuring the enzymatic activity of IPPI is challenging because substrate (IPP) and product (DMAPP) are isomers, excluding the possibility of using spectrophotometric assays. The most common assay for IPPI is a radiometric technique based on the acid lability of DMAPP and 14C radiolabeled precursors (Satterwhite, 1985). Alternatively, IPPI activity has been determined by acidic hydrolysis of DMAPP, which is converted into isoprene, a volatile compound that can be analyzed in the head space of a vial by adsorption in alumina and subsequent desorption in the GC equipment (Lehning et al., 1999; Fisher et al., 2001; Bruggemann and Schnitzler, 2002). We developed a novel method that takes advantage of the differential liability of DMAPP under acidic conditions as compared with IPP, as well as the efficacy of SPME fibers to adsorb the volatile isoprene (Hyspler et al, 2000; Ignea et al., 2011). The method is much simpler and the use of the fiber avoids the necessity of a GC instrument equipped with a headspace auto sampler or similar devices in the injector to get desorption of adsorbed isoprene (Bruggemann and Schnitzler, 2002).
The SPME-GC protocol was employed to assay the effects of three metal cofactors and an irreversible inhibitor on the AaIPPI. For every IPPI type I studied, addition of Mn2+ results in a stronger activation than addition of Mg2+ (Ramos-Valdivia et al., 1997; Zheng et al., 2007). AaIPPI activity was also stimulated by addition of Mn2+ or Mg2+, with Mn2+ having a stronger activation than Mg2+. The maximum activity was detected at 100 μM Mn2+, a value similar to the optimum concentration of manganese for full activity of a partially purified IPPI from Bombyx mori (Koyama et al., 1985). Interestingly, the enzyme’s catalytic activity did not reach a plateau after passing an optimal cofactor concentration threshold, but its activity decreased at higher concentrations of cofactor. This observation may be explained through the formation of a complex between the metal cofactor and the substrate, thus the availability of the substrate is reduced with increasing concentrations of cofactor, as reported in other enzymes requiring metal cofactors (Hallcher and Sherman, 1980). A stimulatory effect of Zn2+ on IPPI has so far only described for E. coli type I (Lee and Poulter, 2006). Our studies showed that Zn2+ is not a required cofactor for mosquito IPPI.
Iodoacetamide has been previously described as a robust inhibitor of IPPIs (Reardon and Abeles, 1986); with the mechanism of inhibition occurring from protonation of the thiol group of the catalytic cysteine residue (Reardon and Abeles, 1986). It was also very effective on AaIPPI, with complete inhibition at 0.1 mM, a concentration in the range previously described for other IPPIs (Ramos-Valdivia, et al., 1997). IPPIs are critical enzymes very well conserved during evolution, with identical structure and mechanism of action; therefore is not surprising that metal requirement and inhibitor activity are also conserved in insect IPPIs. Most reported Km values for IPP isomerases fall in the range of 1–10 μM for a diverse group of organisms that include Claviceps purpurea, Penicillium sp., Narcissus pseudonarcissus, Cinchona robusta, as well as samples from porcine and avian livers (Ramos-Valdivia et al., 1997), however, higher Km values in the range of 22–74 μM have been reported for partially purified IPPI (Reardon and Abeles, 1986; Koyama et al., 1996; Bruggemann and Schnitzler, 2002; Philips et al., 2008). Comparisons of Km between purified and recombinant enzymes are not always straightforward. Discrepancies between the kinetic properties of purified and recombinant IPPI from the same species have been reported (Ramos-Valdivia et al., 1997).
The equilibrium constant of the isomerization of IPP to DMAPP by AaIPPI (Keq = 0.6) suggest that 60% of the isopentenyl pyrophosphate was converted to dimethylallyl pyrophosphate. Keq values ranging from 0.36 to 6.69 have been reported from a variety of taxa (Shah et al., 1965; Lutzow and Beyer, 1988; Bruggemann and Schnitzler, 2002; Jonnalagadda et al., 2012), although comparisons are difficult since they were obtained using different experimental conditions.
4.2. Role of IPPI on JH synthesis in mosquitoes
IPPI is a key enzyme of the mevalonate pathway, a metabolic route that gives origin to a great diversity of compounds in insects, including heme, ubiquinone, dolichol and isopentenyl adenine (Belles et al., 2005). In addition, isoprenoids from the mevalonate pathway are involved in the prenylation of membrane-bound proteins that play an important role in cell signaling; as well as in synthesis of defensive secretions, pheromones and JH (Belles et al., 2005). AaIPPI transcripts were expressed in all adult female mosquito tissues evaluated; suggesting that AaIPPI might be involved in many metabolic pathways. The higher levels of IPPI mRNA were detected in the CA; because transcript levels are expressed as the ratio IPPI/ribosomal proteins, and the CA has very low cell number when compared to other tissues, the remarkable differences in the IPPI/rpl32 ratios between CA and the rest of the tissues should not be misinterpreted as AaIPPI strict specificity of expression in the CA (Nouzova et al., 2011). Transcript levels were also markedly elevated in the reproductive tissues of female and male. IPPI mRNA levels are expressed almost exclusively in the CA of Bombyx mori 4th instar larvae, with relative low levels in other tissues (Kinjoh et al., 2007).
We found a good correlation between AaIPPI mRNA expression in the CA and JH synthesis (Li et al., 2003). AaIPPI mRNA levels were very low in the CA of early pupae and JH synthesis is suppressed during pupae development. In adult females, CA AaIPPI transcript levels and JH synthesis reached maximum values during the first day after eclosion. In addition, changes in AaIPPI transcripts in CA of sugar-fed and blood-fed female mosquito were also in good agreement with the changes in JH synthesis in the CA (Li et al., 2003). Studies on Bombyx mori also showed a good agreement between JH biosynthesis and expression of IPPI in the CA of larvae, pupae and adult female (Kinjoh et al., 2007).
In summary, this is the first functional characterization of an isopentenyl diphosphate isomerase involved in the production of juvenile hormone in the CA of an insect. AaIPPI has the typical structure and functional features of members of the family described in other groups of organisms. Changes in IPPI mRNA levels in the CA in the pupa and adult female mosquito correspond well with the changes in JH synthesis, suggesting that AaIPPI transcript fluctuations are at least partially responsible for the changes of JH biosynthesis observed.
Supplementary Material
We characterized an isopentenyl diphosphate isomerase from the mosquito Aedes aegypti.
The protein catalyzes the isomerization of IPP into DMAPP.
AaIPPI is highly expressed in the corpora allata (CA).
The pattern of changes of AaIPPI mRNA in the CA resembles the changes in JH biosynthesis.
Acknowledgments
The authors thank Mario Perez, Mark Clifton and John Mackemson for comments on the manuscript. This work was supported by NIH grant AI 45545 to FGN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL Workspace: A web-based environment for protein structure homology modeling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Bellés X, Martin D, Piulachs M-D. The mevalonate pathway and the synthesis of juvenile hormone in insects. Ann Rev Entomol. 2005;50:181–190. doi: 10.1146/annurev.ento.50.071803.130356. [DOI] [PubMed] [Google Scholar]
- Benkert P, Biasini M, Schwede T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics. 2011;27:343–350. doi: 10.1093/bioinformatics/btq662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JB, Edo C, Eswar N, Pieper U, Romanwski MJ, Ilyin V, Gerchman SE, Kycia H, Studier FW, Sali A, Burley SK. Structural genomics of enzymes involved in sterol/isoprenoid biosynthesis. Proc Natl Acad Scien USA. 2001;98:12896–12901. doi: 10.1073/pnas.181466998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann N, Schnitzler JP. Relationship of isopentenyl Diphosphate (IDP) isomerase activity to isoprene emission of oak leaves. Tree Physiol. 2002;22:1011–1018. doi: 10.1093/treephys/22.14.1011. [DOI] [PubMed] [Google Scholar]
- Carrigan CN, Poulter CD. Zinc is an essential cofactor for type I isopentenyl diphosphate:dimethylallyl diphosphate isomerase. J Am Chem Soc. 2003;125:9008–9009. doi: 10.1021/ja0350381. [DOI] [PubMed] [Google Scholar]
- Durbecq V, Sainz G, Oudjama Y, Clantin B, Bompard-Gilles C, Tricot C, Caillet J, Stalon V, Droogmans L, Villeret V. Crystal structure of Isopentenyl diphosphate: dimethylallyl diphosphate isomerase. EMBO J. 2001;20:1530–1537. doi: 10.1093/emboj/20.7.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: An approach using bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Rosenstiel TN, Shirk MC, Fall R. Nonradioactive Assay for Cellular Dimethylallyl Diphosphate. Analyt Biochem. 2001;292:272–279. doi: 10.1006/abio.2001.5079. [DOI] [PubMed] [Google Scholar]
- Goodman WG, Granger NA. The juvenile Hormones. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 6. Elsevier; 2005. pp. 55–115. [Google Scholar]
- Hallcher LM, Sherman WR. The Effects of Lithium Ion and Other Agents on the Activity of myo-Inositol-1-phosphatase from Bovine Brain. J Biol Chem. 1980;255:10896–10901. [PubMed] [Google Scholar]
- Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyspler R, Crhova S, Gasparic J, Zadak Z, Cizkova M, Balasova V. Determination of isoprene in human expired breath using solid-phase microextraction and gas chromatography-mass spectrometry. J Chromat. 2000;739:183–190. doi: 10.1016/s0378-4347(99)00423-5. [DOI] [PubMed] [Google Scholar]
- Ignea C, Cvetkovic I, Loupassaki S, Kefalas P, Johnson CB, Kampranis SC, Makris AM. Improving yeast strains using recyclable integration cassettes, for the production of plant terpenoids. Microb Cell Factories. 2011 doi: 10.1186/1475-2859-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnalagadda V, Toth K, Richard JP. Isopentenyl diphosphate isomerase catalyzed reactions in D2O: Product release limits the rate of this sluggish enzyme-catalyzed reaction. J Am Chem Soc. 2012;134:6568–6570. doi: 10.1021/ja302154k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjoh T, Kaneko Y, Itoyama K, Mita K, Hiruma K, Shinoda T. Control of juvenile hormone biosynthesis in Bombyx mori: cloning of the enzymes in the mevalonate pathway and assessment of their developmental expression in the corpora allata. Insect Biochem Molec Biol. 2007;37:808–818. doi: 10.1016/j.ibmb.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Koyama T, Matsubara M, Ogura K. Isoprenoid Enzyme Systems of Silkworm. I. Partial Purification of Isopentenyl Pyrophosphate Isomerase, Farnesyl Pyrophosphate Synthetase, and Geranylgeranyl Pyrophosphate Synthetase. J Biochem. 1985;98:449–456. doi: 10.1093/oxfordjournals.jbchem.a135299. [DOI] [PubMed] [Google Scholar]
- Koyama T, Wititsuwannakul D, Asawatreratanakul K, Wititsuwannakul R, Ohya N, Tanaka Y, Ogura K. Isopentenyl diphosphate isomerase in rubber latex. Phytochemistry. 1996;43:769–772. [Google Scholar]
- Lawson D, Arensburger P, Atkinson P, Besansky NJ, Bruggner RV, Butler R, et al. VectorBase: a data resource for invertebrate vector genomics. Nucleic Acids Res. 2009;37:583–587. doi: 10.1093/nar/gkn857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Poulter CD. Escherichia coli Type I Isopentenyl Diphosphate Isomerase: Structural and Catalytic Roles for Divalent Metals. J Am Chem Soc. 2006;128:11545–11550. doi: 10.1021/ja063073c. [DOI] [PubMed] [Google Scholar]
- Lehning A, Zimmer I, Steinbrecher R, Bruggemann R, Schnitzler JP. Isoprene synthase activity and its relation to isoprene emission in Quercus robur L. leaves. Plat, Cell and Environment. 1999;22:495–504. [Google Scholar]
- Li YP, Hernandez-Martinez S, Unnithan GC, Feyereisen R, Noriega FG. Activity of the corpora allata of adult female Aedes aegypti: effects of mating and feeding. Insect Biochem Mol Biol. 2003;33:1307–1315. doi: 10.1016/j.ibmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Lin J, Tian B, Hua Y. Structural and functional diversity of Nudix fold. Prot Pept Letters. 2008;15:108–112. doi: 10.2174/092986608783330350. [DOI] [PubMed] [Google Scholar]
- Lutzow M, Beyer P. The isopentenyl-diphosphate delta isomerase and its relation to the phytoene synthase complex in daffodil chromoplasts. Biochim Biophys Acta. 1988;959:118–126. [Google Scholar]
- Mildvan AS, Xia Z, Azurmendi HF, Sarswat V, Legler PM, Massiah MA, Gabelli SM, Bianchet MA, Kang L-W, Amzel LM. Structure and mechanisms of Nudix hydrolases. Arch Biochem Biophys. 2005;433:129–143. doi: 10.1016/j.abb.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Mayoral JG, Nouzova M, Yoshiyama M, Shinoda T, Hernandez-Martinez S, Dolghih E, Turjanski AG, Roitberg AR, Priestap H, Perez M, Mackenzie L, Li Y, Noriega FG. Molecular and functional characterization of a juvenile hormone acid methyltransferase expressed in the corpora allata of mosquitoes. Insect Bioch Mol Biol. 2009;39:31–37. doi: 10.1016/j.ibmb.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FG, Wells MA. A comparison of three methods for isolating RNA from mosquitoes. Insect Molec Biol. 1993;2:21–24. doi: 10.1111/j.1365-2583.1993.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Colonna AE, Wells MA. Increase in the size of the amino acid pool is sufficient to activate translation of early trypsin mRNA in Aedes aegypti midgut. Insect Biochem Mol Biol. 1999;29:243–247. doi: 10.1016/s0965-1748(98)00132-5. [DOI] [PubMed] [Google Scholar]
- Noriega F, Ribeiro JMC, Koener JF, Valenzuela JG, Hernandez-Martinez S, Pham VM, Feyereisen R. Comparative genomics of insect juvenile hormone biosynthesis. Insect Biochem Mol Biol. 2006;36:366–374. doi: 10.1016/j.ibmb.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouzova M, Edwards M, Mayoral JG, Noriega FG. A coordinated expression of biosynthetic enzymes controls the flux of juvenile hormone precursors in the corpora allata of mosquitoes. Insect Biochem Molec Biol. 2011;41:660–669. doi: 10.1016/j.ibmb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips MA, D’Auria JC, Gershenzon J, Pichersky E. The Arabidopsis thaliana Type I isopentenyl diphosphate isomerases are targeted to multiple subcellular compartments and have overlapping functions in isoprenoid biosynthesis. The Plant Cell. 2008;20:677–696. doi: 10.1105/tpc.107.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Valdivia AC, van der Heijden R, Verpoorte R. Isopentenyl diphosphate isomerase: a core enzyme in isoprenoid biosynthesis. A review of its biochemistry and function. Natural Product Reviews. 1997;14:591–603. doi: 10.1039/np9971400591. [DOI] [PubMed] [Google Scholar]
- Reardon JE, Abeles RH. Mechanism of action of isopentenyl phosphate isomerase: evidence for a carbonium ion intermediate. Biochem. 1986;25:5609–5616. doi: 10.1021/bi00367a040. [DOI] [PubMed] [Google Scholar]
- Rohmer M. The discovery of a mevalonate-independent pathway for Isoprenoid biosynthesis in bacteria, algae and higher plants. Natural Product Reviews. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- de Ruyck J, Pouyez J, Rothman SC, Poulter D, Wouters J. Crystal structure of a type 2 isopentenyl diphosphate isomerase from Thermus thermophiles in complex with inorganic pyrophosphate. Biochem. 2008;47:9051–9053. doi: 10.1021/bi801159x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchettini JC, Poulter CD. Creating isoprenoid diversity. Science. 1997;277:1788–1789. doi: 10.1126/science.277.5333.1788. [DOI] [PubMed] [Google Scholar]
- Satterwhite DM. Isopentenyldiphosphate delta-isomerase. Method Enzymol. 1985;110:92–99. doi: 10.1016/s0076-6879(85)10064-9. [DOI] [PubMed] [Google Scholar]
- Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah DH, Cleland WW, Porter JW. The partial purification, properties, and mechanism of action of pig liver isopentenyl pyrophosphate isomerase. J Biol Chem. 1965;240:1946–1956. [PubMed] [Google Scholar]
- Spurgeon SL, Porter JW. Biosynthesis of Isoprenoid Compounds. 1981;1:1–46. [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2 MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony. Methods Molec Biol Evol. 2011 doi: 10.1093/molbev/msr121. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Sun F, Bartlam M, Li X, Li R, Rao Z. The crystal structure of human isopentenyl diphosphate isomerase at 1.7 A resolution reveals its catalytic mechanism in isoprenoid biosynthesis. J Mol Biol. 2007;366:1447–1458. doi: 10.1016/j.jmb.2006.12.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.