Abstract
Tumors often display mechanisms to avoid or suppress immune recognition. One such mechanism is the shedding of gangliosides into the local tumor microenvironment, and a high concentration of circulating gangliosides is associated with poor prognosis. In this study, we identify ganglioside GD3, which was isolated from the polar lipid fraction of ovarian cancer-associated ascites, as an inhibitory factor that prevents innate immune activation of natural killer T (NKT) cells. Purified GD3 displayed a high affinity for both human and mouse CD1d, a molecule involved in the presentation of lipid antigens to T cells. Purified GD3, as well as substances within the ascites, bound to the CD1d antigenic binding site and did not require additional processing for its inhibitory effect on NKT cells. Importantly, in vivo administration of GD3 inhibited α-GalCer- induced NKT cell activation in a dose dependent manner. These data therefore indicate that ovarian cancer tumors may use GD3 to inhibit the anti-tumor NKT cell response as an early mechanism of tumor immune evasion.
Introduction
In the United States, ovarian cancer ranks fifth as a cause of cancer related deaths among women (1). Unfortunately, the majority of cases are diagnosed at an advanced stage, leading to poor overall survival. By the time of presentation, ovarian cancer has often undergone successive accumulation of multiple molecular alterations. Therefore, each tumor tends to be molecularly distinct, increasing the difficulty of identifying a common molecular target with prognostic or therapeutic potential.
Ovarian cancer patients often present with ovarian cancer-associated ascites, which contains cellular components of the immune system such as lymphocytes and NKT cells, regulatory factors, such as cytokines, and potential immune inhibitory factors. It has been reported that patients with advanced ovarian cancer have higher levels of gangliosides in their plasma and ascites compared to plasma ganglioside levels in controls (2). Furthermore, abnormal ganglioside expression is strongly associated with clinically aggressive malignancies. Thus cancer patients that have high circulating ganglioside levels at the time of clinical diagnosis exhibit a faster rate of disease progression and a decreased survival rate (3).
One of the earliest pathways in immune activation is the presentation of phospho/glycolipid antigens on CD1d molecules to natural killer T (NKT) cells. NKT cells are primed cells that have large reservoirs of cytokines such as IFN-γ and TNF-α. These cells can, if appropriately activated, induce the development of a robust adaptive immune response. Many studies have characterized the adaptive T cell immune response in ovarian cancer (4–8). However, mechanisms of immune evasion by ovarian cancers, specifically those affecting the NKT cell/CD1d system, remain to be elucidated.
Here, we identified the ganglioside GD3 as a major factor in ovarian cancer ascites fluid that inhibited NKT cell activation. Mechanistically, we found that antigen processing was not required as CD1d-Ig dimers loaded with α-galactosylceramide (α-GalCer) were no longer recognized by NKT cells following treatment with GD3 and that pulsing CD1d-Ig based aAPC with GD3 or ascites fluid led to inhibition of NKT activation. Furthermore, GD3 bound with high affinity to both human and mouse CD1d, and in vivo treatment with GD3 inhibited α-GalCer mediated NKT cell activation. These data indicate that ganglioside shedding may be an early mechanism of immune evasion used by ovarian cancer and indicate that GD3 could be an important diagnostic and/or therapeutic target in the treatment of ovarian cancer.
Materials and Methods
Tumor Associated Ascites
Ovarian cancer-associated ascites was collected from patients undergoing primary cytoreductive surgery by the Kelly Gynecologic Oncology Service at Johns Hopkins Medical Institutions. All donors gave written informed consent before enrolling in the study. The Institutional Review Board of Johns Hopkins Medical Institutions approved this investigation.
Mice
Six–eight-week old C57BL/6 mice were purchased from the Jackson Laboratory or Janvier and were maintained in the animal facility at New York University School of Medicine and at the University of Bonn under pathogen-free conditions.
Cell Lines
Murine L cells transfected with WT cd1d1 cDNA (LCD1dwt) were kindly provided by R.R. Brutkiewicz (Indiana University School of Medicine, Indianapolis, IN) (9) in 2005. The cell lines used have been tested and authenticated routinely by staining for stable cell surface expression of CD1d, compared to isotype control staining, and also compared to control cells stably transfected with the empty control vector.
NKT Cells
The Vα14Jα18+Vβ8.2+ NKT cell hybridoma cell lines DN32.D3 & N38-3C3, and the CD1d-specific NKT cell hybridoma N37-1A12 (Vα5+Vβ7+) have all been described (10–12) and were graciously provided by R.R. Brutkiewicz. A mouse iNKT hybridoma, 1.2, which co-expresses mouse invariant Vα14-Jα18 and Vβ8.2 chains, was kindly provided by Dr. Mitchell Kronenberg (La Jolla Institute of Allergy and Immunology, San Diego, CA). The NKT cells are tested for specificity to CD1d in each experiment via functional T assay.
Lipid antigens
18:1 Biotinyl PE (1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine-N-(Biotinyl)) and 18:1 Caproylamine PE (1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine-N-(hexanoylamine)) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). α-galactosylceramide (α-GalCer) was purchased from Enzo/Axxora.
Generation of human iNKT cell lines
Human iNKT cell lines expressing Vα24 T cell receptor were generated, as previously reported with a slight modification (13). Briefly, Vα24+ cells were isolated from PBMCs by using anti-mouse IgG magnetic beads (Miltenyi Biotec) coupled to a mouse anti-human Vα24 TCR antibody (Beckman Coulter, Fullerton, CA). Vα24+ cells were then co-cultured with mitomycin-C (Sigma-Aldrich, St. Louis, MO) treated autologous immature DCs for 24 hours in the presence of 100 ng/ml of α-GalCer and 10 IU/ml of a recombinant human IL-2 (R&D system), and further cultured for 7–10 days in the presence of 10 IU/ml of human IL-2 alone. After two cycles of stimulations, more than 95% of NKT cells were shown to be Vα24+ cells by FACS.
Generation of Mouse iNKT cell lines
Mouse iNKT cell lines were generated as described with some modifications (14, 15). Thymocytes were co-cultured with autologous immature DCs in the presence of 100 μg/ml α-GalCer for 10 days. After being purified with lympholyte-M (Accurate Chemical, Westbury, NY), cells were re-stimulated with immature DCs in the presence of 100 μg/ml α-GalCer and 10 μg/ml mouse IL-2 (Cell Sciences, Canton, MA) for 1 week. After three cycles of stimulations, more than 95% of NKT cells were shown to react with mouse CD1d-dimer loaded with α-GalCer by FACS.
Generation of artificial Antigen Presenting Cells
The preparation of CD1d-Ig based aAPC was performed according to the previously described method (16). The hCD1d-aAPC were loaded with lipid antigen, α-galactosylceramide (α-GalCer), (5 μg/ml in 1 ml PBS containing 5 × 107 beads) (Axxora, LLC).
Treatment of Cells with Tumor Associated Ascites
The ascites was cleared of cellular debris by centrifugation as previously described (17). The antigen presenting cells were treated with the clarified supernantants for 4 h at 37°C, unless otherwise indicated. The antigen presenting cells were subsequently washed extensively with PBS, and cocultured with NKT hybridomas for 20–24 h at 37°C. Cytokine released (IL-2) was measured as an indication of NKT cell activation and was measured by standard sandwich ELISA. For the conditioned medium from ascites derived cells, 50 ml of ascites was centrifuged at 1500 rpm for 5 min. The ascites fluid was removed and the remaining cell pellet was cultured in 50 ml of RPMI supplemented with 2 mM L-glutamine (BioWhittaker), 10% FBS (HyClone), and ciprofloxacin (Serological Proteins) in a T-175 flask for 4–7 days, until the adherent cells reached confluency, then the cultured supernatant was harvested, centrifuged to remove cellular debris and used for experiments.
Extraction of the polar lipid fraction from the ascites
The polar lipid fraction was isolated from the ascites of the indicated ovarian cancer patients. Methanol then chloroform were added to give chloroform–methanol–water (4 : 8 : 3), and the samples were extracted by stirring the mixture at ambient temperature. Insoluble material was removed by centrifugation, and water was then added to the supernatant to give chloroform–methanol–water (4 : 8 : 5.6). The resulting phases were separated by centrifugation and the upper polar lipid phase was desalted using a Sep-Pak C18 cartridge(18). The extracted lipids were analyzed by TLC (HPTLC Silica Gel 60; Merck, Darmstadt, Germany) with chloroform–methanol–0.25% aqueous KCl (60 : 35 : 8) as running solvent. Gangliosides were detected with a resorcinol–HCl Cu2+ reagent. Bovine brain gangliosides were used as standards. The commercially available, purified gangliosides used in the NKT cell assays: Gg3Cer, GM2, GM3, GD3 (Matreya) and GD2 (Calbiochem) were reconstituted in either methanol or chloroform-methanol as suggested by the manufacturer.
Determination of Serum Cytokine Concentrations
The serum concentrations of IFN-γ, IL-12 p70 and IL-4 were measured 2, 6, 12, 24, 48, and 72 h after treatment with α-GalCer, and different concentrations of GD3 by standard sandwich ELISA as previously described (19).
Staining of human iNKT cells with CD1d-mIgG dimers
One microgram of human or mouse CD1d-mouse IgG dimer was incubated with 1.4 μg α-GalCer, GD3, or ascites in 50 μL of PBS at 37 °C for overnight to load the lipid onto hCD1d-mIgG dimer and used to stain 2 × 105 Human iNKT cells on ice for 30 min. Then the cells were washed with PBS containing 5% FBS twice and incubated with phycoerythrin-labeled rat anti-mouse IgG1 antibody (A85-1) and allophycocyanin-labeled anti-human CD3ε antibody (BD Biosciences, San Diego, CA, USA) on ice for 30 min. After washing, the stained cells were analyzed with FACSCalibur System (BD Biosciences, San Diego, CA, USA). Flow cytometry data was analyzed with FlowJo v8.8 software (Tree Star, Inc, Ashland, OR).
Competitive ELISA assay
The assay was performed as previously described (20). Briefly, Nunc MaxiSorp flat-bottom 96 well plates (Thermo Fisher Scientific, Waltham, MA, USA) were coated with 100 μL of Goat anti-mouse IgG Fc gamma antibody (Biomedia, New York, NY, USA). The plates were washed three times with PBS/Tween and 100 μL of lipid-CD1 dimer mixture was added to the plates immediately after washing. The mixture solution was prepared by mixing CD1dimer (5 μg/mL) and lipids in the presence of Biotinyl PE (2 μg/mL) in PBS. The CD1-Biotinyl PE complex was detected with HRP-labeled Avidin (eBioscience, San Diego, CA, USA). Kd of Biotinyl PE was determined by titrating the amount of Biotinyl PE to reach the maximum binding in the absence of competitors and applying the following equation to the data; Y = (Bmax * X)/(Kd + X). Kd of Biotinyl PE and Ki of PE and α-GalCer were calculated using GraphPad Prism (Ver.4.02) (GraphPad Software, Inc., La Jolla, CA, USA).
Intracellular cytokine staining
Two hours after the injection of lipids, liver MNCs were isolated (21) and cultured ex vivo in the presence of GolgiStop (BD Pharmingen) for 4 h. Cells were stained with anti-TCRb-FITC, anti-NK1.1-PE, anti-CD45-APC-Cy7 (eBioscience), fixed, permeabilized (PermWash, BD) and stained intracellularly for IFN-γ. Dead cells were excluded (LIVE/DEADR Fixable Violet Dead Cell Stain Kit, Invitrogen).
Statistical analysis
Data analysis was performed by Prism software (version 5.02 for Windows; GraphPad). Parametric statistics were used to analyze differences between experimental groups, when needed. Where multiple groups existed within a single experiment multiple between group comparisons were made by ANOVA with Bonferroni’s post-test. A p value <0.05 was considered significant. The error bars in the bar graphs show the SEM.
Results
Pretreatment with tumor-associated ascites rapidly inhibits CD1d-mediated activation of NKT cells
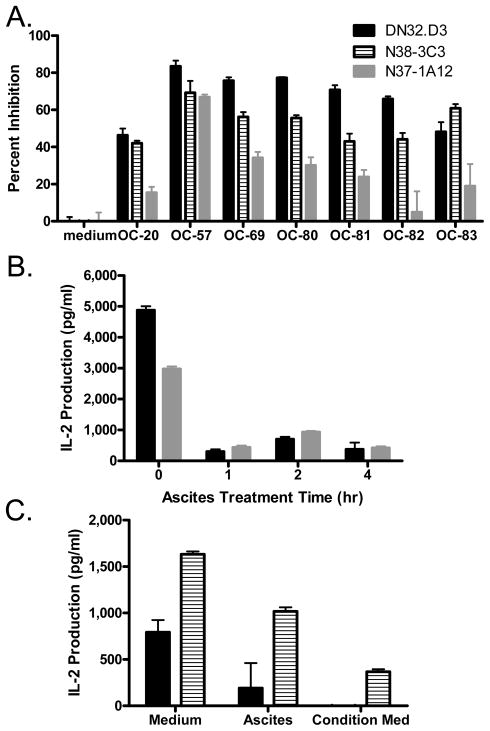
Incubation of CD1d− expressing L cells with ovarian cancer ascites fluid inhibited stimulation of NKT hybridomas, as measured by the release of IL-2 (Fig. 1A). Treatment with ascites for 1 h maximally inhibited the ability of LCD1dwt to stimulate NKT cells (Fig. 1B). Conditioned medium from cultured cells isolated from ascites also inhibited antigen presentation to both canonical (Vα14+, DN32.D3) and non-canonical (Vα5+, N37-1A12) NKT cell hybridomas (Fig. 1C). These studies suggest that cells present in the ascites actively produce substances that abrogate CD1d-mediated activation of NKT cells. To determine whether incubation with ascites-treated cells resulted in a permanent functional defect in NKT cell activation, fresh untreated LCD1dwt cells were added to NKT cells previously exposed to ascites-pretreated APCs. Addition of untreated cells activated NKT cells to produce cytokines (data not shown), demonstrating that stimulation of NKT cells with ascites-treated LCD1dwt cells did not permanently impair NKT cell function.
Figure 1. Tumor Ascites Inhibits CD1d-mediated NKT cell activation.
(A) LCD1dwt cells were treated with control medium or with ovarian cancer ascites fluid from patients for 4 h, then washed extensively and cocultured with a panel of NKT cell hybridomas (DN32.D3, N38-3C3 and N37-1A12). After 20–24 h, IL-2 was measured as an indication of NKT cell activation using standard cytokine ELISA. (B) The effect of ascites on NKT cell recognition of CD1d1 molecules is rapid. LCD1d1wt cells were incubated with ascites for the indicated times. The cells were then co-cultured with NKT cell hybridomas and IL-2 release was measured. (C) Conditioned medium from ascites-derived cells is inhibitory. LCD1d1wt cells were incubated for 4 h with fresh cell culture medium, ascites fluid, or conditioned medium from ascites-derived cells from patient OC-95. Treated APCs were then co-cultured with NKT cell hybridomas and IL-2 release was measured. Data are shown as mean ±S.E.M. of one experiment set up in triplicate. The experiments were performed at least twice with each ascites sample.
Polar lipids present in ascites inhibit NKT cell recognition of endogenous antigen
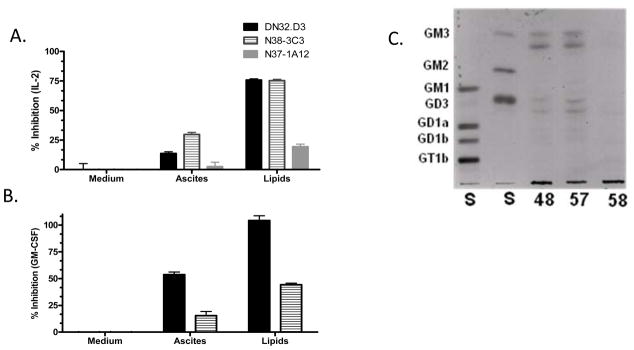
Since ovarian cancer patient ascites is rich in gangliosides, we hypothesized that these lipids are responsible for the inhibition of NKT cell activation. The polar lipid fraction was extracted from the ascites, and found to significantly reduce the ability of the APC to stimulate NKT cells (Figs. 2A & 2B). In fact, the reduction by the polar lipid fraction was greater than the inhibition caused by ascites pretreatment. Analysis of the polar lipid fraction by thin-layer chromatography (TLC) showed that the majority of patient samples contained the gangliosides GM3, GD3 and minor amounts of more complex gangliosides (Fig. 2C).
Figure 2. The Polar Lipid Fraction of Ascites inhibits NKT cell activation.
The polar lipid fraction was isolated from the ascites of ovarian cancer patients. LCD1dwt cells were treated with ascites or the polar lipid fraction of the ascites for 4 h, then washed extensively and cocultured with a panel of NKT cell hybridomas. (A) IL-2 production was measured as an indication of NKT cell activation. Data are shown as mean ±S.E.M. In addition, treatment with the polar lipid fraction significantly inhibited all hybridomas examined (p<0.001). (B) GM-CSF production was measured as an indication of NKT cell activation. Data shown as mean ±S.E.M. (C) TLC of the Polar Lipid Fraction. The extracted lipids were analyzed by TLC. Gangliosides were detected with a resorcinol–HCl Cu2+ reagent. Bovine brain gangliosides were used as standards as indicated at the left. Tumor gangliosides typically appear as doublets due to heterogeneity in their ceramide lipid moieties.
Identification of GD3 as an inhibitory lipid in ovarian cancer ascites
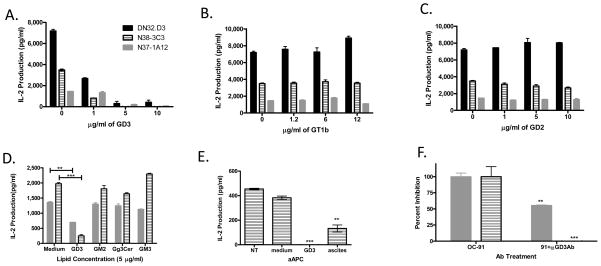
To test which of the observed gangliosides is responsible for the inhibition associated with ascites, antigen presenting cells were incubated with increasing doses of purified GD3 and the related gangliosides including GT1b, and GD2 (see Fig. S1 for structures) (22, 23), washed and cocultured with NKT cells (Fig 3A–C). Only GD3 inhibited CD1d-mediated activation of NKT cells in a dose-dependent fashion. Other tumor-associated glycolipids, such as GM3 (the biosynthetic precursor of GD3) and the neutral glycosphingolipid gangliotriaosylceramide (Gg3Cer), have been reported to inhibit canonical NKT cell activation when used at high concentrations (9, 24). Therefore, we also compared the ability of GD3, GM3, Gg3Cer, and GM2 to inhibit NKT cells (Fig. 3D). We found that only incubation with GD3 resulted in a significant reduction in NKT cell activation.
Figure 3. Ganglioside treatment alters CD1d-mediated NKT cell activation.
(A) GD3 (B) GT1b or (C) GD2 at the indicated concentrations for 4 h, washed extensively and co-cultured with NKT cell hybridomas, DN32.D3, N37-1A12, and N38-3C3 overnight. (D) LCD1dwt cells were treated with the indicated gangliosides (5μg/ml) for 4 h, washed, and co-cultured with NKT cell hybridomas. Pretreatment with the indicated purified gangliosides altered CD1d-mediated NKT cell activation (E) Treatment of α-GalCer loaded CD1d-Ig aAPC with GD3 inhibits antigen presentation. α-GalCer - loaded aAPC were incubated for 4 h with either medium, GD3 (5μg/ml), or ascites fluid. The aAPC were washed extensively and then cocultured with the Vα14+ NKT cell hybridoma, N38-3C3. (F) Addition of anti-GD3 mAb to tumor associated ascites restores NKT cell recognition of CD1d molecules. LCD1dwt cells were treated with ascites (OC-91) or the ascites pretreated with an antibody specific for GD3 (clone R24) for 4 h, then washed extensively and cocultured with a panel of NKT cell hybridomas. IL-2 was measured, as an indication of NKT cell activation, by standard cytokine ELISA. Data are shown as mean ±S.E.M. of one experiment set up in triplicate. ** P≤0.01, *** P≤0.001. The experiments were performed three times.
In order to determine whether the inhibition observed following pretreatment with GD3 required antigen processing, we used CD1d-Ig based artificial antigen presenting cells (aAPC). CD1d-Ig based aAPC were, loaded with α-GalCer (16) and the loaded aAPCs were then treated with control medium, ascites fluid, or purified gangliosides. Treatment with GD3 completely inhibited α-GalCer presentation by aAPCs while treatment with control medium had only minimal effects on aAPC-mediated NKT cell activation (Fig. 3E). To further demonstrate that the presence of GD3 in ovarian cancer-associated ascites was responsible for the loss of NKT cell activation, we pretreated ascites with a monoclonal Ab specific for GD3. The presence of antibodies against GD3 significantly blocked the ascites-mediated NKT cell inhibition (Fig. 3F). Taken together, these data show that GD3 in ovarian cancer ascites fluid inhibits NKT activation and does not require further processing for its inhibitory effects.
In vivo treatment with GD3 abrogates α–GalCer- induced cytokine secretion by NKT cells
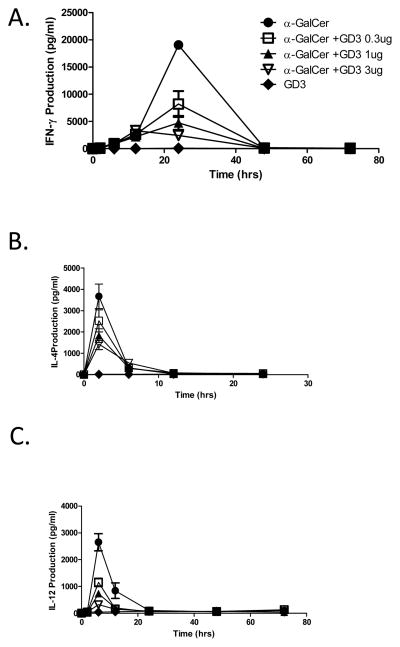
To test the effects of GD3 in vivo, we used an in vivo NKT cell activation model in which α-GalCer injection results in cytokine release by NKT cells. Mice were injected with α-GalCer, in the presence or absence of different concentrations of GD3. Blood samples were obtained at various time points, and serum cytokine levels were assessed. IFN-γ production was detectable at 2 h, with the peak production at 24 h after treatment and returned to baseline by 48 h (Fig. 4A). Treatment with GD3 resulted in a dose dependent inhibition of α–GalCer-induced IFN-γ production with half-maximal inhibition seen at 0.3μg GD3. In vivo administration of GD3 also inhibited IL-4 (Fig. 4B) and IL-12 production by α-GalCer stimulated NKT cells, as shown in Figure 4C.
Figure 4. GD3 inhibits α-GalCer-induced cytokine production in vivo.
(A) WT C57BL/6 mice were treated intravenously with 1 μg of either α-GalCer in the presence or absence of the indicated concentrations of GD3, and serum samples were obtained at the indicated time points after injection for ELISA analyses of IFN-γ, (B) IL-4, and (C) IL-12 concentrations. The data are expressed as the mean ± SD of two different dilutions of pooled sera. In all figures, the data represent one of two or more experiments with similar results.
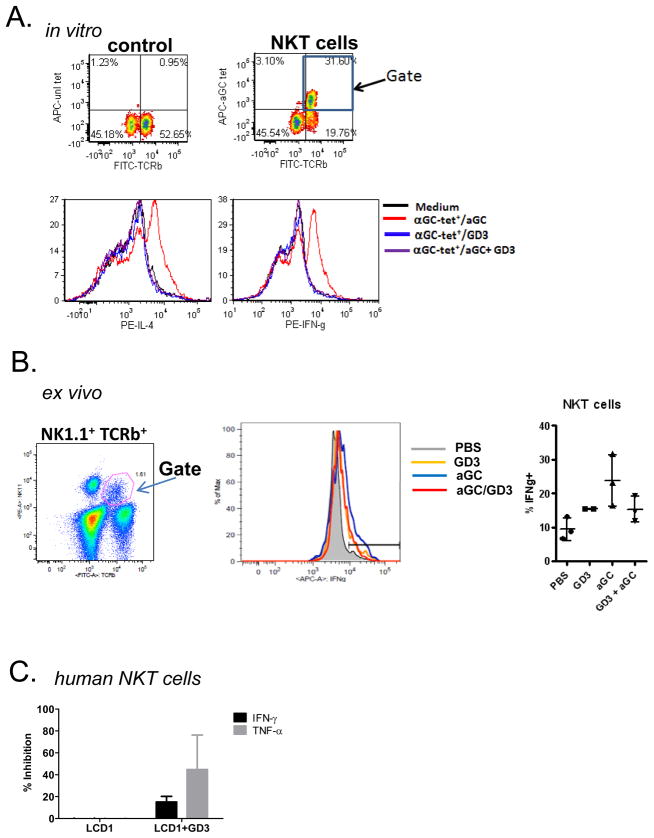
In order to determine if the GD3-mediated inhibition of α–GalCer-induced cytokine production was directly due to the suppression of NKT cell function, we next sought to determine whether GD3 could inhibit freshly prepared bulk NKT cells. Liver MNCs were isolated from C57BL/6 wildtype mice and pulsed with antigen or cultured in medium alone as a control and cytokine production assessed by ICS (Fig. 5A). To confirm whether reduced serum cytokine levels were indeed due to impaired production by NKT cells in the presence of GD3, liver MNCs were harvested 2 h after injection of α-GalCer and GD3 or the combination of both. Assessment of IFN-γ production by intracellular cytokine staining revealed that co-administration of GD3 with aGC reduced IFN-γ production by NKT cells (Fig 5B). Primary human NKT cells were also co-cultured with antigen presenting cells which had been pretreated with GD3 (Fig. 5C). α–GalCer-induced cytokine production was reduced in primary murine and human NKT cells following the addition of GD3.
Figure 5. Ganglioside GD3 treatment inhibits the activation of primary NKT cells.
(A) Liver MNC were harvested from C57BL/6 mice and were stained with either unloaded tetramer or α-GalCer loaded tetramer and mAbs specific for TCRβ (top panels). The CD1d-tetramer+TCRβ+ population was gated following stimulation withα–GalCer and induction of IL-4 (bottom right) or IFN-γ (bottom left) by NKT cells was assessed by flow cytometry. Data are from one experiment, representative of three independent experiments. (B) Ex vivo studies examining the effects of GD3 on α–GalCer mediated NKT cell activation. C57BL/6 mice were injected with 0.1 μg aGC, 2 μg GD3, or both. The liver MNCs were isolated and stained for intracellular IFN-γ production. (C) Primary human NKT cells were co-cultured with LCD1dwt cells pretreated with GD3.
GD3 binds with high affinity to CD1d molecules
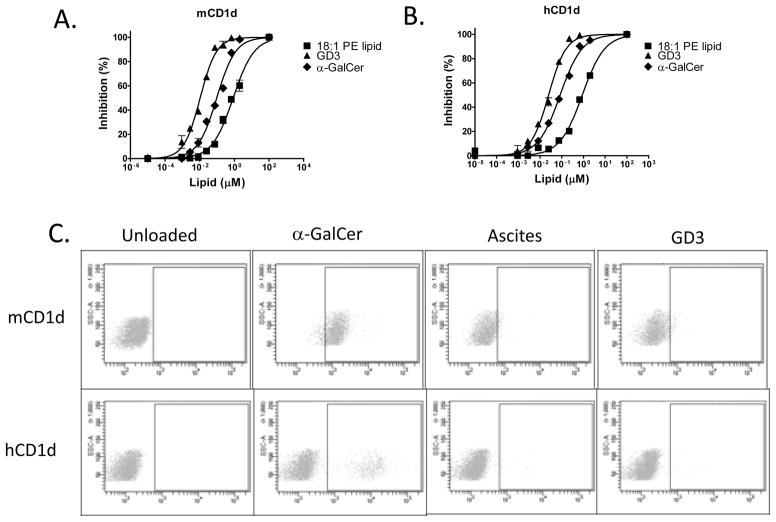
To analyze the mechanism of the inhibition, we studied the binding of GD3 to CD1d molecules using a competitive binding assay (20). A dose-dependent competition by GD3 to the tagged lipid 18:1 biotinyl-PE was observed for both human and mouse CD1d molecules (Figs. 6A & B). The IC50’s (24 nM for hCD1d and 11 nM for mCD1d) indicate that purified GD3 binds with significantly higher affinity to CD1d when compared to PE (IC50 >800 nM, Table 1). Notably the apparent affinity of GD3 was higher than that of α-GalCer (84 nM for hCD1d and 95 nM for mCD1d), this could be due to intrinsic differences in the affinities or differences in solubility. As this assay has been shown to reflect lipid-selectivity by CD1 molecules, these data indicate that GD3 binds strongly to both human and mouse CD1d molecules.
Figure 6. GD3 competes with PE for binding to CD1d.
(A, B) Nunc MaxiSorp flat-bottom 96 well plates were coated with goat anti-mouse IgG Fc gamma antibody and the plates were washed and blocked. Then the wells were coated with CD1d:IgG1 dimer, after washing, serially-diluted lipids were added into the wells in the presence of 2 μg/ml Biotinyl PE. The plates were then washed and the amounts of CD1d:mIgG1-Biotinyl PE complex was detected by adding HRP-labeled Avidin. Comparisons were done among all of the lipids (GD3, 18:1PE and α-GalCer) and the binding affinity between any two lipids are significantly different (C) GD3-loaded CD1d dimeric molecules do not stain NKT cells. Mouse CD1d:Ig dimer was incubated with α-GalCer, ascites, or GD3 to load the lipid onto the dimer, then used to stain NKT cells. The cells were washed and incubated with phycoerythrin-labeled rat anti-mouse IgG1 antibody (A85-1). After washing, the stained cells were analyzed by flow cytometry. Human CD1d:Ig dimer was incubated with α-GalCer, ascites, or GD3 to load the lipid onto the dimer, then used to stain primary NKT cells. The differences observed in binding between α–GalCer loaded mouse and human CD1d:Ig dimers may be due to the use of primary human T cells, rather than NKT cell hybridomas. In all figures, the data represent one of two or more experiments with similar results.
Table 1.
Relative binding affinity of GD3 to human and mouse CD1d molecules
| Ligand (Competitor) | IC50 in hCD1d (nM) | IC50 in mCD1d (nM) |
|---|---|---|
| 18:1 PE lipid | 882 ± 125 | 843 ± 181 |
| GD3(CHCl3+CH3OH) | 24.6 ± 3.2 | 10.5 ± 1.5 |
| α-GalCer | 84.0 ± 10.0 | 95.1 ± 18.3 |
We next examined whether CD1d-Ig dimers loaded with GD3 or ascites fluid could bind to NKT cells (Fig. 6C). Whereas the controls, α-GalCer loaded human and mouse CD1d-Ig dimers, bound to NKT cells, CD1d-Ig dimers loaded with GD3 or ascites did not. These data may explain the inhibitory effect mediated by ascites treatment. While GD3 binds with high affinity to CD1d molecules, those complexes do not bind to or activate NKT cells; thus GD3 present in the ascites may compete with and displace the endogenous stimulatory ligands for binding to CD1d molecules and thereby inhibit activation of NKT cells by their natural stimulatory ligands.
Discussion
Previous studies implicated various roles for specific T cell subsets in ovarian cancer (4–8). Preclinical studies and clinical trials have demonstrated that immunotherapy can effectively treat cancer; however, ovarian cancer immunotherapy needs to also focus on removing negative factors that could inhibit the immune response in order to maximize its therapeutic effects. In the current study we identified an inhibitory CD1d-binding ligand present in ovarian cancer ascites as the ganglioside GD3. Mechanistically, we found that GD3 inhibited the binding of CD1d-Ig dimers loaded withα-GalCer to NKT cells. Furthermore, GD3 bound with an “apparent” high affinity to both human and mouse CD1d. Finally in vivo treatment with GD3 inhibited α-GalCer mediated NKT cell activation. These studies thus identify GD3 as the first high affinity, competitive ligand that inhibits the activation of NKT cells in vitro and in vivo.
Specific gangliosides, such as GD3 and GM3, have been previously suggested to play a role in regulating NKT cell function. GD3 expression is upregulated on human melanomas and when mice were immunized with GD3 loaded DC, low levels of NKT specific GD3 response have been observed (25). A small fraction of the GD3-specific NKT cells can also cross-react with GM3. Analysis of the GM3 response showed that it partially inhibited some of the NKT-specific GD3 response, furthermore it had minimal effect on the α-GalCer induced IL-4 response, and no effect on α-GalCer induced IFN-γ responses. In contrast, we did not find a GD3-specific NKT cell response but rather that spontaneously secreted GD3 was a natural inhibitor of canonical, as well as non-canonical NKT cells. The difference between the two systems could be the fact that in the previous work, the goal was to see if GD3 could serve as a target for immunotherapy and in those experiments animals were immunized with DC-loaded GD3. In contrast, we looked at α-GalCer driven NKT cell responses in vitro and in vivo and found inhibition by GD3. This is consistent with the potential role of GD3 in the setting of ovarian cancer, where it could inhibit the endogenous NKT cell response and is consistent with the finding that ganglioside secretion is associated with decreased survival rates.
Using a quantitative binding assay, we measured the affinity of GD3 for CD1d molecules. The affinity of GD3 was high at 84nM and 95nM for human and mouse, respectively. In contrast most other naturally occurring ligands such as isoglobotrihexosylceramide (iGb3) are thought to be relatively low affinity ligands for CD1d, as determined by functional assays. Even glycolipids isolated from foreign pathogenic bacteria such as Sphingomonas are not as active as α-GalCer and are thought to be lower affinity ligands (13, 26, 27). The high affinity of GD3 for human and mouse CD1d may enable it to displace most NKT activating ligands, making it a uniquely potent immunomodulatory substance.
The proliferative index and metastatic status of malignant melanomas, renal cell carcinoma, and head and neck tumors are correlated with excessive synthesis of GD3 (28, 29). Although GD3 is expressed at a low level on normal melanocytes and a few other cell types, its highly restricted expression select tumor types makes it an attractive therapeutic target. In fact, it has been used for passive and active immunotherapy of melanomas and small cell lung cancers (30–32) (33). Our data suggest that therapeutic strategies targeting GD3 may also be useful for the treatment of ovarian cancer.
To our knowledge, this is the first report demonstrating a mechanism by which ovarian cancer-derived ascites inhibit NKT cell activation. Furthermore, GD3 is the only known endogenous ligand with such a high affinity for CD1d. Therefore, the presence of GD3 in the ascites, and its impact on the activation of NKT cells, may be a prognostic factor for ovarian cancer. While additional work is necessary, these studies indicate that GD3 may be important for early detection and monitoring of ovarian cancer.
Supplementary Material
Acknowledgments
The authors would like to thank Joan Glick Bieler for critical reading of the manuscript, Ophelia Rogers and Aaron Selya for technical assistance. The authors also would like to thank the NIH Tetramer Core Facility for providing CD1d tetramers. The authors (TJW, MO, & JPS) have filed a patent application on the use of GD3 as a biomarker or therapeutic target for ovarian cancer.
Grant Support
This work was supported by grants from the HERA foundation, American Cancer Society, and the NIH K01 131487 to T.J. Webb, 2004 Gynecologic Cancer Foundation/Ann Schreiber Ovarian Cancer Research Grant to R.L. Giuntoli, II, NIH AI 70258 to M. Tsuji and the NIH AI 44129, CA 108835, & P01 AI072677 to J.P. Schneck. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Abbreviations used
- α-GalCer
α-galactosylceramide
- aAPC
artificial antigen presenting cells
- Kd
dissociation constant
- Ki
binding affinities
- MNCs
mononuclear cells
Footnotes
The authors disclose no zpotential conflicts of interest
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Santin AD, Ravindranath MH, Bellone S, Muthugounder S, Palmieri M, O’Brien TJ, et al. Increased levels of gangliosides in the plasma and ascitic fluid of patients with advanced ovarian cancer. Bjog. 2004;111:613–8. doi: 10.1111/j.1471-0528.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 3.Valentino L, Moss T, Olson E, Wang HJ, Elashoff R, Ladisch S. Shed tumor gangliosides and progression of human neuroblastoma. Blood. 1990;75:1564–7. [PubMed] [Google Scholar]
- 4.Santin AD, Bellone S, Ravaggi A, Pecorelli S, Cannon MJ, Parham GP. Induction of ovarian tumor-specific CD8+ cytotoxic T lymphocytes by acid-eluted peptide-pulsed autologous dendritic cells. Obstet Gynecol. 2000;96:422–30. doi: 10.1016/s0029-7844(00)00916-9. [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishna V, Ross MM, Petersson M, Gatlin CC, Lyons CE, Miller CL, et al. Naturally occurring peptides associated with HLA-A2 in ovarian cancer cell lines identified by mass spectrometry are targets of HLA-A2-restricted cytotoxic T cells. Int Immunol. 2003;15:751–63. doi: 10.1093/intimm/dxg074. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 7.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 8.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sriram V, Cho S, Li P, O’Donnell PW, Dunn C, Hayakawa K, et al. Inhibition of glycolipid shedding rescues recognition of a CD1+ T cell lymphoma by natural killer T (NKT) cells. Proc Natl Acad Sci U S A. 2002;99:8197–202. doi: 10.1073/pnas.122636199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brutkiewicz RR, Bennink JR, Yewdell JW, Bendelac A. TAP-independent, β2-microglobulin-dependent surface expression of functional mouse CD1.1. J Exp Med. 1995;182:1913–9. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, et al. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J Immunol. 1998;161:3271–81. [PubMed] [Google Scholar]
- 13.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 14.Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc. 2008;3:70–8. doi: 10.1038/nprot.2007.515. [DOI] [PubMed] [Google Scholar]
- 15.Molling JW, Moreno M, van der Vliet HJ, von Blomberg BM, van den Eertwegh AJ, Scheper RJ, et al. Generation and sustained expansion of mouse spleen invariant NKT cell lines with preserved cytokine releasing capacity. J Immunol Methods. 2007;322:70–81. doi: 10.1016/j.jim.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Webb TJ, Bieler JG, Schneck JP, Oelke M. Ex vivo induction and expansion of natural killer T cells by CD1d1-Ig coated artificial antigen presenting cells. J Immunol Methods. 2009;346:38–44. doi: 10.1016/j.jim.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webb TJ, Giuntoli RL, 2nd, Rogers O, Schneck J, Oelke M. Ascites specific inhibition of CD1d-mediated activation of natural killer T cells. Clin Cancer Res. 2008;14:7652–8. doi: 10.1158/1078-0432.CCR-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnaar RL, Needham LK. Thin-layer chromatography of glycosphingolipids. Methods Enzymol. 1994;230:371–89. doi: 10.1016/0076-6879(94)30025-9. [DOI] [PubMed] [Google Scholar]
- 19.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631–41. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiratsuchi T, Schneck J, Kawamura A, Tsuji M. Human CD1 dimeric proteins as indispensable tools for research on CD1-binding lipids and CD1-restricted T cells. J Immunol Methods. 2009;345:49–59. doi: 10.1016/j.jim.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tupin E, Kronenberg M. Activation of natural killer T cells by glycolipids. Methods Enzymol. 2006;417:185–201. doi: 10.1016/S0076-6879(06)17014-7. [DOI] [PubMed] [Google Scholar]
- 22.Svennerholm L. Designation and schematic structure of gangliosides and allied glycosphingolipids. Prog Brain Res. 1994;101:XI–XIV. doi: 10.1016/S0079-6123(08)61935-4. [DOI] [PubMed] [Google Scholar]
- 23.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Marth JD, et al. Symbol nomenclature for glycan representation. Proteomics. 2009;9:5398–9. doi: 10.1002/pmic.200900708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JE, Wu DY, Prendes M, Lu SX, Ragupathi G, Schrantz N, et al. Fine specificity of natural killer T cells against GD3 ganglioside and identification of GM3 as an inhibitory natural killer T-cell ligand. Immunology. 2008;123:145–55. doi: 10.1111/j.1365-2567.2007.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–81. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu D, Zajonc DM, Fujio M, Sullivan BA, Kinjo Y, Kronenberg M, et al. Design of natural killer T cell activators: structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc Natl Acad Sci U S A. 2006;103:3972–7. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 28.Portoukalian J, David MJ, Shen X, Richard M, Dubreuil C. Tumor size-dependent elevations of serum gangliosides in patients with head and neck carcinomas. Biochem Int. 1989;18:759–65. [PubMed] [Google Scholar]
- 29.Ravindranath MH, Tsuchida T, Morton DL, Irie RF. Ganglioside GM3:GD3 ratio as an index for the management of melanoma. Cancer. 1991;67:3029–35. doi: 10.1002/1097-0142(19910615)67:12<3029::aid-cncr2820671217>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Nasi ML, Meyers M, Livingston PO, Houghton AN, Chapman PB. Anti-melanoma effects of R24, a monoclonal antibody against GD3 ganglioside. Melanoma Res. 1997;7 (Suppl 2):S155–62. [PubMed] [Google Scholar]
- 31.Forero A, Shah J, Carlisle R, Triozzi PL, LoBuglio AF, Wang WQ, et al. A phase I study of an anti-GD3 monoclonal antibody, KW-2871, in patients with metastatic melanoma. Cancer Biother Radiopharm. 2006;21:561–8. doi: 10.1089/cbr.2006.21.561. [DOI] [PubMed] [Google Scholar]
- 32.Giaccone G, Debruyne C, Felip E, Chapman PB, Grant SC, Millward M, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study) J Clin Oncol. 2005;23:6854–64. doi: 10.1200/JCO.2005.17.186. [DOI] [PubMed] [Google Scholar]
- 33.Lo AS, Ma Q, Liu DL, Junghans RP. Anti-GD3 chimeric sFv-CD28/T-cell receptor zeta designer T cells for treatment of metastatic melanoma and other neuroectodermal tumors. Clin Cancer Res. 2010;16:2769–80. doi: 10.1158/1078-0432.CCR-10-0043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.