Abstract
Recombinant immunodominant mycobacterial antigens are needed for the development of new vaccines and immunodiagnostic tools for use against tuberculosis. Ubiquitous exposure to mycobacteria in tropical countries could influence vaccine-induced immunity and the specificity of tuberculosis immunodiagnosis. For this study conducted in The Gambia, cellular immune responses to recombinant mycobacterial antigens were characterized in Mycobacterium bovis BCG-vaccinated and nonvaccinated infants, adult community controls, household contacts, health care workers, and tuberculosis patients. Neonatal BCG vaccination induced gamma interferon (IFN-γ) responses to Mtb8.4, Mtb32-C, Mtb39A, Mtb9.9A, and Mtb32-N, but not CFP-10 (Mtb11) and α-crystallin (Mtb16). Exposure to Mycobacterium tuberculosis in household contacts and health care workers was associated with high responses to CFP-10 and α-crystallin. Generally, low IFN-γ responses were found in tuberculosis patients. These results suggest that Mtb8.4, Mtb32-C, Mtb39A, Mtb9.9A, and Mtb32-N may be used in a subunit vaccine to boost BCG-induced immunity. While CFP-10 and α-crystallin are promising candidates for the immunodiagnosis of M. tuberculosis infection or for vaccine use, disease-associated immunosuppression may prevent IFN-γ immunodiagnosis of more advanced tuberculosis.
Ubiquitous exposure to mycobacteria, starting early in life in tropical countries, poses a number of problems for the development of tuberculosis control tools. Mycobacterium tuberculosis remains one of the world's most serious public health threats, with about two billion people infected worldwide and three million annual deaths from tuberculosis (18). The attenuated strain of Mycobacterium bovis bacillus Calmette-Guérin (BCG), so far the only tuberculosis vaccine available for human use, has failed to control M. tuberculosis transmission. Clinical trials conducted in different countries have reported protective efficacy ranging from 0 to 80% (12, 19). Among the factors that may explain this variable protection, prevaccination immunity to environmental mycobacteria can prevent the induction of protective immunity by BCG vaccination, as demonstrated in animals (20, 31). In humans, it was recently shown in Malawi that preexisting responsiveness to mycobacterial antigens is associated with lower gamma interferon (IFN-γ) responses to BCG vaccination in adults (8, 9). These data may partly explain why neonatal BCG vaccination induces higher levels of protection than does adult vaccination (12).
Exposure to environmental mycobacteria and BCG vaccination are also responsible for the poor predictive value of the tuberculin skin test. The tuberculin skin test is the only available test used to confirm clinical suspicion of tuberculosis, to identify recently exposed individuals for administration of preventive chemotherapy, and to estimate the prevalence and risk of infection in epidemiological studies (24). Indeed, tuberculin purified protein derivative (PPD) is a soluble protein preparation containing more than 200 different mycobacterial antigens, many of which are expressed by a wide range of mycobacteria including M. bovis BCG, resulting in frequent cross-reactivity (11).
Important efforts have been made to identify immunodominant mycobacterial antigens, with the objective of developing new vaccine and immunodiagnostic candidates (2, 3). Well-characterized immunodominant antigens have the potential to circumvent some of the disadvantages associated with the use of widely cross-reactive antigen preparations of poorly defined specificity. As a vaccine, a recombinant antigen may be used to boost preexisting immune responses induced by BCG or environmental mycobacteria or may be used to initiate a primary immune response. Used in an immunodiagnostic assay, a recombinant antigen should detect a response induced specifically by M. tuberculosis, but not by environmental mycobacteria or BCG, and the recombinant antigen should be absent from any new vaccine preparation in order to avoid confusion between disease, environmental mycobacterial exposure, and past vaccination. The analysis of the immune response induced by BCG vaccination, exposure to the environment, exposure to pathogenic mycobacteria, and tuberculosis disease to an antigen candidate for vaccine and immunodiagnostic development should help in the rational selection of antigens. Such information is especially needed in developing countries, where BCG vaccination is generalized, exposure to environmental mycobacteria is most intense, and new tuberculosis control tools are most needed.
A series of soluble purified recombinant mycobacterial antigens has been identified recently (1, 4-7, 13-17, 23, 25-27, 34, 40-42). As shown in Table 1, the expression profiles of antigens across different species of mycobacteria have been determined. In this field study in The Gambia, the immune response to these antigens was investigated in non-BCG-vaccinated infants, whom we assume to be free of previous mycobacterial exposure; BCG-vaccinated infants, documenting “BCG only” induced immune responses; adult community controls who had been exposed to environmental mycobacteria, BCG, and M. tuberculosis; tuberculosis patients; household contacts known to have been exposed recently to infectious tuberculosis cases; and health care workers who were chronically exposed to infectious patients but remained free of tuberculosis. Selected comparisons among these different population groups were made in order to analyze potential use in subunit vaccine or immunodiagnostic development.
TABLE 1.
Characteristics of the antigens used in this study
| Antigen | Gene | Molecular mass (kDa) | Secreted antigen | Present in:
|
Reference(s) | |||
|---|---|---|---|---|---|---|---|---|
| M. tuberculosis | BCG | M. avium | Othersa | |||||
| CFP-10 (Mtb11) | Rv3874 | 11 | Yes | Yes | No | No | No | 4-7, 16, 25-27, 32 |
| α-Crystallin (Mtb16) | Rv2031c | 16 | No | Yes | Yes | —c | —c | 15, 23, 41, 42 |
| Mtb8.4 | Rv1174c | 8.4 | Yes | Yes | Yes | Yes | No | 13-14 |
| Mtb12 | Rv2376c | 12.5 | Yes | Yes | Yes | No | —b | 40 |
| Mtb39A | Rv1196 | 49 | No | Yes | Yes | No | No | 17 |
| Mtb9.9A | Rv1793 | 9.9 | Yes | Yes | Yes | No | No | 1 |
| Mtb32-Nd | Rv0125 | 20 | Yes | Yes | Yes | No | No | 34 |
| Mtb32-Cd | Rv0125 | 14 | Yes | Yes | Yes | No | No | 34 |
Mycobacterium smegmatis, Mycobacterium gordonae, Mycobacterium chelonae, Mycobacterium fortuitum, Mycobacterium scrofulaceum, Mycobacterium vaccae, and Mycobacterium leprae.
Present in M. leprae; presence in other strains is unknown.
Sequence homology with the 18-kDa antigen present in M. leprae; similar genes were present in other mycobacteria (39).
Mtb32-N and Mtb32-C are the N- and C-terminal fractions, respectively, of the Mtb32 transcript.
MATERIALS AND METHODS
Study populations.
The Medical Research Council (MRC) guidelines for good research practices were followed in the conduct of this study, which was approved by the MRC Ethics Committee of the Gambian government. Informed consent was obtained from adults or mothers of infants enrolled in this study. Twenty-nine healthy newborns were enrolled at Serrekunda Health Centre. Seventeen infants were randomly assigned to receive intradermal BCG (0.1 ml; Statens Serum Institute, Copenhagen, Denmark) vaccination at birth, and 12 were scheduled for vaccination at the age of 2 months. At the age of 2 months, a venous blood sample was collected. Adult tuberculosis cases and controls were recruited within the frame of a case-control study investigating potential host-related and environmental factors in susceptibility to tuberculosis (29). Only human immunodeficiency virus-seronegative individuals were included in the study.
Thirty-three patients with pulmonary tuberculosis were enrolled at the chest clinic of the Serrekunda Health Center after confirmation of disease by sputum microscopy. After blood sampling for immunological analysis, antituberculosis therapy was started as soon as the diagnosis was confirmed. Twenty-one individuals who had not had tuberculosis in the past and who shared living quarters with a tuberculosis patient agreed to join the study as household contacts. Twenty-two randomly selected individuals living in the vicinity of the tuberculosis patients' houses, having had neither tuberculosis in the past nor any history of known contact, agreed to take part in the study as external community controls. In addition, 23 heavily exposed health care workers working closely with pulmonary tuberculosis patients for at least 2 years were included in the study. These individuals had no history of tuberculosis on the basis of personal history, physical examination, and chest X ray. All adults had a venous blood sample taken and a tuberculin skin test (using 2 tuberculin units of intradermal PPD) result recorded after 72 h, and results were considered positive if the skin induration measured 10 mm or more.
Laboratory assays.
Peripheral blood mononuclear cells (PBMC) were freshly isolated from heparinized venous blood samples by density gradient centrifugation. The cells were washed twice and then suspended at a concentration of 106 cells per ml in complete medium containing 10% heat-inactivated pooled human AB serum (Sigma, St. Louis, Mo.). A total of 2 × 105 PBMC per well was cultured in triplicate in medium alone or in the presence of 10 μg of recombinant mycobacterial antigens per ml, 10 μg of PPD (PPD RT48; Statens Serum Institute) per ml, 2 μg of tetanus toxoid (Chiron Behring, Marburg, Germany) per ml, or 5 μg of phytohemagglutinin (PHA) (PHA-L; Sigma) per ml, and the mixture was incubated at 37°C in a 5% CO2 atmosphere. After 2 days, 75 μl of supernatants from the PHA-stimulated wells was harvested and pooled. After 6 days, 75 μl of supernatants from the nonstimulated and antigen-stimulated samples were harvested, pooled, and frozen. Cells were pulsed with 1 μCi of [3H]thymidine (Amersham Life Science, Little Chalfont, United Kingdom) per well. After an additional 16 h, cells were harvested and tritium incorporation was measured by liquid scintillation. IFN-γ, interleukin 5 (IL-5) (BioSource Europe, Fleurus, Belgium), and IL-13 (Diaclone, Besançon, France) concentrations in supernatants were determined by using commercially available enzyme-linked immunosorbent assay kits. Detection limits were 8 pg/ml for all cytokines studied.
Recombinant mycobacterial antigen production.
The full-length open reading frames of the cloned genes or the mature form (in the case of a secreted antigen with a putative signal sequence) were PCR amplified by using sequence-specific oligonucleotides followed by subcloning into pET17b expression vector as previously described (35). Immediately following the ATG translation initiation codon, the 5′ oligonucleotide was designed to contain nucleotide sequences coding for six histidine residues for ease of purification by affinity chromatography over a nickel column. Ligated products were then transformed into Escherichia coli. Correct insertion and orientation were identified by restriction digestion and DNA sequencing. For expression of the recombinant antigens, plasmids were transformed into the E. coli expression host BL-21(pLysE). The recombinant (His tag) antigens were purified by affinity chromatography using the one-step QIAexpress Ni-nitrilotriacetic acid agarose matrix (QIAGEN, Chatsworth, Calif.) in urea. The yield of purified recombinant protein varied from 10 to 75 mg per liter of induced bacterial culture, with over 98% purity. Endotoxin levels were typically <10 EU of protein per mg (i.e., <1 ng of lipopolysaccharide/mg).
Statistical analysis.
Specific hypotheses were tested by comparison of both proportions of responders and of medians among study groups. A positive response was defined as a cytokine concentration or counts per minute (CPM) in stimulated wells of more than three times the cytokine concentration or CPM measured in nonstimulated wells. Fisher's exact test or χ2 test, when applicable, was used to compare proportions. The possibility of whether age and BCG vaccination status (for adults) and gender could confound the results was tested, but no effect of these variables was found. For the comparison of median cytokine responses, cytokine concentrations in nonstimulated wells were subtracted from those measured in stimulated wells. For the comparison of median proliferation responses, stimulation indices (CPM in stimulated wells/CPM in nonstimulated wells) were calculated. The Mann-Whitney-Wilcoxon test was used to compare medians.
RESULTS
As IFN-γ is an important effector cytokine in mycobacterium-induced immune responses, IFN-γ production was the main focus of this study (2, 3). Cell proliferation was also studied, but as differences in cell proliferative responses among groups were similar to those observed with IFN-γ production, they are not presented. Because Th-2 cytokines may be involved in disease susceptibility or immunoregulatory mechanisms, antigen-specific IL-5 and IL-13 production was also measured. IL-5 responses to mycobacterial antigens were found to be low or not detected, with no variation between groups, and are therefore also not shown. IFN-γ and IL-13 responses are presented in this report. Unstimulated background IFN-γ and IL-13 responses were low and similar in all study groups (medians ranged from 13 to 28 and 8 to 14 pg/ml, respectively). No statistically significant differences in background cytokine and proliferative responses were found in all specific group comparisons (data not shown).
Immune responses to mycobacterial antigens induced by neonatal BCG vaccination.
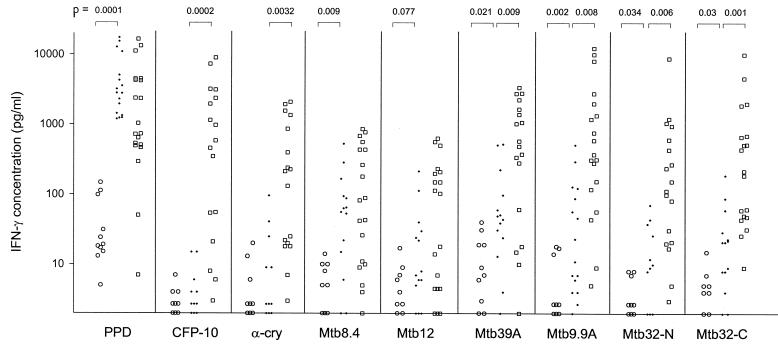
BCG vaccination at birth was associated with increased IFN-γ responses to PPD as well as, to various extents, to Mtb8.4, Mtb39A, Mtb9.9A, Mtb32-N, and Mtb32-C, but not to CFP-10 and α-crystallin. The difference for Mtb12 did not reach the significance level (Fig. 1). Three out of 12 unvaccinated babies responded to PPD, but none responded to the purified antigens. In contrast, the numbers of vaccinated babies (out of 17) responding to PPD, Mtb8.4, Mtb39A, Mtb9.9A, Mtb32-N, Mtb32-C, Mtb12, CFP-10, and α-crystallin were 17, 8, 4, 6, 2, 3, 2, 0, and 1, respectively. Neonatal BCG vaccination induced a significant IL-13 response to PPD but not to the other mycobacterial antigens (data not shown).
FIG. 1.
IFN-γ production (values are given in picograms per milliliter) by PBMC stimulated with mycobacterial antigens in infants not vaccinated with BCG (○), BCG-vaccinated infants (·), and adult community controls (□). P values are indicated (the Mann-Whitney-Wilcoxon test was used). α-cry, α-crystallin.
Immune responses to mycobacterial antigens are modulated by exposure to the environment in countries where tuberculosis is endemic (Fig. 1).
Similar IFN-γ responses to PPD were found in vaccinated infants and adult community controls. We have previously reported that, despite the immaturity of the immune system at birth, BCG vaccination of neonates induces high levels of IFN-γ production by CD4+ T cells (37). Higher IFN-γ responses to Mtb32-C, Mtb39A, Mtb9.9A, CFP-10, α-crystallin, and Mtb32-N were found in community controls than those found in BCG-vaccinated infants. These increased responses are probably related to exposure to environmental mycobacteria and M. tuberculosis during childhood and adulthood. BCG-vaccinated infants and community controls had similar IL-13 responses to all mycobacterial antigens studied (data not shown).
IFN-γ responses to mycobacterial antigens are associated with M. tuberculosis exposure (Tables 2 and 3).
TABLE 2.
IFN-γ and tuberculin skin test responses in this studya
| Antigen | Data for subsets of study population:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC
|
HC
|
HCW
|
TB
|
|||||||||
| No. of responders/no. of participants | IFN-γ concn (pg/ml)
|
No. of responders/no. of participants | IFN-γ concn (pg/ml)
|
No. of responders/no. of participants | IFN-γ concn (pg/ml)
|
No. of responders/no. of participants | IFN-γ concn (pg/ml)
|
|||||
| Median | 10-90%ile | Median | 10-90%ile | Median | 10-90%ile | Median | 10-90%ile | |||||
| TST | 12/22 | ND | 17/19 | ND | 22/23 | ND | 27/30 | ND | ||||
| PPD | 21/22 | 1,688 | 293-16,540 | 21/21 | 6,195 | 28-119,822 | 23/23 | 3,515 | 995-16,669 | 30/33 | 1,968 | 113-9,004 |
| CFP-10 | 14/22 | 202 | 10-3,189 | 19/21 | 498 | 31-2,111 | 23/23 | 287 | 67-1,754 | 20/33 | 68 | 0-3,004 |
| α-Cry | 11/22 | 24 | 0-1,563 | 17/21 | 260 | 4-1,911 | 21/23 | 190 | 46-1,401 | 12/33 | 12 | 0-318 |
| Mtb8.4 | 10/22 | 43 | 0-684 | 13/21 | 64 | 0-1,401 | 11/23 | 28 | 3-571 | 11/33 | 10 | 0-294 |
| Mtb12 | 8/22 | 16 | 0-502 | 9/21 | 15 | 0-132 | 14/23 | 118 | 8-757 | 8/33 | 1 | 0-64 |
| Mtb39A | 17/22 | 533 | 3-3,325 | 19/21 | 687 | 45-11,902 | 21/23 | 469 | 55-1,152 | 23/33 | 120 | 0-5,264 |
| Mtb9.9A | 17/22 | 315 | 0-8,142 | 18/21 | 676 | 11-2,853 | 21/23 | 320 | 54-1,309 | 18/33 | 34 | 0-1,200 |
| Mtb32-N | 15/22 | 91 | 0-1,062 | 16/21 | 192 | 0-1,164 | 18/23 | 200 | 3-1,182 | 14/33 | 11 | 0-278 |
| Mtb32-C | 15/22 | 125 | 1-1,995 | 13/21 | 208 | 9-1,313 | 15/23 | 91 | 0-400 | 12/33 | 6 | 0-264 |
| Tet tox | 10/22 | 69 | 0-2,007 | 11/21 | 49 | 0-1,373 | 17/23 | 130 | 5-1,221 | 6/23 | 0 | 0-291 |
| PHA | 22/22 | 5,138 | 600-50,077 | 21/21 | 6,966 | 273-22,197 | 21/23 | 6,810 | 1,263-25,607 | 31/33 | 1,384 | 70-8,595 |
Abbreviations: TST, tuberculin skin test; α-Cry, α-crystallin; Tet tox, tetanus toxoid; CC, community controls; HC, household contacts; HCW, health care workers; TB, tuberculosis patients; 10-90%ile, 10 to 90 percentile; ND, not determined.
TABLE 3.
P value comparisons of responder proportions and median values between groups used in this studya
| Antigen |
P values for groups obtained by:
|
|||||
|---|---|---|---|---|---|---|
| χ2 or Fisher's exact testb
|
Mann-Whitney-Wilcoxon testc
|
|||||
| HC vs CC | HCW vs CC | TB vs CC | HC vs CC | HCW vs CC | TB vs CC | |
| TST | 0.001 | 0.001 | 0.001 | ND | ND | ND |
| PPD | 1 | 0.49 | 0.64 | 0.16 | 0.08 | 0.89 |
| CFP-10 | 0.067 | 0.001 | 0.82 | 0.24 | 0.41 | 0.5 |
| α-Cry | 0.033 | 0.003 | 0.31 | 0.085 | 0.051 | 0.17 |
| Mtb8.4 | 0.28 | 0.87 | 0.36 | 0.28 | 0.73 | 0.16 |
| Mtb12 | 0.66 | 0.1 | 0.33 | 0.66 | 0.287 | 0.092 |
| Mtb39A | 0.41 | 0.24 | 0.53 | 0.34 | 0.54 | 0.14 |
| Mtb9.9A | 0.7 | 0.24 | 0.086 | 0.64 | 0.86 | 0.02 |
| Mtb32-N | 0.55 | 0.45 | 0.077 | 0.34 | 0.29 | 0.066 |
| Mtb32-C | 0.66 | 0.83 | 0.021 | 0.92 | 0.33 | 0.002 |
| Tet tox | ND | ND | ND | 0.87 | 0.29 | 0.022 |
| PHA | ND | ND | ND | 0.81 | 0.96 | 0.0025 |
The influence of exposure of household contacts and health care workers to M. tuberculosis was first evaluated by measuring the skin response to tuberculin. The tuberculin skin test was more frequently positive in household contacts and health care workers than in community controls. In contrast, IFN-γ responses to PPD in household contacts and health care workers were similar to those in community controls. Increased IFN-γ responses to CFP-10 and α-crystallin were observed in household contacts and health care workers compared with those of community controls, whereas the response to the other purified antigens was similar in the three groups. As shown in Tables 4 and 5, community controls and household contacts had similar mycobacterial antigen-specific IL-13 responses. Surprisingly, higher concentrations of IL-13 were induced by all mycobacterial antigens studied (except Mtb8.4) in health care workers than those induced in community controls. This difference was specific for mycobacterial antigens, as it was not observed with tetanus toxoid or PHA stimulation.
TABLE 4.
IL-13 responses in this studya
| Antigen | Data for subsets of study population:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC
|
HC
|
HCW
|
TB
|
|||||||||
| No. of responders/no. of participants | IL-13 concn (pg/ml)
|
No. of responders/no. of participants | IL-13 concn (pg/ml)
|
No. of responders/no. of participants | IL-13 concn (pg/ml)
|
No. of responders/no. of participants | IL-13 concn (pg/ml)
|
|||||
| Median | 10-90%ile | Median | 10-90%ile | Median | 10-90%ile | Median | 10-90%ile | |||||
| PPD | 9/22 | 9 | 0-97 | 7/21 | 14 | 0-81 | 20/23 | 47 | 5-335 | 14/32 | 11 | 0-60 |
| CFP-10 | 1/22 | 0 | 0-15 | 2/21 | 5 | 0-15 | 8/23 | 9 | 1-34 | 4/32 | 1 | 0-16 |
| α-Cry | 2/22 | 1 | 0-12 | 2/21 | 0 | 0-8 | 9/23 | 12 | 3-40 | 2/32 | 0 | 0-16 |
| Mtb8.4 | 2/22 | 2 | 0-14 | 2/21 | 0 | 0-8 | 3/23 | 4 | 0-18 | 1/32 | 1 | 0-10 |
| Mtb12 | 2/22 | 1 | 0-10 | 0/21 | 0 | 0-8 | 1/23 | 5 | 1-13 | 1/32 | 0 | 0-4 |
| Mtb39A | 2/22 | 1 | 0-13 | 2/21 | 3 | 0-11 | 5/23 | 5 | 1-36 | 4/32 | 1 | 1-13 |
| Mtb9.9A | 1/22 | 0 | 0-6 | 1/21 | 0 | 0-3 | 5/23 | 9 | 1-29 | 0/32 | 0 | 0-5 |
| Mtb32-N | 2/22 | 0 | 0-9 | 2/21 | 0 | 0-10 | 3/23 | 6 | 0-22 | 2/32 | 0 | 0-8 |
| Mtb32-C | 1/22 | 0 | 0-6 | 1/21 | 0 | 0-8 | 2/23 | 2 | 0-13 | 0/32 | 0 | 0-7 |
| Tet tox | 7/22 | 2 | 0-158 | 6/21 | 3 | 0-50 | 6/23 | 7 | 0-115 | 6/23 | 2 | 0-97 |
| PHA | 20/22 | 327 | 22-1,889 | 18/21 | 302 | 70-1,754 | 20/23 | 862 | 6-1,822 | 23/32 | 137 | 0-622 |
Abbreviations: CC, community controls; HC, household contacts; HCW, health care workers; TB, tuberculosis patients; 10-90%ile, 10 to 90 percentiles; α-Cry, α-crystallin; Tet tox, tetanus toxoid.
TABLE 5.
P values of median IL-13 concentrations of groups used in this studya
| Antigen |
P values for groups obtained by the Mann-Whitney-Wilcoxon testb
|
||
|---|---|---|---|
| HC vs CC | HCW vs CC | TB vs CC | |
| PPD | 0.42 | 0.004 | 0.89 |
| CFP-10 | 0.27 | 0.006 | 0.81 |
| α-Cry | 0.33 | 0.0001 | 0.6 |
| Mtb8.4 | 0.23 | 0.36 | 0.53 |
| Mtb12 | 0.27 | 0.041 | 0.11 |
| Mtb39A | 0.28 | 0.01 | 0.79 |
| Mtb9.9A | 0.82 | 0.0002 | 0.54 |
| Mtb32-N | 0.63 | 0.008 | 0.74 |
| Mtb32-C | 0.77 | 0.007 | 0.68 |
| Tet tox | 0.85 | 0.31 | 0.75 |
| PHA | 0.65 | 0.008 | 0.008 |
Low cytokine responses are found in tuberculosis patients.
The tuberculin skin test was more frequently positive among tuberculosis patients than among community controls. In contrast, IFN-γ responses to PPD were similar between the two groups, and tuberculosis patients had low IFN-γ responses to some of the purified mycobacterial antigens (Mtb9.9A and Mtb12) as well as to tetanus toxoid and PHA. Low IL-13 responses to mycobacterial antigens were observed both in tuberculosis patients and in controls. The production of IL-13 in response to PHA was lower in tuberculosis patients (Table 4).
DISCUSSION
This study was undertaken to characterize the immune response to recently described mycobacterial antigens in a population living in a country where tuberculosis is endemic. We endeavored to evaluate their potential value as candidates for vaccine development or for the immunodiagnosis of tuberculosis. In areas with a low incidence of tuberculosis, these antigens had been previously shown to stimulate the production of IFN-γ in tuberculin skin test-positive but not in tuberculin skin test-negative individuals (1, 4, 14, 16, 17, 34, 40, 41). In this study, we confirm that all antigens are able to stimulate IFN-γ production, but important differences were observed among groups exposed to different levels and types of mycobacteria, especially in the responses to CFP-10 and α-crystallin.
CFP-10 is a low-molecular-weight culture filtrate protein whose gene is cotranscribed with that of the ESAT-6 antigen. Both antigens are expressed by mycobacteria from the M. tuberculosis complex and only a restricted number of environmental mycobacteria. CFP-10 is not expressed by M. bovis BCG, as the coding region has been deleted during the process of virulence attenuation (7, 16). Previous studies have suggested a potential use of CFP-10 in tuberculosis immunodiagnosis (4). Here, we show that neonatal BCG vaccination induces no response to CFP-10. Further exposure to the Gambian environment results in significant responses, as 60% of adult community controls responded to CFP-10, a proportion close to that of ESAT-6 responders in a similar population (38). The use of the highly specific CFP-10 and ESAT-6 antigens suggests a high prevalence of infection, as was shown previously in India (26). The higher IFN-γ responses to CFP-10 observed in health care workers compared with those of community controls and the trend toward higher responses in household contacts both support the potential use of this antigen as a marker of M. tuberculosis infection. Cross-reactivity due to previous BCG vaccination or exposure to environmental mycobacteria should not be expected. The higher IFN-γ responses to CFP-10 observed in health care workers who remained free of disease suggest that this antigen may also be involved in protective immunity against tuberculosis, confirming recent animal vaccine studies (32). In humans, IFN-γ production by CFP-10-specific CD4+ and CD8+ cytotoxic lymphocytes in M. tuberculosis-infected individuals has been demonstrated (16, 25, 27). Used in a vaccine, CFP-10 may present the advantage of being devoid of the influence of prevaccination exposure to environmental mycobacteria.
In contrast to CFP-10, α-crystallin is expressed by M. bovis BCG, and the gene that encodes it has some homology with sequences present in other mycobacteria (39). Surprisingly, while increased IFN-γ responses to α-crystallin were observed for adults with a history of past BCG vaccination (41), no significant response to this antigen was detected in BCG-immunized infants 2 months after vaccination. The expression of α-crystallin and the development of a primary immune response to this antigen may be slower than that of other mycobacterial antigens in vivo (15). Time and further exposure to mycobacteria resulted in increased IFN-γ and proliferative responses to α-crystallin in community controls compared to those of BCG-immunized infants. High proportions of IFN-γ responses to α-crystallin were observed in household contacts (80%) and in health care workers (90%) compared with those of community controls (50%). Although this finding suggests a potential use for M. tuberculosis infection immunodiagnosis, the role of previous BCG vaccination deserves further investigation.
The increased IFN-γ responses to α-crystallin observed in health care workers suggest that this antigen may be involved in protective immunity. Anti-α-crystallin immunity may prevent M. tuberculosis survival during latent infection and the development of pathogenic lesions, as the production of α-crystallin has been shown to be essential for the survival of M. tuberculosis in macrophages, especially under hypoxic conditions such as those present in tuberculous granuloma and caseum (15, 42). The use of this antigen in immunotherapeutic vaccination approaches deserves consideration. Concerns may be raised about the safety of this strategy, as α-crystallin is a member of the Acr family of heat shock proteins—molecules that are involved in the maintenance of the transparency of the vertebrate eye (23). Our data indicate that cell-mediated immune responses to this antigen are ubiquitous in countries where tuberculosis is endemic, with no apparent autoimmune consequences.
The other antigens, Mtb8.4, Mtb39A, Mtb9.9A, Mtb12, and Mtb32 (N- and C-terminal fractions) are all expressed in M. tuberculosis and BCG but not in most other mycobacteria (Table 1). IFN-γ responses to these antigens were induced by BCG vaccination in newborns and furthermore by exposure to mycobacteria through the environment, as shown in the community controls. The similar IFN-γ responses to these antigens observed in community controls, household contacts, health care workers, and tuberculosis patients suggest that these antigens will be of limited utility for the immunodiagnosis of tuberculosis but indicates some degree of immunogenicity. A recent study demonstrated that, in C57BL/6 mice vaccinated with Mtb8.4 either as a protein mixed with IFA (an original experimental wash) or in a DNA format, IFN-γ and cytotoxic T-cell responses were induced, conferring a level of protection against virulent M. tuberculosis challenge similar to that of BCG (13). These antigens may therefore be interesting candidates as subunit tuberculosis vaccines, used alone or in combination. The fact that neonatal BCG vaccination induces IFN-γ responses to these antigens demonstrates that they may be used to boost BCG-induced immunity, with potentially stronger and longer protection (33). Given the low level of IFN-γ responses to some of these antigens in BCG-vaccinated infants, specific strategies of antigen presentation may be needed to promote immunogenicity in early life.
Previous studies in industrialized countries suggested that the IFN-γ response to purified mycobacterial antigens such as ESAT-6 or CFP-10 may be used for the immunodiagnosis of active tuberculosis (3-6). For Gambian tuberculosis patients, we generally found decreased responses to mycobacterial antigens, PHA, and tetanus toxoid compared with those of healthy individuals. All patients included in this study had tuberculous lesions extending to several lung regions (data not shown). These results confirm a previous demonstration that severe tuberculosis is associated with suppressed immune responses (36). The mechanism(s) involved could include the production of immunosuppressive cytokines such as IL-10 and TGF-β, preferential expansion of Th2-type responses, and T-cell apoptosis (10, 21, 22, 28, 30). This phenomenon could compromise the use of immunodiagnostic tests based on IFN-γ production in developing countries.
Interestingly, we observed that health care workers had increased IL-13 responses to mycobacterial antigens compared with those of community controls or household contacts. This difference appeared specific for mycobacterial antigens, as IL-13 production was not increased in health care workers following stimulation by tetanus toxoid or PHA. Remaining free of disease despite chronic exposure to and frequent reinfection with M. tuberculosis may require the development of anti-inflammatory immune responses to prevent excessive self-tissue damage. The role of IL-13 responses in antimycobacterial immunity deserves further investigation.
We conclude that ubiquitous exposure to mycobacteria in tropical countries induces marked immune responses to a broad range of recently identified mycobacterial antigens. The association between immunity to CFP-10 and α-crystallin and exposure to M. tuberculosis suggests that these antigens may be used for the immunodiagnosis of tuberculosis infection and/or for the development of vaccine strategies that avoid the immunodeviating consequences of exposure to environmental mycobacteria.
Acknowledgments
This study was conducted within the framework of the MRC Tuberculosis Programme and a collaborative study funded by EC-DGXII, grant no. IC18CT980375. J.V. is supported by the Belgian Fond National de la Recherche Scientifique.
This work would not have been possible without the assistance of the tuberculosis epidemiology team at the MRC in The Gambia in the field work and the collaboration of the medical staff of Serrekunda Health Centre. We are grateful to all the individuals who agreed to take part in the study and to the mothers who agreed to the enrollment of their babies in the study.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Alderson, M. R., T. Bement, C. H. Day, L. Zhu, D. Molesh, Y. A. Skeiky, R. Coler, D. M. Lewinsohn, S. G. Reed, and D. C. Dillon. 2000. Expression cloning of an immunodominant family of Mycobacterium tuberculosis antigens using human CD4+ T cells. J. Exp. Med. 191:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P. 2001. TB vaccines: progress and problems. Trends Immunol. 22:160-168. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 4.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 5.Arend, S. M., A. Geluk, K. E. van Meijgaarden, J. T. van Dissel, M. Theisen, P. Andersen, and T. H. Ottenhoff. 2000. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect. Immun. 68:3314-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arend, S. M., T. H. Ottenhoff, P. Andersen, and J. T. van Dissel. 2001. Uncommon presentations of tuberculosis: the potential value of a novel diagnostic assay based on the Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:680-686. [PubMed] [Google Scholar]
- 7.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 8.Black, G. F., H. M. Dockrell, A. C. Crampin, S. Floyd, R. E. Weir, L. Bliss, L. Sichali, L. Mwaungulu, H. Kanyongoloka, B. Ngwira, D. Warndorff, and P. E. Fine. 2001. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J. Infect. Dis. 184:322-329. [DOI] [PubMed] [Google Scholar]
- 9.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 10.Boussiotis, V. A., E. Y. Tsai, E. J. Yunis, S. Thim, J. C. Delgado, C. C. Dascher, A. Berezovskaya, D. Rousset, J. M. Reynes, and A. E. Goldfeld. 2000. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Investig. 105:1317-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaparas, S. D., C. J. Maloney, and S. R. Hedrick. 1970. Specificity of tuberculins and antigens from various species of mycobacteria. Am. Rev. Respir. Dis. 101:74-83. [DOI] [PubMed] [Google Scholar]
- 12.Colditz, G. A., C. S. Berkey, F. Mosteller, T. F. Brewer, M. E. Wilson, E. Burdick, and H. V. Fineberg. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29-35. [PubMed] [Google Scholar]
- 13.Coler, R. N., A. Campos-Neto, P. Ovendale, F. H. Day, S. P. Fling, L. Zhu, N. Serbina, J. L. Flynn, S. G. Reed, and M. R. Alderson. 2001. Vaccination with the T cell antigen Mtb 8.4 protects against challenge with Mycobacterium tuberculosis. J. Immunol. 166:6227-6235. [DOI] [PubMed] [Google Scholar]
- 14.Coler, R. N., Y. A. Skeiky, T. Vedvick, T. Bement, P. Ovendale, A. Campos-Neto, M. R. Alderson, and S. G. Reed. 1998. Molecular cloning and immunologic reactivity of a novel low molecular mass antigen of Mycobacterium tuberculosis. J. Immunol. 161:2356-2364. [PubMed] [Google Scholar]
- 15.Cunningham, A. F., and C. L. Spreadbury. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J. Bacteriol. 180:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon, D. C., M. R. Alderson, C. H. Day, D. M. Lewinsohn, R. Coler, T. Bement, A. Campos-Neto, Y. A. Skeiky, I. M. Orme, A. Roberts, S. Steen, W. Dalemans, R. Badaro, and S. G. Reed. 1999. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect. Immun. 67:2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. W. H. O. Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 19.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Pando, R., L. Pavon, K. Arriaga, H. Orozco, V. Madrid-Marina, and G. Rook. 1997. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect. Immun. 65:3317-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch, C. S., Z. Toossi, C. Othieno, J. L. Johnson, S. K. Schwander, S. Robertson, R. S. Wallis, K. Edmonds, A. Okwera, R. Mugerwa, P. Peters, and J. J. Ellner. 1999. Depressed T-cell interferon-γ responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J. Infect. Dis. 180:2069-2073. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch, C. S., Z. Toossi, G. Vanham, J. L. Johnson, P. Peters, A. Okwera, R. Mugerwa, P. Mugyenyi, and J. J. Ellner. 1999. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J. Infect. Dis. 179:945-953. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz, J. 1992. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 89:10449-10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huebner, R. E., M. F. Schein, and J. B. Bass, Jr. 1993. The tuberculin skin test. Clin. Infect. Dis. 17:968-975. [DOI] [PubMed] [Google Scholar]
- 25.Lalvani, A., R. Brookes, R. J. Wilkinson, A. S. Malin, A. A. Pathan, P. Andersen, H. Dockrell, G. Pasvol, and A. V. Hill. 1998. Human cytolytic and interferon γ-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 95:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalvani, A., P. Nagvenkar, Z. Udwadia, A. A. Pathan, K. A. Wilkinson, J. S. Shastri, K. Ewer, A. V. Hill, A. Mehta, and C. Rodrigues. 2001. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J. Infect. Dis. 183:469-477. [DOI] [PubMed] [Google Scholar]
- 27.Lewinsohn, D. M., L. Zhu, V. J. Madison, D. C. Dillon, S. P. Fling, S. G. Reed, K. H. Grabstein, and M. R. Alderson. 2001. Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J. Immunol. 166:439-446. [DOI] [PubMed] [Google Scholar]
- 28.Lienhardt, C., A. Azzurri, A. Amedei, K. Fielding, J. Sillah, O. Y. Sow, B. Bah, M. Benagiano, A. Diallo, R. Manetti, K. Manneh, P. Gustafson, S. Bennett, M. M. D'Elios, K. P. McAdam, and G. Del Prete. 2002. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur. J. Immunol. 32:1605-1613. [DOI] [PubMed] [Google Scholar]
- 29.Lienhardt, C., S. Bennett, G. Del Prete, O. Bah-Sow, M. Newport, P. Gustafson, K. Manneh, V. Gomes, A. Hill, and K. P. McAdam. 2002. Investigation of environmental and host-related risk factors for tuberculosis in Africa. I. Methodological aspects of a combined design. Am. J. Epidemiol. 155:1066-1073. [DOI] [PubMed] [Google Scholar]
- 30.Marchant, A., A. Amedei, A. Azzurri, J. Vekemans, M. Benagiano, C. Tamburini, C. Lienhardt, T. Corrah, K. P. McAdam, S. Romagnani, M. M. D'Elios, and G. Del Prete. 2001. Polarization of PPD-specific T-cell response of patients with tuberculosis from Th0 to Th1 profile after successful antimycobacterial therapy or in vitro conditioning with interferon-α or interleukin-12. Am. J. Respir. Cell Mol. Biol. 24:187-194. [DOI] [PubMed] [Google Scholar]
- 31.Orme, I. M., and F. M. Collins. 1984. Efficacy of Mycobacterium bovis BCG vaccination in mice undergoing prior pulmonary infection with atypical mycobacteria. Infect. Immun. 44:28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pym, A. S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K. E. Griffiths, G. Marchal, C. Leclerc, and S. T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9:533-539. [DOI] [PubMed] [Google Scholar]
- 33.Ramshaw, I. A., and A. J. Ramsay. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Immunol. Today 21:163-165. [DOI] [PubMed] [Google Scholar]
- 34.Skeiky, Y. A., M. J. Lodes, J. A. Guderian, R. Mohamath, T. Bement, M. R. Alderson, and S. G. Reed. 1999. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect. Immun. 67:3998-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skeiky, Y. A., P. J. Ovendale, S. Jen, M. R. Alderson, D. C. Dillon, S. Smith, C. B. Wilson, I. M. Orme, S. G. Reed, and A. Campos-Neto. 2000. T cell expression cloning of a Mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection. J. Immunol. 165:7140-7149. [DOI] [PubMed] [Google Scholar]
- 36.Sodhi, A., J. Gong, C. Silva, D. Qian, and P. F. Barnes. 1997. Clinical correlates of interferon γ production in patients with tuberculosis. Clin. Infect. Dis. 25:617-620. [DOI] [PubMed] [Google Scholar]
- 37.Vekemans, J., A. Amadei, M. O. Ota, M. M. D'Elios, T. Goetghebuer, J. Ismaili, M. J. Newport, G. Del Prete, M. Goldman, K. P. McAdam, and A. Marchant. 2001. Neonatal bacillus Calmette-Guérin vaccination induces adult-like IFN-γ production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 38.Vekemans, J., C. Lienhardt, J. S. Sillah, J. G. Wheeler, G. P. Lahai, M. T. Doherty, T. Corrah, P. Andersen, K. P. McAdam, and A. Marchant. 2001. Tuberculosis contacts but not patients have higher gamma-interferon responses to ESAT-6 than do community controls in The Gambia. Infect. Immun. 69:6554-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verbon, A., R. A. Hartskeerl, A. Schuitema, A. H. Kolk, D. B. Young, and R. Lathigra. 1992. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the α-crystallin family of low-molecular-weight heat shock proteins. J. Bacteriol. 174:1352-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webb, J. R., T. S. Vedvick, M. R. Alderson, J. A. Guderian, S. S. Jen, P. J. Ovendale, S. M. Johnson, S. G. Reed, and Y. A. Skeiky. 1998. Molecular cloning, expression, and immunogenicity of MTB12, a novel low-molecular-weight antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 66:4208-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson, R. J., K. A. Wilkinson, K. A. De Smet, K. Haslov, G. Pasvol, M. Singh, L. Svarcova, and J. Ivanyi. 1998. Human T- and B-cell reactivity to the 16-kDa α-crystallin protein of Mycobacterium tuberculosis. Scand. J. Immunol. 48:403-409. [DOI] [PubMed] [Google Scholar]
- 42.Yuan, Y., D. D. Crane, R. M. Simpson, Y. Q. Zhu, M. J. Hickey, D. R. Sherman, and C. E. Barry. 1998. The 16-kDa α-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc. Natl. Acad. Sci. USA 95:9578-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]