Abstract
We previously interrogated the transcriptome in heart tissue from LmnaH222P/H222P mice, a mouse model of cardiomyopathy caused by lamin A/C gene (LMNA) mutation, and found that the extracellular signal-regulated kinase 1/2 and Jun N-terminal kinase branches of the mitogen-activated protein (MAP) kinase signaling pathway were abnormally hyperactivated prior to the onset of significant cardiac impairment. We have now used an alternative gene expression analysis tool to reanalyze this transcriptome and identify hyperactivation of a third branch of the MAP kinase cascade, p38α signaling. Biochemical analysis of hearts from LmnaH222P/H222P mice showed enhanced p38α activation prior to and after the onset of heart disease as well as in hearts from human subjects with cardiomyopathy caused by LMNA mutations. Treatment of LmnaH222P/H222P mice with the p38α inhibitor ARRY-371797 prevented left ventricular dilatation and deterioration of fractional shortening compared with placebo-treated mice but did not block the expression of collagen genes involved in cardiac fibrosis. These results demonstrate that three different branches of the MAP kinase signaling pathway with overlapping consequences are involved in the pathogenesis of cardiomyopathy caused by LMNA mutations. They further suggest that pharmacological inhibition of p38α may be useful in the treatment of this disease.
INTRODUCTION
Mutations in the lamin A/C gene (LMNA) encoding nuclear A-type lamins cause dilated cardiomyopathy with or without associated skeletal muscular dystrophy (1,2). LMNA may be the most prevalent dilated cardiomyopathy gene, as mutations appear to be responsible for ∼8% of inherited cases (3,4). LMNA-dilated cardiomyopathy is associated with arrhythmias, sudden death, myocardial remodeling and dilatation of left ventricle (LV) ultimately resulting in poor cardiac performance and heart failure. While sudden death from arrhythmias may be prevented by implantation of a pacemaker and defibrillator, progressive heart failure eventually becomes resistant to treatment, such as angiotensin converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, diuretics and aldosterone antagonists. No drugs are curative and heart transplantation is frequently necessary.
Despite the fact that the etiology and the symptoms of LMNA-dilated cardiomyopathy have been extensively investigated, little is known about the underlying pathogenic mechanisms. The LmnaH222P/H222P mouse, which develops cardiomyopathy that recapitulates the human disease, has been a useful small animal model (5). We previously interrogated the transcriptome in heart tissue from LmnaH222P/H222P mice and identified the extracellular signal-regulated kinase 1/2 (ERK1/2) and Jun N-terminal kinase (JNK) branches of the mitogen-activated protein (MAP) kinase signaling pathway as abnormally hyperactivated prior to the onset of significant cardiac impairment (6). We then hypothesized that small molecule inhibitors of ERK1/2 and JNK signaling would prevent LV dilatation and improve cardiac function in LmnaH222P/H222P mice and have seen these beneficial effects when administering such drugs (7–10).
Microarray analysis of RNAs generates large lists of differentially expressed genes; however, biological interpretation can be challenging. Bioinformatics tools that use functional information accumulated in public databases make it possible to dissect large gene lists to identify the most pertinent biological processes related to the microarray data. Our initial analysis of the transcriptome of hearts from LmnaH222P/H222P mice used ErmineJ, a tool based on gene set enrichment analysis (GSEA) that implements multiple algorithms including over-representation and resampling-based methods that focus on gene scores or correlation of gene expression profiles (11). Since then, additional analysis tools have emerged providing different approaches to analyze microarray data that could potentially identify pathways or functional groups of genes that were not detected using another bioinformatics tool. We therefore reanalyzed our microarray data sets from hearts of LmnaH222P/H222P mice using the Database for Annotation, Visualization and Integrated Discovery (DAVID), a program that uses different methodological and statistical approaches (12,13). Based on this reanalysis, we have identified a third branch of the MAP kinase cascade, p38α signaling, to be hyperactivated in LMNA cardiomyopathy. We further show that pharmacological inhibition of p38α prevents LV dilatation and prevents deterioration in LV fractional shortening (FS) in LmnaH222P/H222P mice.
RESULTS
Abnormal p38α signaling in hearts of LmnaH222P/H222P mice and humans with cardiomyopathy caused by LMNA mutations
We previously examined differential expression of mRNAs isolated from hearts of 10-week wild-type (n= 8) and LmnaH222P/H222P (n= 6) male mice using Affymetrix Mouse Genome 430 2.0 Arrays (6). These data are publicly available at Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) with accession numbers GSE6397 and GSE6398. We then used ErmineJ to analyze data from over 45 000 probe sets on the arrays to identify functional groups of genes, based on gene ontology (GO), that were differentially expressed in hearts of the LmnaH222P/H222P mice compared with wild-type mice. This analysis revealed alterations in the expression of genes in several signaling pathways, among them MAP kinases (6). However, ErmineJ has several limitations, including its incomplete coverage annotating the large database of existing signaling pathways. We therefore reanalyzed our data sets of genes differentially expressed in hearts of LmnaH222P/H222P mice using both ErmineJ and the DAVID program. These are based on different key computational methods: GSEA for ErmineJ and singular enrichment analysis (SEA) for DAVID (12,13).
After analysis of only the 7805 differentially expressed genes that met a false discovery rate threshold of q< 0.05 (see Material and Methods), we identified 16 signaling pathways using ErmineJ, 13 signaling pathways using the KEGG database in DAVID and 12 using the BIOCARTA database in DAVID (Table 1). Overall, there was consistency in the results using ErmineJ and DAVID, as in a previous study that ran the same data sets with DAVID versus ErmineJ (14). For example, Wnt, TGFβ, MAP kinase, JAK-STAT and insulin signaling were identified using both DAVID and ErmineJ. However, only ErmineJ identified the I-kappaB kinase/NF-kappaB signaling pathway and only DAVID identified some signaling pathways, including ErbB, calcineurin/calcium, PDGF, AKT/mTOR and PPAR. In ErmineJ and both the KEGG and BIOCARTA pathway databases in DAVID, the GO term ‘MAP kinase signaling pathway’ considers the expression of genes in ERK1/2, JNK and p38α branches. However, the BIOCARTA database in DAVID includes a separate GO term that specifically considers only genes in the p38α branch, which was found to be significantly different in LmnaH222P/H222P mouse hearts using this analysis method.
Table 1.
GO analysis using ErmineJ and DAVID of genes differentially expressed in hearts from LmnaH222P/H222P mice at 10 weeks of age
| GSEA | Rank | Signaling pathway (GO term) | P-value |
| -ErmineJ- | |||
| 1 | Wnt receptor signaling pathway | 4.14E−10 | |
| 2 | JNK kinase signaling pathway | 7.21E−10 | |
| 3 | Receptor serine/threonine kinase signaling pathway | 1.04E−07 | |
| 4 | MAP kinase signaling pathway | 1.15E−05 | |
| 5 | Transforming growth factor β receptor signaling pathway | 1.57E−05 | |
| 6 | Regulated secretory signaling pathway | 2.67E−04 | |
| 7 | G-protein signaling pathway | 6.93E−04 | |
| 8 | JAK-STAT signaling pathway | 6.97E−04 | |
| 9 | I-kappaB kinase/NF-kappaB signaling pathway | 1.81E−03 | |
| 10 | Frizzled signaling pathway | 0.02308889 | |
| 11 | Intracellular receptor-mediated signaling pathway | 0.02352453 | |
| 12 | Complement activation signaling pathway | 0.02534146 | |
| 13 | Insulin receptor signaling pathway | 0.02737485 | |
| 14 | Insulin-like growth factor receptor signaling pathway | 0.03702819 | |
| 15 | Cytokine- and chemokine-mediated signaling pathway | 0.04445473 | |
| 16 | Steroid hormone receptor signaling pathway | 0.04874929 | |
| SEA | |||
| -DAVID- | |||
| KEGG | |||
| 1 | Wnt signaling pathway | 5.20E−05 | |
| 2 | TGF beta-signaling pathway | 2.03E−04 | |
| 3 | MAP kinase signaling pathway | 2.18E−04 | |
| 4 | Insulin signaling pathway | 2.61E−04 | |
| 5 | GnRH signaling pathway | 6.42E−04 | |
| 6 | Neurotrophin signaling pathway | 0.00121274 | |
| 7 | T cell receptor signaling pathway | 0.001278196 | |
| 8 | Calcium signaling pathway | 0.022151913 | |
| 9 | Jak-STAT signaling pathway | 0.024891802 | |
| 10 | ErbB signaling pathway | 0.025551016 | |
| 11 | Adipocytokine signaling pathway | 0.02786599 | |
| 12 | PPAR signaling pathway | 0.03570115 | |
| 13 | mTOR signaling pathway | 0.043633556 | |
| BIOCARTA | |||
| 1 | Wnt signaling pathway | 0.000151627 | |
| 2 | TGF beta signaling pathway | 0.003815475 | |
| 3 | CXCR4 signaling pathway | 0.007966109 | |
| 4 | AKT/mTOR signaling pathway | 0.008654229 | |
| 5 | p38 MAPK signaling pathway | 0.010403168 | |
| 6 | IGF-1 signaling pathway | 0.012208631 | |
| 7 | PDGF signaling pathway | 0.012444725 | |
| 8 | MAP kinase signaling pathway | 0.015196888 | |
| 9 | T-cell receptor signaling pathway | 0.015464509 | |
| 10 | Integrin signaling pathway | 0.016080005 | |
| 11 | JNK signaling pathway | 0.044987126 | |
| 12 | Calcineurin signaling pathway | 0.046356356 | |
The most significantly changed GO terms in hearts from LmnaH222P/H222P mice were identified by canonical pathways analysis using either a GSEA enrichment method (ErmineJ) or a SEA enrichment method (DAVID). For each bioinformatics tool, signaling pathways identified were ranked according to their P-value. DAVID allows the identification of pathways from either the KEGG or BIOCARTA databases.
Many individual genes in the ERK1/2, JNK and p38α branches of the MAP kinase signaling cascade were differentially expressed in hearts from LmnaH222P/H222P mice compared with wild-type mice (Fig. 1A). Differences in expression of 10 genes in the p38α branch were detected on the Affymetrix arrays (Fig. 1B). To validate the altered expression of four selected transcripts of genes encoding proteins in the p38α signaling branch detected on the arrays, we performed real-time quantitative RT–PCR using mRNA extracted from cardiac tissue of male LmnaH222P/H222P and Lmna+/+ mice. We detected increased expression of Atf2, Elk-1, Creb and Chop10 as early as 8 weeks of age in hearts from LmnaH222P/H222P mice and their expression becomes gradually more increased until 20 weeks of age (Fig. 1C).
Figure 1.
Altered expression of genes in the p38α branch of the MAP kinase signaling cascade in hearts from LmnaH222P/H222P compared with wild-type mice at 10 weeks of age. (A) Representation of genes in the MAP kinase signaling pathway (MAPK signaling pathway) identified by the KEGG visual pathway resource, which includes the ERK1/2 (ERK signaling), JNK (JNK signaling) and p38α (p38 signaling) branches within this GO term. Genes with statistically significant differences (q< 0.05) in expression detected on Affymetrix Mouse Genome 430 2.0 Arrays in hearts of LmnaH222P/H222P mice compared with wild-type mice are indicated by asterisks; red asterisks indicate genes in the p38α branch. (B) Matrices showing array data of corresponding probe sets corresponding to 10 genes in the in the p38α branch with statistically significant differences (q< 0.05) in expression between wild-type (Lmna+/+) and LmnaH222P/H222P mouse hearts. In the matrices, each probe set is visualized as a row of colored squares and each column is a biological replicate (sample form a different mouse heart). Darker color means lower expression and brighter color higher expression. The genes correspond to those indicated with red asterisks in (A) [DDIT3/CHOP10 is the same as GADD153 and MAP2K6/MEK6 the same as MKK6 in (A)]. (C) Validation of differential expression in hearts from LmnaH222P/H222P mice of four selected genes in the p38α MAP signaling pathway using real-time quantitative RT–PCR. RNA was obtained from hearts of mice at different ages as indicated on the x-axis. White bars show relative RNA expression levels in hearts of Lmna+/+ mice and black bars in hearts of LmnaH222P/H222P mice. Values are means ± SEM for n= 3 samples per group. The real-time quantitative RT–PCR was performed in triplicate with the different RNA samples. *P< 0.05, **P< 0.005, ***P< 0.0005.
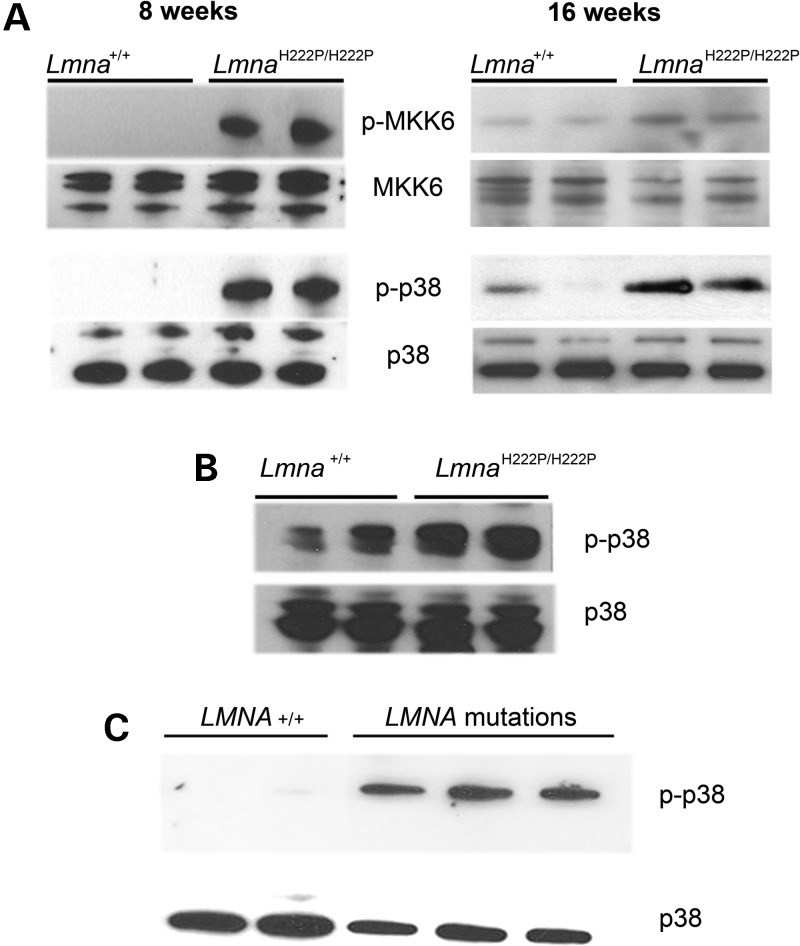
We next examined activation of p38α signaling in cardiac tissue from male LmnaH222P/H222P mice at 8 weeks of age, when heart function is normal, and at 16 weeks of age, when the mice have LV dilatation and decreased ejection fraction. We performed immunoblotting to detect p38α phosphorylation (activation) as well as phosphorylation of MAP kinase kinase (MKK) 6, the kinase that activates p38α. There was increased phosphorylation of both p38α and MKK6 in extracts of whole hearts from LmnaH222P/H222P mice compared with wild-type mice at both 8 and 16 weeks of age (Fig. 2A). In cardiomyocytes isolated from LmnaH222P/H222P mouse hearts, there was a similar hyperactivation of p38α MAP kinase signaling compared with cardiomyocytes from wild-type mice (Fig. 2B). To determine whether the activation in p38α MAP kinase signaling in hearts of LmnaH222P/H222P mice occurs in the corresponding human disease, we examined the levels of phosphorylated and total p38α in heart tissue from human subjects with cardiomyopathy caused by LMNA mutations. A significant increase in phosphorylated p38α was detected in LV tissue from three patients with LMNA mutations compared with controls without LMNA mutations (Fig. 2C). These results show that p38α MAP kinase signaling is hyperactivated in LMNA cardiomyopathy.
Figure 2.
p38α signaling is hyperactivated in hearts of mice and humans with cardiomyopathy and lamin A/C gene mutaitons. (A) Immunoblots showing phosphorylated p38α (p-p38α), total p38α, phosphorylated MKK6 (p-MKK6) and total MKK6 in protein extracts of hearts of wild-type (Lmna+/+) and LmnaH222P/H222P mice at 8 weeks (left panel) and 16 weeks (right panel) of age. Each lane contains protein extracts from a different mouse. (B) Immunoblot showing p-p38α and total p38α in protein extracts of cardiomyocytes isolated from hearts of wild-type (Lmna+/+) and LmnaH222P/H222P mice at 16 weeks. Each lane contains protein extracts from a different mouse. (C) Immunoblots showing p-p38α and total p38α in protein extracts of heart tissue from two human controls (LMNA+/+) and three individuals with dilated cardiomyopathy caused by LMNA mutations.
Inhibition of p38α signaling prevents dilatation and deterioration of LV function in LmnaH222P/H222P mice
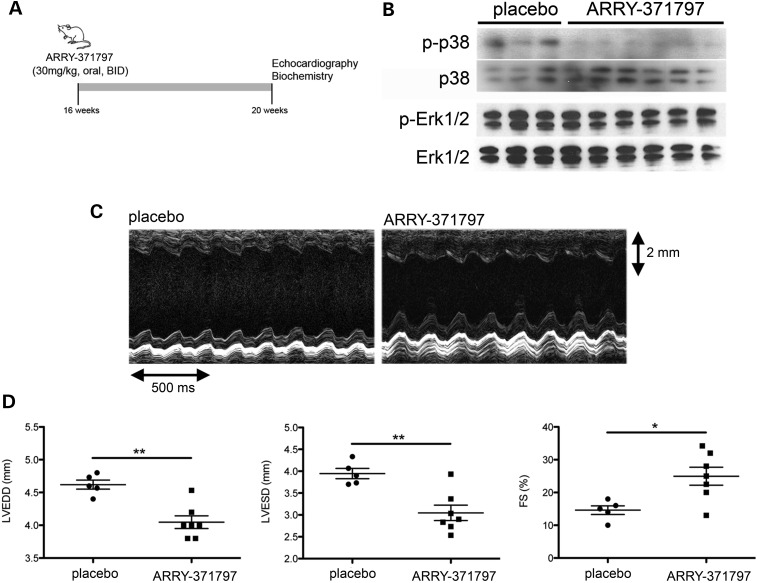
Given the enhanced p38α MAP kinase signaling in hearts of LmnaH222P/H222P mice, we asked whether we could improve the LV structure and performance in these mice. We blocked p38α MAP kinase signaling in male LmnaH222P/H222P mice with twice daily oral doses of ARRY-371797 (30 mg/kg), a highly selective, potent inhibitor of p38α under investigation in a Phase II clinical trial for acute inflammatory pain (15). A treatment for 4 weeks was initiated at 16 weeks of age, when there were significant increases in LV end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD) as well as a decrease in FS, a parameter directly proportional to the LV ejection fraction. Following 4 weeks of treatment, the mice were analyzed by echocardiography and then sacrificed for biochemical studies (Fig. 3A). ARRY-371797 reduced phosphorylated p38α in hearts compared with placebo, as shown by immunoblotting of proteins in tissue homogenates (Fig. 3B). ARRY-371797 did not reduce the phosphorylation of ERK1/2, suggesting that it is a selective inhibitor of p38α (Fig. 3B). We used M-mode echocardiography to image hearts in vivo and assess LV diameters (Fig. 3C). It showed that LVEDD and LVESD in LmnaH222P/H222P mice treated with ARRY-371797 were significantly smaller and FS was significantly increased compared with the placebo-treated mice (Fig. 3D; see also Table 2).
Figure 3.
ARRY-371797 prevents LV dilatation and deterioration of FS in LmnaH222P/H222P mice. (A) Schematic representation of the treatment protocol of LmnaH222P/H222P mice with ARRY-371797. (B) Representative immunoblots using antibodies against phosphorylated p38α (p-p38α), total p38α, phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (ERK1/2) to probe proteins extracted from hearts from LmnaH222P/H222P mice treated with placebo or ARRY-371797. Each lane contains protein extracts from a different mouse. (C) Representative M-mode transthoracic echocardiographic tracings from 20-week-old male LmnaH222P/H222P mice treated with placebo or ARRY-371797. (D) Graphs showing mean LVEDD, mean LVESD and FS in 20-week-old male LmnaH222P/H222P mice treated with placebo (n= 5) or ARRY-371797 (n= 7). Values for each individual mouse receiving placebo (circles) or ARRY-371797 (squares) as well SEMs of means (bars) are shown. *P< 0.05, **P< 0.005.
Table 2.
Effect of ARRY-371797 on LV diameters and FS in LmnaH222P/H222P mice
| Treatment | Heart rate (bpm) | LVEDD (mm) | LVESD (mm) | FS (%) |
|---|---|---|---|---|
| Placebo (n= 5) | 509.0 ± 4.5 | 4.6 ± 0.1 | 3.9 ± 0.1 | 14.6 ± 1.3 |
| ARRY-371797 (n= 7) | 496.0 ± 2.7 | 4.0 ± 0.1* | 3.0 ± 0.2* | 25.0 ± 2.7** |
**P< 0.05, *P< 0.005 between placebo-treated and ARRY-371797-treated mice.
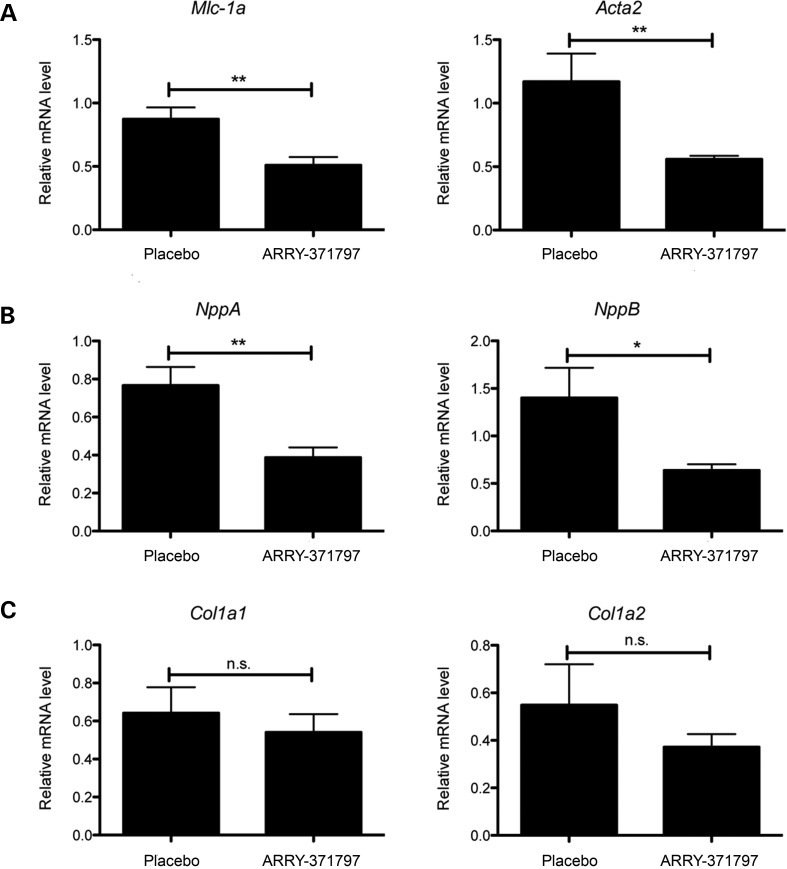
We also examined the effects of treatment with ARRY-371797 on the expression of genes encoding proteins involved in sarcomere organization and compensatory responses to LV dilatation such as natriuretic peptides and collagens. Hearts from the ARRY-371797-treated LmnaH222P/H222P mice had significantly reduced expression of Mlc-1a and Acta2, which encode proteins involved in sarcomere organization (Fig. 4A). They also had reduced expression of Nppa and NppB, respectively, encoding atrial natriuretic peptide A and brain natriuretic peptide B (Fig. 4B). ARRY-371797 treatment did not, however, reduce the expression of Col1a1 and Col1a2 mRNAs encoding collagens in hearts of LmnaH222P/H222P mice (Fig. 4C). These results show that inhibition of p38α signaling in hearts of LmnaH222P/H222P mice attenuates the expression of sarcomeric proteins and natriuretic peptides secreted in response to LV dilatation but may not prevent the myocardial fibrosis that occurs in these mice (5,8–10).
Figure 4.
ARRY-371797 decreases expression of genes encoding proteins involved in sarcomere organization and natriuretic peptides but not collagens in LmnaH222P/H222P mice. (A) Relative expression of mRNAs from genes encoding myosin light chain (Mlc-1a) and cardiac-specific actin (Acta2) in LmnaH222P/H222P mice treated with placebo or ARRY-371797. (B) Relative expression of mRNAs from genes encoding atrial natriuretic peptide A (NppA) and brain natriuretic peptide B (NppB) in LmnaH222P/H222P mice treated with placebo or ARRY-371797. (C) Relative expression of mRNAs from genes encoding type 1 collagen isoforms (Col1a1 and Col1a2) in LmnaH222P/H222P mice treated with placebo or ARRY-371797. Real-time quantitative RT–PCR, performed in triplicate on three separate samples, was used to measure mRNAs in hearts from LmnaH222P/H2222P mice treated with placebo (n= 5) or ARRY-371797 (n= 7). Values shown are means ± SEM. *P< 0.05, **P< 0.005, n.s., not significant.
DISCUSSION
We previously used the gene expression analysis tool ErmineJ to analyze mRNA isolated from hearts of LmnaH222P/H222P mouse model of cardiomyopathy caused by LMNA mutation and identified the ERK1/2 and JNK branches of the MAP kinase signaling pathway as abnormally hyperactivated prior to the onset of cardiac disease (6). In the present study, we used an alternative analysis tool, DAVID, with two separate pathway databases, to obtain evidence for hyperactivation of the p38α branch of the MAP kinase signaling pathway using the same data sets. These results were confirmed by biochemical assays on isolated heart tissue showing hyperactivation of the p38α signaling branch. Our reanalysis using DAVID but not ErmineJ also detected differences in the AKT/mTOR signaling pathway in hearts from LmnaH222P/H222P mice, which prior to performing this analysis we found to be upregulated (16). DAVID additionally identified TGFβ signaling as being perturbed, which has been demonstrated previously using cell biological methods in hearts from older LmnaH222P/H222P mice and is consistent with the presence of cardiac fibrosis (5). Both analysis tools identified Wnt signaling as the highest ranked altered pathway by P-value; the role of Wnt in LMNA cardiomyopathy therefore may warrant further study. Overall, while for the most part ErmineJ and DAVID gave consistent results in analyzing alterations in cell signaling pathways, analysis with these two different bioinformatics tools identified non-overlapping alterations in additional pathways that were confirmed by biochemical methods.
Our reanalysis of this microarray data prompted us to examine the possible pathophysiological role of p38α hyperactivation in the development of dilated cardiomyopathy caused by LMNA mutation. Our experimental results provide several lines of evidence for such a role. First, activation of p38α is detectable as early as 8 weeks of age in LmnaH222P/H222P mice, before any measurable structural alterations or functional deterioration in the heart. Second, we observed hyperactivation p38α, albeit at a later stage, in heart tissue from human subjects with dilated cardiomyopathy caused by LMNA mutation. Third, pharmacological inhibition of p38α prevented LV dilatation and deterioration in LV function in LmnaH222P/H222P mice. The p38α hyperactivation in cardiomyopathy caused by LMNA mutation appears to occur concurrently with hyperactivation of ERK1/2 and JNK, two other MAP kinases
MAP kinases are activated by binding of mitogens, growth factors and cytokines to cell surface receptors and also by osmotic, mechanical and chemical stresses, such as reactive oxygen species, independent of such receptors (17,18). There is considerable crosstalk between different MAP kinase branches, particularly between JNK and p38α (Fig. 1A), and between MAP kinase and other signaling pathways. In dilated failing hearts, there is activation of multiple signaling pathways including ERK1/2, JNK and p38a MAP kinases as well as AKT (19). Given the crosstalk and overlap between these and other pathways, deciphering the specific pathogenic roles of any individual MAP kinase branch has been extremely complicated. Indeed, despite being the focus of extensive investigation, contradictive results have led to a perception that activated MAP kinases can have both protective and detrimental effects in the heart (20,21). With regards to p38α signaling, experimental studies have yet generated a clear picture regarding its role in cardiac pathogenesis. Ventricular-specific transgenic expression of MKK3 or MKK6 that activated p38α has been reported to cause systolic contractile depression, increased fibrosis and restrictive diastolic abnormalities in the absence of significant hypertrophy (22). Mice with cardiac-specific transgenic expression of dominant-negative mutants of p38α, MKK3 or MKK6 have been reported to have baseline cardiac hypertrophy (23). In contrast, mice with cardiac-selective depletion have been reported to have a normal life span and normal global cardiac structure and function (24). However, in response to pressure overload, these mice develop hypertrophy similar to controls but also develop LV dilatation and fibrosis (24). Treatment of hearts from hamsters with dilated cardiomyopathy with inhibitors of p38α has been reported to have beneficial effects, including decreased LV dimensions, increased ejection fraction and decreased fibrosis (25). The first two of these are consistent with our findings in LmnaH222P/H222P mice. However, we did not see a decrease in expression of genes encoding collagens that function in laying down fibrous tissue.
Overall, the hyperactivation of ERK1/2, JNK, AKT and p38α signaling that occurs prior to clinically detectable dilated cardiomyopathy in hearts of mice with Lmna mutation mimics what appears to occur later in dilated failing hearts (19). How this apparently detrimental pattern of cell signaling arises as a consequence of abnormalities in A-type lamins remains a mystery. MAP kinases can be activated by mechanical stress and A-type lamins are necessary to maintain proper mechanical stiffness as well as nuclear and cytoplasmic integrity in cells (26–28). Hence, defects in A-type lamins could predispose cells, especially contractile ones, to more readily activate stress–response signaling pathways in response to mechanical strain. Along these lines, cardiomyocyte nuclei in LmnaH222P/H222P mice are more elongated than in wild-type mice and treatment with MEK1/2 and JNK inhibitors reverses this alteration (7–10). Whether similar effects on nuclear structure occur with ARRY-371797 treatment remains to be determined. Lamin A also binds directly to ERK1/2, suggesting that defects in A-type lamins could affect its activation (29). Regardless of the mechanism responsible, the fact that ERK1/2, JNK, AKT and p38α are all hyperactivated in a mouse model of LMNA cardiomyopathy prior to the onset of clinical disease lead us to hypothesize that inhibiting their activities to restore a more physiological balance would be beneficial. In LmnaH222P/H222P mice, pharmacological inhibition of ERK1/2 prevents LV dilation, improves LV ejection fraction/FS, inhibits fibrosis and produces a modest survival benefit (7–9). Inhibition of JNK appears to have similar beneficial effects on LV structure, ejection fraction/FS and fibrosis (8). Inhibition of mTOR, a downstream target activated by AKT, correlates with enhancement of macroautophagy, prevention of LV dilation and improvement FS but does not appear to inhibit fibrosis (16). The present results show that p38α inhibition prevents LV dilatation and improves FS. Further preclinical studies in mice, including effects on lifespan of each of these drugs, could allow for the optimization of protocols that inhibit these signaling pathways to treat human subjects with cardiomyopathy caused by LMNA mutations.
MATERIALS AND METHODS
Microarray data analysis
Data files were obtained through the National Center for Biotechnology Information's Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), accessible through accession numbers GSE6397 and GSE6398. Genes were identified as being differentially expressed if they met a false discovery rate threshold of q< 0.05 in a two-tail Student's t-test, using GeneSpring GX software (Agilent Technologies). Gene expression changes related to functional groups were analyzed using the class score method in the bioinformatics tools DAVID (http://david.abcc.ncifcrf.gov/) and ErmineJ (http://www.chibi.ubc.ca/ermineJ/) to provide a statistical confidence to groupings. These bioinformatics tools take as input the q-values of differentially expressed genes and identify statistically significant functional groupings (GO terms) using modified Fisher exact test in DAVID and Wilcoxon rank-sum test in ErmineJ. Significant GO terms were identified using a false discovery rate of P< 0.05.
Mice
LmnaH222P/H222P mice were bred and genotyped as previously described (5). Mice were fed chow and housed in a disease-free barrier facility with 12 h/12 h light/dark cycles. The Institutional Animal Care and Use Committee at Columbia University Medical Center approved the use of animals and the study protocols.
Isolation of mouse cardiomyocytes
Lmna+/+ and LmnaH222P/H222P mice (16 weeks of age) were anesthetized with pentofurane. Ventricular cardiomyocytes were isolated as described in the Alliance for Cellular Signaling procedure protocol PP00000125 (http://www.signaling-gateway.org/data/ProtocolLinks.html). Briefly, hearts were removed and the aorta cannulated. After Ca2+-free buffer was perfused for 2min, 0.25 mg/ml collagenase I/II (Roche) solution was perfused through the coronary arteries for 6 min with 12.5 μm Ca2+. Left ventricular tissue was teased apart and pipetted to release individual cells. After enzymatic dispersion, Ca2+ concentration in the buffer containing bovine serum albumin was elevated in three steps up to 500 μm.
Human tissue samples
Sections of explanted hearts from human subjects with LMNA mutations were obtained from Myobank-AFM of l'lnstitut de Myologie. The subjects were a 23-year-old man with dilated cardiomyopathy associated with muscular dystrophy (LMNA delK61 mutation), a 47-year-old woman with isolated dilated cardiomyopathy (LMNA R60G mutation) and from a 62-year-old woman with dilated cardiomyopathy associated with muscular dystrophy (LMNA c.IVS9 + 1g sup a mutation). Control human heart samples from a 57-year-old man who died from an intracranial bleed and a 15-year-old woman who died of drug overdose were obtained from the National Disease Research Interchange. All tissue samples were obtained with appropriate approvals and consent (not specifically for this study) from l'lnstitut de Myologie and the National Disease Research Interchange and provided without patient identifiers.
Quantitative real-time RT–PCR analysis
Total RNA was extracted using the Rneasy isolation kit (Qiagen). cDNA was synthesized using superscript first strand synthesis system according to the manufacturer's instructions (Invitrogen) on total RNA. For each replicate in each experiment, RNA from tissue samples of different animals was used. Primers were designed corresponding to mouse RNA sequences using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) (9). Real-time quantitative RT–PCR reactions contained HotStart-IT SYBR green qPCR Master Mix (Affymetrix), 200 nm of each primer and 0.2 µl of template in a 25 µl reaction volume. Amplification was carried out using the ABI 7300 Real-Time PCR System (Applied Biosystems) as described previously (9). Relative levels of mRNA expression were calculated using the ΔΔCT method (30). Individual expression values were normalized by comparison to Gapdh mRNA.
Protein extraction and immunoblotting
Human or mouse heart tissue was homogenized in sample extraction buffer (Cell Signaling) as previously described (10). Extracted proteins were separated by SDS–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and blotted with primary antibodies against p38α (No 9212, Cell Signaling), phosphorylated p38α (No 9216, Cell Signaling), MKK6 (No 9264, Cell Signaling) and phosphorylated MKK6 (No 9236, Cell Signaling). Secondary antibodies were horseradish peroxidate-conjugated (GE Healthcare). Recognized proteins were visualized by enhanced chemiluminescence (GE Healthcare).
Mouse treatment protocols
ARRY-371797 (Array BioPharma) was dissolved in Water for Injection (WFI) (Gibco) at a concentration of 0.5 mg/ml. The placebo control consisted of the same volume of WFI. ARRY-371797 was delivered at a dose of 30 mg/kg, twice a day. ARRY-371797 and WFI were administered orally by gavage starting when mice were 16 weeks of age and continuing until 20 weeks of age.
Thansthoracic echocardiography
LmnaH222P/H222P mice were anesthetized with 1.5% isoflurane in O2 and placed on a heating pad (37°C). Echocardiography was performed using a Visualsonics Vevo 770 ultrasound with a 30 MHz transducer applied to the chest wall. Cardiac ventricular dimensions and FS were measured in 2D mode and M-mode three times for the number of animals indicated.
Statistics
Values for real-time quantitative RT–PCR were compared using an unpaired Student t-test. Comparisons of echocardiographic parameters between ARRY-371797-treated and placebo-treated LmnaH222P/H222P mice were performed using a Welch t-test; to validate these results, a non-parametric test (Mann–Whitney) was performed and concordance checked. Statistical analyses were performed using GraphPad Prism software.
FUNDING
This work was supported by grants from the NIH/NIHMS (R01AR048997) and Muscular Dystrophy Association (MDA172222) to H.J.W. and a grant from the Association Francaise Contre les Myopathies to A.M.
ACKNOWLEDGEMENTS
We thank Dr Gisèle Bonne (Institut de Myologie) for providing LmnaH222P/H222P mice and Array BioPharma for providing ARRY-371797.
Conflict of Interest statement. H.J.W. and A.M. are inventors on a pending patent application (PCT/US09/42614) on methods for treating and/or preventing cardiomyopathies by ERK and JNK inhibition filed by the Trustees of Columbia University in the City of New York.
REFERENCES
- 1.Bonne G., Di Barletta M.R., Varnous S., Bécane H.M., Hammouda E.H., Merlini L., Muntoni F., Greenberg C.R., Gary F., Urtizberea J.A., et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 2.Fatkin D., MacRae C., Sasaki T., Wolff M.R., Porcu M., Frenneaux M., Atherton J., Vidaillet H.J., Jr, Spudich S., De Girolami U., et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 3.Taylor M.R., Fain P.R., Sinagra G., Robinson M.L., Robertson A.D., Carniel E., Di Lenarda A., Bohlmeyer T.J., Ferguson D.A., Brodsky G.L., et al. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J. Am. Coll. Cardiol. 2003;41:771–780. doi: 10.1016/s0735-1097(02)02954-6. [DOI] [PubMed] [Google Scholar]
- 4.Parks S.B., Kushner J.D., Nauman D., Burgess D., Ludwigsen S., Peterson A., Li D., Jakobs P., Litt M., Porter C.B., et al. Lamin A/C mutation analysis in a cohort of 324 unrelated patients with idiopathic or familial dilated cardiomyopathy. Am. Heart J. 2008;156:161–169. doi: 10.1016/j.ahj.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arimura T., Helbling-Leclerc A., Massart C., Varnous S., Niel F., Lacène E., Fromes Y., Toussaint M., Mura A.M., Keller D.I., et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- 6.Muchir A., Pavlidis P., Decostre V., Herron A.J., Arimura T., Bonne G., Worman H.J. Activation of MAPK pathway links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J. Clin. Invest. 2007;117:1282–1293. doi: 10.1172/JCI29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muchir A., Shan J., Bonne G., Lehnart S.E., Worman H.J. Inhibition of extracellular signal-regulate kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum. Mol. Genet. 2009;18:241–247. doi: 10.1093/hmg/ddn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu W., Shan J., Bonne G., Worman H.J., Muchir A. Pharmacological inhibition of c-Jun N-terminal kinase signaling prevents cardiomyopathy caused by mutation in LMNA gene. Biochim. Biophys. Acta. 2010;1802:632–638. doi: 10.1016/j.bbadis.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu W., Muchir A., Shan J., Bonne G., Worman H.J. Mitogen activated protein kinase inhibitors improve heart function and prevent fibrosis in cardiomyopathy caused by lamin A/C gene mutation. Circulation. 2011;123:53–61. doi: 10.1161/CIRCULATIONAHA.110.970673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muchir A., Reilly S.A., Wu W., Iwata S., Homma S., Bonne G., Worman H.J. Treatment with selumetinib preserves cardiac function and improves survival in cardiomyopathy caused by mutation in the lamin A/C gene. Cardiovasc. Res. 2012;93:311–319. doi: 10.1093/cvr/cvr301. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.K., Braynen W., Keshav K., Pavlidis P. ErmineJ: tool for functional analysis of gene expression data sets. BMC Bioinformatics. 2005;6:269. doi: 10.1186/1471-2105-6-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennis G., Sherman B.T., Hosack D.A., Yang J., Gao W., Lane H.C., Lempicki R.A. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 13.Huang D.W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang D.W., Sherman B.T., Tan Q., Collins J.R., Alvord W.G., Roayaei L., Stephens R., Baseler M.W., Lane H.C., Lempicki R.A. The DAVID gene functional classification tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulthard L.R., White D.E., Jones D.L., McDermott M.F., Burchill S.A. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009;15:369–379. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi J.C., Muchir A., Wu W., Iwata S., Homma S., Morrow J.P., Worman H.J. Temsirolimus activates autophagy and ameliorates cardiomyopathy caused by lamin A/C gene mutation. Sci. Transl. Med. 2012 doi: 10.1126/scitranslmed.3003875. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seger R., Krebs E.G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 18.Chang Y., Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 19.Haq S., Choukroun G., Lim H., Tymitz K.M., del Monte F., Gwathmey J., Grazette L., Michael A., Hajjar R., Force T., et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007;116:1413–1423. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose B.A., Force T., Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol. Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao P., Georgakopoulos D., Kovacs A., Zheng M., Lerner D., Pu H., Saffitz J., Chien K., Xiao R.P., Kass D.A., Wang Y. The in vivo role of p38 MAP kinases in cardiac remodelling and restrictive cardiomyopathy. Proc. Natl Acad. Sci. USA. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braz J.C., Bueno O.F., Liang Q., Wilkins B.J., Dai Y.S., Parsons S., Braunwart J., Glascock B.J., Klevitsy R., Kimball T.F., et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida K., Yamaguchi O., Hirotani S., Hikosa S., Higuchi Y., Watanabe T., Takeda T., Osuka S., Morita T., Kondah G., et al. p38a mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol. Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyoi S., Otami H., Matsuhisa S., Akita Y., Tatsumi K., Enoki C., Fujiwara H., Imamura H., Kamihata H., Iwasaka T. Opposing effect of p38 MAP kinase and JNK inhibitors on the development of heart failure in the cardiomyopathic hamster. Cardiovasc. Res. 2006;69:888–898. doi: 10.1016/j.cardiores.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Lammerding J., Schulze P.C., Takahashi T., Kozlov S., Sullivan T., Kamm R.D., Stewart C.L., Lee R.T. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broers J.L., Peeters E.A., Kuijpers H.J., Endert J., Bouten C.V., Oomens C.W., Baaijens F.P., Ramaekers F.C. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum. Mol. Genet. 2004;13:2567–2580. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 28.Dahl K.N., Ribeiro A.J., Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ. Res. 2008;102:1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González J.M., Navarro-Puche A., Casar B., Crespo P., Andrés V. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J. Cell Biol. 2008;183:653–666. doi: 10.1083/jcb.200805049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponchel F., Toomes C., Bransfield K., Leong F.T., Douglas S.H., Field S.L., Bell S.M., Combaret V., Puisieux A., Mighell A.J., et al. Real-time PCR based on SYBR-Green I fluorescence: an alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol. 2003;3:18. doi: 10.1186/1472-6750-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]