Abstract
The Xenopus tadpole has the capacity fully to regenerate its tail after amputation. Previously, we have established that this regeneration process requires the operation of several signaling pathways including the bone morphogenic protein, Wnt, and Fgf pathways. Here, we have addressed the signaling requirements for spinal cord and muscle regeneration in a tissue-specific manner. Two methods were used namely grafts of transgenic spinal cord to a wild type host, and the use of the Tet-on conditional transgenic system to express inhibitors in the individual tissues. For the grafting experiments, the tail was amputated through the graft, which contained a temperature inducible inhibitor of the Wnt-β-catenin pathway. For the Tet-on experiments, treatment with doxycycline was used to induce cell autonomous inhibitors of the Wnt-β-catenin or the Fgf pathway in either spinal cord or muscle. The results show that both spinal cord and muscle regeneration depend on both the Wnt-β-catenin and the Fgf pathways. This experimental design also enables us to observe the effect of inhibition of regeneration of one tissue on the regeneration of the others. Regardless of the method of inhibition, we find that reduction of spinal cord regeneration reduces regeneration of other parts of the tail, including the myotomal muscles. In contrast, reduction of muscle regeneration has no effect on the regeneration of the spinal cord. In common with other regeneration systems, this indicates that soluble factors from the spinal cord are needed to promote the regeneration of the other tissues in the tail.
Keywords: Xenopus tadpole, tail, regeneration, Wnt, Fgf, spinal cord, muscle, tissue interaction
INTRODUCTION
The African clawed frog Xenopus has an interesting range of regeneration behaviors, including the complete regeneration of the tadpole tail and the early developing limb (Dent, 1962; Filoni and Bosco, 1981; Mochii et al., 2007; Slack et al., 2008; Beck et al., 2009). In recent years, Xenopus has emerged as a leading model for regeneration research largely because it has the advantage for experimental work of being a standard laboratory model organism (Sive et al., 2000). The combination of a good probe inventory, a whole range of microsurgical techniques and a set of well-established transgenic techniques provides a powerful opportunity to investigate the cellular and molecular mechanism for organ regeneration in Xenopus. Findings from regeneration research in Xenopus has already greatly contributed to our understanding of regeneration capacity and mechanisms in other animals (Poss, 2010).
The tail of the Xenopus tadpole regenerates completely during the tadpole’s life, except for a brief refractory period before feeding (Beck et al., 2003a), and a decline as metamorphosis approaches (Franchini and Bertolotti, 2011). The tail consists of three main axial structures: the spinal cord, the notochord, and the segmented muscles. These are surrounded by connective tissue and epidermis. Previous work in our laboratory has shown that the spinal cord and notochord regenerate from their corresponding tissues in the stump; and that the regeneration of the muscle comes from muscle satellite cells (Gargioli and Slack, 2004; Chen et al., 2006). Other cell types, such as pigment cells, also regenerate from their precursors in the stump (Lin et al., 2007), so there is limited de-differentiation and no metaplasia during regeneration. This phenomenon of lineage restriction, or tissue memory, during regeneration has recently been shown also to be conserved in other vertebrate animals, including urodele amphibians (Kragl et al., 2009), teleost fish (Tu and Johnson, 2011), and mammals (Rinkevich et al., 2011). This shows that there is an assemblage of distinct zones of regenerating tissues in these various situations. Thus it is of interest to ask what mechanisms are required to initiate and sustain regeneration in each of these distinct regenerating tissues, and the extent to which one tissue type is required for the regeneration of others.
Our laboratory work has previously established that the bone morphogenic protein (BMP) and Notch signaling pathways are critical for both embryonic tail bud outgrowth and tadpole tail regeneration (Beck and Slack, 1999; Beck et al., 2003b, 2006). The TGFβ pathway is also required (Ho and Whitman, 2008). More recently, we have demonstrated that both Wnt and Fgf signaling pathways are required for Xenopus tadpole tail regeneration, and the Wnt signal operates upstream of the Fgf signal (Lin and Slack, 2008). Evidence suggests that both canonical and noncanonical Wnt signaling pathways are involved in Xenopus tadpole tail regeneration (Sugiura et al., 2009). This molecular mechanism is conserved in other regenerating systems, such as the zebrafish fin and axolotl limbs, despite their clearly different anatomies (Poss et al., 2000; Kawakami et al., 2006; Stoick-Cooper et al., 2007; Yokoyama et al., 2007). It has also been shown that other processes, such as ion channels, cell death, and hyaluronan, are all needed for tail regeneration, but how these relate to the signaling pathways is not currently clear (Adams et al., 2007; Tseng et al., 2007; Contreras et al., 2009). In this work, we have further investigated the roles of Wnt and Fgf signals by establishing their role in two of the individual tissue types in the tail: the spinal cord and the segmented muscle.
We have used two different procedures in this work. The first, used for spinal cord alone, is to graft transgenic tissue bearing a temperature-inducible antagonist, to a wild type host. The second is to set up an inducible system such that antagonists of Wnt and Fgf signals can be expressed in each individual tissue during regeneration. We have used the N-tubulin promoter to drive expression in the spinal cord (Marsh-Armstrong et al., 1999), and the Cardiac actin promoter (Mohun et al., 1986) to drive expression in the segmental muscles. In each case, the tissue-specific promoter was used to drive expression of a Tet activator, such that expression of the Wnt or FGF inhibitor could be induced in a tissue specific manner, by addition of doxycycline. The inhibitors themselves were cell autonomous. ΔTcf3 is a truncated form of the transcription factor TCF, which prevents the normal form from binding to β-catenin, and thereby inhibits the consequences of Wnt signaling (Molenaar et al., 1996). Xfd is a truncated form of the FGF receptor, which forms unproductive dimers with the normal form and prevents signal transduction following binding of the FGF ligand (Amaya et al., 1991).
The results show that both spinal cord and muscle regeneration require both Wnt and Fgf signals. They also indicate that there are interactions between the tissues during regeneration, but the nature of the dependence differs in the two cases. Interventions that inhibit the regeneration of the spinal cord also reduce the volume of muscle regenerated. On the other hand, inhibition of Wnt and Fgf signaling in muscles reduces the size of regenerated muscle and the length of the regenerated tail, but it does not lead to a failure of spinal cord regeneration.
MATERIALS AND METHODS
Transgene Constructs
HGEM-xtDkk1 was used to generate donors for spinal cord transplantation experiment. The construction of HGEM-xtDkk1 has been described previously (Lin and Slack, 2008). Doxycycline inducible constructs pCar-rtTA, pNtub-rtTA, and pCS2-TetOn-GFP were obtained from Dr. Brown’s lab (Das and Brown, 2004). The pCar promoter (Mohun et al., 1986) drives gene expression in all tadpole tail muscles (Das et al., 2002). The pNtub promoter drives gene expression in neural tissues including the spinal cord in the tail (Marsh-Armstrong et al., 1999). These constructs were modified in this work as described below. For better identification of transgenic tadpoles before doxycycline treatment, the fluorescent protein tdTomato was incorporated to generate pCar-tdTom-2A-rtTA and pNtub-tdTom-2A-rtTA. First, the H2B-GFP fragment in pCAG-tdTom-2A-H2BGFP (Trichas et al., 2008) was replaced by a PCR amplified fragment containing the rtTA2S-M2 coding sequence at BglII/EcoRV sites. The fragment containing tdTom-2A-rtTA was then subcloned into pCar-rtTA and pNtub-rtTA, replacing the original rtTA sequence, at HindIII/NotI sites. pCS2-TetOn-GfpΔTcf and pCS2-TetOn-XfdGfp were used to manipulate activities of Wnt and Fgf signaling. pCS2-TetOn-GfpΔTcf was generated by replacing GFP in pCS2-TetOn-GFP with GfpΔTcf fragment from pHS-GFPΔTcf (a gift from Dr. Richard Dorsky). pCS2-TetOn-XfdGfp was generated by replacing GFP in pCS2-TetOnGFP with XfdGfp, a mycGFP fused version of Xfd (Amaya et al., 1991).
Tadpoles, Spinal Cord Transplantation, and Tail Amputation
Wild type tadpoles were raised from natural mating of wild type frogs. Transgenic tadpoles were created as described (Kroll and Amaya, 1996) except that the restriction enzyme was omitted. Cyan fluorescent protein (CFP) tadpoles were bred from transgenic frogs established in the lab with a CMV-CFP construct. HGEM-xtDkk1: CMV-dsRed double transgenic tadpoles were generated using HGEM-xtDkk1 and CMV-dsRed constructs. Tadpoles were raised in 0.1×MMR and staged according to the NF stage series (Nieuwkoop and Faber, 1967).
For spinal cord transplantation, stage 49 or older wild type and transgenic tadpoles were anesthetized in 0.02% MS222 in 0.5×MMR (Sigma), and were kept in the anesthetic solution during the operation. A small piece of spinal cord in the middle tail was removed by two small incisions with a sharp microsurgery knife, cutting through the lateral muscle. The donor spinal cord piece was inserted into the wild type host, and the tadpoles were kept in 0.5×MMR for 15 minutes before being transferred to tadpole water for recovery. Three days post-transplantation, well-healed tadpoles were anesthetized in 0.02% MS222 and their tails were amputated through the spinal cord graft. Heat shock activation of the Hsp70 promoter (34°C, 30 minutes each day) was performed as described (Beck et al., 2003b) and carried out every day until 7 days post amputation when specimens were fixed for analysis.
To generate tadpoles that are doxycycline responsive, one rtTA construct plus one ptetOn construct was used in the same transgenesis reaction. To induce gene expression, doxycycline (25 mg/L, Sigma) was added to the tadpole water, starting 1 day before tail amputation, and continued until 7 days post tail amputation, when specimens were fixed for analysis. Nontransgenic siblings, or pTetOn-Gfp transgenic tadpoles were treated together with transgenic pTetOn-GfpΔTcf and pTetOn-XfdGfp tadpoles, as controls. Some transgenic tadpoles not treated with doxycycline were also included as control tadpoles.
Histology and Immunohistochemistry
For morphological analysis of the regenerated tadpole tails, hematoxylin and eosin (HE) staining was performed on paraffin sections prepared from specimens fixed with MEMFA (0.1 M MOPS, 2 mM EGTA, 1 mM MgSO4, 3.7% formaldehyde, pH 7.4). To analyze muscle regeneration in the regenerate tadpole tails, whole mount immunohistochemistry was performed as described (Chen et al., 2006). The monoclonal antibody 12/101 (Kintner and Brockes, 1984) was used for the detection of muscle (original cells from Dr. E. Jones, Warwick University). Alkaline phosphatase conjugated secondary antibody (1:500, Vector lab) was used and BM purple (Roche) was used for color development. Stained tails were refixed and sectioned after paraffin embedding, and sections were counterstained with Nuclear Fast Red (Vector Labs).
Photography and Microscopy
Tail regeneration and expression of fluorescent gene products was observed in tadpoles under anesthesia in 0.02% MS222 using a Leica Fluo III fluorescent dissecting microscope. Stained sections were visualized with a Leica DMRB microscope. Images were captured using a Retiga 2000R digital camera (Qimaging) and processed with Photoshop software (Adobe).
RESULTS
Spinal Cord Regeneration in Tadpoles Transplanted with Dkk1 Spinal Cord
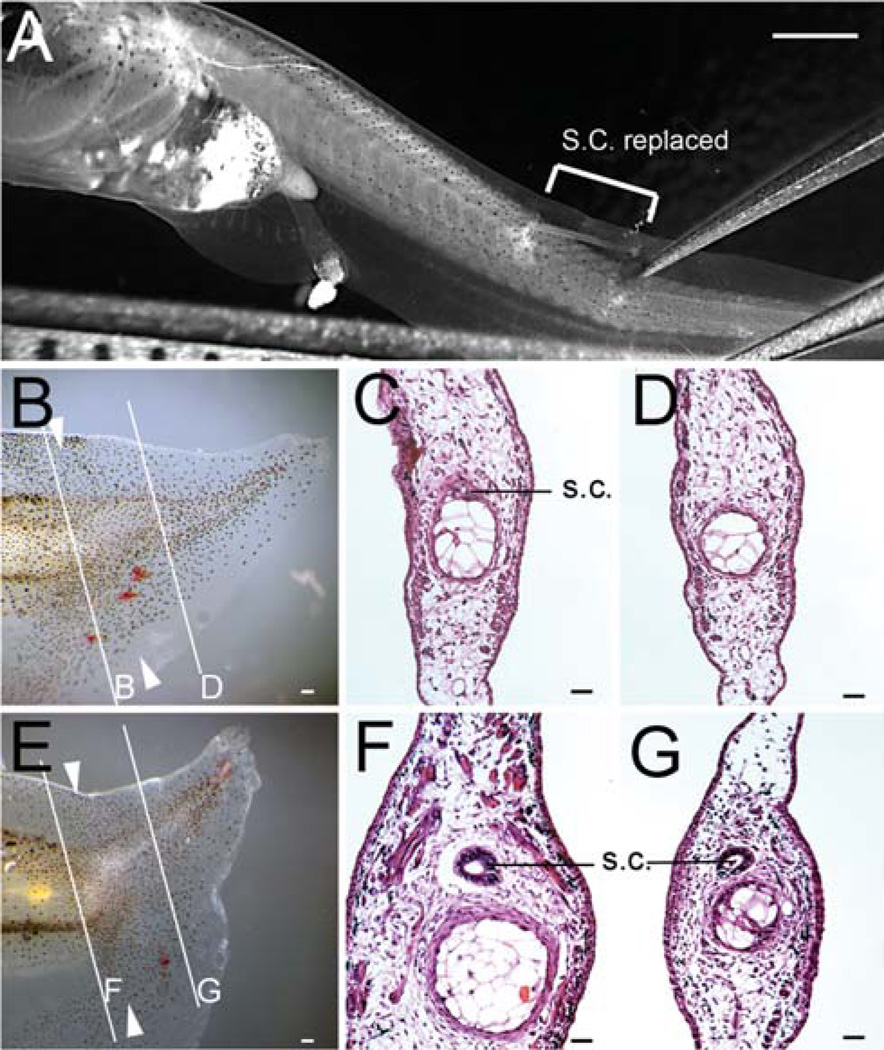
To investigate whether Wnt/beta-catenin signaling is required in the regeneration of spinal cord during Xenopus tadpole tail regeneration, we used grafts of Dickkopf1 (Dkk1) expressing spinal cord transplanted to wild type tadpoles. Dkk1 antagonizes Wnt/beta-catenin signaling (Glinka et al., 1998) by binding to the LRP coreceptor for the Wnt ligand (Mao et al., 2001). We initially used HGEM-xtDkk1 transgenic tadpoles as donors in this experiment and followed the procedure illustrated in Fig. 1A. After recovery from the transplantation, we induced expression of Dkk1 in a warm bath, amputated the operated tadpoles’ tails through the graft, and observed the subsequent tail regeneration. The induction of Dkk1 was maintained long term by a 30 minute warm bath treatment each day. Our previous results on mRNA level following temperature shocks indicates that this is sufficient to maintain an active level of mRNA (Beck et al., 2006). We found that all operated tadpoles regenerated their tails. But when we checked the morphology, we found that the majority (71%) did not contain spinal cords (Table 1, Fig. 1), indicating that Dkk1 production in the spinal cord alone inhibits spinal cord regeneration. It also compromises, but does not suppress, regeneration of other parts of the tail, leading to a wavy appearance.
Fig. 1.
Tail regeneration in HGEM-Dkk1 spinal cord transplanted tadpoles. (A) Example of a stage 51 tadpole with a piece of spinal cord (shown in bracket) replaced. (B–G) Examples of regenerated tails in tadpoles transplanted with Dkk1 spinal cord. (B, E) Whole tail image. (C, D, F, G) H-E staining of regenerated tail, showing some tadpoles have spinal cord regenerated (G), some not (D), even though grafted spinal cord can been seen at the amputation stump (C, F). Scale bars in (A, B, E): 500 µm. Scale bars in (C, D, F, G): 100 µm.
TABLE 1.
Transgenic experiments on spinal cord and muscle regeneration in Xenopus tadpoles
| Experiment | N |
a: Normal regenerate |
b: Partial regenerate |
c: No regenerate |
SC normal in a+b |
SC reduced in a+b |
M normal in a+b |
M reduced in a+b |
|---|---|---|---|---|---|---|---|---|
| 1. CFP spinal cord grafta | 14 | 14 (100%) | 14 (100%) | 14 (100%) | ||||
| 2. Dkk spinal cord graft | 21 | 6 (29%) | 15 (71%) | 6 (29%) | 15 (71%) | 9 (43%) | 12 (57%) | |
| 3. Dkk:dsRed spinal cord graft | 18 | 9 (50%) | 9 (50%) | 9 (100%) | 1 (11%) | 8 (89%) | ||
| 4. pNtub-rtTA: ptetOn-GFPb | 20 | 20 (100%) | 20 (100%) | 20 (100%) | ||||
| 5. pNtub-tdTom-2a-rtTA: ptetOn–GfpΔTcf | 16 | 14 (88%) | 2 (12%) | 10 (71%) | 4 (29%) | 3 (21%) | 11 (79%) | |
| 6. pNtub-tdTom-2a-rtTA: ptetOn–XfdGfp | 16 | 6 (37%) | 10 (63%) | 6 (37%) | 10 (63%) | 16 (100%) | ||
| 7. pCar-rtTA: ptetOn–GFPc | 23 | 23 (100%) | 23 (100%) | 23 (100%) | ||||
| 8. pCar-tdTom-2a-rtTA: ptetOn–GfpΔTcf | 33 | 8 (24%) | 14 (43%) | 11 (33%) | 22 (100%) | 8 (36%) | 14 (64%) | |
| 9. pCar-tdTom-2a-rtTA: ptetOn–XfdGfp | 36 | 2 (5%) | 29 (81%) | 5 (14%) | 31 (100%) | 2 (6%) | 29 (94%) |
Tails of stage 49+ tadpoles were amputated after spinal cord transplantation or doxycycline induction of ΔTcf or Xfd. Specimens were collected 7 days after tail amputation and analyzed by fluorescence visualization, immunohistochemistry or histology examination. Numbers are pooled from several rounds of experiments.
N, total number; SC, spinal cord; M, muscle.
Control for experiments 1–3.
Control for experiments 4–6.
Control for experiments 7–9.
In the previous experiments, the donor spinal cord was not labeled, making it difficult to follow the regeneration process visually. To make sure that the transplanted spinal cord survived in the host and to better observe it during regeneration, we generated HGEM-xtDkk1:CMV-dsRed double transgenic tadpoles, so that the donor spinal cord could be identified and traced during regeneration with red fluorescence (Fig. 2). In tadpoles transplanted with HGEM-xtDkk1:CMV-dsRed spinal cords, we found that all donor spinal cords survived transplantation (Fig. 2C, D). In this series 50% (9/18) of the tadpoles failed to regenerate their tails (Table 1, Fig. 2E), although all control tadpoles with CFP spinal cord grafts did so (Fig. 2J). In the other 50% (9/18) tadpoles that did regenerate their tails, two thirds (6/9) of them only partially regenerated their spinal cord, as indicated by the red fluorescence (Fig. 2F, G). The remainder (3/9, 17% of total) had red fluorescent spinal cord along the full length of the regenerating tail (Fig. 2H). When these tadpoles were subjected to a second tail amputation, they continued to regenerate their spinal cord from the transplanted spinal cord (Fig. 2I).
Fig. 2.
Tail regeneration in Xenopus tadpoles with HGEM-Dkk1:dsRed spinal cord transplantation. (A, B) Example of a tadpole used as donor in this study. This tadpole is transgenic for both heat shock inducible Dkk1 (HGEM-Dkk1) and dsRed (red fluorescence in (B) driven by human cytomegalovirus (CMV) promoter. Arrow in (A) indicates the green lens of the eye, which marks the integration of the HGEM transgene. (C, D) Example of a tadpole with a piece of spinal cord transplanted (shown in red) 3 days after grafting (C) and immediately after tail amputation though the graft (D). (E–H) Examples of tadpole tail regeneration in HGEM-Dkk1:dsRed spinal cord transplantation, 7 dpa. (E) An example of nonregenerating tail. (F, G) Examples of regenerating tadpole tail with partial regenerating spinal cord, as indicated by dsRed shown in red. (H) An example of fully regenerating spinal cord in a partially regenerating tail. This tadpole was subjected to a second tail amputation and fully regenerated its tail with spinal cord (I). (J) Tail regeneration in a tadpole transplanted with CFP transgenic spinal cord, 7 dpa. White arrowheads in (E–J) indicate amputation levels. dpa: day post amputation. s.c.: spinal cord. Scale bars: 500 µm.
In this type of experiment different individual tadpoles may have different transgene insertion sites and copy numbers, therefore we expect to see some variation in the results. Clearly Dkk1 does inhibit spinal cord regeneration. In many cases, the rest of the tail does regenerate to some extent indicating that the presence of spinal cord is not absolutely obligatory. However in these cases there was a reduction of muscle mass (Table 1) and also the notochord, and often the whole tail, had a wavy appearance. In those cases where all regeneration was blocked it may be that the level of Dkk1 expression was so high that it was present at an inhibitory level across the whole of the amputation surface.
Using a Doxycycline Inducible System in Xenopus Tadpole Tail Regeneration
As shown above, transplantation is a useful technique in probing the necessity of Wnt signaling for study of spinal cord. But for muscle, tadpole stage transplantation is not feasible. We have previously made many grafts of presomite plate to neurula stage hosts but these generally yield a region of mosaic host/donor muscle and furthermore this often lies at too proximal a position in the tail to be compatible with survival after amputation. To resolve this problem, we adapted the doxycycline inducible system that has been previously shown to work in Xenopus transgenic tadpoles (Das and Brown, 2004).
Firstly, we made transgenic tadpoles with inducible production of GFP either in the neural tissue (pNtub-rtTA: pTetOn-Gfp) or in muscle tissues (pCar-rtTA: pTetOn-Gfp) and examined tail regeneration. As previously reported, adding doxycycline to the tadpoles’ water rapidly induced Gfp expression and did so according to the expected specificity of the promoter (Fig. 3A–D). Following amputation, all these control tadpoles regenerated their tails completely (Table 1).
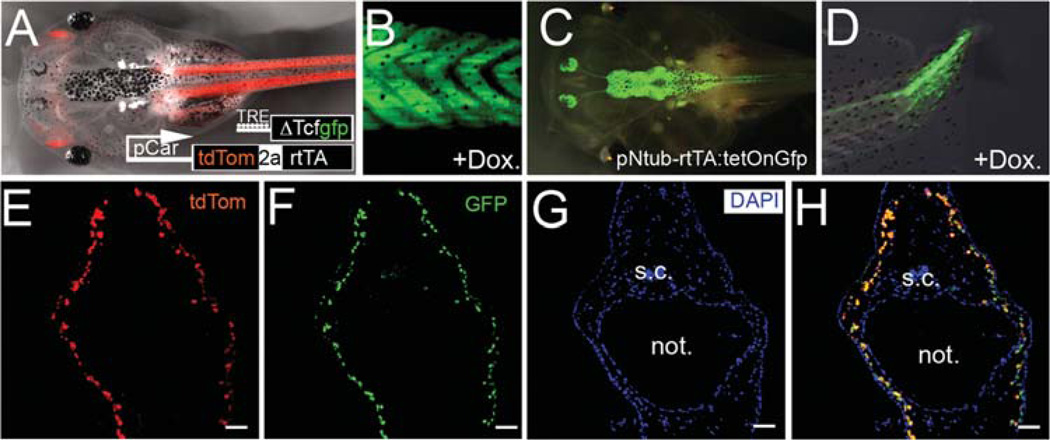
Fig. 3.
Doxycycline-inducible transgenic tadpoles. (A, B) Example of transgenic tadpoles with muscle specific expression of rtTA, indicated here by expression of tdTomato shown in red (A), and integration of transgene GfpΔTcf, by expression of GFP after doxycycline treatment (B). (C, D) Example of transgenic tadpoles with neural tissue specific expression of GFP, in the central nervous system (C) and the regenerating tadpole tail (D) after doxycycline treatment. Labeling of axons is apparent in the regenerating tail. (E–H) Cross sections of tadpole tail were collected for visualization of tdTomato (E) to indicate expression of rtTA, and immunoreacted to GFP antibody (F), followed by nuclear staining with DAPI (G). Merged image shows the colocalized expression of tdTomato and GFP (H). s.c.: spinal cord. not.: notochord. Dox.: doxycycline. Scale bars in (B, G): 500 µm. Scale bars in (C–F, H): 100 µm.
For better visualization of double transgenic tadpoles and for control of experimental variance, we modified the transgene constructs so that expression of Tet transactivator rtTA2S-M2 was marked by the expression of tdTomato, using a viral 2A peptide link (de Felipe et al., 2003; Trichas et al., 2008). Unlike bicistronic constructs using the internal ribosomal entry site (IRES) in which gene production of downstream is lower then that of upstream, bicistronic peptides linked with the 2A sequence are intrinsically self-cleaved to produce equal molar amounts of the products (Ibrahimi et al., 2009). This allows evaluation of the production of rtTA in live specimens by directly observing tdTomato under the fluorescence microscope.
We next checked both the tissue specificity and the correlation of the Tet transactivator with expression of our gene of interest, by comparing the expression of tdTomato against GFPΔTcf in pCar-tdTom-2A-rtTA: pTetOn-GfpΔTcf tadpole tails. As shown in Fig. 3, tdTomato was highly expressed in the tail muscles (red in Fig. 3A). In the presence of doxycycline, GFP was also induced in the tail muscle (Fig. 3B). Cross sections through the tail confirmed that tdTomato and GFP were expressed in the same tissues (Fig. 3E–H). This observation confirms that the doxycycline inducible system is reliable and can be used for the study of Xenopus tadpole regeneration. Similar results were obtained with the N-tubulin system (Fig. 3C, D). For the whole series of experiments with both promoters the correspondence of the GFP expression with the rtTA promoter specificity, and the tdTomato signal was generally good, but in 5–10% of cases, some ectopic GFP was observed.
Spinal Cord Regeneration in Doxycycline Inducible ΔTcf and Xfd Transgenic Tadpoles
Using the doxycycline system, we first set out to confirm the requirement of Wnt/beta-catenin signals in the regeneration of spinal cord which had been established using the transgenic spinal cord grafts. We generated pNtub-tdTom-2A-rtTA: pTetOn-GfpΔTcf tadpoles and followed their tail regeneration after doxycycline treatment. ΔTcf is a N-terminus deletion, dominant negative form of Tcf that has been shown to block Wnt signaling (Molenaar et al., 1996). We found that induced ΔTcf expression in the spinal cord abolished spinal cord regeneration in some (4/14, 29%) but not all the transgenic tadpoles (Table 1). These tadpoles show more cases of muscle reduction than of spinal cord reduction, but this may be because the morphology of the tails classified as “partial” in this series was a short stubby tail in which the muscle content is proportionately low.
To investigate the requirement for Fgf signaling in spinal cord regeneration, we examined the tail regeneration in pNtub-tdTom-2a-rtTA: pTetOn-XfdGfp transgenic tadpoles. Again, we observed that some tadpoles failed to regenerate their spinal cord when Xfd was induced. The inhibitory effects are less marked than those shown by the Dkk1 expressing spinal cord transplants, but it is likely that the level of expression of the gene of interest induced in the Tet-on system is considerably lower than that obtained directly from a heat shock promoter. The muscle content in this series was mostly normal.
Taken together, the results do demonstrate that Xenopus tadpole spinal cord regeneration requires both Wnt/beta-catenin and Fgf signaling, and that partial regeneration of other tissues is possible in the absence of the spinal cord. As for the Dkk1 cases, regenerates which had lost spinal cord were defective, tending to be short tails with a wavy notochord.
Muscle Regeneration in Doxycycline Inducible ΔTcf and Xfd Transgenic Tadpole Tails
To see whether Wnt and Fgf signaling pathways are needed for muscle regeneration in Xenopus tails, we examined tail regeneration in pCar-tdTom-2a-rtTA: pTetOn-GfpΔTcf and pTetOn-XfdGfp tadpoles. Two-thirds of tadpoles with ΔTcf over-expression in their muscles regenerated their tails, and all these contained spinal cord and muscle tissue (Table 1). However these regenerates were smaller compared to controls (i.e., than the pCar-rtTA:pTetOn-GFP tadpoles) (Fig. 4G–I), and this was mostly due to a deficit of muscle as shown in sections such as Fig. 4H. The spinal cords were all normal. One-third of the tadpoles failed to regenerate their tail altogether (Table 1).
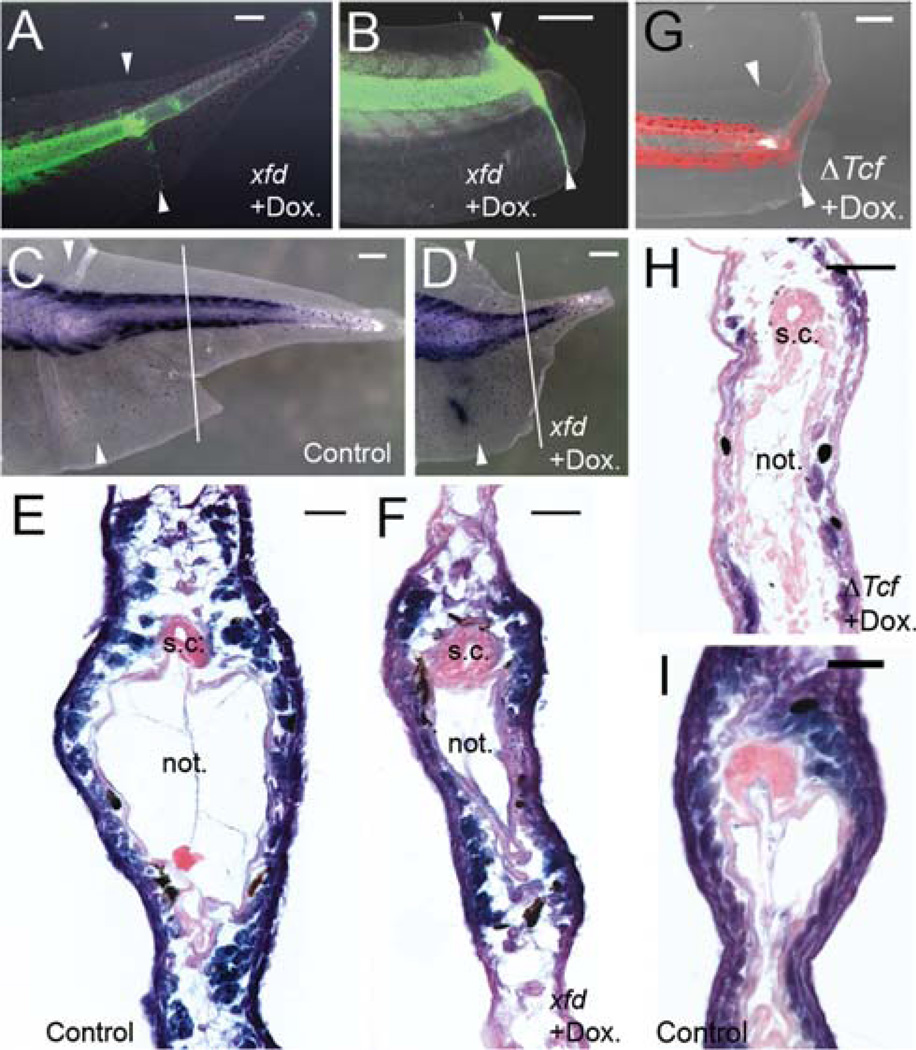
Fig. 4.
Muscle regeneration in doxycycline-inducible Xfd and GfpΔTcf transgenic tadpole tails. (A, B) Example of a regenerating tails in transgenic tadpoles with muscle specific expression of Xfd, indicated here by expression of GFP shown in green. (A) Example of a tadpole tail fully regenerated but with reduced muscle. (B) Example of a tadpole tail which failed to regenerate after Xfd induction with doxycycline treatment. (C, D) Whole mount immunohistochemistry of regenerating tails with 12/101 to detect muscle tissues. (C) Example of control tadpole tail after staining. (D) Example of immunostaining of a transgenic tadpole tail with muscle specific induction of Xfd. Note the short tail regenerate. White arrows in (A–D) indicate amputation levels. White lines in (C, D) indicated the level of cross sections represented by (E, F). (E, F) Cross sections of immunostained tadpole tail counterstained with Nuclear Fast Red, showing normal and reduced muscle content. (G) Example of regenerating tail in a tadpole with muscle specific expression of ΔTcf, after doxycycline treatment. Expression of tdTomato is shown in red and overlaid to bright-field image of the regenerating tail. White arrows indicated amputation level. (H) Example of cross section through the regenerating tail of a tadpole with muscle specific expression of ΔTcf. Cross sections were obtained after whole mount immunohistochemistry with 12/101 antibody for muscle, and counterstained with Nuclear Fast Red. (I) A cross section from a wild type tadpole tail regenerate. s.c.: spinal cord. not.: notochord. Dox.: doxycycline. Scale bars in (A–D, G): 500 µm. Scale bars in (E, F, H, I): 100 µm.
The muscle-specific Xfd transgenic tadpoles gave similar, but more pronounced, results. Most tadpoles regenerated their tails when Xfd expression was induced in the tail muscle (Fig. 4A). But most cases had a much smaller tail compared to untreated controls (Fig. 4C, D), and cross sections of the regenerated tail revealed that the transgenic tadpoles regenerated much less muscle compared to controls (Fig. 4E, F). The spinal cords in these cases were normal. The notochords were fairly normal although it is difficult to exclude some small effects. As in the case of the ΔTcf tadpoles, over-expression of Xfd in tail muscles sometimes blocked tail regeneration completely, especially when there was strong expression of Xfd at the amputation surface (Fig. 4B). These results demonstrate that Fgf signaling is critical for muscle regeneration in Xenopus tadpole tail.
Tissue Interactions
The effect of the reduction of one tissue type on the regeneration of the others can be seen more clearly when the data from Table 1 is aggregated, as shown in Table 2. Here, all of the spinal cord inhibition experiments are pooled and all of the muscle inhibition experiments are pooled. For the spinal cord inhibitions, 31 of 38 cases showing absent or reduced spinal cord also show a reduction of muscle content. This strongly suggests that the spinal cord releases factors that are necessary for, or promote, the regeneration of the muscle. Conversely, in all of the 43 cases of muscle inhibition resulting in regenerates with reduced muscle, the spinal cord was normal. So it seems that factors from muscle are not needed to promote the regeneration of the spinal cord.
TABLE 2.
Aggregated results for inhibition of spinal cord and muscle regeneration
| Experiment | N |
a: Normal regenerate |
b: Partial regenerate |
c: No regenerate |
SC normal in a+b |
SC reduced in a+b |
M normal in a+b |
M reduced in a+b |
Pa |
|---|---|---|---|---|---|---|---|---|---|
| 2+3+5+6 sc inhibition Experiments | 71 | 12 (17%) | 48 (68%) | 11 (15%) | 22 (37%) | 38 (63%) | 29 (48%) | 31 (52%) | >0.05 |
| 8+9 m inhibition Experiment | 69 | 10 (15%) | 43 (62%) | 16 (23%) | 53 (100%) | 10 (19%) | 43 (81%) | <0.01 |
Numbers are aggregated from the indicated rows of Table 1.
SC, spinal cord; M, muscle.
Statistics performed with χ2 contingency table test. H0: spinal cord experiments and muscle experiments regulate spinal cord and muscle regeneration in the same manner. The results show that spinal cord inhibition also reduces muscle regeneration. But muscle inhibition does not affect spinal cord regeneration.
DISCUSSION
Tissue Specificity in Xenopus Tadpole Tail Regeneration
Up to now the studies performed on zebrafish and Xenopus tail and limb buds have all involved uniform treatment of the organism, either with small molecule inhibitors, or with temperature inducible transgenes. Our work presented here addresses the issue of the requirements of each of the individual tissues for signaling pathways during the regeneration process. The Xenopus tadpole tail, when looked at in cross section, consists of several anatomically distinct tissue compartments. There is a notochord in the central region, the spinal cord dorsally, the segmented myotomes on both sides, and these axial structures are surrounded by loose connective tissue and the epidermis, which is drawn into dorsal and ventral fins containing neural crest-derived mesenchyme. Each of these tissue types is formed by a specific process in early embryonic development: mesoderm induction forms the notochord and muscle (Woodland and Jones, 1988), neural induction forms the spinal cord (Kelly and Melton, 1995) and neural crest induction forms the fins (Tucker and Slack, 2004). Also gene expression studies have shown that the embryonic tail bud can be anatomically divided into gene expression zones corresponding to these tissue types (Gont et al., 1993; Tucker and Slack, 1995b; Beck and Slack, 1998; Gawantka et al., 1998). Studies of the cell lineage of tail regeneration have shown that each of the principal tissues regenerates independently, without metaplasia or exchange of cells between the tissues (Gargioli and Slack, 2004; Chen et al., 2006; Lin et al., 2007). For these reasons we can predict that each tissue is likely to have specific signaling requirements for the initiation and progression of regeneration.
Our results presented here have focused on two tissue types in the tadpole tail, the spinal cord and myotome; and on two signaling pathways, the Wnt-β-catenin and the Fgf pathways. We used two methods: grafts carrying a temperature inducible Dkk1 gene, and a conditional transgenic system enabling us to induce expression of ΔTcf or of Xfd by treatment with doxycycline. In principle the latter method is preferable because the inhibitors used are cell autonomous and so avoid ambiguities arising from diffusion of Dkk1 protein away from the graft. Also the transgenic method avoids any anatomical disturbance of the tail due to the prior surgery. However the former method also has its advantages because the heat shock promoter provides a robust high level expression and Dkk1 is a very effective inhibitor of the Wnt pathway.
In transgenic experiments it is important to be able to monitor events and to prove that the transgenes are present and expressed at the time and place, that is, expected. Variation may arise between individuals because the tadpoles used are primary transgenics in which each individual may carry the transgenes in different locations and at different copy numbers. To enable accurate monitoring we built two fluorescent labels into each pair of constructs. A gene for a red fluorescent protein, tdTomato, was driven by the tissue specific promoter, and was linked to the Tet activator through a 2A sequence. So observation of a red central nervous system, or myotomes, enabled us to know that this tadpole contains a functional transgene and that the specificity and level of expression of the Tet activator is suitable for the experiment. The two inhibitor transgenes, ΔTcf and Xfd, were prepared such that the product is a fusion protein with GFP. So following treatment with doxycycline it is possible to know that the inhibitory protein is being produced, in response to doxycycline induction, with the correct tissue specificity, and at a suitable level. The Tet-on system does have a significant disadvantage which is that the level of expression of the induced gene is often much lower than can be produced with a constitutive promoter such as CMV, or by the heat shock promoter used in our spinal cord grafts. So, although the Tet-on system is the more elegant, we feel that both methods are of value and both can contribute useful results.
The results showed that both tissue types have a common requirement for both signaling pathways in tadpole tail regeneration. In the Dkk1 inhibition experiments, 24/30 cases showed reduced or absent spinal cord, with a further nine cases showing complete inhibition of tail regeneration. The ΔTcf should also inhibit the effects of Wnt signaling but had a less severe inhibitory effect with only 4/14 cases showing reduced or absent spinal cord and two failing to regenerate. The reason for this difference is probably a combination of higher expression of Dkk1 compared to ΔTcf, and the greater intrinsic effectiveness of the Dkk1. There may also be additional effects arising from the diffusion of Dkk1 protein. In the Xfd experiments, 10/16 cases showed reduced or absent spinal cord, showing that receipt of FGF signaling by the spinal cord is also necessary for its regeneration.
The Tet-on experiments also showed that the regeneration of muscle depends on these two pathways. Although the Cardiac actin promoter is not active in muscle satellite cells, it will become active as the cells start to differentiate to form the new muscle. Because the doxycycline induction is maintained during the regeneration process, the differentiating muscle cells will be exposed to the inhibitory reagents as they form. Fourteen of twenty-two cases inhibited with ΔTcf showed reduced muscle, with a further 11 not regenerating at all. Twenty-nine of thirty-one cases inhibited with the Xfd showed reduction of muscle, with a further five not regenerating at all. Since the ΔTcf and the Xfd are cell autonomous components we can be confident that for both tissue types and pathways the requirement is for the receipt of the signal by the tissue concerned, rather than for its generation.
In the experiments we cannot be sure that pathways are 100% inhibited, and indeed we expect some variability because different individual transgenic tadpoles will contain different copy numbers of the insert and have different insertion sites. However, all of the corresponding control groups: the CFP-labeled grafts of the spinal cord, and GFP induction by Tet activator driven by either the N-tubulin or the Cardiac actin promoter, showed 100% regeneration. So we can be confident that none of the inhibitory effects are due to nonspecific factors to do with surgery, heat shock, transgenesis or doxycycline induction. In summary, this part of the work shows that receipt of both Wnt and FGF signals are required for regeneration both of the spinal cord and of the myotomal muscle.
Tissue Interactions During Xenopus Tadpole Tail Regeneration
Tissue interactions during the early embryonic development is critical for the morphogenesis of the Xenopus tail bud. Embryonic tail bud will not form in the first place unless the neural plate, posterior mesoderm and posterior notochord all meet at one point (Tucker and Slack, 1995a). This tissue interaction leads to the activation of BMP and Notch signaling pathways (Beck and Slack, 1999, 2002; Beck et al., 2001). Here in this work, our results suggest that tissue interactions are also required during tail regeneration.
Our results show that loss of the spinal cord does not totally prevent regeneration of the other parts of the tail, but it is associated with defects in muscle and notochord. This result is comparable to previous experiments in which regeneration was examined following surgical removal of the spinal cord (Taniguchi et al., 2008) or by localized ablation by laser (Mondia et al., 2011). These both showed that regeneration does proceed in the absence of the spinal cord, but that tails are shorter, with a wavy notochord, and the formation of the nonspinal tissues is defective. By contrast our experiments on muscle that result in reduction of muscle regeneration do not affect the spinal cord at all, and the tails generally have good morphology although they may be short. This indicates that some factor or factors from the spinal cord is necessary for normal regeneration of the rest of the tail, but there is no such requirement for a factor from the muscle. A requirement for neural tissues is well known in other regenerating systems, including amphibian limbs, the lizard tail and annelid worms (Singer, 1952; Carlson, 2007), so the fact that it exists also for the Xenopus tadpole tail is not surprising. Work on the neural requirement for amphibian limb regeneration have suggested both the neuregulin group of growth factors and a substance called AG (anterior gradient) as candidates (Brockes and Kintner, 1986; Kumar et al., 2007). Elucidation of the nature of the factor(s) in the Xenopus tadpole tail will be a task for future work.
ACKNOWLEDGEMENT
The authors thank Dr. R. Dorsky, Dr. D. Brown, and Dr. E. Jones for sharing regents with us. The research supported by NIH NIGMS was approved by the University of Minnesota Institutional Animal Care and Use Committee according to the NIH guidelines for the care of lab animals.
Grant sponsor: NIH NIGMS Eureka grant; Grant number: R01GM088500.
LITERATURE CITED
- Adams DS, Masi A, Levin M. H+ pump dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, Barker D, Slack JMW. Temporal requirement for bone morphogenetic proteins in regeneration of the tail and limb of Xenopus tadpoles. Mech Dev. 2006;123:674–688. doi: 10.1016/j.mod.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, Slack JMW. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003a;5:429–439. doi: 10.1016/s1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, Slack JMW. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003b;5:429–439. doi: 10.1016/s1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Beck CW, Izpisúa Belmonte JC, Christen B. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev Dyn. 2009;238:1226–1248. doi: 10.1002/dvdy.21890. [DOI] [PubMed] [Google Scholar]
- Beck CW, Slack JMW. Analysis of the developing Xenopus tail bud reveals separate phases of gene expression during determination and outgrowth. Mech Dev. 1998;72:41–52. doi: 10.1016/s0925-4773(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Beck CW, Slack JMW. A developmental pathway controlling outgrowth of the Xenopus tail bud. Development. 1999;126:1611–1620. doi: 10.1242/dev.126.8.1611. [DOI] [PubMed] [Google Scholar]
- Beck CW, Slack JMW. Notch is required for outgrowth of the Xenopus tail bud. Int J Dev Biol. 2002;46:255–258. doi: 10.1387/ijdb.011489. [DOI] [PubMed] [Google Scholar]
- Beck CW, Whitman M, Slack JMW. The role of BMP signaling in outgrowth and patterning of the Xenopus tail bud. Dev Biol. 2001;238:303–314. doi: 10.1006/dbio.2001.0407. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kintner CR. Glial growth factor and nerve dependent proliferation in the regeneration blastema of urodele amphibians. Cell. 1986;45:301–306. doi: 10.1016/0092-8674(86)90394-6. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Principles of regenerative biology. Burlington MA: Academic Press; 2007. [Google Scholar]
- Chen Y, Lin G, Slack JMW. Control of muscle regeneration in the Xenopus tadpole tail by Pax7. Development. 2006;133:2303–2313. doi: 10.1242/dev.02397. [DOI] [PubMed] [Google Scholar]
- Contreras EG, Gaete M, Sanchez N, Carrasco H, Larrain J. Early requirement of Hyaluronan for tail regeneration in Xenopus tadpoles. Development. 2009;136:2987–2996. doi: 10.1242/dev.035501. [DOI] [PubMed] [Google Scholar]
- Das B, Brown DD. Controlling transgene expression to study Xenopus laevis metamorphosis. Proc Natl Acad Sci USA. 2004;101:4839–4842. doi: 10.1073/pnas.0401011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Schreiber AM, Huang H, Brown DD. Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. Proc Natl Acad Sci USA. 2002;99:12230–12235. doi: 10.1073/pnas.182430599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe P, Hughes LE, Ryan MD, Brown JD. Co-translational, intraribosomal cleavage of polypeptides by the foot-and-mouth disease virus 2A peptide. J Biol Chem. 2003;278:11441–11448. doi: 10.1074/jbc.M211644200. [DOI] [PubMed] [Google Scholar]
- Dent JN. Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. J Morphol. 1962;110:61–78. doi: 10.1002/jmor.1051100105. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bosco L. Comparative analysis of the regenerative capacity of caudal spinal cord in larvae of several anuran amphibian species. Ann Embryol Morphol Exp. 1981;2:199–226. [PubMed] [Google Scholar]
- Franchini A, Bertolotti E. Tail regenerative capacity and iNOS immunolocalization in Xenopus laevis tadpoles. Cell Tissue Res. 2011;344:261–269. doi: 10.1007/s00441-011-1136-3. [DOI] [PubMed] [Google Scholar]
- Gargioli C, Slack JMW. Cell lineage tracing during Xenopus tail regeneration. Development. 2004;131:2669–2679. doi: 10.1242/dev.01155. [DOI] [PubMed] [Google Scholar]
- Gawantka V, Pollet N, Delius H, Vingron M, Pfister R, Nitsch R, Blumenstock C, Niehrs C. Gene expression screening in Xenopus identifies molecular pathways, predicts gene function and provides a global view of embryonic patterning. Mech Dev. 1998;77:95–141. doi: 10.1016/s0925-4773(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gont LK, Steinbeisser H, Blumberg B, de Robertis EM. Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development. 1993;119:991–1004. doi: 10.1242/dev.119.4.991. [DOI] [PubMed] [Google Scholar]
- Ho DM, Whitman M. TGF-beta signaling is required for multiple processes during Xenopus tail regeneration. Dev Biol. 2008;315:203–216. doi: 10.1016/j.ydbio.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahimi A, Vande Velde G, Reumers V, Toelen J, Thiry I, Vandeputte C, Vets S, Deroose C, Bormans G, Baekelandt V, Debyser Z, Gijsbers R. Highly efficient multicistronic lentiviral vectors with peptide 2A sequences. Hum Gene Ther. 2009;20:845–860. doi: 10.1089/hum.2008.188. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Rodriguez Esteban C, Raya M, Kawakami H, Marti M, Dubova I, Izpisua Belmonte JC. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 2006;20:3232–3237. doi: 10.1101/gad.1475106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly OG, Melton DA. Induction and patterning of the vertebrate nervous system. Trends Genet. 1995;11:273–278. doi: 10.1016/s0168-9525(00)89074-5. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772–777. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Chen Y, Slack JMW. Regeneration of neural crest derivatives in the Xenopus tadpole tail. BMC Dev Biol. 2007;7:56. doi: 10.1186/1471-213X-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Slack JMW. Requirement for Wnt and FGF signaling in Xenopus tadpole tail regeneration. Dev Biol. 2008;316:323–335. doi: 10.1016/j.ydbio.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Berry DL, Brown DD. Germline transmission of transgenes in Xenopus laevis. Proc Natl Acad Sci USA. 1999;96:14389–14393. doi: 10.1073/pnas.96.25.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochii M, Taniguchi Y, Shikata I. Tail regeneration in the Xenopus tadpole. Dev Growth Differ. 2007;49:155–161. doi: 10.1111/j.1440-169X.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- Mohun TJ, Garrett N, Gurdon JB. Upstream sequences required for tissue-specific activation of the cardiac actin gene in Xenopus laevis embryos. Embo J. 1986;5:3185–3193. doi: 10.1002/j.1460-2075.1986.tb04628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Mondia JP, Levin M, Omenetto FG, Orendorff RD, Branch MR, Adams DS. Long-distance signals are required for morphogenesis of the regenerating Xenopus tadpole tail, as shown by femtosecond-laser ablation. PLoS One. 2011;6:e24953. doi: 10.1371/journal.pone.0024953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North-Holland; 1967. [Google Scholar]
- Poss KD. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat Rev Genet. 2010;11:710–722. doi: 10.1038/nrg2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Shen J, Nechiporuk A, McMahon G, Thisse B, Thisse C, Keating MT. Roles for Fgf signaling during zebrafish fin regeneration. Dev Biol. 2000;222:347–358. doi: 10.1006/dbio.2000.9722. [DOI] [PubMed] [Google Scholar]
- Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476:409–413. doi: 10.1038/nature10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. The influence of the nerve in the regeneration of the amphibian extremity. Q Rev Biol. 1952;27:169–200. doi: 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis: A laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Slack JMW, Lin G, Chen Y. The Xenopus tadpole: a new model for regeneration research. Cell Mol Life Sci. 2008;65:54–63. doi: 10.1007/s00018-007-7431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Tazaki A, Ueno N, Watanabe K, Mochii M. Xenopus Wnt-5a induces an ectopic larval tail at injured site, suggesting a crucial role for noncanonical Wnt signal in tail regeneration. Mech Dev. 2009;126:56–67. doi: 10.1016/j.mod.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Sugiura T, Tazaki A, Watanabe K, Mochii M. Spinal cord is required for proper regeneration of the tail in Xenopus tadpoles. Dev Growth Differ. 2008;50:109–120. doi: 10.1111/j.1440-169X.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- Trichas G, Begbie J, Srinivas S. Use of the viral 2A peptide for bicistronic expression in transgenic mice. BMC Biol. 2008;6:40. doi: 10.1186/1741-7007-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Adams DS, Qiu DY, Koustublian P, Levin M. Apoptosis is required during early stages of tail regeneration in Xenopus laevis. Dev Biol. 2007;301:62–69. doi: 10.1016/j.ydbio.2006.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S, Johnson SL. Fate restriction in the growing and regenerating zebrafish fin. Dev Cell. 2011;20:725–732. doi: 10.1016/j.devcel.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AS, Slack JMW. Tail bud determination in the vertebrate embryo. Curr Biol. 1995a;5:807–813. doi: 10.1016/s0960-9822(95)00158-8. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Slack JMW. The Xenopus laevis tail-forming region. Development. 1995b;121:249–262. [Google Scholar]
- Tucker AS, Slack JMW. Independent induction and formation of the dorsal and ventral fins in Xenopus laevis. Dev Dyn. 2004;230:461–467. doi: 10.1002/dvdy.20071. [DOI] [PubMed] [Google Scholar]
- Woodland HR, Jones EA. Mesoderm induction in the future tail region of Xenopus. Roux Arch Dev Biol. 1988;197:441–446. doi: 10.1007/BF00398996. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Ogino H, Stoick-Cooper CL, Grainger RM, Moon RT. Wnt/beta-catenin signaling has an essential role in the initiation of limb regeneration. Dev Biol. 2007;306:170–178. doi: 10.1016/j.ydbio.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]