Abstract
Two models, Z Dosage and Dominant W, have been proposed to explain sex determination in birds, in which males are characterized by the presence of two Z chromosomes, and females are hemizygous with a Z and a W chromosome. According to the Z Dosage model, high dosage of a Z-linked gene triggers male development, whereas the Dominant W model postulates that a still unknown W-linked gene triggers female development. Using 33 polymorphic microsatellite markers, we describe a female triploid Kentish plover Charadrius alexandrinus identified by characteristic triallelic genotypes at 14 autosomal markers that produced viable diploid offspring. Chromatogram analysis showed that the sex chromosome composition of this female was ZZW. Together with two previously described ZZW female birds, our results suggest a prominent role for a female determining gene on the W chromosome. These results imply that avian sex determination is more dynamic and complex than currently envisioned.
Keywords: sex determination, birds, microsatellites, aneuploidy
1. Introduction
Birds show striking sexual dimorphism with pronounced phenotypic differences between males and females. Sex in birds is determined genetically; males are ZZ and females are ZW. However, precisely how the phenotypic sexual dimorphism is initiated, is debated [1–3]. Two models have been proposed to explain sex determination in birds [4]. The Z Dosage model postulates that the main determinant for sex is located on the Z chromosome. This sex determinant interacts with an autosomal gene and, depending on the ratio between copies of Z chromosomes and autosomes (Z : A ratio), the embryo develops as male or female. Z Dosage is based on the observed ineffective dosage compensation for Z genes, i.e. their expression is proportional to the copy number in birds [5,6]. The model is supported by experimental RNA inhibition of the Z-linked DMRT1 gene, a major sex determinant in vertebrates [2]. When DMRT1 was inhibited early in development, ZZ chicken Gallus gallus embryos subsequently developed ovaries but no testes. By contrast, the Dominant W model postulates that the main determinant for females is located on the W chromosome. For example, the presence of a gene located on the W chromosome may antagonistically interact with DMRT1 by altering methylation of the male hypermethylated region adjacent to DMRT1 in chicken [1]. However, such a ‘female gene’ has yet to be described in birds.
Chromosomal aberrations such as aneuploidy can help to clarify the sex determination mechanism although they are often already lethal at the embryonic stage in birds [7]. Triploid chickens with an ZWW genotype are not viable, whereas triploid ZZZ chickens develop a male phenotype but produce only abnormal sperm. Triploid ZZW chickens initially develop female phenotypes but before sexual maturity they develop male phenotypes [8]. Importantly, these intersexual chickens fail to produce viable gametes [8].
Here, we report a female putative triploid Kentish plover Charadrius alexandrinus that reproduced successfully in a natural population. We explore the type of its sex chromosome aneuploidy and discuss the implications of this case for models of avian sex determination.
2. Material and methods
The female in question was a regular breeder captured during incubation in 1997 and 1999 at Tuzla, Turkey (36°42′ N, 35°03′ E). The first clutch in 1997 was predated, but in 1999 the entire clutch hatched and all family members were sampled for blood. Twenty-five microlitres of blood were taken from either the brachial vein (adults) or metatarsal vein (chicks) and stored in Queen's lysis buffer [9]. The female and her mate were sexed in the field based on plumage characteristics and sex-specific pattern of incubation in this species [10–12]. Molecular sexing using P2/P8 primers to amplify W- and Z-specific chromohelicase DNA binding protein fragments [13] confirmed the phenotypic sexing results of adults and showed that all three chicks were male. The family was genotyped using 33 microsatellite markers, including two Z-linked and one W-linked locus [14–17]. Genotypes were checked for consistency across two runs. Because no shorebird genome is yet available we mapped microsatellite locations to the chicken (WSHUC2) and zebra finch Taeniopygia guttata (taeGut3.2.4) genome databases following [16].
The three sex-linked markers (two Z-linked and one W-linked) had low polymorphism and the female was monomorphic at all of them (electronic supplementary material, S1). Therefore, we performed a peak height ratio analysis to establish composition and number of sex chromosomes [18]. We amplified products for W-linked Calex-31 and Z-linked Calex-26 together in a single PCR with 35 cycles and established the W/Z peak height ratio of the putative triploid female and 22 females from the same population that had the same genetic profiles at the sex-linked markers. We then compared the W/Z peak height ratio of the female in question to those of the control females.
3. Results
For 17 of the 33 markers, we identified homologues on nine zebra finch and nine chicken chromosomes (electronic supplementary material, S1). The female had triallelic genotypes at 14 markers and all three maternal alleles were represented in the offspring at six markers (electronic supplementary material, S1; for example, electronic supplementary material, S2). Eight triallelic markers were mapped to six zebra finch and eight chicken autosomes. All alleles of the chicks were assigned to their social parents. Neither of the chicks nor the male showed triallelic genotypes. The peak height ratio analysis revealed that the triploid female differed from the mean peak height ratio of the 22 control females by 4.47 s.d. The W product was under-represented and reached only 45–66% of the ratio of the control females, consistent with an ZZW sex chromosome aneuploidy (figure 1).
Figure 1.
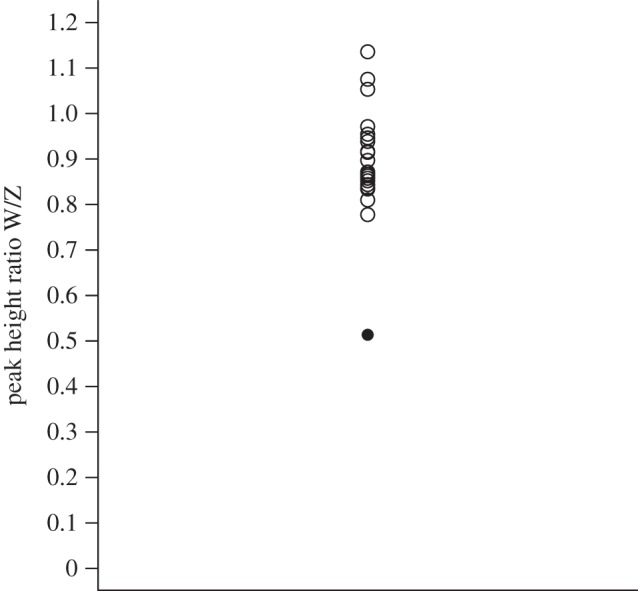
Peak height ratio of one putative triploid (black circle) and 22 diploid (open circles) females for Calex-26 (Z-linked) and Calex-31 (W-linked).
4. Discussion
Triploidy is usually lethal at the embryonic stage in birds [7]. We report a triploid ZZW Kentish plover that behaved as a female and produced viable diploid offspring in the wild.
The Z : A ratio is an important feature of the Z Dosage model [4]. Triploid ZZW chickens that have an intermediate Z : A ratio of 2 : 3 are sex changers that start as females but assume phenotypic characteristics of males before reaching sexual maturity. In contrast to our plover female these chicken sex changers do not produce viable gametes [8]. During a period of 3 years, we observed two reproduction attempts of this female with the same male. The age of the female was at least 3 years when it reproduced successfully and was last seen alive. We consider it unlikely that she changed her sex subsequently, long after onset of sexual maturity and successful reproduction.
The observation of a reproducing ZZW female has implications for avian sex determination. Despite the recent support for an important role of DMRT1 in the sex determination cascade in a bird [2], an effect of a W-linked gene that triggers femaleness should not be discarded [3]. This still unknown gene could antagonistically interact with DMRT1, for example, through changes of methylation patterns [1]. In amphibians with a ZW sex determination system, DM-W, a recently identified truncated paralogue of DMRT1 on the W chromosome, interacts antagonistically with DMRT1 and is known to trigger femaleness [19]. DM-W has no known homologue in chickens, although the current lack of sequence information for the W chromosome from other birds does not rule out the presence of a DMRT1 paralogue or other potentially female-determining genes in other avian lineages.
We suggest that more than one sex determination mechanism may have evolved in birds and that the current description of DMRT1-driven male determination in birds is incomplete or overly simplistic. In most vertebrate groups, the mechanism of sex determination is not fully conserved [20]. For example, switches between environmental and genetic sex determination (ZW or XY) have occurred frequently during the evolutionary history of reptiles [21–23]. Previously, two cases of adult ZZW females were reported in blue-and-yellow macaws Ara ararauna and great reed warblers Acrocephalus arundinaceus [24,25], two other non-galliform species. However, in both previous studies aneuploidy could not be established for the gonads. The females either did not have offspring [24] or transmitted only alleles of one Z chromosome to the offspring [25]. By contrast, we showed that the triploid plover female transmitted all three alleles to the offspring for at least six loci. Therefore, we conclude that her gonads were also triploid.
Observations of ZZW females exclusively in non-galliform birds suggest that an alternative sex determination mechanism may have evolved in this group. This is further supported by the large interspecific size variation of bird sex chromosomes [26], and expression differences of Z-linked genes between galliform and non-galliform birds [27]. Only recently, for example, a neosex chromosome was discovered through linkage analyses that arose from the fusion of the sex chromosomes with chromosome 4a in the warbler family Sylvidae [28]. Taken together, these reports suggest that avian sex determination is more complex and dynamic than currently recognized. We suggest that future studies should focus not only on chickens but also include a phylogenetically broad range of bird species to better understand the sex determination pathway in birds.
Acknowledgements
Permission for field work and blood sampling was provided by the Turkish Ministry of National Parks, Tuzla Municipality and Mr E. Karakaya, the Governor of Karatas District.
We are grateful for the comments of Jennifer Marshall Graves and three anonymous reviewers that helped to improve the manuscript. Genetic analyses were carried out at NBAF Sheffield and funded by NERC, UK. C.K. was supported by DAAD and by NSF grant no. IOS-0922640 to Gabrielle Nevitt and S.V.E. J.A. was supported by Wilhelm och Martina Lundgrens Vetenskapsfond, Helge Ax:son Johnsons Stiftelse and Rådman och Fru Ernst Collianders Stiftelse. A.K. was supported by the Hungarian Scientific Research Fund (OTKA, K81953 to Ádám Miklósi).
References
- 1.Teranishi M., et al. 2001. Transcripts of the MHM region on the chicken Z chromosome accumulate as non-coding RNA in the nucleus of female cells adjacent to the DMRT1 locus. Chromosome Res. 9, 147–165 10.1023/a:1009235120741 (doi:10.1023/a:1009235120741) [DOI] [PubMed] [Google Scholar]
- 2.Smith C. A., Roeszler K. N., Ohnesorg T., Cummins D. M., Farlie P. G., Doran T. J., Sinclair A. H. 2009. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461, 267–271 10.1038/nature08298 (doi:10.1038/nature08298) [DOI] [PubMed] [Google Scholar]
- 3.Ellegren H. 2011. Sex-chromosome evolution: recent progress and the influence of male and female heterogamety. Nat. Rev. Genet. 12, 157–166 10.1038/nrg2948 (doi:10.1038/nrg2948) [DOI] [PubMed] [Google Scholar]
- 4.Clinton M. 1998. Sex determination and gonadal development: a bird's eye view. J. Exp. Zool. 281, 457–465 (doi:10.1002/(sici)1097-010x(19980801)281:5<457::aid-jez10>3.0.co;2-6) [DOI] [PubMed] [Google Scholar]
- 5.Itoh Y., et al. 2007. Dosage compensation is less effective in birds than in mammals. J. Biol. 6, 2. 10.1186/jbiol53 (doi:10.1186/jbiol53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mank J. E., Ellegren H. 2009. All dosage compensation is local: gene-by-gene regulation of sex-biased expression on the chicken Z chromosome. Heredity 102, 312–320 10.1038/hdy.2008.116 (doi:10.1038/hdy.2008.116) [DOI] [PubMed] [Google Scholar]
- 7.Forstmeier W., Ellegren H. 2010. Trisomy and triploidy are sources of embryo mortality in the zebra finch. Proc. R. Soc. B 277, 2655–2660 10.1098/rspb.2010.0394 (doi:10.1098/rspb.2010.0394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin M., Thorne M. H., Martin I. C. A., Sheldon B. L., Jones R. C. 1995. Development of the gonads in the triploid (ZZW and ZZZ) fowl, Gallus domesticus, and comparison with normal diploid males (ZZ) and females (ZW). Reprod. Fertil. Dev. 7, 1185–1197 10.1071/rd9951185 (doi:10.1071/rd9951185). [DOI] [PubMed] [Google Scholar]
- 9.Seutin G., White B. N., Boag P. T. 1991. Preservation of avian blood and tissue samples for DNA analyses. Can. J. Zool. 69, 82–90 10.1139/z91-013 (doi:10.1139/z91-013). [DOI] [Google Scholar]
- 10.Kosztolányi A., Székely T. 2002. Using a transponder system to monitor incubation routines of snowy plovers. J. Field Ornithol. 73, 199–205 10.1648/0273-8570(2002)073[0199:uatstm]2.0.co;2 (doi:10.1648/0273-8570(2002)073[0199:uatstm]2.0.co;2) [DOI] [Google Scholar]
- 11.AlRashidi M., Kosztolányi A., Küpper C., Cuthill I. C., Javed S., Székely T. 2010. The influence of a hot environment on parental cooperation of a ground-nesting shorebird, the Kentish plover Charadrius alexandrinus. Front. Zool. 7, 1. 10.1186/1742-9994-7-1 (doi:10.1186/1742-9994-7-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cramp S., Simmons K. E. L. 1983. Handbook of the birds of Europe, the Middle East and North Africa. New York, NY: Oxford University Press [Google Scholar]
- 13.Griffiths R., Double M. C., Orr K., Dawson R. J. G. 1998. A DNA test to sex most birds. Mol. Ecol. 7, 1071–1075 10.1046/j.1365-294x.1998.00389.x (doi:10.1046/j.1365-294x.1998.00389.x) [DOI] [PubMed] [Google Scholar]
- 14.Dawson D. A., et al. 2010. New methods to identify conserved microsatellite loci and develop primer sets of high cross-species utility—as demonstrated for birds. Mol. Ecol. Resour. 10, 475–494 10.1111/j.1755-0998.2009.02775.x (doi:10.1111/j.1755-0998.2009.02775.x) [DOI] [PubMed] [Google Scholar]
- 15.Küpper C., Horsburgh G. J., Dawson D. A., Ffrench-Constant R., Székely T., Burke T. 2007. Characterization of 36 polymorphic microsatellite loci in the Kentish plover (Charadrius alexandrinus) including two sex-linked loci and their amplification in four other Charadrius species. Mol. Ecol. Notes 7, 35–39 10.1111/j.1471-8286.2006.01517.x (doi:10.1111/j.1471-8286.2006.01517.x) [DOI] [Google Scholar]
- 16.Küpper C., Burke T., Székely T., Dawson D. A. 2008. Enhanced cross-species utility of conserved microsatellite markers in shorebirds. BMC Genomics 9, 502. 10.1186/1471-2164-9-502 (doi:10.1186/1471-2164-9-502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Küpper C., Augustin J., Kosztolányi A., Figuerola J., Burke T., Székely T. 2009. Kentish versus snowy plover: phenotypic and genetic analyses of Charadrius alexandrinus reveal divergence of Eurasian and American subspecies. Auk 126, 839–852 10.1525/auk.2009.08174 (doi:10.1525/auk.2009.08174) [DOI] [Google Scholar]
- 18.Young D. R., Tun Z., Honda K., Matoba R. 2001. Identifying sex chromosome abnormalities in forensic DNA testing using amelogenin and sex chromosome short tandem repeats. J. Forensic Sci. 46, 346–348 10.1520/jfs14969j (doi:10.1520/jfs14969j) [DOI] [PubMed] [Google Scholar]
- 19.Yoshimoto S., Ikeda N., Izutsu Y., Shiba T., Takamatsu N., Ito M. 2010. Opposite roles of DMRT1 and its W-linked paralogue, DM-W, in sexual dimorphism of Xenopus laevis: implications of a ZZ/ZW-type sex-determining system. Development 137, 2519–2526 10.1242/dev.048751 (doi:10.1242/dev.048751) [DOI] [PubMed] [Google Scholar]
- 20.Graves J. A. M., Peichel C. L. 2010. Are homologies in vertebrate sex determination due to shared ancestry or to limited options? Genome Biol. 11, 205. 10.1186/gb-2010-11-4-205 (doi:10.1186/gb-2010-11-4-205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janes D. E., Organ C. L., Edwards S. V. 2009. Variability in sex-determining mechanisms influences genome complexity in reptilia. Cytogenet. Genome Res. 127, 242–248 10.1159/000293283 (doi:10.1159/000293283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezaz T., Stiglec R., Veyrunes F., Graves J. A. M. 2006. Relationships between vertebrate ZW and XY sex chromosome systems. Curr. Biol. 16, R736–R743 10.1016/j.cub.2006.08.021 (doi:10.1016/j.cub.2006.08.021) [DOI] [PubMed] [Google Scholar]
- 23.Sarre S. D., Ezaz T., Georges A. 2011. Transitions between sex determining systems in reptiles and amphibians. Ann. Rev. Genomics Hum. Gen. 12, 391–406 10.1146/annurev-genom-082410-101518 (doi:10.1146/annurev-genom-082410-101518) [DOI] [PubMed] [Google Scholar]
- 24.Tiersch T. R., Beck M. L., Douglass M. 1991. ZZW autotriploidy in a blue-and-yellow macaw. Genetica 84, 209–212 10.1007/bf00127249 (doi:10.1007/bf00127249) [DOI] [Google Scholar]
- 25.Arlt D., Bensch S., Hansson B., Hasselquist D., Westerdahl H. 2004. Observation of a ZZW female in a natural population: implications for avian sex determination. Proc. R. Soc. Lond. B 271, S249–S251 10.1098/rsbl.2003.0155 (doi:10.1098/rsbl.2003.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiglec R., Ezaz T., Graves J. A. M. 2007. A new look at the evolution of avian sex chromosomes. Cytogenet. Genome Res. 117, 103–109 10.1159/000103170 (doi:10.1159/000103170) [DOI] [PubMed] [Google Scholar]
- 27.Itoh Y., Replogle K., Kim Y. H., Wade J., Clayton D. F., Arnold A. P. 2010. Sex bias and dosage compensation in the zebra finch versus chicken genomes: general and specialized patterns among birds. Genome Res. 20, 512–518 10.1101/gr.102343.109 (doi:10.1101/gr.102343.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pala I., Naurin S., Stervander M., Hasselquist D., Bensch S., Hansson B. 2012. Evidence of a neo-sex chromosome in birds. Heredity 108, 264–272 10.1038/hdy.2011.70 (doi:10.1038/hdy.2011.70) [DOI] [PMC free article] [PubMed] [Google Scholar]


