Abstract
Pancreatic ductal adenocarcinoma (PDA) is a common and lethal malignancy, resulting in over 250,000 deaths per year world-wide. Despite extensive efforts, cytotoxic and targeted therapies have provided only limited efficacy for PDA patients to date. One contributing factor to the failure of systemic therapies may be the abundant tumor stromal content that is characteristic of PDA. The PDA stroma, aptly termed the tumor microenvironment (TME), occupies the majority of the tumor mass and consists of a dynamic assortment of extracellular matrix components and non-neoplastic cells including fibroblastic, vascular and immune cells. Recent work has revealed that the PDA stroma supports tumor growth and promotes metastasis, and simultaneously serves as a physical barrier to drug delivery. Accordingly, methods that alter stromal composition or function, for instance interference with the vasculature via Notch/Hedgehog pathway inhibition or relief of vascular compression by hyaluronidase, are under active investigation. Here we will review our current understanding of the PDA tumor microenvironment, and highlight opportunities for further exploration that may benefit patients.
Keywords: pancreatic cancer, GEMM, desmoplasia, vasculature, immunotherapy
Tumor microenvironment – Achilles heel of pancreatic cancer?
Pancreatic ductal adenocarcinoma (PDA) is an aggressive malignant disease of the exocrine pancreas with a 5-year survival rate of less than 5% (1). In the United States, it represents the fourth-leading cause of cancer-related deaths, with an estimated 43,920 new cases and 37,390 deaths in 2012 (2). The majority of patients initially present with advanced and metastatic disease, with only 10-15% of patients being candidates for surgical resection. Unfortunately, post-surgically most patients still relapse despite adjuvant systemic therapies (3). This dismal prognosis is a result of the late diagnosis of the disease, the lack of biomarkers allowing early screening, the early metastatic dissemination and ultimately the resistance to systemic therapies.
Recent years have seen significant advances in the treatment for many tumor types, including melanoma, lung and colorectal cancer based on the rational design of targeted therapies directed at molecular alterations arising in cancer cells (4). Unfortunately, similar success has not occurred in PDA, which remains a lethal disease. Gemcitabine, the current standard-of-care chemotherapeutic was approved mainly on the basis of patient benefit and produced only a modest increase in survival (5). Even targeted therapy approaches have had limited success so far. Indeed, the only other drug approved is the EGFR tyrosine kinase inhibitor Erlotinib (Tarceva), that when combined with gemcitabine increased overall the survival from 5.91 to 6.24 months (6). A promising classical combination chemotherapy approach recently reported is FOLFIRINOX (oxaliplatin, irinotecan, leucovorin and 5-fluorouracil), which achieved a significant survival benefit for patients with metastatic PDA compared to gemcitabine (11.1 versus 6.8 months) (7). Unfortunately, FOLFIRINOX is only suitable for patients with good performance status due to increased toxicity. Therefore, new approaches are sorely needed for the vast majority of PDA patients. What are the reasons that most conventional and targeted therapies fail to provide substantial response rates in pancreatic cancer? The challenges faced by oncologists in the treatment of pancreatic cancer may in part be explained by the diverse influences exerted by the microenvironment on cancer cells. Intriguingly, there is a huge discrepancy between the relative success and effectiveness of therapies, including gemcitabine reported in preclinical assays (cell culture, xenograft mouse models) and subsequent failure in human PDA (8). Revealing the underlying molecular mechanisms of the microenvironment-tumor cell cross-talk is challenging due to the heterogeneous nature of the PDA stroma. Importantly, the generation of genetically engineered mouse models (GEMMs) for pancreatic cancer that faithfully recapitulate the human disease, including resistance to gemcitabine, has enabled new approaches to understand the importance of the TME in disease pathogenesis and therapeutic response (9-11). These GEMMs are founded on early genetic analyses that discovered the presence of a single point mutation in the KRAS oncogene in over 90% of human PDA specimens (12). Subsequent genetic manipulation of the orthologous gene in mice demonstrated that this mutation was sufficient to initiate the formation of premalignant ductal transformation (pancreatic intraepithelial neoplasia, PanIN). Further studies showed that the loss or mutation of tumor suppressor genes commonly acquired during human disease progression (Tp53 and Ink4a/Arf) cooperate with Kras in mice to promote invasive cancer (13-15). More insight into the underlying genetic alterations in pancreatic cancer is provided in the review by Iacobuzio-Donahue et al. in this issue (16).
Pancreatic ductal adenocarcinoma is one of the most stroma-rich cancers. It is not uncommon for stromal components to outnumber cancer cells as illustrated in Fig.1. PDA stroma is very heterogeneous and comprised of cellular and acellular components, such as fibroblasts, myofibroblasts, pancreatic stellate cells, immune cells, blood vessels, extracellular matrix and soluble proteins such as cytokines and growth factors. The TME is not a static entity but is constantly changing in composition especially in the progression from preneoplastic PanIN to invasive PDA. We aim to outline the current evidence for TME influences on multiple aspects of PDA. These include proliferation and survival, metastasis, resistance to therapy and escape from immune control. We have limited our analysis to studies carried out in the most relevant GEMMs or orthotopic tumor models as well as clinical data because xenograft/allograft models have a fundamentally different composition and functionality of the microenvironment including dramatic differences in ECM components, immune cells and tumor vasculature. This fact must be considered in the design of future research and interpretation of published data.
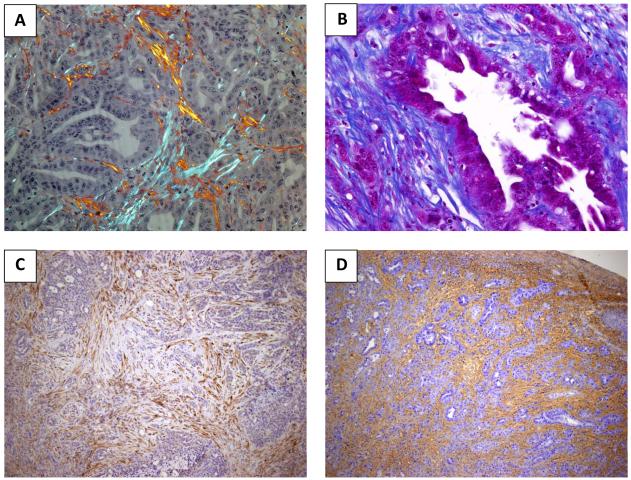
Fig.1.
PDA contains an abundant stroma. A Mouse PDA tissue was stained with Picrosirius Red and imaged under polarized light to visualize collagen fibres (20x magnification). B Masson’s Trichrome staining highlights connective tissue distribution in an example of mouse PDA. Collagen fibres are staining in blue with the cytoplasm appearing in red (40x magnification). C Immunohistochemistry for SPARC on murine PDA (α-SPARC polyclonal antibody, Protein tech catalogue number: 15274-1-AP, 10x magnification). D Histochemical staining of hyaluronan (biotinylated hyaluronan binding protein, Calbiochem 385911, 10x magnification) reveals pan-stromal deposition in murine PDA.
Stromal fibroblasts - the pancreatic stellate cell
Pancreatic stellate cells (PaSCs) were identified in 1998 as a rare stromal cell type in the healthy pancreas (17, 18). Their peri-acinar star shaped morphology, characteristic marker protein expression and storage of fat droplets rich in vitamin A resembled hepatic stellate cells and inspired the name. Under homeostatic conditions PaSCs are quiescent and their physiological role has yet to be delineated. Acute and chronic inflammatory conditions cause activation of PaSCs which is characterized by morphological changes, increased proliferation, deposition of extracellular matrix and expression of alpha-smooth muscle actin (α-SMA) as well as the loss of the fat droplets (19). Based on the observation that activated PaSC are detected in areas with high collagen content, it was postulated that PaSC may be causally involved in the pathogenesis of pancreatic fibrosis (20). While the embryonic origin of PaSCs has not yet been addressed mesenchymal cells in the bone marrow are a likely source for PaSC in adult mice after injury (pancreatitis, partial pancreatectomy) and in DMBA-initiated sarcomatoid pancreatic tumors (21-23).
The scarcity of PaSCs and their limited life span in culture has prompted the generation of immortalized PaSC lines from human, rat and mouse pancreata (24-30) (Table 1). Such immortalized PaSCs have enabled the dissection of important cross-talk pathways between PaSCs and neoplastic PDA cells by co-culturing in monolayers or three-dimensional models. Indeed, it was recently reported that all-trans-retinoic acid (ATRA) induced the quiescence of PaSCs and this led to decreased proliferation and survival of pancreatic cancer cell in three-dimensional co-culture and in a GEMM (31). Therefore, PaSCs represent a resource that may be harnessed to explore the tumor-promoting aspects of tumor fibroblasts in PDA.
Table 1.
Overview of published pancreatic stellate cell lines of either human, mouse or rat origin.
| Name of cell line |
Source | Method of immortalization |
Reference |
|---|---|---|---|
| RLT-PaSC | Primary human from chronic pancreatitis |
SV40 large T antigen + hTERT |
26 |
| 25HPaSC | Human PDA | SV40 large T antigen + hTERT |
25 |
| PS-1 | Human pancreas (transplantation tissue) |
hTERT | 24 |
| irPaSC | Sprague-Dawley rat |
SV40 large T antigen | 28 |
| SAM-K | Male Wistar rat | SV40 large T antigen | 29 |
| SIPS | Male Wistar rat | Spontaneous immortalization |
27 |
| LTC-7 and LTC-14 |
Male LEW.1W | SV40 large T antigen | 30 |
| imPaSC | C57BL/6 mouse | SV40 large T antigen | 28 |
Co-cultures of PaSCs and PDA cells have generally demonstrated an enhancement of pancreatic cancer cell proliferation and migration by release of growth factors and cytokines (32). In vivo studies corroborate those findings, revealing that the co-injection of pancreatic stellate cells with tumor cells in orthotopic models of PDA increases tumor size and causes a higher incidence of metastasis (25, 33). A subsequent study from the Apte group investigated the role of PaSCs in the metastatic process and found that they orchestrate metastatic dissemination by co-migrating with neoplastic cells to potentially establish the appropriate metastatic niche or “soil” (34). Two recent publications may provide an additional explanation for the enhanced tumorigenicity of tumor cells/PaSC co-transplants. In vitro experiments demonstrated that PaSC increase the stem cell phenotype of pancreatic cancer cells and suggest that pharmacological targeting of PaSC could have unrecognized additional benefits (35, 36). The identification of major signalling pathways activated in PaSCs in response to contact with cancer cells will be an interesting platform on which to develop therapies targeting PaSCs. For instance, the MAP kinase pathway plays a prominent role in the response to mitogenic stimuli of which PDGF seems most potent (37, 38). Other potential targets include stimulators of the fibrinogenic program such as FGF, downstream effectors generated by transforming growth factor β (TGFβ), connective tissue growth factor (CTGF) and epidermal growth factor (EGF). Additional insights may be gleaned by investigating pathways known to be relevant for hepatic stellate cell activation (39). Finally, targeting specific pathways germane for PaSC-neoplastic cell cross-talk may also modulate other aspects of the TME including the vascular and immune system.
Extracellular matrix as an obstacle to therapy
Pancreatic ductal adenocarcinoma is histologically characterized by the abundance of of extracellular matrix (ECM), commonly also referred to as desmoplasia (Fig. 1A-D). Extracellular matrix components include collagen, fibronectin, proteoglycans and hyaluronic acid, as well as catalytically active enzymes and proteinases. The accumulation of ECM components distorts the normal architecture of pancreatic tissue inducing an abnormal configuration of blood and lymphatic vessels (40-42). One factor potentially contributing to therapeutic resistance in PDA may be the rigidity of the ECM that compresses blood vessels, leading to reduced perfusion that ultimately impedes the delivery of drugs to neoplastic cells. Indeed, we previously reported that the concentration of active intracellular metabolite of gemcitabine, 2′,2-difluorodeoxycytidine triphosphate (dFdCTP), was high in stroma-poor subcutaneous or orthotopic xenografts/syngeneics but hardly detectable in stroma-rich PDA tumors in a GEMM (11). Further analysis revealed that transplanted tumors exhibited an increased vascular content and function as compared to primary GEMM tumors and human PDA. Since sonic hedgehog (SHH) signaling has been shown to be restricted to the stromal compartment and enhance the desmoplastic reaction (43, 44) our laboratory reasoned that pharmacological inhibition of the SHH pathway may positively impact on gemcitabine delivery. As predicted, the combination of a Smoothened inhibitor (IPI-926) with gemcitabine caused a depletion of tumor stroma and resulted in increased mean vessel density and patency (11). This alteration of the TME was paralleled by significantly enhanced intratumoral concentrations of dFdCTP, transient disease stabilization and a survival benefit (11). Several clinical trials have been initiated as a result of this and are recruiting patients to investigate the mechanism and treatment effect of pharmacological SHH-inhibitors in pancreatic cancer patients (http://clinicaltrials.gov; NCT01195415, NCT01064622, NCT01130142, NCT01096732). Unfortunately, Infinity Pharmaceuticals announced in January 2012 that it was halting its phase II trial of the Smoothened inhibitor IPI-926 plus gemcitabine (NCT01130142). This is very surprising in light of the encouraging results of 31% partial response rate (10% for gemcitabine only) reported in a previous phase Ib trial (ASCO 2011, abstract #4114). An analysis of this trial is underway by the investigators conducting this trial.
Secreted protein acidic and rich in cysteine (SPARC) represents another proposed target to facilitate depletion of the tumor stroma in pancreatic cancer. SPARC is overexpressed by fibroblasts in the TME of human and murine PDA (Figure 1C) and has been shown to inversely correlate with survival (45, 46). A novel drug formulation consisting of paclitaxel associated with albumin (Abraxane or nab-paclitaxel) has been hypothesized to accumulate in and potentially deplete PDA tumor stroma via binding of albumin to SPARC-positive fibroblasts thus representing a mechanism for targeting a specific cell type within the PDA tumor microenvironment (47). The first clinical trial of gemcitabine in combination with nab-paclitaxel showed a promising overall survival of 12.2 months, and the subset of patients with elevated SPARC expression correlated with increased survival in this study. The potential role of SPARC as a predictive biomarker for positive responsive to nab-paclitaxel and gemcitabine contrasts with a separate report that showed a poor prognosis for PDAC patients who had SPARC enriched tumors resected and received standard adjuvant therapy (45, 46). In this trial patients with high SPARC levels had a mean overall survival of 17.8 months as compared to 8.1 for low SPARC (48). A preclinical study in a GEMM from our laboratory confirmed the remarkable efficacy of nab-paclitaxel in combination with gemcitabine, although in contrast to data originating from patient derived xenografts (PDXs), we did not observe stromal depletion in our preclinical setting. Instead, we reported a mechanism involving impaired gemcitabine metabolism due to reactive oxygen species (ROS)-mediated degradation of cytidine deaminase (49). Further in depth investigations are required to elucidate the exact role of SPARC as a novel biomarker for PDA patients, in particular whether treatment with nab-paclitaxel represents the sole determinant for its prognostic impact.
Another possible strategy to relieve vessel compression and aid drug delivery is to enzymatically break down the ECM scaffold. Many cancers are rich in hyaluronan (HA), a megadalton glycosaminoglycan that retains water due to its high colloid osmotic pressure (Figure 1D) (50). This provides elasticity to connective tissue in healthy organs but excessive HA accumulation in solid tumors raises interstitial fluid pressure and compresses blood vessels. We and others have recently shown in a GEMM of PDA that enzymatic remodelling of the ECM is indeed a promising avenue. Hyaluronan degradation by hyaluronidase PEGPH20 decreased interstitial fluid pressure in KPC tumors (51), as shown previously in a prostate cancer xenograft model (52). Consequently increased vessel patency, drug delivery and survival were observed (51, 53). It will be interesting to see if this concept holds true in human pancreatic cancer when the results of an ongoing Phase I/II trial of hyaluronidase (PEGPH20) plus gemcitabine will be released (NCT01453153).
The conundrum of hypovascularity in pancreatic cancer
As alluded to in the previous paragraph, the vasculature in PDA is profoundly affected by the excessive desmoplasia. As a consequence, vascular dysfunction represents a major obstacle to pharmacodelivery. Moreover, how cancer cells maintain their nutrient demands to fuel the rapid growth despite the lack of adequate perfusion is elaborated upon in the accompanying review by Le et al. in this issue (54). The discovery of the hypovascularity and perfusion impairment has broken with the general assumption of an “angiogenic switch” required for tumor progression (55, 56). Unlike pancreatic neuroendocrine tumors, which are clearly dependent on angiogenic factors their exocrine counterparts seem to thrive without the requirement for excessive angiogenesis (11, 57). In fact, hypovascularity and perfusion impairment have long served as diagnostic tools in the imaging of pancreatic masses (58, 59), but mechanisms behind these histopathological features have not been fully elucidated.
Transcriptional analysis has previously demonstrated a gradient of angiogenic activation from normal pancreas to PDA (60). Consistent with this finding, components of the prototypically angiogenic VEGF pathway are highly expressed in tumor cells and associated endothelia (61, 62). Despite this, tumor samples show substantially lower microvessel densities (MVD) than that of normal pancreas (Figure 2) (11, 57). While VEGF immunostaining is positively correlated with MVD, it has limited association with patient survival (63, 64). Despite these contradictory findings anti-angiogenic therapy was tested in PDA. Initial approaches targeted matrix metalloproteinases using Marimastat and BAY 12-9566, as well as the integrins αVβ3 and αVβ5 using Cilengitide. These compounds did not provide any clinical benefit in trials (65-67). More recently targeted agents such as Bevacizumab, an anti-VEGFA monoclonal antibody, have been investigated in advanced pancreatic cancer in combination with gemcitabine and did not improve survival compared to gemcitabine plus placebo in a randomized phase III trial (5.8 vs 5.9 months) (68). In addition, there was no significant benefit to overall survival in combining Bevacizumab with erlotinib and gemcitabine compared to the combination of the latter two compounds (7.1 vs 6.0 months) (69). Bevacizumab has also been evaluated in other combinations including with docetaxel and with concurrent capecitabine and radiation without any proven benefit, although certain studies are still underway (70, 71). VEGF receptor inhibition using Axitinib in combination with gemcitabine also had no beneficial effect on overall survival (72). In addition, the kinase inhibitor Sorafenib, which targets VEGFR as well as PDGFR, c-KIT, Raf1 and FLT3 was found to be inactive in advanced pancreatic cancer (73). Likewise, discouraging results testing sunitinib in a preclinical trial in the LSL-KrasG12D;p53R172H/+;Ptf1a-Cre model add to mounting evidence of PDA’s angiogenic independence and dominance of tumor-driven angiostasis (57). This phenotype suggests that endogenous inhibitors in the microenvironment might exert an overriding angiostatic effect during PDA’s natural history. Many of these factors are generated from plasma and extracellular matrix proteins by proteases, which are frequently upregulated in tumor and stellate cells (PaSCs) (74). For example, angiostatin and endostatin are produced by PDA and detected at high concentrations in patients’ circulation (75, 76). Moreover, while activated PaSCs are ostensibly proangiogenic, their co-culture with tumor cells robustly increases endostatin levels, demonstrating the angiostatic potential of such heterotypic interactions (77).
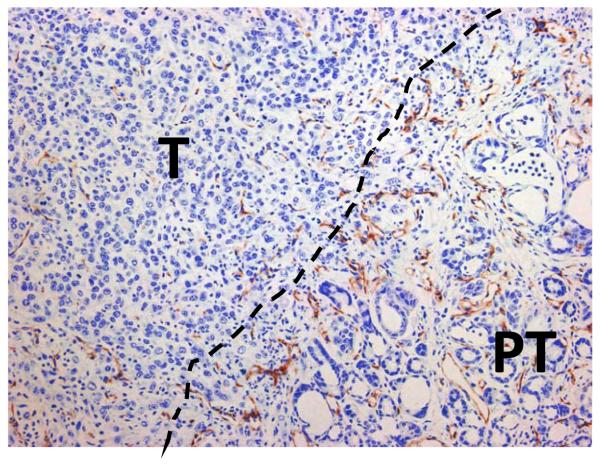
Fig. 2.
Murine PDA is characterized by hypovasularity. In this example of CD31 immunohistochemistry the dotted line denotes the boundary between tumor (T) and peritumoral diseased pancreas (PT) (20x magnification).
On the whole anti-angiogenic therapies have proven not to be viable option for pancreatic cancer, and while therapeutic delivery may contribute to these failures, alternative approaches targeting the PDA vasculature remain attractive and potentially feasible. Two scenarios are possible: If PDA maintains itself on frugal use of restricted resources as a consequence of limited perfusion it may be conceivable that impairing perfusion even more could tip the balance towards widespread hypoxic necrosis (78). In contrast, increasing tumor perfusion may seem counterintuitive but could synergise with cytotoxic therapy to increase the intratumoral drug delivery and response.
Is PDA hypoxic?
The previous paragraph laid the groundwork for this question. Most solid tumors contain areas of below optimal oxygen concentration (hypoxia). This occurs as a result of inefficient tumor vascular supply and a high metabolic need for oxygen (79). Many studies have provided evidence that hypoxic cells are more resistant to both chemotherapy and radiotherapy, can increase their invasive and metastatic potential, ultimately creating a more aggressive disease (80, 81). The ability of cancer cells to survive under these hypoxic conditions results from ability to co-opt pathways necessary for embryonic development under hypoxic conditions. The main pathway involved in the hypoxic response is the hypoxia inducible factor (HIF) pathway (82). HIF can induce a wide range of gene products controlling cellular metabolism and energetics, cell survival, migration and pH (83). The HIF transcription factors also direct the transcription of many angiogenic growth factors (84).
Considering that the hypovascular nature of PDA has a significant impact on perfusion and drug delivery (11) it would be reasonable to assume a hypoxic state. However, direct evidence is sparse and the majority of publications have used surrogate markers for measuring hypoxia, such as necrosis or expression of HIF target genes (85-87). Only one small study involving 7 patients with pancreatic cancer has directly measured the oxygen pressure during panreaticoduodenectomy by inserting needle electrodes. It found a dramatic reduction in oxygenation of tumor tissue versus normal pancreas (88). Interestingly, pre-clinical work in an orthotopic model of pancreatic cancer has shown a lack of correlation between microvessel density and hypoxia, perhaps suggesting that a hypovascular pancreatic tumor is not directly linked to hypoxia (89). Nevertheless, this work did predict more aggressive behaviour, including a more metastatic phenotype in hypoxic tumors. Clinical work is underway to assess the prognostic significance of these results in pancreatic cancer patients, using the hypoxia probe, pimonidazole, administered 24 hours prior to surgery (NCT01248637).
The reason hypoxia poses a challenge to the field of anticancer therapeutics is that it provides a niche for slow-cycling, highly drug-resistant cells, which may be identical to the proposed cancer stem cells (see review by Penchev et al. in this issue, (90, 91). Thus standard chemotherapy agents fail because they are unsuccessful at targeting the cell within the hypoxic TME, which might be those that most need to be eliminated (92). Additionally, hypoxic conditions are also known to stimulate the Notch signaling pathway and it has recently been shown that pancreatic cancer cells can be sensitised by Notch inhibition (78). Thus, the hypovascular state of PDA could be exploited by novel therapeutic approaches such as hypoxia activatable prodrugs (93). Further investigations are required into the downstream Notch targets that are important for tumor cell survival under hypoxic conditions. Currently, a clinical trial is investigating the benefit of combining Notch pathway inhibition and gemcitabine (NCT01232829, NCT01098344).
Out of balance - immune cells in PDA
Similar to other cancer types, inflammation seems to be crucially linked to PDA development as exemplified by chronic pancreatitis being a major risk factor (94). However, the molecular details are still obscure and are just beginning to be elucidated (95, 96). A comprehensive analysis of the immune cell composition of PanIN and PDA in the LSL-KrasG12D;Pdx1-Cre mice defined an important baseline for future studies (97). Using enzymatic tumor digestion followed by FACS analysis it revealed that immune cells make up roughly 50% of the tumor cell mass (Figure 3 illustrates similar findings by immunofluorescence). From this study it is apparent that immunosuppressive cell types such as regulatory T cells and myeloid derived suppressor cells (MDSCs) are predominant with hardly any cytotoxic T lymphocytes (CTLs) infiltrating the tumors. This paints a picture of a striking imbalance in pro-tumorigenic and anti-tumorigenic immune cells. To add to the complexity, a recent study revealed a new immunosuppressive cell type in the stroma of PDA and other cancers. This cell expresses fibroblast activation protein α (FAPα), and FAPα cell ablation resulted in immunological control of tumor growth in several subcutaneous tumor models (98).
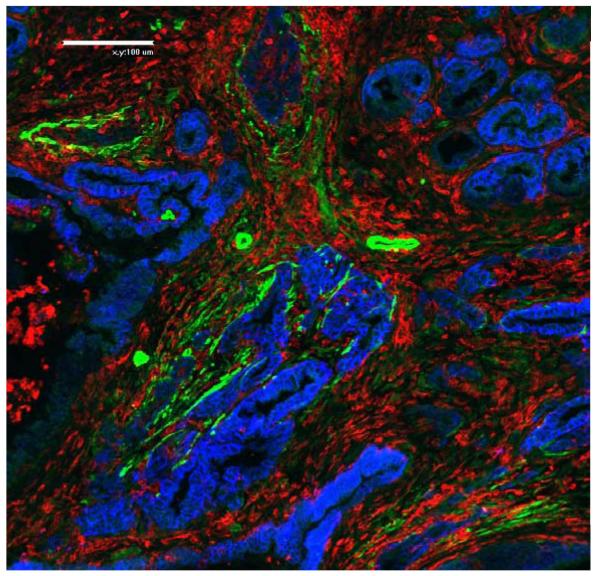
Fig.3.
Prominent immune cell infiltration exists in mouse PDA. This immunofluorescence staining illustrates the abundance of immune cells marked by CD45 expression (red) between neoplastic glandular structures (stained for EpCam in blue) and α-SMA positive stromal fibroblasts and perivascular cells that likely represent pericytes (green) (20x magnification).
Successful immunotherapy depends on the cancer cells expressing proteins that can be recognized as altered by the immune system. These fall in two categories: tumor associated antigens (TAA) are non-mutated self proteins that are aberrantly regulated (overexpressed or expressed in other tissues or oncofetal antigens), while tumor specific antigens (TSA) are generated as a consequence of the mutational events in neoplastic cells and are de novo antigens. The goal is to induce high-affinity cytotoxic T cells (CTL or CD8 T cells) without causing autoimmunity. Antigens targeted in immunotherapy clinical trials in PDA have included Muc1, mesothelin, Kras, carcinoembryonic antigen (CEA), survivin and telomerase as well as whole tumor cells engineered to express GM-CSF (reviewed in (99)).
The first phase I trial using irradiated allogeneic granulocyte-macrophage colony-stimulating factor (GM-CSF) expressing tumor cell vaccines was well tolerated and found to be safe for use in humans (100). This warranted a larger phase II trial to investigate the disease free and overall survival after surgical resection followed by chemoradiation and vaccination, which reported a median survival of 24.8 months (101). Another approach is to pulse dendritic cells with tumor antigens ex vivo and re-infuse them into patients. Muc1-pulsed dendritic cells were evaluated in a phase I/II trial in patients with resected pancreatic and biliary tumors. The vaccine transiently increased the percentages of functional CD4 and CD8 T-cells as well as regulatory T-cells. 4/12 (33%) of patients in this study were alive five years post-surgery with a median survival time of 26 months (range of 13-69 months) (102). Both of these studies compare favourably in terms of the median survival for resected pancreatic cancer, which is normally between 11-20 months. A third approach is the use of blocking/neutralizing antibodies such as Ipilimumab, which targets CTLA-4, a surface protein expressed by activated T cells that confers inhibitory signals. Unfortunately, Ipilimumab as a single agent was found to be ineffective in a phase II trial in locally advanced and metastatic pancreatic cancer. However, one of the patients on this study experienced significant delayed regression of the primary tumor and 20 hepatic metastases, which may merit further investigation (103).
GEMMs are an ideal system to evaluate immune therapeutic approaches in PDA, however few reports exist to date on this topic. This may be because the tumor antigens for PDA are unknown making the tracing of immune responses very difficult. Nonetheless, a recent immunotherapy study employed the “KPC” GEMM (LSL-KrasG12D;LSL-p53R172H/+;Pdx1-Cre) to evaluate whether activation of antigen presenting cells via stimulation of CD40 would result in increased tumor antigen presentation and priming of effector T cells (104). Treatment with agonistic anti-CD40 achieved tumor stabilization and even regression in KPC mice but was surprisingly T cell independent. Instead, tumor control was exerted by the activated macrophages targeting the fibrotic stroma. Furthermore, an early phase clinical trial with anti-CD40 antibody showed promising results in patients (104).
Conclusions and future outlook
The influences of the stroma in pancreatic cancer are as manifold as its components (Figure 4). But this curse may be turned into a blessing as this complexity also provides numerous avenues for therapeutic exploration (clinical trials mentioned in this review are summarized in Table 2). Accumulating evidence suggests that the extensive desmoplastic reaction may be at least partly responsible for the innate chemoresistance in pancreatic tumors by creating barriers that fence off tumor cells from circulating active therapeutic compounds. Breaching this stromal barrier represents a promising strategy to improve the delivery and efficacy of cytotoxic drugs in the future. Therapeutic benefit may be gained by strategies aimed at depleting the desmoplastic stroma, exploiting the poor vasculature or activating the immune system to target tumor cells. We anticipate that future therapies will have to be tailored to target several of the described components of the microenvironment to achieve long lasting therapeutic response.
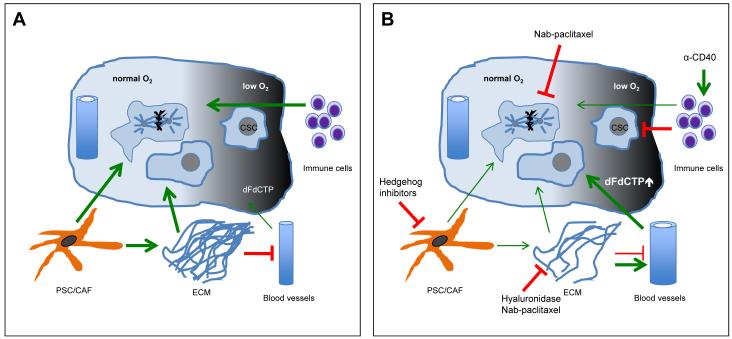
Fig.4.
Schematic of the TME network, crosstalk and interdependence in PDA with a focus on therapeutic intervention points. A Activated pancreatic stellate cells lay down vast amounts of ECM, which causes a constriction/collapse of the sparse vessel network. This impedes on gemcitabine delivery. Hypoxia generates niches for slow-cycling cells that are not targeted by chemotherapeutics. Also, an immunosuppressive microenvironment further supports tumor growth. B Hedgehog pathway inhibition causes stromal depletion accompanied by reduced ECM. The ECM can also be enzymatically targeted and both interventions lead to increased vessel patency and intra-tumoral gemcitabine delivery. The immune system can be stimulated to turn against cancer cells for instance by anti-CD40 antibody treatment.
Table 2.
Summary of past and current clinical trials targeting components of the TME
| Intervention | Trial Status | Outcome | Reference | ||
|---|---|---|---|---|---|
|
| |||||
| Current Phase |
Trial Identifier |
Status | Median Survival |
||
| Erlotinib + Gemcitabine | III | NCT00026338 | Completed | 6.24 mo vs 5.91 mo |
6 |
| Oxaliplatin + Irinotecan + Leucovorin + 5-FU | II/III | NCT00112658 | Completed | 11.1 mo vs 6.8 mo |
7 |
| GDC-0449 + Gemcitabine | II | NCT01064622 | Active | ||
| IPI-926 + Gemcitabine | I/II | NCT01130142 | Stopped | N/A | |
| nab-Paclitaxel + Gemcitabine | I/II | NCT00398086 | Completed | 12.2 mo | 48 |
| PEGPH20 + Gemcitabine | I/II | NCT01453153 | Active | ||
| Marimastat + Gemcitabine | III | N/A | Completed | 5.44 mo vs 5.39 mo |
65 |
| Cilengitide + Gemcitabine | II | N/A | Completed | 6.7 mo vs 7.7 mo |
66 |
| BAY 12-9566 | III | N/A | Completed | 3.7 mo vs 6.6 mo |
67 |
| Bevacizumab + Gemcitabine | III | NCT00088894 | Completed | 5.8 mo vs 5.9 mo |
68 |
| Bevacizumab + Erlotinib + Gemcitabine | III | N/A | Completed | 7.1 mo vs 6.0 mo |
69 |
| Bevacizumab + Docetaxel Radiotherapy + Capecitabine + Bevacizumab, Bevacizumab + Gemcitabine |
II | N/A | Completed | 4.1 mo vs 5.4 mo |
70 |
| II | NCT00114179 | Completed | 11.9 | 71 | |
| Axitinib + Gemcitabine | III | NCT00471146 | Completed | 8.5 mo vs 8.3 mo |
72 |
| Sorafenib + Gemcitabine | II | N/A | Completed | 4.0 mo | 73 |
| RO4929097 | II | NCT01232829 | Active | ||
| MK0752 + Gemcitabine | I/II | NCT01098344 | Active | ||
| Irradiated Allogeneic GM-CSF-Secreting Tumour Vaccine | II | NCT00084383 | Completed | 24.8 mo | 101 |
| MUC1 peptide-loaded Dendritic Cell Vaccine | I/II | N/A | Completed | 26 mo | 102 |
| Ipilimumab | II | NCT00112580 | Completed | N/A | 103 |
| CP-870,893 + Gemcitabine | I | NCT00711191 | Completed | 7.4 mo | 104 |
|
| |||||
| Mechanistic Studies | |||||
|
| |||||
| GDC-0449 + Gemcitabine | 0 | NCT01195415 | Active | ||
| GDC-0449 | II | NCT01096732 | Active | ||
| Preoperative Pimonidazole | N/A | NCT01248637 | Active | ||
Acknowledgments
Financial support: This research was supported by the University of Cambridge and Cancer Research UK, The Li Ka Shing Foundation and Hutchison Whampoa Limited, the NIHR Cambridge Biomedical Research Centre and the European Community Grant EPC-TM-Net 256974. CF was supported by the EMBO long term fellowship and by a Marie Curie Intra European Fellowship within the 7th European Community Framework Programme. AN was supported by Deutsche Krebshilfe Mildred Scheel Postdoctoral Fellowship. CD is funded by the Frank Edward Elmore Fund. DAT is a Senior Group Leader at the Cancer Research UK Cambridge Research Institute.
Footnotes
Conflict of interest: The authors declare no conflict of interest
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010 Apr 29;362(17):1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP. Adjuvant treatment of pancreatic cancer. Eur J Cancer. 2011 Sep;47(Suppl 3):S378–80. doi: 10.1016/S0959-8049(11)70210-6. [DOI] [PubMed] [Google Scholar]
- 4.Yauch RL, Settleman J. Recent advances in pathway-targeted cancer drug therapies emerging from cancer genome analysis. Curr Opin Genet Dev. 2012 Feb 7; doi: 10.1016/j.gde.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997 Jun;15(6):2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 6.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007 May 20;25(15):1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011 May 12;364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001 May 18;84(10):1424–31. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol. 2010 Jun;28(6):585–93. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- 10.Gopinathan A, Tuveson DA. The use of GEM models for experimental cancer therapeutics. Dis Model Mech. 2008 Sep-Oct;1(2-3):83–6. doi: 10.1242/dmm.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY. 2009 Jun 12;324(5933):1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–54. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 13.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer cell. 2003 Dec;4(6):437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 14.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005 May;7(5):469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes & development. 2003 Dec 15;17(24):3112–26. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iacobuzio-Donahue CA, Velculescu VE, Wolfgang CL, Hruban RH. The genetic basis of pancreas cancer development and progression: insights from whole-exome and whole-genome sequencing. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-12-0315. xx-xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998 Jul;43(1):128–33. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998 Aug;115(2):421–32. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- 19.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. The Journal of clinical investigation. 2007 Jan;117(1):50–9. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. The American journal of pathology. 1999 Oct;155(4):1087–95. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han F, Wang CY, Yang L, Zhan SD, Zhang M, Tian K. Contribution of Murine Bone Marrow Mesenchymal Stem Cells to Pancreas Regeneration after Partial Pancreatectomy in Mice. Cell Biol Int. 2012 May 11; doi: 10.1042/CBI20110680. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe T, Masamune A, Kikuta K, Hirota M, Kume K, Satoh K, et al. Bone marrow contributes to the population of pancreatic stellate cells in mice. Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297(6):G1138–46. doi: 10.1152/ajpgi.00123.2009. [DOI] [PubMed] [Google Scholar]
- 23.Scarlett CJ, Colvin EK, Pinese M, Chang DK, Morey AL, Musgrove EA, et al. Recruitment and activation of pancreatic stellate cells from the bone marrow in pancreatic cancer: a model of tumor-host interaction. PLoS ONE. 2011;6(10):e26088. doi: 10.1371/journal.pone.0026088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Froeling FE, Mirza TA, Feakins RM, Seedhar A, Elia G, Hart IR, et al. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. The American journal of pathology. 2009 Aug;175(2):636–48. doi: 10.2353/ajpath.2009.090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer research. 2008 Feb 1;68(3):918–26. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jesnowski R, Furst D, Ringel J, Chen Y, Schrodel A, Kleeff J, et al. Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: deactivation is induced by matrigel and N-acetylcysteine. Lab Invest. 2005 Oct;85(10):1276–91. doi: 10.1038/labinvest.3700329. [DOI] [PubMed] [Google Scholar]
- 27.Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Establishment and characterization of a rat pancreatic stellate cell line by spontaneous immortalization. World J Gastroenterol. 2003 Dec;9(12):2751–8. doi: 10.3748/wjg.v9.i12.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathison A, Liebl A, Bharucha J, Mukhopadhyay D, Lomberk G, Shah V, et al. Pancreatic stellate cell models for transcriptional studies of desmoplasia-associated genes. Pancreatology. 2010;10(4):505–16. doi: 10.1159/000320540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh M, Masamune A, Sakai Y, Kikuta K, Hamada H, Shimosegawa T. Establishment and characterization of a simian virus 40-immortalized rat pancreatic stellate cell line. Tohoku J Exp Med. 2002 Sep;198(1):55–69. doi: 10.1620/tjem.198.55. [DOI] [PubMed] [Google Scholar]
- 30.Sparmann G, Hohenadl C, Tornoe J, Jaster R, Fitzner B, Koczan D, et al. Generation and characterization of immortalized rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004 Jul;287(1):G211–9. doi: 10.1152/ajpgi.00347.2003. [DOI] [PubMed] [Google Scholar]
- 31.Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology. 2011 Oct;141(4):1486–97. e1–14. doi: 10.1053/j.gastro.2011.06.047. 97. [DOI] [PubMed] [Google Scholar]
- 32.Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012 Feb;61(2):172–8. doi: 10.1136/gutjnl-2011-301220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer research. 2008 Apr 1;68(7):2085–93. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 34.Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. The American journal of pathology. 2010 Nov;177(5):2585–96. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012 Apr 1;11(7):1282–90. doi: 10.4161/cc.19679. [DOI] [PubMed] [Google Scholar]
- 36.Hamada S, Masamune A, Takikawa T, Suzuki N, Kikuta K, Hirota M, et al. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochemical and biophysical research communications. 2012 May 4;421(2):349–54. doi: 10.1016/j.bbrc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Schneider E, Schmid-Kotsas A, Zhao J, Weidenbach H, Schmid RM, Menke A, et al. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol. 2001 Aug;281(2):C532–43. doi: 10.1152/ajpcell.2001.281.2.C532. [DOI] [PubMed] [Google Scholar]
- 38.Jaster R, Sparmann G, Emmrich J, Liebe S. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells. Gut. 2002 Oct;51(4):579–84. doi: 10.1136/gut.51.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008 Apr;26(4):431–42. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- 40.Wehr AY, Furth EE, Sangar V, Blair IA, Yu KH. Analysis of the human pancreatic stellate cell secreted proteome. Pancreas. 2011 May;40(4):557–66. doi: 10.1097/MPA.0b013e318214efaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007 Apr;6(4):1186–97. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 42.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011 Jun;60(6):861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 43.Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009 Mar 17;106(11):4254–9. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008 Oct 1;14(19):5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantoni TS, Schendel RR, Rodel F, Niedobitek G, Al-Assar O, Masamune A, et al. Stromal SPARC expression and patient survival after chemoradiation for non-resectable pancreatic adenocarcinoma. Cancer Biol Ther. 2008 Nov;7(11):1806–15. doi: 10.4161/cbt.7.11.6846. [DOI] [PubMed] [Google Scholar]
- 46.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007 Jan 20;25(3):319–25. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 47.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006 Feb 15;12(4):1317–24. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 48.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine Plus nab-Paclitaxel Is an Active Regimen in Patients With Advanced Pancreatic Cancer: A Phase I/II Trial. J Clin Oncol. 2011 Dec 1;29(34):4548–54. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discovery. 2012;(2):260–9. doi: 10.1158/2159-8290.CD-11-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI. Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol. 2008 Aug;18(4):288–95. doi: 10.1016/j.semcancer.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer cell. 2012 Mar 20;21(3):418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson CB, Shepard HM, O’Connor PM, Kadhim S, Jiang P, Osgood RJ, et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther. 2010 Nov;9(11):3052–64. doi: 10.1158/1535-7163.MCT-10-0470. [DOI] [PubMed] [Google Scholar]
- 53.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2012 Mar 30; doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le A, Rajeshkumar NV, Maitra A, Dang CV. Conceptual framework for cutting the pancreatic cancer fuel supply. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-12-0041. xx-xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 56.Xie L, Duncan MB, Pahler J, Sugimoto H, Martino M, Lively J, et al. Counterbalancing angiogenic regulatory factors control the rate of cancer progression and survival in a stage-specific manner. Proceedings of the National Academy of Sciences of the United States of America. 2011 Jun 14;108(24):9939–44. doi: 10.1073/pnas.1105041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olson P, Chu GC, Perry SR, Nolan-Stevaux O, Hanahan D. Imaging guided trials of the angiogenesis inhibitor sunitinib in mouse models predict efficacy in pancreatic neuroendocrine but not ductal carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2011 Dec 6;108(49):E1275–84. doi: 10.1073/pnas.1111079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freeny PC, Traverso LW, Ryan JA. Diagnosis and staging of pancreatic adenocarcinoma with dynamic computed tomography. Am J Surg. 1993 May;165(5):600–6. doi: 10.1016/s0002-9610(05)80443-x. [DOI] [PubMed] [Google Scholar]
- 59.Sofuni A, Iijima H, Moriyasu F, Nakayama D, Shimizu M, Nakamura K, et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. Journal of gastroenterology. 2005 May;40(5):518–25. doi: 10.1007/s00535-005-1578-z. [DOI] [PubMed] [Google Scholar]
- 60.Abdollahi A, Schwager C, Kleeff J, Esposito I, Domhan S, Peschke P, et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jul 31;104(31):12890–5. doi: 10.1073/pnas.0705505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Itakura J, Ishiwata T, Shen B, Kornmann M, Korc M. Concomitant over-expression of vascular endothelial growth factor and its receptors in pancreatic cancer. Int J Cancer. 2000 Jan 1;85(1):27–34. doi: 10.1002/(sici)1097-0215(20000101)85:1<27::aid-ijc5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 62.Dallas NA, Gray MJ, Xia L, Fan F, van Buren G, 2nd, Gaur P, et al. Neuropilin-2-mediated tumor growth and angiogenesis in pancreatic adenocarcinoma. Clin Cancer Res. 2008 Dec 15;14(24):8052–60. doi: 10.1158/1078-0432.CCR-08-1520. [DOI] [PubMed] [Google Scholar]
- 63.Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto Y, Buchler MW, et al. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997 Aug;3(8):1309–16. [PubMed] [Google Scholar]
- 64.Ellis LM, Takahashi Y, Fenoglio CJ, Cleary KR, Bucana CD, Evans DB. Vessel counts and vascular endothelial growth factor expression in pancreatic adenocarcinoma. Eur J Cancer. 1998 Feb;34(3):337–40. doi: 10.1016/s0959-8049(97)10068-5. [DOI] [PubMed] [Google Scholar]
- 65.Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002 Jul 15;87(2):161–7. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friess H, Langrehr JM, Oettle H, Raedle J, Niedergethmann M, Dittrich C, et al. A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer. 2006;6:285. doi: 10.1186/1471-2407-6-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003 Sep 1;21(17):3296–302. doi: 10.1200/JCO.2003.02.098. [DOI] [PubMed] [Google Scholar]
- 68.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. Aug 1;28(22):3617–22. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009 May 1;27(13):2231–7. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 70.Astsaturov IA, Meropol NJ, Alpaugh RK, Burtness BA, Cheng JD, McLaughlin S, et al. Phase II and coagulation cascade biomarker study of bevacizumab with or without docetaxel in patients with previously treated metastatic pancreatic adenocarcinoma. Am J Clin Oncol. 2011 Feb;34(1):70–5. doi: 10.1097/COC.0b013e3181d2734a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crane CH, Winter K, Regine WF, Safran H, Rich TA, Curran W, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009 Sep 1;27(25):4096–102. doi: 10.1200/JCO.2009.21.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kindler HL, Ioka T, Richel DJ, Bennouna J, Letourneau R, Okusaka T, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011 Mar;12(3):256–62. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 73.Kindler HL, Wroblewski K, Wallace JA, Hall MJ, Locker G, Nattam S, et al. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs. 2012 Feb;30(1):382–6. doi: 10.1007/s10637-010-9526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamamoto H, Itoh F, Iku S, Adachi Y, Fukushima H, Sasaki S, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J Clin Oncol. 2001 Feb 15;19(4):1118–27. doi: 10.1200/JCO.2001.19.4.1118. [DOI] [PubMed] [Google Scholar]
- 75.Kisker O, Onizuka S, Banyard J, Komiyama T, Becker CM, Achilles EG, et al. Generation of multiple angiogenesis inhibitors by human pancreatic cancer. Cancer research. 2001 Oct 1;61(19):7298–304. [PubMed] [Google Scholar]
- 76.Ohlund D, Ardnor B, Oman M, Naredi P, Sund M. Expression pattern and circulating levels of endostatin in patients with pancreas cancer. Int J Cancer. 2008 Jun 15;122(12):2805–10. doi: 10.1002/ijc.23468. [DOI] [PubMed] [Google Scholar]
- 77.Erkan M, Reiser-Erkan C, Michalski CW, Deucker S, Sauliunaite D, Streit S, et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009 May;11(5):497–508. doi: 10.1593/neo.81618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cook N, Frese KK, Bapiro TE, Jacobetz MA, Gopinathan A, Miller JL, et al. Gamma secretase inhibition promotes hypoxic necrosis in mouse pancreatic ductal adenocarcinoma. The Journal of experimental medicine. 2012 Mar 12;209(3):437–44. doi: 10.1084/jem.20111923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004 Jun;4(6):437–47. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 80.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998 Apr 1;58(7):1408–16. [PubMed] [Google Scholar]
- 81.Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004 Aug-Dec;23(3-4):293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- 82.Brahimi-Horn MC, Pouyssegur J. HIF at a glance. J Cell Sci. 2009 Apr 15;122(Pt 8):1055–7. doi: 10.1242/jcs.035022. [DOI] [PubMed] [Google Scholar]
- 83.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006 May 25;441(7092):437–43. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 84.Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res. 2010 Dec 15;16(24):5928–35. doi: 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shibaji T, Nagao M, Ikeda N, Kanehiro H, Hisanaga M, Ko S, et al. Prognostic significance of HIF-1 alpha overexpression in human pancreatic cancer. Anticancer research. 2003 Nov-Dec;23(6C):4721–7. [Research Support, Non-U.S. Gov’t] [PubMed] [Google Scholar]
- 86.Kitada T, Seki S, Sakaguchi H, Sawada T, Hirakawa K, Wakasa K. Clinicopathological significance of hypoxia-inducible factor-1alpha expression in human pancreatic carcinoma. Histopathology. 2003 Dec;43(6):550–5. doi: 10.1111/j.1365-2559.2003.01733.x. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- 87.Hiraoka N, Ino Y, Sekine S, Tsuda H, Shimada K, Kosuge T, et al. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. British journal of cancer. 2010 Sep 28;103(7):1057–65. doi: 10.1038/sj.bjc.6605854. [Evaluation Studies Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000 Nov 1;48(4):919–22. doi: 10.1016/s0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 89.Chang Q, Jurisica I, Do T, Hedley DW. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer research. 2011 Apr 15;71(8):3110–20. doi: 10.1158/0008-5472.CAN-10-4049. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 90.Penchev VR, Rasheed ZA, Maitra A, Matsui W. Heterogeneity and targeting of pancreatic cancer stem cells. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-11-3112. xx-xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007 May 4;129(3):465–72. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milas L, Hittelman WN. Cancer stem cells and tumor response to therapy: current problems and future prospects. Semin Radiat Oncol. 2009 Apr;19(2):96–105. doi: 10.1016/j.semradonc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Denny WA. Hypoxia-activated prodrugs in cancer therapy: progress to the clinic. Future Oncol. 2010 Mar;6(3):419–28. doi: 10.2217/fon.10.1. [DOI] [PubMed] [Google Scholar]
- 94.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993 May 20;328(20):1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 95.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer cell. 2011 Apr 12;19(4):456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 96.Fukuda A, Wang SC, Morris JPt, Folias AE, Liou A, Kim GE, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer cell. 2011 Apr 12;19(4):441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer research. 2007 Oct 1;67(19):9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 98.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. Nov 5;330(6005):827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 99.Dodson LF, Hawkins WG, Goedegebuure P. Potential targets for pancreatic cancer immunotherapeutics. Immunotherapy. 2011 Apr;3(4):517–37. doi: 10.2217/imt.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001 Jan 1;19(1):145–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 101.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011 Feb;253(2):328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6(B):955–64. [PMC free article] [PubMed] [Google Scholar]
- 103.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010 Oct;33(8):828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science (New York, NY. 2011 Mar 25;331(6024):1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]