Abstract
Brevetoxins are a family of ladder-frame polyether toxins produced during blooms of the marine dinoflagellate Karenia brevis. Inhalation of brevetoxins aerosolized by wind and wave action can lead to asthma-like symptoms in beach goers. Consumption of either shellfish or finfish exposed to K. brevis blooms can lead to the development of neurotoxic shellfish poisoning. The toxic effects of brevetoxins are due to activation of voltage-sensitive sodium channels (VSSCs) in cell membranes. Binding of brevetoxin analogs and competitors to site 5 on these channels has historically been measured using a radioligand competition assay that is fraught with difficulty, including slow analysis time, production of radioactive waste, and cumbersome and expensive methods associated with the generation of radioactive labeled ligands. In this study, we describe the development of a novel fluorescent synaptosome binding assay for the brevetoxin receptor. BODIPY®-conjugated to PbTx-2 was used as the labeled ligand. The BODIPY®-PbTx-2 conjugate was found to displace [3H]-PbTx-3 from its binding site on VSSCs on rat brain synaptosomes with an equilibrium inhibition constant of 0.11 nM. We have shown that brevetoxin A and B analogs are all able to compete for binding with the fluorescent ligand. Most importantly, this assay was validated against the current site 5 receptor binding assay standard, the radioligand receptor assay for the brevetoxin receptor using [3H]-PbTx-3 as the labeled ligand. The fluorescence based assay yielded equilibrium inhibition constants comparable to the radioligand assay for all brevetoxin analogs. The fluorescence based assay was quicker, far less expensive, and did not generate radioactive waste or need radioactive facilities. As such, this fluorescence-based assay can be used to replace the current radioligand assay for site 5 on voltage-sensitive sodium channels and will be a vital tool for future experiments examining the binding affinity of various ligands for site 5 on sodium channels.
Keywords: Brevetoxin, Fluorescence Assay, Competition Binding Assay, BODIPY®
1. Introduction
The dinoflagellate responsible for the majority of Florida red tides, Karenia brevis (formerly known as Gymnodinium brevi and Ptychodiscus brevis), produces numerous ladder framed polyether compounds, the most abundant of which are the brevetoxins (PbTxs) (Baden and Tomas, 1988). PbTxs bind to site 5 of voltage-sensitive sodium channels (VSSCs) resulting in persistent activation of the channel (Jeglitsch et al., 1998; Purkerson et al., 1999; Baden et al., 2005). The PbTxs are potent neurotoxins that are known to cause massive fish kills and have caused a large number of mortalities in seabirds, sea turtles, and marine mammals (O’Shea et al., 1991; Bossart et al., 1998; Flewelling et al., 2005; Fleming et al., 2011). In addition, humans who consume shellfish contaminated with PbTxs may develop neurotoxic shellfish poisoning (NSP). Symptoms of NSP include gastroenteritis, sensory abnormalities, cranial nerve dysfunction, and other neurotoxic effects. In cases of severe acute cases of NSP, victims require treatment in the emergency room and intensive care units to prevent respiratory failure (Watkins et al., 2008). Furthermore, aerosolized PbTxs in sea spray have been found to induce respiratory dysfunction and distress in beach visitors, especially those with preexisting conditions such as asthma. The most common symptoms of exposure to aerosolized brevetoxins are eye irritation and acute respiratory irritation of both the upper and lower respiratory tracts, including, nasal congestion, throat irritation, cough, chest tightness, wheezing, and shortness of breath (Backer et al., 2003). People that are particularly susceptible, such as asthmatics and those with COPD, may develop more severe symptoms and require hospitalization following exposure to inhaled brevetoxins (Kirkpatrick et al., 2006; Fleming et al., 2007). This is not surprising because brevetoxins have been found to induce bronchoconstriction and airway hypersensitivity in asthmatic sheep, with effects that are larger and lasting longer in susceptible animals as compared to control animals (Abraham et al., 2005a, b).
The search for a treatment to alleviate the symptoms of brevetoxosis and NSP has led to the development of a competitive radioligand binding assay to examine the binding activity of various compounds in relation to brevetoxin (Poli et al., 1986). This type of assay was instrumental in characterizing the binding properties of the newly discovered antagonist to brevetoxin known as brevenal (Bourdelais et al., 2004, 2005). Since 1981, various assays have been developed to aid in detection and elucidation of the effects of brevetoxins during red tide events, including the development of a brevetoxin ELISA (Naar et al., 2002), LC-MS analysis and fluorescence monitoring of synapto-neruosomal membrane potentials (David et al., 2003) and calcium oscillations (Dravid et al., 2004). However, the standard for measuring binding affinity for compounds to the brevetoxin receptor remains the radioligand assay. With increasing restrictions placed on the use of radioactive materials and concomitant concerns regarding radioactive waste and contamination, in addition to cumbersome and expensive methodology associated with the generation of radioactive ligands, there is a great need for the development of a non-radioactive assay to examine the affinity of various compounds for the VSSC PbTx receptor.
In recent years, a variety of fluorescence techniques have revolutionized the study of receptor-ligand interactions. The development of fluorescent probes has allowed researchers to develop vital techniques for life science research, including fluorescence correlation spectroscopy, fluorescence polarization spectroscopy, and in vivo fluorescence imaging. In addition, many studies have investigated the use of fluorescent probes as a substitute for radioligands in competition assays (as reviewed in Leopoldo, et al., 2009; Middleton and Kellam, 2005). These techniques are of particular importance for therapeutically important receptors involved in drug discovery research where the ability to measure and quantify receptor-ligand binding remains important. Fluorescent competition assays historically have the advantage of lower non-specific binding and background, which plagues radioligand assays. In particular, fluorescent based assays may be able to detect interactions between receptors and natural product ligands which may be overlooked in the conventional radioligand assays (Pramanik, 2004). The use of fluorescent probes and fluorescence-based competition assays, for example, has revolutionized the study of G-protein coupled receptors (GPCRs) in drug discovery and has allowed researchers to elucidate complex receptor interactions (Verkaar et al., 2008; Daly and McGrath, 2003; Arterburn et al., 2009).
As fluorescent ligands and probes offer safer, less expensive, more powerful, and more versatile alternatives to radioligands, it was our intent to develop an effective fluorescence-based assay for the study of inhibition of binding at the brevetoxin receptor. This assay allows specific detection of compounds that bind to site 5 of VSSCs and allows for development of novel compounds that may be used as a treatment for the effects of these neurotoxins. In this report, we describe the development and validation of a non-radioactive fluorescence-based receptor assay for site 5 of the VSSC.
2. Materials and Methods
2.1 Reagents and Materials
Reagent grade sucrose, sodium phosphate, Trizma base, HEPES, choline chloride, glucose, EGTA, bovine serum albumin (BSA), protease inhibitor cocktail, and polyethyleneimine solution (PEI) were purchased from Sigma-Aldrich (St. Louis, MO). Reagent grade potassium chloride, magnesium sulfate, and ethyl alcohol were purchased from ThermoFisher Scientific. Alkamuls detergent was purchased from Rhone-Poulenc (Cranbury, NJ).
2.2 Isolation of Brevetoxins
PbTx-1, PbTx-2, PbTx-3, and PbTx-9 were purified from unialgal cultures of Karenia brevis (Wilson strain) as previously described (Bourdelais et al., 2004; Truxal et al. 2008). Brevetoxins were extracted from K. brevis cultures using 1 L of choroform/10 L culture through liquid:liquid extraction. The chloroform was added to the carboy containing K. brevis culture and homogenized using an IKA ultra turrex. The homogenate was allowed to stand until the two layers separated. The chloroform layer (lower) was removed, filtered and dried under vacuum. The polyethers were then separated from lipophilic pigments and cell debris using petroleum ether partitioning with a 90:10:1 (petroleum ether:methanol:water) mixture. The methanol layer containing the target compounds was collected and washed with petroleum ether (2X). The petroleum ether layer was backwashed with 90% MeOH (2X). A Kromaton Fast Centrifugal Partition Chromatograph (Kromaton, France) was then used on the methanol:water extract to effect a rapid liquid:liquid partitioning into 72 fractions based on compound polarity. The fractions containing the brevetoxins were identified (by thin layer chromatography) and further purified using a series of two different HPLC columns.
The fractions from the Kromaton containing PbTx-1, PbTx-2, PbTx-3 and PbTx-9 were purified using two different reversed phase HPLC columns. The first step in HPLC purification used a Phenomenex™ phenyl-hexyl column, 10 × 250 mm, 100A, 5 µm column with isocratic elution using MeOH/H2O; 98:2; with UV detection at 215 nm. The fractions eluting from the column containing PbTx-1, PbTx-2, PbTx-3, and PbTx-9 were collected separately and run individually on a second column: Varian, MVP C18, 10×250 mm, 100A, 5 µm column using isocratic elution with MeOH/H2O; 90:10; with UV detection at 215 nm. After the second HPLC separation the compounds had a purity of >99% by LC/MS and NMR analysis.
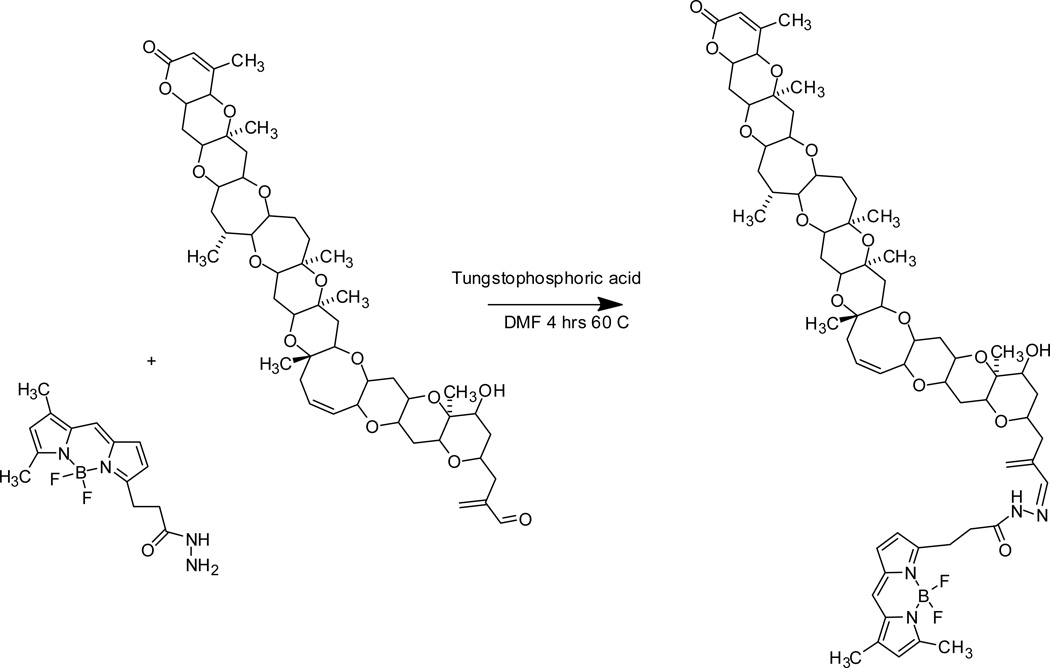
2.3 Synthesis of the BODIPY®-PbTx-2 conjugate
BODIPY® FL hydrazide (D-2371, Invitrogen, Eugene, OR) was conjugated to PbTx-2 using a modified Fischer reaction (Heydari et al., 2007). A one pot reaction was performed using a 1:1 molar ratio of PbTx-2:BODIPY® FL hydrazide in DMF. The reaction conditions were carried out using a catalytic amount of tungstophosphoric acid at 60 °C for four hours with stirring. The DMF was evaporated to dryness under vacuum at 60 °C then resuspended in a small amount of methanol and then filtered (0.2 mM nylon filter). After filtration the reaction mixture was dried again under vacuum to a volume of about 1 mL and then the BODIPY®-PbTx-2 conjugate was purified from the starting materials using a two step reversed phase HPLC procedure. The first step utilized a Phenomenex™ phenyl-hexyl column, 10 × 250 mm, 100A, 5 µm column with isocratic elution using ACN:H2O; 55:45, 3.4 mL/min; UV detection at 215 nm and 360 nm was used. The fraction eluting from the column containing the BODIPY®-PbTx-2 conjugate was collected and further purified using a Varian, MVP C18, 10 × 250 mm, 100A, 5 µm column using isocratic elution with MeOH:H2O; 74:26; and detected using an UV detector set to 215 nm and 360 nM. The purified PbTx-BODIPY® hydrazone derivative was confirmed using LC/MS and 1D and 2D-NMR, as described below.
2.4 MS and NMR spectra measurements
A liquid chromatography–tandem mass spectrometry method was used to confirm the mass (M + 1) of PbTx-2 and BODIPY®-PbTx-2. The sample was run under acidic conditions (MP 80:20:0.1% acetonitrile:H20:Formic acid) 100 µL/min over three minutes using an Agilent 1100 LC (Agilent Technologies, Santa Clara, USA) coupled to a 2000 QTRAP mass spectrometer via an atmosphere pressure ionization electrospray ion source (Turbospray) (Applied Biosystems, Foster City, CA, USA). The parameters for MS detection were: scan type Q1 MS scanning from 115 to 1700 amu:positive mode; cycle time:1.0 sec; duration:2.965 mins; 177 cycles; delay time:0.0 sec; scan mode:profile; step size:0.1 amu; resolution Q1:unit; settling time:0.0 ms; pause between mass ranges:5.007 ms; curtain gas:25 psi; ion spray voltage:5000 V (positive); temperature:300 °C; ion source gas 1:35 psi; ion source gas 2:50 psi; interface heater: on; declustering potential:100 V; entrance potential:11; collision cell potential range: 8.9–63 V. Analyst software v1.4.1 was used for the entire MS method, instrument control, data acquisition and data analysis.
To determine the structure of the BODIPY®-PbTx-2 conjugate 1D (1H, 13C, 13C DEPT) and 2D .(1H-1H COSY, H-13C ROESY, 1H-1H TOCSY, 1H-13C HSQC, 1H-13C HMBC) NMR experiments were performed using a Bruker Avance 500 MHz spectrometer equipped with a 1.7 mm TXI probe at 298 °K in C6D6,
2.5 Preparation of Synaptosomes
Frozen whole adult male Sprague-Dawley rat brains were purchased from Lampire Biological Laboratories (Pipersville, PA). The preparation of synaptosomes was modified from Poli and coworkers (1985). All equipment in contact with the brains/homogenates was at ice temperature. Twelve to eighteen frozen rat brains were thawed on ice and homogenized in 10 mL of ice-cold homogenization buffer (0.32 M sucrose, 0.005 M sodium phosphate, 0.02% protease inhibitor cocktail and brought to pH 7.4 with Trizma base) with ten up-and-down passes of a motor driven Teflon/glass homogenizer. The resulting homogenate was sedimented at 700 × g for 10 min at 4 °C. The supernatant was saved and the pellet was resuspended in 10 mL homogenization buffer and homogenized again. Sedimentation was repeated at 700 × g as described above. The second supernatant was saved, and the pellet was resuspended, homogenized, and sedimented for a third time as described above. All supernatants were combined, and the pellet was discarded. The supernatant mixture was layered over 3 mL of 1.2 M sucrose solution containing protease inhibitors in 10 mL polyallomer centrifuge tubes and centrifuged at 105,000 × g for 30 min at 4 °C. The material at the interface, between the 0.32 M and 1.2 M sucrose solutions, was collected, minimizing the amount of 1.2 M sucrose solution included. The interface material was diluted with homogenization buffer to a final volume of approximately 17 mL, layered over 0.8 M sucrose solution containing protease inhibitors in 5 mL polyallomer centrifuge tubes and centrifuged at 140,000 × g for 35 min at 4 °C. The supernatant was discarded and the final pellet containing synaptosomes was resuspended in standard binding medium (50 mM HEPES, 130 mM choline chloride, 5.4 mM potassium chloride, 0.8 mM magnesium sulfate, 5.5 mM glucose, 1 mM EGTA, 0.02% protease inhibitor cocktail and brought to pH 7.4 with Trizma base). The resulting suspension was diluted to 1 mg protein/mL and aliquots were stored at -80 °C for use in subsequent assays. Protein content was assayed using a modified Lowry Protein Assay (Bio-Rad, Hercules, CA). Synaptosomes can be used only once after transferring to a filter plate, and appear to retain optimal binding for up to 12 hours after thawing if kept cold. Synaptosomes retain consistent binding for up to 6 months if kept at −80°C.
2.6 Radioligand binding
[3H]-PbTx-3 was synthesized from PbTx-2 as previously described (Poli et al., 1985). Tritiated sodium borohydride (NaB3H4: 80 Ci/mmol) was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). Binding of [3H]-PbTx-3 to rat brain synaptosomes was measured independently using a rapid filtration technique (similar to that described in detail below) as previously described (Truxal et al., 2010). All buffers, reagents, and plastic-ware were at ice temperature throughout each experiment except during filtration. All experiments were performed in standard binding medium with 1 mg/mL BSA without detergent.
2.7 Fluorescent-ligand binding
Equilibrium binding of BODIPY®-PbTx-2 was determined on rat brain synaptosomes as follows. A stock solution of BODIPY®-PbTx-2 (0.1 mM) was prepared in ethanol and then diluted in assay buffer (standard binding medium with 1 mg/mL BSA, and 0.02% Alkamuls detergent). Rat brain synaptosomes (50 µg: 50 µL at 1 mg/mL) were added to 400 µL of assay buffer in a deep well polystyrene 96-well assay plate. To assess the time-course of binding, 50 µL of diluted BODIPY®-PbTx-2 (final concentration of 1 nM) was added to wells, and incubation times ranged from 5 seconds to 2 hours. All reactions were incubated on ice in the dark. After incubation, reaction mixtures were transferred to Unifilter-96 GF/B filter plates (PerkinElmer, Waltham, MA) using a FilterMate Harvester vacuum apparatus (PerkinElmer, Waltham, MA). Filters on the filter plates were exposed to assay buffer prior to transfer. Plates were dried thoroughly on the FilterMate Harvester. Fluorescence intensity was measured using a Flex Station III microplate reader (Molecular Devices, Sunnyvale, CA) using the SoftMax Pro 5.2 software (Molecular Devices, Sunnyvale, CA). Relative fluorescence units (RFUs) were analyzed against time by non-linear regression curve-fit analysis by GraphPad Prism v4.03 (GraphPad Prism version 4.03 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com).
2.8 Saturation binding
To determine total binding (specific + non-specific) of BODIPY®-PbTx-2, a plate was prepared in which each well assayed contained 50 µg of synaptosomes suspended in 245 µL of assay buffer plus 5 µL of ethanol (vehicle control). Wells for determining non-specific binding were prepared in the same manner except that the 5 µL of ethanol contained 1 µM PbTx-2. This concentration of unlabeled PbTx-2 was sufficient to displace the BODIPY®-PbTx-2 bound to the VSSCs, leaving the non-specifically bound molecules on synaptosomes. Next, a stock solution of 5 nM BODIPY®-PbTx-2 was prepared in assay buffer and serially diluted (1:2) in the wells until the final concentration was below 0.05 nM. Each dilution was run in quadruplicate for both total and non-specific binding treatments. The reactions were allowed to incubate on ice in the dark for over one hour with gentle mixing on a shaker platform. Reaction mixtures were then transferred to filter plates and fluorescence was measured as described above.
Specific binding was calculated as the difference between total binding and non-specific binding at each concentration of fluorescent ligand. The specific binding curves were analyzed by non-linear regression analysis by GraphPad Prism v4.03 to yield equilibrium dissociation constants (Kd) and binding maxima (Bmax) using equation 1:
2.9 Determination of Bmax from relative fluorescence units
A conversion factor was determined to convert units of maximal specific binding (Bmax) from relative fluorescence units (RFUs) to number of receptor sites bound (pmol per mg of synaptosomes). Concentrations of fluorescent ligand (BODIPY®-PbTx-2) ranging from 1 × 10−5 M to 1 × 10−12 M were diluted in assay buffer and placed into the wells of a filter plate. Fluorescence intensity was measured in the filter plate without filtering to ensure the total amount of fluorescent ligand would be detected. A standard curve was constructed whereby the amount of BODIPY®-PbTx-2 was calculated in terms of pmoles, and then graphed against the respective RFU measured. The resultant curve was analyzed by linear regression analysis by GraphPad Prism v4.03 to yield a slope that could then be used to convert RFU from Bmax into pmol of receptor per mg of protein using equation 2:
This equation assumes that only one BODIPY®-PbTx-2 binds per receptor site. This assumption is based on the finding that only one [3H]-PbTx-3 binds per receptor site consistent with other binding data for VSSCs, and that unconjugated BODIPY® alone does not exhibit binding to synaptosomes (data not shown).
2.10 Inhibition of BODIPY®-PbTx-2 binding
The inhibition of binding of the fluorescent ligand (BODIPY®-PbTx-2) was determined in the presence of various competitors ranging in concentration from 1 × 10−12 M to 1 × 10−5 M. Serial dilutions (1:10) of unlabeled PbTx-2 (control) and natural PbTx derivatives (PbTx-1, PbTx-3, and PbTx-9) were prepared in ethanol. The appropriate concentration of the competitor solution was added (5 µL) to each well, followed by 195 µL of assay buffer, followed by 50 µg of synaptosomes, followed by 50 µL of the fluorescent ligand (0.75 or 1 nM final concentration), followed by 200 µL of assay buffer (500 µL total volume). Reaction mixtures were incubated on ice in the dark for over one hour with gentle mixing on a shaker platform, then transferred to a filter plate and fluorescence was measured as described above. The percent total binding curves were analyzed by non-linear regression analysis by GraphPad Prism v4.03.
The percent specific binding was determined by subtracting the non-specific binding from the total binding. Curves were analyzed by non-linear regression analysis, and equilibrium inhibition constants (Ki) were determined using the Kd value for BODIPY®-PbTx-2 obtained in saturation experiments, by GraphPad Prism v4.03 using equation 3:
2.11 Statistical Analysis
The specific binding curves for saturation binding were analyzed by non-linear regression analysis by GraphPad Prism v4.03 to compare the fit of the data to one-site and two-site models using the extra sum-of-squares F test. Ki values determined for the various competitors were compared for the fluorescence-based assay and the radio-based assay using Student’s t-test SAS v9.1.3 software (SAS Institute Inc., Cary, NC). In all experiments, results are presented as the mean +/− SEM and were considered statistically significant if a p-value of less than 0.05 was obtained.
3. Results
3.1 Synthesis of the BODIPY®-PbTx-2 Conjugate
Reaction of the BODIPY® FL hydrizide with PbTx-2 using a modified Fischer reaction resulted in the production of a single product with a 98% yield (Figure 1). A comparison of 1H and 13C NMR chemical shifts in ppm for BODIPY®-PbTx-2 and PbTx-2 (C6D6, 500 mHz) can be viewed in the supplementary material (Table S1), for the structure and associated carbon position numbers for PbTx-2 and BODIPY®-PbTx-2 see supplementary material (Figure S1A and S1B respectively). From the NMR experiments, it was determined that the reaction resulted in the formation of a stable hydrazide as the major BODIPY®-PbTx-2 conjugate; this was confirmed by the mass spectral data (BODIPY®-PbTx-2: M+H = 1184.4, C64H85BF2N4O14 requires 1183.19).
Figure 1. Reductive amination reaction of PbTx-2 and BODIPY®-FL hydrazide.
3.2 Determination of optimal excitation and emission wavelengths of BODIPY®-PbTx-2
In order to determine the optimal excitation and emission wavelengths for recording fluorescence of BODIPY®-PbTx-2, the fluorescent ligand was diluted to a concentration of 10 µM in assay buffer and a range of excitation and emission ranges were measured on the Flex Station III. Invitrogen gives the excitation and emission maxima for BODIPY® FL hydrazide as 503 nm and 512 nm, respectively (D-2371, Invitrogen, Eugene, OR). Excitation of the BODIPY®-PbTx-2 conjugate increased from 410 nm to 506 nm before falling sharply to 530 nm. To avoid bleed over into fluorescence from absorption, we chose an excitation wavelength of 495 nm to measure fluorescence. An emission wavelength of 525 nm was chosen for sufficient distance from excitation to measure fluorescence without interference following experimentation with multiple wavelengths on both BODIPY®-PbTx-2 and a blank filter plate (data not shown).
3.3 Assessment of binding equilibrium of BODIPY®-PbTx-2 on rat brain synaptosomes
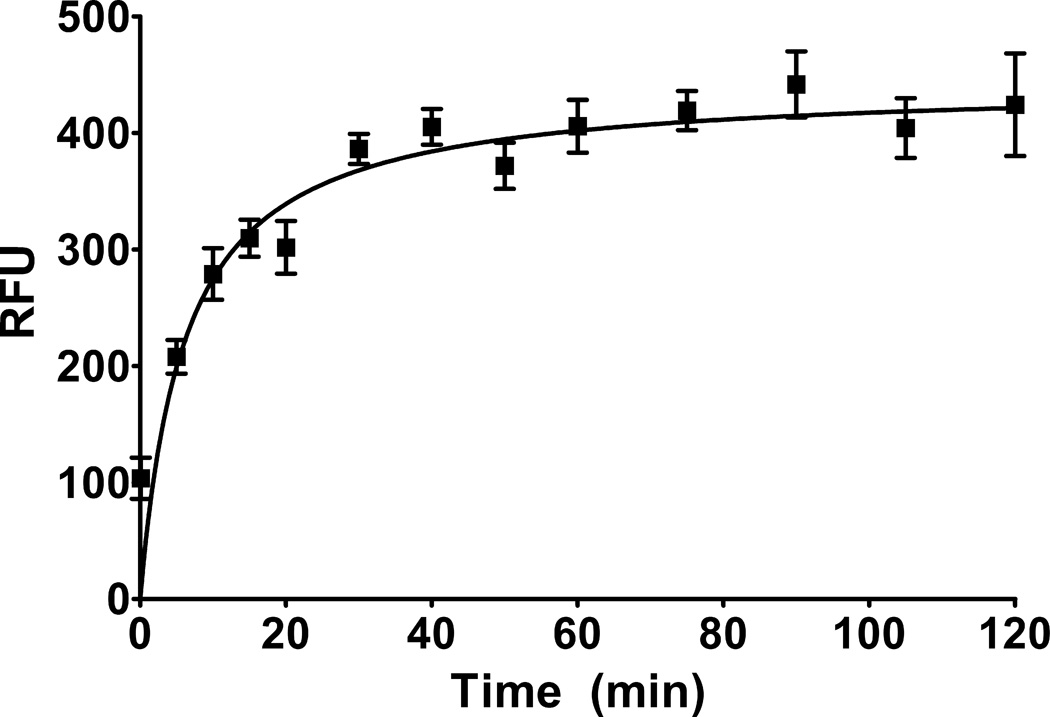
To determine the length of time necessary for binding of the fluorescent ligand to equilibrate on rat brain synaptosomes, we performed a time-course assay with BODIPY®-PbTx-2. Synaptosomes were incubated with 1 nM BODIPY®-PbTx-2 for time points ranging from 5 seconds to 120 minutes. As shown in Figure 2, binding equilibrated after approximately 30–40 minutes and did not deteriorate following at least 2 hours (n = 12).
Figure 2. Time course of BODIPY®-PbTx-2 binding to rat brain synaptosomes.
Simple binding of BODIPY®-PbTx-2 (1 nM) was assayed on rat brain synaptosomes. Timing of exposure of rat brain synaptosomes to BODIPY®-PbTx-2 ranged from 5 seconds to 120 minutes to determine binding equilibrium (n = 12). Equilibrium was determined to be achieved in less than 1 hr.
3.4 Equilibrium dissociation constant and maximal binding of BODIPY®-PbTx-2
In order to determine the equilibrium dissociation constant (Kd) and maximal binding (Bmax) of BODIPY®-PbTx-2 saturation binding experiments were performed using serial dilutions of BODIPY®-PbTx-2 in the presence and absence of PbTx-2 (1 µM). As shown in Figure 3, total binding of BODIPY®-PbTx-2 was concentration-dependent and non-linear. Non-specific binding was concentration-dependent and linear. Subtraction of non-specific binding revealed the specific saturation binding component. Non-linear regression analysis of the specific binding component indicated that BODIPY®-PbTx-2 bound to rat brain synaptosomes with a Kd of 0.17 +/− 0.02 nM (n = 13), which was lower than the Kd value for [3H]-PbTx-3 of approximately 1 nM determined previously in our lab.
Figure 3. Saturation binding of BODIPY®-PbTx-2 to rat brain synaptosomes.
Rat brain synaptosomes were exposed to a range of concentrations of BODIPY®-PbTx-2 (5 nM to less than 0.05 nM). Total binding (without competition) and non-specific binding (with competition from 1 µM PbTx-2) was measured using fluorescence. Specific binding was calculated from the difference between mean total binding and mean non-specific binding at each indicated concentration of BODIPY®-PbTx-2. Representative experiment shown (n = 4 replicates per BODIPY®-PbTx-2 concentration).
Non-linear regression analysis of the specific binding component of the saturation assay determined that BODIPY®-PbTx-2 had a Bmax value of 377.0 +/− 38.0 relative fluorescent units (RFU) (n = 13). To calculate the value of Bmax in terms of pmol/mg protein, a conversion factor was determined by measuring total fluorescence of concentrations of BODIPY®-PbTx-2 ranging from 10 µM to 1 nM, and then converted to pmoles of BODIPY®-PbTx-2 and graphed against the respective RFUs measured to obtain a slope of the line. Bmax was thus calculated to 8.55 +/− 0.86 pmol/mg (n = 13), which compares favorably to the estimated radioligand Bmax values for [3H]-PbTx-3 of 6–13.5 pmol/mg protein (Poli et al. 1986), and not statistically different from what was determined for the radioligand assay previously in our lab at 7.1 +/− 0.2 pmol/mg (p > 0.05; Student’s t-test).
To find the equilibrium inhibition constant (Ki) value of BODIPY®-PbTx-2, a radioligand binding assay was run versus [3H]-PbTx-3. The Ki value of BODIPY®-PbTx-2 was determined to be 0.11 +/− 0.05 nM (n = 5). This was found to be statistically significant when compared to the Ki value of 1.09 +/− 0.06 obtained for unconjugated PbTx-2 on the radioligand assay (n = 6; p < 0.05; Student’s t-test).
3.5 Inhibition binding experiments and comparison to radioligand binding assay
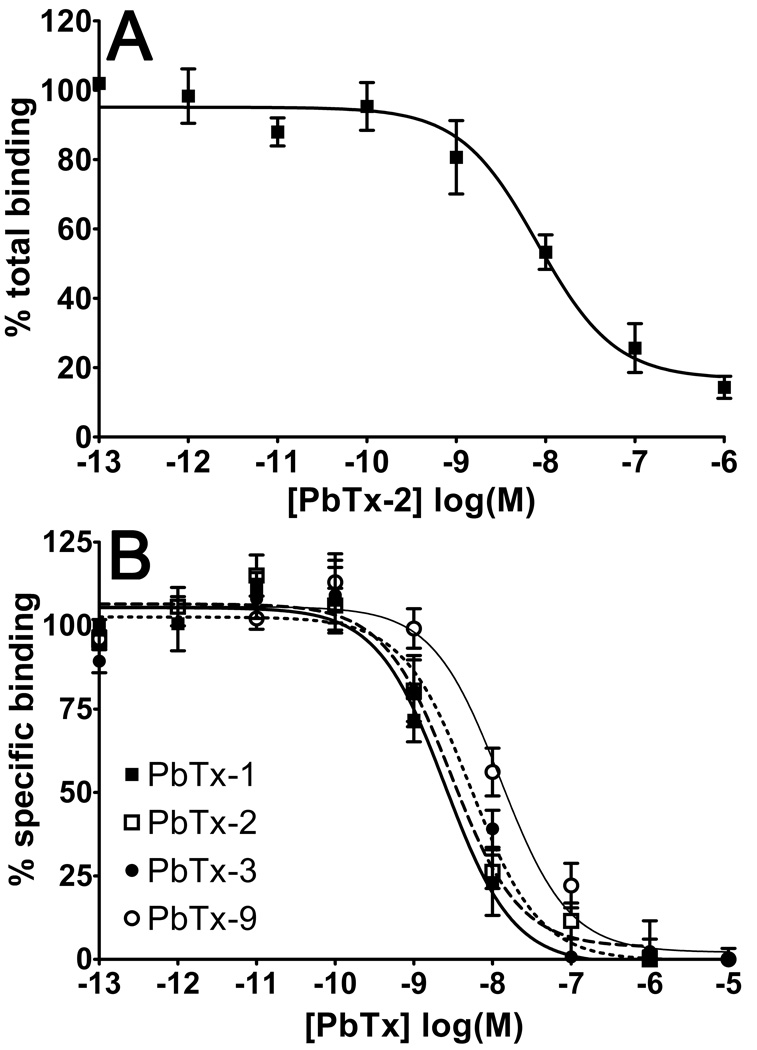
To determine the degree of non-specific binding of BODIPY®-PbTx-2, we measured the percent of total binding for PbTx-2 displacement of BODIPY®-PbTx-2 (1 nM). As shown in Figure 4A, BODIPY®-PbTx-2 exhibited low non-specific binding. Non-specific binding for BODIPY®-PbTx-2 was 14.3% +/− 1.4% (n = 5), and was comparable to the radioligand binding curve (less than or equal to 20% non-specific binding).
Figure 4. Inhibition of BODIPY®-PbTx-2 binding by various PbTx analogs.
Panel A: Competition for binding was assayed using BODIPY®-PbTx-2 (1 nM) against varying concentrations of non-fluorescent PbTx-2 (1 pM to 1 µM) to determine inhibition of BODIPY®-PbTx-2 binding to rat brain synaptosomes. Representative experiment shown (n = 3 replicates per PbTx-2 concentration). Non-specific binding of BODIPY®-PbTx-2 averaged 14.3% +/− 3.2% (n = 5 independent experiments). Panel B: Competitive binding was assayed using BODIPY®-PbTx-2 against varying concentrations of non-fluorescent ligands: PbTx-1, PbTx-2, PbTx-3, and PbTx-9 (1 pM to 10 µM). The thick solid line denotes PbTx-1 binding, the longer hashed line denotes PbTx-2 binding, the shorter hashed line denotes PbTx-3 binding, and the thin solid line denotes PbTx-9 binding (n = 8). Non-specific binding was less than 20% in all cases.
In order to confirm the accuracy and precision of the fluorescent ligand binding assay, a series of inhibition binding experiments were performed using various naturally-occurring PbTx analogs (PbTx-1, PbTx-3, and PbTx-9) compared to PbTx-2 itself. As shown in Figure 4B, all analogs competed with BODIPY®-PbTx-2 for binding to synaptosomes. Using the Kd of BODIPY®-PbTx-2 determined from saturation experiments, Ki values were calculated for each competitive ligand. These Ki values are reported in Table 1. In addition, a series of radioligand binding experiments were also performed on the same day, using the same batch of synaptosomes and dilutions of analogs, in order to validate the fluorescent binding assay. As shown in Table 1, there was no significant difference in Ki values calculated for each of the brevetoxin analogs between the fluorescent ligand and radioligand binding assays.
Table 1.
Comparison between radioligand and fluorescent ligand brevetoxin receptor assays.
| Compound | Ki: Fluorescent-ligand Assay | Ki: Radioligand Assay |
|---|---|---|
| PbTx-1 | 0.68 +/− 0.12 | 0.66 +/− 0.10 |
| PbTx-2 | 1.03 +/− 0.30 | 1.09 +/− 0.06 |
| PbTx-3 | 1.31 +/− 0.30 | 1.55 +/− 0.11 |
| PbTx-9 | 3.63 +/− 0.40 | 3.80 +/− 0.47 |
A series of experiments were performed to compare the Ki values obtained for PbTx-1, PbTx-2, PbTx-3, and PbTx-9 between the radioligand and fluorescent ligand PbTx-2 receptor assays. Experiments were performed on the same day, using the same batch of synaptosomes, and assaying the same dilutions of compound (1pm to 1µM). Each value represents the mean Ki +/− SEM from 6 independent tandem experiments. There was no significant difference in Ki values between assays for any competitor shown (p < 0.05).
4. Discussion
The standard for measuring binding affinity for compounds to the many potential targets in drug discovery research, including the brevetoxin receptor, remains the radioligand assay. However, with increasing restrictions placed on the use of radioactive material and greater concerns of radioactive waste pollution and contamination, there is a great need for the development of non-radioactive labeled compound assays to examine the binding affinity of various ligands in drug discovery. Indeed, the advent of various fluorescence-based techniques has allowed researchers to replace radioactive probes and to elucidate complex receptor interactions for a variety of important target receptors, such as the GPCRs (Verkaar et al., 2008; Daly and McGrath, 2003; Arterburn et al. 2009). Fluorescent competition assays typically have the advantage of lower non-specific binding and background, which plagues radioligand assays, and they may be able to detect interactions between receptors and natural product ligands, which may be overlooked in the conventional radioligand assays (Pramanik, 2004).
The recently discovered natural product brevenal, which acts as an antagonist to the PbTx-2 receptor (Bourdelais et al., 2004, 2005), illustrates the need for a fast, safe, and accurate alternative to the typical radioligand assay. As more natural products are discovered from K. brevis strains or other marine dinoflagellates producing ligands for site 5 on VSSCs, it is vital to test for the binding affinity of these compounds to the PbTx-2 receptor. The development of a fluorescence-based binding assay to determine binding constants of these compounds allows for much faster throughput than could be accomplished with a radioligand assay. The radioligand competition assay has been the standard for determining binding affinities of various ligands to the PbTx-2 receptor (Poli et al., 1986; Bourdelais et al., 2004, 2005; Truxal et al., 2010). However, it would be optimal to be able to replace the radioligand assay with a fully developed fluorescence-based assay for the PbTx-2 receptor. In the present study, we have shown the development of a competition binding assay based on the fluorescent probe BODIPY®-conjugated PbTx-2. Using this probe, we established a quicker and safer alternative binding assay to the traditional radioligand assay for the PbTx-2 receptor binding.
We have demonstrated that BODIPY®-PbTx-2 binds to the PbTx-2 receptor through competitive inhibition studies. BODIPY®-PbTx-2 inhibited binding of [3H]-PbTx-3 with an equilibrium inhibition constant of approximately 10 fold lower than PbTx-2 alone. In addition, BODIPY®-PbTx-2 yielded an equilibrium dissociation constant in saturation assays approximately 10 fold lower than what was found for [3H]-PbTx-3. This indicates that BODIPY®-PbTx-2 binds with a slightly higher binding affinity to the receptor site than PbTx-2 or [3H]-PbTx-3. This may seem counterintuitive in that a large and cumbersome molecule, such as BODIPY®, attached to PbTx-2 might be expected to exhibit a decreased affinity compared to PbTx-2 alone for its receptor. However, the above results are consistent with other binding data observed in this laboratory (data not shown). Furthermore, other studies have shown that conjugating BODIPY® to various ligands may slightly increase or decrease binding affinity for the receptors, though still remain effective in establishing binding constants (Dumont et al., 2005; Schoebel et al., 2010).
Finally, we have shown that the fluorescence-based competition assay generates inhibition constant values that are very similar to and not significantly different from those generated by the radioligand assay. This is an important step in the eventual reliance on the fluorescence-based assay and phasing out of the radioligand assay for determining the binding affinities of natural product ligands and derivatives from K. brevis. It is important to note that while equilibrium binding constants may not always be exactly parallel between the two assays, relative binding affinity of various compounds will be able to be determined when using either assay and should be consistent within the assay itself. There is the potential for greater variation within the fluorescence-based assay because of the novelty of the assay and the lack of history regarding the generation of Ki values with this assay. As such, it may be preferable to assay compounds in triplicate, or otherwise increase replicates, until this assay has had a chance to be fully validated among several laboratories.
At this time, the fluorescence-based assay for site 5 of VSSCs has only been used to investigate the binding affinities of various ligands in the search for agonists and antagonists for the brevetoxin receptor. We have not examined the binding characteristics of BODIPY®-PbTx-2 in anything other than a clean synaptosome preparation with purified compounds. Such investigation is needed, and ongoing studies in our laboratory are determining the binding characteristics and utility of this assay for quantification of brevetoxins and ciguatoxins in a variety of biological matrices such as water and shellfish extracts.
5. Conclusions
We have developed and described a fluorescence-based binding assay for the brevetoxin receptor in rat brain synaptosomes. Not only does this assay generate binding constants comparable to the radioligand assay, but it is also faster (with an assay and analysis time of less than 3 hours vs. overnight), less expensive, safer, and generates far less harmful waste. This assay has show utility in screening for selective agonists and antagonists for the brevetoxin receptor. The discovery of antagonists that are capable of blocking binding of brevetoxin and thus preventing the harmful effects of brevetoxins during red tide blooms remains an important area of research in both this laboratory and in other institutions. As such, this fluorescence assay will be a vital tool for future experiments examining the binding affinity of various ligands for the brevetoxin receptor.
Supplementary Material
Highlights.
A fluorescence-based binding assay was developed for the brevetoxin receptor.
The fluorescent ligand displaced known ligands for site 5 of VSSCs.
The assay was validated against the current standard radioligand assay for site 5.
The fluorescence-based assay was quicker and far less expensive.
The fluorescence-based assay did not generate radioactive waste.
Acknowledgements
This work was supported by NIH grants PO1 ES10594-10 and R21 NS067503-A101, NOAA CIOERT NA09OAR4320073. Portions of this work were sponsored by MARBIONC, an economic development program located in North Carolina at UNCW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WM, Bourdelais AJ, Sabater JR, Ahmed A, Lee TA, Serebriakov I, Baden DG. Airway responses to aerosolized brevetoxins in an animal model of asthma. Am. J. Respir. Crit. Care Med. 2005a;171(1):26–34. doi: 10.1164/rccm.200406-735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WM, Bourdelais AJ, Ahmed A, Serebriakov I, Baden DG. Effects of inhaled brevetoxins in allergic airways: toxin-allergen interactions and pharmacologic intervention. Environ. Health Perspect. 2005b;113:632–637. doi: 10.1289/ehp.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arterburn JD, Oprea TI, Prossnitz ER, Edwards BS, Sklar LA. Discovery of selective probes and antagonists for G protein coupled receptors FPR/FPRL1 and GPR30. Curr. Top. Med. Chem. 2009;9(13):1227–1236. doi: 10.2174/156802609789753608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce RH, Zaias J, Bean J, Bossard GD, Johnson D, Quimbo R, Baden DG. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae. 2003;2:19–28. [Google Scholar]
- Baden DG, Tomas CR. Variations in major toxin composition for six clones of Ptychodiscus brevis . Toxicon. 1988;26(10):961–963. doi: 10.1016/0041-0101(88)90261-9. [DOI] [PubMed] [Google Scholar]
- Baden DG, Bourdelais AJ, Jacocks H, Michelliza S, Naar J. Natural and derivative brevetoxins: historical background, multiplicity, and effects. Environ. Health Perspect. 2005;113:621–625. doi: 10.1289/ehp.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart GD, Baden DG, Ewing RY, Roberts B, Wright SD. Brevetoxicosis in manatees (Trichechus manatus latirostris) from the 1996 epizootic: gross, histologic, and immunohistochemical features. Toxicol. Pathol. 1998;26:276–282. doi: 10.1177/019262339802600214. [DOI] [PubMed] [Google Scholar]
- Bourdelais AJ, Campbell S, Jacocks H, Naar J, Wright JLC, Carsi J, Baden DG. Brevenal is a natural inhibitor of brevetoxin action insodium channel receptor binding assays. Cell. Mol. Neurobiol. 2004;24(4):553–563. doi: 10.1023/B:CEMN.0000023629.81595.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdelais AJ, Jacocks HM, Wright JLC, Bigwarfe PM, Baden DG. A new polyether ladder compound produced by the dinoflagellate Karenia brevis. J. Nat. Prod. 2005;68:2–6. doi: 10.1021/np049797o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly CJ, McGrath JC. Fluorescent ligands, antibodies, and proteins for the study of receptors. Pharmacol. Ther. 2003;100:101–118. doi: 10.1016/j.pharmthera.2003.08.001. [DOI] [PubMed] [Google Scholar]
- David LS, Plakas SM, El Said KR, Jester ELE, Dickey RW, Nicholson RA. A rapid assay for the brevetoxin group of sodium channel activators based on fluorescence monitoring of synaptoneurosomal membrane potential. Toxicon. 2003;42:191–198. doi: 10.1016/s0041-0101(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Dravid SM, Baden DG, Murray TF. Brevetoxin activation of voltage-gated sodium channels regulates Ca2+dynamics and ERK1/2 phosphorylation in murine neocortical neurons. J. Neurochem. 2004;89:739–749. doi: 10.1111/j.1471-4159.2004.02407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont Y, Gaudreau P, Mazzuferi M, Langolis D, Chabot JG, Fournier A, Simonato M, Quirion R. BODIPY®-conjugated neuropeptide Y ligands: new fluorescent tools to tag Y1, Y2, Y4 and Y5 receptor subtypes. Br. J. Pharmacol. 2005;146(8):1069–1081. doi: 10.1038/sj.bjp.0706425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Reich A, Zaias J, Cheng YS, Pierce R, Naar J, Abraham WM, Baden DG. Aerosolized red-tide toxins (brevetoxins) and asthma. Chest. 2007;131:187–194. doi: 10.1378/chest.06-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Walsh CJ, Nierenberg K, Clark J, Reich A, Hollenbeck J, Benson J, Chen YS, Naar J, Pierce R, Bourdelais AJ, Abraham WM, Kirkpatrick G, Zaias J, Wanner A, Mendes E, Shalat S, Hoagland P, Stephan W, Bean J, Watkins S, Clarke T, Byrne M, Baden DG. Review of Florida red tide and human health effects. Harmful Algae. 2011;10:224–233. doi: 10.1016/j.hal.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewelling LJ, Naar JP, Abbot JP, Baden DG, Barros NB, Bossart GD, Bottein MD, Hammond DG, Haubold EM, Heil CA, Henry MS, Jacocks HM, Leighfield TA, Pierce RH, Pitchford TD, Rommel SA, Scott PS, Steidinger KA, Truby EW, Van Dolah FM, Landsberg JH. Red tides and marine mammal mortalities: unexpected brevetoxin vectors may account for deaths long after or remote from an algal bloom. Nature. 2005;435:755–756. doi: 10.1038/nature435755a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeglitsch G, Rein K, Baden DG, Adams DG. Brevetoxin-3 (PbTx-3) and its derivatives modulate single tetrodotoxin-sensitive sodium channels in rat sensory neurons. J. Pharmacol. Exp. Ther. 1998;284(2):516–525. [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Backer LC, Bean JA, Tamer R, Kirkpatrick G, Kane T, Wanner A, Dalpra D, Reich A, Baden DG. Environemtnal exposures to Florida red tides: effects on emergency room respiratory diagnoses admissions. Harmful Algae. 2006;5(5):526–533. doi: 10.1016/j.hal.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari A, Khaksar S, Akbari J, Esfandyari M, Pourayoubi M, Tajbakhsh M. Direct reductive amination and selective 1,2-reduction of, α,β-unstaturated aldehydes and ketone by NaBH4using H3W12O40as catalyst. Tet. Lett. 2007;48:1135–1138. [Google Scholar]
- Leopoldo M, Lacivita E, Berardi F, Perrone R. Developments in fluorescent probes for receptor research. Drug Discov. Today. 2009;14(13–14):706–712. doi: 10.1016/j.drudis.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Middleton RJ, Kellam B. Fluorophore-tagged GPCR ligands. Curr. Opin. Chem. Biol. 2005;9:517–525. doi: 10.1016/j.cbpa.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Naar J, Bourdelais AJ, Tomas C, Kubanek J, Whitney PL, Flewelling L, Steidinger K, Lancaster J, Baden DG. A competitive ELISA to detect brevetoxins from Karenia brevis (formerly Gymnodinium breve) in seawater, shellfish, and mammalian body fluid. Environ. Health Perspect. 2002;110(2):179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea TJ, Rathbun GB, Bonde RK, Buergelt CD, Odell DK. An epizootic of Florida manatees associated with a dinoflagellate bloom. Mar. Mam. Sci. 1991;7:165–179. [Google Scholar]
- Purkerson SL, Baden DG, Fieber LA. Brevetoxin modulates neuronal sodium cannels in two cell lines derived from rat brain. Neurotoxicology. 1999;6:909–920. [PubMed] [Google Scholar]
- Poli MA, Mende TJ, Baden DG. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol. Pharmacol. 1986;30:129–135. [PubMed] [Google Scholar]
- Pramanik A. Ligand-receptor interactions in live cells by fluorescence correlation spectroscopy. Curr. Pharm. Biotechnol. 2004;5(2):205–212. doi: 10.2174/1389201043377002. [DOI] [PubMed] [Google Scholar]
- Schoebel S, Blankenfeldt W, Goody RS, Itzen A. High-affinity binding of phosphatidylinositol 4-phosphate by Legionella pneumophila DrrA. EMBO Rep. 2010;11(8):598–604. doi: 10.1038/embor.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truxal LT, Bourdelais AJ, Jacocks H, Abraham WM, Baden DG. Characterization of Tamulamides A and B, polyethers isolated from the marine dinoflagellate Karenia brevis. J. Nat. Prod. 2010;73:536–540. doi: 10.1021/np900541w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkaar F, van Rosmalen JWG, Blomenrohr M, van Koppen CJ, Blankesteign WM, Smits JFM, Zaman GJR. G protein-independent cell-based assay for drug discovery on seven-transmembrane receptors. Biotechnol. Annu. Rev. 2008;14:253–274. doi: 10.1016/S1387-2656(08)00010-0. [DOI] [PubMed] [Google Scholar]
- Watkins S, Reich A, Fleming L, Hammond R. Neurotoxic shellfish poisoning. Marine Drugs. 2008;6:431–455. doi: 10.3390/md20080021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.