Abstract
Triacylglycerol (TAG) levels and oil bodies persist in sucrose (Suc)-rescued Arabidopsis (Arabidopsis thaliana) seedlings disrupted in seed oil catabolism. This study set out to establish if TAG levels persist as a metabolically inert pool when downstream catabolism is disrupted, or if other mechanisms, such as fatty acid (FA) recycling into TAG are operating. We show that TAG composition changes significantly in Suc-rescued seedlings compared with that found in dry seeds, with 18:2 and 18:3 accumulating. However, 20:1 FA is not efficiently recycled back into TAG in young seedlings, instead partitioning into the membrane lipid fraction and diacylglycerol. In the lipolysis mutant sugar dependent1and the β-oxidation double mutant acx1acx2 (for acyl-Coenzyme A oxidase), levels of TAG actually increased in seedlings growing on Suc. We performed a transcriptomic study and identified up-regulation of an acyltransferase gene, DIACYLGLYCEROL ACYLTRANSFERASE3 (DGAT3), with homology to a peanut (Arachis hypogaea) cytosolic acyltransferase. The acyl-Coenzyme A substrate for this acyltransferase accumulates in mutants that are blocked in oil breakdown postlipolysis. Transient expression in Nicotiana benthamiana confirmed involvement in TAG synthesis and specificity toward 18:3 and 18:2 FAs. Double-mutant analysis with the peroxisomal ATP-binding cassette transporter mutant peroxisomal ABC transporter1 indicated involvement of DGAT3 in the partitioning of 18:3 into TAG in mutant seedlings growing on Suc. Fusion of the DGAT3 protein with green fluorescent protein confirmed localization to the cytosol of N. benthamiana. This work has demonstrated active recycling of 18:2 and 18:3 FAs into TAG when seed oil breakdown is blocked in a process involving a soluble cytosolic acyltransferase.
In many plant species, triacylglycerols (TAGs) are the major storage lipids, serving as an important energy reserve in seeds for subsequent germination and seedling development. TAGs are also essential for pollen development and sexual reproduction (Slocombe et al., 1994; Wolters-Arts et al., 1998; Zheng et al., 2003). Plant-derived storage lipids are a major feedstock for the food and feed industries as well as the oleochemical and biofuel industries and there continues to be much interest in understanding the regulation of their synthesis in planta (Weselake, 2005; Durrett et al., 2008; Dyer et al., 2008).
In oilseeds, TAG bioassembly is catalyzed by the membrane-bound enzymes of the Kennedy pathway that operate in the endoplasmic reticulum (Stymne and Stobart, 1987). Diacylglycerol acyltransferase (DGAT; EC 3.2.1.20) catalyzes the final acylation of sn-1,2 diacylglycerol (DAG) to give TAG and has been suggested as one of the rate-limiting steps in plant storage lipid accumulation (Ichihara et al., 1988). The first DGAT gene, a member of the DGAT1 family, was isolated from mouse and was followed by the identification of other DGAT1 members from a number of plant species, including Arabidopsis (Arabidopsis thaliana; Hobbs et al., 1999; Routaboul et al., 1999; Zou et al., 1999; Bouvier-Navé et al., 2000). A second family of DGAT genes (DGAT2) was first identified in the oleaginous fungus Morteriella ramanniana, but these have no sequence similarity with DGAT1 (Lardizabal et al., 2001) and appear to have a nonredundant function in TAG biosynthesis. Two enzyme activities catalyzing acyl-CoA-independent synthesis of TAG have also been described: phospholipid:DGAT (PDAT; Dahlqvist et al., 2000) and DAG:DAG transacylase, which catalyzes a transacylation reaction in which the free hydroxyl group of a DAG molecule is acylated with a fatty acid (FA) moiety from a second DAG molecule, forming monoacylglycerol and TAG (Mancha and Stymne, 1997; Stobart et al., 1997). To date, however, a DAG:DAG transacylase gene has not been isolated from any source. In addition to the membrane-bound pathway for TAG synthesis, an alternate pathway was proposed in peanut (Arachis hypogaea). This pathway takes place in the cytosol and involves the acylation of monoacylglycerol to DAG and the further acylation of DAG to TAG by the action of a cytosolic DGAT (Tumaney et al., 2001; Saha et al., 2006).
Upon germination and early postgerminative growth, FAs derived from storage TAG are converted to Suc to provide metabolic energy and carbon skeletons for seedling growth. The mobilization of storage oil involves the coordinated induction of a number of biochemical pathways in different subcellular locations. The first step in oil breakdown is catalyzed by lipases that hydrolyze TAG to produce free FA and glycerol (Huang, 1992). The FA then enters single membrane-bound organelles termed peroxisomes (glyoxysomes) where β-oxidation and part of the glyoxylate cycle occurs (Cooper and Beevers, 1969). β-Oxidation converts FA to acetyl-CoA, which is subsequently condensed into four-carbon compounds via the glyoxylate cycle. These four-carbon compounds are then transported to the mitochondria, where they can either be converted to malate and transported to the cytosol for gluconeogenesis, or used as substrates for respiration.
Disruption of a number of genes involved in oil catabolism results in a partial or complete block in TAG breakdown, defects in seed germination, and an inability to establish photosynthetic competence (Graham, 2008). These genes include SUGAR DEPENDENT1 (SDP1), a patatin-like TAG lipase associated with oil body membranes (Eastmond, 2006), PEROXISOMAL ABC TRANSPORTER1 (PXA1), involved in the transport of free FAs (Fulda et al., 2004; van Roermund et al., 2012) and/or acyl-CoA esters across the peroxisome membrane (Zolman et al., 2001), and those encoding any of the core reactions of the peroxisomal β-oxidation pathway. The first step of β-oxidation is carried out by the acyl-CoA oxidase (ACX) family, which comprises isoenzymes with distinct FA chain-length specificities. Six ACX genes have been identified in the Arabidopsis genome (Graham and Eastmond, 2002). Single mutants disrupted in each of the six ACX genes have been described and seedling establishment and lipid breakdown were unaffected, probably due to the overlapping substrate specificities of the gene products (Eastmond et al., 2000; Rylott et al., 2003; Adham et al., 2005). However, the acx1acx2 double mutant, which lacks the medium- to long-chain and the long-chain ACXs, respectively, shows a Suc-dependent seedling establishment phenotype, indicating that long-chain ACXs are essential for seedling establishment (Pinfield-Wells et al., 2005). The second step of peroxisomal β-oxidation consists of four separate reactions, two of which (2-trans enoyl-CoA hydratase and l-3-hydroxyacyl-CoA dehydrogenase) are core activities required for the catabolism of all FA. Both activities are contained on MULTIFUNCTIONAL PROTEIN (MFP). Arabidopsis contains two isoforms of MFP. AIM1 is expressed at a low level in germinating seedlings and the aim1 mutant exhibits normal germination and seedling establishment but does have an altered meristem mature plant phenotype (Richmond and Bleecker, 1999). In contrast, MFP2, the gene encoding the second isoform, is strongly induced during postgerminative seedling growth (Eastmond and Graham, 2000). The Arabidopsis mfp2 mutant requires an exogenous supply of Suc for seedling establishment and is compromised in storage oil breakdown (Rylott et al., 2006). The enzyme 3-ketoacyl-CoA thiolase (KAT; EC 2.3.1.16) catalyzes the last step of FA β-oxidation. The Arabidopsis genome contains three loci that encode KAT enzymes, annotated as KAT1, KAT2, and KAT5. KAT2 is the only one of the three KAT genes expressed at significant levels during seed germination in Arabidopsis, and the kat2 mutant is blocked in storage oil breakdown and is dependent on exogenous Suc for seedling establishment (Germain et al., 2001).
TAG oil bodies persist in Suc-rescued seedlings of these various mutants but it is not known if the total amount and composition of TAG molecular species in the oil bodies is the same as those in mature seeds or if they reflect the FA composition of green tissues as is the case with de novo synthesized TAG in leaves (Kunz et al., 2009; Slocombe et al., 2009). If there is feedback inhibition of lipolysis then TAG composition reflecting that in mature seed would be expected to persist in Suc-rescued seedlings. If, on the other hand there is a mechanism for TAG recycling distinct from de novo synthesis in developing seeds, TAG composition might be expected to change. We report here that FA recycling does operate when FA breakdown is blocked postlipolysis and this recycling is mediated to a significant extent by a previously uncharacterized cytosolic route for TAG synthesis involving an acyl-CoA-dependent cytosolic acyltransferase.
RESULTS
TAG Synthesis and Recycling in Suc-Rescued Mutant Seedlings
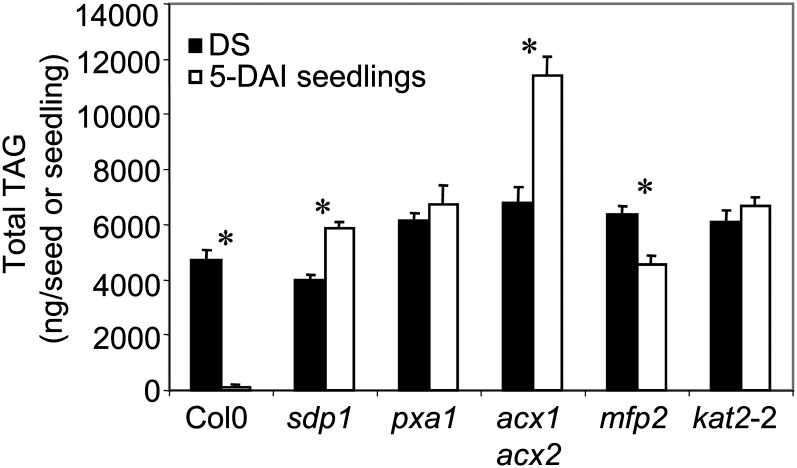
Total TAG content in Suc-rescued mutants disrupted in TAG breakdown (sdp1; Eastmond, 2006), FA transport across the peroxisome membrane (pxa1; Zolman et al., 2001), and the core reactions of β-oxidation (acx1acx2, Pinfield-Wells et al., 2005; mfp2, Rylott et al., 2006; and kat2-2, Eastmond, 2006) are shown in Figure 1. While TAG was almost completely catabolized in Columbia-0 (Col-0) seedlings maintained on Suc at 5 d after imbibition (DAI), the total TAG content in 5-DAI Suc-rescued mutant seedlings showed smaller changes compared with dry seed (DS) and in some cases increases (Fig. 1). These results are consistent with published data on total FA composition and eicosenoic acid levels (a marker for seed storage TAG) and confirm these mutants are severely impaired in their ability to catabolize seed storage TAG (Germain et al., 2001; Zolman et al., 2001; Pinfield-Wells et al., 2005; Eastmond, 2006; Rylott et al., 2006). The increased TAG levels in sdp1 and acx1acx2 5-DAI seedlings suggest de novo TAG synthesis is occurring in these mutant seedlings. The slight decrease in mfp2 5-DAI seedlings compared with DS is consistent with this mutant not being completely blocked in FA breakdown as previously reported based on eicosenoic acid levels (Rylott et al., 2006).
Figure 1.
Total TAG content of Col-0 and mutant DS and 5-DAI seedlings. Values are means ± sd of measurements from five separate batches of 50 seeds or 30 seedlings. Significant differences (Student’s t test, P < 0.05) are indicated by an asterisk.
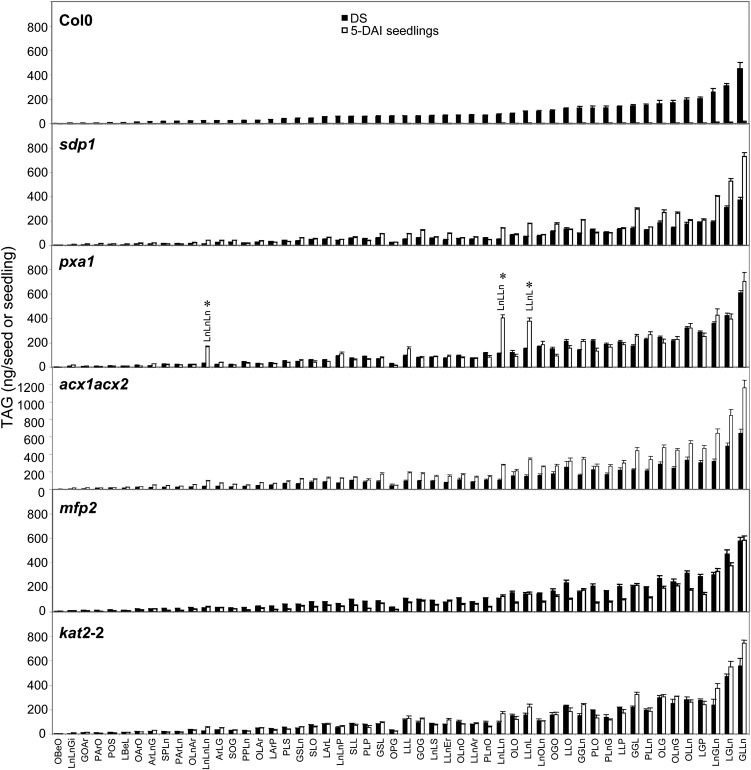
Compositional analysis of individual TAG species confirmed that for Col-0 all of the identified TAGs decreased to near-undetectable levels in 5-DAI Suc-rescued seedlings compared with DS, in marked contrast to the oil breakdown mutants (Fig. 2). In sdp1 and acx1acx2 Suc-rescued 5-DAI seedlings the levels of most of the TAG species including those containing 20:1 actually increased. In kat2-2 Suc-rescued 5-DAI seedlings the majority of TAG species were at similar levels compared with the corresponding DS and in mfp2 TAG species content was slightly lower than in DS. Interestingly, in pxa1 Suc-rescued 5-DAI seedlings we observed an increase in TAG species containing 18:3 and to a lesser extent 18:2 (Fig. 2C). These results indicate that blocking different steps in TAG catabolism affects not only TAG content in 5-DAI seedlings, but also TAG composition.
Figure 2.
TAG composition of Col-0 and mutant DS and 5-DAI seedlings. Values are means ± sd of measurements from five separate batches of 50 seeds or 30 seedlings. TAG molecular species are given as three concatenated FA codes, with FAs coded as follows: P = 16:0, H = 16:3, S = 18:0, O = 18:1, L = 18:2, Ln = 18:3, Ar = 20:0, G = 20:1, Gi = 20:2, Be = 22:0, and Er = 22:1. Coding order reflects molecular composition only and does not imply regiospecific arrangements. Significant differences (Student’s t test, P < 0.05) were performed for selected pxa1 TAGs and are indicated by an asterisk.
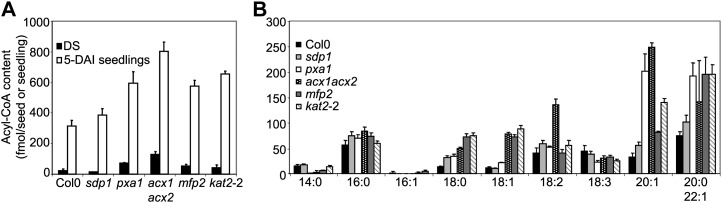
In germinating seedlings the FAs released from oil bodies are activated to acyl-CoAs before they are catabolized by peroxisomal FA β-oxidation (Fulda et al., 2004). To investigate how the acyl-CoA pool size and composition changes in concert with the apparent decrease in TAG breakdown observed in the mutants, we analyzed the acyl-CoA content in seedlings. In sdp1 the total acyl-CoA content in 5-DAI seedlings was similar to Col-0 (Fig. 3A). In contrast, we observed that in pxa1 and all β-oxidation mutants there was an accumulation of acyl-CoAs in 5-DAI seedlings compared with Col-0. Furthermore, the acyl-CoA profile showed a relative enrichment in long-chain acyl-CoAs, especially 20:1-CoA, in pxa1 and β-oxidation mutants (Fig. 3B). These results confirm that sdp1 is impaired in lipolysis, as has been reported by Eastmond (2006), while in pxa1 and the β-oxidation mutants lipolysis is not impaired and FAs are activated to acyl-CoAs but further catabolism is compromised. In the case of pxa1, the data suggests efficient incorporation of de novo or recycled 18:2 and 18:3 FA/acyl-CoAs into TAG but poor utilization of accumulated 20:1 acyl-CoA.
Figure 3.
Total acyl-CoA content in Col-0 and mutant DS and 5-DAI seedlings (A) and acyl-CoA composition in 5-DAI seedlings (B). Values are means ± sd of measurements from five separate batches of 30 seedlings.
An Alternative Pathway for TAG Synthesis in pxa1 during Seedling Establishment
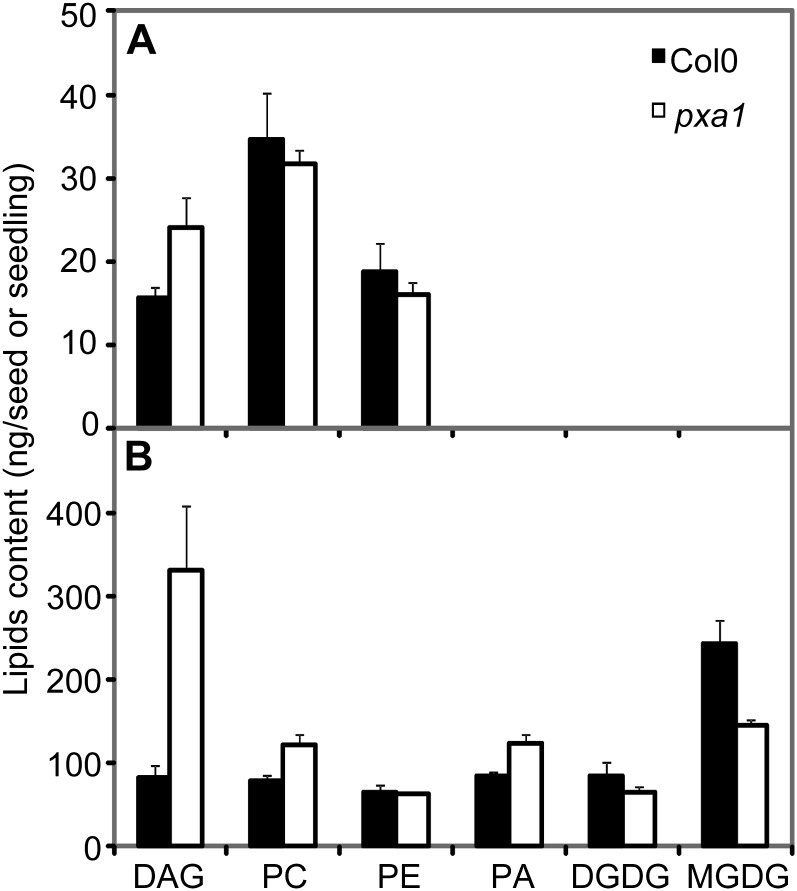
The observation that impairing the peroxisomal ATP-binding cassette transporter function affects TAG and acyl-CoA pool content and composition suggests that other lipid pools may also be affected. To investigate this we analyzed DAG and polar lipid composition in Col-0 and pxa1 DS and 5-DAI seedlings. The only difference we observed in DS was a slightly higher DAG content in pxa1 relative to Col-0 (Fig. 4A). However, in 5-DAI seedlings there was a general increase in microsomal lipids and a decrease in galactolipids in pxa1 compared with Col-0 (Fig. 4B). The lipid FA composition in pxa1 5-DAI seedlings showed an increase in 20:1 content in DAG, phosphatidic acid, phosphatidylcholine, and phosphatidylethanolamine compared with Col-0, while there were no significant differences in 18:2 and 18:3 content between pxa1 and Col-0 in any of the polar lipids analyzed (Table I).
Figure 4.
Glycerolipid content of Col-0 and pxa1 DS (A) and 5-DAI seedlings (B). Values are means ± sd of measurements from five separate batches of 200 seeds or 100 seedlings. Lipid classes are abbreviated as follows: PC, Phosphatidylcholine; PE, phosphatidylethanolamine; PA, phosphatidic acid; DGDG, digalactosyldiacylgalactolipid; MGDG, monogalactosyldiacylgalactolipid.
Table I. DAG and polar lipid FA composition in 5-DAI seedlings.
Values are means ± sd of measurements from five separate batches of 100 seedlings. Asterisk (*) indicates significantly different from Col-0 (P < 0.05) by ANOVA test. ND, Not detected.
|
FA Composition |
||||||||
|---|---|---|---|---|---|---|---|---|
| Lipid Class | Seed Type | 16:0 | 16:3n3 | 18:0 | 18:1n9c | 18:2n6c | 18:3n3 | 20:1n9 |
| % | ||||||||
| DAG | Col-0 | 19.19 ± 1.66 | ND | ND | 3.62 ± 0.19 | 42.02 ± 1.01 | 33.60 ± 2.12 | 1.57 ± 0.48 |
| pxa1 | 7.92 ± 0.39 | ND | ND | 6.23 ± 1.19 | 40.95 ± 1.10 | 35.36 ± 1.81 | 9.54 ± 0.90* | |
| Phosphatidylcholine | Col-0 | 26.29 ± 2.28 | 0.38 ± 0.18 | 7.82 ± 3.92 | 5.34 ± 0.64 | 31.85 ± 1.62 | 17.63 ± 0.82 | 2.74 ± 0.24 |
| pxa1 | 19.17 ± 1.55 | 0.16 ± 0.11 | 9.32 ± 2.66 | 7.54 ± 0.54 | 28.18 ± 1.50 | 17.74 ± 1.40 | 10.14 ± 1.14* | |
| Phosphatidylethanolamine | Col-0 | 27.42 ± 1.59 | 1.07 ± 0.45 | 5.63 ± 0.56 | 2.17 ± 0.11 | 31.13 ± 1.62 | 12.66 ± 0.33 | 2.27 ± 0.28 |
| pxa1 | 21.82 ± 0.99 | 0.14 ± 0.21 | 9.20 ± 1.19 | 2.52 ± 0.25 | 31.63 ± 0.86 | 11.72 ± 0.52 | 6.10 ± 0.37* | |
| Phosphatidic acid | Col-0 | 24.83 ± 1.88 | 0.45 ± 0.39 | 6.97 ± 0.86 | 4.63 ± 0.12 | 31.77 ± 0.12 | 15.43 ± 0.55 | 2.83 ± 0.35 |
| pxa1 | 17.65 ± 1.50 | 0.15 ± 0.04 | 8.39 ± 1.25 | 6.18 ± 0.61 | 29.71 ± 1.01 | 17.58 ± 1.16 | 11.04 ± 0.49* | |
| Digalactosyldiacylgalactolipid | Col-0 | 13.45 ± 2.25 | 1.43 ± 0.42 | 4.48 ± 1.26 | 1.32 ± 0.17 | 3.62 ± 0.13 | 57.88 ± 6.14 | ND |
| pxa1 | 11.35 ± 1.70 | 0.73 ± 0.18 | 5.85 ± 3.07 | 0.52 ± 0.14 | 1.89 ± 0.05 | 61.58 ± 6.75 | ND | |
| Monogalactosyldiacylgalactolipid | Col-0 | 2.95 ± 0.50 | 18.78 ± 1.27 | 1.28 ± 0.35 | 1.17 ± 0.18 | 4.25 ± 0.22 | 66.04 ± 3.62 | ND |
| pxa1 | 3.15 ± 0.28 | 7.51 ± 0.44 | 2.26 ± 0.74 | 0.38 ± 0.06 | 2.56 ± 0.18 | 72.19 ± 0.88 | ND | |
In Arabidopsis, DGAT1 has been described as the main enzyme responsible for TAG synthesis and accumulation during seed development (Lu et al., 2003; Lung and Weselake, 2006) and 20:1 is specifically found in TAG (Lemieux et al., 1990). However, our results imply that in pxa1 germinating seeds, where FA are released from TAG and activated to acyl-CoA but not catabolized via β-oxidation, 20:1-CoA is transferred to microsomal lipids, while 18:2-CoA and 18:3-CoA are preferentially incorporated into TAG. This finding suggests that an alternative pathway that prefers 18:2 and 18:3 over 20:1 for TAG synthesis is operating during pxa1 seedling establishment.
To further investigate the putative alternative pathway for TAG synthesis during pxa1 seedling establishment, we performed a transcriptomic study of Col-0 and pxa1-1 germinating seeds at 12 h after imbibition (HAI) and 5-DAI seedlings using the Affymetrix ATH1 microarray. Table II shows the expression levels of genes identified to be involved in lipid synthesis and catabolism (Li-Beisson et al., 2010). Only three genes show a greater than 2-fold change in pxa1 compared with Col-0: LPEAT2, ACX1, and DGAT3. Of these the DGAT3 gene (At1g48300) is potentially interesting since it shows greater homology to a peanut cDGAT, than to AtDGAT1 or AtDGAT2 (Supplemental Fig. S1). Quantitative real-time (qRT)-PCR analysis showed that while DGAT1, DGAT2, and PDAT1 gene expression levels in pxa1 were similar to Col-0 during seed germination and seedling establishment, DGAT3 expression in pxa1 12-HAI seeds was 2-fold more abundant than in Col-0 (Supplemental Fig. S2). These data suggest that DGAT3 could have a role in an alternative pathway for TAG synthesis during pxa1 seedling establishment. Analysis of publicly available Affymetrix data revealed DGAT3 expression throughout seed development and seedling establishment in Col-0 (Supplemental Fig. S3A). Although DGAT1 showed the highest expression levels in developing seeds during the period of maximum TAG accumulation, DGAT3 expression levels were higher than DGAT1, DGAT2, or PDAT1 during seed germination and the latter stages of seedling establishment. Therefore it is possible that in pxa1 the block in the import of FA into the peroxisome leads to an increase of acyl-CoA substrate for the putative DGAT3, and the incorporation of acyl-CoA into TAG, with a preference for 18:2 and 18:3 over 20:1. We used the Genevestigator development tool (Hruz et al., 2008) to compare the expression levels of DGAT1, DGAT2, DGAT3, and PDAT1 in Affymetrix datasets in the public domain. We found that DGAT3 shows strong expression across 10 development stages that is consistently higher than the other three genes apart from the senescence stage, where DGAT1 is more strongly expressed (Supplemental Fig. S3B).
Table II. Expression of lipid synthesis and mobilization-related genes in 12-HAI seeds and 5-DAI seedlings using Affymetrix ATH1 microarray data.
AGI, Arabidopsis Genome Initiative.
| Average Signal Value |
Fold Change |
||||||
|---|---|---|---|---|---|---|---|
| AGI Code | Name | Col-0 12-HAI | pxa1 12-HAI | Col-0 5-DAI | pxa1 5-DAI | 12-HAI | 5-DAI |
| At1g12640 | LPLAT1 | 455.4 ± 90.3 | 743.8 ± 83.8 | 675.7 ± 50.8 | 609.9 ± 41.3 | 1.63 | 0.90 |
| At1g63050 | LPLAT2 | 1,587.4 ± 451.7 | 867.2 ± 128.4 | 616.9 ± 36.9 | 522.5 ± 124.9 | 0.55 | 0.85 |
| At1g80950 | LPEAT1 | 1,604.9 ± 100.2 | 1,603.6 ± 120.4 | 837.2 ± 123.6 | 773.4 ± 63.5 | 1.00 | 0.92 |
| At2g45670 | LPEAT2 | 55.7 ± 11.3 | 198.7 ± 30.3 | 308.6 ± 65.2 | 329.6 ± 32 | 3.57 | 1.07 |
| At3g12120 | FAD2 | 4,060.1 ± 245.4 | 7,008 ± 1,098.8 | 8,598.6 ± 1,206.7 | 8,021.5 ± 190.8 | 1.73 | 0.93 |
| At2g29980 | FAD3 | 3,955.9 ± 283.1 | 2,862.5 ± 1,789.7 | 902.2 ± 175.3 | 1,003.3 ± 56.5 | 0.72 | 1.11 |
| At3g57650 | LPAAT2 | 5275.5 ± 623.2 | 3,285.4 ± 930.5 | 2,517.9 ± 24.4 | 2,672.4 ± 31.5 | 0.62 | 1.06 |
| At1g51260 | LPAAT3 | 3.5 ± 1.2 | 3.4 ± 0.9 | 60.8 ± 23.3 | 14.5 ± 10.8 | 0.96 | 0.24 |
| At1g75020a | LPAAT4 | 285 ± 33.1 | 279 ± 26.6 | 270.2 ± 51.4 | 309.1 ± 52.3 | 0.98 | 1.14 |
| At3g18850 | LPAAT5 | 83.0 ± 14.6 | 35.9 ± 16.5 | 331.9 ± 22.9 | 178.8 ± 20.3 | 0.43 | 0.54 |
| At2g19450 | DGAT1 | 1,672.0 ± 102.4 | 1,939.2 ± 382.7 | 252.2 ± 81.3 | 199.2 ± 44.8 | 1.16 | 0.79 |
| At3g51520 | DGAT2 | 494.8 ± 64.1 | 582.9 ± 81.4 | 1,244.3 ± 81.2 | 1,116.6 ± 14.1 | 1.18 | 0.90 |
| At1g48300 | DGAT3 | 2,937.5 ± 525.4 | 7,057.7 ± 208.3 | 2,385 ± 333.6 | 2,201.1 ± 234.2 | 2.40 | 0.92 |
| At5g13640 | PDAT1 | 514.5 ± 36.8 | 415.3 ± 35.8 | 856.2 ± 20.3 | 753 ± 52.7 | 0.81 | 0.88 |
| At5g04040 | SDP1 | 2,618.5 ± 62.2 | 3,432.3 ± 1,417.3 | 524.8 ± 37.3 | 535.5 ± 44.9 | 1.31 | 1.02 |
| At3g57140 | SDP1-like | 303.6 ± 84 | 205.1 ± 52.2 | 43.2 ± 34.1 | 14.5 ± 9.8 | 0.68 | 0.34 |
| At3g05970 | LACS6 | 3,883.5 ± 135.5 | 4,030.3 ± 396.9 | 661.1 ± 40.5 | 703.4 ± 95.2 | 1.04 | 1.06 |
| At5g27600 | LACS7 | 2,737.4 ± 174.6 | 2,338 ± 352.5 | 272.8 ± 43.6 | 297.3 ± 71.1 | 0.85 | 1.09 |
| At4g16760 | ACX1 | 2,112.3 ± 152.3 | 4,655 ± 1,440.7 | 1,142.2 ± 57.6 | 1,333.5 ± 97.1 | 2.20 | 1.17 |
| At5g65110 | ACX2 | 6,873.6 ± 570.2 | 6,129.4 ± 589 | 1,213 ± 188.8 | 1,382.6 ± 383.1 | 0.89 | 1.14 |
| At1g06290 | ACX3 | 3,057.9 ± 381.6 | 3,821.4 ± 386.7 | 840.6 ± 123 | 988.8 ± 228.9 | 1.25 | 1.18 |
| At3g51840 | ACX4 | 3,345.4 ± 132.1 | 5,761.4 ± 1,340.8 | 2,509.6 ± 180.5 | 2,303.2 ± 298.5 | 1.72 | 0.92 |
| At3g06860 | MFP2 | 12,811.6 ± 870.2 | 12,611.5 ± 891.8 | 909.2 ± 183.4 | 1,288 ± 285.6 | 0.98 | 1.42 |
| At2g33150 | KAT2 | 17,323.7 ± 1,055.1 | 19,731.8 ± 2,221.9 | 4,485.6 ± 545.3 | 5,040.1 ± 25.5 | 1.14 | 1.12 |
A Novel Cytosolic DGAT Is Encoded by At1g48300
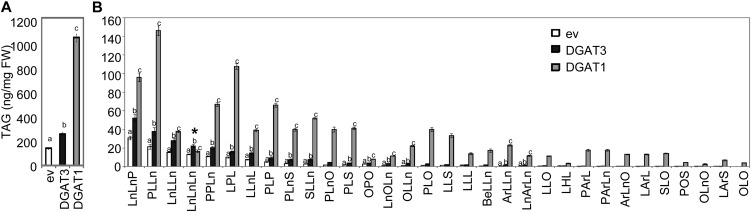
To test whether the putative DGAT3 gene encodes for a cytosolic DGAT, we performed Agrobacterium-mediated transient expression in Nicotiana benthamiana leaves, using AtDGAT1 as a positive control. Five days after Agrobacterium infiltration, leaves were sampled and TAGs isolated and analyzed directly from fresh-frozen tissue. The results showed a significant increase in TAG content in N. benthamiana leaves expressing either DGAT3 or DGAT1 genes, compared with leaves from the empty vector (ev) negative control (Fig. 5A). These results support our homology-based assumption that the DGAT3 gene (At1g48300) encodes a DGAT enzyme, although its overall activity appears lower than DGAT1. Trilinolenin stood out in this analysis as the only TAG accumulating to higher levels in DGAT3 than DGAT1 (Fig. 5B). Indeed, TAG composition analysis revealed an enrichment of TAGs containing 18:3 for DGAT3 compared with DGAT1, with the latter appearing to preferentially incorporate FA other than 18:3 into TAG (Fig. 5B).
Figure 5.
Total TAG content (A) and composition (B) of N. benthamiana leaves transiently expressing DGAT1 or DGAT3 compared with the ev negative control. Values are means ± sd of measurements from five samples of approximately 40 mg leaf tissue. Total TAGs (A) were analyzed by ANOVA followed by pairwise t tests with Bonferroni correction; significantly different groupings (P < 0.05) are indicated by letters above the bars. For TAG composition (B), the same tests were performed for each individual TAG; only those species where DGAT3 was significantly different from the ev control are labeled. The triolein TAG species denoted by an asterisk is the only one that is higher in DGAT3 compared with DGAT1.
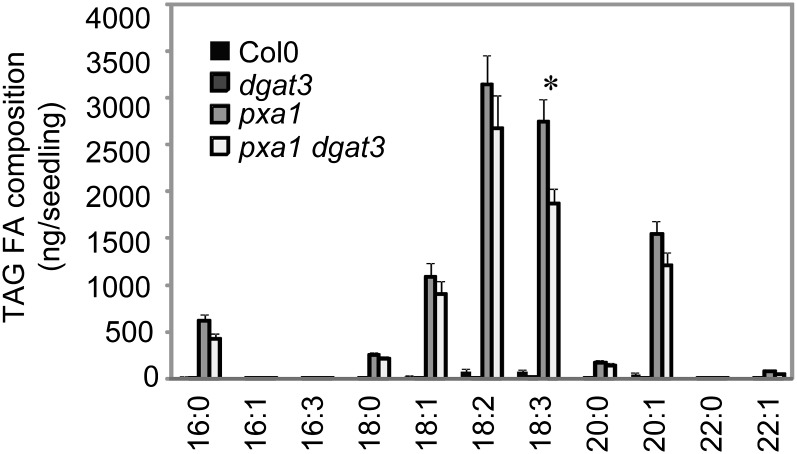
We obtained, from the Nottingham Arabidopsis Stock Centre, an Arabidopsis GABI-Kat insertion line (GABI_696F08) that carries a T-DNA at the position 336 of the DGAT3 open reading frame. Homozygous plants were obtained by self pollination and confirmed by PCR. No obvious morphological or developmental differences compared with Col-0 were observed under standard growth room conditions. Quantification of TAG, DAG, and galactolipids in DS and 5-DAI Suc-grown seedlings of this mutant, which we now refer to as dgat3, showed no differences compared with Col-0 (Supplemental Fig. S4). However, compared with pxa1, a pxa1dgat3 double mutant did show a significant decrease in the amount of 18:3 FA derived from TAGs in 5-DAI Suc-grown seedlings (Fig. 6), providing further evidence that DGAT3 contributes to recycling 18:3 into TAG.
Figure 6.
FA composition calculated from TAG in Col-0, dgat3, pxa1, and pxa1dgat3 5-DAI seedlings. Values are means ± sd of measurements from five separate batches of 30 seedlings. Significant differences (P < 0.05) between pxa1 and pxa1dgat3 are indicated by an asterisk.
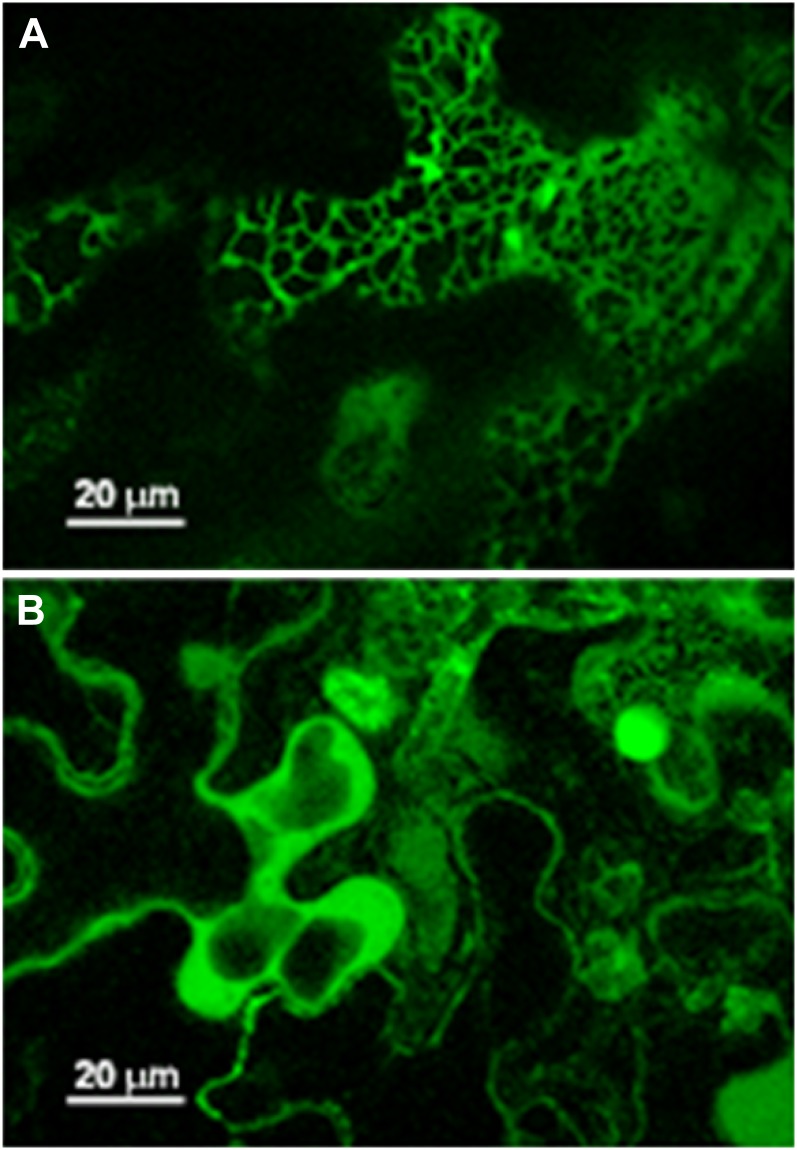
To compare the localization of DGAT3 with that of DGAT1, which is known to be localized to the endoplasmic reticulum (Browse and Somerville, 1991), the genes were cloned and fused at their C termini with GFP. The fused genes were transiently expressed in N. benthamiana leaves, which were analyzed for fluorescence patterns using confocal microscopy. As shown in Figure 7, the transiently expressed gene products presented a different pattern. While leaves expressing DGAT1-GFP displayed strong fluorescence that localized to a reticular network characteristic of the endoplasmic reticulum, the more diffuse fluorescence pattern observed for the transiently expressed DGAT3-GFP can be taken as evidence that this enzyme is mainly cytosolic. There is precedence for this endoplasmic reticulum versus cytosolic labeling pattern in N. benthamiana leaves (Grefen et al., 2008).
Figure 7.
Subcellular localization of DGAT1-GFP (A) and DGAT3-GFP (B) fusion proteins by transient expression in N. benthamiana leaves. Scale as indicated.
These results indicate that there is a cytosolic route for TAG synthesis, where the DGAT3, encoded by At1g48300, preferentially incorporates 18:3 and to a lesser extent 18:2 into TAG.
DISCUSSION
Storage oil mobilization during seed germination involves the action of TAG lipases, FA transport across the peroxisome membrane, and peroxisomal FA β-oxidation to produce acetyl-CoA, which ultimately provides the carbon skeletons and energy necessary to drive postgerminative growth (Graham, 2008). It is well established that Arabidopsis mutants disrupted in lipolysis, FA transport into the peroxisome, and peroxisomal β-oxidation are unable to catabolize TAG and require exogenous Suc for postgerminative growth and seedling establishment (Hayashi et al., 1998; Eastmond, 2006). This article investigates how the disruption in different steps in the storage oil mobilization process affects not only overall TAG levels, but also TAG composition.
For this purpose, we analyzed Arabidopsis mutants with defects in TAG and FA breakdown during seed germination and seedling establishment. All the mutants studied were severely impaired in their ability to catabolize TAG as reported previously (Germain et al., 2001; Zolman et al., 2001; Pinfield-Wells et al., 2005; Eastmond, 2006; Rylott et al., 2006). Among the various mutants analyzed we only observed a slight decrease in TAG levels in mfp2 seedlings grown for 5 d on Suc compared with DS. This partial block in TAG breakdown in mfp2 is likely because additional hydratases and dehydrogenases are contributing to MFP activity during seedling establishment (Rylott et al., 2006). In sdp1 the patatin-like TAG lipase associated with oil body membranes is blocked, and therefore TAGs are not hydrolyzed (Eastmond, 2006). In the pxa1 and β-oxidation mutants FA breakdown is impaired due to FA transport into the peroxisome or FA β-oxidation inside the peroxisome being disrupted, respectively.
Inhibition of lipolysis is one obvious explanation for the persistence of TAGs in Suc-rescued mutant seedlings. TAG-lipase inhibition has been reported previously by Eastmond (2007) in the sdp2 mutant, which is disrupted in the peroxisomal membrane isoform of monodehydroascorbate reductase (MDAR4). The main role of MDAR4 appears to be to prevent hydrogen peroxide from escaping beyond the outer surface of the peroxisomal membrane; the consequence of hydrogen peroxide escape being inactivation of SDP1. While we cannot rule out some degree of posttranscriptional feedback inhibition at the level of lipolysis of TAGs, our results clearly demonstrate that available FA are being actively sequestered into TAG in Suc-rescued mutant seedlings.
The first indication that de novo TAG synthesis is occurring during postgerminative seedling growth on Suc came from our finding that TAG levels actually increased in 5-DAI seedlings compared with DS in both the sdp1 and acx1acx2 mutants (Fig. 1A). This TAG synthesis may use a combination of FA and acyl-CoA substrates made available from partially catabolized TAGs and turnover of other glycerolipids, and de novo FA synthesis. Suc has been shown to induce DGAT activity in cell suspension cultures of Brassica napus, with relatively little alteration in FA composition (Weselake et al., 1998; Nykiforuk et al., 2002). In addition, Glc has been reported to up-regulate DGAT1 gene expression levels when Arabidopsis seedlings are grown on supplemented medium (Lu et al., 2003). Sugar-induced TAG synthesis could be operating here but it is not clear why the effect is specific to sdp1 and acx1acx2 and is not seen in pxa1 and kat2-2 that are similarly blocked in FA breakdown.
Evidence for TAG recycling comes from the modifications that we see in pxa1 5-DAI seedling TAG composition, which indicates that FAs are released from and reincorporated into TAG during seed germination and postgerminative growth, possibly with additional de novo synthesized 18:2 and 18:3 FA. Although long-chain acyl-CoA levels were increased in pxa1 and the β-oxidation mutants we did not observe evidence, in the form of an altered TAG composition, for FA recycling in the latter. This result could be explained by the assumption that β-oxidation mutants accumulate acyl-CoAs inside the peroxisome, and therefore they are not available for TAG synthesis, while pxa1 accumulates acyl-CoAs in the cytosol. Graham and Eastmond (2002) proposed that the increase in peroxisome size in ped1 and kat2-2 mutants might be due specifically to a peroxisomal accumulation of acyl-CoAs. This is in agreement with Pinfield-Wells et al. (2005) and Rylott et al. (2006), who observed an accumulation of long-chain acyl-CoAs and an increase in peroxisome size in acx1acx2 and mfp2 seedlings, respectively. On the other hand, the peroxisome size and structure remains normal in cts and ped3 seedlings (Footitt et al., 2002; Hayashi et al., 2002), which are allelic to pxa1, further supporting the assumption that accumulation of acyl-CoA in pxa1 occurs in the cytosol, where they are available for TAG synthesis.
The most striking observation with regard to specificity is that 18:3, and to a lesser extent 18:2, are preferentially incorporated into TAG in pxa1 seedlings, whereas the acyl-CoA pool in the cytosol is enriched in 20:1 acyl-CoA. Interestingly, this 20:1 acyl-CoA is instead incorporated into microsomal lipids, which are increased in pxa1 seedlings compared with Col-0. One possible explanation for this could be that in pxa1 seedlings an enzyme that prefers 18:3 over 20:1 is actively incorporating cytosolic acyl-CoAs into TAG. Lung and Weselake (2006) described that DGAT1 is the main enzyme responsible for TAG synthesis during seed development, with DGAT1 gene expression peaking during the period of maximum TAG accumulation. However, DGAT1 gene expression is not restricted to developing seeds as it is also expressed albeit at lower levels during seed germination and seedling establishment and it has been shown to be involved in the production of TAG in mature leaf tissue (Slocombe et al., 2009). In Arabidopsis, 20:1 is specifically found in storage TAG in seeds (Lemieux et al., 1990), indicating that DGAT1 preferentially incorporates these FAs into TAG. In addition, in as11, which carries an ethyl methanesulfonate mutation in the TAG1 gene, the reduction in TAG accumulation during seed development is accompanied by decreased 20:1 and 18:1 levels and an increase in 18:3 (Katavic et al., 1995). In agreement with these results, Andrianov et al. (2010) reported that the overexpression of AtDGAT1 in tobacco (Nicotiana tabacum) leaves leads to an increase in TAG content and a shift in the FA composition, with an increase in 18:1 and a decrease in 18:3 levels. Taken together, these data suggest that an alternative pathway for TAG synthesis is responsible for the incorporation of 18:3 into TAG, since DGAT1 shows a preference for FAs other than 18:3.
Transcriptomic analysis in Col-0 and pxa1 revealed an up-regulation of the AtDGAT3 (At1g48300) gene in 12-HAI seeds of pxa1. This gene is a homolog of the gene encoding the cytosolic DGAT from peanut (Saha et al., 2006) and is therefore a candidate for involvement in partitioning acyl-CoAs to TAG in pxa1 seedlings. In peanut, the cytosolic DGAT catalyzes the acylation of sn-1,2 DAG (Saha et al., 2006). AtDGAT3 is expressed in Col-0 germinating seed and young seedlings at higher levels than other genes involved in TAG synthesis, such as DGAT1, DGAT2, and PDAT1. In addition, we observed that the transiently expressed cytosolic DGAT in N. benthamiana leaves has higher preference for 18:2 and 18:3, the main FA in young seedlings, than DGAT1. Consistent with this, the pxa1dgat3 double mutant had significantly decreased levels of 18:3 in TAG compared with pxa1 in 5-d-old seedlings grown on sugar. The remaining levels of 18:3 in pxa1dgat3 were still significant, suggesting an additional pathway for incorporation into TAG is also working. Zhang et al. (2009) showed that PDAT1 is the gene responsible for most of the TAG synthesis in the dgat1-1 mutant, indicating that DGAT1 and PDAT1 have overlapping functions for TAG synthesis in seed and pollen of Arabidopsis. The fact that the dgat1-1 mutant showed a 20% to 30% decrease in oil content (Katavic et al., 1995) while no changes of oil in pdat1-1 were observed (Mhaske et al., 2005) might suggest that DGAT1 can completely compensate for the lack of PDAT1 function, whereas PDAT1 only partially complements the function of DGAT1 in developing seeds. Partial complementation of DGAT3 by PDAT1 could be occurring in the pxa1dgat3 double mutant, where PDAT1 would be responsible for the incorporation of 18:3 into TAG in germinating seeds and young seedlings. Despite numerous attempts, we were unable to isolate pdat1pxa1 double mutants by selfing plants that were either homozygous for pxa1 and heterozygous for pdat1 or vice versa. We previously showed that DGAT1 plays a major role in the partitioning of FAs to TAG in mature and senescing leaves of Col-0 and pxa1 plants (Slocombe et al., 2009). The double mutant disrupted in DGAT1 and PXA1 is severely compromised in vegetative growth, highlighting the negative effect that perturbation of these pathways can have on plant growth (Kunz et al., 2009; Slocombe et al., 2009). We have been able to uncover the in vivo function of DGAT3 because pxa1 seedlings accumulate acyl-CoAs and this together with an induction of DGAT3 expression, leads to the synthesis of TAG species containing 18:3 and 18:2 FAs. The incorporation of 20:1 into microsomal lipids in young seedlings of pxa1 suggests that DGAT1 activity is not operating as it does in developing seeds or mature leaves. It therefore appears that the relative contribution of the different routes for incorporation of FAs into TAG varies in different tissues.
Taken together these data allow us to propose an in vivo role for the cytosolic DGAT in recycling of 18:3 to TAG via a previously uncharacterized cytosolic pathway. The consistently high level of expression of DGAT3 across various developmental stages suggests a housekeeping function associated with regulating flux between the cytosolic acyl CoA pool and TAG. In young seedlings the biosynthetic machinery is directed primarily toward the production of membrane lipids. In such a case we propose that TAGs are involved as a dynamic fatty acyl pool with DGAT3 playing a role in regulating acyl-CoA pool size and composition in response to the needs of membrane lipid biosynthesis. Cytosolic oil droplets are well documented in the literature including for example a field survey of 302 angiosperm species, which found that 24% had conspicuous cytosolic oil droplets in leaves (Lersten et al., 2006) and we have also reported TAG present in oil droplets in Arabidopsis leaves (Slocombe et al., 2009). A role for cytosolic leaf TAG in carbon storage and/or membrane lipid remodeling has previously been proposed (Murphy and Parker, 1984; Murphy, 2001; Kaup et al., 2002; Lin and Oliver, 2008). Our data suggest a cytosolic pathway to TAG involving DGAT3 plays a key role in this important aspect of lipid metabolism.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type Arabidopsis (Arabidopsis thaliana) ecotype Col-0 and mutant (Col-0 background) seeds were surface sterilized and germinated in one-half-strength Murashige and Skoog medium (Murashige and Skoog, 1962) containing 1% (w/v) agar and 20 mm Suc. After a cold treatment of 72 h at 4°C in the dark, plates were transferred to a growth room at 20°C with continuous light (70 μm m−2 s−1). Twelve hours after imbibition seeds and 5-d-old seedlings were harvested, frozen with liquid nitrogen, and stored at −80°C. sdp1 and kat2 in the Col-0 background (herein referred to as kat2-2) were isolated in a sugar-dependent screen (Eastmond, 2006). pxa1-1 and acx1-2acx2-1 seeds were kindly donated by Dr. Bonnie Bartel and mfp2 was obtained from the Salk collection (http://signal.salk.edu/cgi-bin/tdnaexpress). dgat3 is a GABI-kat insertion line (GABI_696F08) obtained from Nottingham Arabidopsis Stock Centre, which carries a T-DNA in the At1g48300 gene. The homozygous pxa1-1dgat3 double mutant was confirmed for the pxa1 mutation by the pxa1 phenotype of failure to undergo successful seedling establishment without Suc (Zolman et al., 2001) and for the dgat3 mutation by PCR using DGAT3-specific primers LH9 and LH10 (Supplemental Table S1) and T-DNA-specific primer GABI-left: 5′-CCCATTTGGACGTGAATGTAGACAC-3′.
Lipid Analysis
Lipid extraction and neutral lipid analysis by liquid chromatography/tandem mass spectrometry were performed as previously described (Burgal et al., 2008). Polar lipids were separated by two-dimensional thin layer chromatography (Hernández et al., 2008). FA methyl esters of individual lipids classes were produced by acyl-catalyzed transmethylation (Browse et al., 1986) and analyzed by gas chromatography with flame ionization detection (GC8000 Top, Thermoquest Separation Products), fitted with a 30-m-long 0.25-mm ID SGE BPX70 column (SGE). Helio was used as a carrier gas at 1 mL min−1 with a 30:1 split ratio. The oven was run isothermally at 110°C for 1 min, then ramped to 180°C at 20°C min−1 then to 221°C at 2.5°C min−1.
The acyl-CoA profile was measured using the method of Larson and Graham (2001) with modifications described in Larson et al. (2002).
RNA Purification and cDNA Synthesis
Total RNA isolation from dry and imbibed seeds was performed using solutions previously treated with diethyl pyrocarbonate to inhibit RNases. Approximately 200 seeds were ground with liquid nitrogen using a blue pestle. After homogenization, 150 μL of extraction buffer (0.2 m sodium borate decahydrate, 30 mm EGTA, 1% [w/v] SDS, and 1% [w/v] sodium deoxycholate), 10 mm dithiothreitol, 2% (w/v) polyvinyl pyrrolidone, and 1% (v/v) IGEPAL were added. After adding 6 μL of proteinase K (Roche Diagnostics), samples were mixed and incubated at 42°C for 90 min. After the incubation, 12 μL of 2 m KCl were added, samples were mixed, and incubated on ice for 60 min. To remove debris samples were centrifuged at 15,000g for 20 min at 4°C. The supernatant was transferred to a fresh tube and 54 μL of 8 m LiCl were added. Samples were mixed and incubated at −20°C for 3 h. After the incubation, samples were centrifuged at 15,000g for 20 min at 4°C and the RNA pellet was dissolved in 100 μL RNase-free water. RNA was purified by RNeasy plant mini kit (QIAGEN).
Total RNA was isolated from 5-d-old seedling using the RNeasy plant mini kit (QIAGEN).
The quality of RNA was verified by demonstration of intact ribosomal bands following agarose gel electrophoresis in addition to the absorbance ratios (A260/280 and A260/030). Contaminating DNA was removed from RNA samples (1 μg) using the TURBO DNA-free kit (Ambion). First-strand cDNA was synthesized from 0.5 μg DNA-free total RNA using the SuperScript III first-strand synthesis system (Invitrogen) with oligo(dT)20 primer, following the manufacturer’s instructions.
Affymetrix Genechip Experiment and Data Analysis
Isolated RNA was used for cDNA synthesis and biotin-modified RNA amplification using the MessageAmp III RNA amplification kit (Ambion). Three biological replicates per sample were hybridized independently to the Affymetrix ATH1 array, washed, stained, and scanned following the procedures described in the Affymetrix technical manual. The expression levels of genes were measured by signal intensities using the Micro Array Suite 5.0 software with a target signal of 500. Public domain Affymetrix ATH1 data sets were obtained from NascArrays (http://affymetrix.arabidopsis.info/narrays/experimentbrowse.pl) and The Arabidopsis Information Resource (http://www.arabidopsis.org/). The Genevestigator V4 classic metaprofile analysis tool (available at https://www.genevestigator.com) was used to compare expression levels of DGAT1 (At2g19450), DGAT2 (At3g51520), DGAT3 (At1g48300), and PDAT1 (At5g13640) across 10 different developmental stages.
qRT-PCR
Gene expression analysis was performed by qRT-PCR using an ABI Prism 7000 thermal cycler and the SYBR-green PCR master mix (Applied Biosystems). Primers for gene-specific amplification (Supplemental Table S1) were designed using the Primer3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) to generate a product of 100 to 200 bp, and to have a melting temperature of 60°C ± 1°C and a length of 19 to 23 bp. Reaction mix (25 μL per well) contained 1× SYBR-green PCR master mix, 400 nm forward and reverse primers, and 1 μL of cDNA diluted 10 times, which was selected according to the primers amplification efficiency. The thermal cycling conditions consisted of an initial denaturation step of 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The specificity of the PCR amplification was monitored by melting curve analysis following the final step of the PCR. PCR efficiencies (E) of all primers were calculated using dilution curves with four dilution points, and the equation E = [10(−1/slope)] − 1. The CITRATE SYNTHASE3 gene was used as the endogenous reference since it has similar levels of expression as the genes of interest in both the wild type and pxa1. The qRT-PCR data were calibrated relative to the corresponding gene expression level in Col-0, following the 2−ΔΔCt method for relative quantification (Livak and Schmittgen, 2001). The data are presented as means ± sd of three biological replicates, each having three replicates per plate.
Transient Expression in Nicotiana benthamiana
For Agrobacterium-mediated cauliflower mosaic virus 35S-driven transient expression, the DGAT3 and DGAT1 coding sequences were PCR amplified using the specific primers LH47, LH48, LH35, and LH36, respectively (Supplemental Table S1), and subcloned into the GATEWAY-compatible binary vector pH2GW7 (for TAG analysis) or pK7FWG2 (for subcellular localization; Karimi et al., 2002). The resulting constructs and the ev used as a control were introduced into Agrobacterium tumefaciens strain GV3101 by electroporation. Nicotiana benthamiana leaves were infiltrated with A. tumefaciens cultures (OD600 approximately 0.8) according to Voinnet et al. (2003) omitting the acetosyringone treatment. Samples were collected 5 d after infiltration, ground in liquid nitrogen, and stored at −80°C until TAG analysis. For subcellular localization, fresh leaf imaging was carried out on a Zeiss LSM 510 META laser-scanning confocal equipped with a Zeiss Axioplan 2 microscope (Carl Zeiss Ltd). Images were acquired using a Plan-Neofluar 20×/0.5 or a Plan-Apochromat 63×/1.4 oil immersion differential interference contrast objective. Imaging of GFP emission was performed by sequential scanning. GFP was excited with the 488-nm line of a 30-mW argon laser and the emission collected through a 505 to 530 bp emission filter. Images were taken at Nyquist resolution with eight line averaging.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Comparison of the deduced amino acid sequences of peanut DGAT3 and Arabidopsis DGAT1 (At2g19450), DGAT2 (At3g51520), and DGAT3 (At1g48300) genes.
Supplemental Figure S2. Quantitative gene expression profiles of TAG synthesis genes during seed germination and seedling establishment in pxa1-1 relative to Col-0.
Supplemental Figure S3. Affymetrix ATH-1 selected TAG synthesis gene expression levels in Col-0.
Supplemental Figure S4. Total TAG, DAG, and monogalactosyldiacylgalactolipid content from Col-0 and dgat3 DS and 5-DAI seedlings.
Supplemental Table S1. Locus names and sequences of primers pairs used for gene expression analysis by qRT-PCR in this study.
Supplementary Material
Acknowledgments
We thank Dr. Peter Eastmond for providing seeds of sdp1 and Dr. Bonnie Bartel for providing seeds of pxa1-1 and acx1-2acx2-1. We thank the University of York Department of Biology Technology Facility for assistance with confocal microscopy.
Glossary
- TAG
triacylglycerol
- FA
fatty acid
- DAG
diacylglycerol
- Col-0
Columbia-0
- DAI
d after imbibition
- DS
dry seed
- HAI
h after imbibition
- qRT
quantitative real-time
- ev
empty vector
References
- Adham AR, Zolman BK, Millius A, Bartel B. (2005) Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in β-oxidation. Plant J 41: 859–874 [DOI] [PubMed] [Google Scholar]
- Andrianov V, Borisjuk N, Pogrebnyak N, Brinker A, Dixon J, Spitsin S, Flynn J, Matyszczuk P, Andryszak K, Laurelli M, Golovkin M, Koprowski H. (2010) Tobacco as a production platform for biofuel: overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol J 8: 277–287 [DOI] [PubMed] [Google Scholar]
- Bouvier-Navé P, Benveniste P, Oelkers P, Sturley SL, Schaller H. (2000) Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferase. Eur J Biochem 267: 85–96 [DOI] [PubMed] [Google Scholar]
- Browse J, McCourt PJ, Somerville CR. (1986) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152: 141–145 [DOI] [PubMed] [Google Scholar]
- Browse J, Somerville C. (1991) Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol 42: 467–506 [Google Scholar]
- Burgal J, Shockey J, Lu C, Dyer J, Larson T, Graham I, Browse J. (2008) Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J 6: 819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Beevers H. (1969) β oxidation in glyoxysomes from castor bean endosperm. J Biol Chem 244: 3514–3520 [PubMed] [Google Scholar]
- Dahlqvist A, Stahl U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Stymne S. (2000) Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97: 6487–6492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrett TP, Benning C, Ohlrogge J. (2008) Plant triacylglycerols as feedstocks for the production of biofuels. Plant J 54: 593–607 [DOI] [PubMed] [Google Scholar]
- Dyer JM, Stymne S, Green AG, Carlsson AS. (2008) High-value oils from plants. Plant J 54: 640–655 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ. (2006) SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 18: 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ. (2007) MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell 19: 1376–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ, Graham IA. (2000) The multifunctional protein AtMFP2 is co-ordinately expressed with other genes of fatty acid β-oxidation during seed germination in Arabidopsis thaliana (L.) Heynh. Biochem Soc Trans 28: 95–99 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ, Hooks MA, Williams D, Lange P, Bechtold N, Sarrobert C, Nussaume L, Graham IA. (2000) Promoter trapping of a novel medium-chain acyl-CoA oxidase, which is induced transcriptionally during Arabidopsis seed germination. J Biol Chem 275: 34375–34381 [DOI] [PubMed] [Google Scholar]
- Footitt S, Slocombe SP, Larner V, Kurup S, Wu Y, Larson T, Graham I, Baker A, Holdsworth M. (2002) Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J 21: 2912–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda M, Schnurr J, Abbadi A, Heinz E, Browse J. (2004) Peroxisomal Acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell 16: 394–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain V, Rylott EL, Larson TR, Sherson SM, Bechtold N, Carde JP, Bryce JH, Graham IA, Smith SM. (2001) Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid β-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J 28: 1–12 [DOI] [PubMed] [Google Scholar]
- Graham IA. (2008) Seed storage oil mobilization. Annu Rev Plant Biol 59: 115–142 [DOI] [PubMed] [Google Scholar]
- Graham IA, Eastmond PJ. (2002) Pathways of straight and branched chain fatty acid catabolism in higher plants. Prog Lipid Res 41: 156–181 [DOI] [PubMed] [Google Scholar]
- Grefen C, Stadele K, Ruzicka K, Obrdlik P, Harter K, Horak J. (2008) Subcellular localization and in vivo interactions of the Arabidopsis thaliana ethylene receptor family members. Mol Plant 1: 308–320 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nito K, Takei-Hoshi R, Yagi M, Kondo M, Suenaga A, Yamaya T, Nishimura M. (2002) Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid beta-oxidation. Plant Cell Physiol 43: 1–11 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Nishimura M. (1998) 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell 10: 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández ML, Guschina IA, Martínez-Rivas JM, Mancha M, Harwood JL. (2008) The utilization and desaturation of oleate and linoleate during glycerolipid biosynthesis in olive (Olea europaea L.) callus cultures. J Exp Bot 59: 2425–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs DH, Lu C, Hills MJ. (1999) Cloning of a cDNA encoding diacylglycerol acyltransferase from Arabidopsis thaliana and its functional expression. FEBS Lett 452: 145–149 [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinforma 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AHC. (1992) Oil bodies and oleosins in seeds. Annu Rev Plant Physiol Plant Mol Biol 43: 177–200 [Google Scholar]
- Ichihara K, Takahashi T, Fujii S. (1988) Diacylglycerol acyltransferase in maturing safflower seeds: its influences on the fatty acid composition of triacylglycerol and on the rate of triacylglycerol synthesis. Biochim Biophys Acta 958: 125–129 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Katavic V, Reed DW, Taylor DC, Giblin EM, Barton DL, Zou J, Mackenzie SL, Covello PS, Kunst L. (1995) Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol 108: 399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup MT, Froese CD, Thompson JE. (2002) A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiol 129: 1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz HH, Scharnewski M, Feussner K, Feussner I, Flügge UI, Fulda M, Gierth M. (2009) The ABC transporter PXA1 and peroxisomal β-oxidation are vital for metabolism in mature leaves of Arabidopsis during extended darkness. Plant Cell 21: 2733–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardizabal KD, Mai JT, Wagner NW, Wyrick A, Voelker T, Hawkins DJ. (2001) DGAT2 is a new diacylglycerol acyltransferase gene family: purification, cloning, and expression in insect cells of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity. J Biol Chem 276: 38862–38869 [DOI] [PubMed] [Google Scholar]
- Larson TR, Edgell T, Byrne J, Dehesh K, Graham IA. (2002) Acyl CoA profiles of transgenic plants that accumulate medium-chain fatty acids indicate inefficient storage lipid synthesis in developing oilseeds. Plant J 32: 519–527 [DOI] [PubMed] [Google Scholar]
- Larson TR, Graham IA. (2001) Technical Advance: a novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J 25: 115–125 [DOI] [PubMed] [Google Scholar]
- Lemieux B, Miquel M, Somerville C, Browse J. (1990) Mutants of Arabidopsis with alterations in seed lipid fatty acid composition. Theor Appl Genet 80: 234–240 [DOI] [PubMed] [Google Scholar]
- Lersten NR, Czlapinski AR, Curtis JD, Freckmann R, Horner HT. (2006) Oil bodies in leaf mesophyll cells of angiosperms: overview and a selected survey. Am J Bot 93: 1731–1739 [DOI] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, Franke RB, Graham IA, et al. (2010) Acyl-lipid metabolism. The Arabidopsis Book 8: e0133, doi:10.1199/tab.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Oliver DJ. (2008) Role of triacylglycerols in leaves. Plant Sci 175: 233–237 [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lu CL, de Noyer SB, Hobbs DH, Kang J, Wen Y, Krachtus D, Hills MJ. (2003) Expression pattern of diacylglycerol acyltransferase-1, an enzyme involved in triacylglycerol biosynthesis, in Arabidopsis thaliana. Plant Mol Biol 52: 31–41 [DOI] [PubMed] [Google Scholar]
- Lung SC, Weselake RJ. (2006) Diacylglycerol acyltransferase: a key mediator of plant triacylglycerol synthesis. Lipids 41: 1073–1088 [DOI] [PubMed] [Google Scholar]
- Mancha M, Stymne S. (1997) Remodelling of triacylglycerols in microsomal preparations from developing castor bean (Ricinus comunis L.) endosperm. Planta 203: 51–57 [Google Scholar]
- Mhaske V, Beldjilali K, Ohlrogge J, Pollard M. (2005) Isolation and characterization of an Arabidopsis thaliana knockout line for phospholipid: diacylglycerol transacylase gene (At5g13640). Plant Physiol Biochem 43: 413–417 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15: 473–496 [Google Scholar]
- Murphy DJ. (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40: 325–438 [DOI] [PubMed] [Google Scholar]
- Murphy GJP, Parker ML. (1984) Lipid composition and carbon turnover of wheat leaf oleosomes. J Exp Bot 35: 348–355 [Google Scholar]
- Nykiforuk CL, Furukawa-Stoffer TL, Huff PW, Sarna M, Laroche A, Moloney MM, Weselake RJ. (2002) Characterization of cDNAs encoding diacylglycerol acyltransferase from cultures of Brassica napus and sucrose-mediated induction of enzyme biosynthesis. Biochim Biophys Acta 1580: 95–109 [DOI] [PubMed] [Google Scholar]
- Pinfield-Wells H, Rylott EL, Gilday AD, Graham S, Job K, Larson TR, Graham IA. (2005) Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J 43: 861–872 [DOI] [PubMed] [Google Scholar]
- Richmond TA, Bleecker AB. (1999) A defect in β-oxidation causes abnormal inflorescence development in Arabidopsis. Plant Cell 11: 1911–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routaboul JM, Benning C, Bechtold N, Caboche M, Lepiniec L. (1999) The TAG1 locus of Arabidopsis encodes for a diacylglycerol acyltransferase. Plant Physiol Biochem 37: 831–840 [DOI] [PubMed] [Google Scholar]
- Rylott EL, Eastmond PJ, Gilday AD, Slocombe SP, Larson TR, Baker A, Graham IA. (2006) The Arabidopsis thaliana multifunctional protein gene (MFP2) of peroxisomal β-oxidation is essential for seedling establishment. Plant J 45: 930–941 [DOI] [PubMed] [Google Scholar]
- Rylott EL, Rogers CA, Gilday AD, Edgell T, Larson TR, Graham IA. (2003) Arabidopsis mutants in short- and medium-chain acyl-CoA oxidase activities accumulate acyl-CoAs and reveal that fatty acid beta-oxidation is essential for embryo development. J Biol Chem 278: 21370–21377 [DOI] [PubMed] [Google Scholar]
- Saha S, Enugutti B, Rajakumari S, Rajasekharan R. (2006) Cytosolic triacylglycerol biosynthetic pathway in oilseeds. Molecular cloning and expression of peanut cytosolic diacylglycerol acyltransferase. Plant Physiol 141: 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe SP, Cornah J, Pinfield-Wells H, Soady K, Zhang Q, Gilday A, Dyer JM, Graham IA. (2009) Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol J 7: 694–703 [DOI] [PubMed] [Google Scholar]
- Slocombe SP, Piffanelli P, Fairbairn D, Bowra S, Hatzopoulos P, Tsiantis M, Murphy DJ. (1994) Temporal and tissue-specific regulation of a Brassica napus stearoyl-acyl carrier protein desaturase gene. Plant Physiol 104: 1167–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart K, Mancha M, Lenman M, Dahlqvist A, Stymne S. (1997) Triacylglycerols are synthesised and utilized by transacylation reactions in microsomal preparations of developing safflower (Carthamus tinctorius L.) seeds. Planta 203: 58–66 [Google Scholar]
- Stymne S, Stobart AK. (1987) Triacylglycerol biosynthesis. In PK Stumpf, ed, The Biochemistry of Plants, Vol 9. Academic Press, New York, pp 175–214
- Tumaney AW, Shekar S, Rajasekharan R. (2001) Identification, purification, and characterization of monoacylglycerol acyltransferase from developing peanut cotyledons. J Biol Chem 276: 10847–10852 [DOI] [PubMed] [Google Scholar]
- van Roermund CWT, Ijlst L, Majczak W, Waterham HR, Folkerts H, Wanders RJ, Hellingwerf KJ. (2012) Peroxisomal fatty acid uptake mechanism in Saccharomyces cerevisiae. J Biol Chem 287: 20144–20153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O, Rivas S, Mestre P, Baulcombe D. (2003) An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33: 949–956 [DOI] [PubMed] [Google Scholar]
- Weselake R. (2005) Storage lipids. In DJ Murphy, ed, Plant Lipid. Blackwell Publishing, Oxford, pp 162–206
- Weselake RJ, Byers SD, Davoren JM, Laroche A, Hodges DM, Pomeroy MK, Furukawa-Stoffer TL. (1998) Triacylglycerol biosynthesis and gene expression in microspore-derived cell suspension cultures of oilseed rape. J Exp Bot 49: 33–39 [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C. (1998) Lipids are required for directional pollen-tube growth. Nature 392: 818–821 [DOI] [PubMed] [Google Scholar]
- Zhang M, Fan J, Taylor DC, Ohlrogge JB. (2009) DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 21: 3885–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Xia Q, Dauk M, Shen W, Selvaraj G, Zou J. (2003) Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 15: 1872–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Silva ID, Bartel B. (2001) The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol 127: 1266–1278 [PMC free article] [PubMed] [Google Scholar]
- Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor DC. (1999) The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J 19: 645–653 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.