Abstract
Pancreatic cancer has the poorest prognosis among all human malignant solid tumors, mainly due to its high invasive and metastatic biological features. microRNAs (miRNAs) are a group of endogenous and small non-coding RNA molecules 18–25 nucleotides in length, functioning as either tumor-suppressor genes or oncogenes. Evidence has shown that regulation of miRNAs in pancreatic cancer is associated with tumor growth, invasion, metastasis and resistance to therapy. Over the last decade, many studies have also found that there is a close relationship between miRNAs and biological characteristics of pancreatic cancer invasion and metastasis, such as the presence of cancer stem cells, epithelial-mesenchymal transition (EMT) phenotype, DNA methylation or epigenetic alteration, and the activation of some specific signaling pathways. Therefore, better understanding of the complex role of miRNAs in the development and progression of pancreatic cancer metastasis may provide new insights that could be of therapeutic consequence. In this brief review, we discuss the literature concerning the correlation between miRNAs and pancreatic cancer, focusing on miRNAs that contribute to pancreatic cancer invasion and metastasis, particularly on cancer stem cell characteristics, the EMT process, epigenetic modifications and tumor-associated signaling pathways.
Keywords: microRNAs, metastasis, pancreatic cancer
Contents
Introduction
microRNAs contribute to pancreatic cancer invasion and metastasis
microRNAs and epithelial to mesenchymal transition
microRNAs and cancer stem cells or tumor initiating cells
microRNAs and tumor-associated signaling pathways
microRNAs and epigenetic alterations
Conclusion
1. Introduction
Pancreatic cancer is the fourth most common cause of cancer-related death with 43,140 new diagnoses and 36,800 deaths during 2010 in the US (1). Surgical resection is currently the only curative option for pancreatic cancer, however, due to unusual biological property, high invasive ability and early metastatic feature, only ∼15 to 20% of tumors can be surgically removed when diagnosed. Even after radical resection, recurrence and metastasis occur within 1–2 years in the majority of cases and the prognosis remains poor (2). Therefore, there is a growing need to deeply understand the mechanisms of the invasive and metastatic profile of pancreatic cancer, which may ultimately lead to an improvement in the prognosis of this fatal disease.
microRNAs (miRNAs) are a class of natural small non-coding RNAs that target protein-coding mRNAs at the post-transcriptional level (3,4). Wightman and coworkers discovered the first miRNA in Caenorhabditis elegants in 1993 (5), and in 2000 the second miRNA, let-7, was found in the same species (6). Abnormal expression of miRNAs is fundamental to the development and progression of various cancers based on their involvement in basic cellular functions. The link between miRNAs and cancer was firstly demonstrated in 2002 by Calin et al (7), who found that miR-15 and miR-16 were involved in the pathogenesis of chronic lymphocytic leukemia. Subsequently, the critical role of miRNA in cancer initiation and progression has been reported in various cancers including pancreatic cancer (8–10). It was also demonstrated that there is a significantly different miRNA expression profile in metastatic carcinomas compared to non-metastatic tumors, and these metastasis-related miRNAs also significantly correlate with the survival of patients (11). Therefore, interference with the expression of these miRNAs may affect tumor metastasis and improve prognosis. In this review, we summarize several important miRNAs in pancreatic cancer progression, highlighting recent advances in elucidating the role of miRNAs in pancreatic cancer invasion and metastasis.
2. microRNAs contribute to pancreatic cancer invasion and metastasis
The role of miRNAs in the development of tumor invasion and metastasis was not discovered until 2007. Ma et al (12) reported that miR-10b initiated breast cancer invasion and metastasis. Subsequently, the important role of miRNA in cancer invasion and metastasis in various human malignant tumors, including liver, prostate, lung and colorectal cancers has been reported (13–17). In recent years, various miRNAs have also been found to play a significant role in pancreatic cancer invasion and metastasis. miR-21, which is strongly overexpressed in pancreatic cancer, has been proven to be an ‘oncogene’ miRNA and its high expression was found to contribute to poor overall survival and chemotherapy resistance (18,19). It has also been reported that miR-21 modulates pancreatic cancer cell growth and invasion. Pancreatic cancer cells transfected with miR-21 precursor were found to exhibit apparently increased cancer cell proliferation and invasion. Conversely, inhibition of miR-21 has an opposite outcome. Furthermore, the level of metastasis-related genes, matrix metalloproteinase-2 and -9, and vascular endothelial growth factor were positively correlated with miR-21 expression, suggesting that MMP-2, MMP-9 and VEGF are ‘indirect’ target genes of miR-21 (20).
miR-146a has been found to be silent in multiple human cancers, and restoration of its expression can reduce the meta-static potential of cancer cells through suppression of IRAK-1 and subsequent inhibition of NF-κB activity (15,21,22), which is activated in a number of human cancers and involved in promoting tumor development, invasion and metastasis (23,24). In pancreatic cancer, miR-146a is also downregulated, and re-expression of miR-146a inhibits the invasive capacity of pancreatic cancer cells. Notably, a number of studies have indicated that the natural dietary compounds 3,3′-diindolyl-methane (DIM) or isoflavone may upregulate miR-146a and inhibit cancer cell invasion. Further mechanistic analysis suggests that miR-146a regulates a set of genes and suppresses cancer cell invasion and migration through targeting EGFR and IRAK-1 (25). Another study also revealed that miR-146b-5p was significantly downregulated in human pancreatic cancer cells, and overexpressed miR-146b-5p notably reduced the abilities of invasion and migration of MIAPaCa-2 pancreatic cancer cells by targeting MMP-16 (26).
In addition, miR-27a is abnormally upregulated in pancreatic adenocarcinoma and inhibition of miR-27a was found to suppress the growth, colon formation and migration of pancreatic cancer cells by targeting Sprouty2, which is an antagonist of the RAS/MAPK signaling pathway in cancers (27). More recently, Yu et al (28) reported that re-expression of miR-200c appeared to be associated with upregulation of E-cadherin, a gene known to be involved in inhibiting the invasion of pancreatic cancer cells. Wang et al (29) identified that miR-520h downregulated ABCG2 in pancreatic cancer cells to inhibit migration, invasion and side population, and indicated that it could be a potential therapeutical target for pancreatic cancer. Preis et al (30) found that miR-10b was overexpressed in pancreatic ductal adenocarcinoma tissues and lower levels of miR-10b were linked with improved response to neoadjuvant therapy, delayed time to metastasis and increased survival. Srivastava et al (31) reported that the level of miR-150 was significantly lower in pancreatic tumors compared with matched normal pancreas tissue. Furthermore, ectopic expression of miR-150 significantly inhibited pancreatic cancer cell invasion and migration as well as tumor growth by suppressing the MUC4 gene in vitro. Other miRNAs have also been reported to play an important role in pancreatic cancer cell invasion and migration, such as miR-17-5p, miR-29a and miR-20a (32–36). Results from these studies may provide an opportunity to carry out miRNA-based therapeutic intervention for pancreatic cancer metastasis (Table I).
Table I.
Pancreatic cancer invasion and metastasis-related miRNAs.
| miRNA | Up/downregulated | Potential target | Materials | Reference |
|---|---|---|---|---|
| miR-21 | Up | MMP-2, MMP-9, VEGF | PANC-1, AsPC-1, CFPAC-1, SUIT-2 | (20) |
| miR-146a | Down | EGFR, NF-κB, MTA-2, IRAK-1 | Colo357, PANC-1 | (25) |
| miR-146b-5p | Down | MMP16 | MIA-PaCa and FFPE | (26) |
| miR-27a | Up | Spry2 | PANC-1, MIA-PaCa-2, FFPE | (27) |
| miR-200c | Down | E-cadherin | FFPE, SUIT-2, KP-3, PANC-1 | (28) |
| miR-520h | Up | ABCG2 | PANC-1 | (29) |
| miR-10b | Up | nc | FFPE, EUS-FNA sample | (30) |
| miR-150 | Down | MUC4 | Panc10.05, HPAF, Colo357 | (31) |
| miR-17-5P | Up | nc | FFPE, SUIT-2, KP-2 | (32) |
| miR-29a | Down | nc | PANC-1 | (33) |
| miR-20a | Uown | Stat3 | PANC-1, BxPC-3 | (34) |
| miR-126 | Down | ADAM9 | PANC-1, ASPC-1, FFPE, FNA | (35) |
| miR-26a | Down | HMGA1 | PANC-1, Sw1990, nude mice | (36) |
| miR-96 | Down | KRAS | MIA-PaCa-2, PANC-1, FFPE, nude mice | (78) |
MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor; NF-κB, nuclear factor κ B; MTA-2, metastasis-associated protein 2; IRAK-1, interleukin 1 receptor associated kinase 1; MMP16, matrix metalloproteinase-16; Spry2, Sprouty2; ABCG2, breast cancer resistance protein; MUC4, mucin 4; Stat3, transcription proteins 3; ADAM9, disintegrin and metalloproteinase domain-containing protein 9; HMGA1, high mobility group A 1; KRAS, v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; nc, not clear.
3. microRNAs and epithelial to mesenchymal transition
The epithelial to mesenchymal transition (EMT) is a process by which epithelial cells lose their polarity and are converted to a mesenchymal phenotype, which is regarded as a critical event in morphogenetic changes during embryonic development, wound healing and malignant tumor progression (37,38). This process is accompanied by detachment of cells from each other and subsequent increased cell movement and dissemination. Increasing evidence shows that aberrant activation of EMT is a trigger of malignant tumor invasion and metastasis in various human cancers (39–41). The transcriptional repressor zinc-finger E-box binding homeobox 1 (ZEB1), a crucial inducer of EMT, was recently shown to promote cancer invasion and metastasis in vitro and in vivo (42). Furthermore, members of the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141) induce epithelial differentiation through direct targeting of ZEB1 and ZEB2 (43) whereas ZEB1 was reported to directly suppress the transcription of two members of the miR-200a family (miR-200c and miR-141). Therefore, several researchers suggest that there is a feedforward loop between the miR-200 family and ZEB1, which promotes EMT and invasion in cancer cells (44).
Notably, this feedforward loop also exists in pancreatic cancer. Wellner et al (45) reported that expression of miR-200c and miR-203 is low in pancreatic cancer, while ZEB1 is overexpressed. In vitro, silencing of ZEB1 by RNA interference in PANC-1 and MIA-PaCa-2 cell lines resulted in an epithelial transition. In orthotopic xenograft models, injection of ZEB1-knockdown cell clones resulted in smaller primary tumors and less invasion and distant metastasis than the control group. In another study, the level of the miR-200 family was found to be significantly low in gemcitabine-resistant cancer cells, which showed a typical EMT characteristic, and re-expression of miR-200 could be essential for the reversal of the EMT phenotype, and thus inhibit cancer cell invasion and metastasis through increasing the epithelial marker E-cadherin expression and suppressing the expression of ZEB1, slug and vimentin. Furthermore, the natural agent B-DIM and isoflavone may also significantly upregulate the expression of let-7, which is known as a tumor-suppressor miRNA (46,47), consequently inhibiting pancreatic cancer progression by reversing EMT characteristics (48) (Fig. 1).
Figure 1.
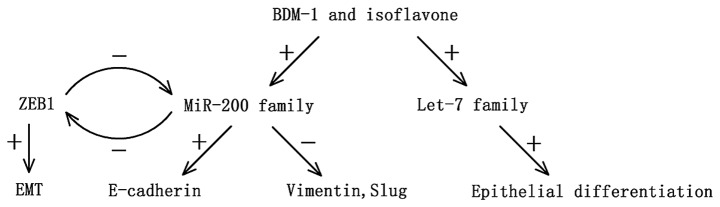
The feedforward loop between miRNAs and ZEB1 in pancreatic cancer.
In summary, the expression of various miRNAs may affect EMT characteristics, whereas the EMT process is necessary for the invasion and metastasis of pancreatic cancer. Therefore, identification of the aberrant expression of these miRNAs may provide insight concerning important mechanisms that contribute to pancreatic cancer development and progression.
4. microRNAs and cancer stem cells or tumor-initiating cells
Cancer stem cells (CSCs) or tumor-initiating cells are a small population of cells within a tumor that initiate proliferation, possess the capacity for self-renewal and ensure multilineage differentiation. The presence of CSCs is considered as the origination of various malignant biological behaviors in human cancers, especially involved in cancer cell migration, invasion and metastasis (49). CSCs were initially discovered in myeloid leukemia cells by Bonnet and Dick (50) in 1997. Subsequently, the existence of CSCs was verified in various cancers, including breast, colorectal and brain cancer (51–53). In 2007, a small subset of highly tumorigenic cancer cells with the cell surface markers CD44, CD24, epithelial-specific antigen (ESA) triple-positive expression, was found in human pancreatic cancer cells (54), which was proven to possess the characteristics of CSCs. Meanwhile, a subpopulation of CD133+, CXCR4+ pancreatic cancer stem cells was verified and confirmed to be essential for tumor metastasis (55). Furthermore, CD133+ pancreatic cancer cells were also found to be related with increased cell proliferation, migration and invasion (56). All of these findings indicate that the presence of CSCs is responsible for the high invasive and metastatic phenotype of pancreatic cancer.
As discussed above, there is a feedforward loop between the miR-200 family and ZEB1 that promotes EMT and invasion in pancreatic cancer cells. It has also been reported that ZEB1 promotes EMT activation and maintains the stemness of cancer cells by suppressing the miR-200 family and miR-203, which is also known as a stemness-inhibiting miRNA, consequently promoting pancreatic cancer invasion and metastasis. In addition, the miR-200 family and miR-203 were found to suppress the expression of ‘stem cell factors’ such as Sox2, Kif4 and the polycomb repressor Bmi-1 in cancer cells (45). These data suggest that the miR-200 family and miR-203 may prevent pancreatic cancer invasion and metastasis not only by reversion of epithelial to mesenchymal transition, but also by suppressing cancer stem cells.
DCAMKL-1, a microtubule-associated kinase expressed in post-mitotic neurons, is upregulated in human pancreatic cancer and was identified as a putative cancer stem cell marker (57). Silencing of DCAMK-1 in pancreatic cancer cells resulted in suppression of snail, slug, twist and upregulation of miR-200a, which inhibits EMT by repressing the transcription factor ZEB1 and ZEB2 with subsequent rescue of E-cadherin, a marker of epithelial lineage. It was also suggested that DCAMKL-1 regulates epithelial-mesenchymal transition through a miR-200a-dependent mechanism, contributing to pancreatic cancer development, invasion and metastasis. In addition, it was found that DCAMKL-1 knockdown resulted in downregulation of c-Myc and KRAS through a let-7a miRNA-dependent mechanism, and inhibition of the Notch-1 pathway by upregulation of miR-144 (58). These results suggest that miRNAs may modulate tumor stem cell properties by regulating their target or related genes.
miR-34 was identified as a p53 target and a tumor-suppressor miRNA (59,60). It has been reported that miR-34 is involved in pancreatic cancer stem cell self-renewal and differentiation. In addition, restoration of miR-34 significantly inhibited the p53-mutant human pancreatic cancer cell growth and invasion and finally led to an 87% reduction in the CD133+/CD44+ tumor-initiating cell population. Furthermore, miR-34 was found to inhibit its target genes Notch and Bcl-2, ‘stem cell genes’ to achieve a reduction in cancer stem cells (61). A similar function of miR-34 has also been reported in gastric cancer (62). In addition, it was reported that the curcumin analogue CDF may attenuate EZH2 and other cancer stem cell marker genes, such as Nanog, CD44 and EpCAM through upregulation of tumor-suppressive miRNAs (miR-26a, miR-101, miR-146a, miR-200b and c, let-7a, b, c and d), thus decreasing cell growth, clonogenicity, cell migration and the elimination of CSCs (63).
It is possible that there is a unique miRNA signature responsible for the maintenance and enrichment of cancer stem cells. These stem cell-specific miRNAs may influence pancreatic cancer invasion and metastasis by regulating the ‘stemness’ and also may provide a potential therapeutic target for pancreatic cancer metastasis (Table II).
Table II.
miRNAs and pancreatic cancer stem cell markers.
| miRNA | Up/downregulated | Related stem cell markers | Materials | Reference |
|---|---|---|---|---|
| miR-200 family and miR-203 | Down | ZEB1 | MIA-PaCa-2, BxPC-3, PANC-1 | (45) |
| miR-200a, let-7 and miR-144 | Down | DCAMKL-1 | AsPC-1, BxPC-3 | (58) |
| miR-34 | Down | CD133/CD44 | MIA-PaCa-2, BxPC-3 | (61) |
| miR-101 | Down | EZH2, EpCAM, Nanog, Sox2, Oct4 | MIA-PaCa-2 | (63) |
ZEB1, zinc finger E-box binding homeobox 1; DCAMKL-1, doublecortin and CaM kinase-like-1; EZH2, enhancer of zeste homolog 2; EpCAM, epithelial cell adhesion molecule; Nanog, Nanog homeobox; Sox2, SRY-box containing gene 2.
5. microRNAs and tumor-associated signaling pathways
It is well known that various signaling pathways such as Notch, hedgehog and Wnt/β-catenin are dysregulated in human carcinomas including pancreatic cancer consequently contributing to tumor development and progression. It has also been shown that several miRNAs appear to possess various essential functions in cancer invasion and metastasis through regulating important signaling pathways. Therefore, the interaction between miRNAs and tumor progression-related signaling pathways in human cancer must be elucidated in detail.
The Notch signaling pathway has been well-documented to be involved in the regulation of numerous cellular process, including cell proliferation, apoptosis, differentiation, invasion and metastasis (64,65). The Notch pathway has been reported to play both oncogenic and tumor-suppressor roles in multiple human cancers (66–68). In pancreatic cancer, the Notch signaling pathway is frequently deregulated with upregulated expression of Notch receptors and their ligands, and functions as an oncogene during tumor growth and progression (69). This is thought to be related to the absence of miR-34. As discussed above, in CD44+/CD133+ MIA-PaCa-2 cancer stem cells with high Notch/Bcl-2 levels, miR-34 restoration significantly inhibits tumor growth and formation in vitro and in vivo. It has also been reported that miR-34 is silent in pancreatic cancer, and restoration of miR-34 expression in pancreatic cancer cell lines MIA-PaCa-2 and Bxpc-3 downregulates the expression of Bcl-2 and Notch1/2, subsequently inhibiting clonogenic cell growth and cell invasion (61). Recently, Nalls et al (70) reported that upregulation of miR-34a by 5-Aza-dC and HDAC inhibitor vorinostat (SAHA) in pancreatic cancer cells suppressed the cell proliferation, cell cycle progression, cell self-renewal, cell invasion and EMT process. Moreover, re-expression of miR-34 by SAHA in pancreatic cancer stem cells inhibited the mRNA expression of all the components of the Notch pathway, including Notch receptor, Notch-1, Notch-3, its ligand Jagged 1 and Notch target gene Hes1; thus miR-34a may inhibit cancer invasion and progression by targeting the Notch signaling pathway (70). In addition, overexpression of Notch-1 in AsPC-1 cells leads to increased cell growth, invasion, clonogenicity, migration, induction of EMT phenotype and decreased expression of miR-200a, miR-200c, let7a, let7b and let7c, while re-expression of miR-200b attenuates the acquisition of the EMT phenotype in large part due to the downregulation of the expression of Notch-1 in Notch-1-overexpressed AsPC-1 cells (71).
The Sonic hedgehog (Shh) pathway is aberrantly activated and plays a decisive role in the development and progression of multiple human cancers (72). In contrast, the blockage of the hedgehog pathway could inhibit cancer invasion and metastasis in vivo (73). Increasing evidence supports the possibility that there is an inner link between miRNAs and the hedgehog signaling pathway in human cancers (74,75). It was reported that certain miRNAs activate the hedgehog signaling pathway by targeting Smo, Cos2 and Fu in Drosophila, which are antagonistic components of the hedgehog pathway (76). Moreover, Tsuda et al (77) reported that synthetic Gli-1-miRNA-3548 and its corresponding Duplex-3548 inhibited cell division and stimulated apoptosis of MIA-PaCa-2 cells through targeting of the Gli-1 gene which controls the pathway of Shh signaling; this suggests that Gli-1 inhibition with miRNA may be a potentially novel approach to pancreatic cancer therapy.
Moreover, miRNAs promote pancreatic cancer invasion and metastasis through the regulation of other tumor associated signaling pathways including RAS, TGF-β and NF-κB. For example, ectopic expression of miR-96 inhibited pancreatic cancer invasion and migration with KRAS down-regulation in vitro and in vivo, and miR-96 was identified as a core regulator of the KRAS/AKT pathway (78).
These studies suggest that various miRNAs play key roles in multiple signal pathways that are responsible for pancreatic cancer invasion and metastasis, and these miRNAs are essential for the unique metastatic characteristics of pancreatic cancer.
6. microRNAs and epigenetic alterations
Epigenetic modification such as DNA hypermethylation could have a great impact on the expression of miRNAs, and thus contribute to cancer development and progression. Frequent DNA methylation of cytosine-phospho-guanine (-CpG) island regions adjacent to miR-34a and miR-34b/c, the direct p53 target genes, was observed in various human malignant tumors, including colorectal, prostate and pancreatic cancer, which may mediate cancer cell apoptosis, cell cycle arrest and senescence (79). Epigenetic silencing of miR-107 contributes to MIA-PaCa-2 and PANC-1 cell growth through regulating the expression of cyclin-dependent kinase 6 (CDK-6), which is a cyclin-D1-dependent kinase facilitating cell cycle progression by regulating the activity of tumor-suppressor protein Rb (80). In addition, the epigenetic inactivation of miRNAs can be reversed by treatment with the DNA demethylating agent, 5-aza-2,-deoxycytidine (DAC) and the histone deacetylase inhibitor, trichostatin A (TSA) (81). Thus, it is possible that aberrant miRNA epigenetic alterations could also play a vital role in pancreatic cancer invasion and metastasis. Recently, miR-224 and miR-486 were found to be significantly overexpressed with epigenetic alterations in highly invasive and metastatic pancreatic cancer, while protein expression of the cell surface marker CD40, a member of the tumor necrosis factor family related to anti-tumor immune responses, was low (82,83). Thus, miRNA-regulated CD40 expression seems to be essential for pancreatic cancer invasion and the process of metastasis (84).
EP-300 is a group of proteins that function as transcriptional coactivators and are also involved in tumor development and progression (85,86). By using 16 human PDAC cell lines in a murine orthotopic PDAC model, Mees et al (87) reported that epigenetic alterations with upregulation of miR-184, miR-200b, miR-200c and miR-429, which target EP300, are likely to cause downregulation of EP300 mRNA and protein expression in highly metastatic PDAC cell lines. The authors also indicated that miRNAs may be able to affect metastatic behavior of pancreatic cancer by modulating the expression of metastasis-related suppressor genes, such as EP-300 (87).
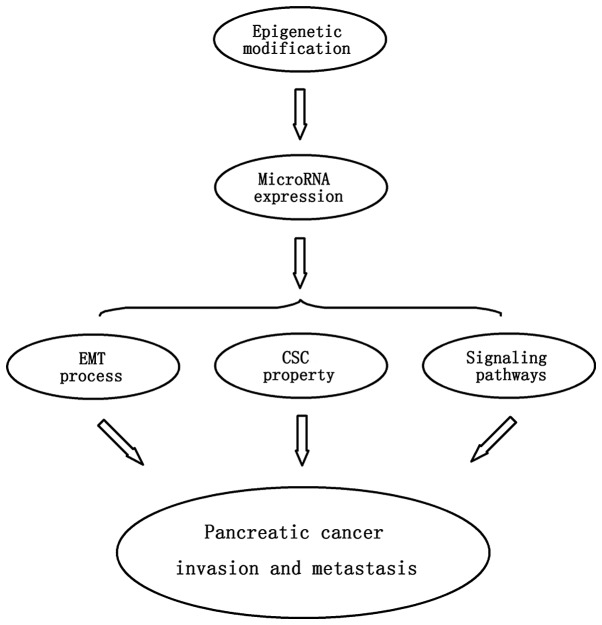
In summary, the expression of various specific miRNAs functioning as tumor-suppressor genes or oncogenes may be regulated by epigenetic modifications such as DNA methylation, finally contributing to pancreatic cancer progression and metastasis. As previously mentioned, differential expression of miRNAs may affect the EMT process, cancer stem cell properties and specific tumor signaling pathways, which may ultimately modulate cancer invasion and metastasis. Therefore, the epigenetic modification responsible for abnormal expression of miRNAs in pancreatic tumors may be a crucial mechanism contributing to cancer invasion and metastasis (Fig. 2).
Figure 2.
Abnormal expression of miRNAs with epigenetic modification contributes to pancreatic cancer invasion and metastasis through regulation of the EMT process, cancer stem cell properties and signaling pathways.
7. Conclusion
Although knowledge concerning the role of miRNA in the regulation of cancer development and progression has been greatly advanced since the first discovery of miRNA nearly 18 years ago, the details of miRNA-specific molecular pathogenesis of pancreatic cancer still remain to be elucidated. Recent studies suggest that a variety of miRNAs are frequently dysregulated in pancreatic cancer and have a crosstalk with various important biological processes including DNA methylation, the presence of cancer stem cells, the process of EMT and associated signaling pathways, which may be crucial in tumorigenesis and metastasis. Moreover, research concerning the targeting of miRNAs in vitro and in vivo has demonstrated amazing results in altering the features of pancreatic cancer invasion and metastasis. Various related miRNAs affect pancreatic cancer invasion and metastasis through the regulation of the EMT process, cancer stem cell properties, and tumor-associated signaling pathways, while epigenetic modification may be an important mechanism causing dysregulation of miRNA expression. Therefore, detailed exploration of miRNA molecular mechanisms not only contributes to the understanding of the origin of the highly metastatic properties of pancreatic cancer, but may also provide insight for the improvement of the prognosis for this fatal disease.
Acknowledgments
This study was supported by National Natural Science Foundation of China grants (Nos. 81172276 and 81001058).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Zuckerman DS, Ryan DP. Adjuvant therapy for pancreatic cancer: a review. Cancer. 2008;112:243–249. doi: 10.1002/cncr.23174. [DOI] [PubMed] [Google Scholar]
- 3.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 4.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 5.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 6.Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 7.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloomston M, Frankel WL, Petrocca F, et al. microRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;229:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 9.Lee JW, Choi CH, Choi JJ, et al. Altered microRNA expression in cervical carcinomas. Clin Cancer Res. 2008;14:2535–2542. doi: 10.1158/1078-0432.CCR-07-1231. [DOI] [PubMed] [Google Scholar]
- 10.Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 11.Budhu A, Jia HL, Forgues M, et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 12.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 13.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 15.Lin SL, Chiang A, Chang D, Ying SY. Loss of mir-146a function in hormone-refractory prostate cancer. RNA. 2008;14:417–424. doi: 10.1261/rna.874808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford M, Brawner E, Batte K, et al. MicroRNA-126 inhibits invasion in non-small cell lung carcinoma cell lines. Biochem Biophys Res Commun. 2008;373:607–612. doi: 10.1016/j.bbrc.2008.06.090. [DOI] [PubMed] [Google Scholar]
- 17.Huang Q, Gumireddy K, Schrier M, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 18.Hwang JH, Voortman J, Giovannetti E, et al. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PloS One. 2010;5:e10630. doi: 10.1371/journal.pone.0010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovannetti E, Funel N, Peters GJ, et al. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70:4528–4538. doi: 10.1158/0008-5472.CAN-09-4467. [DOI] [PubMed] [Google Scholar]
- 20.Moriyama T, Ohuchida K, Mizumoto K, et al. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol Cancer Ther. 2009;8:1067–1074. doi: 10.1158/1535-7163.MCT-08-0592. [DOI] [PubMed] [Google Scholar]
- 21.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–5647. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kogo R, Mimori K, Tanaka F, Komune S, Mori M. Clinical significance of miR-146a in gastric cancer cases. Clin Cancer Res. 2011;17:4277–4284. doi: 10.1158/1078-0432.CCR-10-2866. [DOI] [PubMed] [Google Scholar]
- 23.Fujioka S, Sclabas GM, Schmidt C, et al. Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res. 2003;9:346–354. [PubMed] [Google Scholar]
- 24.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Vandenboom TG, II, Wang Z, et al. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin F, Wang X, Jie Z, et al. Inhibitory effects of miR-146b-5p on cell migration and invasion of pancreatic cancer by targeting MMP16. J Huazhong Univ Sci Technolog Med Sci. 2011;31:509–514. doi: 10.1007/s11596-011-0481-5. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, Yu S, Zhao W, Lu Z, Chen J. miR-27a regulates the growth, colony formation and migration of pancreatic cancer cells by targeting Sprouty2. Cancer Lett. 2010;298:150–158. doi: 10.1016/j.canlet.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Ohuchida K, Mizumoto K, et al. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer. 2010;9:169. doi: 10.1186/1476-4598-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Xue X, Wei J, et al. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer. 2010;103:567–574. doi: 10.1038/sj.bjc.6605724. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Preis M, Gardner TB, Gordon SR, et al. MicroRNA-10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17:5812–5821. doi: 10.1158/1078-0432.CCR-11-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava SK, Bhardwaj A, Singh S, et al. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32:1832–1839. doi: 10.1093/carcin/bgr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Ohuchida K, Mizumoto K, Fujita H, Nakata K, Tanaka M. MicroRNA miR-17-5p is overexpressed in pancreatic cancer, associated with a poor prognosis, and involved in cancer cell proliferation and invasion. Cancer Biol Ther. 2010;10:748–757. doi: 10.4161/cbt.10.8.13083. [DOI] [PubMed] [Google Scholar]
- 33.Muniyappa MK, Dowling P, Henry M, et al. MiRNA-29a regulates the expression of numerous proteins and reduces the invasiveness and proliferation of human carcinoma cell lines. Eur J Cancer. 2009;45:3104–3118. doi: 10.1016/j.ejca.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Yan H, Wu J, Liu W, et al. MicroRNA-20a overexpression inhibited proliferation and metastasis of pancreatic carcinoma cells. Hum Gene Ther. 2010;21:1723–1734. doi: 10.1089/hum.2010.061. [DOI] [PubMed] [Google Scholar]
- 35.Hamada S, Satoh K, Fujibuchi W, et al. MiR-126 acts as a tumor suppressor in pancreatic cancer cells via the regulation of ADAM9. Mol Cancer Res. 2012;10:3–10. doi: 10.1158/1541-7786.MCR-11-0272. [DOI] [PubMed] [Google Scholar]
- 36.Li W, Yuan Y, Huang L, Qiao M, Zhang Y. Metformin alters the expression profiles of microRNAs in human pancreatic cancer cells. Diabetes Res Clin Pract. 2012;96:187–195. doi: 10.1016/j.diabres.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 37.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelialmesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 39.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 40.Xiong H, Hong J, Du W, et al. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J Biol Chem. 2012;287:5819–5832. doi: 10.1074/jbc.M111.295964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, Wirth T. NF-kappaB promotes epithelialmesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett. 2010;295:214–228. doi: 10.1016/j.canlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Spaderna S, Schmalhofer O, Wahlbuhl M, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 43.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burk U, Schubert J, Wellner U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 46.Torrisani J, Bournet B, du Rieu MC, et al. let-7 microRNA transfer in pancreatic cancer-derived cells inhibits in vitro cell proliferation but fails to alter tumor progression. Hum Gene Ther. 2009;20:831–844. doi: 10.1089/hum.2008.134. [DOI] [PubMed] [Google Scholar]
- 47.Ali S, Almhanna K, Chen W, Philip PA, Sarkar FH. Differentially expressed miRNAs in the plasma may provide a molecular signature for aggressive pancreatic cancer. Am J Transl Res. 2010;3:28–47. [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, VandenBoom TG, II, Kong D, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea - a paradigm shift. Cancer Res. 2006;66:1883–1890. 1895–1896. doi: 10.1158/0008-5472.CAN-05-3153. discussion. [DOI] [PubMed] [Google Scholar]
- 50.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 51.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 52.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Scie USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 54.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 55.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Moriyama T, Ohuchida K, Mizumoto K, et al. Enhanced cell migration and invasion of CD133+ pancreatic cancer cells cocultured with pancreatic stromal cells. Cancer. 2010;116:3357–3368. doi: 10.1002/cncr.25121. [DOI] [PubMed] [Google Scholar]
- 57.May R, Sureban SM, Lightfoot SA, et al. Identification of a novel putative pancreatic stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas. Am J Physiol Gastrointest Liver Physiol. 2010;299:G303–310. doi: 10.1152/ajpgi.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sureban SM, May R, Lightfoot SA, et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71:2328–2338. doi: 10.1158/0008-5472.CAN-10-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarasov V, Jung P, Verdoodt B, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 60.Bommer GT, Gerin I, Feng Y, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 61.Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PloS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ji Q, Hao X, Meng Y, et al. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bao B, Ali S, Banerjee S, et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72:335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Z, Li Y, Banerjee S, Sarkar FH. Emerging role of Notch in stem cells and cancer. Cancer Lett. 2009;279:8–12. doi: 10.1016/j.canlet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demarest RM, Ratti F, Capobianco AJ. It’s T-ALL about Notch. Oncogene. 2008;27:5082–5091. doi: 10.1038/onc.2008.222. [DOI] [PubMed] [Google Scholar]
- 66.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27:5115–5123. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 68.Strizzi L, Hardy KM, Seftor EA, et al. Development and cancer: at the crossroads of Nodal and Notch signaling. Cancer Res. 2009;69:7131–7134. doi: 10.1158/0008-5472.CAN-09-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ristorcelli E, Lombardo D. Targeting Notch signaling in pancreatic cancer. Expert Opin Ther Targets. 2010;14:541–552. doi: 10.1517/14728221003769895. [DOI] [PubMed] [Google Scholar]
- 70.Nalls D, Tang SN, Rodova M, Srivastava RK, Shankar S. Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells. PloS One. 2011;6:e24099. doi: 10.1371/journal.pone.0024099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bao B, Wang Z, Ali S, et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Matthaios D, Zarogoulidis P, Balgouranidou I, Chatzaki E, Kakolyris S. Molecular pathogenesis of pancreatic cancer and clinical perspectives. Oncology. 2011;81:259–272. doi: 10.1159/000334449. [DOI] [PubMed] [Google Scholar]
- 73.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferretti E, De Smaele E, Miele E, et al. Concerted microRNA control of Hedgehog signalling in cerebellar neuronal progenitor and tumour cells. EMBO J. 2008;27:2616–2627. doi: 10.1038/emboj.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Northcott PA, Fernandez LA, Hagan JP, et al. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69:3249–3255. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Friggi-Grelin F, Lavenant-Staccini L, Therond P. Control of antagonistic components of the hedgehog signaling pathway by microRNAs in Drosophila. Genetics. 2008;179:429–439. doi: 10.1534/genetics.107.083733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuda N, Ishiyama S, Li Y, Ioannides CG, Abbruzzese JL, Chang DZ. Synthetic microRNA designed to target glioma-associated antigen 1 transcription factor inhibits division and induces late apoptosis in pancreatic tumor cells. Clin Cancer Res. 2006;12:6557–6564. doi: 10.1158/1078-0432.CCR-06-0588. [DOI] [PubMed] [Google Scholar]
- 78.Yu S, Lu Z, Liu C, et al. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70:6015–6025. doi: 10.1158/0008-5472.CAN-09-4531. [DOI] [PubMed] [Google Scholar]
- 79.Vogt M, Munding J, Gruner M, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–322. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 80.Tashiro E, Tsuchiya A, Imoto M. Functions of cyclin D1 as an oncogene and regulation of cyclin D1 expression. Cancer Sci. 2007;98:629–635. doi: 10.1111/j.1349-7006.2007.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee KH, Lotterman C, Karikari C, et al. Epigenetic silencing of MicroRNA miR-107 regulates cyclin-dependent kinase 6 expression in pancreatic cancer. Pancreatology. 2009;9:293–301. doi: 10.1159/000186051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 83.Costello RT, Gastaut JA, Olive D. What is the real role of CD40 in cancer immunotherapy? Immunology Today. 1999;20:488–493. doi: 10.1016/s0167-5699(99)01507-8. [DOI] [PubMed] [Google Scholar]
- 84.Mees ST, Mardin WA, Sielker S, et al. Involvement of CD40 targeting miR-224 and miR-486 on the progression of pancreatic ductal adenocarcinomas. Ann Surg Oncol. 2009;16:2339–2350. doi: 10.1245/s10434-009-0531-4. [DOI] [PubMed] [Google Scholar]
- 85.Kouzarides T. Histone acetylases and deacetylases in cell proliferation. Curr Opin Genet Dev. 1999;9:40–48. doi: 10.1016/s0959-437x(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 86.Ida K, Kitabayashi I, Taki T, et al. Adenoviral E1A-associated protein p300 is involved in acute myeloid leukemia with t(11;22) (q23;q13) Blood. 1997;90:4699–4704. [PubMed] [Google Scholar]
- 87.Mees ST, Mardin WA, Wendel C, et al. EP300 - a miRNA-regulated metastasis suppressor gene in ductal adenocarcinomas of the pancreas. Int J Cancer. 2010;126:114–124. doi: 10.1002/ijc.24695. [DOI] [PubMed] [Google Scholar]