Abstract
Meningococcal lipopoly(oligo)saccharide (LOS) is a major inflammatory mediator of fulminant meningococcal sepsis and meningitis. Highly purified wild-type meningococcal LOS and LOS from genetically defined mutants of Neisseria meningitidis that contained specific mutations in LOS biosynthesis pathways were used to confirm that meningococcal LOS activation of macrophages was CD14/Toll-like receptor 4 (TLR4)-MD-2 dependent and to elucidate the LOS structural requirement for TLR4 activation. Expression of TLR4 but not TLR2 was required, and antibodies to both TLR4 and CD14 blocked meningococcal LOS activation of macrophages. Meningococcal LOS α or β chain oligosaccharide structure did not influence CD14/TLR4-MD-2 activation. However, meningococcal lipid A, expressed by meningococci with defects in 3-deoxy-d-manno-octulosonic acid (KDO) biosynthesis or transfer, resulted in an ∼10-fold (P < 0.0001) reduction in biologic activity compared to KDO2-containing meningococcal LOS. Removal of KDO2 from LOS by acid hydrolysis also dramatically attenuated cellular responses. Competitive inhibition assays showed similar binding of glycosylated and unglycosylated lipid A to CD14/TLR4-MD-2. A decrease in the number of lipid A phosphate head groups or penta-acylated meningococcal LOS modestly attenuated biologic activity. Meningococcal endotoxin is a potent agonist of the macrophage CD14/TLR4-MD-2 receptor, helping explain the fulminant presentation of meningococcal sepsis and meningitis. KDO2 linked to meningococcal lipid A was structurally required for maximal activation of the human macrophage TLR4 pathway and indicates an important role for KDO-lipid A in endotoxin biologic activity.
Neisseria meningitidis is a devastating human pathogen that causes fulminant, rapidly fatal sepsis and meningitis worldwide, often in large epidemics (30). The morbidity and mortality of meningococcal bacteremia has been directly correlated with circulating meningococcal endotoxins (lipopoly[oligo]saccharides [LOS]) (2, 4, 45). The engagement of meningococcal LOS with the human Toll-like receptor 4 (TLR4) on human macrophages and other host cells is proposed to trigger signaling events that ultimately result in the production of proinflammatory cytokines and chemokines. Meningococcemia is predicted in large part to be a direct result of the broad stimulation of TLR4 receptors on macrophages and other host cells by circulating meningococcal LOS (2-4), inducing a cascade of events that leads clinically to acute inflammation, hypotension, organ failure, necrosis, coma, and death.
Meningococcal LOS lacks the repeating O antigens of enteric lipopolysaccharide (LPS) but has a conserved region composed of heptose (Hep) and two molecules of unphosphorylated 3-deoxy-d-manno-2-octulosonic acid (KDO) attached to lipid A. Attached to Hep2-KDO2-lipid A are variable α and β chain saccharides (13). The LOS structure is common among other mucosal pathogens 2, including Bordetella pertussis, Campylobacter jejuni, and Haemophilus species. Other differences between meningococcal LOS and enteric LPS that may be biologically important occur in the composition and attachment of the lipid A acyl chains and phosphorylation patterns of lipid A (13).
The structure of endotoxin from gram-negative bacteria has been shown to influence human macrophage activation. Lipid A has long been recognized as the active moiety for endotoxin biologic activity (9, 10, 16, 20, 29). Structural variations in lipid A (14), degree of lipid A phosphorylation (36), net charge of the lipid A molecule (34), and symmetry, number, and length of fatty acyl chains (33, 35) influence biologic activity. Variations in saccharide content of endotoxin have been reported as structural determinants of macrophage activation by different endotoxins. However, the role of inner or outer core oligosaccharides remains controversial (25, 43).
In this study, highly purified, structurally defined LOS from genetically defined and novel mutants of N. meningitidis (12, 28, 37, 39, 41, 42, 52) were used to confirm the role of CD14/TLR4-MD-2 pathway and to determine the meningococcal endotoxin structure required for activation of human and murine macrophages.
MATERIALS AND METHODS
Reagents.
RPMI 1640 medium, Dulbecco's Eagle medium, fetal bovine serum, penicillin-streptomycin, sodium pyruvate, and nonessential amino acids were obtained from Cellgro Mediatech (Herndon, Va.). Phorbol myristate acetate (PMA) was from GibcoBRL (Grand Island, N.Y.). Tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), IL-10, and IL-8 enzyme-linked immunosorbent assay (ELISA) kits were from R&D Systems (Minneapolis, Minn.). Polystyrene latex beads, zymosan, endotoxin-free albumin, synthetic KDO, and lucigenin were from Sigma (St. Louis, Mo.). RAW 264.7 and THP-1 cell lines were provided by Fred Quinn (Centers for Disease Control and Prevention, Atlanta, Ga.). The U937 cell line was from Yusof Abu Kwaik (University of Kentucky School of Medicine, Lexington). The C3H/HeJ (TLR4−/−) cell line was from Bruce Beutler (Scripps Research Institute, La Jolla, Calif.). N. meningitidis LOS were obtained from genetically defined meningococcal mutants (Table 1) and were purified and quantitated as described below (Carbohydrate Research Center, University of Georgia, Athens). E. coli LPS 0111:B4 was from Sigma and also further purified. Murine anti-human TLR4-MD-2 monoclonal antibodies (HTA125 and HTA1216) were a gift from Kensku Miyake (Saga Medical School, Nabeshima, Saga, Japan). CD14 monoclonal antibody (clone 134620) obtained from R&D Systems is produced in sheep immunized with purified, CHO cell-derived, recombinant human CD14. CD14-specific IgG was purified by human CD14 affinity chromatography.
TABLE 1.
Genotype and structure of the serogroup B N. meningitidis LOS used in this study
| Genotype | Enzyme deficiency | LOS structurea | Reference |
|---|---|---|---|
| Wild type (strain NMB) | NeuNAc-Galβ-GlcNAc-Galβ-Glcβ-Hep2 (GlcNAc, Glcα) PEA-KDO2-lipid A; 1,4′ bisphosphorylated | 28 | |
| synA::Tn916 | N-Acetyl-d-glucosamine-6-phosphate-2-epimerase | Galβ-GlcNAc-Galβ-Glcβ-Hep2 (GlcNAc, Glcα) PEA-KDO2-lipid A; 1,4′ bisphosphorylated | 28 |
| lst::Ω(sp)b | 2-3 Sialyltransferase | Galβ-GlcNAc-Galβ-Glcβ-Hep2 (GlcNAc, Glcα) PEA-KDO2-lipid A; 1,4′ bisphosphorylated | 13 |
| pgm::Tn916 | Phosphoglucomutase | Hep2 (GlcNAc) PEA-KDO2-lipid A; 1,4′ bisphosphorylated | 52 |
| rfaK::Ω(sp)b | α1-2-N-Acetyl glucosamine transferase | Hep2 PEA-KDO2-lipid A; 1,4′ bisphosphorylated | 12 |
| gmhX::Tn916 | 2-d-Glycero-manno-heptose phosphatase | KDO2-lipid A; 1,4′ bisphosphorylated | 37 |
| kdtA::aphA-3 | KDO transferase | Lipid A; 1,4′ bisphosphorylated | 41 |
| kpsF::aphA-3 | Arabinose 5-phosphate isomerase | Lipid A; 4′monophosphorylated | 42 |
| lpxA::aphA-3 | UDP-GlcNAc acyltransferase | LOS deficient | Unpublishedc |
| lpxL1::aphA-3 | Lauroyl acyltransferase | NeuNAc-Galβ-GlcNAc-Galβ-Glcβ-Hep2 (GlcNAc, Glcα) PEA-KDO2-lipid A(penta-acylated); 1,4′ bisphosphorylated | Unpublishedc |
| gmhX::Tn916/lpxL1::aphA-3 | Lauroyl acyltransferase | KDO2-lipid A (penta-acylated); 1,4′ bisphosphorylated | Unpublishedc |
LOS structure linkages: NeuNAc-Galβ1-4 GlcNAcβ1-3 Galβ1-4 Glcβ1-4 Hep2 (GlcNAcβ1-2, Glcα1-3) PEAO6-KDO2-lipid A; 1,4′ bisphosphorylated. (Phosphoethanolamine group is located at O6 position on HepII.)
sp, spectinomycin.
Y.-L. Tzeng and D. S. Stephens.
LOS purification and quantitation.
LOS from the serogroup B N. meningitidis strain NMB (encapsulated, L2 immunotype) and genetically defined mutants of this strain (listed in Table 1) were initially extracted by the phenol-water method (28). Residual membrane phospholipids (unsaturated fatty acyl residues, C18:0) were removed by repeated extraction of the dry LOS samples with 9:1 ethanol-water. The expected LOS fatty acyl components of 3-OH C12:0, 3-OH C14:0, and C12:0 and the absence of membrane phospholipids was assessed by mass spectroscopy. No fatty acyl residues characteristic of phospholipids could be detected following the 9:1 ethanol-water extraction. LOS preparations were examined by silver staining and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12); no proteins were visualized. No nucleic acids were detected in the purified LOS samples when measured at UV wavelengths of 260 and 280 nm. No muramic acid was detected by mass spectroscopy, which suggested the absence of peptidoglycan. Purified LOS samples were quantitated and standardized based on the number of lipid A molecules per sample (49). The amount of lipid A was quantified based on the fact that there are two molecules of β-hydroxymyristic acid per molecule of meningococcal lipid A and four molecules per Escherichia coli lipid A; thus, the molar concentration of each LOS preparation could be determined by quantifying the amount of this fatty acid. Briefly, the β-hydroxymyristic acid in each LOS preparation was released by methanolysis in methanolic 1 M HCl at 80°C for 4 h and trimethylsilylated. The resulting methyl β-trimethylsilylmyristate was then quantified by gas chromatography-mass spectroscopy analysis. All LOS samples were resuspended in pyrogen-free water with 0.5% triethylamine, vortexed for at least 5 min, boiled for 1 h at 65°C, and then sonicated for 30 min in a water bath sonicator (L&R Transistor/Ultrasonic T-14) to disperse and enhance solubility of LOS. To ensure solubility of all LOS and lipid A stock solutions optical densities were measured at 600 and 630 nm versus water blank. All LOS stock solutions were made in pyrogen-free water at a concentration of 10 nmol/ml and further diluted with phosphate-buffered saline (PBS) to 1 nmol/ml and 100 pmol/ml with extensive vortex and sonication prior to any dilution. All LOS and lipid A samples were soluble, standardized at equal molar basis of lipid A content, not dry weight, and used at final concentrations of 0.056, 0.56, or 5.6 pmol/ml, which is equivalent to approximately 0.1, 1, or 10 ng/ml of lipid A. N. meningitidis lipid A molecular mass is 1,740 Da.
Cell cultures.
Cells of U937 and THP-1, human macrophage-like cell lines, were grown in RPMI 1640 with l-glutamate supplemented with 10% fetal bovine serum, penicillin (50 IU/ml), streptomycin (50 μg/ml), 1% sodium pyruvate, and 1% nonessential amino acids. Culture flasks were incubated at 37°C with humidity under 5% CO2. Murine macrophage cells (RAW 264.7 and C3H/HeJ) were grown in Dulbecco's Eagle medium supplemented and incubated as mentioned above.
Cytokine induction by LOS.
U937 and THP-1 human monocytes were differentiated into macrophage-like cells using PMA at a final concentration of 10 ng/106 cell and incubated at 37°C for at least 24 h. Differentiated U937 cells express TLR4 but not TLR2 on the surface, while THP-1 cells express both receptors (48). Freshly differentiated macrophages were washed with PBS, counted and adjusted to 106 cells/ml, transferred into a 24-well tissue culture plate (1 ml/well), stimulated with LOS at a final concentration of 0.56 pmol/ml, and incubated overnight at 37°C with 5% CO2. Cell culture supernatants were harvested and saved at −20°C. For dose-response experiments, cells were stimulated with LOS at concentrations of 0.056, 0.56, or 5.6 pmol/ml (∼0.1, 1.0, and 10 ng/ml) and incubated overnight. For time course experiments, macrophages were stimulated with LOS at a concentration of 0.56 pmol/ml and incubated for 1, 2, 3, 5, and 24 h.
TLR4 and CD14 inhibition.
TLR4 and CD14 receptors were blocked with specific monoclonal antibodies prior to stimulation with LOS. U937 and THP-1 cells were differentiated and prepared as mentioned earlier. One million cells were resuspended in 200 μl of PBS to which 5 or 10 μg of anti-TLR4 or anti-CD14 was added alone or in combination, respectively, and the suspension was incubated for 30 min at 37°C with gentle shaking. Cells were then centrifuged at 2,000 rpm (500 × g) for 5 min and resuspended in 1 ml of RPMI 1640 medium, stimulated with LOS at a concentration of 0.56 pmol/ml, and incubated overnight.
Competitive inhibition assay.
THP-1 and RAW 264.7 cells (106/ml) were stimulated with increasing concentrations of the unglycosylated (KDO-deficient) lipid A (kdtA) ranging from 0.56 to 16 pmol/ml in the presence of a fixed concentration (0.56 pmol/ml) of the glycosylated LOS (KDO2-lipid A) and incubated overnight. In other experiments equal concentrations (0.56 pmol/ml) of glycosylated and unglycosylated LOS were added simultaneously to stimulate cells, or LOS were mixed and incubated together overnight prior to use in stimulating cells. Supernatants were harvested and saved for cytokine and nitric oxide quantification.
Quantitation of TNF-α and other cytokines by ELISA.
Human TNF-α, IL-8, IL-1β, and IL-10 Duoset kits (R&D Systems) were used for cytokine quantification according to the manufacturer's instructions. Maxisorp ELISA plates were obtained from Nalge Nunc International, Rochester, N.Y. Supernatants were diluted in reagent diluent (0.1% bovine serum albumin-0.05% Tween 20 in Tris-buffered saline).
Nitric oxide induction in RAW macrophages.
Freshly grown RAW 246.7 macrophages adherent to the flask were washed with PBS and incubated with 5 ml of trypsin for 5 min at 37°C. Harvested cells were washed and resuspended in Dulbecco's complete medium. Macrophages (106/ml) were transferred into a 24-well tissue culture plate, stimulated with LOS at a concentration of 0.56 pmol/ml, and incubated overnight. RAW macrophages were indirectly stimulated with 100 μl of cell culture supernatants from previously stimulated U937 or THP-1 human macrophage-like cells exposed to LOS at a concentration of 0.56 pmol/ml. Induced RAW macrophages were incubated overnight at 37°C with 5% CO2, and supernatants were harvested and saved.
Nitric oxide quantitation.
The Griess chemical method (27) was used to detect nitrite (NO2) accumulated in supernatants of induced RAW macrophages. Nitrite and nitrate (NO3) are the end products of nitric oxide. Nitrate was not detected in these experiments; thus, nitrite was reflecting the amount of nitric oxide released. Griess reagent was freshly prepared by mixing equal volumes of 1% sulfanilamide and 0.1% N-(1-naphthylethylenediamine) solutions. One hundred microliters of cell supernatants was transferred into a 96-well plate to which 100 μl of Griess reagent was added. The plate was mixed gently, incubated for 10 min at room temperature, and read at 540 nm using a microplate reader (EL 312e; BIO-TEK Instruments, Winooski, Vt.). The optical densities were correlated to the concentration of nitrite. Nitrite was quantitated using the standard curve of NaNO2 (1 mM stock concentration in distilled water further diluted to the highest standard at 100 μM followed by serial dilutions to 1.56 μM).
Cellular respiratory burst (oxidative burst) activity.
Freshly grown THP-1 cells were adjusted to 2 × 106/ml, transferred to a small tissue culture flask, and incubated with LOS at 5.6 pmol/ml (∼10 ng/ml) overnight at 37°C under 5% CO2. Unprimed cells were incubated in the same way but without LOS. The cells were washed twice with culture medium and resuspended in standard buffer (4.58 mM KH2PO4, 8.03 mM NaHPO4, 0.5 mM MgCl2, 0.45 mM CaCl2, 1% glucose, 0.033% KCl, 0.76% NaCl, and 0.1% endotoxin-free albumin [pH 7.3]) at 2 × 106/ml. The chemiluminescence probe lucigenin was added to the cell suspension (25 μl/ml of cells from a 1.0 mM stock solution) and mixed gently. Aliquots (150 μl) of the mixture were transferred into at least quadruplicate wells of a white 96-well plate (FluoroNunc-PolySorp; Nalge Nunc International). The respiratory burst was triggered with 50 μl of PMA (1 μM) or with opsonized zymosan (500 μg/ml). Chemiluminescence was measured in relative light units (a measure of the number of photons generated by the reaction at each time point). Chemiluminescence was measured with a luminometer (ML3000; Dynatech Laboratories Inc. Chantilly, Va.), and the plate was read immediately and then at 5-min intervals for 90 min (54).
KDO mild acid hydrolysis.
LOS from wild type with hexa-acyl lipid A and/or with penta-acyl lipid A, LOS from the gmhX, a KDO2-lipid A mutant (hexa- and pentacyl lipid A), LOS from the KDO-deficient mutant kdtA with hexa-acyl lipid A and E. coli 111:B4 LPS were hydrolyzed with mild or harsh acid conditions. The mild acid hydrolysis method was adapted and modified based on a previously described method (15). Briefly, 50 μl of LOS (stock concentration 10 nmol/ml) was mixed with 450 μl of 1 M sodium acetate (pH 4.3), 12 mM acetic acid (pH 3.4), 1% acetic acid (pH 2.8) or PBS (pH 7.4), all pyrogen-free solutions, to give a final LOS concentration of 1 nmol/ml. After vigorous mixing, all tubes were incubated at 90°C for 45 min and then dried in a SpeedVac (Savant, Farmingdale, N.Y.). The dried pellets were resuspended in 500 μl of pyrogen-free water, vortexed vigorously, and saved for further use to stimulate nitric oxide induction in RAW macrophages or cytokine induction in THP-1 cells. Lipid A structures were confirmed after mild acid hydrolysis using the thin-layer chromatography method (8) with the following solvent system ratio (vol/vol): chloroform-methanol-water-trimethylamine, 30:12:20.1.
Statistical analysis.
Mean values ± standard deviations (SD) and P values (Student t test) of at least four independent determinations were calculated with Microsoft Excel software.
RESULTS
Meningococcal LOS structure and cytokine release from macrophages.
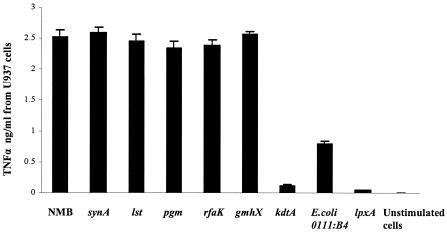
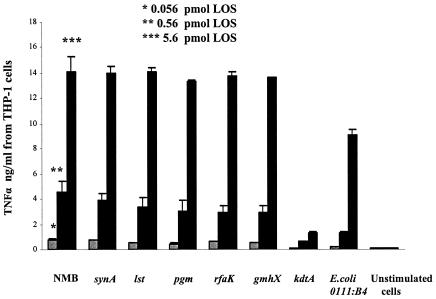
Structurally confirmed LOS from genetically defined mutants of N. meningitidis (Table 1) were used to investigate the role of LOS sialylation, oligosaccharide length and lipid A structure on the activation of the macrophage CD14/TLR4-MD-2 receptor complex. The LOS were standardized based on lipid A content. TNF-α release from differentiated U937 and THP-1 human macrophage-like cells or RAW264.7 murine macrophages stimulated with endotoxin (0.56 pmol/ml, or approximately 1 ng/ml) was consistently two- to fourfold higher for meningococcal LOS than for equal molar amounts of E. coli LPS 0111:B4 (P < 0.0001) (Fig. 1). The TNF-α release with meningococcal LOS was similar when wild-type meningococcal LOS (NMB), oligosaccharide-truncated meningococcal LOS (pgm, rfaK, and gmhX), or unsialylated meningococcal LOS (synA and lst) were used (Table 1; Fig. 1). These meningococcal LOS also induced similar cytokine release profiles for IL-8, IL-1β, and IL-10 (data not shown). The kinetics of TNF-α and other cytokine induction were similar in both dose-response (Fig. 2) and time course assays (data not shown).
FIG. 1.
The effect of meningococcal LOS structure on TNF-α release by human macrophages. Purified meningococcal LOS containing lipid A at a concentration of 0.56 pmol/ml (1 ng/ml) was used to stimulate 106 differentiated U937 macrophages/ml overnight and assayed for TNF-α release. LOS from parent strain NMB and synA, lst, pgm, rfaK, gmhX, and kdtA mutants were used (Table 1). LPS from E. coli 0111:B4 was used as a control. As a control for cellular components retained by the purification procedure used to obtain LOS, a preparation from the LOS-deficient meningococcal mutant (lpxA) (Table 1) was used at 100 ng/ml (dry weight). Unstimulated macrophages incubated simultaneously were also used as control. Error bars represent SD from the mean, and the data are from eight independent determinations.
FIG. 2.
Dose-dependent induction of TNF-α by meningococcal LOS. LOS at a concentration of 0.056 pmol/ml (0.1 ng/ml), 0.56 pmol/ml (1 ng/ml), or 5.6 pmol/ml (10 ng/ml) from the parent strain NMB and synA, lst, pgm, rfaK, gmhX, and kdtA mutants (Table 1) were used to stimulate 106 differentiated THP-1 macrophages/ml overnight and assayed for TNF-α release. Decreased TNF-α release was seen with the KDO2-deficient meningococcal lipid A (kdtA). E. coli LPS (0111:B4), a LOS-deficient (lpxA) preparation, and unstimulated cells were used as controls.
In contrast, TNF-α release from macrophages was markedly attenuated for meningococcal lipid A deficient in KDO2 (kdtA [Fig. 1 and 2] and kpsF [data not shown]) when equal molar amounts of lipid A were used to stimulate macrophages (P < 0.0001). Cytokine induction by all meningococcal LOS structures was neutralized with polymyxin B (2 μg/ml) when added during the induction assay (data not shown). No significant cytokine release was observed when differentiated cells were exposed to a preparation from a meningococcal LOS-deficient mutant (lpxA) (P < 0.0001) extracted in a manner identical to the other LOS preparations (Fig. 1). These data indicate that cytokine release from macrophages was due to meningococcal LOS and that KDO2-deficient meningococcal LOS showed marked attenuation in biologic activity (Fig. 1 and 2).
The role of TLR4-MD-2 and CD14 in cytokine release from macrophages by meningococcal LOS.
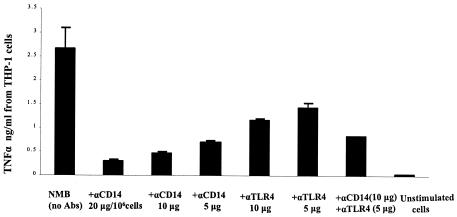
To confirm that the interaction of meningococcal LOS with macrophages was CD14 and TLR4 dependent, a monoclonal antibody (clone 134620, R&D Systems) to CD14 (when used alone or in combination with anti-TLR4-MD-2) preincubated with cells significantly reduced the effect of LOS cytokine induction in human THP-1 (Fig. 3) and U937 cells. The blocking of cytokine release by anti-CD14 (P < 0.001) and anti-TLR4 (P < 0.004) was dose dependent (Fig. 3). Thus, in both THP-1 and U937 macrophages, meningococcal LOS cytokine induction was mCD14 and TLR4 mediated.
FIG. 3.
TNF-α induction in human macrophages by meningococcal LOS was CD14 and TLR4-MD-2 mediated. THP-1 macrophages (106/ml) were incubated with 10 or 5 μg of anti-CD14 and anti-TLR4, respectively, or with anti-CD14 or anti-TLR4 in a dose-dependent manner prior to stimulation with 0.56 pmol (1 ng/ml) of meningococcal LOS from parent strain NMB (Table 1). Unstimulated cells were included as a control. Error bars represent SD from the average of four different experiments.
Meningococcal LOS structure and nitric oxide release.
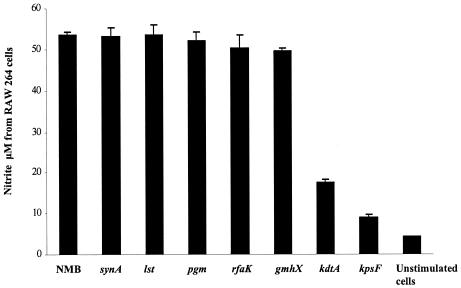
The release of nitric oxide from RAW macrophages stimulated with wild-type meningococcal LOS or meningococcal LOS with oligosaccharide truncations was similar (Fig. 4). However, the KDO2-deficient meningococcal lipid A's showed markedly attenuated release of nitric oxide (P < 0.0001). A similar attenuation in nitric oxide release with KDO2-deficient meningococcal lipid A was seen when RAW macrophages were indirectly stimulated with cell-free supernatants from previously induced THP-1 cells (data not shown). Nitric oxide release was dose-dependent for meningococcal LOS. No response was seen in TLR4-deficient cells (C3H/HeJ) stimulated with purified meningococcal LOS (data not shown). In addition, nitric oxide was not released when RAW macrophages were indirectly induced with supernatants from differentiated U937 cells or THP-1 cells previously blocked (prior to stimulation by LOS) with anti-CD14 or anti-TLR4-MD-2. These data indicate that the attenuated activity of meningococcal lipid A was not species specific since meningococcal lipid A had attenuated activity in both human and murine cell lines.
FIG. 4.
The effect of meningococcal LOS structure on nitric oxide induction in macrophages. RAW macrophages (106 cells/ml) were stimulated overnight with meningococcal LOS NMB and synA, lst, pgm, rfaK, gmhX, kdtA, and kpsF mutants at 0.56 pmol/ml (Table 1). A preparation from the LOS-deficient mutant (lpxA) and unstimulated cells were used as controls. Nitrite concentration in cell supernatants was detected with the Griess method. Error bars represent SD from the mean, and the data are from four different readings and are representative of other similar experiments.
Meningococcal LOS structure and oxidative burst.
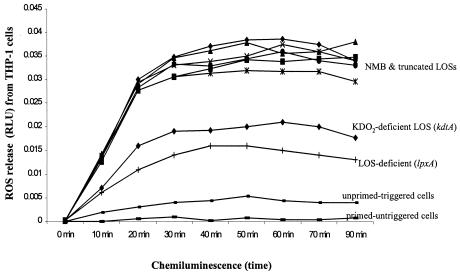
To further assess meningococcal LOS structure-function relationships, oxidative burst (reactive oxygen species [ROS] release) of THP-1, U937, and RAW macrophages primed overnight with meningococcal LOS was investigated using cellular chemiluminescence. No significant differences in ROS release were seen between the glycosylated meningococcal LOS structures (Fig. 5). However, KDO2-deficient lipid A and the LOS-deficient preparation showed significantly attenuated ROS release (P < 0.0001). The results again indicated that oligosaccharide chain length or structure did not affect meningococcal LOS priming of macrophages to release ROS but that KDO2-lipid A was required for maximal agonist activity.
FIG. 5.
The role of meningococcal LOS structure in priming human macrophages for oxidative burst. A representative graph of THP-1 (106 macrophages/ml) which were primed with 5 pmol (10 ng/ml) of meningococcal LOS (Table 1). Oxidative burst was triggered with 2 μM PMA, and ROS release was detected with the lucigenin chemiluminescent probe. Primed untriggered macrophages, unprimed triggered macrophages, and macrophages exposed to the preparation from the LOS-deficient mutant (lpxA) at 1,000 ng/ml (dry weight) were used as controls. THP-1 cells express both TLR4 and TLR2 (46).
Role of KDO in meningococcal LOS interactions with macrophages.
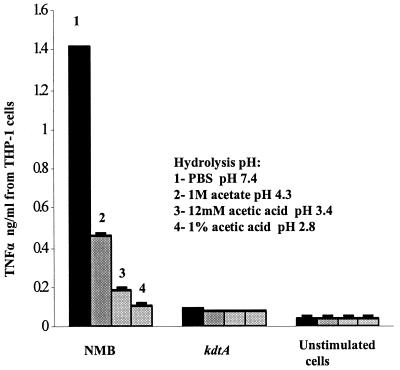
To further determine the importance of the KDO attached to meningococcal lipid A in activation of TLR4, meningococcal LOS was subjected to mild acid hydrolysis to cleave KDO2 and other oligosaccharides from lipid A. Compared to controls, cytokine and nitric oxide release were significantly attenuated (P < 0.0001) when wild-type meningococcal LOS or oligosaccharide-truncated gmhX LOS were subjected to acid hydrolysis (Fig. 6). The activity of hydrolyzed LOS was comparable to that of the KDO2-deficient lipid A. LOS hydrolyzed with 1% acetic acid (pH 2.8) showed the greatest attenuation in TNF-α release (Fig. 6). Although acid hydrolysis might be predicted to affect lipid A phosphate head groups and contribute to attenuation, no significant difference in activity was seen between the KDO2-deficient lipid A hydrolyzed and unhydrolyzed (P = 0.083). Synthetic KDO alone did not activate the CD14/TLR4-MD-2 receptor complex at dose ranges from 10 ng to 100 μg (data not shown). These results support the importance of KDO2 linked to meningococcal lipid A for maximal stimulation of macrophages via the CD14/TLR4-MD-2 receptor complex.
FIG. 6.
Effect of removal of meningococcal KDO2 by acid hydrolysis on TNF-α release by human macrophages. Meningococcal LOS of parent strain NMB (Table 1) was hydrolyzed with 1% acetic acid (pH 2.8) or with 12 mM acetic acid (pH 3.4) or 1 M acetate buffer (pH 4.3) or was treated with PBS (pH 7.4). TNF-α release from THP-1 cells induced with hydrolyzed LOS at a concentration of 0.56 pmol/ml was measured. Lipid A of the kdtA mutant and unstimulated cells were used as controls. Error bars represent SD from the mean.
Glycosylated and unglycosylated lipid A binds to the CD14/TLR4-MD-2 receptor complex.
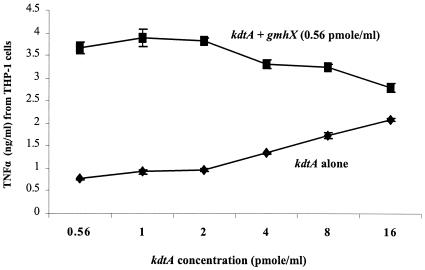
The attenuated activity of the KDO2-deficient meningococcal lipid A was not due to decreased binding to the CD14/TLR4-MD-2 receptor complex. In a competitive binding inhibition assay using glycosylated and unglycosylated LOS, meningococcal KDO2-lipid A induction of nitric oxide or TNF-α was competitively inhibited by increasing concentrations of unglycosylated meningococcal lipid A. In contrast to the dose-response increase in biologic activity of KDO2-lipid A observed in Fig. 2, the addition of glycosylated (gmhX, KDO2-lipid A) and unglycosylated meningococcal lipid A (kdtA) simultaneously to THP-1 macrophages resulted in a significant decrease in TNF-α (P < 0.001) release compared to KDO2-lipid A alone at each concentration of lipid A (Fig. 7). When RAW macrophages were stimulated with KDO2-lipid A and unglycosylated lipid A, intermediate levels of nitrite (15 ± 0.45 μM) were detected in supernatants compared to KDO2-lipid A alone (25 ± 0.47 μM) or unglycosylated lipid A alone (6 ± 0.32 μM). The overnight incubation of glycosylated lipid A (KDO2-lipid A) and unglycosylated lipid A (KDO-deficient lipid A) prior to cell induction resulted in a further decrease in nitric oxide release or TNF-α release (data not shown). These assays suggested that glycosylated and unglycosylated lipid A bind equally well or share similar binding sites on the CD14/TLR4-MD-2 receptor complex and that lipid A aggregation was not the cause of decreased biologic activity.
FIG. 7.
Competitive inhibition of glycosylated lipid A by unglycosylated lipid A on TNF-α release by human macrophages. TNF-α released from 106 THP-1 macrophages/ml stimulated with 0.56 pmol of KDO2-lipid A (gmhX)/ml in the presence of increasing concentrations of the KDO-deficient lipid A (kdtA).
The role of meningococcal lipid A acyl chain structure on macrophage CD14/TLR4-MD-2 activation.
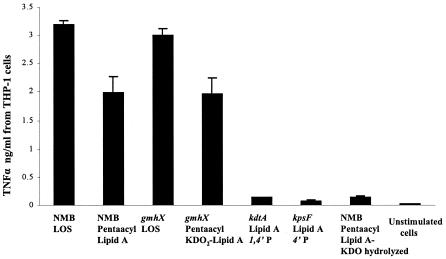
Meningococcal LOS structures with penta-acylated lipid A (Table 1) NMB-lpxL1 and gmhX-lpxL1 were used to induce cytokine, nitric oxide, and ROS release from macrophages. Penta-acylated meningococcal LOS induced ∼70% of the TNF-α release (P < 0.0001) compared to the corresponding glycosylated hexa-acylated meningococcal LOS (Fig. 8). By comparison, ≤10% of TNF-α activity was noted when KDO2-deficient hexa-acylated lipid A was used. Similar results were seen for nitric oxide or ROS release when cells were stimulated with penta or with hexa-acylated LOS. The induction of TNF-α was dramatically decreased (P < 0.0001) to levels similar to those of the KDO2-deficient lipid A when penta-acylated LOS was subjected to mild acid hydrolysis (pH 4.3) (Fig. 8). Thus, loss of a fatty acyl chain from lipid A resulted in a modest reduction in biological activity. However, the loss of KDO2 from penta-acylated meningococcal lipid A resulted in a dramatic attenuation in biologic activity.
FIG. 8.
Effect of meningococcal lipid A acyl chain number on TNF-α induction by human macrophages. Penta-acylated meningococcal LOS, with otherwise-complete oligosaccharide structure, or penta-acylated KDO2-lipid A (gmhX-lpxL1) (Table 1) was used to stimulate 106 THP-1 macrophages/ml. The activity was compared to LOS from the corresponding parent strains NMB and the gmhX mutant with hexa-acylated lipid A structures (Table 1). KDO-deficient (kdtA) and (kpsF) with hexa-acylated lipid A were also used to show the role of KDO2 on LOS biological activity. Penta-acylated LOS hydrolyzed with mild acid and unstimulated cells were used as controls. Error bars represent SD from the mean of three independent experiments.
DISCUSSION
Meningococcal LOS is a major inflammatory mediator of meningococcemia and meningococcal meningitis (45). Meningococcal LOS levels in serum of ≥1 ng/ml are associated with shock and death in meningococcemia (1, 2, 4). The interaction of meningococcal LOS with the CD14/TLR4-MD-2 receptor complex is predicted to result in macrophage activation and subsequent release of cytokines, chemokines, nitric oxide, and ROS. The goal of this study was to define the relationship of meningococcal LOS structure with the biological activity initiated through the human CD14/TLR4-MD-2 receptor.
The importance of CD14 and TLR4-MD-2 in macrophage activation by meningococcal LOS was demonstrated. When CD14 was efficiently blocked with specific monoclonal antibody, TNF-α production was markedly reduced. When TLR4-MD-2 was blocked and CD14 was available, a significant reduction in cytokine release was also observed. Further, highly purified meningococcal LOS did not stimulate TLR2 in our experimental models when C3H/HeJ (TLR4−/−) cells were induced, supporting the model that CD14/TLR4-MD-2 is the meningococcal endotoxin receptor.
Meningococcal LOS α and β chain oligosaccharide structure and length had no effect on CD14/TLR4-MD-2 receptor activation. However, KDO2 linked to meningococcal lipid A was required for maximal agonist activation. Consistent results were seen in this study with cytokine induction, nitric oxide or ROS release and in time course or dose-response manner. Meningococcal KDO2-lipid A was recognized by the CD14/TLR4-MD-2 receptors of both human and murine cells. Thus, the KDO2 structure was not the determinant of the species-specific differences noted with other LPS structures (19).
Decreased solubility of meningococcal lipid A was not the explanation for attenuation in biological activity. Muller et al. have recently shown that endotoxin aggregates are significantly more biologically active than monomers (24). Meningococcal lipid A's were soluble at the concentrations and procedures used, and unglycosylated meningococcal lipid A was a dose-dependent competitive inhibitor of glycosylated meningococcal LOS. In addition, polystyrene beads coated with meningococcal LOS or lipid A showed similar differences in cytokine, nitric oxide, and ROS release (data not shown). The importance of meningococcal KDO2-lipid A was confirmed when meningococcal LOS was subjected to acid hydrolysis to cleave KDO2 from lipid A. Thus, the loss of KDO2 from lipid A attenuated meningococcal LOS biologic activity. Mild acid hydrolysis does not alter lipid A structure or cleave lipid A phosphate head groups (53).
The importance of KDO2 linked to lipid A for maximal meningococcal endotoxin biological activity and for endotoxin activity in general is supported by other studies. Schromm et al. (34) found that the number, nature, and location of negatively charged molecules modulate the molecular conformation of E. coli LPS and lipid A and are linked to IL-6 inducing capacity. Recently, synthetic lipid A with two KDO molecules was found to have enhanced agonist activity compared to one KDO molecule or none (18, 50). A decreased number or lack of KDO and hydroxymyristic acid is proposed as a contributor to low endotoxic activity of Leptospira interrogans (46), Francisella tularensis (47), Legionella pneumophila, and different Rhizobium species LPSs (32, 51).
While KDO2 linked to meningococcal lipid A was essential for maximal activation of CD14/TLR4-MD-2, the negatively charged lipid A phosphate head groups appeared to be less important. Monophosphorylated meningococcal KDO2-deficient lipid A was only minimally less active than the bis-phosphorylated meningococcal lipid A, and glycosylated meningococcal LOS structures with variable 1 and 4′ phosphorylation were equal agonists. The ability of meningococcal LOS to clot Limulus amebocyte lysate is related to the amount of bis-phosphorylated lipid A expressed by meningococcal isolates (31). However, Limulus amebocyte lysate activity may not correlate to LPS biological activity (23). Phosphate and pyrophosphoethanolamine substitution of enteric lipid A head groups do affect enteric endotoxic activity (6, 7, 16, 20, 29), and the phosphate group of meningococcal lipid A is quite variable. The precise contribution of the lipid A phosphate head groups in meningococcal LOS activation of CD14/TLR4-MD-2 will require mutation of the genes that add phosphate to lipid A, an area of active investigation.
The number, structure, length, branches, symmetry, chirality, and saturation of fatty acyl chains in lipid A are important determinants of biological activity of lipid A's (23, 33, 35, 40, 51). For example, synthetic tetra-acylated lipid A (lipid IVa) is an antagonist of LPS activation of human macrophages (9, 21) and penta-acylated LPS extracted from Porphyromonas gingivalis containing extended and branched fatty acyl chains has attenuated activity (26). Hawkins et al. (11), using synthetic simplified lipid A-like structures, showed that isomers with R,R,R,R-acyl chain configuration were strongly agonistic, whereas similar compounds with R,S,S,R-acyl chain configuration were much weaker in biologic activity.
Meningococcal fatty acyl chain number was a contributor to macrophage activation via the CD14/TLR4-MD-2 receptor. Meningococcal LOS with penta-acylated lipid A but an otherwise-intact oligosaccharide structure showed a reduction in cytokine induction compared to the corresponding hexa-acylated lipid A. The attenuation in agonist activity of LOS with penta-acyl lipid A was seen in both human and murine macrophages. van der Ley et al. (44) also showed that a penta-acylated meningococcal mutant (lpxL1) had reduced toxicity as measured in a TNF-α induction assay. There is considerable experimental support that tetra-acyl lipid A's have no activity and act as antagonists in human cells but are agonists in murine cells (19); thus, KDO linked to a tetra-acyl lipid A has no activity in human macrophages (22). Kitchens and Munford also reported that enzymatically deacylated E. coli LPS and Salmonella LPS (i.e., tetra-acylated LPS structures with KDO) were inactive in human THP-1 cells and inhibit IL-8 release (17).
Seydel and Schromm and colleagues (33-35) have proposed that the biological activity of endotoxin is determined by the three dimensional structure of lipid A. Lipid A's with a conical-concave shape, the cross-section of the hydrophobic region being larger than that of the hydrophilic region, have strong IL-6-inducing activity. A cylindrical molecular shape of lipid A correlates with antagonistic activity. Preliminary data indicate that meningococcal KDO2-lipid A phase transition temperature is much lower than that of the unglycosylated meningococcal lipid A. Transition temperature is inversely correlated with fatty acyl chain fluidity and conical/concave shape.
Meningococcal hexa-acylated KDO2-lipid A (with symmetrical acylation of fatty acyl chain length of C12 and C14) appears to be the optimal agonist of the CD14/TLR4-MD-2 pathway. Meningococcal LOS like enteric LPS is likely transferred via LBP-sCD14 to membrane bound CD14/TLR4-MD-2 (5, 38), bringing the molecule into a close proximity to TLR4-MD-2. The negatively charged KDO sugars of meningococcal lipid A may facilitate binding to the leucine-rich repeats (most likely to residues 190 to 194 that contain positively charged amino acids (6) of the TLR4 ectodomain in the presence of MD-2 to initiate a conformational change that is sufficient to trigger intracellular signaling. In contrast, unglycosylated lipid A ineffectively induces conformational change in TLR4-MD-2.
In conclusion, meningococcal LOS is a potent activator of the macrophage TLR4 pathway. This may help explain the role of meningococcal endotoxin in acute meningococcal sepsis and meningitis. Meningococcal oligosaccharide α or β chain structure or length was not a contributor to human or murine TLR4 activation. KDO2 linked to lipid A was structurally required for maximal meningococcal endotoxin agonist activity.
Acknowledgments
This work was supported by grant 2 R01 AI033517-10 from the National Institutes of Health to D.S.S.
We thank Larry Martin for technical assistance and Lane Pucko for administrative assistance.
Editor: B. B. Finlay
REFERENCES
- 1.Brandtzaeg, P., K. Bryn, P. Kierulf, R. Ovstebo, E. Namork, B. Aase, and E. Jantzen. 1992. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy J. Clin. Investig. 89:816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandtzaeg, P., P. Kierulf, P. Gaustad, A. Skulberg, J. N. Bruun, S. Halvorsen, and E. Sorensen. 1989. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease J. Infect. Dis. 159:195-204. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg, P., R. Ovstebo, and P. Kierulf. 1995. Bacteremia and compartmentalization of LPS in meningococcal disease. Prog. Clin. Biol. Res. 392:219-233. [PubMed] [Google Scholar]
- 4.Brandtzaeg, P., R. Ovsteboo, and P. Kierulf. 1992. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J. Infect. Dis. 166:650-652. [DOI] [PubMed] [Google Scholar]
- 5.da Silva Correia, J., K. Soldau, U. Christen, P. S. Tobias, and R. J. Ulevitch. 2001. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex: transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 26:26. [DOI] [PubMed] [Google Scholar]
- 6.Frecer, V., B. Ho, and J. L. Ding. 2000. Interpretation of biological activity data of bacterial endotoxins by simple molecular models of mechanism of action. Eur. J. Biochem. 267:837-852. [DOI] [PubMed] [Google Scholar]
- 7.Frecer, V., B. Ho, and J. L. Ding. 2000. Molecular dynamics study on lipid A from Escherichia coli: insights into its mechanism of biological action. Biochim. Biophys. Acta 1466:87-104. [DOI] [PubMed] [Google Scholar]
- 8.Fukuoka, S., H. Kamishima, Y. Nagawa, H. Nakanishi, K. Ishikawa, Y. Niwa, E. Tamiya, and I. Karube. 1992. Structural characherization of lipid A component of Erwinia carotovora lipopolysaccharide. Arch. Microbiol. 157:311-318. [Google Scholar]
- 9.Galanos, C., V. Lehmann, O. Luderitz, E. T. Rietschel, O. Westphal, H. Brade, L. Brade, M. A. Freudenberg, T. Hansen-Hagge, T. Luderitz, et al. 1984. Endotoxic properties of chemically synthesized lipid A part structures. Comparison of synthetic lipid A precursor and synthetic analogues with biosynthetic lipid A precursor and free lipid A. Eur. J. Biochem. 140:221-227. [DOI] [PubMed] [Google Scholar]
- 10.Galanos, C., O. Luderitz, E. T. Rietschel, O. Westphal, H. Brade, L. Brade, M. Freudenberg, U. Schade, M. Imoto, H. Yoshimura, et al. 1985. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 148:1-5. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins, L. D., S. T. Ishizaka, P. McGuinness, H. Zhang, W. Gavin, B. DeCosta, Z. Meng, H. Yang, M. Mullarkey, D. W. Young, D. P. Rossignol, A. Nault, J. Rose, M. Przetak, J. C. Chow, and F. Gusovsky. 2002. A novel class of endotoxin receptor agonists with simplified structure, Toll-like receptor 4-dependent immunostimulatory action, and adjuvant activity. J. Pharmacol. Exp. Ther. 300:655-661. [DOI] [PubMed] [Google Scholar]
- 12.Kahler, C. M., R. W. Carlson, M. M. Rahman, L. E. Martin, and D. S. Stephens. 1996. Two glycosyltransferase genes, lgtF and rfaK, constitute the lipooligosaccharide ice (inner core extension) biosynthesis operon of Neisseria meningitidis. J. Bacteriol. 178:6677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahler, C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24:281-334. [DOI] [PubMed] [Google Scholar]
- 14.Kanegasaki, S., K. Tanamoto, T. Yasuda, J. Y. Homma, M. Matsuura, M. Nakatsuka, Y. Kumazawa, A. Yamamoto, T. Shiba, S. Kusumoto, et al. 1986. Structure-activity relationship of lipid A: comparison of biological activities of natural and synthetic lipid A's with different fatty acid compositions. J. Biochem. (Tokyo) 99:1203-1210. [DOI] [PubMed] [Google Scholar]
- 15.Kiang, J., S. C. Szu, L. X. Wang, M. Tang, and Y. C. Lee. 1997. Determination of 2-keto-3-deoxyoctulosonic acid (KDO) with high-performance anion-exchange chromatography (HPAEC): survey of stability of KDO and optimal hydrolytic conditions. Anal. Biochem. 245:97-101. [DOI] [PubMed] [Google Scholar]
- 16.Kirikae, T., F. U. Schade, U. Zahringer, F. Kirikae, H. Brade, S. Kusumoto, T. Kusama, and E. T. Rietschel. 1994. The significance of the hydrophilic backbone and the hydrophobic fatty acid regions of lipid A for macrophage binding and cytokine induction. FEMS Immunol. Med. Microbiol. 8:13-26. [DOI] [PubMed] [Google Scholar]
- 17.Kitchens, R. L., and R. S. Munford. 1995. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J. Biol. Chem. 270:9904-9910. [DOI] [PubMed] [Google Scholar]
- 18.Kusumoto, S., K. Fukase, Y. Fukase, M. Kataoka, and Y. Suda. 2002. Structural basis for endotoxic and antagonistic activities. Investigation with novel synthetic lipid A analogs. J. Endotoxin Res. 8:195. [DOI] [PubMed] [Google Scholar]
- 19.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loppnow, H., H. Brade, I. Durrbaum, C. A. Dinarello, S. Kusumoto, E. T. Rietschel, and H. D. Flad. 1989. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J. Immunol. 142:3229-3238. [PubMed] [Google Scholar]
- 21.Luderitz, O., K. Tanamoto, C. Galanos, G. R. McKenzie, H. Brade, U. Zahringer, E. T. Rietschel, S. Kusumoto, and T. Shiba. 1984. Lipopolysaccharides: structural principles and biologic activities. Rev. Infect. Dis. 6:428-431. [DOI] [PubMed] [Google Scholar]
- 22.Matsuura, M., M. Kiso, and A. Hasegawa. 1999. Activity of monosaccharide lipid A analogues in human monocytic cells as agonists or antagonists of bacterial lipopolysaccharide. Infect. Immun. 67:6286-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuyama, N., T. Kirikae, F. Kirikae, M. Hashimoto, K. Amanot, S. Hayashi, Y. Hirai, T. Kubota, and M. Nakano. 2001. Non-standard biological activities of lipopolysaccharide from Helicobacter pylori. J. Med. Microbiol. 50:865-869. [DOI] [PubMed] [Google Scholar]
- 24.Muller, M. R., B. Lindner, and U. Seydel. 2002. Endotoxin aggregates are significantly more active than monomers. J. Endotoxin Res. 8:167-168. [Google Scholar]
- 25.Muroi, M., and K. Tanamoto. 2002. The polysaccharide portion plays an indispensable role in Salmonella lipopolysaccharide-induced activation of NF-κB through human Toll-like receptor 4. Infect. Immun. 70:6043-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa, T., Y. Asai, M. Hashimoto, O. Takeuchi, T. Kurita, Y. Yoshikai, K. Miyake, and S. Akira. 2002. Cell activation by Porphyromonas gingivalis lipid A molecule through Toll-like receptor 4- and myeloid differentiation factor 88-dependent signaling pathway. Int. Immunol. 14:1325-1332. [DOI] [PubMed] [Google Scholar]
- 27.Park, E., M. R. Quinn, C. E. Wright, and G. Schuller-Levis. 1993. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J. Leukoc. Biol. 54:119-124. [DOI] [PubMed] [Google Scholar]
- 28.Rahman, M. M., D. S. Stephens, C. M. Kahler, J. Glushka, and R. W. Carlson. 1998. The lipooligosaccharide (LOS) of Neisseria meningitidis serogroup B strain NMB contains L2, L3, and novel oligosaccharides, and lacks the lipid-A 4′-phosphate substituent. Carbohydr. Res. 307:311-324. [DOI] [PubMed] [Google Scholar]
- 29.Rietschel, E. T., T. Kirikae, F. U. Schade, U. Mamat, G. Schmidt, H. Loppnow, A. J. Ulmer, U. Zahringer, U. Seydel, F. Di Padova, et al. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8:217-225. [DOI] [PubMed] [Google Scholar]
- 30.Robbins, A., and P. Freeman. 1988. Obstacles to developing vaccines for the Third World. Sci. Am. 259:126-133. [DOI] [PubMed] [Google Scholar]
- 31.Roth, R. I., R. Yamasaki, R. E. Mandrell, and J. M. Griffiss. 1992. Ability of gonococcal and meningococcal lipooligosaccharides to clot Limulus amebocyte lysate. Infect. Immun. 60:762-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russa, R., T. Urbanik-Sypniewska, A. Choma, and H. Mayer. 1991. Identification of 3-deoxy-lyxo-2-heptulosaric acid in the core region of lipopolysaccharides from Rhizobiaceae. FEMS Microbiol. Lett. 68:337-343. [DOI] [PubMed] [Google Scholar]
- 33.Schromm, A. B., K. Brandenburg, H. Loppnow, A. P. Moran, M. H. Koch, E. T. Rietschel, and U. Seydel. 2000. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur. J. Biochem. 267:2008-2013. [DOI] [PubMed] [Google Scholar]
- 34.Schromm, A. B., K. Brandenburg, H. Loppnow, U. Zahringer, E. T. Rietschel, S. F. Carroll, M. H. Koch, S. Kusumoto, and U. Seydel. 1998. The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J. Immunol. 161:5464-5471. [PubMed] [Google Scholar]
- 35.Seydel, U., M. Oikawa, K. Fukase, S. Kusumoto, and K. Brandenburg. 2000. Intrinsic conformation of lipid A is responsible for agonistic and antagonistic activity. Eur. J. Biochem. 267:3032-3039. [DOI] [PubMed] [Google Scholar]
- 36.Seydel, U., A. B. Schromm, R. Blunck, and K. Brandenburg. 2000. Chemical structure, molecular conformation, and bioactivity of endotoxins. Chem. Immunol. 74:5-24. [DOI] [PubMed] [Google Scholar]
- 37.Shih, G. C., C. M. Kahler, R. W. Carlson, M. M. Rahman, and D. S. Stephens. 2001. gmhX, a novel gene required for the incorporation of L-glycero-D-manno-heptose into lipooligosaccharide in Neisseria meningitidis. Microbiology 147:2367-2377. [DOI] [PubMed] [Google Scholar]
- 38.Smith, P. D., L. E. Smythies, M. Mosteller-Barnum, D. A. Sibley, M. W. Russell, M. Merger, M. T. Sellers, J. M. Orenstein, T. Shimada, M. F. Graham, and H. Kubagawa. 2001. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J. Immunol. 167:2651-2656. [DOI] [PubMed] [Google Scholar]
- 39.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 40.Suda, Y., Y. M. Kim, T. Ogawa, N. Yasui, Y. Hasegawa, W. Kashihara, T. Shimoyama, K. Aoyama, K. Nagata, T. Tamura, and S. Kusumoto. 2001. Chemical structure and biological activity of a lipid A component from Helicobacter pylori strain 206. J. Endotoxin Res. 7:95-104. [PubMed] [Google Scholar]
- 41.Tzeng, Y. L., A. Datta, V. K. Kolli, R. W. Carlson, and D. S. Stephens. 2002. Endotoxin of Neisseria meningitidis composed only of intact lipid A: inactivation of the meningococcal 3-deoxy-d-manno-octulosonic acid transferase. J. Bacteriol. 184:2379-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzeng, Y. L., A. Datta, C. Strole, V. S. Kolli, M. R. Birck, W. P. Taylor, R. W. Carlson, R. W. Woodard, and D. S. Stephens. 2002. KpsF is the arabinose-5-phosphate isomerase required for 3-deoxy-D-manno-octulosonic acid biosynthesis and for both lipooligosaccharide assembly and capsular polysaccharide expression in Neisseria meningitidis. J. Biol. Chem. 277:24103-24113. [DOI] [PubMed] [Google Scholar]
- 43.Ulmer, A. J., H. Heine, W. Feist, M. H. Wang, H. Loppnow, T. Kirikae, F. Kirikae, S. Kusumoto, T. Kusama, H. Brade, et al. 1994. Biological activity of lipid A partial structures. Prog. Clin. Biol. Res. 388:71-83. [PubMed] [Google Scholar]
- 44.van der Ley, P., L. Steeghs, H. J. Hamstra, J. ten Hove, B. Zomer, and L. van Alphen. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 69:5981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Deuren, M., P. Brandtzaeg, and J. W. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13:144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinh, T., B. Adler, and S. Faine. 1986. Ultrastructure and chemical composition of lipopolysaccharide extracted from Leptospira interrogans serovar copenhageni. J. Gen. Microbiol. 132:103-109. [DOI] [PubMed] [Google Scholar]
- 47.Vinogradov, E., M. B. Perry, and J. W. Conlan. 2002. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 269:6112-6118. [DOI] [PubMed] [Google Scholar]
- 48.Yang, S., R. Tamai, S. Akashi, O. Takeuchi, S. Akira, S. Sugawara, and H. Takada. 2001. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect. Immun. 69:2045-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.York, W. S., A. G. Darvill, M. McNeil, T. T. Stevenson, and P. Albersheim. 1985. Isolation and characterization of plant cell walls and cell wall components. Methods Ezymol. 118:3-40. [Google Scholar]
- 50.Yoshizaki, H., N. Fukuda, K. Sato, M. Oikawa, K. Fukase, Y. Suda, and S. Kusumoto. 2001. First total synthesis of the re-type lipopolysaccharide. Angew. Chem. Int. Ed. 8:1475-1480. [DOI] [PubMed] [Google Scholar]
- 51.Zahringer, U., Y. A. Knirel, B. Lindner, J. H. Helbig, A. Sonesson, R. Marre, and E. T. Rietschel. 1995. The lipopolysaccharide of Legionella pneumophila serogroup 1 (strain Philadelphia 1): chemical structure and biological significance. Prog. Clin. Biol. Res. 392:113-139. [PubMed] [Google Scholar]
- 52.Zhou, D., D. S. Stephens, B. W. Gibson, J. J. Engstrom, C. F. McAllister, F. K. Lee, and M. A. Apicella. 1994. Lipooligosaccharide biosynthesis in pathogenic Neisseria. Cloning, identification, and characterization of the phosphoglucomutase gene. J. Biol. Chem. 269:11162-11169. [PubMed] [Google Scholar]
- 53.Zhou, Z., K. A. White, A. Polissi, C. Georgopoulos, and C. R. Raetz. 1998. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J. Biol. Chem. 273:12466-12475. [DOI] [PubMed] [Google Scholar]
- 54.Zughaier, S. M., H. C. Ryley, and S. K. Jackson. 1999. A melanin pigment purified from an epidemic strain of Burkholderia cepacia attenuates monocyte respiratory burst activity by scavenging superoxide anion. Infect. Immun. 67:908-913. [DOI] [PMC free article] [PubMed] [Google Scholar]