Abstract
The number of overweight and obese individuals has dramatically increased in the US and other developed nations during the past 30 years. While type II diabetes and cardiovascular disease are well recognized co-morbid conditions associated with obesity, recent reports have demonstrated a greater severity of illness in obese patients due to influenza during the 2009 H1N1 pandemic. Consistent with these reports, diet-induced obesity has been shown to impair anti-viral host defense in murine models of influenza infection. However, the impact of obesity on the risk of community-acquired and nosocomial pneumonia in human patients is not clear. Relatively few studies have evaluated the influence of diet-induced obesity in murine models of bacterial infections of the respiratory tract. Obese leptin deficient humans and leptin and leptin-receptor deficient mice exhibit greater susceptibility to respiratory infections suggesting a requirement for leptin in the pulmonary innate and adaptive immune response to infection. In contrast to these studies, we have observed that obese leptin receptor signaling mutant mice are resistant to pneumococcal pneumonia highlighting the complex interaction between leptin receptor signaling and immune function. Given the increased prevalence of obesity and poor responsiveness of obese individuals to vaccination against influenza, the development of novel immunization strategies for this population is warranted. Additional clinical and animal studies are needed to clarify the relationship between increased adiposity and susceptibility to community-acquired and nosocomial pneumonia.
Introduction
The prevalence of obesity among adults living in the US has increased to alarming levels with 34% obese (BMI≥30) and 68% overweight (BMI=25-29.9) 1. Moreover, 46-54% of hospitalized patients are overweight, 32% are obese, and 5% are extremely obese with a BMI (BMI≥40) 2. Similar trends have been observed in other developed nations raising global concerns about the chronic health consequences of obesity 3. While type II diabetes, cardiovascular disease, and non-alcoholic fatty liver disease are well known co-morbid conditions associated with obesity, the evidence that excess white adipose tissue suppresses pulmonary host defense against infection is emerging 4-7. The recent observation that the obese were uniquely susceptible to and suffered more severe outcomes from the 2009 H1N1 influenza pandemic underscores the need to elucidate the effects of obesity on innate and adaptive immune responses against respiratory infections 8. This review will examine the evidence that obesity contributes to a greater severity of respiratory infections in humans and in animal models and will also discuss potential mechanisms underlying these responses.
I. Co-morbid conditions associated with obesity compromise pulmonary host defense
Obesity is a complex condition that is characterized by excess white adipose tissue and is often accompanied by other co-morbid conditions, such as gastroesophageal reflux disease (GERD) and type II diabetes, known to compromise host defense against infection 9. Both GERD and type II diabetes, even in the absence of excess adiposity, have been shown to compromise host defense by impairing innate and adaptive immunity 10-11.
Obesity and gastroesophageal reflux disease (GERD)
Obesity is associated with larger gastric volumes and the accumulation of visceral adipose tissue known to increase gastric pressure. The combination of these factors compromise lower esophageal sphincter closure. As a consequence, reflux of gastric fluid occurs and this fluid can be aspirated into the respiratory tract resulting in pneumonia 12. GERD is common in patients with abdominal obesity who are at a greater risk for aspiration pneumonia 10. Positioning these patients in a semi-upright position during sleep and hospitalization can decrease symptoms associated with GERD and aspiration pneumonia 13-14. It is also important to note that GERD is very common among those recovering from bariatric surgery and these patients should be monitored for reflux to prevent aspiration pneumonia 15-16.
Host defense and diabetes
Type II diabetes is fairly common among those who are overweight or obese and this condition is a well established risk factor for infectious disease for many reasons 17. Diabetes is known to delay wound healing, impair host defense against skin and cutaneous infections, and is associated with nosocomial infections and infectious complications of surgery 17. Interestingly, a recent study by O'Brien et al. demonstrated impaired wound healing in obese hyperglycemic mice after infection with the influenza virus 18. It is also an important risk factor for pneumonia and influenza 11 and contributes to a higher risk of death from community-acquired pneumonia 19.
Adipose tissue inflammation, nutrient excess, and immune suppression
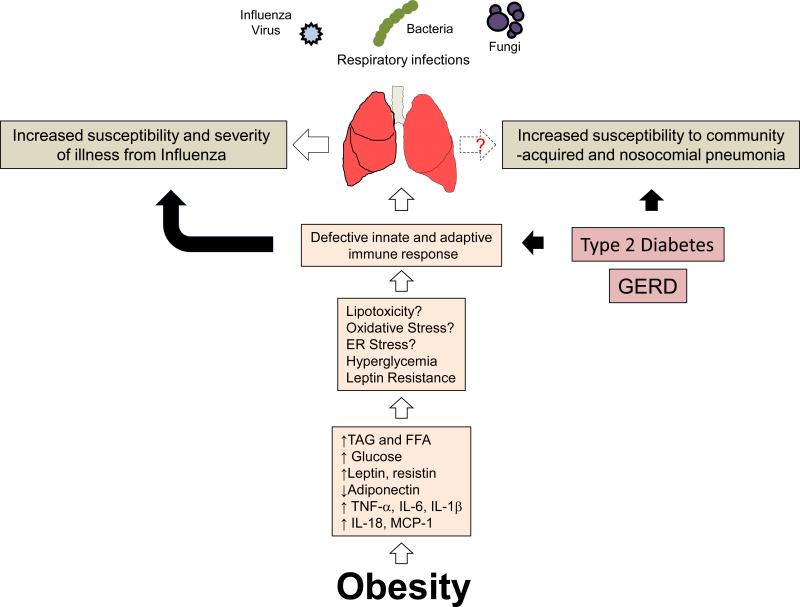
The accumulation of adipose tissue during obesity may attenuate pulmonary host defense through metabolic disturbances that often accompany this condition. Adipose plays an important role in storing excess calories in the form of triacylglycerol (TAG). In addition to serving as a buffer for dietary TAG, adipose tissue is an endocrine gland. As this tissue expands, its ability to buffer dietary lipids declines resulting in elevated blood TAG and free fatty acids (FFA). As a consequence, these lipids increase in the peripheral circulation and accumulate in ectopic sites such as skeletal muscle, the liver, and islets of the pancreas which leads to insulin resistance and hyperglycemia 20. In addition, adipose tissue elaborates proinflammatory cytokines (TNF-α, IL-6, IL-1β, IL-18, MCP-1), proinflammatory adipokines such as leptin and resistin, and produces less anti-inflammatory adipokines such as adiponectin. The consequence of these events is systemic inflammation which may ultimately impair innate and adaptive immune function by inducing endoplasmic reticulum stress, lipotoxicity, oxidative stress, and leptin resistance 21-22. (Summarized in Figure 1).
Figure 1.
Obesity weighs down host defense against pulmonary infections. Obesity diminishes pulmonary host defense against influenza and possibly bacterial and fungal pathogens. Excess adipose tissue in the obese results in numerous metabolic disturbances that contributes to a chronic state of low grade inflammation. The cause of which is due to elevated serum triacylglycerol (TAG), free fatty acids (FFA), hyperglycemia, and elevated proinflammatory adipokines such as leptin and resistin, and lower amounts of the anti-inflammatory adipokine, adiponectin, and classical cytokines, TNF-α, IL-6, IL-1β, IL-18, and MCP-1 are produced in greater quantities as adipose tissue expands. Collectively, these alterations result in defective innate and adaptive immune function that impair host against influenza infection. Whether or not obesity impairs host defense against bacterial pathogens is not clear. In addition, complications of obesity such as type II diabetes and gastroesophageal reflux disease (GERD) are known to impair host defense against viral, bacterial, and fungal pathogens.
II. Obesity is a risk factor for the severity of illness from influenza
The H1N1 influenza pandemic of 2009 provides the strongest evidence that the obese exhibit greater susceptibility to pulmonary viral infections 5, 23. A number of reports indicated that the obese and morbidly obese appeared to be more susceptible to and exhibited a greater severity of illness from the H1N1 influenza pandemic of 20095, 23-25. Additional studies confirmed these associations indicating that obesity and morbid obesity were independent risk factors for hospitalization 26, admission to an intensive care unit 27, and critical illness and death 23, 28 associated with H1N1 infection in the US29. Similar reports from many other countries also indicate a greater severity of illness and death from pandemic H1N1 in obese patients 30-35 (Summarized in Table 1). While this information suggests that obese individuals should receive annual seasonal influenza vaccinations, obesity is associated with a greater decline in influenza vaccine antibody titers and defective influenza-specific CD8+T cell function 36. A novel influenza vaccine strategy may be required to protect obese humans from seasonal influenza. Finally, since mortality from influenza is often associated with secondary bacterial pneumonia, future studies should investigate potential associations between obesity and the possibility that these individuals may be more likely to develop secondary bacterial pneumonia.
Table 1.
Association of obesity with severity of illness and death from pandemic H1N1 influenza of 2009
| Reference Year | Country/year | Number of subjects | Comparisons | Odds ratios (95% confidence interval) | Association |
|---|---|---|---|---|---|
| MMWR3 2009 | United States | 10 patients, 3 deaths | None | NA | 9 of 10 ICU patients admitted for severe H1N1 infection were obese |
| Vaillant23 2009 | World wide | 574 deaths | None | NA | Metabolic condition (including obesity) a risk factor for death |
| Jain26 2009 | United States | 272 | General US population | NA | Obesity was an independent risk factor for hospitalization |
| Fuhrman30 2010 | France | 758 patients hospitalized with H1N1 | Severe disease vs non-severe hospitalized adult cases | 9.1 (4.4-18.7) | Obesity associated with severe disease |
NA: Not applicable
III. Diet induced obese mice exhibit increased mortality and impaired host defense against influenza-infection
Diet Induced Obese (DIO) mice and influenza A infection
The recent obesity epidemic is most likely due to a positive energy balance that commonly occurs as a consequence of an energy rich diet and limited physical activity. Many investigators have taken a similar approach in producing DIO mice by feeding these animals diets consisting of 40-60% fat, usually in the form of saturated fatty acids, to induce obesity within a relatively short period of time (100-120 days) 37. Using the DIO model, a number of studies that have examined the effects of obesity on mechanisms of host defense against influenza infection 18, 21, 38-40. In the first study to demonstrate that obesity increases the risk of death from influenza infection, Smith et al. observed that DIO mice exhibited greater mortality, increased lung pathology scores, and a defective cytokine response following infection with a mouse adapted influenza virus (Influenza A/PR8/34) 38. The impairment in host defense in DIO mice was associated with a decrease in type I IFNs (IFN-α and IFN-β), a delay in the expression of IL-6 and TNF-α that eventually increased to levels greater than that observed in lean animals, and impaired natural killer (NK) cell cytotoxicity. Subsequent studies revealed that DIO also impairs the ability of dendritic cells to present antigens to T cells, attenuating monocyte and CD8+T cell recruitment, and diminishing IL-2 and IL-12 production following influenza infection 39. Following a primary challenge with non-lethal influenza H3N2, Karlsson et al. demonstrated that DIO mice exhibited increased morbidity and mortality following a secondary infection with influenza A/PR/8 that was associated with reduced CD8+ T cell, IFN-γ production, and defective antigen presentation by dendritic cells 21, 41. This impairment was due to an inability of DIO mice to generate and maintain functional antigen specific memory CD8+ T cells.
DIO mice and pandemic H1N1 infection
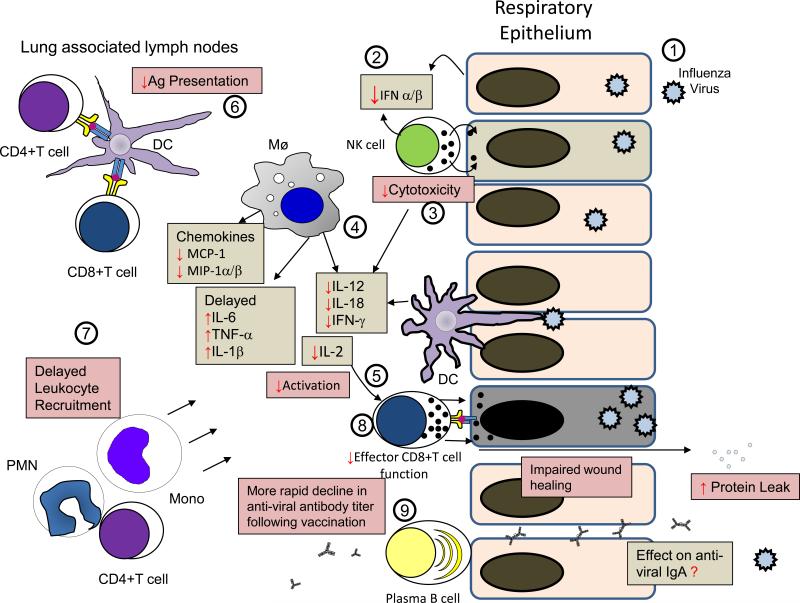
Recent studies by Easterbook et al. and O'Brien et al. showed that DIO mice experience greater mortality despite similar viral loads following infection with the 2009 pandemic H1N1 influenza virus 40, 42. In the report by Easterbrook, the authors observed that pulmonary IFN-β and proinflammatory cytokine production in DIO mice were lower than in lean control animals. Interestingly, serum cytokine levels were elevated in DIO and this response did not occur after influenza infection in lean mice. In a study by O'Brien et al., increased mortality in DIO and ob/ob mice following H1N1 infection was associated with increased lung pathology, impaired wound repair and subsequent pulmonary edema 42. The results of these studies are summarized in Figure 2, a picture adapted and updated from a review published by Karlsson et al. 41. While these reports provide valuable insights into mechanisms by which obesity may impair host defense against influenza infection, future studies should examine the direct effects of nutrient excess (i.e. elevated blood lipids and hyperglycemia), endoplasmic reticulum and oxidative stress, and leptin resistance on intracellular signaling pathways and effector functions in NK cells, macrophages, CD4+ T cells, effector CD8+T cells, plasma B cells, DCs, and other cells known to participate in the anti-viral response 21. The use of cells recovered from obese patients and mice that become obese due to hyperphagia, a more physiologically relevant model, rather than a high fat diet, are also encouraged.
Figure 2.
Mechanisms by which obesity impairs host defense against influenza infection. This figure has been adapted and updated from a similar one published by Karlsson et al.21. Following infection of respiratory epithelial cells with the influenza virus (1), the elaboration of type I interferons (IFN-α/β) are reduced and delayed (2) and the cytotoxic response of natural killer (NK) cells (3) is attenuated in obese mice. The elaboration proinflammatory cytokines (IL-1β, IL-6, and TNF-α) (4) are delayed and increased and the production of cytokines (IL-12, IL-18, IFN-γ) produced by NK cells, macrophages (Mø), dendritic cells (DC) and IL-2 produced by CD4+ T cells, known to enhance the adaptive immune response is also reduced (5). Antigen presentation by DCs to CD4+ T helper and CD8+ T cells is also impaired (6). A delay in chemokine (MCP-1 and MIP-1α/β) production results in postponed recruitment of PMNs (PMN), CD4+ T cells, and monocytes (Mono) (7). The ability of effector CD8+ T cells to kill influenza infected cells is diminished and healing of pulmonary epithelial cells is also impaired resulting in increased microvascular permeability and protein leak (8). While a more rapid decline in antibody titers of obese humans following vaccination against influenza has been reported 36, it is not known if obesity affects IgA levels in the lung (9). The grey boxes indicate changes in cytokines and the pink boxes represent aberrant immune responses in the obese. Red arrows ( or
or  ) indicate responses that are impaired or enhanced in the obese host.
) indicate responses that are impaired or enhanced in the obese host.
IV. Does obesity increase the risk of community-acquired or nosocomial pneumonia?
Obesity and risk of community-acquired pneumonia
While there is ample evidence that shows an increased risk of bacterial infections of the feet, surgical and catheter sites, gingival and periodontal tissues, gastrointestinal tract, and of the skin in obese patients, the impact of obesity on bacterial pneumonia is less certain 14. Community-acquired pneumonia is most frequently caused by bacterial pathogens. Paradoxically, three studies have demonstrated a protective association between obesity and mortality from pneumonia 43-45. Corrales-Medina et al. 43 demonstrated that increasing BMI was negatively correlated with 30-day mortality in patients with proven pneumococcal or Haemophilus community-acquired pneumonia. Similarly, LaCroix et al. 44 reported a negative relationship between mortality from pneumonia and BMI with increased mortality in men in the lowest BMI quartile compared with the highest BMI quartile. This study also suggests that the obese were protected from pneumonia as a cause of death. In a study that evaluated protective factors against death from pneumonia in 110,000 Japanese subjects, Inoue et al. 45 reported that low BMI (<18) was associated with an increased risk of death while the opposite was true for subjects with a high BMI (25-30.9). The reported associations between lower BMI and an increased risk of death from pneumonia 43-45 probably reflects the greater frequency of chronic diseases associated with malnutrition, e.g. emphysema, known to increase one's susceptibility to pneumonia. In contrast to these, a study by Baik et al., which included 26,429 men aged 44 to 79 years from the Health Professionals Follow-up Study and 78,062 women aged 27 to 44 years from the Nurses’ Health Study II, demonstrated a significant association between a 40-lb weight gain and a twofold increased risk of community-acquired pneumonia 46. Only one study, at the time that this review was written, has demonstrated an association between childhood obesity and respiratory infections 47. In this study, the authors reported that overweight and obese children (BMI in the 90th percentile) experienced twice as high a risk for acute respiratory infections (the cause of which was not identified) than children with a low BMI. At present, the effect of obesity on susceptibility to community-acquired bacterial pneumonia is uncertain. However, the disparity in these reports may be due to the endpoints reported. For example, obesity was associated with a lower risk of mortality from community-acquired pneumonia in the studies by Corrales-Medina, LaCroix, and Innoe 43-45 and a higher risk of respiratory infection in the studies by Baik and Jedrychowski 46-47. Based on these reports, it is possible that obesity increases susceptibility to community-acquired pneumonia while reducing the risk of mortality. However, additional research is needed to reach an appropriate conclusion.
Obesity and risk of nosocomial pneumonia
Obese patients experience more complications while hospitalized for critical illness and after surgery requiring greater hospital and ICU lengths of stay. Although there are numerous studies demonstrating that obesity is associated with a greater risk of surgical site infections 48, wound infections, catheter and blood stream infections 49, and infections of the urinary tract 50, the data on the association between obesity and the risk of nosocomial pneumonia, which is most often caused by bacteria, are mixed. For example, a prospective study by Bochicchio et al. involving 1167 critically ill trauma patients demonstrated that obesity was associated with increased hospital and ICU lengths of stay and a twofold increased risk of urinary tract and blood stream infections, and pneumonia 4. Similarly, Newell et al. also observed an increase in hospital and ICU lengths of stay, increased urinary tract infections, a longer period of ventilator support, as well as an increased risk of pneumonia in obese and severely obese critically injured blunt trauma patients 6. A retrospective chart review of patients admitted to medical ICUs conducted by Yaegashi et al. 7 revealed that morbidly obese patients, defined as BMI ≥40, had higher rates of mortality, acute respiratory distress syndrome, catheter infections, acute renal failure, nosocomial pneumonia, and sepsis than obese patients with a BMI in the range of 30-39.9. The morbidly obese also required a longer period of time on ventilatory support. Finally, in a retrospective study which included patients admitted to a Level 1 trauma center, Serrano et al. observed an increased risk of nosocomial pneumonia among obese patients 51. In contrast to the studies mentioned above, studies by Brant et al. 52 and Moulton 53 did not find a significant association between obesity and an increased risk of nosocomial pneumonia in patients undergoing heart surgery. In agreement with Brandt and Moulton, Dossett et al. did not find a significant association of BMI with pulmonary complications (such as pneumonia) in a cohort study of critically injured adults 54. The lack of agreement of these studies may be due to the reason for admission. For example, the increased risk of pneumonia may be observed among obese trauma patients rather than those who have had surgery. In total, based on available evidence, an association between obesity and community-acquired or nosocomial pneumonia is not clear and additional research is needed. In the future, investigators should identify the cause of infectious pneumonia since it is likely that obesity selectively impairs the immune response to some but not all infectious agents. Table 2 provides a summary of some of the studies mentioned above.
Table 2.
Effect of obesity on community-acquired and nosocomial pneumonia.
| Reference Year | Country | Number of patients | Comparisons | Odds ratios (95% confidence interval) or other | Association |
|---|---|---|---|---|---|
| Baik46 2000 | United States | 26,429 Men 78,062 Women |
WT maintained vs 40 lb WT gain |
M 1.46 (1.00-2.14) W 1.55 (1.15-2.10) |
Increased risk of community acquired pneumonia |
| Jedrychowski 1998 47 | Poland | 1129 children | Low BMI with BMI≥20 (overweight children) | 2.02 (1.13-3.59) | Increased risk of acute respiratory infection |
| Corrales-Medena43 2011 | United States | 317 | BMI (under WT, normal WT, over WT, obese) | 0.91 (0.84–0.98) | Increased BMI associated with lower 30-day mortality |
| Bochicchio4 2006 | United States | 1167 | Non-obese vs obese | 2.0 (1.02–3.76) | Increased risk of nosocomial pneumonia |
| Newell6 2007 | United States | 1543 | BMI 18.5-24.9 vs 30-39.9, and ≥ 40 | 1.7 (1.21-2.44) 2.5 (1.48- 4.30) |
Increased risk of nosocomial pneumonia |
| Yaegashi7 2005 | United States | 63 | BMI 30-39.9 vs BMI ≥ 40 | 3% vs 33% | Increased risk for morbidly |
| Brant52 2001 | Germany | 500 | Normal BMI 18.5-25 vs BMI≥30 | 3% vs 5% | No differences in risk of nosocomial pneumonia |
| Moulton53 1996 | United States | 2299 | BMI | NA | No differences in risk of nosocomial pneumonia |
| Dossett54 2008 | United States | 1291 | Normal BMI vs BMI 25- 29.9, 30-39.9, and ≥ 40 | Obese 0.96 (0.64–1.4) Severely obese 0.89 (0.46–1.7) | No differences in risk of nosocomial pneumonia |
WT: Weight, BMI: Body Mass Index
V. Effect of leptin and leptin receptor deficiency on susceptibility to infection in mice and humans
Leptin and leptin receptor deficiency impairs host defense against pulmonary bacterial infections
Most investigators have used leptin (ob/ob) and leptin receptor (db/db) deficient mice which are not only obese but exhibit many immune and endocrine abnormalities that are caused by both leptin deficiency and obesity which complicates the interpretation of these studies 42, 55-58. Leptin is essential for normal development and function of cells of the myeloid and lymphoid linage affecting both innate and adaptive immune responses and the absence of this hormone or its receptor results in severe immune abnormalities and greater susceptibility to viral 42, 59, bacterial 55-56, mycobacterial 57-58, and fungal infections60. In general, leptin promotes TH1 cytokine production and the elaboration of proinflammatory lipid mediators 61-63. It's also been shown to promote immune cell survival 64. Studies conducted in our laboratory have shown that ob/ob mice exhibit increased pulmonary bacterial burdens and reduced survival following an intratracheal challenge with either K. pneumoniae or S. pneumoniae 55-56. In comparison with WT animals, ob/ob mice produced proinflammatory mediators (TNF-α, IL-6, IL-12, and MIP-2) that were not different 55, reduced 57, 65, or elevated (MIP-2, PGE2, TNF-α) 56 following intrapulmonary bacterial challenge. In a study conducted in by Hsu et al., neutrophil (PMN) recruitment to the lung in response to S. pneumoniae challenge was enhanced in ob/ob mice. The host defense impairment in these animals was related to defective alveolar macrophage and PMN phagocytosis and killing of bacteria in vitro 56. These defects suggest an essential role for this adipokine in leukocyte antibacterial effector functions. Leptin is known to induce actin polymerization 66, upregulate complement receptor (CD11b/CD18) expression in monocytes 67 and PMNs 68, and enhance the production of reactive oxygen intermediates by inducing the assembly of the NADPH oxidase complex 69-70. Leptin deficiency is also associated with reduced leukotriene synthesis which is known to contribute to impaired pulmonary host defense against bacterial pneumonia 55, 63, 71-73. Interestingly, the provision of exogenous leptin restored host defense and leukotriene synthesis in states of leptin deficiency 55, 71. The ability of leptin to enhance macrophage leukotriene synthesis has also been demonstrated in cells from wild type animals and this might be an additional mechanism by which it promotes host defense in the lung 63, 74-75.
In regard to mycobacterial infections, Wieland et al. reported higher lung M. tuberculosis counts in ob/ob compared with WT mice that was associated with reduced levels of IFN-γ in a murine model of tuberculosis 58. Ordway reported similar results in that IFN-γ+ CD4+ T cell recruitment to the lungs was delayed in ob/ob, compared with WT mice challenged with M. abcessus 57. Lung M. abcessus burdens were higher and mycobacterial clearance was delayed in ob/ob mice. Similarly, Ob/ob and db/db mice also exhibit impaired host defense against many other bacterial, fungal, and viral infections of the CNS, liver, paw, stomach, heart, gut, and peritoneum59, 76-81. While human leptin deficiency is rare, individuals with this genetic defect are known to exhibit greater susceptibility to respiratory infections indicating an important role for leptin in the human immune response to infectious disease as well 82. Despite these advances in pulmonary host defense and leptin deficiency, further evaluation of the mechanisms by which this adipokine regulates innate and adaptive immunity is warranted.
Effects of leptin receptor signaling and leptin resistance in infection
While there is disagreement in the literature regarding the susceptibility of the obese to community-acquired and nosocomial pneumonia, obesity appears to be a risk factor for influenza and possibly other viral infections. As mentioned above, obesity is associated with a chronic state of systemic inflammation and one might infer that this condition would lead to a heightened state of host defense. However, there is more agreement that obesity impairs host defense against influenza infection and this may occur via leptin resistance and metabolic dysfunction. Leptin resistance is a condition by which cells become insensitive to leptin as a consequence of prolonged exposure to elevated levels of this adipokine 83. This effect is mediated through the down regulation of LepRb in immune cells and the prolonged activation of STAT3 signaling resulting in the accumulation of intracellular SOCS3 which inhibits further leptin receptor signaling84. Leptin resistance has been demonstrated in NK cells 85, T cells 86, and peripheral blood monocytes 87 and this might contribute to suboptimal responses in the obese during influenza infection. Lastly, it is interesting to note that polymorphisms in the human leptin receptor gene have not only been associated with obesity but with susceptibility to infectious disease as well 88-89. It is envisioned that leptin receptor mutations in humans may be related to impairments in pulmonary host defense that may or may not be associated with an obese phenotype. Alternatively, leptin receptor mutations can lead to obesity and a protective immune phenotype against pulmonary infections 75. In total, more research is needed to determine the role of leptin receptor dysfunction in infectious disease.
VI. Role of adiponectin and other adipokines in pulmonary infections
Besides leptin, very little is known regarding the importance of other adipokines in host defense against infection. Adiponectin, an adipokine with anti-inflammatory properties, has been shown to play a role in alveolar macrophage activation and the lungs of knockout mice exhibit an emphysematous phenotype 90. Exogenous administration of adiponectin has been shown to suppress leukocyte recruitment and it plays an anti-inflammatory role in allergic airway disease in murine models 91-92. Adiponectin levels are reduced in obese subjects and its role in infectious disease is unknown 93. Blood adiponectin levels are elevated in human patients with M. avium-intracellulare complex pulmonary infection 94 and in mice following influenza infection 95. Interestingly, Uji et al. observed that adiponectin-knockout mice exhibit greater mortality in a murine model of polymicrobial sepsis and that pharmacologically induced increases in serum adiponectin improved survival96. Adiponectin also facilitates the uptake of apoptotic cells 97 by macrophages and this response is critical for reducing inflammation in the lungs 98-99. Additional studies that evaluate the role of adiponectin in pulmonary host defense against infection are warranted since the levels of this adipokine are reduced in obese humans 100.
In regard to other adipokines and host defense against respiratory infections, lipocalin 2, which is produced in abundance by adipose tissue of obese mice and humans, has been shown to play a protective role against Klebsiella and E.coli pneumonia 101-102. As discussed above, it is uncertain whether or not the obese are more susceptible to bacterial pneumonia. Evaluating a potential association between elevated serum lipocalin 2 levels and respiratory infections in obese human subjects may provide more insight into physiologic role of adipokines and host defense. Finally, nothing is known regarding the role of resistin, retinoic acid binding protein 4, and other proinflammatory adipokines produced in abundance in the obese during infections of the respiratory tract.
Conclusions
Health care professionals are well acquainted with the association between obesity, type II diabetes, athlerosclerosis, and ischemic heart disease. However, overweight and obese adults and children may also be especially susceptible to respiratory infections and this was evident during the recent H1N1 influenza pandemic of 2009. Since the prevalence of obesity is likely to be stable within the foreseeable future, this condition should be recognized as a chronic medical condition known to increase the risk of influenza-related complications requiring vaccination against seasonal influenza. Only a small number of studies have characterized the mechanisms by which obesity increases the risk of influenza in animal models and this research should be expanded to increase our understanding of the mechanistic underpinnings of this association. Even less is known regarding the susceptibility of the obese to bacteria and other respiratory pathogens and this warrants further investigation. Finally, future studies should also determine if therapeutic strategies employed to prevent obesity-related metabolic and cardiovascular disease, such as weight loss, would also improve immune function against respiratory infections.
Acknowledgements
Support for the author was provided by a grant from the National Institutes of Health, HL077417, and a Clinical Innovator Award (CIA-103071) from the Flight Attendants Medical Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010 Jan 20;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Doyle SL, Lysaght J, Reynolds JV. Obesity and post-operative complications in patients undergoing non-bariatric surgery. Obes Rev. 2010 Dec;11(12):875–886. doi: 10.1111/j.1467-789X.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- 3.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011 Feb 12;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bochicchio GV, Joshi M, Bochicchio K, Nehman S, Tracy JK, Scalea TM. Impact of obesity in the critically ill trauma patient: a prospective study. J Am Coll Surg. 2006 Oct;203(4):533–538. doi: 10.1016/j.jamcollsurg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Intensive-care patients with severe novel influenza A (H1N1) virus infection - Michigan, June 2009. MMWR Morb Mortal Wkly Rep. 2009 Jul 17;58(27):749–752. [PubMed] [Google Scholar]
- 6.Newell MA, Bard MR, Goettler CE, et al. Body mass index and outcomes in critically injured blunt trauma patients: weighing the impact. J Am Coll Surg. 2007 May;204(5):1056–1061. doi: 10.1016/j.jamcollsurg.2006.12.042. discussion 1062-1054. [DOI] [PubMed] [Google Scholar]
- 7.Yaegashi M, Jean R, Zuriqat M, Noack S, Homel P. Outcome of morbid obesity in the intensive care unit. J Intensive Care Med. 2005 May-Jun;20(3):147–154. doi: 10.1177/0885066605275314. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrman C, Bonmarin I, Bitar D, et al. Adult intensive-care patients with 2009 pandemic influenza A(H1N1) infection. Epidemiol Infect. 2010 Oct 26;:1–8. doi: 10.1017/S0950268810002414. [DOI] [PubMed] [Google Scholar]
- 9.Ahima RS. Digging deeper into obesity. The Journal of Clinical Investigation. 2011;121(6):2076–2079. doi: 10.1172/JCI58719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001 Apr;321(4):249–279. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Valdez R, Narayan KM, Geiss LS, Engelgau MM. Impact of diabetes mellitus on mortality associated with pneumonia and influenza among non-Hispanic black and white US adults. Am J Public Health. 1999 Nov 1;89(11):1715–1721. doi: 10.2105/ajph.89.11.1715. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol. 2011;8(6):340–347. doi: 10.1038/nrgastro.2011.73. [DOI] [PubMed] [Google Scholar]
- 13.Marik P, Varon J. The obese patient in the ICU. Chest. 1998;113:492–498. doi: 10.1378/chest.113.2.492. [DOI] [PubMed] [Google Scholar]
- 14.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006 Jul;6(7):438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 15.Carter PR. Association between gastroesophageal reflux disease and laparoscopic sleeve gastrectomy. Surgery for obesity and related diseases. 2011;7(5):569–572. doi: 10.1016/j.soard.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Tutuian R. Obesity and GERD: pathophysiology and effect of bariatric surgery. Current gastroenterology reports. 2011;13(3):205–212. doi: 10.1007/s11894-011-0191-y. [DOI] [PubMed] [Google Scholar]
- 17.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003 Feb 1;26(2):510–513. doi: 10.2337/diacare.26.2.510. 2003. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien KB, Vogel P, Duan S, et al. Impaired Wound Healing Predisposes Obese Mice to Severe Influenza Virus Infection. Journal of Infectious Diseases. 2012 Jan 15;205(2):252–261. doi: 10.1093/infdis/jir729. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yende S, van der Poll T, Lee M, et al. The influence of pre-existing diabetes mellitus on the host immune response and outcome of pneumonia: analysis of two multicentre cohort studies. Thorax. 2010 Oct 1;65(10):870–877. doi: 10.1136/thx.2010.136317. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008 May 23;94(2):206–218. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity in mice reduces the maintenance of influenza-specific CD8+ memory T cells. J Nutr. 2010 Sep;140(9):1691–1697. doi: 10.3945/jn.110.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011 Feb;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 23.Vaillant L, La Ruche G, Tarantola A, Barboza P. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009;14(33) doi: 10.2807/ese.14.33.19309-en. [DOI] [PubMed] [Google Scholar]
- 24.La Ruche G, Tarantola A, Barboza P, Vaillant L, Gueguen J, Gastellu-Etchegorry M. The 2009 pandemic H1N1 influenza and indigenous populations of the Americas and the Pacific. Euro Surveill. 2009;14(42) doi: 10.2807/ese.14.42.19366-en. [DOI] [PubMed] [Google Scholar]
- 25.Gill JR, Sheng ZM, Ely SF, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010 Feb;134(2):235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized Patients with 2009 H1N1 Influenza in the United States, April–June 2009. New England Journal of Medicine. 2009;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 27.Fezeu L, Julia C, Henegar A, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011 Apr 4; doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 28.Dominguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009 Nov 4;302(17):1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 29.Jhung MA, Swerdlow D, Olsen SJ, et al. Epidemiology of 2009 Pandemic Influenza A (H1N1) in the United States. Clinical Infectious Diseases. 2011 Jan 1;52(suppl 1):S13–S26. doi: 10.1093/cid/ciq008. 2011. [DOI] [PubMed] [Google Scholar]
- 30.Fuhrman C, Bonmarin I, Paty AC, et al. Severe hospitalised 2009 pandemic influenza A(H1N1) cases in France, 1 July-15 November 2009. Euro Surveill. 2010 Jan 14;15(2) doi: 10.2807/ese.15.02.19463-en. [DOI] [PubMed] [Google Scholar]
- 31.Sigurdsson GH, Moller AD, Kristinsson B, et al. [Intensive care patients with influenza A (H1N1) infection in Iceland 2009]. Laeknabladid. 2010 Feb;96(2):83–90. doi: 10.17992/lbl.2010.02.09. [DOI] [PubMed] [Google Scholar]
- 32.Mayoral Cortes JM, Ruiz Fernandez J, Pachon Diaz J, et al. [Infection by the pandemic virus (H1N1) 2009 in Andalusia]. Rev Esp Salud Publica. 2010 Sep-Oct;84(5):517–528. doi: 10.1590/s1135-57272010000500006. [DOI] [PubMed] [Google Scholar]
- 33.Dee S, Jayathissa S. Clinical and epidemiological characteristics of the hospitalised patients due to pandemic H1N1 2009 viral infection: experience at Hutt Hospital, New Zealand. N Z Med J. 2010 Apr 9;123(1312):45–53. [PubMed] [Google Scholar]
- 34.Gauzere BA, Bussienne F, Bouchet B, et al. [Severe cases of A(H1N1)v2009 infection in Reunion Island in 2009 and 2010.]. Bull Soc Pathol Exot. 2011 May;104(2):97–104. doi: 10.1007/s13149-011-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardenosa N, Rodes A, Follia N, et al. Epidemiological analysis of severe hospitalized 2009 pandemic influenza A (H1N1) cases in Catalonia, Spain. Hum Vaccin. 2011 Jan 1;7:226–229. doi: 10.4161/hv.7.0.14609. [DOI] [PubMed] [Google Scholar]
- 36.Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2011 Oct 25; doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buettner R, Scholmerich J, Bollheimer LC. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents[ast]. Obesity. 2007;15(4):798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 38.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007 May;137(5):1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 39.Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology. 2009 Feb;126(2):268–279. doi: 10.1111/j.1365-2567.2008.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Easterbrook JD, Dunfee RL, Schwartzman LM, et al. Obese mice have increased morbidity and mortality compared to non-obese mice during infection with the 2009 pandemic H1N1 influenza virus. Influenza Other Respi Viruses. 2011 doi: 10.1111/j.1750-2659.2011.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010 Mar 15;184(6):3127–3133. doi: 10.4049/jimmunol.0903220. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien KB, Vogel P, Duan S, et al. Impaired wound healing predisposes obese mice to severe influenza virus infection. J Infect Dis. 2012 Jan;205(2):252–261. doi: 10.1093/infdis/jir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrales-Medina VF, Valayam J, Serpa JA, Rueda AM, Musher DM. The obesity paradox in community-acquired bacterial pneumonia. Int J Infect Dis. 2011 Jan;15(1):e54–57. doi: 10.1016/j.ijid.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 44.LaCroix AZ, Lipson S, Miles TP, White L. Prospective study of pneumonia hospitalizations and mortality of U.S. older people: the role of chronic conditions, health behaviors, and nutritional status. Public Health Rep. 1989 Jul-Aug;104(4):350–360. [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue Y, Koizumi A, Wada Y, et al. Risk and protective factors related to mortality from pneumonia among middleaged and elderly community residents: the JACC Study. J Epidemiol. 2007 Nov;17(6):194–202. doi: 10.2188/jea.17.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000 Nov 13;160(20):3082–3088. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 47.Jedrychowski W, Maugeri U, Flak E, Mroz E, Bianchi I. Predisposition to acute respiratory infections among overweight preadolescent children: an epidemiologic study in Poland. Public Health. 1998 May;112(3):189–195. doi: 10.1038/sj.ph.1900438. [DOI] [PubMed] [Google Scholar]
- 48.Choban PS, Heckler R, Burge JC, Flancbaum L. Increased incidence of nosocomial infections in obese surgical patients. Am Surg. 1995 Nov;61(11):1001–1005. [PubMed] [Google Scholar]
- 49.Dossett LA, Dageforde LA, Swenson BR, et al. Obesity and site-specific nosocomial infection risk in the intensive care unit. Surg Infect (Larchmt) 2009 Apr;10(2):137–142. doi: 10.1089/sur.2008.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canturk Z, Canturk NZ, Cetinarslan B, Utkan NZ, Tarkun I. Nosocomial infections and obesity in surgical patients. Obes Res. 2003 Jun;11(6):769–775. doi: 10.1038/oby.2003.107. [DOI] [PubMed] [Google Scholar]
- 51.Serrano PE, Khuder SA, Fath JJ. Obesity as a Risk Factor for Nosocomial Infections in Trauma Patients. J Am Coll Surg. 2010;211(1):61–67. doi: 10.1016/j.jamcollsurg.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Brandt M, Harder K, Walluscheck KP, et al. Severe obesity does not adversely affect perioprative mortality and morbidity in coronary artery bypass surgery. Eur J Cardiothorac Surg. 2001;19(5):662–666. doi: 10.1016/s1010-7940(01)00647-9. [DOI] [PubMed] [Google Scholar]
- 53.Moulton MJ, Creswell LL, Mackey ME, Cox JL, Rosenbloom M. Obesity is not a risk factor for significant adverse outcomes after cardiac surgery. Circulation. 1996 Nov 1;94(9 Suppl):II87–II92. [PubMed] [Google Scholar]
- 54.Dossett LA, Heffernan D, Lightfoot M, et al. Obesity and pulmonary complications in critically injured adults. Chest. 2008 Nov;134(5):974–980. doi: 10.1378/chest.08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in gram-negative pneumonia. J. Immunol. 2002 Apr 15;168(8):4018–4024. doi: 10.4049/jimmunol.168.8.4018. 2002. [DOI] [PubMed] [Google Scholar]
- 56.Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol. 2007 Nov;150(2):332–339. doi: 10.1111/j.1365-2249.2007.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ordway D, Henao-Tamayo M, Smith E, et al. Animal model of Mycobacterium abscessus lung infection. J Leukoc Biol. 2008 Jun;83(6):1502–1511. doi: 10.1189/jlb.1007696. [DOI] [PubMed] [Google Scholar]
- 58.Wieland CW, Florquin S, Chan ED, et al. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int. Immunol. 2005 Nov 1;17(11):1399–1408. doi: 10.1093/intimm/dxh317. 2005. [DOI] [PubMed] [Google Scholar]
- 59.Webb SR, Loria RM, Madge GE, Kibrick S. Susceptibility of mice to group B coxsackie virus is influenced by the diabetic gene. J Exp Med. 1976 May 1;143(5):1239–1248. doi: 10.1084/jem.143.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandez-Riejos P, Najib S, Santos-Alvarez J, et al. Role of leptin in the activation of immune cells. Mediators Inflamm. 2010;2010:568343. doi: 10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 62.Loffreda S, Yang S, Lin H, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 63.Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2γ) protein expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L497–L502. doi: 10.1152/ajplung.00010.2004. [DOI] [PubMed] [Google Scholar]
- 64.Bruno A, Conus S, Schmid I, Simon H-U. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005 Jun 15;174(12):8090–8096. doi: 10.4049/jimmunol.174.12.8090. 2005. [DOI] [PubMed] [Google Scholar]
- 65.Wieland CW, Stegenga ME, Florquin S, Fantuzzi G, van der Poll T. Leptin and host defense against gram-positive and gram-negative pneumonia in mice. Shock. 2006;25:414–419. doi: 10.1097/01.shk.0000209524.12873.da. April. [DOI] [PubMed] [Google Scholar]
- 66.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J. Immunol. 2005 Jun 1;174(11):6820–6828. doi: 10.4049/jimmunol.174.11.6820. 2005. [DOI] [PubMed] [Google Scholar]
- 67.Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 68.Moore SI, Huffnagle GB, Chen GH, White ES, Mancuso P. Leptin modulates neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun. 2003 Jul;71(7):4182–4185. doi: 10.1128/IAI.71.7.4182-4185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J Leukoc Biol. 2001 Mar;69(3):414–418. [PubMed] [Google Scholar]
- 70.De Minicis S, Seki E, Oesterreicher C, Schnabl B, Schwabe RF, Brenner DA. Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology. 2008 Dec;48(6):2016–2026. doi: 10.1002/hep.22560. [DOI] [PubMed] [Google Scholar]
- 71.Mancuso P, Huffnagle GB, Olszewski MA, Phipps J, Peters-Golden M. Leptin corrects host defense defects following acute starvation in murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2006 Jan 15;173:212–218. doi: 10.1164/rccm.200506-909OC. [DOI] [PubMed] [Google Scholar]
- 72.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter RM, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J. Immunol. 1996;157:5221–5224. [PubMed] [Google Scholar]
- 73.Mancuso P, Lewis C, Serezani CH, Goel D, Peters-Golden M. Intrapulmonary administration of leukotriene B4 enhances pulmonary host defense against pneumococcal pneumonia. Infect Immun. 2010;78:2261–2271. doi: 10.1128/IAI.01323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maya-Monteiro CM, Almeida PE, D'Avila H, et al. Leptin induces macrophage lipid body formation by a phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent mechanism. J Biol Chem. 2008 Jan 25;283(4):2203–2210. doi: 10.1074/jbc.M706706200. [DOI] [PubMed] [Google Scholar]
- 75.Mancuso P, Peters-Golden M, Goel D, et al. Disruption of leptin receptor-STAT3 signaling enhances leukotriene production and pulmonary host defense against pneumococcal pneumonia. J Immunol. 2011;186:1081–1090. doi: 10.4049/jimmunol.1001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tschop J, Nogueiras R, Haas-Lockie S, et al. CNS leptin action modulates immune response and survival in sepsis. J Neurosci. 2010 Apr 28;30(17):6036–6047. doi: 10.1523/JNEUROSCI.4875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ikejima S, Sasaki S, Sashinami H, et al. Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes. 2005 Jan 1;54(1):182–189. doi: 10.2337/diabetes.54.1.182. 2005. [DOI] [PubMed] [Google Scholar]
- 78.Park S, Rich J, Hanses F, Lee JC. Defects in innate immunity predispose C57BL/6J-Leprdb/Leprdb mice to infection by Staphylococcus aureus. Infect Immun. 2009 Mar;77(3):1008–1014. doi: 10.1128/IAI.00976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wehrens A, Aebischer T, Meyer TF, Walduck AK. Leptin receptor signaling is required for vaccine-induced protection against Helicobacter pylori. Helicobacter. 2008 Apr;13(2):94–102. doi: 10.1111/j.1523-5378.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 80.D'Andrea BJ, Wilson GL, Craighead JE. Effect of genetic obesity in mice on the induction of diabetes by encephalomyocarditis virus. Diabetes. 1981 May;30(5):451–454. doi: 10.2337/diab.30.5.451. [DOI] [PubMed] [Google Scholar]
- 81.Guo X, Roberts MR, Becker SM, et al. Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol. 2011 May;4(3):294–303. doi: 10.1038/mi.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999 Oct;84(10):3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 83.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998 Oct 22;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 84.Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG, Jr., Xu AW. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes. 2010 Apr;59(4):894–906. doi: 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nave H, Mueller G, Siegmund B, et al. Resistance of Janus kinase-2 dependent leptin signaling in natural killer (NK) cells: a novel mechanism of NK cell dysfunction in diet-induced obesity. Endocrinology. 2008 Jul;149(7):3370–3378. doi: 10.1210/en.2007-1516. [DOI] [PubMed] [Google Scholar]
- 86.Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006 Jun 15;176(12):7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- 87.Tsiotra PC, Pappa V, Raptis SA, Tsigos C. Expression of the long and short leptin receptor isoforms in peripheral blood mononuclear cells: implications for leptin's actions. Metabolism. 2000;49(12):1537–1541. doi: 10.1053/meta.2000.18519. [DOI] [PubMed] [Google Scholar]
- 88.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007 Sep 7;317(5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duggal P, Guo X, Haque R, et al. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. The Journal of Clinical Investigation. 2011;121(3):1191–1198. doi: 10.1172/JCI45294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Summer R, Little FF, Ouchi N, et al. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2008 Jun;294(6):L1035–1042. doi: 10.1152/ajplung.00397.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006 Aug;118(2):389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 92.Medoff BD, Okamoto Y, Leyton P, et al. Adiponectin-deficiency Increases Allergic Airway Inflammation and Pulmonary Vascular Remodeling. Am J Respir Cell Mol Biol. 2009 Jan 23; doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011 Feb;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tasaka S, Hasegawa N, Nishimura T, et al. Elevated serum adiponectin level in patients with Mycobacterium avium-intracellulare complex pulmonary disease. Respiration. 2010;79(5):383–387. doi: 10.1159/000231975. [DOI] [PubMed] [Google Scholar]
- 95.Clinthorne JF, Adams DJ, Fenton JI, Ritz BW, Gardner EM. Short-term re-feeding of previously energy-restricted C57BL/6 male mice restores body weight and body fat and attenuates the decline in natural killer cell function after primary influenza infection. J Nutr. 2010 Aug;140(8):1495–1501. doi: 10.3945/jn.110.122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uji Y, Yamamoto H, Tsuchihashi H, et al. Adiponectin deficiency is associated with severe polymicrobial sepsis, high inflammatory cytokine levels, and high mortality. Surgery. 2009 May;145(5):550–557. doi: 10.1016/j.surg.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 97.Takemura Y, Ouchi N, Shibata R, et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007 Feb;117(2):375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saijo S, Nagata K, Nakano Y, Tobe T, Kobayashi Y. Inhibition by adiponectin of IL-8 production by human macrophages upon coculturing with late apoptotic cells. Biochem Biophys Res Commun. 2005 Sep 9;334(4):1180–1183. doi: 10.1016/j.bbrc.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 99.Nakanishi S, Yamane K, Kamei N, Nojima H, Okubo M, Kohno N. A protective effect of adiponectin against oxidative stress in Japanese Americans: the association between adiponectin or leptin and urinary isoprostane. Metabolism. 2005 Feb;54(2):194–199. doi: 10.1016/j.metabol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 100.Sowers MR, Wildman RP, Mancuso P, et al. Change in adipocytokines and ghrelin with menopause. Maturitas. 2008 Feb 20;59(2):149–157. doi: 10.1016/j.maturitas.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu H, Santoni-Rugiu E, Ralfkiaer E, et al. Lipocalin 2 is protective against E. coli pneumonia. Respir Res. 2010;11(1):96. doi: 10.1186/1465-9921-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chan YR, Liu JS, Pociask DA, et al. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009 Apr 15;182(8):4947–4956. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]