Summary
P66 is a Borrelia burgdorferi surface protein with β3 integrin binding and channel forming activities. In this study, the role of P66 in mammalian and tick infection was examined. B. burgdorferiΔp66 strains were not infectious in wild-type, TLR2−/− or MyD88−/− deficient mice. Strains with p66 restored to the chromosome restored near wild-type infectivity, while complementation with p66 on a shuttle vector did not restore infectivity. Δp66 mutants are cleared quickly from the site of inoculation, but analyses of cytokine expression and cellular infiltrates at the site of inoculation did not reveal a specific mechanism of clearance. The defect in these mutants cannot be attributed to nutrient limitation or an inability to adapt to the host environment in vivo as Δp66 bacteria were able to survive as well as wild-type in dialysis membrane chambers in the rat peritoneum. Δp66 bacteria were able to survive in ticks through the larva to nymph molt, but were non-infectious in mice when delivered by tick bite. Independent lines of evidence do not support any increased susceptibility of the Δp66 strains to factors in mammalian blood. This study is the first to define a B. burgdorferi adhesin as essential for mammalian, but not tick infection.
Introduction
Lyme disease is the most common arthropod-borne disease in the United States and is caused by Borrelia burgdorferi, a spirochete bacterium. This bacterium exists in a zoonotic cycle between ticks of the Ixodes genus and small mammals or birds. Successful infection of an immunocompetent host requires appropriate expression of an elaborate display of molecules, including some that function as adhesins in vitro, some that interact with the host immune system, and some with as yet unidentified functions (Radolf et al., 2012). Several plasmid-encoded proteins have been demonstrated to be required for in vivo infectivity in mammals, including PncA and OspC (Purser et al., 2003, Grimm et al., 2004), and other proteins have been identified as being involved in specific tissue tropism, e.g. DbpA, DbpB (Weening et al., 2008), BmpA and BmpB (Pal et al., 2008). A seroreactive band used for diagnosis of Lyme disease (Dressler et al., 1993) corresponds to the outer membrane protein, P66, encoded on the linear chromosome by gene bb0603 (Bunikis et al., 1995, Probert et al., 1995). P66 has been shown to mediate the interaction between B. burgdorferi and β3 chain integrins, which are found on a variety of activated immune cells, platelets, and endothelial cells (Coburn et al., 1999, Coburn and Cugini, 2003, Defoe and Coburn, 2001). The manipulation of host integrins and downstream signaling by bacterial proteins has been documented in several species, including Yersinia pseudotuberculosis. Y. pseudotuberculosis encodes an integrin ligand, invasin, which binds with high affinity to β1 integrins to initiate bacterial uptake (Alrutz et al., 2001, Dersch and Isberg, 1999, Hamburger et al., 1999, Isberg and Van Nhieu, 1995, Leong et al., 1990, Rankin et al., 1992, Saltman et al., 1996, Young et al., 1992). Previous work has shown that P66 is necessary and sufficient for non-infectious B. burgdorferi to bind to β3 chain integrins and that the host cell responses to the bacteria are affected by P66 (Coburn and Cugini, 2003, LaFrance et al., 2011). In addition to integrin binding activity, P66 is also a channel forming protein/porin (Barcena-Uribarri et al., 2010, Pinne et al., 2007, Skare et al., 1997). The next step in evaluating the importance of P66 as an integrin ligand and a porin became possible with advances in the ability to manipulate the B. burgdorferi genome in infectious strain backgrounds. The goal of this work was to examine the relevance of P66 to the tick-mouse infectious cycle of B. burgdorferi.
Results
P66 is critical to B. burgdorferi infection in mice
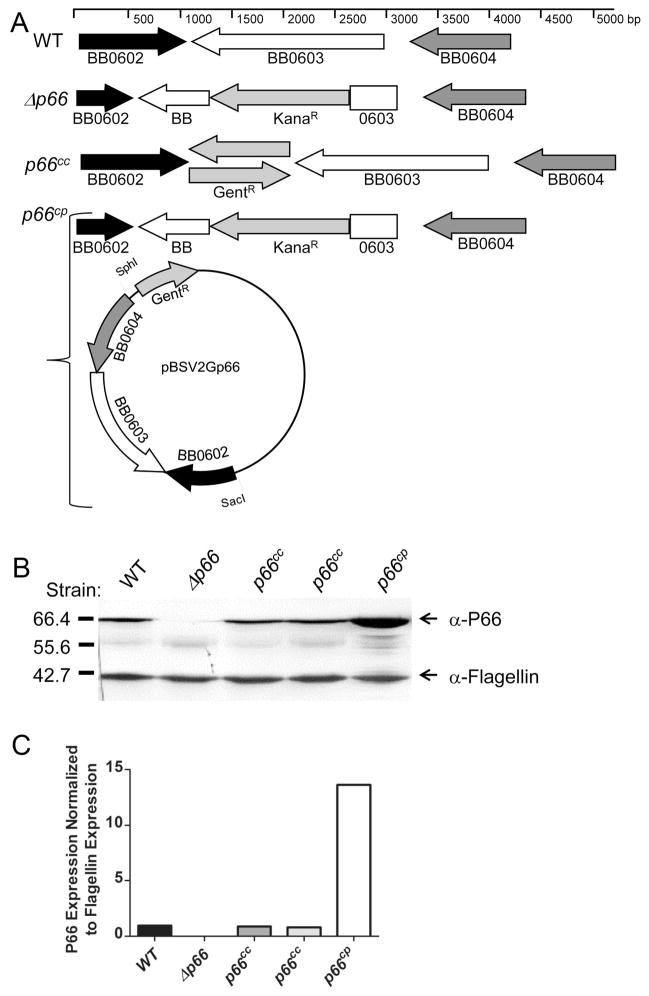
To examine the effects of a loss of P66 expression in vivo, three separateΔp66 clones were generated in the infectious strain background B31-A3 (wild type) (Elias et al., 2002), in independent transformations, with the resulting locus depicted in Figure 1a. All threeΔp66 clones grew to similar densities and with similar kinetics in laboratory culture as the parental strain at 23°C, 33°C, and 37°C and have no discernible changes in protein expression (with the exception of P66, Figure 1b) in vitro (data not shown). Clone 2.3 is missing plasmids lp21 and cp32-3 (neither is critical for murine infection [Purser and Norris, 2000]) relative to the parental strain, so a plasmid-matched control strain intact at the p66 locus was also isolated and tested and is designated B31-MC. The two other Δp66 clones have all plasmids present in the parental strain, and are identical in phenotype in all experiments performed.
Figure 1. Genotypes and phenotypes of p66 strains used in this study.
(A) Schematic representation of p66 loci of wild-type (WT), mutant (Δp66), and complemented (p66cc and p66cp) B. burgdorferi. The scale indicates the size and spacing of the genes in base pair units. GentR refers to the gentamicin resistance cassette, aacC1 from Tn1696, driven by the flgB promoter (Elias et al., 2003). KanaR refers to the kanamycin resistance cassette, aphI from Tn903, driven by the flaB promoter (Bono et al., 2000). (B) Western blot of whole-cell lysates from in vitro grown B. burgdorferi of indicated strains. Two clones of p66cc are shown to represent a clone with each orientation of the gentamicin resistance cassette with respect to p66. Lysates were run on an SDS-PAGE gel and transferred to an Immobilon membrane that was probed with antibodies detecting P66 and flagellin (as a loading control). Colorimetric methods were used for visualization. The values on the left represent molecular size in kilodaltons. (C) Quantification by densitometry of a parallel blot of that shown in panel B with chemiluminescence used for detection. Panels B and C represent one of three independent clones with similar phenotypes. P66 expression was normalized to flagellin expression.
The phenotype of Δp66 strains was examined by performing ID50 determinations. To prepare bacteria for inoculation, bacterial cells were suspended in PBS + 0.2% normal mouse serum, a method that was used to avoid any host response to components of the protein-rich B. burgdorferi culture medium, and injected intradermally or subcutaneously into mice. Tissues were harvested at 2 or 4 weeks post inoculation; length of time or inoculation route did not affect the results regarding the abilities of the strains tested to cause infection. All ID50 determinations were based on the culture results and generated as previously described (Reed and Muench, 1938). As shown in Table 1, the Δp66 strains were significantly attenuated in murine infection with ID50s greater than 108 bacteria. In all of the mice injected with the Δp66 strains (a total of 270 mice), only two ever yielded positive cultures. These positive cultures were from the site of inoculation and no bacteria were recovered at disseminated sites. In contrast, the wild-type strain had ID50s of 2.4 × 101 to 6.8 × 103 bacteria per mouse. This variation is likely due to the differential loss of B. burgdorferi genome segments in different populations used in different experiments, or slight differences in growth phase of the individual cultures, but the attenuation of the Δp66 strains is at minimum four logs.
Table 1.
ID50 determinations of B. burgdorferi strains
| B. burgdorferi strain | Doses (# motile bacteria per mouse) | Number of mice per group for each dose | ID50, 4 weeks post-inoculationa |
|---|---|---|---|
| B31-A3 (wild-type) | 101–104 | 25 | 2.4 × 101 – 6.8 × 103b |
| B31-A3 MCc | 101–104 | 15 | 1.8 × 103–4.8 × 103 b |
| Δp66 clone 2.3 | 104–108 | 10 | >108 |
| 2.3cc Clone 2d | 101–109 | 5 | 104 |
| 2.3cc Clone 49d | 101–109 | 5 | 104 |
| Δp66 clone C3-14 | 104–109 | 20 | >109 |
| C3-14cc Clone 5d | 101–109 | 10 | 1 × 104–1.8 × 104b |
| C3-14cc Clone 23d | 101–109 | 15 | 1.8 × 104– 4.6 × 104b |
| C3-14cp Clone 13 | 101–109 | 5 | >109 |
| C3-14cp Clone 25 | 101–109 | 5 | >109 |
| C3-14cp Clone 34 | 101–109 | 5 | >109 |
| Δp66 clone C6-6 | 101–109 | 10 | >109 |
| C6-6cc Clone 11d | 101–109 | 5 | 1 × 104 |
| C6-6cc Clone 46d | 101–109 | 5 | 1.8 × 104 |
The ID50 values reported here are based on culture of tissues, and were calculated as previously described (Reed and Muench, 1938).
When ranges are provided, different individual experiments yielded different ID50 values.
MC=matched control for Δp66 clone 2.3 and derivatives, B31-A3 strain missing plasmids lp21 and cp32-3
p66cc clones have different p66+gentR orientations specified in Table S1
Although three independent Δp66 clones were all significantly attenuated in mice, and p66 is monocistronic (Bunikis et al., 1995, Casjens et al., 2000, Fraser et al., 1997, Medrano et al., 2010), to ensure that the defect was the result of disruption of the p66 locus, we complemented the mutants by restoring p66 using two different approaches (Figure 1a). In the first approach, p66 and a gentamicin resistance cassette (gentR) were restored to the endogenous locus on the chromosome (complemented on chromosome, p66cc strains). These p66cc strains, with p66 and the gentR cassette in the same or opposite direction relative to each other yielded the same results in all three Δp66 strain backgrounds. Restoration of p66 to the chromosome resulted in P66 expression levels similar to wild-type (Figure 1b, c) and ID50s within one log of that of the wild-type strain within the same experiment (Table 1).
The second complementation strategy was to introduce p66 on the shuttle vector pBSV2G (complemented on plasmid, p66cp strains), which can be used to complement mutants in B. burgdorferi (Elias et al., 2003, Tilly et al., 2007, Tilly et al., 2006). In these constructs, p66 is under the control of its endogenous promoter, but P66 protein levels are approximately 13-fold higher than that of the wild-type strain (Figure 1b, c). This overexpression may be due to the expression of P66 from multiple copies of the shuttle vector present relative to the chromosome, or possibly due to disruption of an as yet unknown regulatory mechanism. Interestingly, as assessed with three independent clones using this complementation strategy, wild-type infectivity is not restored, and these strains have an ID50 comparable to the knock-out strain, greater than 109 spirochetes (Table 1). Loss of the shuttle vector expressing P66 would be hypothesized to result in a loss in virulence, as seen with Δp66 strains; however, the population of bacteria retaining the shuttle vector after inoculation could not be assessed due to the fact that no bacteria were recovered at four weeks post-inoculation. Further investigation is required to elucidate the cause of this deficiency, but in additional (unpublished) experiments, a plasmid carrying the same origin of replication is stable when the bacteria are in mice for at least 7 days, and the parental shuttle vector, pBSV2, has been demonstrated to be stable in the absence of selective pressure in vitro for at least 25 generations (Stewart et al., 2001). If the defect is related to overexpression of P66, current hypotheses include that overexpression of the surface exposed protein is causing an envelope stress response, or that the ability of the bacteria to successfully evade the mammalian immune system has been compromised. Either of these hypotheses may also account for the infectivity defect in the Δp66 strains.
Δp66 mutants are rapidly cleared from the site of inoculation
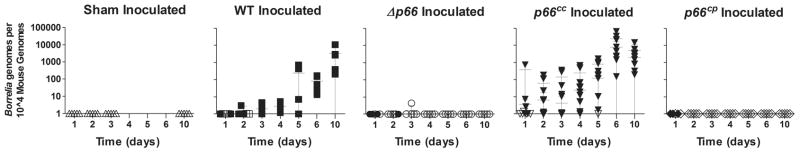
To further investigate the deficiency of Δp66 in murine infection, we analyzed the survival of mutant bacteria in mice throughout a short time course. Mice were inoculated in parallel with wild-type, Δp66, p66cc or p66cp at 105 bacteria per mouse and harvested at various time points, including 6 hours, 1, 2, 3, 4, 5, 6, 7, 10 and 14 days. The site of inoculation was harvested and split into 3 pieces for separate analyses: infection status by culture, qPCR for bacterial load, and assessment of cytokine expression. One piece was placed in culture medium, and infection results in Table 2 were based on culture results. These results indicate that Δp66 strains are cleared from the site of inoculation within two days, before the adaptive immune response can be activated. The serologic response to wild-type or Δp66 strains at 14 days post-inoculation was examined by western blot against whole B. burgdorferi lysates, using individual mouse sera as the primary antibody (Figure S1). These results confirm that there is no specific antibody response mounted against theΔp66 strains. A second piece of the site of inoculation was used in qPCR analyses to confirm culture results, as we considered the possibility that Δp66 mutants may be defective in transition from mouse tissue to laboratory culture. qPCR was performed on the site of inoculation for all experiments in this study, and these results corroborate the culture results, confirming thatΔp66 strains are cleared within 48 hours from mice (Figure 2 and data not shown).
Table 2.
Short-term B. burgdorferi infections
| B. burgdorferi strain | Bacterial dose | Day post injection Positive culturesa/mice injected | Sero-positiveb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 10 | 14 | |||
| WT | 105 | 6/10 | 7/10 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 skin 5/5 bladder | 5/5 |
| Δp66 C3-14 | 105 | 4/10 | 3/10 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 skin 0/5 bladder | 0/5 |
| C3-14cc clone 5 | 105 | 3/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | ND | 5/5 | ND | ND |
| C3-14cc clone 23 | 105 | 2/5 | 4/5 | 4/5 | 5/5 | 4/5 | 5/5 | ND | 5/5 | ND | ND |
| C3-14cp clone 13 | 105 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | ND | 0/5 | ND | ND |
Cultures assessed from skin at inoculation site unless otherwise noted.
Seropositivity based on IgM or IgG reactivity of individual mouse sera from day 14 to whole B. burgdorferi lysate (Figure S1)
ND = Not determined
Figure 2. Bacterial loads at the site of inoculation.
The site of inoculation from C3H/HeN mice inoculated with wild-type, Δp66, p66cc (clones representing both orientations of the gentamicin resistance cassette with respect to p66) or p66cp bacteria at a dose of 105 spirochetes injected subcutaneously was collected and analyzed for bacterial load by qPCR. Tissues were collected from mice at days 1, 2, 3, 4, 5, 6 and 10 post-inoculation. Sham inoculated mice were used as a control at days 1, 2, 3 and 10. Results from qPCR are represented as B. burgdorferi genomes per 104 mouse genomes, with the limit of detection being 6 B. burgdorferi genomes. Symbols represent the infection status for each mouse based on culture results: filled symbol = murine infection detected by culture, unfilled symbol = murine infection not detected by culture. Mean bacterial load is indicated with error bars representing standard deviation.
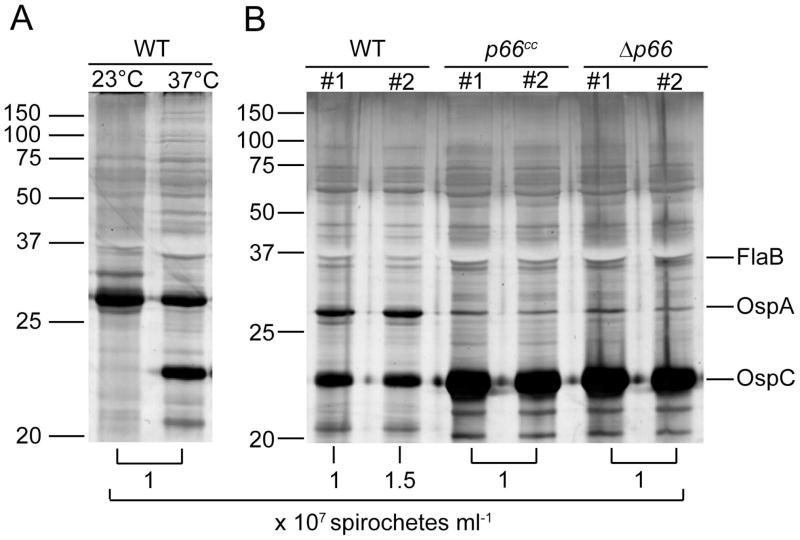
Rapid clearance of Δp66 strains in vivo may be due to a nutritional deficiency if the porin activity of P66 is critical. This defect may not be apparent in the rich growth medium in vitro. Alternatively, the innate immune system, rather than nutrient sequestration may be responsible for elimination of the Δp66 strains. To test the ability of the bacteria to survive in vivo in the absence of effectors of the immune system, the bacteria were introduced to the mammalian milieu in the protected environment of a dialysis membrane chamber, as previously described (Akins et al., 1998). After fourteen days in the peritoneum of rats, the chambers were removed and both protein expression and survival of the bacteria was assessed. Upregulation of ospC and downregulation of ospA have previously been demonstrated to indicate successful adaptation to the host environment (Akins et al., 1998, de Silva and Fikrig, 1997, de Silva et al., 1996, Montgomery et al., 1996, Schwan et al., 1995). In vitro cultivated wild-type bacteria are shown as controls for assessment of the shift in protein expression (Figure 3a). Δp66 and p66cc bacteria adapted to the host environment, as assessed by the comparison of OspC to OspA production (Figure 3b). The Δp66 and p66cc strains both replicated to concentrations equal to that of the wild-type bacteria. While the wild-type bacteria did not produce OspC to the levels seen in the two other strains, there is a noticeable shift compared to in vitro grown bacteria at room temperature. The results were consistent in two independent experiments and demonstrate that the Δp66 strains survive in a mammalian host in this protected environment.
Figure 3. B. burgdorferi survival in dialysis membrane chambers.
3x103 B. burgdorferi of each strain, wild-type, p66cc and Δp66, were inoculated in duplicate into DMCs that were implanted in the peritoneum of rats. Organisms were harvested after 14 days and counted by dark-field microscopy. In vitro cultivated wild-type bacteria grown at 23°C or 37°C were used as controls. Whole cell lysates were run on 12.5% separating polyacrylamide gels. Silver stain was used for visualization. (A) In vitro grown wild-type bacteria. (B) DMC cultivated bacteria. Molecular weight markers are indicated on the left in kilodaltons. Proteins whose regulation is known are indicated on the right; FlaB is consistently expressed, OspA is downregulated upon adaptation to the mammalian environment, and OspC is upregulated. Concentration of spirochetes recovered from each chamber is indicated below the respective lane.
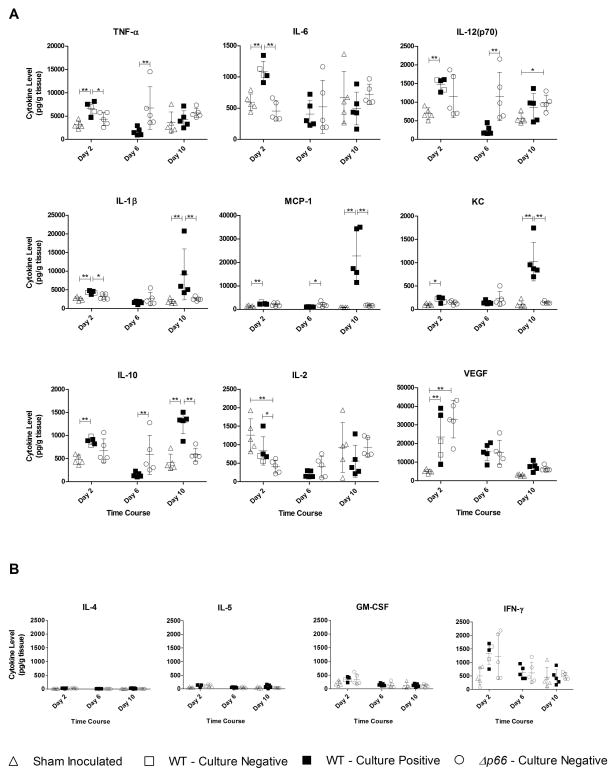
Survival of Δp66 strains within DMCs, combined with the quick clearance of the Δp66 strains outside of the protected environment, led to the hypothesis that innate immune system effectors were responsible for clearance of the mutant bacteria. To begin to investigate the mechanism of clearance of the Δp66 strains, the third piece of the site of inoculation from the short time course was used to examine cytokine expression levels at the site of inoculation. The skin piece was homogenized in a buffer with protease inhibitors, and the lysate was analyzed in a multiplex cytokine assay. Results of cytokine analyses are presented in Figure 4, with cytokine levels in picograms per milliliter normalized to individual tissue weights, resulting in pg gram tissue−1. Pro-inflammatory cytokines analyzed, including TNF-α, IL-6, and IL-12, were increased soon after inoculation, by day 2. IL-1β, another pro-inflammatory cytokine had a different profile, as it correlated to bacterial numbers (see Figure 2), similar to chemokines responsible for recruiting monocytes and neutrophils, MCP-1 and KC, respectively, in support of previously published results (Antonara et al., 2010). The levels of anti-inflammatory cytokine IL-10 also tended to increase with bacterial numbers. Vascular endothelial growth factor (VEGF) does not correlate with bacterial load, as VEGF levels are maximal on day 2 in Δp66 inoculated mice despite the bacterial load being below the limit of detection. Significantly higher levels of VEGF in wild-type inoculated mice compared to sham inoculated controls at early time points has been previously reported (Antonara et al., 2010), and Δp66 inoculated mice followed that trend. The level of IL-2, a T-cell stimulant was lower in mice inoculated with either strain of bacteria compared to the sham inoculated mice, as previously reported for inoculations with wild-type bacteria (Zeidner et al., 1997). Several cytokines analyzed showed no difference between infection conditions at any time point, including IL-4, IL-5, GM-CSF and IFN-γ (Figure 4b). For all cytokines with significantly different expression levels, the cytokine levels in all mice inoculated with Δp66 bacteria remained similar throughout the time course, indicating that the mice did not initiate a significant cytokine-driven response to clear the mutant bacteria. Consistent with this, histologic analyses of the site of inoculation at 4 weeks did not show any statistically significant differences in cell infiltrate between mice inoculated with wild-type, Δp66 or p66cc strains, except a significant increase in mast cells in Δp66 inoculated compared to sham inoculated mice (data not shown).
Figure 4. Cytokine expression during short-term infection.
The site of inoculation from C3H/HeN mice inoculated with wild-type or Δp66 bacteria at a dose of 105 spirochetes injected subcutaneously was collected and analyzed for cytokine expression. Results were collected from days 1, 2, 3, 4, 5, 6, and 10, with days 2, 6, and 10 shown. Sham inoculated mice were used as controls at days 1, 2, 3 and 10. (A) Results from cytokine analyses with significant differences in expression between the infection conditions, including pro-inflammatory cytokines TNF-α, IL-6, IL-12(p70), and IL-1β, anti-inflammatory cytokine IL-10, and chemokines MCP-1 and KC. Results from vascular endothelial growth factor (VEGF) and IL-2, a T-cell stimulant are also shown. (B) Analyses of IL-4, IL-5, IFN-γ and GM-CSF did not show significant differences between infection conditions throughout the time course. Cytokine expression is represented as picograms per milliliter, normalized to the weight of the individual tissue (pg gram tissue−1). Symbols represent the infection status for each mouse based on culture results: filled symbol = murine infection detected by culture, unfilled symbol = murine infection not detected by culture. Infection as assessed by culture was not detected in any sham inoculated mice or in Δp66 inoculated mice at the time points shown. The mean cytokine expression is indicated with error bars representing standard deviation. Statistics were performed using a one way ANOVA Kruskal-Wallis test with Dunn’s multiple comparison post-test *= P <0.05, **=P<0.001, n=5.
Δp66 mutants are not infectious in TLR2−/− or MyD88−/− mice
Several innate immune signaling pathways are known to be activated in response to wild-type B. burgdorferi infection. Among these, TLR2 is activated in response to the numerous lipoproteins that the bacteria express, and activation of this pathway controls spirochete burden (Brightbill et al., 1999, Hirschfeld et al., 1999, Lien et al., 1999, Liu et al., 2004, Weis, 2002, Wooten et al., 2002). MyD88 is a downstream adaptor signaling protein for many of the TLR signaling pathways (Kawai et al., 1999, Schnare et al., 2001). To test the hypothesis that these components of the innate immune response might be involved in clearance of the Δp66 bacteria, mutant mice were utilized. TLR2−/− and MyD88−/− mice were inoculated with wild-type orΔp66 bacteria, and tissues were collected two weeks post-inoculation to assess virulence of each strain. Infection status was assessed by culture and qPCR. The results, summarized in Table 3, show that the absence of TLR2 or MyD88 did not rescue infectivity of the mutant bacteria.
Table 3.
B. burgdorferi infections in mutant mice
| Mouse Strain | B. burgdorferi strain | Dose (# motile bacteria per mouse) | Positive cultures/Mice Injected |
|---|---|---|---|
| C57Bl/6J | WT | 105 | 5/5 |
| Δp66 C3-14 | 105 | 0/5 | |
| TLR2−/− | WT | 105 | 5/5 |
| Δp66 C3-14 | 105 | 0/5 | |
| MyD88−/− | WT | 105 | 5/5 |
| Δp66 C3-14 | 105 | 0/5 |
Δp66 mutants do not show an enhanced susceptibility to serum
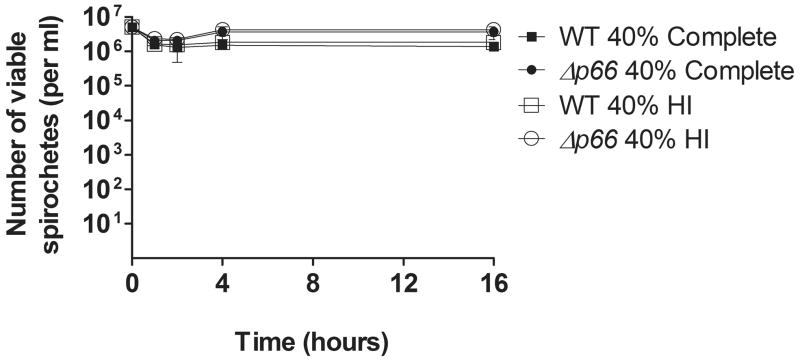
B. burgdorferi is known to encode several proteins that aid in evasion of the innate immune complement pathway, including complement regulator acquiring surface proteins (CRASPs) (reviewed in Kraiczy et al., 2002) and some, but not all, of the OspEF-related proteins (Erps) (reviewed in Brissette et al., 2008). The presence of many of these proteins has been shown to impart the serum resistance seen in many B. burgdorferi strains in vitro, including B31, the background of our strains (reviewed in Kraiczy et al., 2001). To assess the role of complement mediated killing, and the potential of P66 playing a role in complement evasion, wild-type or Δp66 bacteria were subjected to a serum susceptibility test as previously described (Alitalo et al., 2001, Brooks et al., 2005). The serum-resistant phenotype of the wild-type bacteria was maintained in the Δp66 strain, as no differences were seen in survival between wild-type andΔp66 bacteria in this assay (Figure 5).
Figure 5. Serum sensitivity of wild-type or Δp66 B. burgdorferi.
5 × 105 bacteria of the indicated strains were incubated with 40% complete or 40% heat-inactivated (HI) mouse serum for 16 hours in triplicate. Aliquots from each condition were plated in solid BSK II at 1, 2, 4 and 16 hours, and colonies were counted 2 weeks post-plating. The mean concentration of viable bacteria per ml is indicated with error bars representing standard deviation. n=3.
P66 is not critical for B. burgdorferi infection of ticks
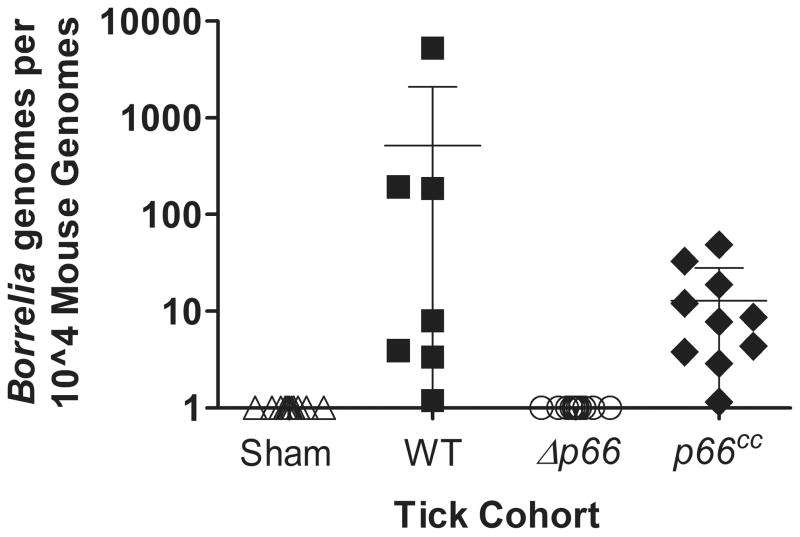
During the infectious cycle that B. burgdorferi maintains between ticks and mammals in nature, there is a major shift in protein expression in the transition from one host to another to allow the bacteria to survive in the different environments. P66 has been shown to be upregulated at the mRNA and protein levels as the bacteria are transmitted into the mammalian host (Cugini et al., 2003, Medrano et al., 2010), which suggests that P66 is important in the mammalian environment and not in the ability of the bacteria to survive within the tick. To confirm the implications of p66 gene regulation, as well as to determine whether the needle inoculation route was responsible for the loss of infectivity by not allowingΔp66 bacteria sufficient time to adapt and thrive in the new environment, Ixodes scapularis larvae were inoculated by immersion feeding with wild-type, Δp66 and p66cc strains to examine the ability of the strains to be maintained within, and be transmitted by, the ticks. After the immersion fed larval ticks were allowed to feed to repletion on mice, and then molt, 10 nymphs were fed on an individual mouse, with at least 5 mice utilized per strain. Ticks were collected after having fed to repletion, generally within six days post-placement. Not all ticks were recovered and subsequently assessed for infection status as not all replete ticks survived encounters with the mice after drop off. Recovered ticks were surface sterilized and homogenized individually in culture medium and monitored for the presence or absence of spirochetes for 8 weeks. Two weeks after drop off of the last tick, mice were sacrificed and tissues collected to analyze by culture and qPCR, with infectivity results for both ticks and mice in Table 4. All mice infested with ticks inoculated with wild-type or p66cc bacteria were found to be infected by culture, which was confirmed by qPCR (Figure 6), and the respective bacterial strains were isolated from recovered replete ticks. The infection status of each mouse is one indicator of the infection status of the ticks that is independent of the infection status of the individual collected replete ticks. This is illustrated in Table 4 by one group of wild-type B. burgdorferi infected ticks having a 0% infection status, yet the mouse on which these ticks had fed being infected, a result that is most likely due to the fact that not all ticks in this cohort were recovered and assessed for infection. Although Δp66 B. burgdorferi was recovered from ticks post-feeding, none of the mice on which they had fed were found to be infected. These results corroborate serum susceptibility results (see Figure 5), and strongly suggest that that the factor(s) responsible for killing Δp66 strains is/are not present in the blood.
Table 4.
B. burgdorferi infection via nymphal tick bite
| B. burgdorferi strain | % ticks infected (for each mouse in group)a | Positive cultures/Infested mice |
|---|---|---|
| WT (7 mice) | 50, 43, 43, 0, 25, 33, 29 | 7/7 |
| Δp66 C3-14 (11 mice) | 100, 40, 60, 67, 63, 86, 80, 100, 100, 100, 80 | 0/11 |
| C3-14cc (2 clones, 5 mice each) | 100, 100, 100, 100, 100, 100, 100, 100, 100, 100 | 10/10 |
Ten ticks were placed on each mouse, and all recovered ticks were cultured to determine the percentage of the cohort that was infected. No mice infested with sham-inoculated ticks were infected (data not shown).
Figure 6. Bacterial load in skin at the site of feeding by nymphal ticks.
Individual C3H/HeN mice were infested with 10 nymphal ticks infected with WT, Δp66 or Δp66cc. Sham inoculated ticks were used as a control. 2 weeks post drop off of the ticks, mice were euthanized and the site of tick feeding was harvested and analyzed by qPCR for bacterial load. Results from qPCR are represented as B. burgdorferi genomes per 104 mouse genomes, with the limit of detection being 6 B. burgdorferi genomes. Symbols represent the infection status for each mouse based on culture results: filled symbol = murine infection detected by culture, unfilled symbol = murine infection not detected by culture. Mean bacterial load is indicated with error bars representing standard deviation.
Discussion
The role of P66 in the life of B. burgdorferi was demonstrated here to be during mammalian infection and not in maintenance in ticks through two blood meals and the molt in between. Previous expression studies support the requirement of P66 in the mammalian host, as P66 is not produced in unfed ticks, and is increased in response to tick feeding when the bacteria are preparing to enter the mammalian host (Cugini et al., 2003). In many respects, Δp66 strains are very similar to ΔospC strains, including that both strains are cleared quickly from the site of inoculation in wild-type mice and the infectivity defect is not restored in mice deficient in MyD88 or TLR2, but both mutants can be maintained in the tick vector (Grimm et al., 2004, Pal et al., 2004, Stewart et al., 2006). We have not ruled out the possibility that the expression or function of these proteins may be linked, resulting in the overlapping phenotypes, although they do not share regulatory mechanisms as p66 is not part of the RpoS regulon (Bunikis et al., 1995, Caimano et al., 2007, Fisher et al., 2005, Ouyang et al., 2008). Temporal requirements for OspC were identified using an OspC deficient strain complemented with a shuttle vector expressing a wild-type OspC, and assessment of the retention of this plasmid in vivo (Tilly et al., 2006). These experiments demonstrated that OspC is required for establishment, but not maintenance of, infection. P66 strains complemented on the same shuttle vector are not infectious in mice, which prevents analysis of the role of P66 in this manner, but the increasing IgG reactivity of human sera to P66 from early to late Lyme disease, and the increasing repertoire of epitopes recognized, strongly suggest that P66 is expressed throughout mammalian infection (Dressler et al., 1993, Ntchobo et al., 2001). Despite the overexpression of P66 in p66cp strains, localization of P66 and OspC on the bacterial surface is not affected (unpublished data). The mechanism underlying the defect in these strains is under investigation, as we have not ruled out the possibility that it is due to aberrant expression of bb0604 or a truncated version of bb0602 also encoded on the shuttle vector. We speculate that overexpression of P66 may indicate there is a delicate balance in expression of P66 required for survival of the bacteria, resulting in a virulence defect similar to that of the Δp66 strains. This is not the first example of successful complementation in cis and not in trans, as this has been described for complementation of flaB (Sartakova et al., 2011). Although the mechanism underlying the deficiency in trans complementation is unknown for both p66 and flaB, it is an important consideration in genetic studies of B. burgdorferi.
Functions of P66 have been identified solely in vitro to this point, and include the protein functioning as a β3 chain integrin ligand (Coburn et al., 1999, Defoe and Coburn, 2001, Coburn and Cugini, 2003), as well as a channel-forming porin on the bacterial surface of B. burgdorferi (Barcena-Uribarri et al., 2010, Pinne et al., 2007, Skare et al., 1997). Of these two functions, the dialysis membrane chamber data reported here suggests that the porin function is not the critical role of P66 necessary for infectivity. It appears likely that factors in the mammalian environment that the mutant bacteria are not exposed to within the dialysis membrane chamber affect their ability to survive when introduced via either needle inoculation or tick bite. This sort of factor could include activities unique to the site of inoculation, including anti-microbial peptides within the skin. Additional host factors, such as cells of the innate defense system that cannot enter the dialysis membrane chamber, may be critical to the observed rapid elimination of the Δp66 strains. The adaptive immune system does not seem to be involved in clearing the bacteria, as the time frame to clearance is so short, and there is no antibody response to B. burgdorferi lysates from sera of mice inoculated with Δp66 strains. We propose that the integrin-binding function of P66 is necessary for escape from the site of inoculation and dissemination throughout the mammalian host. If the bacteria are unable to escape the site of inoculation, they may be caught, and perhaps less able to block phagocytosis, by phagocytic cells. Alternatively, we speculate that binding to β3 integrins may normally be a transient interaction required for moving into and out of the vasculature to cause disseminated infection. The strain complemented on the plasmid, which overproduces P66, may bind with higher affinity or avidity to host cells and limit the motility of the bacteria and their ability to escape the site of inoculation and phagocytic cells.
This work is the first demonstration that an in vitro defined B. burgdorferi adhesin is necessary for mammalian infection. Previous work with B. burgdorferi adhesins has shown functionality of the proteins, i.e. BBK32 to bind fibronectin (Probert and Johnson, 1998) and DbpA and DbpB to bind decorin, but the absence of these proteins does not abrogate infectivity of the bacteria in the context of high doses (Guo et al., 1995, Guo et al., 1998, Li et al., 2006, Seshu et al., 2006, Weening et al., 2008) as we have shown with Δp66 strains. The function of P66 appears to be essential very early in infection, but roles throughout dissemination and maintenance of infection cannot be eliminated at this point. Further understanding of the role of P66 may be revealed by defining the cell type responsible for clearing the bacteria and the location where the bacteria are being cleared. Exploration of the integrin binding function of P66 in vivo will lead to a better understanding of the importance of the adhesin activity of P66 in the mammalian host.
Experimental Procedures
Mice and ticks
C3H/HeN mice were purchased at the age of 3 weeks from Charles River Laboratories (Wilmington, MA). C57Bl/6J (Vendor Stock #000664), TLR2−/− (Vendor Stock # 004650) and MyD88−/− (Vendor Stock #009088) mice were purchased at the age of 3 weeks from Jackson Laboratories (Bar Harbor, ME). All were fed and watered ad libitum throughout the experiments. Ixodes scapularis ticks were purchased as egg masses from the Tick Rearing Facility at Oklahoma State University (Stillwater, OK), and all ticks were housed at ambient temperature over saturated KNO3. All animal work was approved by the IACUCs at the Medical College of Wisconsin and the University of Connecticut.
Bacterial strains and growth conditions
E. coli strains JM109, BLR and Top10 were used in standard cloning procedures and cultured in L broth (Maniatis et al., 1982). Borrelia burgdorferi strain B31-A3 (Elias et al., 2002) was grown in BSK II medium (Barbour-Stoenner-Kelly II [Barbour et al., 1983]) and used for generation of Δp66 mutants. For plating, the media were supplemented with 15 g L−1 agar (for E. coli) or 6.8 g L−1 agarose (for B. burgdorferi) (Samuels, 1995). Media were supplemented with selective antibiotics as appropriate. Antibiotic concentrations used for selection in E. coli were ampicillin to 100 μg ml−1, gentamicin to 5 μg ml−1, kanamycin to 50 μg ml−1, and spectinomycin to 50 μg ml−1. Antibiotic concentrations used for selection in B. burgdorferi were gentamicin to 40 μg ml−1, kanamycin to 200 μg ml−1, and streptomycin to 80 μg ml−1. For culture of B. burgdorferi from ticks and mouse tissues, the medium was supplemented with the Borrelia antibiotic mixture, resulting in final concentrations of 50 μg ml−1 rifampicin, 20 μg ml−1 phosphomycin and 2.5 μg ml−1 amphotericin B. All bacterial strains and plasmids used in this work are described in Table S1; oligonucleotides are listed in Table S2.
Construction of B. burgdorferi mutants and complemented derivatives
B. burgdorferi mutants in p66 (bb0603) in the infectious, genetically tractable strain B31-A3 (Elias et al., 2002) were constructed essentially as described previously (Coburn and Cugini, 2003, Samuels, 1995) using the plasmid p66KO4XS1, a derivative of p66K04 in which the bla gene was inactivated. This construct results in replacement of the region encoding the integrin binding domain of P66 with a kanamycin resistance cassette (p66::kanaR). Candidate B. burgdorferi clones were screened by PCR for the presence of the appropriate drug resistance marker, then for appropriate expression of, or lack thereof, P66 by immunoblot using anti-P66 rabbit antiserum (Coburn and Cugini, 2003) as primary antibody. As secondary antibody, either anti-rabbit IgG-alkaline phosphatase conjugates (Promega, Madison, WI) or anti-rabbit IgG-HRP conjugates (Promega) were used for colorimetric visualization or chemiluminescence and quantification by densitometry, respectively. All B. burgdorferi strains used in the work reported here were monitored for the presence of the plasmids identified in the sequenced strain, B31 M1 (Casjens et al., 2000), by PCR as previously described (Elias et al., 2002). Three clones containing the disrupted p66 locus, confirmed as in previous work (Coburn and Cugini, 2003), derived from three independent transformations, were used in the work reported here, and are referred to as Δp66 strains. In all experiments, Δp66 clones were identical in phenotype.
Complemented derivatives of the B. burgdorferiΔp66 mutants were constructed using two approaches. In one approach, a 4.2 kb region of B. burgdorferi DNA encompassing the p66 gene and flanking sequences was amplified using primers obb0602u and obb0604m and cloned in the TA vector pGEM T Easy (Promega, Madison, WI). This construct, pGTEp66, contains a single Mfe I site immediately 3′ of the p66 gene. The gentamicin resistance cassette was amplified from pBSV2G (Elias et al., 2003) using primers flgBMfe and aacC1Mfe and cloned in pGEM T Easy. The gentamicin resistance cassette was excised from this construct by digestion with Mfe I and cloned in pGTEp66 that had been linearized with Mfe I. After selection for growth in the presence of gentamicin, the orientation of the gentamicin resistance cassette with respect to p66 was determined. Several clones for each orientation were selected for sequencing to verify that the wild-type B. burgdorferi sequences were intact. The β-lactamase (ampicillin resistance) gene of the original pGEM T Easy vector was removed by digestion with BspHI, followed by dilution and ligation. Clones were screened for sensitivity to ampicillin and verified by restriction digestion. These constructs were used to replace the p66::kanaR on the chromosomes of the mutant strains with a wild-type copy of p66 and the gentamicin resistance cassette. Strains containing p66 complemented on chromosome are referred to as p66cc. The integrity of the p66 region sequences were verified by sequencing PCR products amplified from the chromosome.
The second approach to complementation was to supply p66 on the shuttle vector pBSV2G (Elias et al., 2003). The construct made for the previous approach, pGTEp66, was digested with SacI and SphI to excise bb0603 and surrounding B. burgdorferi DNA that had been cloned in the pGEM T Easy vector. This product was cloned in pBSV2G digested with the same restriction enzymes and clones were selected in the presence of gentamicin, resulting in pBSV2Gp66. The integrity of the p66 region on the shuttle vector was verified by sequencing. This construct was transformed into Δp66 mutants and restored P66 expression from the autonomously maintained shuttle vector. Strains containing p66 complemented on plasmid are referred to as p66cp.
Mouse infections by injection
For infection of mice by syringe inoculation, bacteria were grown to mid-log phase and plasmid content was assessed the morning of infection by PCR as previously described (Elias et al., 2002). For strains retaining all plasmids, washing and centrifugation steps were performed both to concentrate the bacteria and to remove the protein-rich medium from the solution. All centrifugation steps were performed at ambient temperature. For infectious doses of 107 bacteria or higher, 500 ml cultures of mid-log phase bacteria were prepared, and the bacteria were pelleted by centrifugation for 15 minutes at 6,000 × g. After removal of the supernatant, the pellets were washed with 30 ml PBS + 0.2% heat-inactivated normal mouse serum (NMS) and pelleted for 10 minutes at 6,000 × g. After removal of the supernatant, the pellets were washed with 1 ml PBS + 0.2% NMS and pelleted for 8 minutes at 11,200 × g. After removal of the supernatant, the bacteria were resuspended in 1 ml of PBS + 0.2% NMS, counted and adjusted to 1010 bacteria ml−1, from which appropriate dilutions were made. For infectious doses of 105 bacteria or lower, 1 ml of mid-log phase bacteria was pelleted by centrifugation for 8 minutes at 11,200 × g. After removal of the supernatant, the pellets were washed with 1 ml PBS + 0.2% NMS and pelleted for the same time at the same speed. After removal of the supernatant, the bacteria were resuspended in 1 ml of PBS + 0.2% NMS, counted and adjusted to 106 bacteria ml−1, from which appropriate dilutions were made.
ID50 determinations were performed by injecting laboratory cultured B. burgdorferi intradermally (25 μl) or subcutaneously (100 μl) at doses ranging from 10–109 bacteria per mouse suspended in PBS + 0.2% NMS. The mice were returned to normal housing for 2–4 weeks, as noted for individual experiments, to account for the possibility of differences in dissemination rates between strains. After euthanasia by CO2 narcosis, tissues were collected for culture (bladder, ear snip, and inoculation site skin), histology and cytokine analysis (inoculation site skin), and DNA purification (inoculation site skin, ear, heart, tibiotarsal joint) for quantification of B. burgdorferi. Since infection by the Δp66 strains was never detected by culture at sites other than the inoculation site skin, B. burgdorferi DNA was not quantified and histologic analyses were not performed for other tissues. Blood was collected by cardiac puncture for serologic analyses. The ID50 values reported here are based on culture of tissues, and were calculated as previously described (Reed and Muench, 1938). The cultures were checked for the presence of B. burgdorferi by dark-field microscopy for up to 8 weeks before being designated as negative, but in infected mice the majority were positive within 1–2 weeks post-harvest.
Short-term infections were performed by injecting laboratory cultured B. burgdorferi subcutaneously (100 μl) at 105 bacteria per mouse suspended in PBS + 0.2% NMS. The mice were returned to normal housing for varying time points between 6 hours and 2 weeks, as noted for individual experiments. After euthanasia by CO2 narcosis, tissues were collected for culture (inoculation site skin for all time points, ear snip and bladder at time points greater than or equal to 1 week), cytokine analysis (inoculation site skin), and DNA purification (inoculation site skin) for quantification of B. burgdorferi. Blood was collected by cardiac puncture for serologic analyses. Culture analysis was performed as described for the ID50 experiments.
Mouse infections by tick transmission
Larval I. scapularis ticks were inoculated with B. burgdorferi by immersion feeding, as previously described (Policastro and Schwan, 2003, Battisti et al., 2008). After the larvae had fed on mice, they were allowed to molt into nymphs, which were assessed for B. burgdorferi infection by culture as follows. Randomly chosen ticks were surface sterilized with 3% H202 for 15 min., followed by 70% ethanol for 15 min. (Policastro and Schwan, 2003), then disrupted in B. burgdorferi culture medium containing the Borrelia antibiotic mixture (above). Cohorts of nymphs with infection prevalence of at least 40% were fed on naïve mice to allow assessment of B. burgdorferi infection by tick-mediated transmission.
Mice were prepared for nymphal tick attachment by anesthesia with a cocktail of 100 mg kg−1 Ketamine and 10 mg kg−1 Xylazine. The hair in the area between the scapulae of the mice was trimmed to allow access to the skin. Plastic chambers consisting of the top cylindrical portions of 1.5 ml microcentrifuge tubes were dipped into a warmed mixture of rosin (MP Biomedicals) and beeswax (Hampton Research) (4:1) and immediately placed on the trimmed area of the mouse and held until the mixture hardened and adhered the tube to the mouse. Ten nymphal ticks were placed in each chamber and the open end was sealed with parafilm, which was punctured once with a 27 gauge needle to allow air exchange. Mice were housed individually with paper towel bedding. After 3 days, mice were checked daily for ticks fed to repletion. Recovered ticks were assessed for B. burgdorferi infection as described for unfed nymphal ticks. Two weeks post drop off of the last tick, mice were sacrificed and tissues were harvested for analyses as described in mouse infections by injection.
Serology
To assess the serologic response to B. burgdorferi, blood was collected by cardiac puncture and allowed to clot at ambient temperature, and serum was removed to fresh tubes and stored at −80°C until analysis. Analyses were performed by immunoblot after separation of B. burgdorferi lysates on 12.5% SDS-PAGE gels and transfer to Immobilon (Millipore, Billerica, MA). For detection of IgG, strips were probed with sera diluted 1:1,000 for 1 hour at ambient temperature, followed by anti-mouse IgG conjugated to alkaline phosphatase (Promega, Madison, WI) diluted 1:10,000 and colorimetric development according to standard protocols (Laemmli, 1970, Towbin, 1979). For detection of IgM, strips were probed with sera diluted 1:100 for 1 hour at ambient temperature, followed by anti-mouse IgM conjugated to alkaline phosphatase (Millipore) diluted 1:10,000 and colorimetric development according to standard protocols.
Dialysis Membrane Chambers
To determine whether the Δp66 mutants were able to survive in a protected environment in a mammalian host and attain a host-adapted state, spirochetes were placed in dialysis membrane chambers (DMCs; Spectra-Por, 8000 MW cut-off) in BSK-II medium at a starting number of 3 × 103 spirochetes per ml, and implanted into the peritoneal cavities of female Sprague Dawley rats (two per isolate per experiment) as previously described (Akins et al., 1998). Fourteen days following implantation, rats were euthanized by CO2 narcosis and DMCs aseptically removed. Spirochete densities in the explanted DMC fluid were determined by dark field microscopy using a Petroff-Hauser counting chamber. DMC-cultivated organisms were harvested by centrifugation at 8,500 × g for 20 min. and the resulting pellets washed twice with 10 ml of phosphate-buffered saline (PBS). Equivalent numbers of cells (~1×107 total spirochetes per lane) were resuspended and boiled in reducing Laemmli sample buffer (Bio-Rad, Hercules, CA). Whole cell lysates were analyzed on 12.5% separating polyacrylamide mini-gels, and the separated proteins visualized by silver stain as previously described (Morrissey, 1981).
Histology
For histologic analyses, inoculation site skin samples were placed in Zinc-Formalin (Fisher Scientific, Kalamazoo, MI) at the time of harvest, then embedded, sectioned, and stained with Hematoxylin and Eosin by the Histology Core at the Children’s Research Institute of the Medical College of Wisconsin. Randomly selected slides were viewed by two independent observers blinded to the infection group; skin sections for all mice were scored for several criteria: neutrophils, monocytes, eosinophils and mast cells. Each cell type was scored in 10 high-power fields (400 × magnification) for each sample as previously described (Antonara et al., 2010).
Cytokine analyses
Mouse tissues were prepared for cytokine analysis by dicing the tissue on dry ice. Tissues were placed in preweighed 2 ml microcentrifuge tubes and tissue weights were determined. 1 ml of “Cytokine Buffer” (PBS + 0.1% Igepal, 0.01 TIU ml−1 aprotinin, 1 mM benzamidine-HCl, 1 μg ml−1 leupeptin [Antonara et al., 2010]) was added to each sample, and tissues were homogenized using a Tissue-Tearor (Cat. No. 985-370, Biospec Products, Bartlesville, OK). Samples were run in duplicate following the Bio-plex Pro Assay manufacturer instructions on a Bioplex-200 System (BioRad, Hercules, CA). Cytokines analyzed were IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), KC, MCP-1, TNF-α, VEGF, GM-CSF and IFN-γ, based on a previous study observing statistically significant differences in expression levels between sham inoculated or wild-type inoculated mice (Antonara et al., 2010). Results in pg ml−1 were normalized to the tissue weights determined before homogenization, and are presented as the average of the values from duplicate wells in pg gram tissue−1. Significance was determined using GraphPad software utilizing a one way ANOVA Kruskal-Wallis test with Dunn’s multiple comparison post-test.
Quantitative PCR
Mouse tissues were prepared for quantitative PCR (qPCR) by dicing the tissues on dry ice, and DNA was extracted using the QIAGEN QIAamp DNA Mini Kit. Samples were stored at −80°C until the day of analysis. Sample concentrations were determined by OD260/280 readings, and all samples were diluted to 20 ng μl−1, or 100ng reaction−1. Borrelia burgdorferi DNA was prepared for the standard curve by growing B31-A3 in BSK II culture medium to a concentration of 5x107 spirochetes ml−1 and DNA was extracted with the QIAGEN QIAamp DNA Mini Kit. Mouse DNA for standard curves were prepared by growing LA-4 cells (ATCC #CCL 196) at 37°C in 5% CO2 atmosphere and DNA was extracted with the QIAGEN QIAamp DNA Mini Kit. Single use aliquots of each DNA standard were frozen at −80°C until the day of analysis. 10-fold dilutions of each standard were made the day of use in the elution buffer. The B. burgdorferi standard curve was spiked with 100ng mouse DNA per well to mimic the conditions of the experimental samples. All standards and samples were run in triplicate. A standard curve was generated using Biorad iQCycler5 software, and the numbers of genomes per sample were extrapolated from the Ct values. Data are presented as number of B. burgdorferi genomes per 10,000 mouse genomes.
Serum Sensitivity Assay
Serum sensitivity assays were performed as described (Alitalo et. al, 2001, Brooks et. al, 2005) with mouse serum. Whole blood was collected by cardiac puncture from uninfected mice, allowed to clot at room temperature and centrifuged for 5 min. at 2,500 × g, and sera were pooled. In triplicate, wild-type or Δp66 bacteria were grown to mid-log phase and diluted to a final concentration of 5 × 106 bacteria per ml into BSKII medium with a final concentration of 40% complete mouse serum, 40% heat-inactivated serum or no serum added control. At 1, 2, 4 and 16 hours, an aliquot was taken from each condition and counted by Petroff-Hausser using dark-field microscopy (data not shown). In parallel, an aliquot was plated in solid BSK II medium. Colonies were counted 2 weeks post-plating. Results are presented as number of viable spirochetes per ml.
Supplementary Material
Acknowledgments
We thank Dr. Dorothee Grimm and Angela Tanudra for their contributions to constructing B. burgdorferi mutants and complemented clones, Dr. Tom Schwan and Dr. Paul Policastro for their generosity in providing training, advice and protocols for work with ticks, and Sheryl Konrad for her contributions in optimizing quantitative PCR conditions. PCR primer sequences for plasmid profiling were generously provided by Dr. Darrin Akins. This work was supported by NIH grants AI051407 and AI059505, the Advancing a Healthier Wisconsin program, Department of Medicine, and the Center for Infectious Disease Research at the Medical College of Wisconsin.
References
- Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo A, Meri T, Rämö L, Jokiranta TS, Heikkilä T, Seppälä IJ, Oksi J, Viljanen M, Meri S. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect Immun. 2001;69:3685–3691. doi: 10.1128/IAI.69.6.3685-3691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrutz MA, Srivastava A, Wong KW, D’Souza-Schorey C, Tang M, Ch’Ng LE, Snapper SB, Isberg RR. Efficient uptake of Yersinia pseudotuberculosis via integrin receptors involves a Rac1-Arp 2/3 pathway that bypasses N-WASP function. Mol Microbiol. 2001;42:689–703. doi: 10.1046/j.1365-2958.2001.02676.x. [DOI] [PubMed] [Google Scholar]
- Antonara S, Ristow L, McCarthy J, Coburn J. Effect of Borrelia burgdorferi OspC at the site of inoculation in mouse skin. Infect Immun. 2010;78:4723–4733. doi: 10.1128/IAI.00464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Burgdorfer W, Grunwaldt E, Steere AC. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983;72:504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárcena-Uribarri I, Thein M, Sacher A, Bunikis I, Bonde M, Bergström S, Benz R. P66 porins are present in both Lyme disease and relapsing fever spirochetes: a comparison of the biophysical properties of P66 porins from six Borrelia species. Biochim Biophys Acta. 2010;1798:1197–1203. doi: 10.1016/j.bbamem.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Battisti JM, Bono JL, Rosa PA, Schrumpf ME, Schwan TG, Policastro PF. Outer surface protein A protects Lyme disease spirochetes from acquired host immunity in the tick vector. Infect Immun. 2008;76:5228–5237. doi: 10.1128/IAI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono JL, Elias AF, Kupko JJ, 3rd, Stevenson B, Tilly K, Rosa P. Efficient targeted mutagenesis in Borrelia burgdorferi. J Bacteriol. 2000;182:2445–2452. doi: 10.1128/jb.182.9.2445-2452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Brissette CA, Cooley AE, Burns LH, Riley SP, Verma A, Woodman ME, Bykowski T, Stevenson B. Lyme borreliosis spirochete Erp proteins, their known host ligands, and potential roles in mammalian infection. Int J Med Microbiol. 2008;298(Suppl 1):257–267. doi: 10.1016/j.ijmm.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CS, Vuppala SR, Jett AM, Alitalo A, Meri S, Akins DR. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J Immunol. 2005;175:3299–3308. doi: 10.4049/jimmunol.175.5.3299. [DOI] [PubMed] [Google Scholar]
- Bunikis J, Noppa L, Bergström S. Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme diseaseBorrelia. FEMS Microbiol Lett. 1995;131:139–145. doi: 10.1111/j.1574-6968.1995.tb07768.x. [DOI] [PubMed] [Google Scholar]
- Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser CM. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- Coburn J, Chege W, Magoun L, Bodary SC, Leong JM. Characterization of a candidate Borrelia burgdorferi beta(3)-chain integrin ligand identified using a phage display library. Mol Microbiol. 1999;34:926–940. doi: 10.1046/j.1365-2958.1999.01654.x. [DOI] [PubMed] [Google Scholar]
- Coburn J, Cugini C. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin alpha(v)beta(3) Proc Natl Acad Sci U S A. 2003;100:7301–7306. doi: 10.1073/pnas.1131117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugini C, Medrano M, Schwan TG, Coburn J. Regulation of expression of the Borrelia burgdorferi beta(3)-chain integrin ligand, P66, in ticks and in culture. Infect Immun. 2003;71:1001–1007. doi: 10.1128/IAI.71.2.1001-1007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AM, Fikrig E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Invest. 1997;99:377–379. doi: 10.1172/JCI119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AM, Telford SR, 3rd, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoe G, Coburn J. Delineation of Borrelia burgdorferi p66 sequences required for integrin alpha(IIb)beta(3) recognition. Infect Immun. 2001;69:3455–3459. doi: 10.1128/IAI.69.5.3455-3459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersch P, Isberg RR. A region of the Yersinia pseudotuberculosis invasin protein enhances integrin-mediated uptake into mammalian cells and promotes self-association. EMBO J. 1999;18:1199–1213. doi: 10.1093/emboj/18.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler F, Whalen JA, Reinhardt BN, Steere AC. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- Elias AF, Bono JL, Kupko JJ, 3rd, Stewart PE, Krum JG, Rosa PA. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J Mol Microbiol Biotechnol. 2003;6:29–40. doi: 10.1159/000073406. [DOI] [PubMed] [Google Scholar]
- Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, Bono JL, Akins DR, Radolf JD, Schwan TG, Rosa P. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun. 2002;70:2139–2150. doi: 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, Gherardini FC. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A. 2005;102:5162–5167. doi: 10.1073/pnas.0408536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fuji C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A. 2004;101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BP, Brown EL, Dorward DW, Rosenberg LC, Höök M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- Guo BP, Norris SJ, Rosenberg LC, Höök M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. Hamburger, Z. A., M. S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger ZA, Brown MS, Isberg RR, Bjorkman PJ. Crystal structure of invasin: a bacterial integrin-binding protein. Science. 1999;286:291–295. doi: 10.1126/science.286.5438.291. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- Isberg RR, Van Nhieu GT. The mechanism of phagocytic uptake promoted by invasin-integrin interaction. Trends Cell Biol. 1995;5:120–124. doi: 10.1016/s0962-8924(00)88962-x. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88− deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kraiczy P, Skerka C, Kirschfink M, Zipfel PF, Brade V. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int Immunopharmacol. 2001;1:393–401. doi: 10.1016/s1567-5769(00)00041-2. [DOI] [PubMed] [Google Scholar]
- Kraiczy P, Skerka C, Zipfel PF, Brade V. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: a new protein family involved in complement resistance. Wien Klin Wochenschr. 2002;114:568–573. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LaFrance ME, Pierce JV, Antonara S, Coburn J. The Borrelia burgdorferi integrin ligand P66 affects gene expression by human cells in culture. Infect Immun. 2011;79:3249–3261. doi: 10.1128/IAI.05122-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong JM, Fournier RS, Isberg RR. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990;9:1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu X, Beck DS, Kantor FS, Fikrig E. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect Immun. 2006;74:3305–3313. doi: 10.1128/IAI.02035-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, Carroll JD, Espevik T, Ingalls RR, Radolf JD, Golenbock DT. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- Liu N, Montgomery RR, Barthold SW, Bockenstedt LK. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect Immun. 2004;72:3195–3203. doi: 10.1128/IAI.72.6.3195-3203.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory; New York: 1982. [Google Scholar]
- Medrano MS, Policastro PF, Schwan TG, Coburn J. Interaction of Borrelia burgdorferi Hbb with the p66 promoter. Nucleic Acids Res. 2010;38:414–427. doi: 10.1093/nar/gkp1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RR, Malawista SE, Feen KJ, Bockenstedt LK. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey JH. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Ntchobo H, Rothermel H, Chege W, Steere AC, Coburn J. Recognition of multiple antibody epitopes throughout Borrelia burgdorferi p66, a candidate adhesin, in patients with early or late manifestations of Lyme disease. Infect Immun. 2001;69:1953–1956. doi: 10.1128/IAI.69.3.1953-1956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Blevins JS, Norgard MV. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology. 2008;154:2641–2658. doi: 10.1099/mic.0.2008/019992-0. [DOI] [PubMed] [Google Scholar]
- Pal U, Wang P, Bao F, Yang X, Samanta S, Schoen R, Wormser GP, Schwartz I, Fikrig E. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J Exp Med. 2008;205:133–141. doi: 10.1084/jem.20070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, Norgard MV, Fikrig E. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinne M, Thein M, Denker K, Benz R, Coburn J, Bergström S. Elimination of channel-forming activity by insertional inactivation of the p66 gene in Borrelia burgdorferi. FEMS Microbiol Lett. 2007;266:241–249. doi: 10.1111/j.1574-6968.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- Policastro PF, Schwan TG. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J Med Entomol. 2003;40:364–370. doi: 10.1603/0022-2585-40.3.364. [DOI] [PubMed] [Google Scholar]
- Probert WS, Allsup KM, LeFebvre RB. Identification and characterization of a surface-exposed, 66-kilodalton protein from Borrelia burgdorferi. Infect Immun. 1995;63:1933–1939. doi: 10.1128/iai.63.5.1933-1939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S, Isberg RR, Leong JM. The integrin-binding domain of invasin is sufficient to allow bacterial entry into mammalian cells. Infect Immun. 1992;60:3909–3912. doi: 10.1128/iai.60.9.3909-3912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Saltman LH, Lu Y, Zaharias EM, Isberg RR. A region of the Yersinia pseudotuberculosis invasin protein that contributes to high affinity binding to integrin receptors. J Biol Chem. 1996;271:23438–23444. doi: 10.1074/jbc.271.38.23438. [DOI] [PubMed] [Google Scholar]
- Samuels DS. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol. 1995;47:253–259. doi: 10.1385/0-89603-310-4:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartakova ML, Dobrikova EY, Motaleb MA, Godfrey HP, Charon NW, Cabello FC. Complementation of a nonmotile flaB mutant of Borrelia burgdorferi by chromosomal integration of a plasmid containing a wild-type flaB allele. J Bacteriol. 2001;183:6558–6564. doi: 10.1128/JB.183.22.6558-6564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Höök M, Skare JT. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol. 2006;59:1591–1601. doi: 10.1111/j.1365-2958.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- Skare JT, Mirzabekov TA, Shang ES, Blanco DR, Erdjument-Bromage H, Bunikis J, Bergström S, Tempst P, Kagan BL, Miller JN, Lovett MA. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect Immun. 1997;65:3654–3661. doi: 10.1128/iai.65.9.3654-3661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, Thalken R, Bono JL, Rosa P. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol Microbiol. 2001;39:714–721. doi: 10.1046/j.1365-2958.2001.02256.x. [DOI] [PubMed] [Google Scholar]
- Stewart PE, Wang X, Bueschel DM, Clifton DR, Grimm D, Tilly K, Carroll JA, Weis JJ, Rosa PA. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect Immun. 2006;74:3547–3553. doi: 10.1128/IAI.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Bestor A, Jewett MW, Rosa P. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect Immun. 2007;75:1517–1519. doi: 10.1128/IAI.01725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun. 2006;74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staeheli T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening EH, Parveen N, Trzeciakowski JP, Leong JM, Höök M, Skare JT. Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect Immun. 2008;76:5694–5705. doi: 10.1128/IAI.00690-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis JJ. Host-pathogen interactions and the pathogenesis of murine Lyme disease. Curr Opin Rheumatol. 2002;14:399–403. doi: 10.1097/00002281-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168:348–355. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- Young VB, Falkow S, Schoolnik GK. The invasin protein of Yersinia enterocolitica: internalization of invasin-bearing bacteria by eukaryotic cells is associated with reorganization of the cytoskeleton. J Cell Biol. 1992;116:197–207. doi: 10.1083/jcb.116.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidner N, Mbow ML, Dolan M, Massung R, Baca E, Piesman J. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect Immun. 1997;65:3100–3106. doi: 10.1128/iai.65.8.3100-3106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.