Abstract
The transcription factors MyoD and Myf-5 control myoblast identity and differentiation. MyoD and Myf-5 manifest opposite cell cycle-specific expression patterns. Here, we provide evidence that MyoD plays a pivotal role at the G2/M transition by controlling the expression of p21Waf1/Cip1 (p21), which is believed to regulate cyclin B-Cdc2 kinase activity in G2. In growing myoblasts, MyoD reaccumulates during G2 concomitantly with p21 before entry into mitosis; MyoD is phosphorylated on Ser5 and Ser200 by cyclin B-Cdc2, resulting in a decrease of its stability and down-regulation of both MyoD and p21. Inducible expression of a nonphosphorylable MyoD A5/A200 enhances the MyoD interaction with the coactivator P/CAF, thereby stimulating the transcriptional activation of a luciferase reporter gene placed under the control of the p21 promoter. MyoD A5/A200 causes sustained p21 expression, which inhibits cyclin B-Cdc2 kinase activity in G2 and delays M-phase entry. This G2 arrest is not observed in p21−/− cells. These results show that in cycling cells MyoD functions as a transcriptional activator of p21 and that MyoD phosphorylation is required for G2/M transition.
Myogenic differentiation is under the control of the MyoD family of b-HLH transcription factors (MRFs), which includes MyoD, myogenin, Myf-5, and MRF4 (11, 31, 45). Activation of muscle-specific genes by the MRFs occurs through their heterodimerization with the E protein b-HLH factors (28) and subsequent binding to E-box DNA consensus sequences (CANNTG) (9). MRFs thus transactivate muscle-specific genes and efficiently convert nonmuscle cells to the myogenic lineage (45). p300/CBP and P/CAF coactivators have acetyltransferase activities and regulate transcription, cell cycle progression, and differentiation. They are both required for MyoD activity and muscle differentiation (34, 39). In contrast, histone deacetylation inhibits gene activation, and the interaction between histone deacetylase 1 (HDAC1) and MyoD prevents premature activation of the myogenic program in growing myoblasts (25).
MyoD is expressed in proliferating myoblasts and causes efficient withdrawal from the cell cycle prior to the differentiation process. The cyclin-dependent kinase (Cdk) inhibitors p21 and p57Kip2, which negatively regulate cell cycle progression (19), play an important role in this process. Both of these proteins are highly expressed during muscle differentiation, both in embryos and in tissue culture (37, 50). MyoD is responsible for up-regulating p21 in muscle cells that have been induced to differentiate in vitro (17, 32). The MyoD level appears to be tightly controlled in growing myoblasts (22). Phosphorylation of MyoD at serine 200 plays a crucial role in modulating its half-life and its transcriptional activity during myoblast proliferation (21, 41). MyoD is degraded by the ubiquitin-proteasome pathway (3, 44). Up-regulation of p57Kip2 stabilizes MyoD by blocking cyclin E-Cdk2 activity (36) and by direct interaction with MyoD (37).
Ectopic expression of p21 induces G1 (18) and G2 arrest in mammalian cells (29). p21 may also play a role during the G2/M-phase transition by inducing pause, thereby facilitating the integration of critical G2 checkpoint signals that regulate entry into mitosis (10). Overexpression of p21 induces growth arrest and a senescent phenotype by selectively inhibiting a set of genes involved in mitosis, DNA replication, segregation, and repair (5). Based on the evidence that tumor cells lacking these proteins enter into mitosis with accelerated kinetics (4, 6), p53, p21, and 14-3-3 σ are necessary to maintain a G2 arrest following DNA damage. Nonetheless, the G2 checkpoint is maintained by redundant pathways (33). The transactivation of p21 may dictate the duration of G2 phase through an initial inhibition of cyclin B-Cdc2 activity (12, 40).
Cells isolated from adult MyoD-null mice are unable to progress through the normal differentiation program and are mitotically active under conditions that initiate terminal differentiation in wild-type cells (27). MyoD directly orchestrates multiple subprograms of gene expression. Temporal patterning of gene expression is achieved through promoter-specific regulation of MyoD binding (1). Recent data indicate that MyoD has distinct transcriptional activities in growing and differentiating myoblasts. Moreover, MyoD has a bimodal pattern of expression in the course of cell cycle division (20, 44). Thus, MyoD may control specific myogenic programs in cycling myoblasts.
In the present work we addressed the possibility that periodic expression of MyoD in the cell cycle might constitute a mechanism for regulating its myogenic functions in proliferating cells. We show that MyoD protein levels increase in G2 and that MyoD is phosphorylated on Ser5 and Ser200 by cyclinB-Cdc2, resulting in its destabilization before entry into mitosis. Replacement of these two serines by nonphosphorylable alanine prevents MyoD phosphorylation and delays entry into mitosis. We find that the mutant MyoD A5/A200 displays increased interaction with the coactivator P/CAF, whose binding stimulates the MyoD-mediated transcriptional transactivation of chromatin-integrated p21 promoter-luciferase (p21-Luc). We show that cyclin B-Cdc2-mediated phosphorylation of MyoD destabilizes the MyoD interaction with P/CAF and thus represses the transactivation of p21. These results reveal that proliferation and expression of transcriptionally active MyoD are compatible and that phosphorylation of MyoD can result in its effective functional inactivation.
MATERIALS AND METHODS
Expression vectors and purification of proteins.
Expression vector pCMV-HA-MyoD was generated by cloning three hemagglutinin epitope tags at the amino termini of cDNA MyoD inserts in pcDNA3 (Invitrogen). MyoD mutant derivatives were obtained by oligonucleotide-directed mutagenesis by using a Quick Change site-directed mutagenesis kit (Stratagene) as instructed by the manufacturer. p21-Luc was constructed by inserting the HindIII-EcoRV fragment of pJFCAT (obtained from B. Vogelstein), filled in by the Klenow polymerase fragment and cloned into the SmaI site of the pGL3 basic expression plasmid (Promega). For the Gal4 fusion experiments, we cloned sequences from MyoD and MyoD,A5/A200 into the pM vector (Clontech). The reporter plasmid 5XGal4-E1b-luc was constructed by replacing the CAT reporter gene with the Luc reporter gene in the pG5E1bCAT construct. All constructions were verified by sequencing with an ABI automatic laser fluorescence sequencer.
Cell culture and DNA transfection.
The mouse skeletal muscle cell line C2C12 and the fibroblastic cell line 10T1/2 were maintained in Dulbecco's modified Eagle's medium supplemented, respectively, with 20 or 15% fetal calf serum. T-Rex-293 cells (Invitrogen) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 5 μg of blasticidin/ml. The HCT116 human colon carcinoma cell line and a derivate cell line, HCT116 p21−/−, in which both p21Waf1/Cip1/Sdi1 alleles have been deleted through homologous recombination (4), were kindly provided by Guido Kroemer (Gustave Roussy Institut, Villejuif, France) and maintained in culture in McCoy's (Invitrogen) supplemented with 10% fetal bovine serum.
Myogenic C2C12 cells were transfected by the calcium phosphate procedure, and synchronization was done as previously described (20). 10T1/2 fibroblasts, T-Rex-293, and both HCT116 cell lines were transfected by using JetPEI (Qbiogene). Cells were plated in 6-well plates and transfected 24 h later with a total of 3 μg of various combinations of plasmids as indicated in the legends to the figures. The total amount of DNA used for each plate was normalized with the respective empty expression vehicle. One hundred nanograms of the pEGFP-C1 plasmid (Invitrogen) was included in transfection as an internal control for transfection efficiency and flow cytometry. The stable cell line of 10T1/2 cells expressing the luciferase gene under the control of the p21 promoter (p21-Luc) was obtained by cotransfection of the p21-Luc vector and the pSV2neo vector. T-Rex-293 cells were transiently transfected with pcDNA4-IRES-GFP expression vehicle encoding MyoD and MyoD,A5/A200, and expression of the corresponding vectors was induced by addition of tetracycline to a final volume of 10 μg/ ml. Cells were synchronized for cell division as follows. Twenty-four hours after transfection, T-Rex-293 cells were first treated 17 h with 2 mM thymidine, released for 12 h, and arrested at the G1/S transition with a second treatment with 2 mM thymidine for 17 h. After washing out the thymidine, the cells were cultured 6 h to allow S-phase progression with the addition of tetracycline to a final volume of 10 μg/ml to induce plasmid expression. Finally, the cells were treated for 12 h with 0.1 μg of nocodazole/ml to arrest at the G2/M transition.
Forty-eight hours after transfection, determination of luciferase activity was done in triplicate and repeated at least twice with cell extracts normalized to protein content and normalized for transfection efficiency with a cotransfected pCMV-green fluorescent protein (GFP) reporter and by Western blotting of the MyoD proteins in the cell extract, with similar results.
Cell staining, flow cytometry, and cell sorter experiment.
At the indicated time points, the cells were harvested via trypsinization, washed in PBS 1X, and fixed in 85% (vol/vol) methanol for 30 min at −20°C. Cells were pelleted and resuspended in 100 μl of staining buffer (10 μg of bovine serum albumin/ml, 0.05% saponin, 1× PBS) containing a 1:100 MPM-2 dilution of MPM-2 antibody for 1 h. The cells were then washed and incubated 45 min at room temperature in staining buffer containing a 1:200 dilution of phycoerythrin-conjugated secondary rabbit antibody (Jackson) and 10 μg of Hoechst/ml. Cells were analyzed in a Becton Dickinson FACS VANTAGE SE. Cell cycle data were plotted with Cell Quest software (Becton Dickinson) in a FACS VANTAGE SE (argon laser 333 to 363 nm [COHERENT]), and axis scales were optimized by using the control sample and maintaining that value for each sample. For the cell sorter experiment, approximately 2.106 cells per ml were incubated for 30 min at 37°C with 10 μg of Hoechst (Sigma)/ml in medium with 15% fetal calf serum. Cells from different fractions were collected by centrifugation, washed in PBS 1X, and lysed in immunoprecipitation (IP) buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 10% glycerol, 0.5% NP40, 0.5 mM Na-orthovanadate, 50 mM NaF, 80 mM β-glycerophosphate, 10 mM Na-pyrophosphate, 1 mM dithiothreitol, 1 mM EGTA, 10 μg of leupeptin/ml, 10 μg of pepstatin/ml, and 10 μg of aprotinin/ml).
Immunological reagents and procedures.
Immunoprecipitation and Western blotting were performed as previously described (36, 37) unless otherwise specified. Gel loading was normalized to protein concentration. Signals were quantified by Gelscan. Antibodies used included the following: anti MyoD monoclonal antibody diluted to 1/300 with 5A8 (Pharmingen), anti-MyoD polyclonal antibody diluted to 1/500 with C-20, anti-Cdc2 diluted to 1/1000 with M-2, anti cyclin-B diluted to 1/1,000 with M-20, anti-p21 diluted to 1/250 with F5, and anti-p300 polyclonal antibody diluted to 1/500 with C-20 (all from Santa Cruz). Anti-hemagglutinin (HA) antibody was diluted to 1/1,000 with 12CA5 (Roche). The monoclonal antibody α-tubulin was diluted to 1/5,000 with DM1A, and anti-Flag was diluted to 1/1,000 with M2 (Sigma).
Metabolic labeling.
Thirty-six hours after transfection, cells were labeled with [32P]orthophosphate (0.5 mCi ml−1) in phosphate-free medium for 2 h. Cells were lysed in IP buffer precleared for 30 min with protein-G beads and immunoprecipitated for 3 h with anti-MyoD polyclonal antibody (C-20). Immunoprecipitates were washed four times in IP buffer and then resuspended in sodium dodecyl sulfate (SDS)-sample buffer and resolved on a 10% polyacrylamide gel. Immunoprecipitated proteins were transferred to nitrocellulose and visualized by autoradiography.
Cycloheximide treatment was carried out essentially as described previously (37).
Cyclin B-Cdc2 kinase assays and phosphatase treatment.
Production and purification of full-length murine MyoDwt, MyoDA5, MyoDA200, and MyoD,A5/A200 bacterially produced proteins were purified essentially as described (44). Active cyclin B-Cdc2 was obtained by immunoprecipitation with anti-Cdc2 antibody. Cyclin B-Cdc2 complexes were incubated at 30°C for 30 min with purified recombinant proteins (2 μg) in a 30-μl reaction mixture containing 50 mM HEPES (pH 8.0), 10 mM MgCl2, 2.5 mM EGTA, 1 mM dithiothreitol, 10 μM β-glycerophosphate, 1 mM NaF, 0.1 mM Na3VO4, 0.1 mM phenylmethylsulfonal fluoride, 10 μM ATP, and 150 kBq of [γ32P]ATP (5,000 Ci/mmole; ICN). The reaction products were separated on SDS-polyacrylamide gel electrophoresis (PAGE), and phosphorylated proteins were transferred to nitrocellulose and visualized by autoradiography. Phosphatase treatments were done essentially as described previously (36).
RESULTS
Accumulation of MyoD in G2 phase is followed by phosphorylation in mitosis.
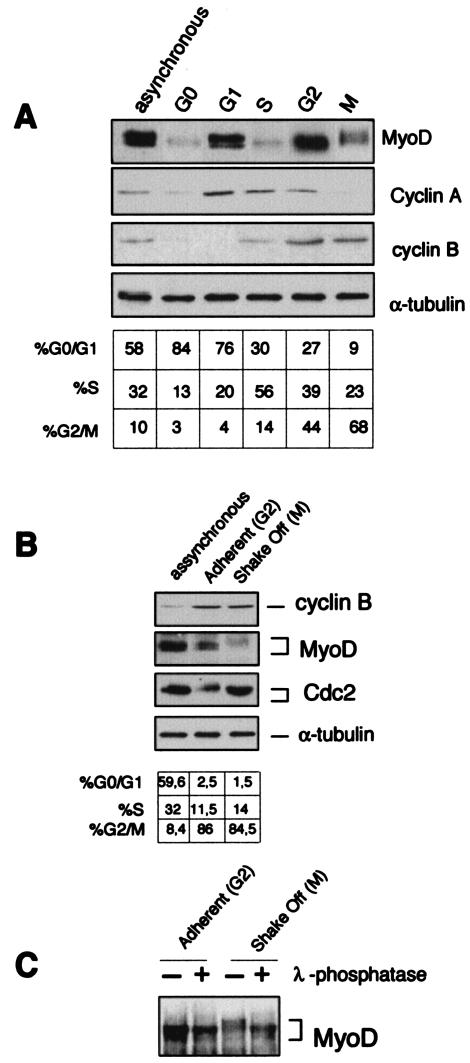
In C2 myoblasts released from the quiescent G0 stage, MyoD manifests a bimodal expression pattern in the course of the cell division cycle with high levels in mid-G1 and in late G2 (20). The destruction of the MyoD protein at late G1, just before the incipient S phase, is mediated by the ubiquitin-proteasome system, a process that is triggered by direct cyclin E-Cdk2-dependent phosphorylation of MyoD on serine 200 (44). As shown in Fig. 1A, the amount of MyoD protein remained low throughout the S phase, rose during G2, and dropped again in mitosis while exhibiting a shift to a slowly migrating form (Fig. 1A). The mitotic arrest of C2C12 myoblasts by nocodazole resulted in a strong phosphorylation of MyoD, which migrated with a slower mobility in the mitotic fraction (shake-off fraction that consisted of ∼85% of cells containing condensed chromatin as judged by propidium iodine labeling of fixed cells) compared to that for the nonmitotic fraction (Fig. 1B). When exposed to lambda phosphatase, MyoD from mitotic cells reacquired a normal gel mobility (Fig. 1C), indicating that its reduced mobility was indeed due to phosphorylation (Fig. 1C). In addition, the total amonts of MyoD appear to be significantly diminished in mitotic cells.
FIG. 1.
Mitotic phosphorylation of MyoD. (A) C2C12 cells were arrested in quiescence (G0) by incubation in methionine-depleted medium containing 1% serum for 36 h. Cells were released from G0 block by addition of serum and medium for 6, 12, 14, and 18 h to obtain cells in G1, S, G2, and M, respectively, and extracts (50 μg) from each time were analyzed by Western blotting for MyoD, cyclin A, cyclin B, and α-tubulin. The cell cycle profile of the different samples was determined by flow cytometry and reported in the table as the percentage of cells in different phases. (B) Proliferating C2C12 myoblasts treated for 12 h with 200 ng of nocodazole/ml were used to generate a mitotic fraction (shake-off fraction, M) and a nonmitotic fraction (adherent, G2). Cells were harvested and analyzed for MyoD, cyclin B, and Cdc2 expression by Western blot. (C) Aliquots of the extracts from mitotic shake-off and adherent C2C12 myoblasts shown in B were incubated for 1 h at 50°C with (+) or without (−) lambda phosphatase (50 units/100 μl) and analyzed by immunoblot with anti-MyoD antibodies.
Cyclin B-Cdc2 mediates phosphorylation of MyoD Ser5 and Ser200.
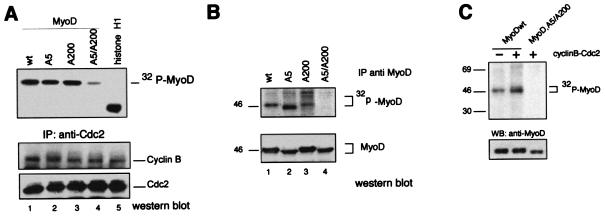
MyoD has previously been shown to be phosphorylated in vitro by cyclin B-Cdc2 on serine 5 (located in the transactivation domain) and serine 200. Only serine 200 was found to be phosphorylated in vivo, in asynchronous myoblasts which contain only a few mitotic cells (21). Because Cdc2 is known to be active at the G2/M transition and during mitosis, we investigated whether cyclin B-Cdc2 kinase would cause the phosphorylation of MyoD Ser5 and Ser200. We first mutated Ser5 to Ala5 (MyoD,A5) or Ser200 to Ala200 (MyoD,A200) and/or both Ser5 and Ser200 to Ala5 and Ala200 (MyoD,A5/A200) and then analyzed recombinant MyoD protein in an in vitro kinase assay using immunoprecipitated cyclin B-Cdc2 complexes from nocodazole-treated cells. As shown in Fig. 2A, cyclin B-Cdc2 isolated from nocodazole-treated myoblasts efficiently phosphorylated MyoDwt, MyoD,A5, and MyoD,A200, whereas phosphorylation of mutant MyoD,A5/A200 was strongly reduced. We then investigated whether Ser5 and Ser200 in MyoD could be phosphorylated in vivo. Expression vectors encoding MyoDwt or mutant derivatives were transfected in 10T1/2 cells, and MyoD phosphorylation was monitored in total extracts of nocodazole-treated cells labeled with [32P]orthophosphate. Immunoprecipitation of MyoD proteins revealed that MyoDwt, MyoDA5, and MyoDA200 but not MyoDA5/A200 were efficiently phosphorylated in vivo (Fig. 2B). Furthermore, in asynchronous cells in which only serine 200 was found phosphorylated in vivo, overexpression of transfected cyclin B-Cdc2 expression vectors increased both the number of cells in G2/M and the serine phosphorylation of MyoDwt, while no specific phosphorylation was observed with the MyoD mutant A5/A200 (Fig. 2C). Altogether, these data indicate that cyclin B-Cdc2 kinase phosphorylates MyoD on Ser5 and Ser200. This prompted us to investigate the effect of mutations on both Ser5 and Ser200 on MyoD during G2/M transition.
FIG. 2.
Serine residues at positions 5 and 200 of MyoD are phosphorylated by cyclin B-Cdc2. (A) Cyclin B-Cdc2 phosphorylates MyoD in vitro. Cyclin B-Cdc2 complexes were immunoprecipitated with anti-Cdc2 antibodies from nocodazole-arrested cells and were assayed for kinase activity using Histone H1, MyoDwt, MyoDA5, MyoDA200 and MyoD,A5/A200 as substrates. Shown is an autoradiogram of the kinase reaction following SDS-PAGE (upper panel). Western blot analysis of cyclin B and Cdc2 after immunoprecipitation (lower panel). (B) 10T1/2 cells were cotransfected with expression vectors encoding MyoDwt, MyoDA5, MyoDA200, and MyoD,A5/A200 in the presence of expression vectors for Cdc2 plus cyclin B. Forty-eight hours after transfection, cells were metabolically labeled with [32P]orthophosphate for two hours. Cells in G2/M were collected and immunoprecipitated with anti-MyoD antibodies (C-20). Shown is an autoradiogram of the radioactivity incorporated into MyoD and a Western blot analysis of the same membrane probed with the anti-MyoD monoclonal antibody (5A8). Note that phosphorylated MyoD is absent from the MyoD,A5/A200 immunoprecipitate. (C) 10T1/2 cells were transfected and treated 24 h later as in panel B but in the absence (−; 12% of cells in G2/M) and in the presence (+; 30 to 33% of cells in G2/M) of cotransfected vectors encoding cyclin B and Cdc2. wt, wild type. WB, Western blot.
Cyclin B-Cdc2 phosphorylation represses MyoD-dependent transactivation.
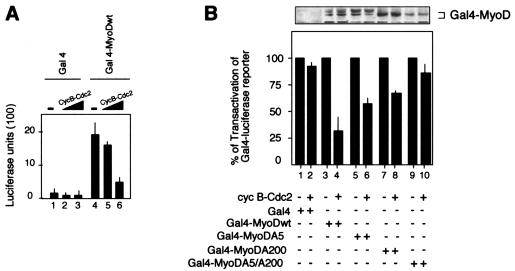
Reversible phosphorylation of the transcriptional machinery represses transcription at mitosis either by inhibiting DNA-binding activity or by suppressing transcription initiation (15, 26, 30, 38). We examined the effect of cyclin B-Cdc2 kinase on the transactivation function of MyoDwt by measuring the ability of Gal4DBD-MyoDwt, Gal4DBD-MyoDA5, Gal4DBD-MyoDA200 and Gal4DBD-MyoDA5/A200 fusion proteins to activate a Gal4-Luc reporter (pG5E1b-luc). The Gal4DBD-MyoDwt-mediated transactivation of the reporter gene was reduced in a dose-dependent manner by coexpression of cyclin B-Cdc2 (Fig. 3A). The impact of the MyoD phosphorylation by cyclin B-Cdc2 was further investigated by cotransfecting Gal4DBD-MyoDwt, Gal4DBD-MyoDA5, Gal4DBD-MyoDA200, and Gal4DBD-MyoDA5/A200 with the Gal4-Luc reporter with or without coexpression vectors coding for cyclin B and Cdc2. Importantly, the mutation of the Cdc2 phosphorylation sites on MyoD prevented the repression of the Gal4-Luc reporter gene by cyclin B-Cdc2 (Fig. 3B). In this assay, the double mutation (Gal4DBD-MyoDA5/A200) had a stronger effect on both single mutations (Gal4DBD-MyoDA5 and Gal4DBD-MyoDA200), emphasizing the importance of both Cdc2 phosphorylation sites in the transcriptional activity of MyoD. Altogether, these results show that cyclin B-Cdc2 phosphorylation significantly represses the transactivation function of MyoD by phosphorylation of Ser5 and Ser200.
FIG. 3.
Cyclin B-Cdc2 complexes repress MyoD transactivation function. (A) Luciferase activity was monitored from whole-cell extracts of 10T1/2 fibroblasts cotransfected with a Gal4x5 reporter construct plus plasmids encoding the Gal4DBD (lanes 1 to 3), Gal4DBD-MyoDwt (lanes 4 to 6) either in the absence (lanes 1 and 4) or in the presence (lanes 2, 3, 5, and 6) of increasing amounts of cyclin B and Cdc2 expression vectors. The minus sign indicates that an empty expression vehicle has been added instead of the corresponding expression plasmids. CycB, cyclin B. (B) 10T1/2 cells were transfected with the Gal4x5 reporter construct together with expression vectors for Gal4DBD, Gal4DBD-MyoDwt, Gal4DBD-MyoDA5, Gal4DBD-MyoDA200, or Gal4DBD-MyoDA5/A200 in the absence (−) or the presence of cyclin B-Cdc2 as indicated. After transfection, cells were synchronized by serum and methionine deprivation. Thirty-six hours later, cells were re-fed, cells in G2/M were purified by cell sorter apparatus, and luciferase activity was monitored from whole-cell extracts. Values shown are means ± standard deviations of three separate readings and represent the ratio of luciferase activity in the absence (−) (set at 100%) and in the presence (+) of cyclin B-Cdc2. cyc B, cyclin B.
Phosphorylation of MyoD inhibits the interaction with P/CAF by recruiting HDAC1.
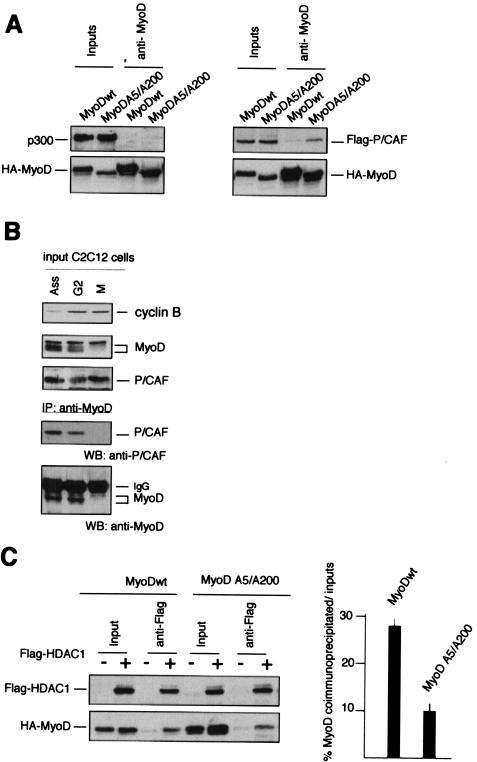
To elucidate the mechanism responsible of the transcriptional repression of MyoD induced by cyclin B-Cdc2, we hypothesized that phosphorylation might regulate the interaction of MyoD with a critical effector molecule involved in its transcriptional activation or silencing. Because P/CAF and p300/CBP coactivators can bind to and mediate MyoD-dependent transcription, likely by histone acetyltransferase activity (35), we determined whether these coactivators would bind to MyoD. 10T1/2 cells were transfected with expression vectors encoding MyoDwt and/or the nonphosphorylable mutant MyoDA5/A200 in the absence and in the presence of p300 or P/CAF, and whole-cell extracts were prepared from G2/M enriched cells obtained by cell sorting. After being normalized for protein content, the extracts were immunoprecipitated with anti-MyoD antibodies. The immunoprecipitates were then examined for the presence of p300 or P/CAF by immunoblotting. As shown in Fig. 4A, there was no association between MyoD or MyoDA5/A200 and p300. In the same conditions, an interaction between P/CAF and MyoDA5/A200 was clearly observable, suggesting that cyclin B-Cdc2 phosphorylation might control the ability of P/CAF to associate with MyoD. MyoD is acetylated in vivo and forms a complex with P/CAF that is readily detectable in proliferating muscle cells (39). To corroborate that the association between MyoD and P/CAF can occur at endogenous levels, synchronized C2C12 myoblasts were harvested at the G2/M block. Total cellular extracts were first immunoprecipitated with anti-MyoD antibodies, and then the immunoprecipitates were examined for the presence of P/CAF by Western blotting. As shown in Fig. 4B, there was hardly any association between MyoD and P/CAF in mitosis. In contrast, in G2 and in asynchronous myoblasts, an interaction between these two proteins was clearly observable. Recently, HDAC1 has been shown to inhibit the ability of P/CAF to augment the MyoD-dependent transcription in vivo, suggesting that HDAC1 normally plays a role in silencing the transcriptional activity of MyoD. As MyoD can associate with HDAC1 in undifferentiated cells and this interaction is direct (25), we investigated whether HDAC1 would interact with MyoD,A5/A200 and MyoDwt to the same extent. Mutation of Ser5 and Ser200 to nonphosphorylable alanine residues led to a ∼50 to 60% reduction in the HDAC1-MyoD coimmunoprecipitation (Fig. 4B), indicating that phosphorylation of MyoD favors its association with HDAC1 in vivo.
FIG. 4.
Phosphorylation represses the association of MyoD and P/CAF and favors interaction with HDAC1. (A) 10T1/2 fibroblasts were cotransfected with plasmids encoding P/CAF or p300 together with MyoDwt or MyoD,A5/A200 as indicated. After transfection, cells were rendered quiescent by serum and methionine deprivation for 36 h. After stimulation with serum and fresh medium, cells in G2/M were purified by FACS, as described in Materials and Methods, and total cell extracts were first immunoprecipitated by anti-MyoD polyclonal antibodies. Western blot analyses for the indicated proteins are shown for either the input cell lysates or for material immunoprecipitated with anti-MyoD. Immunoprecipitates were then analyzed by Western blot with specific monoclonal antibodies for HA-MyoD, p300, or pFlag-P/CAF. (B) C2C12 myoblasts were cultured in high-mitogen medium (20% fetal calf serum containing proliferative medium [Ass]) for 48 h. C2C12 myoblasts were arrested in quiescence (G0) by incubation in methionine-depleted medium containing 1% serum for 30 h. Cells were released by addition of serum for 20 h in the presence of 500 nM nocodazole before shake-off treatment. The shake-off fraction (M, mitotic) and the adherent fraction (G2) were harvested and extracts (200 μg) from each time were analyzed by Western blot for cyclin B, MyoD, and P/CAF expression, respectively (input). Total cellular extracts (1 mg) were immunoprecipitated with anti-MyoD antibodies (C-20). The immunoprecipitates were then subjected to SDS-PAGE and transferred to nitrocellulose membrane. MyoD and p/CAF were detected by Western blot analysis. (C) 10T1/2 cells were transfected with plasmids encoding MyoDwt or MyoD,A5/A200 alone or together with HDAC1 as indicated. Eighteen hours after transfection, cells were rendered quiescent by serum deprivation for 36 h. Cells were restimulated with serum and fresh medium. Cells in G2/M were harvested. Total cellular extracts were prepared and immunoprecipitated with anti-Flag antibodies. The immunoprecipitates were then subjected to SDS-PAGE and transferred to nitrocellulose membrane. MyoD and Flag-HDAC1 were detected by Western blot analysis using anti-HA and anti-Flag monoclonal antibodies, respectively. Quantitative representation of the percentage of coimmunoprecipitated MyoD protein with HDAC1 is shown in the right panel. Values are the means of the results of two separate experiments.
MyoD mediates p21 accumulation at the onset of mitosis.
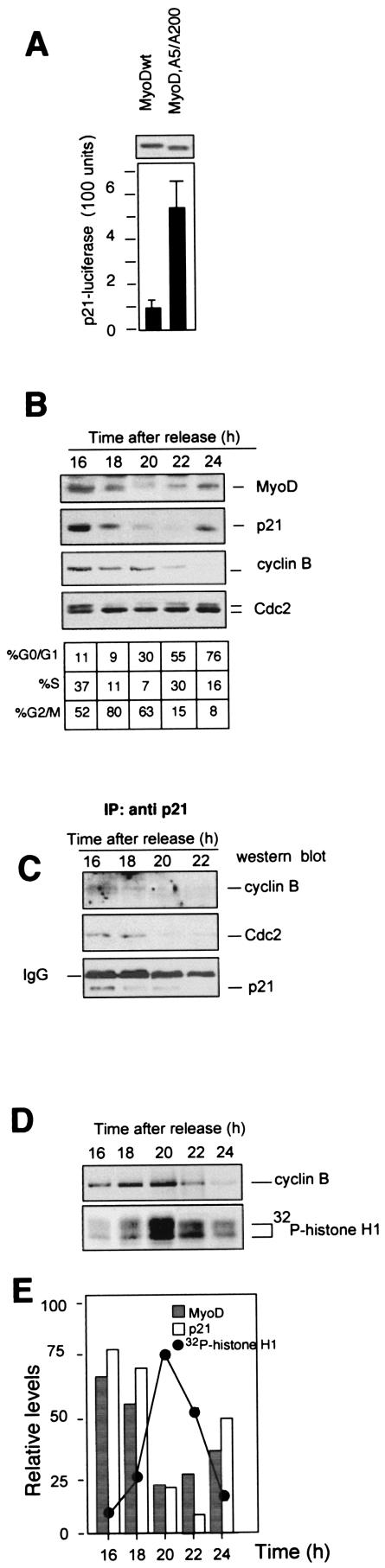
Several redundant pathways maintain a G2 checkpoint in which p21 may initially inhibit cyclin B-Cdc2, thus inducing a pause that regulates entry into mitosis (33). Because p21 is a downstream target of MyoD in myogenic cells (17), we evaluated whether MyoD could affect p21 expression in G2/M transition by examining the activity of a chromatin-integrated p21-responsive reporter (p21-Luc) and by determining the induction of endogenous p21 protein. We stably transfected the luciferase gene under the control of the promoter of p21 (p21-Luc), and we first monitored the activation of the luciferase reporter placed under the control of the p21 promoter by MyoDwt and MyoD,A5/A200. Whole-cell extracts from G2/M-enriched cells were prepared for luciferase activity and Western blot expression of MyoD proteins. As shown in Fig. 5A, we observed a much higher activation of p21-Luc by MyoD,A5/A200 than by MyoDwt. Under the same conditions, a GFP protein placed under the control of the MCK promoter was repressed in cycling cells and induced only in differentiating cells (data not shown).
FIG.5.
Up-regulation of p21 by MyoD represses cyclin B-Cdc2 kinase activity. (A) After release from G0 block, the activity of a chromatin-integrated reporter p21-Luc was measured in 10T1/2 cells transfected with MyoDwt or MyoD,A5/A200. G0-arrested cells were released into the cell cycle. Cells in G2/M were purified by FACS, and luciferase activity was monitored. Values shown are means ± standard deviations of two separate readings and represent the activity of luciferase relative to MyoD protein expression. (B) Total cell extracts from synchronized C2C12 myoblasts during G2-to-G1-phase progression were prepared at the indicated times after serum stimulation. Fifty micrograms was analyzed by SDS-PAGE and immunoblotted with specific antibodies for MyoD, cyclin B, Cdc2, and p21. (C) Two hundred micrograms of the aliquots shown in panel B were first immunoprecipitated with anti-p21 antibodies. The immunoprecipitates were probed by Western blot for their contents in p21, cyclin B, and Cdc2 proteins. IgG, immunoglobulin G. (D) Kinase activity of cyclin B-Cdc2 complexes immunoprecipitated from cell extracts (400 μg) by anti-Cdc2 antibodies was tested by using histone H1 as substrate. Shown is an autoradiograph of the radioactivity incorporated into histone H1 and a Western blot analysis of the same membrane probed for cyclin B. (E) Schematic representation of the data obtained in panels B to D.
To address the biological role of the MyoD-dependent accumulation of p21, we determined whether p21 accumulating during G2 would inhibit the activity of the cyclin B-Cdc2 complex. Cell lysates from synchronously proliferating C2C12 myoblasts were first analyzed by Western blot for MyoD, cyclin B, Cdc2, and p21 levels during G2 to G1 transition. In Fig. 5B, we show that p21 levels rapidly decreased as C2C12 myoblasts entered into mitosis and became detectable again only as cells approached the next G1 phase. To determine whether p21 is associated with active or inactive cyclin B-Cdc2 complexes (47), cell lysates were immunoprecipitated with anti-p21 antibodies and the immunoprecipitates were sequentially analyzed by Western blot for cyclin B, Cdc2, and p21. As shown in Fig. 5C, both cyclin B and Cdc2 coimmunoprecipitated with p21 in G2. Furthermore, samples of the same lysates were immunoprecipitated with anti-cyclin B antibodies, and immunoprecipitates were analyzed for cyclin B-associated kinase using histone H1 as the substrate. During G2 phase progression, a low level of cyclin B-associated kinase activity was observed in the presence of high levels of p21, and entry into mitosis was confirmed by a sharp rise of cyclin B-associated kinase activity (Fig. 5C and E). As expected, the cyclin B-associated kinase activity is high during mitotic progression and declined abruptly when myoblasts reentered G1 (7). Altogether, these results strongly suggest that a MyoD-mediated modulation of p21 protein levels participates in the control of the cyclin B-Cdc2 kinase activity during the G2 phase.
Inducible expression of mutant MyoD,A5/A200 delays mitotic entry.
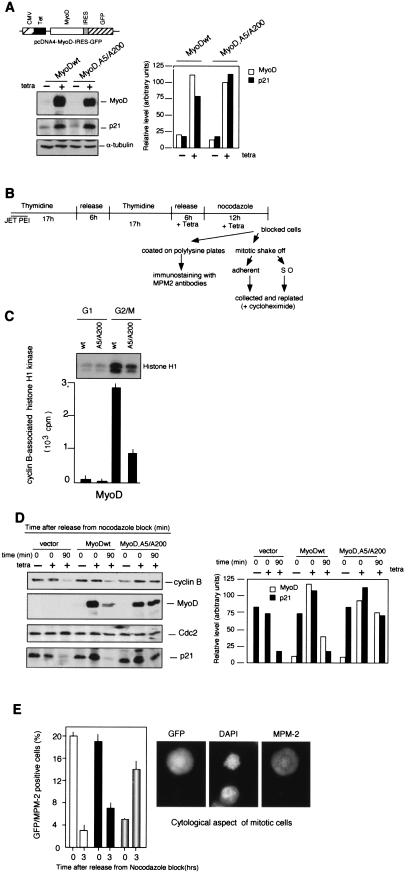
We examined whether loss of phosphorylation of MyoD would delay the entry into mitosis. We first used the tetracyclin-inducible expression system and created pcDNA4-IRES-GFP derivatives containing MyoDwt or MyoDA5/A200 and transfected T-Rex-293 cells that stably express the Tet repressor. Addition of tetracycline to the culture medium induced high levels of MyoD proteins that up-regulated p21 protein expression (Fig. 6A). Because high levels of MyoD protein induce significant G1 arrest, expression vectors encoding the MyoD proteins were activated by tetracycline after the release from G1/S block, in the presence of nocodazole, followed by recovery of the nonmitotic and mitotic fractions according to the experimental scheme illustrated in Fig. 6B. GFP-positive cells in G2/M were purified by fluorescence-activated cell sorter (FACS), and aliquots were first analyzed for the cyclin B-associated histone H1 kinase activity. Comparative analyses revealed that the cyclin B-Ccd2 activity of cells transfected by the nonphosphorylable MyoD-A5/A200 mutant was strongly reduced (by 75%) compared to that for cells transfected with MyoDwt (Fig. 6C). Secondly, cells were coated on poly-l-lysine plates with fresh medium, and 0 or 90 min after release from the G2/M block, we analyzed MyoDwt, MyoD,A5/A200, and p21 protein expression by immunoblotting. Degradation of cyclin B, which occurs in metaphase-anaphase transition (7), was used to control exit from mitosis. Indeed, 90 min after nocodazole release, cells transfected by the vector alone or encoding MyoDwt exited from mitosis and showed a down-regulation of p21 and MyoDwt, respectively (Fig. 6D). In contrast, cyclin B degradation was not observed in MyoD,A5/A200-expressing cells which maintained, to a lesser extent, MyoD and p21 protein expression. These findings suggest that MyoD,A5/A200 may be efficient in blocking entry into mitosis and/or in delaying exit from mitosis, probably by sustaining p21 expression. To answer this question, nocodazole-blocked cells were coated on poly-l-lysine plates, and 0 or 3 h after release from nocodazole block, the frequency of mitotic cells was determined by immunostaining with the MPM-2 antibody (which stains mitotic phosphoproteins) (8) and by cytological examination. In the control cells transfected with vector only, more than 20% of GFP+ cells were MPM-2+. Three hours after release from nocodazole block, the majority of GFP+ cells were in G1, and only a low percentage were in mitosis (3 to 4% GFP/MPM2+). Transfection of 293 cells with expression vector encoding MyoDwt yielded comparable results. At time zero, 20% of GFP+ cells were MPM-2+, and 3 h after release from nocodazole block the great majority of cells (>90%) had entered the G1 phase. In contrast, cells expressing the MyoD A5/A200 mutant showed a delayed passage through the mitosis. At time zero, only 4% of the GFP-positive cells were MPM-2+, and 3 h later more than 15% of GFP positive cells were in mitosis, as judged by positive staining for MPM-2 and the cytological examination (condensed chromosomes and nuclear envelope breakdown) (Fig. 6E). These observations suggest that conditional expression of a nonphosphorylable MyoD,A5A/A200 in T-Rex-293 cells delays entry into mitosis.
FIG.6.
Mutant MyoD A5/A200 delays M-phase entry. (A) T293 T-Rex cells were transfected with pcDNA4-IRES-GFP encoding MyoDwt or the mutant MyoD,A5/A200. Cells were induced by addition of tetracycline for 6 h. MyoD, p21, and alpha-tubulin proteins were analyzed by Western blot (left panel), and graphic display of intensities of MyoD and p21 are shown (right panel). The signals were quantitated by a Gelscan (Pharmacia). (B) Scheme of the protocol applied to T293 T-Rex to obtain a transfected population released into the cell cycle after synchronization in G2/M. Cells were transfected by JET PEI and enriched in G1/S phase by double thymidine treatment (17 h). During the release period in drug-free medium, tetracyclin was added, followed by nocodazole (12 h). When the cells detached from the plate with a rounded phenotype, indicating a G2/M enrichment, the shake-off fraction (mitotic) and adherent fraction (G2) were harvested together and coated on polylysine plates and/or separately replated to be collected at the indicated point. (C) GFP-positive cells gated in G2/M were purified and analyzed for cyclin B-associated histone H1 kinase. Whole-cell extracts (200 μg) were immunoprecipitated with antibodies to cyclin B and assayed for kinase activity by using histone H1 as substrate. Cells in G1 were used as negative controls. (D) GFP-positive cells gated in G2/M as described in panel B were purified and replated in fresh medium in the absence of nocodazole and then harvested at the indicated time. Cells incubated with nocodazole but in the absence of tetracycline were used as controls (−). Total cellular extracts were separated in SDS-PAGE and after transfer on nitrocellulose membranes, proteins were analyzed for cyclin B, MyoD, Cdc2, and p21 expression by immunoblotting (left panel), and a graphic display of intensities of MyoD and p21 are shown (right panel). The signals were quantitated by a Gelscan (Pharmacia). (E) Cells coated on polylysine plates according to the scheme shown in panel B. They were released from nocodazole block by addition of fresh medium and then treated for immunofluorescence microscopy at time zero and 3 h after release. The frequency of mitotic cells was determined after immunostaining of MPM-2 antigens and cytological aspect of GFP-positive cells was also determined. White bars, empty vector; black bars, pcDNA4-MyoDwt-IRES-GFP; partially shaded bars, pcDNA4-MyoDA5/A200-IRES-GFP.
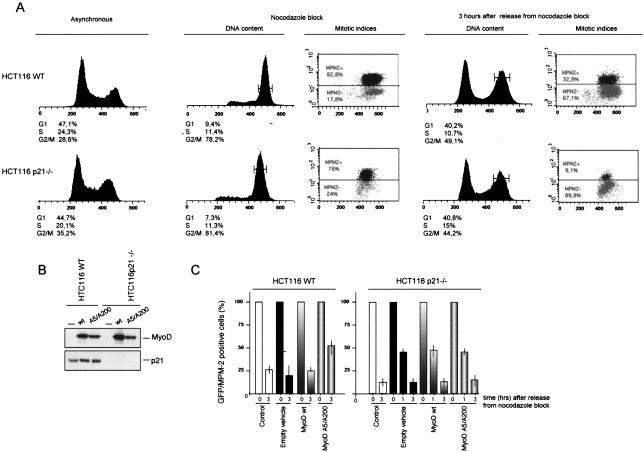
To confirm that MyoD participates via p21 in the G2 checkpoint, we took advantage of the HTC 116 cell line and its p21−/− derivative (4). Both cell lines exhibited mitotic arrest in response to nocodazole treatment (Fig. 7A). pcDNA4-IRES-GFP derivatives containing MyoDwt or MyoDA5/A200 were transfected in HTC116 wild-type and/or HTC116p21−/− cells, and MyoD and p21 protein expression was determined in total extracts from nocodazole-treated cells. As shown in Fig. 7B, enforced expression of MyoDwt and/or MyoD A5/A200 induced a slight increase in p21 expression in the parental HTC116 wild-type cells, while no p21 synthesis occurred in HTC116 p21−/− cells. Transfected cells were released from nocodazole block, and the frequency of mitotic GFP+ cells was determined by MPM-2 staining at time zero and 3 h after nocadazole removal. As described above for T293 cells, expression of vector alone or a vector encoding MyoDwt led to comparable results. Three hours after release from nocodazole block, more than 75% of GFP-positive cells had gone through mitosis and were in G1. Expression of MyoD A5/A200 led to a delay in mitosis in p21-sufficient HTC116 cells. Thus, 3 h after release from nocodazole block, more than 50% of GFP-positive cells were in mitosis (MPM-2+) (Fig. 7C). In strict contrast, p21-deficient HTC116 p21−/− cells expressing either MyoDwt or MyoDA5/A200 entered mitosis with indistinguishable, normal kinetics (Fig. 7C). These results indicate that a nonphosphorylable MyoD delays M-phase entry by sustaining p21 expression.
FIG. 7.
Mutant MyoD A5/A200 has no effect on mitotic time course progression in HCT116 p21−/− cells. (A) Flow cytometry analysis of asynchronous cultures of HCT116 and HCT116 p21−/− cells treated 20 h with nocodazole (100 nM). Cells were harvested at the indicated times, and FACS profiles of DNA content (determined after Hoechst 33352 staining) are shown. G2/M cells were isolated (through cell sorting at the indicated time), and mitotic indices were scored by MPM-2 staining as described in Materials and Methods. The values obtained are an average of the results of two independent experiments with a minimum of 15,000 events for each single determination. (B) GFP-positive cells gated in G2/M were purified, and whole-cell extracts (200 μg) were analyzed by Western blot with antibodies to MyoD and p21, respectively. (C) Schematic representation of mitotic progression of HCT116 wild-type and HCT116 p21−/− cells (white bars), transfected with empty vehicle (black bars), MyoD wild type wild type (bars with graduated shading) or MyoD A5/A200 (bars outlined in gray). After being blocked by nocodazole, G2/M transfected cells were isolated by cell sorter. The frequency of mitotic cells was determined after immunostaining of MPM-2 antigens and cytological aspect of GFP-positive cells was also determined. The shaded bars represent the means of the results for mitotic MPM2 cells determined in at least two independent experiments. The amount of MPM-2-positive cells at time zero was set at 100%.
Phosphorylation of MyoD in late G2 is required for its rapid degradation.
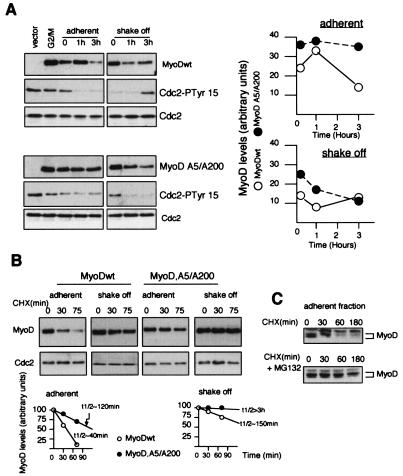
Phosphorylation may be involved in the degradation of MyoD as well as in its transcriptional activity (21, 41, 44). The abundance of MyoDwt and MyoD,A5/A200 was measured in adherent (G2) and mitotic shake-off (mitosis) 293 cells, obtained as schematized in Fig. 6B, after tetracyclin-mediated induction of the protein. Cells from the adherent fractions were recultured in the presence of fresh medium without nocodazole, and total cell lysates were analyzed by Western blot at various times after release of nocodazole block. As shown in Fig. 8A, MyoDwt cells entered into mitosis, as determined by the total disappearance of inactive phosphorylated Cdc2 (Cdc2-PTyr15) and the decrease in MyoD levels. In contrast, MyoD,A5/A200 cells were blocked in G2, as evidenced by high levels of inactive Cdc2-PTyr15. Three hours after replating, a large amount of MyoD,A5/A200 cells entered into mitosis, correlating with the disappearance of Cdc2-PTyr15. During the same period, MyoDwt protein levels were remarkably stable. On the other hand, by releasing the shake-off fractions in fresh medium leading to mitotic exit, we confirmed that MyoDwt cells passed through mitosis. Three hours later, MyoDwt cells were effectively in G1 and showed increasing amounts of MyoD proteins and inactive Cdc2 (Cdc2-PTyr15). In contrast, MyoD,A5/A200 cells exhibited a delayed entry into mitosis, while the abundance of MyoD,A5/A200 protein decreased slowly. It is well known that the sharp increase of cyclin B-Cdc2 activity just before entry in mitosis is essential for proper execution of mitotic events (10). To dissect the mechanisms that determine the abundance of MyoD, we examined the stability of MyoD wild type and MyoD,A5/A200 in adherent and mitotic shake-off fractions. Inhibition of protein synthesis by cycloheximide allowed us to estimate that MyoDwt had a half-life of ∼40 min and that MyoD,A5/A200 had a half-life of ∼120 min (Fig. 8B) in adherent cells. In contrast, in the mitotic shake-off fractions, both MyoDwt and MyoD,A5/A200 were extremely stable, as judged by extended half-lives of up to 3 h. Addition of the proteasome inhibitor MG132 to the culture medium of adherent fractions confirmed that MyoDwt is destabilized in late G2 via the proteasome pathway (Fig. 8C). Altogether, these results suggest that phosphorylation and a phosphorylation-independent pathway, which is probably specific to the G2 phase, could be implicated in MyoD degradation.
FIG. 8.
Increasing stability of MyoD in mitosis. (A) Cells from the shake-off fraction (mitotic) and adherent fraction (G2) were collected separately, replated in fresh medium in the absence of nocodazole, and then harvested at the indicated time. Total cellular extracts were separated in SDS-PAGE, and after transfer to nitrocellulose membranes, proteins were analyzed for MyoD, Cdc2, and the inactive tyrosine-15-phosphorylated Cdc2 (Cdc2-PTyr15) by immunoblotting with anti-MyoD, anti Cdc2, and anti-Cdc2 Tyr15-P antibodies. Quantitative representation of the MyoD protein expression is shown in the right panel. (B) The shake-off fraction (mitotic) and the adherent fraction (G2) were harvested and replated in the presence of cycloheximide (CHX) according to the scheme used for panel B and for the indicated time point (min). Protein stability of MyoD was analyzed by immunoblotting with specific anti-MyoD antibodies. Cdc2 detection was used to normalize for equal gel loading. A graphic display of intensities of MyoD derivatives is shown in B. The results are representative of two independent experiments. For quantitation, the autoradiograms were scanned with a Gelscan. (C) The adherent fraction was treated as in panel B in the presence of 50 μM MG132 (added 30 min before cycloheximide treatment) and analyzed by immunoblot.
DISCUSSION
MyoD and Myf-5 exhibit a distinct profile of cell cycle-specific expression in myoblasts (20, 23, 24). Recently, MyoD has been found to regulate genes expressed at different times during myogenesis by promoter-specific binding, thus contributing to the regulation of gene expression in normal differentiation (1, 2) as well as in undifferentiated proliferating muscle cells (46). In synchronized myoblasts emerging from quiescence, MyoD shows a bimodal pattern of expression (20, 44). In growing myoblasts, the phosphorylation and degradation of MyoD have been shown to represent the regulatory checkpoint that controls the decision to undergo a G1→G0 transition with terminal differentiation (22). The present data are consistent with the notion that reaccumulation of transcriptionally active hypophosphorylated MyoD participates in the control of the G2/M transition by modulating cyclin B-Cdc2 kinase via p21 expression, a process most likely mediated by P/CAF. This data sheds light on a new regulatory pathway in which MyoD is transcriptionally active in proliferating myoblasts.
Phosphorylation of MyoD has been recognized to suppress the MyoD-mediated myogenic differentiation (16, 36). Phosphorylation of Ser200 by cyclin E-Cdk2 kinase is required to induce degradation of MyoD in response to growth factors, whereas neither Cdk4 nor Cdk5 is able to phosphorylate MyoD (21, 44, 48, 49) in spite of the fact that both Ser5 and Ser200 are potential (Ser/Thr-Pro) CDK consensus sites (42). In contrast, cyclin B-Cdc2-mediated phosphorylation of both Ser5 and Ser200 has been observed in vitro (21) (Fig. 2) and in vivo after entry into mitosis when cyclin B-Cdc2 complexes become physiologically active and essential for progression through mitosis (Fig. 1 and 2). Phosphorylation of the transcriptional machinery is implicated in its repression at mitosis, and changes in chromatin structure and occupancy of promoter elements by both general and gene-specific transcription factors also play a role in transcriptional silencing (15, 26, 38). Phosphorylation of Ser200 has been implicated both in the reduction of activity and in the degradation of MyoD (21, 41). The dual phosphorylation of MyoD is observed in vitro only after cyclin B-Cdc2 phosphorylation and in late G2 and mitosis. During mitosis, MyoD has a half-life 2.5 times longer than in interphase (more than 100 min versus 45 min in interphase). Mutations of both Ser5 and Ser200 inhibit the repression of the transcriptional activity of MyoD by cyclin B-Cdc2 (Fig. 3). Together, these data suggest that Ser5, which is located in the transactivation domain of MyoD, could act as a super repressor of transcriptional activity of MyoD once it has been phosphorylated by cyclin B-Cdc2.
Acetylation by the histone acetyltransferase P/CAF and p300 plays a key role in regulating the transcriptional activity of MyoD (34, 35, 39). Association of MyoD with P/CAF has been observed throughout the myogenic process, and the interaction of MyoD with histone acetyltransferases takes place only when MyoD is hypophosphorylated (25). Although p300/CBP and P/CAF are relatively abundant in transfected cells, we did not observe any association between MyoDwt and/or MyoD,A5/A200 and p300 in G2/M-enriched cells. The simplest explanation for this result may be that the epitope recognized by the HA antibody became inaccessible because of the binding of p300, thus precluding a coimmunoprecipitation. However, the histone acetyltransferase activity of P/CAF but not that of p300 has recently been reported to be important for endogenous p21 expression and cell cycle arrest in myogenic cells (35). Preferential interaction between hypophosphorylated MyoD and P/CAF could modulate the specific transcriptional activity of MyoD (Fig. 9). Indeed, the association between endogenous P/CAF and endogenous MyoD was observed in G2 (when MyoD is not phosphorylated). The interaction between P/CAF and nonphosphorylabel MyoD,A5/A200 was stronger than that with wild-type MyoD, again suggesting that phosphorylated MyoD may fail to interact with P/CAF, which would thus preclude its P/CAF-mediated acetylation and activation. HDAC1 can repress transcription by binding to MyoD in growing cells (25). Indeed, we found that phosphorylation of MyoD increases its interaction with HDAC1 (Fig. 4B), which may repress the interaction with P/CAF and hence the transcriptional actvity of MyoD. During the early phases of myogenic differentiation, the level of CKI p57Kip2 increases markedly and induces the accumulation of hypophosphorylated MyoD (36). Thus, phosphorylation and acetylation processes are closely implicated in the control of the transcriptional activity of MyoD.
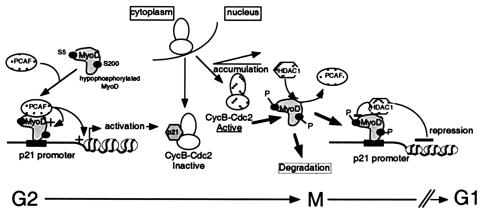
FIG. 9.
Model for in vivo p21 expression by MyoD and HDAC or P/CAF in G2/M muscle cells. During G2 in myoblasts, nuclear accumulation of hypophosphorylated MyoD activates expression of p21 that binds to cyclin B-Cdc2 complexes before late G2. This p21 accumulation may regulate entry into mitosis through the modulation of the activity of the cyclin B-Cdc2 complex. Increasing accumulation of nuclear cyclin B-Cdc2 in late G2 induces phosphorylation of MyoD, leading to p21 down-regulation and destabilization of MyoD until entry into mitosis.
It is presently assumed that the main targets for MyoD are the genes involved in the terminal differentiation process of muscle cells, a process that would depend on proper environmental conditions, such as reduced growth. It remains unclear why MyoD is expressed in undifferentiated myoblasts. MyoD expression is observed immediately following the activation of quiescent satellite cells, prior to the expression of proliferating cell nuclear antigen, or to the induction of cell proliferation. Mice lacking both Myf-5 and MyoD exhibit a complete absence of myoblasts, and MyoD-null mice are defective for satellite-cell activation. These observations suggest that MyoD regulates myoblast genes that are distinct from the MyoD-regulated genes that are necessary for terminal differentiation. Indeed, MyoD exhibits distinct transcriptional activity in growing myoblasts as well as a unique embryonic expression pattern (1, 2, 46). An increase in MyoD protein levels in G2 and a decrease in mitosis raised the possibility that MyoD might play a role in G2/M transition. Our data demonstrate that before the cells enter mitosis MyoD transcriptionally transactivates the p21 gene. Thus, MyoD and p21 exhibit a parallel pattern expression in synchronized myoblasts (Fig. 5 and data not shown). p21 acts primarily as a negative cell cycle regulator of the G1/S arrest (6). Its reaccumulation in G2 phase contributes to the implementation of the late cell cycle checkpoint. Several distinct mechanisms have been postulated for how p21 participates in inhibiting Cdc2 activity and causing G2 arrest (43). p21 may repress CDK activity by binding directly to cyclin B-Cdc2 complexes (2) (Fig. 5). Moreover, p21 reduces the active phosphorylation of Cdc2 of threonine 161 by Cdk-activating kinase (40). Near the end of G2 but before the prophase, cyclin B accumulates in the cytoplasm (7). Abrupt relocalization of cyclin B to the nucleus occurs shortly before chromatin condensation and entry in mitosis (10). In late G2, cyclin B-Cdc2 phosphorylates MyoD, resulting in its destabilization and in the repression of its transcriptional activity. These events suppress the ability of MyoD to activate p21 promoter. In contrast, elevated levels of p21 protein in response to expression of nonphosphorylable mutant MyoD maintain low levels of Cdc2 kinase, conferring a reversible G2 pause in myoblasts. Altogether, this scenario supports the role for cyclin B-Cdc2 phosphorylation of MyoD in the control of its transcriptional activity and stability in G2/M transition.
MyoD protein reaccumulating in the G2 phase must be degraded before cells enter into mitosis. MyoD degradation is triggered by its phosphorylation and presumably occurs in a proteasome-dependent fashion, as suggested by the fact that a proteasome inhibitor prevents degradation of phosphorylated MyoD in G2 (Fig. 8). However, in mitosis MyoD is stabilized irrespective of its phosphorylation status, suggesting that the molecular mechanism implicated in its degradation acts only in late G2. Identification of a functional D-box motif in the b-HLH domain of MyoD leads us to hypothesize that MyoD could be a substrate of the anaphase-promoting complex (APC)-cyclosome complex. Our results indicate that neither the CDC20 nor the CDH1 coactivators of APC interact in vitro or in vivo with MyoD (unpublished data). Recently, a D-box-like motif overlapping the basic domain has been implicated in the constitutive instability of Myf-5 in mitosis (24). However, the degradation of Myf-5 in mitotic cells involves a mechanism distinct from that which regulates known substrates of APC. Furthermore, a version of this motif mutated by substitution of a single residue (Q101), which more closely resembles the homologous motif present in MyoD, also has a stabilizing effect on Myf-5 in mitosis (24). These findings suggest that the b-HLH region of MyoD and Myf-5 might determine their different stability in mitosis and may participate in recognition of phosphorylated MyoD by a different ubiquitin ligase targeting it to proteasome-mediated degradation. Alternatively, it may be speculated that the D-box of Myo D could be recognized by the APC operating at a different stage of the cell cycle than that which has traditionally been assumed (13, 14).
In conclusion, our results establish a novel role for MyoD in the cell cycle. MyoD expressed in the G2 phase can interact with its coactivator P/CAF to stimulate p21, thus controlling cyclin B-Cdc2 kinase activity and hence progression through the G2/M boundary. Phosphorylation of MyoD by cyclin B-Cdc2 kinase results in its inaction. The dropping of MyoD below a threshold level could well represent the critical event required for triggering myoblast mitosis (Fig. 5).
Acknowledgments
We are grateful to Anne Fernandez and Ned Lamb for critical reading of the manuscript.
L. A. Tintignac is a fellow of Ministère de la Recherche et de la Technologie (MRT). V. Sirri is supported by the Institut Gustave Roussy. This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, and grants from Ligue Nationale contre le Cancer, Association pour la Recherche sur le Cancer (A.R.C. no. 5921), and the Institut Gustave Roussy.
REFERENCES
- 1.Bergstrom, D. A., and S. J. Tapscott. 2001. Molecular distinction between specification and differentiation in the myogenic basic helix-loop-helix transcription factor family. Mol. Cell. Biol. 21:2404-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergstrom D. A., B. H. Penn, A. Strand, R. L. Perry, M. A. Rudnicki, and S. J. Tapscott. 2002. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell 9:587-600. [DOI] [PubMed] [Google Scholar]
- 3.Breitschopf, K., E. Bengal, T. Ziv, A. Admon, and A. Ciechanover. 1998. A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J. 17:5964-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, B. Vogelstein, and K. W. Kinzler. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497-1501. [DOI] [PubMed] [Google Scholar]
- 5.Chang, B.-D., K. Watanabe, E. V. Broude, J. Fang, J. C. Poole, T. V. Kalinichenko, and I. B. Roninson. 2000. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc. Natl. Acad. Sci. USA 97:4291-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21Cip1 and p27Kip1 CDK ′inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clute, P., and J. Pines. 1999. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 2:82-87. [DOI] [PubMed] [Google Scholar]
- 8.Davis, F. M., T. Y. Tsao, S. K. Fowler, and P. N. Rao. 1983. Monoclonal antibodies to mitotic cells. Proc. Natl. Acad. Sci. USA 10:2926-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, R. L., P. Cheng, A. Lassar, and H. Weintraub. 1990. The MyoD DNA binding domain contains a recognition code for muscle-specific gene activation. Cell 60:733-746. [DOI] [PubMed] [Google Scholar]
- 10.Dulic, V., G. H. Stein, D. F. Far, and S. I. Reed. 1998. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol. Cell. Biol. 18:546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edmondson D. G., and E. N. Olson. 1993. Helix-loop-helix proteins as regulators of muscle-specific transcription. J. Biol. Chem. 268:755-758. [PubMed] [Google Scholar]
- 12.Flatt, P. M., L. J. Tang, C. D. Scatena, S. T. Szak, and J. A. Pietenpol. 2000. p53 regulation of G2 checkpoint is retinoblastoma protein dependent. Mol. Cell. Biol. 20:4210-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gieffers, C., B. H. Peters, E. R. Kramer, C. G. Dotti, and J. M. Peters. 1999. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc. Natl. Acad. Sci. USA 96:11317-11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.. Glotzer, M., A. W. Murray, and M. W. Kirschner. 1991. Cyclin is degraded by the ubiquitin pathway. Nature 349:132-138. [DOI] [PubMed] [Google Scholar]
- 15.Gottesfeld, J. M., V. J. Wolf, T. Dang, D. J. Forbes, and P. Hartl. 1994. Mitotic repression of RNA polymerase III transcription in vitro mediated by phosphorylation of a TFIIIB component. Science 263:81-84. [DOI] [PubMed] [Google Scholar]
- 16.Guo, K., and K. Walsh. 1997. Inhibition of myogenesis by multiple cyclin-Cdk complexes. Coordinate regulation of myogenesis and cell cycle activity at the level of E2F. J. Biol. Chem. 272:791-797. [DOI] [PubMed] [Google Scholar]
- 17.Halevy, O., B. G. Novitch, D. B. Spicer, S. X. Skapek, J. Rhee, G. J. Hannon, D. Beach, and A. B. Lassar. 1995. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 267:1018-1021. [DOI] [PubMed] [Google Scholar]
- 18.Harper, J. W., S. J. Elledge, K. Keyomarsi, B. Dynlacht, L. H. Tsai, P. Zhang, S. Dobrowolski, S. Bai, L. Connell-Crowley, E. Swindell, et al. 1995. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 6:387-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper, J. W., and S. J. Elledge. 1996. Cdk inhibitors in development and cancer. Curr. Opin. Genet. 6:56-64. [DOI] [PubMed] [Google Scholar]
- 20.Kitzmann, M., G. Carnac, M. Vandromme, M. Primig, N. J. C. Lamb, and A. Fernandez. 1998. The muscle regulatory factors MyoD and Myf-5 undergo distinct cell cycle-specific expression in muscle cells. J. Cell Biol. 142:1447-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitzmann, M., M. Vandromme, V. Schaeffer, G. Carnac, J.-C. Labbe, N. Lamb, and A. Fernandez. 1999. cdk1- and cdk2-mediated phosphorylation of MyoD Ser200 in growing C2 myoblasts: role in modulating MyoD half-life and myogenic activity. Mol. Cell. Biol. 19:3167-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitzmann, M., and A. Fernandez. 2001. Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell. Mol. Life Sci. 58:571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindon C., D. Montarras, and C. Pinset. 1998. Cell cycle-regulated expression of the muscle determination factor Myf5 in proliferating myoblasts. J. Cell Biol. 140:111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindon, C., O. Albagli, P. Domeyne, D. Montarras, and C. Pinset. 2000. Constitutive instability of muscle regulatory factor Myf5 is distinct from its mitosis-specific disappearance, which requires a D-box-like motif overlapping the basic domain. Mol. Cell. Biol. 20:8923-8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mal, A., M. Sturniolo, R. L. Schiltz, M. K. Ghosh, and M. L. Harter. 2001. A role for histone deacetylase HDAC1 in modulating the transcriptional activity of MyoD: inhibition of the myogenic program. EMBO J. 20:1739-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Balbas, M. A., M. Dey, S. K. Rabindran, K. Ozato, and C. Wu. 1995. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell 83:29-38. [DOI] [PubMed] [Google Scholar]
- 27.Megeney, L. A., B. Kablar, K. Garrett, J. E. Anderson, and M. A. Rudnicki. 1996. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 10:1173-1183. [DOI] [PubMed] [Google Scholar]
- 28.Murre, C., P. S. McCaw, H. Vaessin, M. Caudy, L. Y. Jan, Y. N. Jan, C. V. Cabrera, J. N. Buskin, S. D. Hauschka, A. B. Lassar, et al. 1989. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell 58:537-544. [DOI] [PubMed] [Google Scholar]
- 29.Niculescu, A. B., III, X. Chen, M. Smeets, L. Hengst, C. Prives, and S. I. Reed. 1998. Effects of p21Cip1/Waf1 at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell. Biol. 18:629-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connell, M., N. Walworth, and A. Carr. 2000. The G2-phase DNA damage checkpoint. Trends Cell Biol. 7:296-303. [DOI] [PubMed] [Google Scholar]
- 31.Olson, E. N., and W. H. Klein. 1994. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1:1-8. [DOI] [PubMed] [Google Scholar]
- 32.Parker, S. B., G. Eichele, P. Zhang, A. Rawls, A. Sands, A. Bradley, E. N. Olson, J. W. Harper, and S. J. Elledge. 1995. p53-independent expression of p21 CIP1 in muscle and other terminally differentiating cells. Science 267:1024-1027. [DOI] [PubMed] [Google Scholar]
- 33.Passalaris, T. M., J. A. Benanti, L. Gewin, T. Kiyono, and D. A. Galloway. 1999. The G2 checkpoint is maintained by redundant pathways. Mol. Cell. Biol. 19:5872-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polesskaya, A., A. Duquet, I. Naguibneva, C. Weise, A. Vervisch, E. Bengal, F. Hucho, P. Robin, and A. Harel-Bellan. 2000. CREB-binding protein/p300 activates MyoD by acetylation. J. Biol. Chem. 275:34359-34364. [DOI] [PubMed] [Google Scholar]
- 35.Puri, P. L., V. Sartorelli, X. J. Yang, Y. Hamamori, V. V. Ogryzko, B. H. Howard, L. Kedes, J. Y. Wang, A. Graessmann, Y. Nakatani, and M. Levrero. 1997. Differential roles for p300 and PCAF acetyltransferases in muscle differentiation. Mol. Cell 1:35-45. [DOI] [PubMed] [Google Scholar]
- 36.Reynaud, E. G., K. Pelpel, M. Guillier, M.-P. Leibovitch, and S. A. Leibovitch. 1999. p57Kip2 stabilizes the MyoD protein by inhibiting cyclin E-Cdk2 kinase activity in growing myoblasts. Mol. Cell. Biol. 19:7621-7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynaud, E. G., M. P. Leibovitch, L. A. J. Tintignac, K. Pelpel, M. Guillier, and S. A. Leibovitch. 2000. Stabilization of MyoD by direct binding to p57Kip2. J. Biol. Chem. 275:18767-18776. [DOI] [PubMed] [Google Scholar]
- 38.Roberts, S. B., N. Segil, and N. Heintz. 1991. Differential phosphorylation of transcription factor Oct1 during the cell cycle. Science 253:1022-1026. [DOI] [PubMed] [Google Scholar]
- 39.Sartorelli, V., P. L. Puri, Y. Hamamori, V. Ogryzko, G. Chung, Y. Nakatani, J. Y. Wang, and L. Kedes. 1999. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell 4:725-734. [DOI] [PubMed] [Google Scholar]
- 40.Smits, V. A. J., R. Klompmaker, T. Vallenius, G. Rijksen, T. P. Makela, and R. H. Medema. 2000. p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J. Biol. Chem. 275:30638-30643. [DOI] [PubMed] [Google Scholar]
- 41.Song, A., Q. Wang, M. G. Goebl, and M. A. Harrington. 1998. Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol. Cell. Biol. 18:4994-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Songyang, Z., S. Blechner, N. Hoagland, M. F. Hoekstra, H. Piwnica-Worms, and L. C. Cantley. 1994. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr. Biol. 4:973-982. [DOI] [PubMed] [Google Scholar]
- 43.Taylor, W. R, and G. R. Stark. 2001. Regulation of the G2/M transition by p53. Oncogene 20:1803-1815. [DOI] [PubMed] [Google Scholar]
- 44.Tintignac, L. A., M. P. Leibovitch, M. Kitzmann, A. Fernandez, B. Ducommun, L. Meijer, and S. A. Leibovitch. 2000. CyclinE-Cdk2 phosphorylation-dependent degradation of MyoD in muscle cells. Exp. Cell Res. 259:300-307. [DOI] [PubMed] [Google Scholar]
- 45.Weintraub, H. 1993. The MyoD family and myogenesis: redundancy, networks and thresholds. Cell 75:1241-1244. [DOI] [PubMed] [Google Scholar]
- 46.Wyzykowski, J. C., T. I. Winata, N. Mitin, E. J. Taparowsky, and S. F. Konieczny. 2002. Identification of novel MyoD gene targets in proliferating myogenic stem cells. Mol. Cell. Biol. 17:6199-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, H., G. J. Hannon, and D. Beach. 1994. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 147:8-15. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, J.-M., Q. Wei, X. Zhao, and B. M. Paterson. 1999. Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with cdk4. EMBO J. 18:926-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, J.-M., X. Zhao, Q. Wei, and B. M. Paterson. 1999. Direct inhibition of G1 cdk kinase activity by MyoD promotes myoblast cell cycle withdrawal and terminal differentiation. EMBO J. 18:6983-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, P., C. Wong, D. Liu, M. Finegold, J. W. Harper, and S. J. Elledge. 1999. p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes Dev. 13:213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]