Abstract
Drosophila melanogaster from Zimbabwe and nearby regions shows strong but asymmetric sexual isolation from its cosmopolitan counterparts. By creating stable chromosome-substitution lines, earlier studies were able to show that the two major autosomes have very large effects on both male mating success and female mating preference. In this study, we genetically dissect this sexual isolation by recombination analysis between a whole-chromosome substitution line (which carries a Zimbabwe-derived third chromosome) and a strain with seven visible markers on that chromosome. Four loci are responsible for male mating success and three others are found to control female mating preference. Because male and female traits are not closely linked, their strong association among isofemale lines is most likely a reflection of sexual selection in nature. The results suggest that a large number of behavioral loci may evolve concurrently in the incipient stage of speciation before other aspects of reproductive isolation (such as hybrid sterility) have become evident. The results shed light on the population genetic processes underlying the formation of nascent species, as well as modes of speciation.
Genetic studies of speciation in the last decade have made substantial progress in the area of postmating reproductive isolation (see recent reviews in refs. 1–3). In general, even closely related species are extensively differentiated in their genetics of reproductive biology (3–5). The cloning of “speciation genes,” including proteins of gamete recognition in marine invertebrates (6) and genes of hybrid-male sterility in Drosophila (7), has substantiated this genetic perspective. Between closely related species, such genes are often extensively divergent, bearing the signature of positive Darwinian selection. This trend is true even for genes belonging to the most conservative of gene families, as in the case of Odysseus (7).
In contrast to postmating isolation, the genetic basis of premating isolation is still poorly understood. A well delineated genetic architecture is central to the theories of speciation (see Discussion) and is a prerequisite for molecular analysis. One approach to studying premating isolation traits is by means of the F2 analysis (e.g., refs. 8–11 and see review in ref. 2), which is widely used in quantitative trait loci (QTL) mapping. This technique attempts to correlate phenotype with genotype at multiple loci among the F2 progeny. The F2 analysis would work well with traits that can be quantified easily and that have a simple genetic architecture. Recent studies of differences in the morphology of male genitalia among different Drosophila species used such an approach and as many as 19 QTLs were identified (12, 13). In this example, multiple genes with small effects are responsible for morphological divergence among species. These studies suggest that reproductive and morphological divergence may have a “diffuse” genetic basis, with many weak-effect loci that may or may not interact strongly (1).
What then may be the genetic basis of behavioral isolation? The analysis of sexual isolation is much more difficult than either postmating isolation or morphological differentiation because behavioral characters are often labile. In conventional F2 quantitative trait loci mapping, each unique genotype is represented by a single individual. When the phenotype has incomplete penetrance and variable expressivity such as behavior traits, it would be desirable to establish a homogeneous line for each genotype that can be measured repeatedly. An attempt to create such homogeneity is in the Drosophila study by Zouros (10). Zouros used nonrecombining F1 hybrid males as the backcross parent to create F2 segregants, which thus consist of whole-chromosome substitution genotypes. Unfortunately, these whole-chromosome substitutions cannot be made into perpetual lines in the species used. Such construction is feasible only with a whole set of “balancer chromosomes.” Hollocher et al. (14) may be the only study that obtained complete sets of chromosome-substitution lines between two behavioral races. In this report, we study the same system of sexual isolation between the Zimbabwe (Z) and worldwide (M) populations of Drosophila melanogaster (15) by creating a series of recombinant lines at the subchromosomal level.
When given a choice, D. melanogaster females of the Z race (from Zimbabwe and southern Africa) mate only with males from the same race, whereas females from the cosmopolitan M race (M for melanogaster of the common type) mate with males of both races indiscriminately (14, 15). The high level of behavioral polymorphism in many populations (16), together with the asymmetric mating preference and the incomplete postmating isolation (15, 17), indicates that these two races are at an incipient stage of speciation. DNA polymorphism studies showing limited genetic differentiation at autosomal loci between the races corroborate the interpretation of nascent species (18–20). In this study, we focus on the third chromosome because it accounts for more than 50% of the total genetic effect on both male mating success and female preference (14). By increasing the resolution down to the subchromosomal level, we are able to estimate the number of genes and identify possible interactions among loci affecting the behavior of either sex.
Materials and Methods
Fly Stocks.
Two isofemale lines from Zimbabwe, Z30 and Z53, and one from France, FrV3–1 (or Fr), were chosen as the standard Z and M lines, respectively. The mating behaviors of these lines have been described briefly above and the details can be found in studies by Wu et al. (15) and Hollocher et al. (14, 16). A multimarker line was used to construct recombinant lines for genetic analysis. This line carries eight visible markers on the third chromosome: roughoid (ru, 3–0, 61F5–62A3), hairy (h, 3–26.5, 66D15), thread (th, 3–43.2, 72B1), scarlet (st, 3–44.0, 73A3–4), curled (cu, 3–50.0, 86D1–4), stripe (sr, 3–62.0, 90E-F), ebony (e, 3–70.7, 93D2), and claret (ca, 3–100.7, 99B11-C1). The genetic and cytological map positions of the mutations are as described by Lindsey and Zimm (21). This marker line is M type in mating characters. Fly stocks were reared on corn meal medium and kept under a 12-h light/dark cycle in a constant-temperature room (23°C) with humidity control.
Constructing Chromosomal Recombinant Lines.
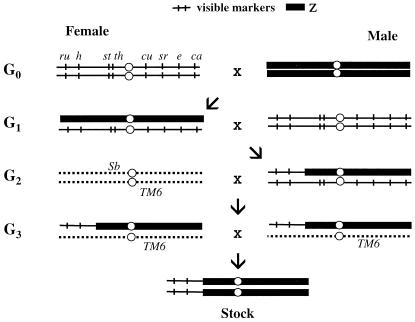
The third chromosome recombinants were generated between the multimarker line described above and an MMZ line, which is a whole-chromosome substitution line constructed by Hollocher et al. (14). This particular MMZ line has the first and the second chromosomes from the Fr line and the third chromosome from Z30. The schematic representation of the crosses for constructing the recombinant lines are given in Fig. 1. A wild-type MMZ line that went through the same scheme without recombining with any of the markers was kept as a control.
Figure 1.
An example of the crosses performed to construct the third chromosome recombinant lines between the MMZ line (with the first and second chromosome from M and third from Z) and the multimarker line (which is MMM behaviorally). Thick bars indicate Z chromosomes and thin bars with ticks denote the marker chromosomes. Dotted lines indicate the balancer chromosome, TM6, which suppresses recombination. Each type of recombinant is selected as multiple G2 males and propagated in subsequent generations. When a recombinant chromosome cannot be established as a homozygous stock (because of linked lethals), it is kept with the balancer, TM6.
Behavior Test and Data Analysis.
The mating design and analysis are described by Wu et al. (15) and Hollocher et al. (14, 16). Briefly, flies, aged 3–7 days, were fed with red- or green-colored food 1 day before the behavior test. Standard double- choice experiments were performed around the time the daily light cycle started and at a constant temperature of 23°C. Normally, about 60–70 flies of each sex and genotype were released into a mating cage (hence 240–280 flies per cage). Copulating pairs were retrieved for typing by the food coloring. In testing male mating success (or Z-maleness), the double-choice experiments were designed to compare the preference of Z females (Z30 or Z53) for α males over β males (α and β represent different test lines) relative to the preference of M females (Fr) for the same two types of males. Similar to male behavior tests, Z female preference (Z-femaleness) was defined as the preference of these α females for Z males (Z30 or Z53) over M males (Fr), relative to the preference of β females in the same mating cage. We used the discrimination index (DI; refs. 14 and 15), to present the relative mating preference for each experiment. For example, DI = −ln [(39 × 28)/(9 × 10)] in Fig. 3T, and its variance is 1/39 + 1/28 + 1/9 + 1/10. In all cases, the two testers (α and β) carry different amounts of the Z genome that correspond to the number of visible markers (from rucuca line, M type) carried by them. In addition to presenting the estimated DI and its standard error, we also give the level of significance by G test. Significant observations were replicated usually.
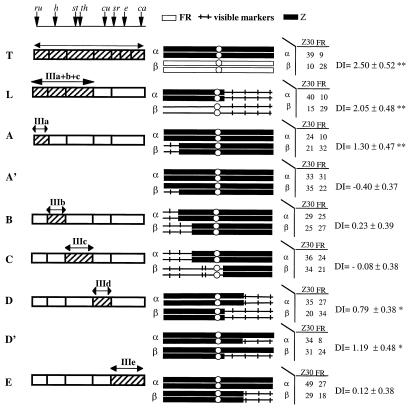
Figure 3.
Mapping of female mating preference (Z femaleness) of the adjacent genotypes. All notations are the same as in Fig. 2.
Results
To detect the genetic differences at a finer scale, we constructed a set of the third chromosome recombinant lines. Seven such lines between a multimarker line of the third chromosome (which is behaviorally M) and the MMZ stock were generated. Thus, all of the third chromosome recombinant lines have the X and the second chromosome from an M source, and their third chromosomes are recombinants designated as [ru-Z], [ru h-Z], [ru h th st-Z], [Z-cu sr e ca], [Z-sr e ca], [Z-e ca], or [Z-ca], where the visible markers are italicized to show the portion bearing the M chromosome (the rest are designated as Z).
The mating results are shown in Fig. 2 (for males) and Fig. 3 (for females). All tests are of the double-choice design, which measures the relative male mating success or female preference between the genotypes compared. The comparisons are between genotypes that differ in one marker at a time. At each marker locus, the allele comes from either the multimarker M line (for instance, ru) or the unmarked Z line (+). Whenever possible, we compared the two homozygotes (ru/ru vs. +/+). In some cases, we could compare only the heterozygote against the homozygote (ru/+ vs. +/+) when homozygotes for the recombinant chromosome are not viable because of linked recessive lethals. An MM[Z] line, which went through the same construction scheme as other recombinant lines but did not recombine, was obtained as a control.
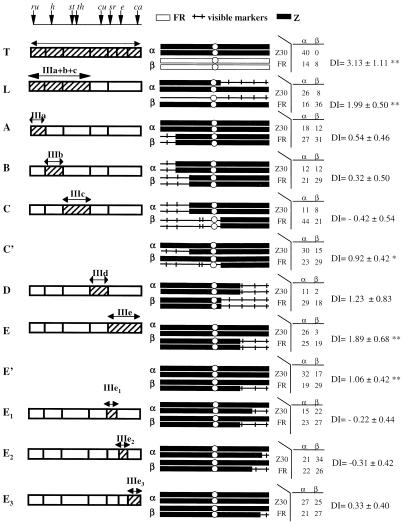
Figure 2.
Mapping of male mating success (Z maleness) of the adjacent genotypes. Each comparison is labeled from A–E in addition to T (for total effect of the third chromosome; ref. 14) and L (for the left arm). C ' and E ' test the same segments as C and E, respectively, but as heterozygotes. E is further decomposed into three subregions. The left column shows the segments tested. Vertical arrows on the top line denote the positions of the morphological markers, and shaded areas indicate the region being tested in each comparison. The middle column shows the α and β genotypes being compared in the behavior test. Solid bars indicate Z chromosomal segments and thin lines indicate M chromosomal segments, with short ticks denoting visible markers. The right column presents results of the mating tests. In general, 60 females (Z30 and Fr) and 60 males (α and β genotypes of the middle column) of each type were released into the mating cage. Each cell denotes the observed number of copulating pairs for each combination (Z30 females × α males, Z30 females × β males, etc). The DI and the significance level by G tests (**, P < 0.01; *, P < 0.05) are also given. Many of these tests with significant differences have been repeated with consistent results.
Male Mating Success.
Fig. 2 shows the behavior tests of six homozygous and five heterozygous comparisons. For the three regions (IIIa, IIIb, and IIId) where both homozygous and heterozygous genotypes showed weak or no effect on male mating success, we present only the data of homozygous comparisons.
The distal part of the right arm (IIIe) shows a significant effect in both homozygous and heterozygous tests (Fig. 2 E and E'). Males with two copies of the Z chromosome from IIIe mate better with Z females than males with either one or no copy. The genetic effect of this region on males' mating success acts in a dosage-dependent manner. To test whether this genetic effect is caused by one single major locus in IIIe, we decomposed this region into three smaller regions (IIIe1, IIIe2, and IIIe3). We used heterozygous genotypes for the tests. Surprisingly, the experiments showed no difference in male mating success in any of the three subregions alone (Fig. 2E 1–3), and we could not detect a significant effect in the combined subregions, IIIe1 + IIIe2 or IIIe2 + IIIe3 (data not shown). The set of five E tests (E, E', and E 1–3) in Fig. 2 suggest that, even at the subchromosomal level, we have not reduced the genetic effect to one single locus. The total effect remains larger than the sum of the parts. The minimal estimate of the number of genes in IIIe would be 2. In that case, the two loci interact nonadditively with each other to manifest the effect on the “Z maleness.” Of course, the number could be higher and the genetic effect may or may not deviate strongly from additivity.
The second region in which we have detected a genetic effect is IIIc of Fig. 2, although, somewhat unexpectedly, it showed only the effect in heterozygotes (Fig. 2C') but not homozygotes (Fig. 2C). Comparing the genetic backgrounds of the two testers, we interpret that another locus on the distal part of chromosome arm 3L (IIIa or IIIb) interacts with this proximal region to enhance the Z maleness. (It seems unlikely that the homozygotes for the st-th double markers could enhance male mating success.) Indeed, the whole left arm (IIIa + IIIb + IIIc) showed a much larger influence on Z maleness than the combined effect of its components, even in the heterozygous condition (Fig. 3L). Thus, the left arm probably contains two interacting loci for Z maleness.
On the third chromosome, our results suggest at least four loci (one in IIIa + IIIb, another one in IIIc, and two others in IIIe) contribute to the mating success of Z males. Overall, there seems to be a pattern in which the total effect is larger than the sum of the parts. Specifically, the whole chromosome exerts a stronger effect (Fig. 2T) on Z maleness than the combined effects of the subregions shown in Fig. 2. Furthermore, each subregion has a stronger effect than further decomposition would show. Implications will be discussed later.
Female Preference.
Fig. 3 shows the results of the behavior tests on five subchromosomal segments of the third chromosome. The tip of chromosome arm 3L (IIIa) shows a strong effect in homozygous females (Fig. 3A), indicating a female-preference locus in this region. Because heterozygous females show the same degree of preference as MM[Z] females (Fig. 3A'), the female-preference allele of the Z type is probably dominant (the concern for the possible marker gene effect will be addressed in Discussion). It is important to point out that the mating results are presented only to highlight the relative preference of the two genotypes. Although it may seem that both the α and β genotypes of Fig. 3A' are indiscriminant in their mate choice (33:31 and 35:22, respectively), a time-course series does show that both types mate with Z30 males in the early part of the experiment (data not shown; available upon request). When the supply of Z30 males was nearly exhausted, both types of females started mating with the less-preferred Fr males. Because we are interested in the relative preference, only the end point of the observation period is given and the information on the absolute female preference is partially lost. This treatment is true for Fig. 3 B–D as well.
Although neither IIIb nor IIIc shows a significant genetic effect in the behavior test (Fig. 3 B and C), the whole 3L (IIIa + IIIb + IIIc) shows a much stronger effect than the IIIa segment alone (Fig. 3 L vs. A). This observation suggests that the regions of IIIb and IIIc cannot be without an effect on female preference. Another female preference locus is mapped to the proximal portion of chromosome arm 3R (IIId) in both homozygous (Fig. 3D) and heterozygous genotypes (Fig. 3D'). Curiously, the heterozygous comparison seems to show a somewhat larger effect. This result may not be unlike the cases of Fig. 2 C and C' (for male behavior), hinting the presence of an interacting gene in the region of IIIe. However, because the difference in the DI value between Fig. 3 D and D' is insignificant, it is prudent to infer only one locus in IIId for female preference, with the Z-type allele being codominant or recessive (Fig. 3D'). In total, at least two (and more likely three) loci on the third chromosome can be inferred to be responsible for the Z female mating preference.
Discussion
At least seven loci on the third chromosome were mapped for either female preference or male mating success at this incipient stage of speciation. Given the low sensitivity in detecting weak genetic effects, this is likely a conservative estimate of the gene number involved. One possible reason for the lower sensitivity is the marker-gene effect. Although the double-choice design permits the calibration of the marker-gene effect on males by the relative preference of Z and M females, increasing the number of markers could reduce the absolute mating success of all males to the point where their relative success is hard to measure. Fig. 2D may be such an example. False identification of Z-maleness genes, on the other hand, is less of a concern because the measurements are all relative between Z and M females, and also because each positive identification was free of the complication of homozygous marker effects (Fig. 2 L, C', and E'). This result is also true for Z-femaleness genes. The only potential marker-gene effect in this study is Fig. 3A, but the combined results of Fig. 3 L, B, and C independently confirm a factor in the interval mapped in Fig. 3A. It should also be noted that the tests on both homozygous and heterozygous genotypes are meant to compensate for the possible marker-gene effects and to confirm the mapping results. The degree of dominance between Z and M alleles cannot be inferred reliably by this experimental design.
In an accompanying analysis of the genetics of the second chromosome (A.T., C.-T.T., and C.-I.W., unpublished data), we made several modifications to improve the sensitivity of the assay. These modifications include the construction of recombinant lines free of visible markers and an additional scheme for measuring mate choice. As a result, a comparable number of loci could be detected for the weaker second chromosome. In light of another earlier study (14), more than 15 loci can be identified to contribute to sexual isolation between the two behavior races.
Another pattern that reiterates itself is that the genetic effect of a whole region is almost always larger than the sum of the parts. A straightforward explanation is that many weak-effect loci exist, but each is too weak to be detected by itself. However, there also may be epistasis among the detected loci, such that the combination of Z alleles from two or more loci often have a synergistic effect. This sort of “weak allele, strong interaction” is the norm of genetic architecture underlying postmating isolation, as has been documented extensively (3). The best example of epistatic interactions in our study is the IIIe region (Fig. 2). The IIIe region exerts a strong genetic effect on male mating success, whereas each of the three subregions (IIIe1, III e2, and IIIe3, as well as their combinations) has little influence. The two possibilities, by no means mutually exclusive, suggest a rather diffuse genetic architecture that characterizes the incipient stage of species differentiation.
How does this conclusion of diffuse genetics compare with other studies of sexual isolation? More interestingly, what may be the consequences of different genetic architectures in terms of the “tempo and mode” of speciation? Although many studies infer the existence of major-effect genes, the results are often equally, if not more, compatible with alternative interpretations. Wu and Palopoli (1) discuss the criteria appropriate for inferring the genetic architecture. The recent study by Doi et al. (22), however, may be the most convincing case of a simple genetic basis for sexual isolation. These authors have been able to demonstrate that a very small region near the marker, Delta, contributes disproportionately to females' preference for Drosophila ananassae males. Females of the sibling species, Drosophila pallidosa, with an introgressed segment near Delta from D. ananassae would mate readily with D. ananassae males. Although the extent of introgression was not determined by molecular means, the 5–10 generations of backcrosses in the absence of an inversion nearby should have narrowed the introgression to a few centimorgans. Although other chromosomes do contribute to the female preference (see figure 2 of ref. 22), the predominant effect exerted by a single region is in dramatic contrast with the genetics of the Z–M divergence. This contrast in the complexity of sexual isolation between the two systems can be seen at the phenotypic level as well. Although courtship songs play a dominant role in the isolation between D. ananassae and D. pallidosa, no single element (visual, acoustic, or olfactory) can be shown to be individually important between the Z and M behavior races (14, 23).
It is striking that the Z and M races of D. melanogaster, which definitely have not achieved the species status, are more divergent with respect to the genetics of sexual isolation than the differentiation between two good species, D. ananassae and D. pallidosa. Although the latter have differentiated in coloration, karyotype, and a certain allozyme loci (24, 25), the molecular divergence between Z and M races for autosomal genes is quite limited (18–20). Morphologically, no diagnostic differences are detectable despite intensive efforts to score them (C.-T.T., A.T., and C.-I.W., unpublished results). Importantly, the southern African populations of D. melanogaster are highly polymorphic for the sexual behavior characters (16) such that the M-type behaviors are not uncommon in most populations in or around Zimbabwe. Why have the Z and M behavior types not evolved into distinct species, whereas D. ananassae and D. pallidosa, with a smaller number of divergent behavior loci, have speciated?
This contrast has led to a hypothesis that the tempo and mode of speciation may be a function of the genetic architecture of reproductive isolation. It is likely that speciation (in the sense of evolving two divergent types with few intermediates) would be harder to achieve if reproductive isolation is based on a large number of loci that potentially can generate many intermediate phenotypes. In the Z–M system, it takes a large number of loci to render a high degree of sexual isolation between the strong Z type and the cosmopolitan M type. As a result, there are a large number of intermediate types that serve as a “glue” to hold the diverging types together. Hollocher et al. (16) did find the intermediate Z type to be prevalent in most southern African populations. On the other hand, the simpler genetic architecture for the behavioral isolation between D. ananassae and D. pallidosa may have permitted the ancestral populations to diverge quickly without generating many intermediate forms. This process may even occur in sympatry (26, 27). In other words, the contrast between these two systems of speciation may be because of the relative ease with which speciation occurred. In the Z–M system, we observed behavioral polymorphisms in most southern African populations instead of speciation. Of course, other elements such as migration and ecology may contribute to the differences, but we have little information on them.
It has been recognized that the genetic architecture would determine the availability of pathways to evolve postmating isolation (28), a concept that has developed since into a comprehensive model (see refs. 29 and 30). It has been less obvious, however, that the genetic architecture also has played an important role in the evolution of behavior isolation—a role that is crucial (but often implicit) in the debate on sympatric speciation. In the extreme example when it is assumed that genotype AA does not mate with aa in a one-locus-two-allele model, then the so-called Fisher's equilibrium will consist only of AA and aa (because Aa is not regenerated), and sympatric speciation is accomplished. If the genetics are as simple as this example, sympatric speciation is almost inevitable. In more general terms, speciation is the state of two phenotypically discontinuous populations that are reproductively incompatible. For speciation to occur in sympatry, the diverging populations have to evolve to the opposite types while the intermediates are absent. However, sexual reproduction and subsequent recombination get in the way by continually producing intermediates. Reducing or removing the production of intermediates is the essence of sympatric speciation. The genetic architecture of species difference is relevant precisely because it determines the genetic nature of the intermediates. This property can be seen in some recent models on sympatric speciation also (26, 27). Arguing from an opposite direction, Goldschmidt (31) reasoned that, if species divergence required a large number of genetic changes cumulatively, sympatric speciation (which he accepted) would have never happened. To circumvent the genetic constraints and realize sympatric speciation, Goldschmidt offered the improbable (and perhaps infamous) “hopeful monsters,” novel species with simple genetic changes.
Finally, we observed that female preference and male mating success mapped to different chromosomal segments that are not tightly linked. This not-unexpected result parallels some previous observations (9–11, 32). The strong correlation between female and male traits among natural isolates collected in various parts of Africa therefore indicates strong linkage disequilibrium among male and female behavior loci (16). As Fisher's runaway process predicts (33–36), female and male traits can be in strong linkage disequilibrium if there is strong mate choice based on the alleles carried by individuals. In other words, the survey by Hollocher et al. (16) and the subchromosomal genetic analysis of this report suggest mate choice [as reported by Wu et al. (15)] must be quite prevalent in nature.
Acknowledgments
We thank M.-L. Wu for helping with the construction of recombination lines and J. Coyne, H. Hollocher, and J. Fay for comments on an earlier draft. This work was supported by a predoctoral fellowship from the Ministry of Education, Taiwan, Republic of China (to C.-T.T.), a Japan Society for the Promotion of Science Fellowship for Young Scientists (to A.T.), and by National Institutes of Health and National Science Foundation grants (to C.-I.W.)
Abbreviation
- DI
discrimination index
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wu C-I, Palopoli M F. Annu Rev Genet. 1994;28:283–308. doi: 10.1146/annurev.ge.28.120194.001435. [DOI] [PubMed] [Google Scholar]
- 2.Coyne J A, Orr H A. Philos Trans R Soc London B. 1998;353:287–305. doi: 10.1098/rstb.1998.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C-I, Hollocher H. In: Endless Forms: Species and Speciation. Howard D, Berlocher S, editors. Oxford: Oxford Univ. Press; 1999. [Google Scholar]
- 4.Coulthart M B, Singh R S. Mol Biol Evol. 1988;5:182–191. doi: 10.1093/oxfordjournals.molbev.a040484. [DOI] [PubMed] [Google Scholar]
- 5.Sawamura K, Davis A W, Wu C-I. Proc Natl Acad Sci USA. 2000;97:2652–2655. doi: 10.1073/pnas.050558597. . (First Published March 7, 2000; 10.1073/pnas.050558597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vacquier V D, Carner K R, Stout C D. Proc Natl Acad Sci USA. 1990;87:1592–1596. doi: 10.1073/pnas.87.15.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting C-T, Tsaur S C, Wu M-L, Wu C-I. Science. 1998;282:1501–1504. doi: 10.1126/science.282.5393.1501. [DOI] [PubMed] [Google Scholar]
- 8.Tan C C. Genetics. 1946;31:558–573. doi: 10.1093/genetics/31.6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zouros E. Evolution (Lawrence, Kans) 1973;27:601–621. doi: 10.1111/j.1558-5646.1973.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 10.Zouros E. Genetics. 1981;97:703–718. doi: 10.1093/genetics/97.3-4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyne J A. Proc Natl Acad Sci USA. 1989;86:5464–5468. doi: 10.1073/pnas.86.14.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng Z B, Liu J, Stam L F, Kao C H, Mercer J M, Laurie C C. Genetics. 2000;154:299–310. doi: 10.1093/genetics/154.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Mercer J M, Stam L F, Gibson G C, Zeng Z B, Laurie C C. Genetics. 1996;142:1129–1145. doi: 10.1093/genetics/142.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollocher H, Ting C-T, Wu M-L, Wu C-I. Genetics. 1997;147:1191–1201. doi: 10.1093/genetics/147.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C-I, Hollocher H, Begun D J, Aquadro C F, Xu Y, Wu M-L. Proc Natl Acad Sci USA. 1995;92:2519–2523. doi: 10.1073/pnas.92.7.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollocher H, Ting C-T, Pollack F, Wu C-I. Evolution (Lawrence, Kans) 1997;51:1175–1181. doi: 10.1111/j.1558-5646.1997.tb03965.x. [DOI] [PubMed] [Google Scholar]
- 17.Alipaz J A, Wu C-I, Karr T L. Proc R Soc London B. 2001;268:789–795. doi: 10.1098/rspb.2000.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andolfatto A, Wall J D, Kreitman M. Genetics. 1999;153:1297–1311. doi: 10.1093/genetics/153.3.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasson E, Wang I N, Zeng L W, Kreitman M, Eanes W F. Mol Biol Evol. 1998;15:756–769. doi: 10.1093/oxfordjournals.molbev.a025979. [DOI] [PubMed] [Google Scholar]
- 20.Tsaur S C, Ting C-T, Wu C-I. Mol Biol Evol. 1998;15:1040–1046. doi: 10.1093/oxfordjournals.molbev.a026002. [DOI] [PubMed] [Google Scholar]
- 21.Lindsey D L, Zimm G G. The Genome of Drosophila Melanogaster. New York: Academic; 1992. [Google Scholar]
- 22.Doi M, Matsuda M, Tomaru M, Matsubayashi H, Oguma Y. Proc Natl Acad Sci USA. 2001;98:6714–6719. doi: 10.1073/pnas.091421598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colegrave N, Hollocher H, Hinton K, Ritchie M G. J Evol Biol. 2000;13:143–150. [Google Scholar]
- 24.Johnson F M, Kanapi C G, Wheeler M R, Stone W S. Proc Natl Acad Sci USA. 1966;63:119–125. doi: 10.1073/pnas.56.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Futch D G. Univ Texas Publ. 1966;6615:79–120. [Google Scholar]
- 26.Dieckmann U, Doebeli M. Nature (London) 1999;400:354–357. doi: 10.1038/22521. [DOI] [PubMed] [Google Scholar]
- 27.Kondrashov A S, Kondrashov F A. Nature (London) 1999;400:351–354. doi: 10.1038/22514. [DOI] [PubMed] [Google Scholar]
- 28.Cabot E L, Davis A W, Johnson N A, Wu C-I. Genetics. 1994;137:175–189. doi: 10.1093/genetics/137.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavrilets S. Evolution (Lawrence, Kans) 2000;54:1126–1134. doi: 10.1111/j.0014-3820.2000.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 30.Orr H A. Genetics. 1995;139:1805–1813. doi: 10.1093/genetics/139.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldschmidt R. The Material Basis of Evolution. New Haven, CT: Yale Univ. Press; 1940. [Google Scholar]
- 32.Coyne J A. Genetics. 1996;143:353–364. doi: 10.1093/genetics/143.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasa Y, Pomiankowski A. Nature (London) 1995;377:420–422. doi: 10.1038/377420a0. [DOI] [PubMed] [Google Scholar]
- 34.Fisher R A. The Genetical Theory of Natural Selection. Oxford: Clarendon; 1930. [Google Scholar]
- 35.Kirkpatrick M. Evolution (Lawrence, Kans) 1982;36:1–12. doi: 10.1111/j.1558-5646.1982.tb05003.x. [DOI] [PubMed] [Google Scholar]
- 36.Lande R. Proc Natl Acad Sci USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]