Abstract
A series of novel 4,4,5,5-tetramethyl-2-(4-substitutedstyrylphenyl)-1,3,2 dioxaborolane derivatives has been synthesized. 4-(4,4,5,5-tetramethyl-1,3,2-dioxaboratophenyl)-methyl triphenylphosphonium bromide (4) was treated with 3 equiv of tBuONa, various aldehydes in the presence of DMF, and stirred at room temperature 4–6 hrs to yield the corresponding boron containing stilbene derivatives in 71–94% yields. A one-pot protocol transformation has also been developed and used to synthesize boron-containing compounds. Several of them, including BF102 and BF175, have the effect of inhibiting lipogenesis by suppressing lipogenic gene expression in mammalian hepatocytes. Moreover, BF102 also inhibits cholesterol biosynthesis by suppressing HMG-CoA reductase gene expression in hepatocytes. Thus, BF102 is a potential lead for the next generation of lipid-lowering drugs.
Introduction
Heart disease is the number one cause of death in the United States. A high level of lipids in blood (hyperlipidemia) greatly increases the risk of heart disease. Thus, understanding the regulation of lipid metabolism is important to public health. Recent studies have emphasized that transcriptional control of lipogenic and cholesterogenic enzymes plays a pivotal role in regulating lipid metabolism1. Among the transcription factors that are known to stimulate lipid biosynthesis, the sterol regulatory element-binding proteins (SREBPs) transcription factors are considered as the “master” regulators, as they can critically activate the transcription of several rate-limiting enzymes, such as fatty acid synthase (FAS) and HMG-CoA reductase (HMGCR), in both fatty acid/triglyceride and cholesterol biosynthesis pathways2,3. In addition to genetic defects, environmental factors, such as diets, can modulate the functions of these regulators, causing changes in lipid metabolism through altering gene expression4. Moreover, gene transcription requires not only transcription factors that bind to the specific DNA sequences, but also transcriptional cofactors that can critically enhance or suppress the activities of transcription factors5. The effectiveness of statins, which inhibit HMGCR activity, in patients with hyperlipidemia suggests that blocking cholesterol biosynthesis is an effective approach to treat hyperlipidemia6.
The current limitations for treating hyperlipidemia are that: 1) The molecular mechanisms of lipid metabolism regulation are still poorly understood; and 2) Some patients with hyperlipidemia are not responsive to statins and the adverse effects of statins have been reported7. Therefore, it is necessary to develop better approaches for treating hyperlipidemia. So we undertook this project to develop novel boron-containing stilbene-based compounds and test their potential as lipogenic inhibitors. Our hypothesis to develop boron-containing stilbene derivatives is based on two expectations as follows.
First, it is expected that the boron atoms introduced into biologically active molecular frameworks may interact with a target protein not only through hydrogen bonds but also through covalent bonds, and this interaction would produce potent biological activity4,5. The use of boron atoms in pharmaceutical drug design possesses a high potential for discovery of new biological activity6. Among various boron compounds synthesized, much attention has been paid to boronic acid containing peptides such as Velcade and DPP-IV inhibitors7,8. In these boropeptides, a carboxylic acid has been replaced by a boronic acid group. These boron-containing compounds could become the basis for novel drugs for the treatment of heart disease and other health problems caused by dysregulation of lipid homeostasis.
Second, identification of interacting proteins could discover novel regulators in the pathways of lipid biosynthesis and thereby improve our basic knowledge on regulation of lipid homeostasis.
Results
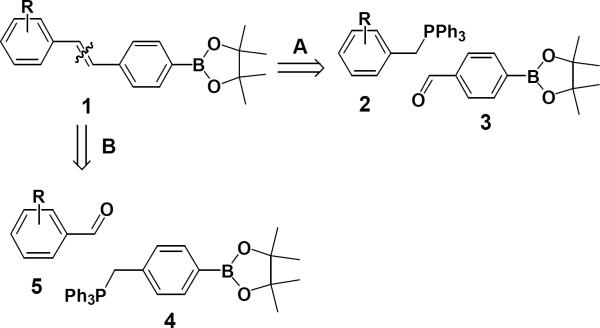
In the process of synthesizing boron-containing lipogenic inhibitors, we developed a synthetic methodology to synthesize boron-containing stilbene derivatives. The most familiar and general strategy for synthesis of boron-containing stilbenes 1 is based on disconnection A (Fig. 1) and involves the Wittig reaction of the various substituted benzyl phosphonium ylide 2 with the pinacol ester of boronate aldehyde 3 10.
Figure 1.
Retrosynthetic approach for pinacolylboronate-substituted stilbene derivatives 1.
To our surprise, literature search failed to uncover any examples of pinacol ester of boronato phosphonium ylide. We report herein the employment of this strategy to prepare novel pinacolylboronate -substituted stilbene derivatives. Although Wittig and Horner-Wadsworth-Emmons reactions have been carried out on aldehyde derivatives of boronate esters11,12,13, the problem with this approach A (Fig. 1) to synthesize boron-containing stilbene derivatives requires various substituted benzyl phosphonium ylides and boron containing aldehydes. Boron-containing aldehydes are very prone to self-dimerization, oxidation and decomposation during storage, so this method is limited in scope. To overcome this problem, we undertook the disconnection B approach (Fig. 1), envisaging the use of the pinacol ester of boronato phosphonium ylide 4, which has not previously been explored.
Compound 4 is stable in air, so different phenyl and alkyl aldehydes are easily available or can be derivatized to synthesize a library of boron-containing stilbene derivatives. Herein, we report the success of this new route to synthesize boron-containing stilbene derivatives based on disconnection B (Fig. 1).
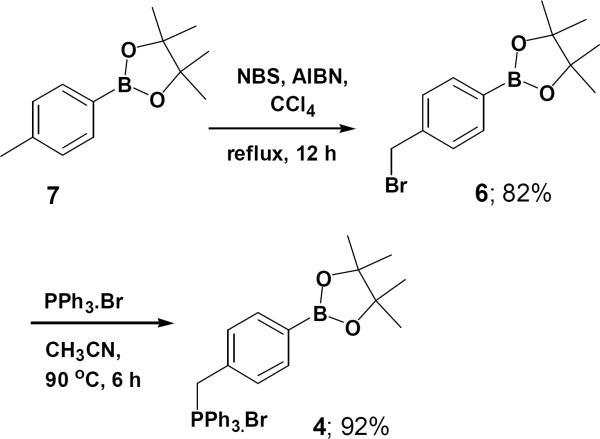
The preparation of pinacol ester of boronato phosphonium ylide 4, followed by the novel synthesis of pinacolylboronate-substituted stilbene derivatives via the direct Wittig bromide 4 from the corresponding 2-[40′-(bromomethyl) phenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 6 in the presence of 1.01 equiv of triphenylphosphine in acetonitrile at reflux condition (Fig. 2).
Figure 2.
Synthesis of 4-(4,4,5,5-tetramethyl-1,3,2-di oxaboratophenyl)-methyl triphenylphosphonium bromide 4.
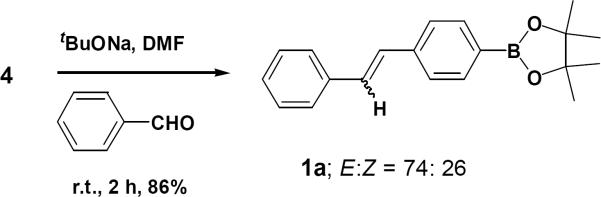
Compound 614 was prepared starting from 4,4,5,5-tetramethyl -2-p-tolyl-1,3,2-dioxaborolane 7, NBS and AIBN in carbon tetrachloride were refluxed for 12 hours. In our initial attempt 4-(4,4,5,5-tetramethyl-1,3,2-dioxaboratophenyl)-methyltriphenylphosphonium bromide 4 was isolated as a white solid in 92% yield. The minor excess of PPh3 was removed from the product by trituration with ether 2–3 times and the product was found to be stable under normal atmospheric conditions. Subsequently, we optimized the Wittig reaction of the ylide derived from this salt using benzaldehyde (Fig. 2).
It is noteworthy that the three equivalents of sodium tert-butoxide in DMF at room temperature led to the highest yield of 1a (Fig. 3). With this optimized condition in hand, we examined the scope of the Wittig reaction for the synthesis of pinacolylboronate -substituted stilbenes using various aryl aldehydes. Detail experimental procedures and analytical data's are presented in reference16,17.
Figure 3.
Proposed synthesis of stibene 1.
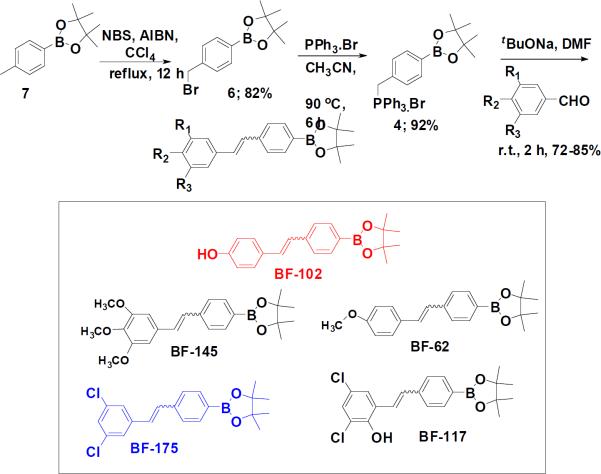
After generalizing the reaction conditions, we synthesized a small library of pinacolatoester substituted stilbene derivatives (Fig. 4). It is noteworthy to mention that, unlike the commonly employed approach of high throughput screening of large libraries to identify lead compounds that elicit a desired phenotypic effect, we are utilizing a Limited Rational Design approach. That means rather than screening 10,000–100,000 arrayed compounds we will generate a small library of only about 50 or less compounds to identify lead compounds. We have perfected this strategy, and used our ongoing chemical biology project for biomarker identification, and developing new therapeutic and diagnostic agents 8–16.
Figure 4.
A small library of boron-containing compounds.
We then tested this small library of boron -containing stilbene derivatives for their biological activity as lipogenic inhibitors using the mRNA level of FAS as the readout. Treatment of HepG2, a line of human hepatoma cells, with some of the compounds at 30μM resulted in a significant decrease of FAS gene expression as measured by quantitative RTPCR (qRT-PCR) (Fig. 5A), although some compounds, such as BF62, were inactive in this assay (Fig. 5A). We chose two compounds, BF102 and BF175, for further analysis, as they appeared to be less toxic to HepG2 cells at the concentrations tested (data not shown).
Figure 5.
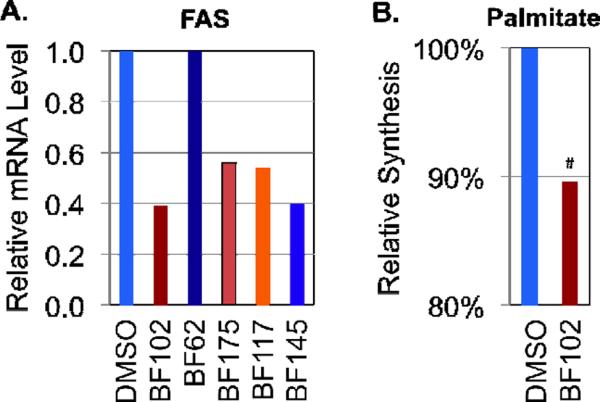
Effects of boron-containing compounds on lipogenic gene expression and de novo lipogenesis. A. Relative mRNA levels of fatty acid synthase (FAS) (detected by quantitative RT-PCR) in HepG2 cells treated with 30μM of the indicated compounds for 6 hours. Cyclophilin B was the invariant control. B. Relative synthesis rate of palmitate (detected by the deuterium enrichment method) in FAO cells after 12 hours of treatment with BF102 (20μM). Data are the averages of three independent samples. #p<0.001 vs DMSO (n=3).
Since FAS is a rate-limiting enzyme in de novo lipogenesis, we decided to determine whether inhibition of FAS gene expression by BF102 could result in a decrease of insulin-induced fatty acid synthesis. Consistent with our gene expression results, using the deuterium enrichment method followed by gas chromatography followed by mass spectrometry (GC/MS), we found that 20μM of BF102 can significantly decrease the synthesis rate of palmitate, the product of FAS, in rat hepatocytes, FAO cells in the presence of 100 nM insulin (Fig. 5B). Thus, BF102 can inhibit de novo lipogenesis.
Next, we wish to test whether BF102 also affect the cholesterol biosynthesis pathway. Using qRT-PCR, we found that BF102 treatment can dose-dependently decrease the mRNA levels of HMGCR, a rate-limiting enzyme for cholesterol biosynthesis and the target of well-known cholesterol lowering drug statins, in HepG2 cells, while BF62 had no effect at all (Fig. 6A and data not shown). Using the deuterium enrichment method followed by GC/MS, we found that 20μM of BF102 can also significantly decrease the synthesis rate of cholesterol in FAO cells induced by 100 nM insulin (Fig. 6B). Thus, BF102 can inhibit de novo cholesterol synthesis.
Figure 6.
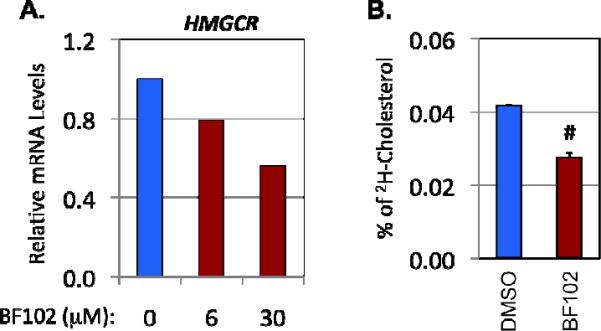
BF102 inhibits cholesterol biosynthesis. A. Relative mRNA levels of HMG-CoA reductase (HMGCR) (detected by quantitative RT-PCR) in HepG2 cells treated with the indicated concentrations of BF102 for 6 hours. Cyclophilin B was the invariant control. B. The synthesis rate of cholesterol (detected by the deuterium enrichment method) in FAO cells after 12 hours of treatment with BF102 (20μM). #p<0.001 vs DMSO (n=3).
Together, our data strongly suggest that BF102 is an inhibitor for both de novo lipogenesis and cholesterogenesis by down-regulating SREBP-target genes.
Discussion
In this study, we have designed and synthesized a series of novel boron-containing stilbene derivatives and tested their biological activity as lipogenic inhibitors in mammalian hepatocytes. From a screen of this small library, we identified one compound BF102 to have potent effects at sub-lethal doses. Treatment with BF102 in hepatocytes resulted in a significant decrease of rate-limiting enzymes in the synthesis pathways for both fatty acids and cholesterol at the mRNA levels. Consistent with gene expression data, BF102 has displayed an inhibitory effect on biosynthesis of both palmitate and cholesterol. Since SREBP transcription factors are the key activators of these genes, it is likely that BF102 functions through interfering SREBP functions. However, the precise mechanism(s) of BF102 actions are currently unclear. Experiments are ongoing in our laboratory to identify the molecular target(s) of BF102. According to our data, the IC50 of BF102 is around 30μM in cultured cells. Currently, we are designing and synthesizing BF102 analogs in order to improve the inhibitory effects on lipogenic gene expression. Interestingly, our preliminary data in vivo suggest that BF102 has no significant toxicity in mice at the highest possible dose we can administrate. Thus, our novel boron-containing compounds may become the next generation of lipid-lowering drugs in treating cardiovascular diseases in humans.
Materials and Methods
Tissue culture
HepG2 and FAO cells were cultured at 37°C and 5 % CO2 in Dulbecco's modified Eagle's medium (DMEM; Sigma), supplemented with 100 mg/ml of penicillin-streptomycin (P/S, GIBCO-BRL), 10% fetal bovine serum (FBS, Hyclone) and 20mM glutamine.
Quantitative RT-PCR assay
Total RNA was extracted from cells with Trizol (Invitrogen) and quantified with an Agilent 2100 Bioanalyzer. For mRNA quantification, 2 μg of RNA was converted to cDNA with a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA), and transcript levels of relevant genes were determined by real-time, quantitative PCR with an ABI 7300 Real Time PCR machine according to the manufacturer's instructions. (PCR primer information is available upon request)
Quantitative measurement of fatty acids and cholesterol synthesis
Fatty acids and cholesterol synthesis rate was determined using the deuterium enrichment method followed by GC/MS analysis. Briefly, cells were cultured in regular DMEM medium containing 10% 2H2O for 18 hours. Fatty acids and cholesterol were purified by extraction with organic solvents chloroform/methanol and quantitatively measured by GC/MS at The Stable Isotope & Metabolomics Core of the Einstein DRTC. The synthesis rates were presented as the percentage of 2H-containing palmitate or cholesterol in total amount of palmitate or cholesterol.
Statistical Analyses
Data are presented as the means ± S.D. or averages. The significance of differences between two groups was evaluated using Student's t test. The difference is considered as significant when the p value is less than 0.05.
Acknowledgements
This work was supported by AECOM start-up funds (to B.F. & F.Y) and a DRTC pilot & feasibility grant (to B.F. & F.Y) under P60 DK020541 (to Einstein DRTC). The authors thank Drs. Bhavapriya Vaitheesvaran and Irwin Kurland for measuring biosynthesis of palmitate and cholesterol, and Dr. Jeffrey E. Pessin for his support and advice.
References
- 1.Sato R. Arch Biochem Biophys. 501:177. doi: 10.1016/j.abb.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. Biochimie. 2004;86:839. doi: 10.1016/j.biochi.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Osborne TF, Espenshade PJ. Genes Dev. 2009;23:2578. doi: 10.1101/gad.1854309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Diabetes. 2005;54:1314. doi: 10.2337/diabetes.54.5.1314. [DOI] [PubMed] [Google Scholar]
- 5.Sato R. FEBS J. 2009;276:622. doi: 10.1111/j.1742-4658.2008.06807.x. [DOI] [PubMed] [Google Scholar]
- 6.Alberts AW. Am J Cardiol. 1988;62:10J. doi: 10.1016/0002-9149(88)90002-1. [DOI] [PubMed] [Google Scholar]
- 7.Golomb BA, Evans MA. Am J Cardiovasc Drugs. 2008;8:373. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das BC, Madhukumar AV, Anguiano J, Kim S, Sinz M, Zvyaga TA, Power EC, Ganellin CR, Mani S. Bioorg Med Chem Lett. 2008;18:3974. doi: 10.1016/j.bmcl.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Das BC, Smith ME, Kalpana GV. Bioorg Med Chem Lett. 2008;18:4177. doi: 10.1016/j.bmcl.2008.05.097. [DOI] [PubMed] [Google Scholar]
- 10.Das BC, Smith ME, Kalpana GV. Bioorg Med Chem Lett. 2008;18:3805. doi: 10.1016/j.bmcl.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Das BC, Madhukumar AV, Anguiano J, Mani S. Bioorg Med Chem Lett. 2009;19:4204. doi: 10.1016/j.bmcl.2009.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das BC, Mahalingam SM, Evans T. Tetrahedron Lett. 2009;50:3031. doi: 10.1016/j.tetlet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das BC, Mahalingam SM, Evans T, Kabalka GW, Anguiano J, Hema K. Chem Commun (Camb) 2009:2133. doi: 10.1039/b823063c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torregroza I, Evans T, Das BC. Chem Biol Drug Des. 2009;73:339. doi: 10.1111/j.1747-0285.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das BC, McCartin K, Liu TC, Peterson RT, Evans T. PLoS One. 2010;5:e10004. doi: 10.1371/journal.pone.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das BC, Mahalingam SM, Panda L, Wang B, Campbell P, Evans T. Tetrahedron Lett. 2011;51:1462. doi: 10.1016/j.tetlet.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]