Abstract
Background
Low vitamin D concentrations are prevalent in chronic kidney disease (CKD) patients. We investigated the relationship between plasma 25-hydroxyvitamin D (25(OH)D) or 1,25-dihydroxyvitamin D (1,25(OH)2D) concentrations with death, cardiovascular events (CVE) and dialysis initiation in patients with advanced CKD. Study Design: The Homocysteine Study was a randomized double-blind trial evaluating the effects of high doses of folic acid on death and chronic dialysis initiation in patients with advanced CKD (stage 4 and 5 not yet on dialysis). 25(OH)D and 1,25(OH)2D concentrations were measured in stored plasma samples obtained 3 months after trial initiation and evaluated at clinically defined cutoffs (<10, 10-30, and >30 ng/mL) and tertiles (< 15, 15-22, and >22 pg/mL), respectively. Cox-proportional hazard models were used to examine the association between vitamin D concentrations and clinical outcomes.
Setting & Participants
1,099 patients with advanced CKD from 36 Veteran Affairs Medical Centers
Predictors
25(OH)D and 1,25(OH)2D concentrations
Outcomes
Death, CVE and time to initiation of chronic dialysis.
Results
After a median follow-up period of 2.9 years, 41% (n=453) died, while 56% (n=615) initiated dialysis. Mean 25(OH)D and 1,25(OH)2D concentrations were 21±10 ng/mL and 20±11 pg/mL, respectively. After adjustment for potential confounders, the lowest tertile of 1,25(OH)2D was associated with death (HR, 1.33; 95% CI, 1.01-1.74) and initiation of chronic dialysis (HR, 1.78; 95% CI, 1.40-2.26), compared to the highest tertile. The association with death and initiation of dialysis was moderately attenuated after adjustment for plasma fibroblast growth factor-23 (FGF23) concentrations (HRs of lower tertiles of 1.20 [95% CI, 0.91-1.58] and 1.56 [95% CI, 1.23-1.99], respectively, compared to highest tertile). There was no association between 25(OH)D concentrations and outcomes.
Limitations
Participants were mostly male.
Conclusions
Low plasma 1,25(OH)2D concentrations are associated with death and initiation of chronic dialysis in advanced CKD. Fibroblast growth factor-23 may attentuate this relationship.
The incidence and prevalence of chronic kidney disease (CKD) continues to rise worldwide. In the United States alone, CKD affects 13% of the population.1 CKD patients have a reduced lifespan compared to the general population and the majority of patients die from cardiovascular disease (CVD).2,3 Furthermore, delaying kidney disease progression is of critical importance. Patients with advanced CKD have a high prevalence of 25-hydroxyvitamin D (25(OH)D) deficiency and 1,25-dihydroxyvitamin D (1,25(OH)2D) concentrations progressively decrease as kidney function declines.4-7 Thus, greater attention has been focused on the abnormalities of vitamin D homeostasis as mechanisms explaining the association of CKD with death, cardiovascular events (CVE) and kidney disease progression.4,5
Multiple mechanisms exist explaining the association between vitamin D deficiency with outcomes in patients with advanced CKD. 1,25(OH)2D has been shown to have autocrine and paracrine effects that can stimulate the secretion and action of insulin,8 regulate blood pressure by modulating renin,9 inhibit cellular proliferation,10 and alter the inflammatory response associated with atherosclerosis.11,12 Prior studies of the association between circulating concentrations of 25(OH)D and 1,25(OH)2D with death and CVE have shown inconsistent results.13,14 In addition, longer-term relationship between 25(OH)D and 1,25(OH)2D concentrations and initiation of dialysis are not well-defined.15 To our knowledge the temporal relationship of plasma 25(OH)D and 1,25(OH)2D concentrations with death, CVE and initiation of chronic dialysis in patients with advanced CKD has not been thoroughly examined.
The present study was designed to evaluate the association between plasma concentrations of 25(OH)D and 1,25(OH)2D with death, CVE and initiation of chronic dialysis in patients with advanced CKD not requiring renal replacement therapy who participated in the Homocysteine in Kidney and End Stage Renal Disease study (HOST).16 We hypothesized that lower plasma 25(OH)D and 1,25(OH)2D concentrations would be associated with death, CVE and initiation of dialysis and that the associations would be independent of other previously recognized confounders including fibroblast growth factor 23 (FGF-23).
METHODS
Homocysteinemia in Kidney and End Stage Renal Disease Study
The details of the HOST Study have been described previously.16 The HOST study was a multicenter, prospective, randomized, double-blind, placebo-controlled trial examining the effects of folate, pyridoxine hydrochloride (vitamin B6) and cyanocobalamin (vitamin B12) on death, CVE and dialysis initiation in patients with advanced CKD and elevated plasma homocysteine concentrations. Each center’s institutional review board approved the study and all participants provided informed consent. A total of 2,056 participants were randomized and inclusion criteria included age greater than or equal to 21 years, either having end stage renal disease (ESRD) and receiving maintenance dialysis (n=751) or having an estimated creatinine clearance (calculated by the Cockcroft-Gault formula) of less than 30 mL/min but not being treated with chronic dialysis (n=1,305), and having a plasma homocysteine concentration of 15 μmol/L or higher. For the purposes of this analysis, only those HOST participants who were not dependent on dialysis were included.
Measurements
We used stored plasma samples, collected in ethylenediaminetetraacetate (EDTA) three months after randomization in the subjects with advanced CKD who were not yet on dialysis, to measure 25(OH)D, 1,25(OH)2D, intact parathyroid hormone (iPTH), and FGF23 concentrations. The mean follow-up duration from the three-month visit until the end of the study in the present cohort was 2.8 ± 1.1 years (the median was 2.9 years).
Plasma 25(OH)D concentrations were measured by a commercially available competitive chemiluminescent immunoassay and a Liaison analyzer and plasma 1,25(OH)2D concentrations was measured by a commercial competitive radioimmunoassay (both from DiaSorin, Stillwater, MN). The analytical measurement range for the 25(OH)D assay is 7-150 ng/mL. The intra-assay coefficients of variations (CVs) were 5.6% and 4.5 % at 11 and 28 ng/mL, respectively. The inter-assay CVs were 9.1% and 5.6% at 16 and 51 ng/mL, respectively. For 1,25(OH)2D, the range of the assay is 5-200 pg/mL. The intra-assay CV was 12.6% and 9.7% at 13 and 45 pg/mL, respectively. The inter-assay CVs were 21.4% and 14.7% at 25 pg/mL and 56 pg/mL, respectively. iPTH was measured by an electrochemiluminescent immunoassay with a reference interval of 15-65 pg/mL. The intra and interassay CVs were both less than 5%. FGF23 concentrations were measured using a second generation two-site ELISA kit (Immutopics, San Clemente, CA) with antibodies directed against two epitopes within the carboxy-terminal region of FGF23.17 The analytical measurement range for the FGF23 assay was 3.0 - 2300 RU/mL. The intra-assay CVs were 2.6% and 1.4% at 32.1 and 299.2 RU/mL respectively. The inter-assay CVs were 3.4% and 4.4 % at 32.1 and 299.2 RU/mL, respectively.
Other Measurements
Information collected at the time of randomization included a complete history and physical examination, demographics, smoking status, etiology of kidney disease, history of hypertension, diabetes and cardiovascular disease, and use of medications including angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), and lipid-modifying drugs. Systolic and diastolic blood pressure was measured in a standardized fashion.16 Serum creatinine was measured using a modified kinetic-Jaffe reaction at each local site.18 Of note, although estimated creatinine clearance (calculated by the Cockcroft-Gault formula) was used for eligibility of the HOST study, for the purpose of this analysis, glomerular filtration rate (GFR) was estimated using the 4-variable Modified Diet Renal Disease (MDRD) Study equation.19
Outcomes
The outcomes for this analysis were: (1) time to death from any cause from three months after randomization; (2) time to any cardiovascular event (composite of myocardial infarction, amputation and stroke) from three months after randomization; and (3) time to initiation of chronic dialysis from three months after randomization. Of note, when time to initiation of dialysis was the outcome participants were censored at death from any cause or at study closure.
The HOST Endpoints Committee reviewed and classified all fatal events by using information from the hospital discharge summary, autopsy report, Medicare End Stage Renal Disease Death Notification or death certificate. Deaths were also tracked with the Beneficiary Identification and Records Locator Subsystem, a VA file used to record death and dates.20 Cardiovascular events were obtained through self-reporting by participants in response to specific queries during quarterly follow-up contacts and by review of the patients’ VA medical record. The endpoint of initiation of chronic dialysis was a pre-specified secondary outcome in the original HOST study. It was obtained through self-reporting by participants in response to specific questions during the follow-up period. The outcome was verified by clinic and hospital records at the local site. Of the 2056 original HOST participants, only 66 (3.2%) withdrew consent for both phone and medical record follow-up
Statistical Analysis
Subjects who had initiated chronic hemodialysis before 3 months after randomization (n=34; 2.6%) or for whom EDTA-plasma sample was not available for vitamin D measurements (n=172, 13.2%) were excluded, resulting in a final sample of 1,099 subjects for the present study. Compared to those excluded because plasma sample was not available, demographics, death rate and cardiovascular event rate were similar to those in subjects who were retained for this analysis (data not shown). Demographic, cardiovascular risk factors, and laboratory values were compared across concentrations of plasma 25(OH)D and 1,25(OH) D through the use of the Chi-square test 2 for categorical data, ANOVA for approximately normally distributed continuous variables, and the Kruskall-Wallis test for skewed continuous variables.
Separate analyses for 25(OH)D and 1,25(OH)2D were performed. Both exposure variables were examined as continuous and also as categorical exposures. For the purpose of this analysis, 25(OH)D concentrations were evaluated by clinically defined cutoffs of <10, 10-30 and >30 ng/mL, with >30 ng/mL as the reference group. All cut points were chosen a priori, based on previous studies. 4,21-24 1,25(OH)2D concentrations were examined as tertiles with the highest tertile being the reference group.6,21 Given the positively skewed distributions, both 25(OH)D and 1,25(OH)2D values were log-transformed. Kaplan-Meier curves were used to compare graphically death, CVE and initiation of chronic dialysis. The associations between vitamin D concentrations with death, CVE and initiation of chronic dialysis after adjustment for potential confounders were evaluated using Cox proportional hazard models. The proportional hazards assumptions were confirmed using log-log plots.
We considered a priori variables that may be associated with vitamin D concentrations, death and kidney disease progression as potential confounders in multivariate models, including important clinical variables, cardiovascular risk factors and biochemical measures. Two sequential sets of covariates were considered. In model 1 we included age, gender, race, season, smoking, HOST treatment assignment, body mass index (BMI), estimated GFR, serum LDL-C, serum HDL-C, hypertension, diabetes, cardiovascular disease, systolic blood pressure, as well as serum albumin, calcium, phosphate and plasma iPTH concentrations. In addition to the variables in Model 1, Model 2 also included plasma FGF23 concentration. All models were also adjusted for the other respective plasma vitamin D concentration. We examined the association of 25(OH)D and 1,25 (OH)2D with death within subgroups based on age, race, diabetes status, CVD history, iPTH and FGF23 concentrations.
Two-tailed values of P<0.05 were considered statistically significant. All statistical analyses were performed with SAS software, version 9.13 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics
The mean age of the 1,099 participants was 69 ±11 (SD) years and 98% of participants were male. During a median follow-up of 2.9 [25th-75th percentile, 2.1-3.7] years, 453 (41%) participants died from any cause (224 participants before the initiation of dialysis and 229 participants after chronic dialysis initiation), 215 (20%) had a cardiovascular event and 615 (56%) initiated chronic dialysis. Demographics and cardiovascular risk factors according to plasma 1,25(OH)2D tertiles and 25(OH)D concentrations subgroups are shown in Table 1 and Table S1 (available as online supplementary material), respectively. Participants with 1,25(OH)2D concentrations in the lowest tertile were more likely to be younger, to have diabetes, to have higher BMI and systolic blood pressure and lower estimated GFR than subjects with 1,25(OH)2D concentrations in the highest tertile. Similar to subjects in the lowest tertile of 1,25(OH)2D, subjects with 25(OH)D concentrations < 10 ng/mL were more likely to be younger, to have diabetes and to have higher BMI than subjects with 25(OH)D concentrations >30 ng/mL. Serum triglycerides and total cholesterol were significantly higher in subjects with 25(OH)D concentrations <10 ng/mL. Baseline estimated GFR was not different across subgroups of 25(OH)D concentrations.
Table 1.
Baseline Characteristics of Study Participants
| 1,25-dihydroxyvitamin D | P-value | |||
|---|---|---|---|---|
| < 15 pg/mL (n=367) |
15-22 pg/mL (n=366) |
> 22 pg/mL (n=366) |
||
| Age (y) | 67 ± 11 | 69 ± 11 | 71 ± 10 | <0.001 |
| Black Race | 31.9 | 29.2 | 34.0 | 0.6 |
| Diabetes | 62.1 | 55.7 | 46.6 | <0.001 |
| Hypertension | 96.5 | 97.2 | 95.6 | 0.5 |
| CVD | 57.5 | 56.0 | 56.7 | 0.8 |
| BMI (kg/m2) | 28.9 ± 5.4 | 27.8 ± 4.5 | 27.1 ± 4.4 | <0.001 |
| Systolic blood pressure (mmHg) | 146 ± 24 | 144 ± 23 | 140 ± 21 | <0.001 |
| eGFR (mL/min/1.73m2) | 16 ± 5.3 | 18 ± 6.2 | 20 ± 6.8 | <0.001 |
| Medication ACE Inhibitor Beta-blockers Lipid-lowering |
43.5 61.8 61.8 |
41.8 60.2 53.8 |
38.0 56.5 54.0 |
0.1 0.1 0.03 |
| Albumin (g/dL) | 3.9 ± 0.5 | 4.1 ± 0.5 | 4.2 ± 0.5 | <0.001 |
| Total Cholesterol (mg/dL) | 174 ± 46 | 171 ± 43 | 176 ± 41 | 0.6 |
| HDL Cholesterol (mg/dL) | 41 ± 12 | 43 ± 17 | 44 ± 15 | 0.01 |
| LDL Cholesterol (mg/dL) | 96 ± 33 | 93 ± 31 | 95 ± 32 | 0.9 |
| Triglycerides (mg/dL) | 193 ± 154 | 179 ± 137 | 181 ± 112 | 0.2 |
Values for continuous variables are expressed as mean ± standard deviation; values for categorical variables given as number (percentage).
CVD= cardiovascular disease; BMI=body mass index; ACE = angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blocker; eGFR= estimated glomerular filtration rate; HDL= high density lipoprotein; LDL= low density lipoprotein; iPTH=intact parathyroid hormone; FGF23=fibroblast growth factor-23.
conversion factors for units: eGFR in ml/min/1.73m2 to mL/s/1.73m2 ×0.01667; serum 25(OH)D in ng/mL to nmol/L ×2.496; 1,25(OH)2D in pg/mL to pmol/L ×2.6; serum calcium in mg/dL to mmol/L × 0.2495; serum phosphorus in mg/dL to mmol/L ×0.3229; Serum hemoglobin and albumin in g/dL to g/L × 10; serum cholesterol mg/dL in mmol/L × 0.02586; serum triglycerides in mg/dL to mmol/L × 0.01129.
Circulating Plasma Concentrations of 25(OH)D and 1,25(OH)2D
In this cohort of 1,099 advanced CKD patients only 17.2% of subjects had 25(OH)D concentrations greater than 30 ng/mL, whereas 69.4% had plasma 25(OH)D concentration ranging between 10-30 ng/ml; the remaining 13.4% were severely 25(OH)D-deficient (<10 ng/mL). There were significant variations in serum 25(OH)D concentrations between races (21.9±10.1 ng/mL in Caucasians vs. 15.9±8.9 ng/mL in African-Americans; p<0.001). Furthermore, diabetics were also more likely to have lower 25(OH)D concentrations than those without diabetes (19.3±9.7 vs 22.3±10.7 ng/mL, p<0.001). The mean plasma concentration of 1,25(OH)2D was 20.1±10.6 pg/mL. Mineral metabolism biomarkers according to 25(OH)D subgroups and 1,25(OH)D tertiles are depicted in Table 2.
Table 2.
Markers of Mineral Metabolism and Kidney Function
| 25-hydroxyvitamin D | ||||
|---|---|---|---|---|
| < 10 ng/mL (n=147) |
10-30 ng/mL (n=763) |
> 30 ng/mL (n=189) |
P-value | |
| Calcium (mg/dL) | 8.8 ± 0.8 | 8.9 ± 0.7 | 8.9 ± 0.6 | 0.06 |
| Phosphorus (mg/dL) | 4.4 ± 1.3 | 4.4 ± 1.4 | 4.2 ± 1.0 | 0.1 |
| 1,25(OH)2D (pg/mL) | 13.9 ± 6.7 | 19.9 ± 10.0 | 26.7 ± 11.7 | <0.001 |
| iPTH (pg/mL) | 192 [123-355] | 149 [91-247] | 114 [66-186] | <0.001 |
| FGF-23 (RU/mL) | 330 [192-984] | 405 [225-947] | 307 [206-809] | 0.9 |
| eGFR (mL/min/1.73m2 ) | 18 ± 7.0 | 18 ± 6.3 | 19 ± 6.4 | 0.1 |
| 1,25-dihydroxyvitamin D | ||||
| < 15 pg/mL (n=367) |
15-22 pg/mL (n=366) |
> 22 pg/mL (n=366) |
P-value | |
| Calcium (mg/dL) | 8.9 ± 0.8 | 8.9 ± 0.7 | 8.9 ± 0.6 | 0.7 |
| Phosphorus (mg/dL) | 4.8 ± 1.3 | 4.3 ± 1.1 | 4.1 ± 1.5 | <0.001 |
| 25(OH)D (ng/mL) | 15.9 ± 8.1 | 20.8 ± 9.9 | 25.4 ± 10.4 | <0.001 |
| iPTH (pg/mL) | 172 [94-291] | 155 [97-245] | 128 [77-208] | <0.001 |
| FGF-23 (RU/mL) | 674 [330-1465] | 345 [218-733] | 256 [170-586] | <0.001 |
| eGFR (mL/min/1.73m2) | 16 ± 5.3 | 18 ± 6.2 | 20 ± 6.8 | <0.001 |
Unless otherwise specified, values are expressed as means ± standard deviation or median [25th-75th percentile]. Abbreviations: 25(OH)D=25-hydroxyvitamin D; 1,25(OH)2=1,25-dihydroxyvitamin D; iPTH=intact parathyroid hormone; FGF23=fibroblast growth factor-23; eGFR=estimated glomerular filtration rate. conversion factors for units: serum calcium in mg/dL to mmol/L ×0.2495; serum phosphorus in mg/dL to mmol/L × 0.3229; Serum 1,25(OH)2D in pg/mL to pmol/L ×2.6; serum 25(OH)D in ng/mL to nmol/L × 2.496; eGFR in ml/min/1.73m2 to mL/s/1.73m2 × 0.01667.
Plasma 25(OH)D concentrations correlated with plasma 1,25(OH)2D (r=0.43) and Plasma iPTH (r=-0.25) concentrations; but did not correlate with serum calcium, serum phosphorus or plasma FGF23 concentrations. In addition to its correlation with 25(OH)D, plasma 1,25(OH)2D concentrations correlated with serum phosphorus (r=-0.32), plasma iPTH (r=-0.15) and, in particular, plasma FGF23 (r= −0.39) concentrations.
Associations of Plasma 25(OH)D Concentrations with Clinical Outcomes
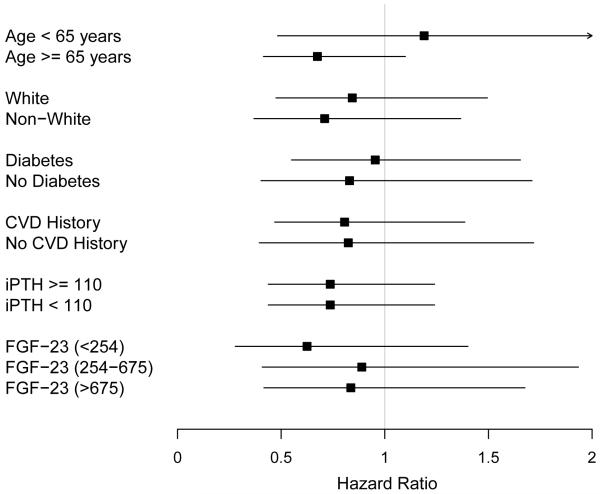
Kaplan-Meier curve analysis followed by log-rank test showed that risk for death did not change (p=0.9) but there was an increase in the risk of chronic dialysis initiation (p=0.01) with decreasing concentrations of plasma 25(OH)D (Figures S1 and S2). Hazard ratios for death, composite of CVE and initiation of chronic dialysis in the various subgroups according to 25(OH)D concentrations are shown in Table 3. After adjusting for multiple covariates in Model 1, there was no increase in the risk of death with decreasing concentrations of 25(OH) D (Table 3). There was no association between decreasing 25(OH)D concentrations with CVE and initiation of chronic dialysis in multivariate models. Almost identical results were obtained for all outcomes when 25(OH)D concentrations were categorized by tertiles (data not shown) or were evaluated linearly (for every log increase in 25(OH)D, adjusted hazard ratio for death was 1.15 [95% CI, 0.69-1.93], p=0.6). There was no association of 1og10 25(OH)D concentrations with death within subgroups defined by age, race, diabetes status, CVD history, iPTH and FGF23 concentrations (Figure 1).
Table 3.
Associations of Plasma 25(OH)D Concentrations with Outcomes
| 25(OH)D < 10 ng/mL | 25(OH)D = 10-30 ng/mL | |
| Death | ||
| Unadjusted | 1.07 (0.76-1.49) | 1.02 (0.80-1.32) |
| Model 1 | 1.04 (0.71-1.54) | 0.97 (0.74-1.27) |
| Model 2 | 1.18 (0.79-1.74) | 0.99 (0.76-1.29) |
| Cardiovascular Events | ||
| Unadjusted | 1.12 (0.68-1.84) | 1.16 (0.80-1.69) |
| Model 1 | 1.15 (0.65-2.06) | 1.13 (0.75-1.68) |
| Model 2 | 1.23 (0.69-2.21) | 1.14 (0.76-1.69) |
| Initation of Chronic Dialysis | ||
| Unadjusted | 1.50 (1.17-2.02) | 1.37 (1.09-1.72) |
| Model 1 | 0.72 (0.51-1.02) | 0.93 (0.72-1.18) |
| Model 2 | 0.79 (0.56-1.12) | 0.96 (0.75-1.23) |
| Composite of Death or Chronic Dialysis Initiation | ||
| Unadjusted | 1.32 (1.03-1.69) | 1.18 (0.98-1.43) |
| Model 1 | 0.75 (0.56-1.01) | 0.85 (0.70-1.05) |
| Model 2 | 0.83 (0.62-1.17) | 0.89 (0.72-1.08) |
Note: values shown are Hazard ratio (95% CI). The reference category is 25(OH)D > 30 ng/mL. 25(OH)D, 25-hydroxyvitamin D.
Model 1: adjusted for age, gender, race, season, smoking, HOST treatment assignment, hypertension, diabetes, cardiovascular disease, systolic blood pressure, body mass index, estimated glomerular filtration rate, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum albumin, serum calcium, serum phosphate, plasma concentrations of 1,25-dihydroxyvitamin D and intact parathyroid hormone.
Model 2: adjusted for covariates in model 1 plus plasma fibroblast growth factor 23.
Figure 1.
Association of baseline plasma 25-hydroxyvitamin D concentrations with death within subgroups. Hazard ratio for every log increase in 25(OH)D. Error bars represent 95% confidence intervals. CVD=cardiovascular disease; iPTH=intact parathyroid hormone; FGF23=fibroblast growth factor 23.
Association of Plasma 1,25(OH)2D Concentrations with Clinical Outcomes
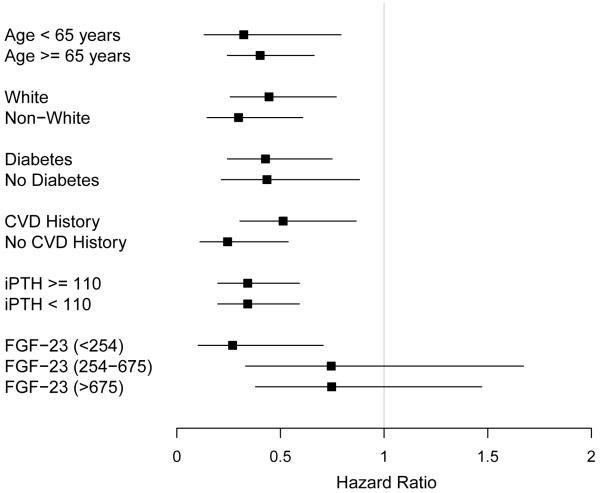
Both the risk of death and progression to initiation of dialysis increased with decreasing 1,25(OH)2D concentrations in unadjusted analyses (Figures S3 and S4 and Table 4). After adjustment for potential confounders in Model 1, the lowest tertile of 1,25(OH)2D concentration remained significantly associated with the risk of death and initiation of chronic dialysis. The associations were stronger for initiation of chronic dialysis than for death, as reflected by greater hazard ratios. Interestingly, the addition of plasma FGF23 concentrations (model 2) diminished the association between 1,25(OH)2D with death and initiation of dialysis. With the adjustment for FGF23 the association was no longer statistically significant (p=0.2) for death but remained statistically significant for initiation of dialysis (p=0.006). When plasma 1,25(OH)2D concentrations were modeled as a continuous variable, the results remained unchanged (for every 1og increase in 1,25(OH)2D, the fully adjusted hazard ratios for death and kidney disease progression were 0.61 [95% CI, 0.35-1.07] and 0.45 [95% CI, 0.28-0.73], respectively). There was no association between decreasing 1,25(OH)2D concentrations with the composite of CVE. The association of 1og(1,25(OH)2D) concentrations with death within subgroups defined by age, race, diabetes status, CVD history, iPTH and FGF23 concentrations were examined. The point estimates were similar and statistically significant within each subgroup, except for increasing FGF23 concentrations (Figure 2). There were no significant interactions between log(1,25(OH)2D) and age, race, diabetes status, CVD history, iPTH and FGF23 concentrations with death.
Table 4.
Associations of Plasma 1,25(OH)2D Concentrations with outcomes.
| 1,25(OH)2D < 15 pg/mL | 1,25(OH)2D = 15-22 pg/mL | |
| Death | ||
| Unadjusted | 1.31 (1.05-1.63) | 0.95 (0.75-1.21) |
| Model 1 | 1.33 (1.01-1.74) | 0.95 (0.74-1.22) |
| Model 2 | 1.20 (0.91-1.58) | 0.95 (0.74-1.22) |
| Cardiovascular Events | ||
| Unadjusted | 1.20 (0.87-1.66) | 1.03 (0.74-1.44) |
| Model 1 | 0.92 (0.62-1.37) | 0.90 (0.63-1.28) |
| Model 2 | 0.85 (0.57-1.27) | 0.89 (0.63-1.27) |
| Initiation of Chronic Dialysis | ||
| Unadjusted | 2.88 (2.35-3.53) | 1.69 (1.37-2.08) |
| Model 1 | 1.78 (1.40-2.26) | 1.36 (1.09-1.70) |
| Model 2 | 1.56 (1.23-1.99) | 1.36 (1.09-1.70) |
| Composite of Death or Progression to Chronic Dialysis | ||
| Unadjusted | 2.44 (2.06-2.89) | 1.45 (1.22-1.73) |
| Model 1 | 1.75 (1.44-2.14) | 1.26 (1.05-1.52) |
| Model 2 | 1.55 (1.26-1.89) | 1.26 (1.05-1.52) |
Note: values shown are Hazard ratio (95% CI). The reference category is 1,25(OH)2D > 22 pg/mL. 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Model 1: adjusted for age, gender, race, season, smoking, HOST treatment assignment, hypertension, diabetes, cardiovascular disease, systolic blood pressure, body mass index, estimated glomerular filtration rate, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum albumin, serum calcium, serum phosphate, plasma concentrations of 1,25-dihydroxyvitamin D and intact parathyroid hormone.
Model 2: adjusted for covariates in model 1 plus plasma fibroblast growth factor 23.
Figure 2.
Association of baseline plasma 1,25-dihydroxyvitamin D concentrations with death within subgroups. Hazard ratio for every log increase in 1,25(OH)2D. Error bars represent 95% confidence intervals. CVD=cardiovascular disease; iPTH=intact parathyroid hormone; FGF23=fibroblast growth factor 23.
Because of the risk that the competing risk of death could lead to informative censoring of incident dialysis, we analyzed the association between 25(OH)D and 1,25(OH)2D with the composite outcome of death or incident chronic dialysis. In sum, 839 subjects reached the composite endpoint of initiation of chronic dialysis (n=615) or death without the initiation of chronic dialysis (n=224). Identical results to the ones obtained for dialysis initiation were obtained when the composite outcome of incident chronic dialysis or all-cause mortality was examined (Table 3 and 4).
DISCUSSION
In this longitudinal study of patients with advanced CKD not yet requiring dialysis, we found that low plasma concentrations of 1,25(OH)2D, not low concentrations of 25(OH)D, were associated with death and progression to chronic dialysis. Interestingly, these associations were modestly attenuated by adjustment of FGF23 concentrations. Yet 1,25(OH)2D remained associated with a 1.2 and 1.6-fold risk of death and progression, although only the outcome of dialysis initiation was statistically significant.
Longitudinal data on the relationship between vitamin D analytes with death in patients with CKD is limited. In a small observational study of 226 patients with stage III and IV CKD, low serum 1,25(OH)2D concentrations were associated with an increased risk of death. In this study, 25(OH)D concentrations were not examined.25 A study of 168 patients with CKD stages 2-5 (mean eGFR of 34 ± 17 ml/min/1.73m2) found that 25(OH)D were superior to 1,25(OH)2D concentrations in predicting death and progression of kidney disease.26 After adjustment for multiple confounders, 1,25(OH)2D no longer predicted death or kidney disease progression in these CKD patients. Our results are in contrast to this published data as we found no association between 25(OH)D concentrations and death in our cohort. None of these previous studies had patients with CKD that was as advanced as in our study (mean eGFR in our cohort of 18.1 ± 6.5 ml/min/1.73m2) nor did they evaluate the effect of FGF23 on the relationship between vitamin D and death. To our knowledge, the data we present herein are novel in that they show a longitudinal link of plasma 1,25(OH)2D concentrations with death and initiation of chronic dialysis in patients with advanced CKD.
The discovery that the vitamin D receptor (VDR) as well as 1α-hydroxylase (which converts 25(OH)D to 1,25(OH)2D locally) are found in most tissues in the body has yielded new insights into the non-skeletal functions of vitamin D and consequences of its deficiency.4 This locally produced 1,25(OH)2D binds to the VDR in an autocrine/paracrine manner and has been found to have several important roles.9 Experimental and clinical studies suggest that calcitriol may exert its effects through alterations in the renin-angiotensin-aldosterone system (RAAS) and inflammatory mediators. Both the RAAS and inflammation are major mediators of death and kidney disease progression. It is also possible that the suggested reno-protective and cardio-protective effects of vitamin D are independent of the effects of the RAAS.
We did not find an association between lower plasma 25(OH)D concentration with death, CVE or kidney disease progression and the absence of an association may be due to higher FGF23 concentration in patients with CKD. Interestingly, FGF23 appears to not only inhibit 1α-hydroxylase in the kidney but also affect extra-renal vitamin D metabolism in patients requiring chronic dialysis.27 In the setting of high FGF23 concentrations, tissues and organs may be unable to convert serum 25(OH)D into 1,25(OH)2D locally. Hence, 25(OH)D may be less important than 1,25(OH)2D concentrations in the setting of high FGF23 concentrations.
Observational studies have found that CKD patients treated with active vitamin D analogs have a lower risk of death than patients who were not treated.28,29 In a study of 520 male VA patients with stage 3-5 CKD, not yet on dialysis, treatment with oral calcitriol was associated with better survival. The incident rate ratio for mortality in patients treated with calcitriol compared to untreated patients was 0.35 (95% CI, 0.23-0.54; p<0.001) and for the combined endpoint of death and dialysis initiation was 0.46 (95% CI, 0.35-0.61).28 Shoben and colleagues29 similarly found improved survival in patients with CKD stage III and IV treated with calcitriol in the Veterans Affairs system. After adjustment, oral calcitriol use was associated with a 26% lower risk for death (P = 0.016) and a 20% lower risk for death or chronic dialysis (P = 0.038). To date, no randomized trials have been performed evaluating whether supplementation with vitamin D decreases mortality in CKD patients.
There is some evidence suggesting that vitamin D may also have reno-protective effects. Observational studies have found a relationship between low 25(OH)D concentrations and kidney disease progression.30,31 These findings are in contrast to our study, in which only low 1,25(OH)2D was associated with progression to chronic dialysis. The reason for the contrary findings may be due to differences in the level of kidney function of the populations included in the studies. 30,31 Small randomized trials have found that treatment with active vitamin D (1,25(OH)2D) analogs decreases proteinuria and delays progression of kidney disease in patients with mild to moderate CKD,32-35 but large definitive randomized trials have not been performed.
In our study, the association between 1,25(OH)2D and death or kidney disease progression was attenuated with the addition of FGF23 to the statistical model, suggesting that FGF23 may be an important effect attenuator in the relationship between 1,25(OH)2D and clinical outcomes. FGF23 inhibits 1-α-hydroxylase and increases renal phosphate excretion. FGF23 concentrations increase early in the course of kidney disease and high concentrations of FGF23 have been associated with death in dialysis patients and with left ventricular hypertrophy, CVE, death and kidney disease progression in pre-dialysis patients.36-40
There are several limitations in the present study. First, this observational study cannot establish a causal relationship between plasma 1,25(OH)2D concentrations with death and kidney disease progression. Second, information on the use of nutritional vitamin D supplements, active vitamin D analogs and dietary intake of vitamin D was not available. However, during the time period in which the HOST study was performed, the use of active vitamin D analogs and nutritional vitamin D supplementation in non-dialysis CKD patients was not prevalent, making the possibility of significant confounding by these covariates less likely. Third, the level of kidney function was estimated by the MDRD Study equation rather than more direct measures of kidney function and measurements of serum creatinine were not performed in a central laboratory. Fourth, data on the degree of proteinuria was not available in the HOST study. Low 25(OH)D and 1,25(OH)2D concentrations have been associated with albuminuria in several studies,44-46 and albuminuria may also lead to low 25(OH)D concentrations.47 Albuminuria is also an independent risk factor for CVD and death in patients with CKD.48 Even though we were unable to adjust for proteinuria, we were able to adjust for blood pressure control, a predictor of kidney disease progression and modulator of proteinuria. Fifth, 1,25(OH)2D concentrations may have cross-reactivity with other vitamin D metabolites. Finally, this cohort included mostly male patients and caution should be used when extrapolating the results to women.
These limitations notwithstanding, our study also has several strengths including the large cohort, the careful collection of clinical outcome data and the relatively long follow-up duration. We were also able to adjust for multiple factors that regulate mineral metabolism and kidney disease progression. Finally, all measurements of vitamin D were performed in a standardized fashion in a single reference laboratory.
In conclusion, low plasma 1,25(OH)2D, not 25(OH)D, concentrations are associated with death and kidney disease progression to chronic dialysis in patients with advanced CKD not requiring dialysis. These associations appeared to be largely attenuated by FGF23. Future studies are necessary to examine if these associations are causal in nature and, if so, to further delineate the mechanisms.
Supplementary Material
Acknowledgements
The HOST Principal Investigators, Co-investigators, and Coordinators are as follows. Ann Arbor: E. Young, K. Belanger; Bay Pines: R. Lopez, N. Grunsten; Birmingham: P. Sanders, MD, D. Killian, N. Shanklin; Boston: J. Kaufman, E. Dibbs; Bronx: E. Langhoff, D. Tyrrell; West Haven: S. Crowley, A. Piexoto, A. DeLorenzo, C. Joncas; Cleveland: R. T. Miller, K. Kaelin, M. Markulin; Dallas: D. Dev, D. Coussirat, A. Sajgure; Dayton: M. Saklayen, H. Neff, S. Shook; Denver: M. Chonchol, M. Levi, L. Clegg, A. Singleton; Detroit: D. Abu-Hamdan, L. Bey-Knight, K. King, E. Jones, Washington, DC: C. Palant, H. Sheriff, C. Simmons, J. Sinks; Gainesville: C. Wingo, B. McAllister, J. Sallustio, N. Smith; Hines: S. Popli, P. Linnerud, A. Zuluaga; Houston: G. Dolson, G. Tasby; Indianapolis: R. Agarwal, R. Lewis, D. Jasinski, N. Sachs, D. Veneck; Jackson: S. Ur Rehman, A. Basu, K. Ingram; Charleston: R. Paul, J. Raymond, K. Holmes; Kansas City: T. Wiegmann, J. Abdallah, A. Nagaria, D. Smith, A. Renft, T. Finnigan; Richmond: G. Feldman, S. Schmid; Memphis: C. Cooke, B. Wall, R. Patterson; Miami: G. Contreras, E. Jaimes, G. O’Brien; Minneapolis: K. Ma, J. Rust, S. Otterness; New Orleans: V. Batuman, C. Kulivan; New York: D. S. Goldfarb, M. Shea, F. Modersitzki; Northport: T. Dixon, E. Pastoriza, L. Titus; Palo Alto: R. Jamison, I. Cook, M. Anderson; Pittsburgh: P. Palevsky, P. Salai; Portland: S. Anderson, J. Walczyk; Seattle: A. Lindner, C. Stehman-Breen, E. Pascual, J. Nugent-Carney; San Diego: S. Thomson, S. Mullaney, J. Dingsdale; San Juan: C. Rosado, C. Azcona-Vilchez; Syracuse: S. Betcher, C. Rewakowski; West Palm Beach: D. Cohen, G. Flanagin, J. Steffe; Buffalo: J. Lohr, N. Fucile, L. Yohe; Milwaukee: S. Blumenthal, D. Schmidt.
Support: The research reported in this study was supported by the Department of Veterans Affairs Cooperative Studies Program and the HOST Executive Committee (Drs. Rex L. Jamison, Pamela Hartigan, James Kaufman, David S. Goldfarb, Stuart R. Warren, Peter D. Guarino, and J. Michael Gaziano). Additional support was provided by the National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK081473, an AMGEN fellowship grant and investigator initiated proposal from Abbott and Genzyme.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Keith DS, Nichols GA, Guillon CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Internal Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D for health and in chronic kidney disease. Semin Dial. 2005;18(4):266–275. doi: 10.1111/j.1525-139X.2005.18402.x. [DOI] [PubMed] [Google Scholar]
- 6.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–8. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 7.Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int. 2007;71(2):134–39. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 8.Chiu KC, Chu A, Go VL, et al. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 9.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carthy EP, Yamashita W, Hsu A, et al. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13(6 Pt 2):954–9. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 12.Oh J, Weng S, Felton SK, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120(8):687–98. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Internal Med. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 14.Jassal SK, Chonchol M, Mühlen DV, Smits G, Barrett-Connor E. Vitamin D, Parathyroid Hormone, and Cardiovascular Mortality in Older Adults: The Rancho Bernardo Study. Am J Med. 2010;123(12):1114–1120. doi: 10.1016/j.amjmed.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remuzzi A. Vitamin D, insulin resistance, and renal disease Kidney Int. 2007;71(2):96–98. doi: 10.1038/sj.ki.5002047. [DOI] [PubMed] [Google Scholar]
- 16.Jamison RL, Hartigan P, Kaufman JS, et al. Veterans Affairs Site Investigators Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298(10):1163–1170. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 18.Walser M. Assessing renal function from creatinine measurements in adults with chronic renal failure. Am J Kidney Dis. 1998;32(1):23–31. doi: 10.1053/ajkd.1998.v32.pm9669420. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Peduzzi P, Hatch HT, Johnson G, et al. Coordinating center follow-up in the Veterans Administration Cooperative Study of Coronary Artery Bypass Surgery. Control Clin Trials. 1987;8(3):190–201. doi: 10.1016/0197-2456(87)90044-4. [DOI] [PubMed] [Google Scholar]
- 21.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 22.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 24.Jacques PF, Felson DT, Tucker KL, Mahnken B, Wilson PW, Rosenberg IH, Rush D. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr. 1997;66(4):929–936. doi: 10.1093/ajcn/66.4.929. [DOI] [PubMed] [Google Scholar]
- 25.Inaguma D, Nagaya H, Hara K, et al. Relationship between serum 1,25-dihydroxyvitamin D and mortality in patients with pre-dialysis chronic kidney disease. Clin Exp Nephrol. 2008;12(2):126–131. doi: 10.1007/s10157-007-0023-4. [DOI] [PubMed] [Google Scholar]
- 26.Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75(1):88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 27.ASN abstract. Baccheta J, Lisee TS, Chin RF, et al. FGF23 inhibits innate immune responses to vitamin D in human monocytes; Poster presented at: American Society of Nephrology 2011 Meeting; Philadelphia, PA. November 8-13, 2011. [Google Scholar]
- 28.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalanar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168(4):397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 29.Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19(8):1613–1619. doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melamed ML, Astor B, Michos ED, Hostetter TH, Power NR, Muntner P. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol. 2009;20(12):2631–2369. doi: 10.1681/ASN.2009030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Boer I, Katz R, Chonchol M, et al. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol. 2011;6(9):2141–2149. doi: 10.2215/CJN.02640311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal R, Acharya M, Tian J, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68(6):2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 33.Alborzi P, Patel NA, Peterson C, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52(2):249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 34.Fishbane S, Chitteni H, Packman M, Dutka P, Ali N, Durie N. Oral paricalcitol in the treatment of patients with CKD and proteinuria: a randomized trail. Am J Kidney Dis. 2009;54(4):647–652. doi: 10.1053/j.ajkd.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 35.de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 36.Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutiérrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu HJ, Wu MS. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci. 2009;37(2):116–122. doi: 10.1097/MAJ.0b013e3181815498. [DOI] [PubMed] [Google Scholar]
- 39.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22(10):1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2007;50(1):69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Verrotti A, Basciani F, Carle F, Morgese G, Chiarelli F. Calcium metabolisim in adolescents and young adults with type 1 diabetes mellitus without and with persistent microalbuminuria. J Endocrinol Invest. 1999;22(3):198–202. doi: 10.1007/BF03343541. [DOI] [PubMed] [Google Scholar]
- 43.Inukai T, Fujiwara Y, Tayama K, Aso Y, Takemura Y. Alterations in serum levels of 1 alpha, 25(OH)2 D3 and osteocalcin in patients with early diabetic nephropathy. Diabetes Res Clin Pract. 1997;38(1):53–59. doi: 10.1016/s0168-8227(97)00090-9. [DOI] [PubMed] [Google Scholar]
- 44.ato KA, Gray RW, Lemann J., Jr. Urinary excretion of 25-hydroxyvitamin D in health and the nephrotic syndrome. J Lab Clin Med. 1982;99(3):325–330. [PubMed] [Google Scholar]
- 45.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(40):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.